Abstract
Background
Increasingly studies reported that the Anti-Müllerian hormone (AMH) seems to be a promising and reliable marker of functional ovarian follicle reserve, even better than the AFC test. Our study aimed to conduct a meta-analysis to assess the predictive value of AMH and AFC for predicting poor or high response in IVF treatment. An electronic search was conducted, and the following databases were used: PubMed, EMBASE, and the Cochrane Library (up to 7 May 2022). The bivariate regression model was used to calculate the pooled sensitivity, specificity, and area under the receiver operator characteristic (ROC) curve. Subgroup analyses and meta-regression also were used in the presented study. Overall performance was assessed by estimating pooled ROC curves between AMH and AFC.
Results
Forty-two studies were eligible for this meta-analysis. Comparison of the summary estimates for the prediction of poor or high response showed significant difference in performance for AMH compared with AFC [poor (sensitivity: 0.80 vs 0.74, P < 0.050; specificity: 0.81 vs 0.85, P < 0.001); high (sensitivity: 0.81 vs 0.87, P < 0.001)]. However, there were no significant differences between the ROC curves of AMH and AFC for predicting high (P = 0.835) or poor response (P = 0.567). The cut-off value was a significant source of heterogeneity in the present study.
Conclusions
The present meta-analysis demonstrated that both AMH and AFC have a good predictive ability to the prediction of poor or high responses in IVF treatment.
Similar content being viewed by others
Background
Controlled ovarian stimulation (COS) is the key to successful assisted reproductive technology (ART). Individualization of COS in in vitro fertilization (IVF) treatments should be based on assessing ovarian reserve and predicting ovarian response for every patient [1]. The starting point is to identify if a patient is likely to have a normal, poor, or high response, and choose the best treatment protocol tailored to this prediction [1]. Patients’ characteristics and biomarkers could accurately predict ovarian response [2]. However, although numerous biochemical measures have been developed to predict IVF outcomes, some biochemical measures, such as estradiol (E2), luteinizing hormone (LH), basal follicle-stimulation hormone (FSH), and inhibin concentrations, fluctuate greatly on the day of the menstrual cycle and do not significantly change with decreasing of ovarian reserve, thus they have limited use owing to a low predictive value [3, 4]. Studies have shown that antral follicle count (AFC) is a better indicator to predict ovarian response than other endocrine markers [5, 6].
AMH, a dimeric glycoprotein, is a member of the extended transforming growth factor-β (TGF-β) family [7, 8]. AMH production diminishes as the follicles become FSH-dependent [8, 9]. Serum levels are not affected during the menstrual cycle, are most probably not manipulated by exogenous steroid administration, and are closely correlated with reproductive age [10]. Therefore, AMH has been used to predict poor and high response in IVF. Several studies argued that the level of AMH is a better predictor of ovarian response than the AFC [11]. However, the data remains conflicting and inconsistent [10]. Furthermore, some studies continue to advocate both AFC and AMH as possible predictors of ovarian response [12]. Although Broer and his colleagues [13, 14] have performed meta-analyses in 2009 and 2011 and demonstrated that AMH has at least the same level of accuracy and clinical value for the prediction of poor or excessive response as AFC, the number of the included studies in their meta-analysis were small (N = 5–12). Therefore, our study aimed to conduct a meta-analysis that included more eligible studies, to assess the diagnostic value of AMH and AFC for predicting poor or high response in IVF treatment.
Methods
The present meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [15].
Search strategy and data sources
The data sources include these electronic databases: PubMed, EMBASE, and the Cochrane Library (up to 1 May 2022). The following keywords were used: in vitro fertilization (IVF), in vitro fertilization, fertilization in vitro, assisted, or intracytoplasmic in combination with Anti-Mullerian Hormone (AMH), Mullerian-Inhibiting Factor, Mullerian-Inhibitory Substance, Mullerian Inhibiting Hormone, or Antral Follicle Count (AFC). There was no language limitation, and we also retrieved articles by manual screening. A complete search strategy for literature search has provided in Supplementary material.
Inclusion and exclusion criteria
The inclusion criteria were based on the Population, Intervention, Comparator, Outcomes, and Study designs (PICOS) structure: P): adult infertile women; I) patients receiving COS for IVF/ICSI; C) AMH or AFC to predict ovarian reserve; O) ovarian response including poor or high response; S) prospective design. Besides, if 2 × 2 tables were constructed from the data presented in the paper, the study was included for final analysis in this meta-analysis. Reviews, conference abstracts, case reports, letters, and animal trials were excluded from this study.
Data extraction
Information was extracted from eligible studies by two authors independently. The following information was included: the authors of the articles, publication year, study location, definition of poor or high response, sample size, true positives (TP), false positives (FP), false negatives (FN), true negatives (TN), and cut-off value. Disagreements were resolved by discussion among all authors.
Study quality assessment
Our study adopted the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) [16] to assess the quality of the included articles, which was the most recommended quality assessment tool for diagnostic accuracy tests. It consists of four main components: patient selection, index test, reference standard, and flow and timing. All components will be assessed for risk of bias, and the first 3 components will also be assessed for clinical applicability. The risk of bias is judged by signature questions, but there are no signature questions for clinical applicability. The “yes”, “no” or “uncertain” answers to the signature questions included in each component may correspond to a bias risk rating of “low”, “high” or “uncertain”. If the answer to all the signature questions in a range is “yes”, then the risk of bias can be assessed as low; If the answer to one of the questions is “no”, the risk of bias is judged to be “high”. The “uncertain” refers to the fact that the literature does not provide detailed information that makes it difficult for the evaluator to make a judgment, and can only be used when the reported data is insufficient.
Statistical analysis
This meta-analysis used Stata V.14.0 (Stata Corp LP) to conduct all statistical analyses. The Cochrane Q and I2 statistics were used to test the heterogeneity among all studies. I2 > 50% indicates the existence of heterogeneity. The bivariate regression model was used to calculate the pooled sensitivity, specificity, and area under the receiver operator characteristic (ROC) curve, and their 95% confidence intervals (CIs). Overall performance was assessed by estimating a pooled ROC curve between AMH and AFC. Furthermore, meta-regression was used to explore the causes of heterogeneity between the studies. Subgroup analyses were performed based on the cut-off value and sample size. Deeks’ funnel plot was used to test publication bias. A two-tailed probability value below 0.05 was regarded as statistically significant.
Results
Study selection and study characteristics
In sum, 7327 articles were identified in electronic and manual searches. However, 1847 articles were excluded for duplication, and another 2698 articles were excluded due to study types (reviews, meeting abstracts, letters, animal trials, and case reports). In addition, 2680 records were excluded after reviewing the title and abstract, and we excluded 60 records after reviewing the full text of 102 articles. Finally, 42 articles [10, 11, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] were included in this meta-analysis (Fig. 1).
The characteristics of the eligible studies are listed in Tables 1 and 2. The sample sizes of participants in each study ranged from 44 to 571, and this meta-analysis included 7190 individuals. Of the 42 studies, all studies were prospective design. The publication year of 42 studies ranged from 2002 to 2021. The included studies were from different countries, including China (n = 3), Spain (n = 4), the UK (n = 7), the USA (n = 4), and so on. AMH was used in 29 studies, and AFC in 15 studies in terms of poor response. As for the high response, AMH was used in 13 studies, and AFC in 6 studies.
Study quality
We adopted the QUADAS-2 to assess the quality of concerning studies (Supplementary material). Regarding risk of bias, 5 studies included consecutive patients, and 37 studies were low risk in index test. Besides, as for applicability of concern, all studies were low risk in both patient selection and index test.
Accuracy of AMH and AFC for predicting poor response
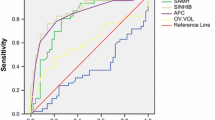
The pooled predictive ability of AMH and AFC for poor response in IVF/ICSI treatments was presented in Table 3. The overall pooled sensitivity and specificity of AMH were 0.80 (95%CI: 0.74–0.85) and 0.81 (95%CI: 0.75–0.85), respectively. The test for heterogeneity demonstrated that there was significant heterogeneity in both sensitivity and specificity (I2 = 68.26% and 92.43%, respectively). The overall ROC curve was presented in Fig. 2A, and AUC was 0.87 (95%CI: 0.84–0.90). The meta-analysis’s overall pooled sensitivity and specificity of AFC were 0.73 (95%CI: 0.62–0.83) and 0.85 (95%CI: 0.78–0.90), respectively. Heterogeneity was found in both sensitivity and specificity (I2 = 85.28% and 91.76%, respectively). The overall ROC curve was presented in Fig. 2B, and AUC was 0.87 (95%CI: 0.84–0.90).
Accuracy of AMH and AFC for predicting high response
Table 3 presented the pooled predictive ability of AMH and AFC for high response in IVF/ICSI treatments. The meta-analysis’s overall pooled sensitivity and specificity of AMH were 0.81 (95%CI: 0.76–0.86) and 0.84 (95%CI: 0.77–0.90), respectively. Heterogeneity was found in both sensitivity and specificity (I2 = 83.00% and95.90%, respectively). The overall ROC curve was presented in Fig. 2C, and AUC was 0.89 (95%CI: 0.86–0.91). The overall pooled sensitivity and specificity of AFC were 0.85 (95%CI: 0.77–0.91) and 0.83 (95%CI: 0.64–0.94), respectively. The test for heterogeneity demonstrated that there was significant heterogeneity in both sensitivity and specificity (I2 = 74.53% and 96.70%, respectively). The overall ROC curve was presented in Fig. 2D, and AUC was 0.90 (95%CI: 0.87–0.92).
Subgroup analysis
Comparison of the summary estimates for the prediction of poor or high response showed significant difference in performance for AMH compared with AFC [poor (sensitivity: 0.80 vs 0.74, P < 0.050; specificity: 0.81 vs 0.85, P < 0.001); high (sensitivity: 0.81 vs 0.87, P < 0.001)]. There were no significant differences between the AUC of AMH and AFC for predicting high (P = 0.835) or poor response (P = 0.567). Besides, in the same definition of poor response (< 4 oocytes), AMH and AFC tests had significant differences in sensitivity (0.78 vs 0.81, P < 0.001) and specificities (0.77 vs 0.80, P < 0.001) (Table 3). However, no significant differences were found between the AUC of AMH and AFC (P = 0.800).
Meta-regression analysis
For AMH, the cut-off value was a significant source of heterogeneity (poor: P = 0.020). For AFC, the cut-off value was a significant source of heterogeneity (poor: P < 0.010; high: P < 0.050). However, sample size was not the significant source of heterogeneity (P > 0.05).
Publication bias
Deek’s plot indicated that there was no publication bias in AMH for predicting poor response (P = 0.510, Fig. 3A) and high response AFC (P = 0.348, Fig. 3C), and AFC for predicting poor (P = 0.396, Fig. 3B) and high response (P = 0.818, Fig. 3D).
Discussion
Main findings
The present meta-analysis summarizes the available evidence about the accuracy of AMH and the AFC for predicting poor or high response to ovarian stimulation in IVF treatments. Although the differences were significant, both AMH and AFC had similar sensitivities and specificities. It seems that both AMH and AFC have a good discriminatory capacity to predict poor or high response in IVF. Besides, the ROC curves did not indicate a better predictive ability for AMH than for AFC, and the difference was not statistically significant. Our results were consistent with previous studies [13, 14, 48, 58]. For example, Broer et al. [13, 14] in their meta-analysis thought that both AMH and AFC are accurate predictors of poor or high response to ovarian hyperstimulation, and both tests appear to have clinical value.
Prior research indicated AFC is better than AMH to predict poor ovarian response [10]. However, several studies argued that the level of AMH is a better predictor of ovarian response than the AFC [11, 43]. In our study, results presented that a comparison of the summary estimates for the prediction of poor or high response showed a significant difference in performance for AMH compared with AFC while there was no significant difference in ROC curves. The discrepancies between studies could be associated with the heterogeneity of the definitions of ovarian response to ovarian stimulation. Therefore, our study conducted a subgroup analysis based on the definition of poor response, and we found that AFC was relatively better than AMH tests in both sensitivity (0.81 vs 0.78, P < 0.001) and specificities (0.80 vs 0.77, P < 0.001) when the poor response was defined as < 4 oocytes. However, although no significant differences were found in ROC curves, AFC seemed to perform slightly better than AMH for predicting poor response (0.87 vs 0.84). Also, Broer et al. [13] had similar findings in AFC and AMH for the prediction of high response.
Our study found that the accuracy of AMH and AFC for the prediction of poor or high response had many different kinds of cut-off values, which is difficult for clinical practice. Therefore, the present study performed a subgroup analysis based on the range of cut-off values. The accuracy threshold value of AFC for predicting high response achieved the highest AUC when the cut-off value was ≥ 15. The corresponding AUC was 0.90 (95%CI: 0.88, 0.93) with a sensitivity of 0.89 and a specificity of 0.94, which indicates the predictive ability with this interval is higher than the range of cut-off value < 15.
The characteristics of patients could predict abnormal ovarian response, including age, menstrual cycle length, and body mass index. However, these factors have limited predictive value. Therefore, emerging studies reported that the multivariate models predicted ovarian response, and found the model could improve the predictive power [17, 59,60,61]. For example, Honnma et al. [60] thought that serum AMH in combination with age is a better indicator than AMH alone. Therefore, clinicians should consider patients’ characteristics and biomarkers together to accurately predict ovarian response in IVF treatments.
Clinical implications
The abnormal response may increase patient discomfort and even decrease the chance of pregnancy. According to the register of the Italian national assisted reproduction technique (ART) in 2010, it reported that 6.7% were canceled due to poor ovarian response, and 1.5% due to ovarian hyperstimulation syndrome (OHSS) in 52,676 IVF cycles [1]. In other words, more than 4300 cycles were canceled every year for an abnormal response to stimulation with gonadotrophins. Furthermore, approximately 35% of couples abandon IVF treatments for physical and psychological burden, and 10% for inadequate ovarian response in the first cycle [62]. Therefore, it is important to reduce the dropout rate in IVF treatments by reducing abnormal responses. Our study found that both AMH and AFC were a good discriminatory capacity to predict poor or high response in IVF. Besides, increasingly studies reported that AMH level is becoming a preferred method for the prediction of ovarian reserve in most women [7, 63]. A multivariable approach, combining patient characteristics and AMH also should be taken into account in the evaluation of ovarian response.
Limitations
Several limitations would be noted in this meta-analysis. First, relatively high heterogeneity still existed. Although we found that the cut-off value was a significant source of heterogeneity in the present study, heterogeneity was caused by other factors, such as study quality characteristics, and study populations among all included studies. In addition, we found that the quality of the included studies was poor, so more high-quality studies are needed to confirm our conclusions in the future. Second, language bias may exist due to the inclusion of only English articles in the meta-analysis. Third, the predictive value of AMH and AFC for ovarian response was not always assessed in a head-to-head comparison in the same study. The accuracy of the results will be affected to some extent due to the differences in cut-off value and sample size. For this issue, we have tried to enhance the persuasiveness of the paper through meta regression and subgroup analysis.
Conclusions
In sum, the present meta-analysis demonstrated that both AMH and AFC have a good predictive ability to predict poor or high responses in IVF treatment.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AMH:
-
Anti-Müllerian hormone
- ROC:
-
Receiver operator characteristic
- COS:
-
Controlled ovarian stimulation
- IVF:
-
In vitro fertilization
- LH:
-
Luteinizing hormone
- FSH:
-
Basal follicle-stimulation hormone
- AFC:
-
Antral follicle count
- TP:
-
True positives
- FP:
-
False positives
- FN:
-
False negatives
- TN:
-
True negatives
- DOR:
-
Diagnostic odds ratio
- CIs:
-
Confidence intervals
- OHSS:
-
Ovarian hyperstimulation syndrome
References
La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update. 2014;20:124–40.
Huang J, Lin J, Gao H, Wang Y, Zhu X, Lu X, et al. Anti-müllerian hormone for the prediction of ovarian response in progestin-primed ovarian stimulation protocol for IVF. Front Endocrinol (Lausanne). 2019;10:325.
Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy C, Englert Y. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837–40.
Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20:370–85.
Nahum R, Shifren JL, Chang Y, Leykin L, Isaacson K, Toth TL. Antral follicle assessment as a tool for predicting outcome in IVF–is it a better predictor than age and FSH? J Assist Reprod Genet. 2001;18:151–5.
Bancsi LF, Broekmans FJ, Eijkemans MJ, de Jong FH, Habbema JD, te Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328–36.
Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20:688–701.
Bedenk J, Vrtačnik-Bokal E, Virant-Klun I. The role of anti-Müllerian hormone (AMH) in ovarian disease and infertility. J Assist Reprod Genet. 2020;37:89–100.
Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83.
Mutlu MF, Erdem M, Erdem A, Yildiz S, Mutlu I, Arisoy O, et al. Antral follicle count determines poor ovarian response better than anti-Müllerian hormone but age is the only predictor for live birth in in vitro fertilization cycles. J Assist Reprod Genet. 2013;30:657–65.
Vembu R, Reddy NS. Serum AMH level to predict the hyper response in women with PCOS and non-PCOS undergoing controlled ovarian stimulation in ART. J Hum Reprod Sci. 2017;10:91–4.
Ocal P, Sahmay S, Cetin M, Irez T, Guralp O, Cepni I. Serum anti-Müllerian hormone and antral follicle count as predictive markers of OHSS in ART cycles. J Assist Reprod Genet. 2011;28:1197–203.
Broer SL, Dólleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJ. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. 2011;17:46–54.
Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91:705–14.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36.
Palhares MB, Martins WP, Romão GS, Ferriani RA, Navarro PA. Combining age, antral follicle count, anti-Müllerian hormone, and follicle-stimulating hormone is more accurate than individual markers in predicting poor ovarian response. J Reprod Med. 2018;63(5):461–6.
Tolikas A, Tsakos E, Gerou S, Prapas Y, Loufopoulos A. Anti-Mullerian hormone (AMH) levels in serum and follicular fluid as predictors of ovarian response in stimulated (IVF and ICSI) cycles. Hum Fertil. 2011;14:246–53.
Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Prediction of high ovarian response to controlled ovarian hyperstimulation: anti-Müllerian hormone versus small antral follicle count (2–6 mm). J Assist Reprod Genet. 2009;26:319–25.
Ashrafi M, Hemat M, Arabipoor A, Salman Yazdi R, Bahman-Abadi A, Cheraghi R. Predictive values of anti-müllerian hormone, antral follicle count and ovarian response prediction index (ORPI) for assisted reproductive technology outcomes. J Obstet Gynaecol. 2017;37(1):82–8.
Baker VL, Glassner MJ, Doody K, Schnell VL, Gracia C, Shin SS, et al. Validation study of the Access antimüllerian hormone assay for the prediction of poor ovarian response to controlled ovarian stimulation. Fertil Steril. 2021;116:575–82.
Baker VL, Gracia C, Glassner MJ, Schnell VL, Doody K, Coddington CC, et al. Multicenter evaluation of the Access AMH antimüllerian hormone assay for the prediction of antral follicle count and poor ovarian response to controlled ovarian stimulation. Fertil Steril. 2018;110:506-13.e3.
Bancsi LFJMM, Broekmans FJM, Looman CWN, Habbema JDF, Te Velde ER. Impact of repeated antral follicle counts on the prediction of poor ovarian response in women undergoing in vitro fertilization. Fertil Steril. 2004;81:35–41.
Eldar-Geva T, Ben-Chetrit A, Spitz IM, Rabinowitz R, Markowitz E, Mimoni T, et al. Dynamic assays of inhibin B, anti-Mullerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Hum Reprod. 2005;20:3178–83.
Fabregues F, Gonzalez-Foruria I, Peñarrubia J, Carmona F. Ovarian response is associated with anogenital distance in patients undergoing controlled ovarian stimulation for IVF. Hum Reprod. 2018;33:1696–704.
Fiçicioglu C, Kutlu T, Baglam E, Bakacak Z. Early follicular antimüllerian hormone as an indicator of ovarian reserve. Fertil Steril. 2006;85:592–6.
Fouda F, Rezk AY, Razik MA, El-Shair O, Salim A. Anti-mullerian hormone level is a reliable predictor for cycle cancellation in ICSI. Middle East Fertil Soc J. 2010;15:194–9.
Frattarelli JL, Levi AJ, Miller BT, Segars JH. A prospective assessment of the predictive value of basal antral follicles in in vitro fertilization cycles. Fertil Steril. 2003;80:350–5.
Gnoth C, Schuring AN, Friol K, Tigges J, Mallmann P, Godehardt E. Relevance of anti-Mullerian hormone measurement in a routine IVF program. Hum Reprod. 2008;23:1359–65.
Heidar Z, Bakhtiyari M, Mirzamoradi M, Zadehmodarres S, Sarfjoo FS, Mansournia MA. Prediction of different ovarian responses using anti-Müllerian hormone following a long agonist treatment protocol for IVF. J Endocrinol Invest. 2015;38:1007–15.
Islam Y, Aboulghar MM, Alebrashy AED, Abdel-Aziz O. The value of different ovarian reserve tests in the prediction of ovarian response in patients with unexplained infertility. Middle East Fertil Soc J. 2016;21(2):69–74.
Izhar R, Husain S, Tahir MA, Kausar M, Sana T, Ghalib F. Antral follicle count and anti-müllerian hormone level as predictors of ovarian hyperstimulation syndrome in women with polycystic ovarian syndrome undergoing controlled ovarian stimulation. J Ultrason. 2021;21:e200–5.
Järvelä IY, Sladkevicius P, Kelly S, Ojha K, Campbell S, Nargund G. Quantification of ovarian power Doppler signal with three-dimensional ultrasonography to predict response during in vitro fertilization. Obstet Gynecol. 2003;102:816–22.
Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Müllerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93:855–64.
Jayaprakasan K, Hilwah N, Kendall NR, Hopkisson JF, Campbell BK, Johnson IR, et al. Does 3D ultrasound offer any advantage in the pretreatment assessment of ovarian reserve and prediction of outcome after assisted reproduction treatment? Hum Reprod. 2007;22:1932–41.
Knez J, Kovačič B, Medved M, Vlaisavljević V. What is the value of anti-Müllerian hormone in predicting the response to ovarian stimulation with GnRH agonist and antagonist protocols? Reprod Biol Endocrinol. 2015;13:58.
Kunt C, Ozaksit G, Keskin Kurt R, Cakir Gungor AN, Kanat-Pektas M, Kilic S, et al. Anti-Mullerian hormone is a better marker than inhibin B, follicle stimulating hormone, estradiol or antral follicle count in predicting the outcome of in vitro fertilization. Arch Gynecol Obstet. 2011;283:1415–21.
La Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, Xella S, et al. Anti-Müllerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod (Oxford, England). 2007;22:766–71.
Lee RK, Wu FS, Lin MH, Lin SY, Hwu YM. The predictability of serum anti-Müllerian level in IVF/ICSI outcomes for patients of advanced reproductive age. Reprod Biol Endocrinol. 2011;9:115.
Lee TH, Liu CH, Huang CC, Wu YL, Shih YT, Ho HN, et al. Serum anti-müllerian hormone and estradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Hum Reprod. 2008;23:160–7.
Li R, Gong F, Zhu Y, Fang W, Yang J, Liu J, et al. Anti-Müllerian hormone for prediction of ovarian response in Chinese infertile women undergoing IVF/ICSI cycles: a prospective, multi-centre, observational study. Reprod Biomed Online. 2016;33:506–12.
Martínez F, Clua E, Carreras O, Tur R, Rodríguez I, Barri PN. Is AMH useful to reduce low ovarian response to GnRH antagonist protocol in oocyte donors? Gynecol Endocrinol. 2013;29:754–7.
McIlveen M, Skull JD, Ledger WL. Evaluation of the utility of multiple endocrine and ultrasound measures of ovarian reserve in the prediction of cycle cancellation in a high-risk IVF population. Hum Reprod. 2007;22:778–85.
Muttukrishna S, Suharjono H, McGarrigle H, Sathanandan M. Inhibin B and anti-Mullerian hormone: markers of ovarian response in IVF/ICSI patients? BJOG. 2004;111:1248–53.
Nakhuda GS, Sauer MV, Wang JG, Ferin M, Lobo RA. Müllerian inhibiting substance is an accurate marker of ovarian response in women of advanced reproductive age undergoing IVF. Reprod Biomed Online. 2007;14:450–4.
Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, et al. Circulating basal anti-Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92:1586–93.
Nelson SM, Yates RW, Fleming R. Serum anti-Müllerian hormone and FSH: Prediction of live birth and extremes of response in stimulated cycles - Implications for individualization of therapy. Hum Reprod. 2007;22:2414–21.
Neves AR, Blockeel C, Griesinger G, Garcia-Velasco JA, Marca A, Rodriguez I, et al. The performance of the Elecsys® anti-Müllerian hormone assay in predicting extremes of ovarian response to corifollitropin alfa. Reprod Biomed Online. 2020;41:29–36.
Peñarrubia J, Fábregues F, Manau D, Creus M, Casals G, Casamitjana R, et al. Basal and stimulation day 5 anti-Müllerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist-gonadotropin treatment. Hum Reprod. 2005;20:915–22.
Sene AA, Ashrafi M, Alaghmand-Fard N, Mohammadi N, Alisaraie MM, Alizadeh A. Anti-müllerian hormone predictive levels to determine the likelihood of ovarian hyper-response in infertile women with polycystic ovarian morphology. Int J Fertil Ster. 2021;15:115–22.
Singh N, Malik E, Banerjee A, Chosdol K, Sreenivas V, Mittal S. “anti-mullerian hormone: marker for ovarian response in controlled ovarian stimulation for IVF patients”: a first pilot study in the Indian population. J Obstet Gynecol India. 2013;63:268–72.
Soldevila PNB, Carreras O, Tur R, Coroleu B, Barri PN. Sonographic assessment of ovarian reserve. Its correlation with outcome of in vitro fertilization cycles. Gynecol Endocrinol. 2007;23:206–12.
Tan X, Xi H, Yang J, Wang W. Development and validation of prediction model for high ovarian response in in vitro fertilization-embryo transfer: a longitudinal study. Comput Math Methods Med. 2021;2021:7822119.
Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-mullerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20–4.
Van Rooij IAJ, Broekmans FJM, Te Velde ER, Fauser BCJM, Bancsi LFJMM, De Jong FH, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–71.
Yong PY, Baird DT, Thong KJ, McNeilly AS, Anderson RA. Prospective analysis of the relationships between the ovarian follicle cohort and basal FSH concentration, the inhibin response to exogenous FSH and ovarian follicle number at different stages of the normal menstrual cycle and after pituitary down-regulation. Hum Reprod. 2003;18:35–44.
Kamel HM, Amin AH, Al-Adawy AR. Basal serum anti-Mullerian hormone (AMH) is a promising test in prediction of occurrence of pregnancy rate in infertile women undergoing ICSI cycles. Clin Lab. 2014;60(10):1717–23.
Polyzos NP, Tournaye H, Guzman L, Camus M, Nelson SM. Predictors of ovarian response in women treated with corifollitropin alfa for in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2013;100:430–7.
Laqqan MM, Yassin MM. Predictive factors of ovarian response to GnRH antagonist stimulation protocol: AMH and age are potential candidates. Middle East Fertil Soc J. 2021;26(1):1–1.
Honnma H, Baba T, Sasaki M, Hashiba Y, Oguri H, Fukunaga T, et al. Different ovarian response by age in an anti-Müllerian hormone-matched group undergoing in vitro fertilization. J Assist Reprod Genet. 2012;29:117–25.
He Y, Xia R, Chen X, Ye D, Tang Y, Li P, et al. Estimation of ovarian response using multiple predictors of ovarian reserve in women undergoing in vitro fertilization-embryo transfer. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:216–20.
Verberg MF, Eijkemans MJ, Heijnen EM, Broekmans FJ, de Klerk C, Fauser BC, et al. Why do couples drop-out from IVF treatment? A prospective cohort study. Hum Reprod. 2008;23:2050–5.
Fleming R, Seifer DB, Frattarelli JL, Ruman J. Assessing ovarian response: antral follicle count versus anti-Müllerian hormone. Reprod Biomed Online. 2015;31:486–96.
Acknowledgements
Not applicable.
Funding
This work was supported by the Yunnan Ten Thousand Youth Talent Program [[2018]73], and the Medical Discipline Leader in Health Commission of Yunnan Province [D-2019004].
Author information
Authors and Affiliations
Contributions
Y L and ZM P carried out the studies, participated in collecting data, and drafted the manuscript. YZ W and JM S performed the statistical analysis and participated in its design. Y L and JS C participated in acquisition, analysis, or interpretation of data and draft the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Pan, Z., Wu, Y. et al. Comparison of anti-Müllerian hormone and antral follicle count in the prediction of ovarian response: a systematic review and meta-analysis. J Ovarian Res 16, 117 (2023). https://doi.org/10.1186/s13048-023-01202-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-023-01202-5