Abstract
Background
Insulin-like peptide 5 (INSL5) is involved in both reproductive and metabolic processes in polycystic ovary syndrome (PCOS). This study aimed to evaluate the relationship between INSL5 and anti-Müllerian hormone (AMH).
Methods
A retrospective case–control study was conducted in a university-based reproductive centre between December 2019 and January 2021. We included 117 women with PCOS and 100 healthy subjects from Zhejiang Province. All subjects were divided into four groups (1st–4th) based on quartiles of serum INSL5 levels. Serum INSL5 concentration was assayed using an enzyme-linked immunosorbent assay.
Results
A significant direct association was observed between serum INSL5 and AMH levels in women with PCOS. The mean AMH level in the 1st–4th INSL5 level quartiles were 4.64, 5.20, 6.46, and 9.48 ng/ml, respectively (P < 0.001). After adjusting for age, body mass index, metabolic indices, and serum levels of oestradiol and total testosterone, AMH levels remained positively and significantly associated with INSL5 levels (P for trend < 0.001). The diagnostic value of AMH was better than that of INSL5.
Conclusions
INSL5 and AMH levels were significantly correlated and elevated in women with PCOS. INSL5 and AMH might be associated with increased androgen secretion and chronic anovulation in PCOS.
Similar content being viewed by others
Introduction
Polycystic ovary syndrome (PCOS) is both a reproductive and metabolic disorder in women. PCOS has a wide variety of clinical presentations and symptoms, including hyperandrogenism, oligo or amenorrhoea and polycystic ovaries (PCO) [1, 2]. The estimated prevalence of PCOS is 8-13% [3]. Familial clustering studies have shown that PCOS has a strong heritable component [4]. Characteristics of the condition are heterogeneous. PCOS features can include cystic ovaries, metabolic disorders, reproductive and physiological abnormalities, and higher risk of cardiovascular disorders [5, 6]. PCOS is also characterised by abnormal follicular development, with the number of growing follicles twice that in typical preovulatory arrest [7].
One of the important biomarkers used to diagnose PCOS is anti-Müllerian hormone (AMH), which is a polypeptide of the transforming growth factor beta family [8]. AMH plays an important role in sexual differentiation in male foetuses and is solely secreted by the granulosa cells of the pre-antral and small antral ovarian follicles in women [9, 10]. AMH inhibits the recruitment of primordial follicles from the resting oocyte pool, which may inhibit the role of follicle-stimulating hormone (FSH), resulting in female ovulation disorders [11]. The changes in AMH precedes modifications in serum FSH, inhibin B, and antral follicle count. AMH secretion drops significantly before menopause due to follicular failure [12]. The levels of AMH in patients with PCOS are significantly correlated with the number of ovarian small follicles, which reflects the presence of an ovarian follicle pool in the early follicular phase of the menstrual cycle and is strongly dependent on the number of antral follicles [13,14,15]. Tata et al. showed that AMH concentrations during pregnancy are two-to-three fold higher in women with PCOS as compared to women with no reproductive defects, and the severity of the reproductive dysfunction is positively correlated with AMH levels [11, 16]. Elevated AMH levels in PCOS were originally thought to be due only to an increase in the number of follicles [17, 18], as a low level of antral follicle count may lead to low serum AMH concentrations. Moreover, the level of serum AMH does not depend on the menstrual cycle phase or exogenous sex steroids [19].
Relaxin peptides are members of a superfamily of peptides including relaxin-1, relaxin-2, insulin-like peptide 3 (INSL3), INSL4, and INSL5, which play a vital physiological role in various diseases [20, 21]. Relaxin peptides interact with four G protein-coupled receptors (GPCRs), which are currently classified as relaxation protein family polypeptide receptors 1–4 (RXFP1–4). INSL5 is mainly expressed in the intestinal endocrine L-cells of the gastrointestinal system, especially the colon and rectum; but also by various tissues such as hypothalamus, uterine and pituitary tissue [22]. The connection between the reproductive system and INSL5 has been observed in INSL5 knockout mice. Animal experiment suggest that knockout INSL5 gene results in a notable decrease in sperm motility and irregular cycle in both sex [23]. INSL3 and AMH levels are significantly associated and elevated in women with PCOS which may reflect the dysfunction of follicles and granulosa cells [24]. Some animal studies have shown that INSL3 plays a vital role in developing ovarian follicular, while impaired fertility is associated with prolonged oestrous cycles and accelerated follicular atresia [25,26,27]. In a cross-sectional study, serum INSL5 levels were significantly higher in women with PCOS compared to women without PCOS (27.63 ± 7.74 vs.19.90 ± 5.85 ng/ml, P < 0.001) [28]. Another study suggested that the expression and localisation of RXFP4 on human sperm and INSL5 may affect male reproductive function [29].
Both INSL5 and AMH are recognised for their importance to reproductive function. They have recently been detected in adulthood in both sexes, where their roles still need to be explored. With this background, we assessed serum levels of AMH and INSL5 in women with PCOS, and we evaluated the relationship of these biochemicals with hormone levels and metabolic parameters.
Methods
Study population
A retrospective case-control study design was used for this study. The trial process took place from December 2019 to January 2021 in the Department of Endocrinology, Hangzhou Women’s Hospital (Hangzhou Maternal and Child Health Hospital) in Zhejiang, China. The study sample included 117 women with PCOS and 100 women with normal menstrual cycles, with ages ranging from 18 to 35 years. A detailed medical assessment (such as height, weight, family history of PCOS, regularity and length of the menstrual cycle, and medical history, etc.) was obtained from all subjects at the initial examination. Written consent was obtained from all participants. This study was approved by the ethics committee of Hangzhou Women’s Hospital (2018-004-No. 03).
Inclusion and exclusion criteria
The subjects in the PCOS group were selected based on the Rotterdam consensus criteria (2018) [1]. Women with other causes of irregular menstrual cycles and/or androgen excess such as hyperprolactinemia, congenital adrenal hyperplasia, Cushing’s syndrome, or other diseases of the galactorrhoea, breast feeding, pregnancy adrenal gland, and thyroid disorders were excluded by laboratory analysis and clinical examination. The use of medications for hormonal contraception and/or anti-androgen therapy within the preceding 6 months, dyslipidaemia, obesity, hypertension, hyperglycaemia and insulin resistance were also criteria for exclusion from this study. The subjects in control group were enrolled with normal menstrual cycle without concomitant health problems or signs of hirsutism, acne or hyperandrogenism. No obvious abnormality in ultrasound imaging.
Laboratory evaluation and biochemical assays
Fasting peripheral venous blood samples (2–3 mL) for fasting glucose, fasting insulin, oestradiol (E2), luteinizing hormone (LH), FSH, prolactin (PRL), progesterone (P), high-density lipoprotein (HDL), low-density lipoprotein (LDL), dehydroepiandrosterone sulfate (DHEA-S), total testosterone (T), sex hormone-binding globulin (SHBG), and AMH were drawn between 07:30 and 09:30 a.m. after an overnight fast. Sex hormone assessments were performed during the early follicular phase of menstrual bleeding (3rd to 5th days) and serum DHEA-S, T, SHBG, AMH were detected using the chemiluminescence method (Beckman Coulter UniCel Dxl-800). The levels of fasting plasma glucose, HDL, and LDL were measured using a Beckman Coulter AU5821 chemistry analyser. After separation, the excess serum samples were stored in a refrigerator at − 80 °C prior to analysis of INSL5. The levels of serum INSL5 were detected using a commercially available human enzyme-linked immunosorbent assay kit (Catalog number: JL19996, Jianglai biological, Hangzhou, China). The free androgen index (FAI) was calculated by the following formula: [FAI = 100x(T/SHBG)]. Insulin resistance was estimated using the homeostatic model assessment index of insulin resistance: HOMA-IR = [fasting insulin (pmol/L) × fasting glucose (mmol/L)] / (22.5 × 6.965).
Statistical methods
The distributions of AMH and INSL5 were both skewed. IBM-SPSS 21.0 statistics software (IBM Inc., Armonk, NY, USA) was used for statistical processing. Data are expressed as mean ± standard deviation (SD). Continuous variables were analysed using analysis of variance (ANOVA) for comparing the characteristics across quartiles of INSL5. Receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to evaluate the performance of INSL5 and AMH in building a risk model to diagnose PCOS. The association between INSL5 and AMH was evaluated using a linear regression model. To evaluate the potential for confounding, six models were built. Model 1 was unadjusted. Model 2 included adjustments for age and body mass index (BMI). Model 3 included the further adjustments of glucose metabolism indexes (fasting glucose and fasting insulin). Model 4 included the adjustments of model 3 and lipid metabolism indexes (total cholesterol and triglyceride). Model 5 included the adjustments of model 4 and lipid metabolism indexes (HDL and LDL). Model 6 included the adjustments of model 5 and sex hormones (E2 and T). Linear trends across the quartiles of INSL5 levels were assessed by modelling the median values of all quartiles as a continuous variable. P value < 0.05 was accepted as statistically significance.
Results
Background characteristics of the study population
The background sample characteristics are shown in the Table 1. The participants were divided into four groups based on quartiles of serum INSL5 levels: 1st (3.92–16.47),2nd (16.47–21.26), 3rd (21.26–30.02) and 4th (30.02–99.84) ng/ml. There was a significant correlation between BMI and INSL5 (P = 0.033). A significant inverse association was observed between INSL5 levels and age across the quartiles (P = 0.02) in Table 1.
INSL5 and hormone levels
The hormone levels, lipid metabolism index and glucose metabolism index in the four groups are presented in Table 1. Significant proportional associations were observed between T levels and INSL5 across the quartiles. The mean T in 1st–4th were 0.71, 0.64, 0.76, and 0.78 ng/mL, respectively (P = 0.014). FAI, FSH and P also showed significant correlations with INSL5 across groups (P = 0.011; P = 0.016; P = 0.033, respectively). There were no significant differences among the groups in E2, LH, PRL, DHEA-S and SHBG (P > 0.05).
INSL5 and AMH
In only-PCOS and the total groups, we found a positive correlation between INSL5 and AMH (P < 0.001). While there was no significant correlation found between INSL5 and AMH in the control group (P = 0.732), as shown in Table 1. Women with PCOS had higher serum AMH levels in the second, third and fourth INSL5 quartiles compared with the PCOS group and total groups (Fig. 1). Significant proportional relationships were observed between AMH levels and INSL5 across the quartiles in all subjects. Linear regression models were designed to reveal the independent relationship between INSL5 and AMH; The mean AMH in 1st–4th were 4.64, 5.20, 6.46, and 9.48, respectively (P < 0.001) in total subjects. The mean AMH in 1st–4th were 6.1, 7.16, 8.24 and 11.37 ng/mL, respectively (P < 0.001) in women with PCOS. In models 1 and model 2, the mean AMH increased with increasing INSL5 levels (Table 2). After further adjustments for glucose metabolism parameters in model 3, this association remained significant (P for trend < 0.001). Adjustment for lipid metabolism parameters had minimal effect on the results, with higher AMH levels remaining significantly associated with higher INSL5. There was a significant correlation between serum AMH and INSL5 in model 6 (P for trend < 0.001).
INSL5 and PCOS
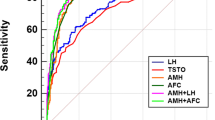
Women with PCOS had significantly higher INSL5 and AMH levels (Fig. 1). The sensitivity and specificity of the serum concentration of INSL5 and AMH were evaluated for the PCOS group by using cut-off values according to the ROC (Fig. 2, Table 3). The AUC of AMH for the diagnosis value of PCOS was 0.902 (95% confidence interval: 0.858–0.945, P < 0.001). The sensitivity for PCOS, with a cut-off value for AMH of 4.56 ng/ml, was 93.2% and the specificity was 77.0%. The AUC of INSL5 for the diagnosis value of PCOS was 0.693 (95% confidence interval:0.623–0.762, P < 0.001). When using a cut-off value for INSL5 of 19.88 ng/ml, the sensitivity was 75.2% and the specificity was 57.0%. While the combination of AMH and INSL5 cut-offs did not significantly improve the accuracy of diagnosis value.
Discussion
In the present study, we investigated the circulating peptide INSL5 levels in women with PCOS and ascertained relationship between INSL5 and AMH. There are few studies about INSL5, although a previous cross-sectional study suggests that the levels of INSL5 are higher in women with PCOS compared to the normal subjects, consistent with our findings [28]. To the best of our knowledge, this is the first study evaluating the association between INSL5 levels and AMH in women PCOS. Our results showed that women with PCOS had elevated serum AMH levels in the second, third and fourth INSL5 level quartiles compared with the overall sample (Table 1). Linear regression analysis revealed that increased INSL5 levels were associated with higher odds of AMH. The results demonstrated that INSL5 level was positively correlated with AMH in women with PCOS. The origin of follicular overgrowth may be the result of primordial ovarian hyperandrogenism in PCOS, mainly caused by thecal cell dysfunction, but its origin is still unclear [7]. AMH, a recognised biomarker of female reproductive potential, is widely used in clinical practice and is a good indicator of female fertility and menopausal health management. The level of AMH reflects the response and number of primary follicles and response to exogenous gonadotrophins [30].
More studies have shown that the offspring of women with PCOS are at increased risk for future metabolic and reproductive dysfunction [31]. The second follicle abnormality in PCOS is follicular accumulation caused by a dominant follicle selection defect or follicular block. Anovulatory infertility in patient with PCOS is the focus concerns. The change of oocyte capacity and decreased endometrial function are considered as a potential pathogenic factor of fertility in women with PCOS [32, 33]. This process in women with PCOS is primarily associated with impaired FSH, which inhibits follicular maturation and also associated with excessive LH and insulin secretion. Although the underlying mechanism of this process remains unclear, the evidence suggests that higher levels of AMH produced in the polycystic ovary may play a negative selection role in follicular development and inhibit FSH-dependent aromatase activity [19, 34].
INSL5, interacting with RXFP4, is widely distributed among different tissues such as colorectal tissues, placenta, thyroid, thymus, kidneys, ovaries and testis [35]. There is some evidence of a link between INSL5 and the reproductive system in both sexes. RXFP4 expression in the testes of INSL5-deficient mice resulted in infertility due to irregular length of the oestrous cycle and spermatogenesis disorders [23]. Another study demonstrated that the expression of RXFP4 and INSL5 in human sperm inhibited the natural decline of sperm motility, which may be related to a decrease in mitochondrial ROS production [29]. The expression of INSL5 in the mouse hypothalamus and testis indicates that INSL5 is influential on the hypothalamic pituitary-gonadal axis (HPG) and has important effects on reproduction [22].
There are few studies on INSL5 and the reproductive system, but many studies have shown that INSL3, another well-known member of the relaxin superfamily, may affect the reproductive function in males and females. Serum LH levels and the number of ovarian follicles determined by ultrasound have been associated with INSL3 levels in women with PCOS [36]. Future clinical may find that the significant correlation between INSL3 and AMH found in this research may be associated with impaired follicular development in women with PCOS [24]. In addition to this, animal studies have already shown that INSL3 plays a vital role in ovarian follicular development [27]. A previous study demonstrated that the impaired fertility in INSL3 knockout mice is related to prolonged oestrus cycle and accelerated follicular atresia [25]. Based on these data, we suggest a possible interaction between the INSL family and ovarian function. On the other hand, these results might indicate that increased INSL5 up-regulate AMH, although the receptor for INSL5 in granulosa cells has not yet been described.
Moreover, an optimal cut-of value of INSL5 for predicting PCOS was found to be ≥19.88 ng/ml (sensitivity 75.2%, specificity 57%) and an optimal cut-of value of AMH was 4.56 ng/ml (sensitivity 93.2%, specificity 77%). In the present study, the results suggest that the diagnosis value of AMH for PCOS is better than that of INSL5. Meanwhile, the combination of INSL5 and AMH did not improve the accuracy of the test and the diagnosis value.
We confirmed that increased INSL5 concentrations are positively correlated with T concentrations in women with PCOS, which also supports the potential role of INSL5 in follicular membrane cell steroidogenesis. Several studies have shown that androgens, whether from the ovary, adrenal glands or exogenous sources, may promote the growth of ovarian follicles through androgen receptors and reduction of granulosa cell apoptosis [37, 38]. Among women with PCOS, the highest level of INSL3 is described as functional ovarian hyperandrogenism, which is characterized by more severe hyperandrogenism and insulin resistance [39]. Another study showed that a positive correlation was between INSL3, LH, androgen levels and ovarian follicular number [36]. An inverse relationship between circulating INSL5 and T levels in 14 obese men has also been found; where levels of T increased but INSL5 decreased after obese men underwent bariatric surgery [40].
The physiological role of INSL5 in the human reproductive system is still unclear although many studies have reported the effects of INSL5 on lipid and glucose metabolism. We found no significant correlation between INSL5 levels and HOMA-IR (P = 0.498), or with fasting insulin and glucose, which does not support some previous studies [28]. Increasing INSL5 has been shown to make no significant difference on food intake, body weight, or glucose control in mice [41]. However, in INSL5 knockout mice, a reduction in the number of beta cells in the pancreas and reduced insulin production was found, which led to a significant increase in blood glucose levels [23]. INSL5 is co-stored with glucagon-like peptide-1 (GLP-1) and peptide YY, and it stimulated insulin, GLP-1 in both vitro and vivo studies [42, 43]. In agreement with some but not all studies, INSL5 did not correlate with total cholesterol, triglycerides, HDL and LDL in our study.
There are some limits in our research. One limitation of this study is that we did not have the data on follicle numbers detected by ultrasound, or INSL3 levels. We didn’t elaborate PCOS phenotypes and explore the effect of PCOS phenotypes and PCOS features. Another important drawback is the lack of accepted international standards for AMH detection, so concentration and cut-off points are method dependent.
The research showed a significant relationship between circulating INSL5 and AMH which may be related to the increased secretion of androgens and chronic anovulation of PCOS. This link may explicate the contribution of this peptide in the development of the PCOS disorder. Moreover, The relationship between INSL5 and female reproductive function may guide clinical applications, such as fertility guidance and drug therapy. The mechanisms responsible for increased concentrations of AMH and particularly INSL5 in the blood of women with PCOS are, however, still unclear and need to be further elucidated in future research.
Availability of data and materials
All data generated or analyzed during this study are included in the manuscript.
Abbreviations
- INSL5:
-
Insulin-like peptide 5
- PCOS:
-
Polycystic ovary syndrome
- AMH:
-
Anti-Müllerian hormone
- PCO:
-
Polycystic ovaries
- GPCRs:
-
G protein-coupled receptors
- RXFP:
-
Relaxin family polypeptide receptor
- LH:
-
Luteinizing hormone
- FSH:
-
Follicle-stimulating hormone
- E2:
-
Estradiol
- PRL:
-
Prolactin
- P:
-
Progesterone
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- DHEA-S:
-
Dehydroepiandrosterone sulfate
- T:
-
Total testosterone
- SHBG:
-
Sex hormone-binding globulin
- FAI:
-
Free androgen index
- HOMA-IR:
-
Homeostatic model assessment index of insulin resistance
- SD:
-
Standard deviation
- ANOVA:
-
Analysed using analysis of variance
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- GLP-1:
-
Glucagon-like peptide-1
References
Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364–79. https://doi.org/10.1016/j.fertnstert.2018.05.004.
Costello MF, Misso ML, Balen A, Boyle J, Devoto L, Garad RM, et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: assessment and treatment of infertility. Hum Reprod Open. 2019:y21. https://doi.org/10.1093/hropen/hoy021.
Moran LJ, Tassone EC, Boyle J, Brennan L, Harrison CL, Hirschberg AL, et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: lifestyle management. Obes Rev. 2020;21:e13046. https://doi.org/10.1111/obr.13046.
Dan S, Jinbiao H, Yitong C, et al. Reproductive health in first-degree relatives of patients with polycystic ovary syndrome: a review and Meta-analysis. J Clin Endocrinol Metab. 2022;107:273–95. https://doi.org/10.1210/clinem/dgab640.
Al Wattar Bassel H, Maria F, Laura B, et al. Clinical practice guidelines on the diagnosis and Management of Polycystic Ovary Syndrome: a systematic review and quality assessment study. J Clin Endocrinol Metab. 2021;106:2436–46. https://doi.org/10.1210/clinem/dgab232.
Okoth K, Chandan JS, Marshall T, Thangaratinam S, Thomas GN, Nirantharakumar K. Association between the reproductive health of young women and cardiovascularb disease in later life: umbrella review. 2020, BMJ;371:m3502. https://doi.org/10.1136/bmj.m3502.
Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–21. https://doi.org/10.1016/s0140-6736(03)14410-8.
Teede H, Misso M, Tassone EC, Dewailly D, Ng EH, Azziz R. Anti-Müllerian hormone in PCOS: a review informing international guidelines. Trends Endocrinol Metab. 2019;30:467–78. https://doi.org/10.1016/j.tem.2019.04.006.
Silva M, Giacobini P. New insights into anti-Müllerian hormone role in the hypothalamic-pituitary-gonadal axis and neuroendocrine development. Cell Mol Life Sci. 2021;78:1–16. https://doi.org/10.1007/s00018-020-03576-x.
Moolhuijsen L, Visser JA. Anti-Müllerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab. 2020;105:3361–73. https://doi.org/10.1210/clinem/dgaa513.
Tata B, Mimouni N, Barbotin AL, Malone SA, Loyens A, Pigny P, et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24:834–46. https://doi.org/10.1038/s41591-018-0035-5.
Xu J, Bishop CV, Lawson MS, Park BS, Xu F. Anti-Müllerian hormone promotes pre-antral follicle growth, but inhibits antral follicle maturation and dominant follicle selection in primates. Hum Reprod. 2016;31:1522–30. https://doi.org/10.1093/humrep/dew100.
Homburg R, Ray A, Bhide P, Gudi A, Shah A, Timms P, et al. The relationship of serum anti-Mullerian hormone with polycystic ovarian morphology and polycystic ovary syndrome: a prospective cohort study. Hum Reprod. 2013;28:1077–83. https://doi.org/10.1093/humrep/det015.
Kostrzewa M, Głowacka E, Stetkiewicz T, Grzesiak M, Szyłło K, Stachowiak G, et al. Is serum anti-Müllerian hormone (AMH) assay a satisfactory measure for ovarian reserve estimation? A comparison of serum and peritoneal fluid AMH levels. Adv Clin Exp Med. 2020;29:853–6. https://doi.org/10.17219/acem/121010.
Dumont A, Robin G, Dewailly D. Anti-müllerian hormone in the pathophysiology and diagnosis of polycystic ovarian syndrome. Curr Opin Endocrinol Diabetes Obes. 2018;25:377–84. https://doi.org/10.1097/MED.0000000000000445.
Cook CL, Siow Y, Brenner AG, Fallat ME. Relationship between serum müllerian-inhibiting substance and other reproductive hormones in untreated women with polycystic ovary syndrome and normal women. Fertil Steril. 2020;77:141–6. https://doi.org/10.1016/s0015-0282(01)02944-2.
Dilaver N, Pellatt L, Jameson E, Ogunjimi M, Bano G, Homburg R, et al. The regulation and signalling of anti-Müllerian hormone in human granulosa cells: relevance to polycystic ovary syndrome. Hum Reprod. 2019;34:2467–79. https://doi.org/10.1093/humrep/dez214.
Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian Hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–520. https://doi.org/10.1210/er.2015-1104.
Lv PP, Jin M, Rao JP, Chen J, Wang LQ, Huang CC, et al. Role of anti-Müllerian hormone and testosterone in follicular growth: a cross-sectional study. BMC Endocr Disord. 2020;20:101. https://doi.org/10.1186/s12902-020-00569-6.
Hsu SY. Cloning of two novel mammalian paralogs of relaxin/insulin family proteins and their expression in testis and kidney. Mol Endocrinol. 1999;13:2163–74. https://doi.org/10.1210/mend.13.12.0388.
Conklin D, Lofton-Day CE, Haldeman BA, Ching A, Whitmore TE, Lok S, et al. Identification of INSL5, a new member of the insulin superfamily. Genomics. 1999;60:50–6. https://doi.org/10.1006/geno.1999.5899.
Dun SL, Brailoiu E, Wang Y, Brailoiu GC, Liu-Chen LY, Yang J, et al. Insulin-like peptide 5: expression in the mouse brain and mobilization of calcium. Endocrinology. 2006;147:3243–8. https://doi.org/10.1210/en.2006-0237.
Burnicka-Turek O, Mohamed BA, Shirneshan K, Thanasupawat T, Hombach-Klonisch S, Klonisch T, et al. INSL5-deficient mice display an alteration in glucose homeostasis and an impaired fertility. Endocrinology. 2012;153:4655–65. https://doi.org/10.1210/en.2012-1161.
Pelusi C, Fanelli F, Pariali M, Zanotti L, Gambineri A, Pasquali R. Parallel variations of insulin-like peptide 3 (INSL3) and antimüllerian hormone (AMH) in women with the polycystic ovary syndrome according to menstrual cycle pattern. J Clin Endocrinol Metab. 2013;98:E1575–82. https://doi.org/10.1210/jc.2013-1107.
Spanel-Borowski K, Schäfer I, Zimmermann S, Engel W, Adham IM. Increase in final stages of follicular atresia and premature decay of corpora lutea in Insl3-deficient mice. Mol Reprod Dev. 2001;58:281–6. https://doi.org/10.1002/1098-2795(200103)58:3<281::AID-MRD6>3.0.CO;2-0.
Irving-Rodgers HF, Bathgate RA, Ivell R, Domagalski R, Rodgers RJ. Dynamic changes in the expression of relaxin-like factor (INSL3), cholesterol side-chain cleavage cytochrome p450, and 3beta-hydroxysteroid dehydrogenase in bovine ovarian follicles during growth and atresia. Biol Reprod. 2002;66:934–43. https://doi.org/10.1095/biolreprod66.4.934.
Satchell L, Glister C, Bleach EC, Glencross RG, Bicknell AB, Dai Y, et al. Ovarian expression of insulin-like peptide 3 (INSL3) and its receptor (RXFP2) during development of bovine antral follicles and corpora lutea and measurement of circulating INSL3 levels during synchronized estrous cycles. Endocrinology. 2013;154:1897–906. https://doi.org/10.1210/en.2012-2232.
Bicer M, Alan M, Alarslan P, Guler A, Kocabas GU, Imamoglu C, et al. Circulating insulin-like peptide 5 levels and its association with metabolic and hormonal parameters in women with polycystic ovary syndrome. J Endocrinol Investig. 2019;42:303–12. https://doi.org/10.1007/s40618-018-0917-x.
Yeganeh IS, Taromchi AH, Fathabadi FF, Nejatbakhsh R, Novin MG, Shokri S. Expression and localization of relaxin family peptide receptor 4 in human spermatozoa and impact of insulin-like peptide 5 on sperm functions. Reprod Biol. 2017;17:327–32. https://doi.org/10.1016/j.repbio.2017.09.004.
Neven A, Laven J, Teede HJ, Boyle JA. A summary on polycystic ovary syndrome: diagnostic criteria, prevalence, clinical manifestations, and management according to the latest international guidelines. Semin Reprod Med. 2018;36:5–12. https://doi.org/10.1055/s-0038-1668085.
Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015;21(5):575–92. https://doi.org/10.1093/humupd/dmv029.
Palomba S. Is fertility reduced in ovulatory women with polycystic ovary syndrome? An opinion paper. Hum Reprod. 2021;36(9):2421–8. https://doi.org/10.1093/humrep/deab181.
Palomba S, Daolio J, La Sala GB. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol Metab. 2017;28(3):186–98. https://doi.org/10.1016/j.tem.2016.11.008.
Rudnicka E, Kunicki M, Calik-Ksepka A, Suchta K, Duszewska A, Smolarczyk K, et al. Anti-Müllerian hormone in pathogenesis, diagnostic and treatment of PCOS. Int J Mol Sci. 2021:22. https://doi.org/10.3390/ijms222212507.
Kay RG, Galvin S, Larraufie P, Reimann F, Gribble FM. Liquid chromatography/mass spectrometry based detection and semi-quantitative analysis of INSL5 in human and murine tissues. Rapid Commun Mass Spectrom. 2017;31:1963–73. https://doi.org/10.1002/rcm.7978.
Gambineri A, Patton L, De Iasio R, Palladoro F, Pagotto U, Pasquali R. Insulin-like factor 3: a new circulating hormone related to luteinizing hormone-dependent ovarian hyperandrogenism in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2066–73. https://doi.org/10.1210/jc.2006-1678.
Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod. 1999;61:353–7. https://doi.org/10.1095/biolreprod61.2.353.
Henríquez S, Kohen P, Xu X, Villarroel C, Muñoz A, Godoy A, et al. Significance of pro-angiogenic estrogen metabolites in normal follicular development and follicular growth arrest in polycystic ovary syndrome. Hum Reprod. 2020;35(7):1655–65. https://doi.org/10.1093/humrep/deaa098.
Gambineri A, Patton L, Prontera O, Fanelli F, Ciampaglia W, Cognigni GE, et al. Basal insulin-like factor 3 levels predict functional ovarian hyperandrogenism in the polycystic ovary syndrome. J Endocrinol Investig. 2011;34:685–91. https://doi.org/10.3275/7726.
Wagner IV, Flehmig G, Scheuermann K, Löffler D, Körner A, Kiess W, et al. Insulin-like peptide 5 interacts with sex hormones and metabolic parameters in a gender and adiposity dependent manner in humans. Horm Metab Res. 2016;48:589–94. https://doi.org/10.1055/s-0042-109869.
Zaykov AN, Gelfanov VM, Perez-Tilve D, Finan B, Dimarchi RD. Insulin-like peptide 5 fails to improve metabolism or body weight in obese mice. Peptides. 2019;120:170116. https://doi.org/10.1016/j.peptides.2019.170116.
Lee YS, De Vadder F, Tremaroli V, Wichmann A, Mithieux G, Bäckhed F. Insulin-like peptide 5 is a microbially regulated peptide that promotes hepatic glucose production. Mol Metab. 2016;5:263–70. https://doi.org/10.1016/j.molmet.2016.01.007.
Billing LJ, Smith CA, Larraufie P, Goldspink DA, Galvin S, Kay RG, et al. Co-storage and release of insulin-like peptide-5, glucagon-like peptide-1 and peptideYY from murine and human colonic enteroendocrine cells. Mol Metab. 2018;16:65–75. https://doi.org/10.1016/j.molmet.2018.07.011.
Funding
Hangzhou Medical and health Science and Technology Project (OO20190291); Zhejiang Health Science and Technology Plan Project (2021KY261); Zhejiang Province Major Science and Technology Program of Medicine and Health (WKJ-ZJ-2010); Hangzhou Agriculture and Social Development Research Project (202004A12); Special Project of Traditional Chinese Medicine Modernization of Zhejiang Province (2021ZX013).
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.C., Formal analysis and investigation: M.D., Z.C., and Q.W.; Writing - original draft preparation: Y.C., and S.H.; Writing - review and editing: J.C., H. Z., and W.L.; Funding acquisition: X.J., and Z. Z.. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Hangzhou Women’s Hospital (2018-004-No. 03).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Y., Deng, M., Chen, Z. et al. Insulin-like peptide 5 (INSL5) positively correlates with anti-Müllerian hormone (AMH) in women with the polycystic ovary syndrome: a case-control study. J Ovarian Res 15, 118 (2022). https://doi.org/10.1186/s13048-022-01052-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-022-01052-7