Abstract
Ovarian aging refers to the process by which ovarian function declines until eventual failure. The pathogenesis of ovarian aging is complex and diverse; oxidative stress (OS) is considered to be a key factor. This review focuses on the fact that OS status accelerates the ovarian aging process by promoting apoptosis, inflammation, mitochondrial damage, telomere shortening and biomacromolecular damage. Current evidence suggests that aging, smoking, high-sugar diets, pressure, superovulation, chemotherapeutic agents and industrial pollutants can be factors that accelerate ovarian aging by exacerbating OS status. In addition, we review the role of nuclear factor E2-related factor 2 (Nrf2), Sirtuin (Sirt), mitogen-activated protein kinase (MAPK), protein kinase B (AKT), Forkhead box O (FoxO) and Klotho signaling pathways during the process of ovarian aging. We also explore the role of antioxidant therapies such as melatonin, vitamins, stem cell therapies, antioxidant monomers and Traditional Chinese Medicine (TCM), and investigate the roles of these supplements with respect to the reduction of OS and the improvement of ovarian function. This review provides a rationale for antioxidant therapy to improve ovarian aging.
Similar content being viewed by others
Introduction
Aging is an irreversible physiological and pathological phenomenon in normal metabolism [1]. Aging is complex and multiple factors are known to contribute towards the overall aging process and phenotype [2]. In the female body, the ovary acts as a natural biological clock, thus controlling the process of aging [3]. Ovarian aging refers to the process of gradual decline and eventual exhaustion of ovarian function.
Two major categories of ovarian aging have been identified: physiological and pathological. Physiological ovarian aging refers to the process by which ovarian function deteriorates naturally with age until menopause. Pathological ovarian aging refers to the premature decline of ovarian function, including diminished ovarian reserve (DOR), premature ovarian insufficiency (POI), and poor ovarian response (POR) in the field of in vitro fertilization-embryo transfer (IVF-ET). Pathological aging can be caused by a variety of pathogenic factors [4]. Ovarian aging is characterized by menstrual disorders (amenorrhea or oligomenorrhea) and reduced reproductive function, accompanied by elevated levels of gonadotropins and decreased levels of estrogen. In terms of pathology, ovarian aging predominantly manifests as a reduction in the number of follicles and a decline in the quality of oocytes [5]. The terminal stage of ovarian aging is menopause. Following menopause, dysfunction of the endocrine, cardiovascular and nervous systems, among others, becomes apparent or aggravated with hormonal changes in female body. Therefore, ovarian aging is considered an important node in the process of female aging.
As a classic theory of aging, free radical theory was first proposed in the 1950s. The important role of free radical has been extensively studied across multiple diseases and is considered a key mechanism of ovarian aging. Free radicals are a high-activity pro-oxidation group of molecules produced during aerobic metabolism, including reactive oxygen species (ROS) and reactive nitrogen species (RNS) [6]. ROS is primarily a byproduct of mitochondrial oxidative phosphorylation. A temperate amount of ROS is essential for the normal physiological functions of the body. ROS are not only involved in the synthesis of active substances, cellular detoxification and immune function [7], they also act as an important second messenger that participates in the intercellular signal transduction and regulation of gene expression, thereby maintaining cellular homeostasis [8].
Under physiological conditions, the oxidative and antioxidant systems are in dynamic equilibrium. The antioxidant system is composed of both enzymes and non-enzymes. Of these, the key enzymes include superoxide dismutase (SOD), catalase (CAT), glutathione (GSH) and glutathione peroxidase (GSH-Px). Non-enzymes mainly include melatonin, vitamins (C, E) and trace elements (copper, zinc and selenium). However, when ROS are overproduced or antioxidant utilization increases, this leads to an imbalance of redox reactions and induces an oxidative stress (OS) state in the body [9]. Extensive studies have shown that the OS state of the ovarian microenvironment can result in pathological damage, including meiotic arrest in oocytes, granulosa cell apoptosis, and corpus luteum dysfunction, thus accelerating the process of ovarian aging. Supplementation with antioxidants can improve the ovarian OS state and enhance ovarian function. This article reviews research progress in the field of OS in relation to ovarian aging.
The physiological role of ROS in the ovary
In the ovary, ROS are involved in the regulation of oocyte growth, meiosis, ovulation and other physiological processes [10]. During follicular growth, increased steroid production leads to the expression of cytochrome P450, thus resulting in ROS formation. Meanwhile, the increased secretion of estradiol (E2) in growing follicles triggers the expression of peroxidase CAT, resulting in a dynamic balance between ROS and antioxidants [11]. The precise regulation of meiotic arrest and recovery of oocytes is essential for female reproductive development. ROS stimulation regulates the progress of meiosis I; in contrast, the progression of meiosis II is mainly controlled by antioxidants in the ovary. This demonstrates the complex relationship between free radicals and antioxidants within the meiotic maturation process of oocytes [12]. The surge of luteinizing hormone (LH) prior to ovulation increases the levels of inflammatory precursors in the ovary, thus leading to the excessive production of ROS. Increased ROS induces apoptosis in the granulosa cells, which further leads to follicular wall rupture and ovulation; therefore, this is regarded as an important ovulation signal [13]. Similarly, the regression of the corpus luteum is also mediated by OS-induced apoptosis of luteinized granulosa cells [14]. The balance of ROS is also critical within the in vitro setting, and can exert influence on oocyte maturation, fertilization, and subsequent embryo implantation and development [15]. The appropriate concentration of ROS in follicular fluid is not only an indicator of good follicular metabolic activity but can also be used as a potential marker for predicting the outcome of IVF-ET [16].
Pathological mechanisms related with OS in ovarian aging
Apoptosis
Apoptosis has been studied extensively in ovarian aging [17, 18]. Apoptosis in ovarian cells can cause extensive follicular atresia or regression and is considered to be one of the most important mechanisms underlying ovarian aging [19]. OS has been shown to induce apoptosis in ovarian cells by various processes, including exogenous pathways, endogenous pathways and by endoplasmic reticulum stress (ERS) [20,21,22]. In the exogenous pathway, excess ROS in ovarian tissue can activate the Fas/FasL pathway and recruit caspase-8 (CASP8) to form a death-inducing signaling complex (DISC) with Fas and FasL. Activated CASP8 then activates CASP3, CASP6, CASP7 and cleaves various downstream intracellular substrates that are necessary for cell survival, thereby inducing apoptosis [23]. In the endogenous pathway, ROS can disrupt mitochondrial homeostasis in ovarian cells, thus resulting in the release of Cytochrome C from the mitochondria. Cytochrome C and apoptotic protease-activating factor (Apaf-1) form a multimeric complex, continue to activate CASP3 and CASP9, and upregulate the Bax/Bcl-2 ratio, thus inducing apoptosis [24]. In addition, OS can activate inositol-requiring enzyme 1 (IRE1) and protein kinase RNA-like ER kinase (PERK), induce unfolded protein response (UPR) factors and ultimately promote apoptosis in granulosa via the ERS pathway [25, 26].
Oocyte apoptosis leads to the loss of germ cells directly. Granulosa cell apoptosis leads to nutrient deprivation in ovarian cells and induces metabolic disorders in the ovarian microenvironment, thus aggravating the decline in ovarian function [27]. Furthermore, OS can lead to apoptosis in female germline stem cells (FGSCs). Phenotypic changes in FGSCs reduce their proliferative capacity and stemness. And the ovary also loses the potential to replenish the primordial follicle pool and produce oocytes, ultimately reducing ovarian reserve [28]. The cell debris generated by apoptosis can continue to affect the ovarian microenvironment. Apoptosis elevated the levels of cell-free DNA in follicular fluid which stimulates the massive production of intracellular ROS and ultimately exacerbates apoptosis [29].
Inflammation
Long-term chronic inflammation accelerates aging in the body [30]. An abundance of evidence has shown that chronic inflammation is closely related with ovarian aging [31]. Clinical studies have found that the serum levels of IL-6 and IL-21 were significantly higher in POI patients than in healthy women of the same age [32]. NLRP3, as an inflammasome in the NLR family, plays an important role in inflammation [33]. NLRP3 was found to be highly expressed in the granulosa cells of DOR patients [34]. After NLRP3 reduction, the levels of pro-inflammatory cytokines were down-regulated while the levels of AMH and the number of primordial follicles increased [34].
OS is closely linked with inflammation. Excessive levels of ROS in the body can trigger the assembly and activation of the NLRP3 inflammasome [35], which subsequently promotes the infiltration of inflammatory cells and the secretion of the pro-inflammatory cytokines IL-1β and IL-18 in tissues [36]. ROS can also directly activate nuclear factor-k-gene binding (NF-κB) to promote inflammation while activation of NF-κB further upregulates NLRP3 expression [37]. Moreover, after immune cells are activated, intracellular ROS are produced in large quantities to participate in the activation of the immune response, further leading to increased OS damage [38].
OS and inflammation produce synergistic disruptive effects on ovarian tissue. A previous study showed that the levels of the products of OS damage (carbonylated and nitrated proteins) and inflammatory markers (TNF-α, IL-1β) were significantly higher in the ovaries of aged mice than in younger mice [39]. Ovarian RNA transcriptome analysis further revealed that OS-related proteins (Nrf2, SOD2, CAT, GSH-px1) and genes encoding NLRP3 inflammatory vesicles could be used as key biomarkers to differentiate between young and aging mice [40]. A variety of models of functional ovarian damage were shown to exhibit high levels of inflammatory factors and OS markers [41, 42]. Furthermore, the use of antioxidants or dietary supplements (quercetin, ginsenoside Rg1, Vitamin B12) has been shown to significantly improve both inflammation and OS, thus enhancing ovarian function [43,44,45]. Therefore, inflammation plays an important role in ovarian aging.
Mitochondria
Mitochondria are important organelles in cells. They can generate ATP through the process of oxidative phosphorylation and are known as the energy production machinery for cells [46]. Mitochondria also have their own genomes that encode polypeptides involved in energy production [47]. Mitochondrial DNA (mtDNA) is vulnerable to ROS attack due to the lack of histone protection and an overlap with the ROS generation site in the mitochondrial inner membrane [48]. Excessive levels of ROS not only induce mtDNA mutations to result in inefficient electron transport chain (ETC) expression, but also mediate abnormal mtDNA-protein cross-linking, thus leading to mitochondrial dysfunction in several ways [49]. Mitochondrial dysfunction further exacerbates the leakage of ROS from the ETC, thereby exacerbating intracellular OS damage [50]. Ultimately, this cascade of amplified injury can have serious adverse effects on ovarian function.
Mitochondria are the most numerous organelles in the oocyte and provide sufficient energy to allow fertilization and maintain embryogenesis [51]. Mitochondria are involved in important processes during oocyte meiosis, including spindle assembly, chromosome segregation and cell maturation [52]. Therefore, the number and distribution of mitochondria, along with alterations of the mtDNA sequence, are closely related to the quality of oocytes and have important impacts on embryonic development [53]. Zhang et al. constructed a secondary oocyte OS injury model and found that the ATP level and mitochondrial membrane potential were both decreased; this was accompanied by spindle damage [54]. Recombinant peroxiredoxin 3 (Prdx3) is localized in the mitochondria and acts as a key regulator of mitochondrial ROS [55]. Global gene expression analysis of aged mouse oocytes revealed reduced Prdx3 mRNA expression and increased sensitivity of oocytes to OS [56].
Granulosa cells represent the largest cell population in the ovary. Their growth, proliferation and division require abundant and stable mitochondria to supply appropriate amounts of energy [57]. During proliferation, granulosa cells experience a significant increase in ROS levels and mtDNA damage [58]. At the same time, granulosa cells exhibit reduced mitochondrial membrane potential and reduced expression levels of mitochondrial-related genes (Nd1, Cytb, Cox1 and ATPase6), ultimately resulting in a poor state of decreased viability and cell cycle arrest [59]. Tanabe et al. reported that the OS damage products 8-OHdG, yH2AX and HEL were significantly elevated and that the proportion of active mitochondria was significantly reduced in an OS injury model of granulosa cells [60]. Mitochondrial dysfunction disrupted the bidirectional interaction between oocytes and granulosa cells, blocking the exchange of important substances such as pyruvate, amino acids and nucleotides, which in turn lead to stagnant cell growth and development [61].
Telomeres
Telomeres are short repetitive sequences that are located at the ends of eukaryotic chromosomes and mainly composed of non-coding DNA and telomere-binding proteins. The telomeres are responsible for maintaining genome integrity and chromosomal stability [62]. Telomere length gradually shortens with an increasing number of cell divisions and are therefore considered to be closely associated with the degree of aging in the human body [63]. The correlation between telomere status and ovarian function in women has also received increasing levels of attention. Clinical studies have also found that the length of telomeres in the granulosa cells of POI patients is significantly shorter, and that telomerase activity is significantly reduced [64]. Telomere damage was also observed in naturally aged ovarian cells, along with reduced expression levels of telomerase (TERC), telomeric reverse transcriptase (TERT) and telomere-related proteins (TRF1, TRF2, POT1) [65, 66]. The relative length of telomeres in cumulus cells is closely related to oocyte and embryo quality and can be used as a potential marker for screening high-quality oocytes in the field of assisted reproductive technology (ART) [67].
Telomeres lack protective proteins and are therefore highly susceptible to ROS attack and shortening [68]. Sirtuin 6 (Sirt6) is thought to play an important role in the stabilization of telomeres in oocytes [69]. As a trans-activator, Sirt6 participates in the regulation of Nrf2, thus maintaining cellular redox homeostasis [70]. Liao et al. found that both the mRNA and protein levels of Sirt6 were significantly lower in aged oocytes and that this was accompanied by a reduction in telomere length [71]. Ge et al. found that Sirt6-specific depletion in oocytes could exacerbate mitochondrial dysfunction and apoptosis in early embryos [72].
Antioxidant supplementation was previously shown to improve OS and telomere status, thus alleviating ovarian aging. A study by Akino et al. showed that the administration of dimethyl fumarate (DMF) led to the activation of the Keap1/Nrf2 signaling pathway in the ovaries of aged mice [73]. DMF supplementation increased mRNA and protein expression levels of telomerase and increased the number of primordial follicles [73]. In another study, Liu et al. found that long-term supplementation with low doses of NAC increased telomere length, elevated telomerase activity, improved oocyte quality and the number of fertilized oocytes, ultimately increasing litter size [74].
Biomacromolecules
Excess ROS can result in oxidative damage to intracellular biomacromolecules such as proteins and lipids [75]. Approximately 70% of oxidized molecules in cells are proteins, indicating that proteins are the main targets of ROS attack [76]. OS can lead to multiple modifications of proteins, including tyrosine nitration, sulfonation, thiol oxidation and 4-hydroxynonenal (4HNE) protein adducts [77]. Impaired protein function further leads to abnormal activation or inactivation of the signaling pathways required for normal ovarian physiology [77]. A number of studies have shown that multiple protein OS damage products can be observed in ovarian cells and follicular fluid, including advanced oxidation protein products (AOPPs), carbonylated and nitrated proteins, 4-HNE [78,79,80,81]. Follicular membranes contain large amounts of unsaturated fatty acids which are susceptible to ROS attack, thus leading to lipid peroxidation (LPO). LPO leads to the formation of acrolein and malondialdehyde (MDA) which can lead to a further increase in ROS production [82]. Oocyte meiosis and granulosa cell proliferation require an adequate supply of protein, carbohydrate and lipid; however, OS can directly damage macromolecular structures and therefore lead to ovarian damage [83].
Factors related to OS that lead to ovarian aging
Aging
Age is the most important intrinsic factor affecting ovarian function and fertility [84]. With increasing age, the number of primordial follicles decreases exponentially, while the frequency of aneuploidy in oocytes increases, eventually leading to a significant reduction in pregnancy and live birth rates [85]. Clinical studies have shown that a woman’s fertility declines linearly by approximately 10% per year after the age of 35, with only 1% fertility by the age of 43 [86].
Aging leads to increased ROS production and reduced activity of antioxidant systems, which together lead to increased intracellular OS damage. Accumulation of DNA damage is one of the key factors in damage of oocyte quality with age [87]. DNA damage accumulation increases chromosomal fragmentation and affects meiosis, spindle assembly and mitochondrial distribution in the oocyte, ultimately affecting embryo development [88]. Prolonged quiescence during meiosis I renders oocytes unusually sensitive to the accumulation of DNA damage [89].
DNA damage occurs frequently in aged oocytes, such as DNA double-strand breaks (DSBs), however, DNA damage response (DDR) repair is inefficient [90]. Studies have confirmed that the expression levels of oocyte DNA repair genes, such as BRCA1, MRE11, and H2AX, decrease significantly with age [91, 92]. BRCA1 recruits various DNA repair proteins to promote the homologous recombination (HR)-based pathway for DNA double-strand break repair (DSBR), and therefore plays an important role in DNA repair in aging ovaries [93, 94]. In addition, BRCA1 is required to prevent abnormal chromosome segregation [95]. Genome-wide association study (GWAS) analysis confirmed that genes associated with DDR, particularly BRCA1, are key determinants of the natural menopausal age in women [96].
Mitochondrial dysfunction due to aging has also been found to be a fundamental factor in the decline of oocyte quality. Mitochondrial dysfunction increases ROS leakage and mtDNA mutations and reduces ATP synthesis, thus affecting meiosis and decreasing oocyte quality [56]. In addition, biomacromolecular damage also accumulates in ovarian cells with age; this represents another cause of ovarian hypofunction [97].
Cigarette smoking
The extensive harm of cigarette smoking to the human body is well established. Smoking has been demonstrated to damage ovarian function and closely related with infertility, pregnancy complications and fetal abortion [98]. Tobacco smoke contains stable pro-oxidants that can directly increase ROS in the body [99]. Tobacco also contains harmful chemicals such as nicotine and tar that can deplete protective antioxidants, ultimately leading to an OS state [100].
Researchers collected follicular fluid from female patients undergoing ART. High levels of tobacco metabolites were found in the follicular fluid of women who smoked, along with increased levels of lipid peroxidation damage and decreased levels of antioxidants [101, 102]. A meta-analysis of 12 studies reported that time to conception, the incidence of infertility, and the number of IVF treatment cycles, were significantly higher in smokers than in non-smokers; this was associated with ovarian OS damage caused by tobacco exposure [98].
The extent of ovarian OS damage is thought to fluctuate with the amount and duration of smoke exposure [103]. Any level of smoke exposure cannot be considered safe, either passively or only at low levels [104]. This raises a warning for women of childbearing age who have a smoking habit or are often passively exposed to cigarette smoke. Moreover, the harm caused by cigarette smoking can also impair ovarian function in female offspring of smokers. Studies investigating the ovaries of female offspring (F1 generation) of smoking mothers showed significantly increased OS levels in oocytes, further leading to apoptosis and the abnormal proliferation of granulosa cells [105]. This effect continued into the F2 generation with impact on ovarian function not declining until the F3 generation [106].
High-sugar diet
High-sugar diets are generally considered to be an unhealthy lifestyle [107]. Abundant clinical studies have confirmed that a high-sugar diet can lead to an OS state in the body, which accelerates the development of multisystem pathologies, such as diabetes, neurodegenerative and cardiovascular diseases [108,109,110]. A high-sugar diet, whether long-term, short-term or fluctuating, can affect the body’s redox status and thus have a negative impact on ovarian function [111].
Excess carbohydrates in the body combine with proteins to form advanced glycation end products (AGEs) [112]. AGEs bind to specific cell surface receptors (RAGE) and promote ROS production via NF-κB and NADPH oxidase [113]. RAGE proteins are abundantly distributed on the membranes of oocytes, stromal cells and granulosa cells. Therefore, prolonged exposure to high concentrations of AGEs can lead to a gradual accumulation of OS damage in the ovary [114].
In addition, ROS are involved in a key step in the modified advanced glycation in AGE production. Thus, the accumulation of AGEs and the increase of ROS form a positive feedback loop that together increase OS [115]. Both AGEs and ROS can interfere with insulin signaling pathways and affect the normal function of ectopic glucose transporters. And this leads to a reduction in the uptake of glucose by ovarian cells, resulting in poor follicle development and an acceleration of ovarian aging [116]. OS damage induced by AGEs may also induce inflammation and hypoxia, damaging the blood vessels of the ovaries and further accelerating ovarian aging [116]. In addition, AGEs can directly stimulate the production of extracellular matrix (ECM) and the abnormal cross-linking of collagen and elastin in the ovary, thus affecting the proliferation and division of granulosa cells and ultimately disrupting ovarian function [117].
Pressure
Females are frequently exposed to multiple forms of pressure, including career, family and childbirth [118]. Both repeated acute stress and long-term persistent psychological stress have been shown to be independent risk factors for pregnancy rates, live birth rates, preterm delivery and low birth weight [119].
Pressure can impair ovarian function in many ways. On the one hand, pressure can induce the release of the stress hormone cortisol, which acts on the hypothalamic–pituitary–adrenal (HPA) and hypothalamic-pituitary-ovarian (HPO) axes to elicit direct negative effects on the ovary [120]. At the same time, the body’s adaptation to pressure leads to a dramatic rise in intracellular OS and an endogenous burst of internal calcium stores [121]. These trigger multiple regulatory mechanisms in ovarian cells, such as autophagy, apoptosis and paraptosis, further leading to cell cycle arrest and a decline in ovarian function [122, 123]. On the other hand, cortisol also reduces the secretion of antioxidants such as estradiol-17β, thus leading to decreased OS defense in ovarian cells [124]. Zhao et al. investigated the effect of chronic unpredictable mild stress (CUMS) on ovarian function in rats. The results showed that the CUMS group had increased ovarian ROS levels, mitochondrial dysfunction and cell apoptosis, along with decreased AMH and E2 levels. [125, 126]. In addition, pressure has been found to decrease levels of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) [127, 128]; thus leading to over-activation of the sympathetic nerves in the ovary, thereby impairing ovarian function [129].
Superovulation
Controlled ovarian hyperstimulation (COH) is widely used in IVF-ET to obtain a sufficient number of oocytes for downstream processing [130]. However, successful ovulation is accompanied by a rapid increase in OS levels and inflammatory response [131]; thus, whether COH can lead to ovarian OS damage has received widespread attention.
Clinical studies have identified significantly lower levels of antioxidants (including alpha-tocopherol, TAA and paraoxonase) in the follicular fluid of women receiving COH intervention when compared to women receiving natural cycle (NC) intervention [132]. Women who receive COH also exhibited an abnormal inflammatory response [133, 134]. OS damage to the ovaries was also significantly correlated with COH cycles. Higher markers of OS damage and lower antioxidant enzymes in the ovaries could be observed in cycles 3–5 when compared to cycles 1–2. This was accompanied by lower quality embryo rates, implantation rates and clinical pregnancy rates [135].
Similarly, related experimental studies have confirmed the oxidative damage to the ovaries by superovulation. Nie et al. performed continuous superovulation intervention in mice and observed a significant increase in OS damage products and oocyte apoptosis in the super-promoting group that was associated with activation of the Sirt1/FoxO1 signaling pathway [136]. Repeated ovulation promotion increased the number of abnormal mitochondria in mouse oocytes, reduced the volume of the primordial follicular pool, decreased serum AMH levels and significantly inhibited embryo development [137]. This process also significantly increases the risk of long-term complications such as osteoporosis and cardiovascular disease [138]. In contrast, oral contraceptives have been shown to alleviate age-related ovarian aging and fertility decline by suppressing ovulation [139].
Chemotherapy
The application of chemotherapy agents has increased the chances of long-term survival for cancer patients. However, the damage that these agents cause to ovarian function has received increasing levels of attention [140]. Chemotherapy not only induces atresia of growing follicles leading to temporary amenorrhea [141], but also accelerates overactivation of the primordial follicular leading to a reduced ovarian reserve [142]. Multiple clinical trials have identified varying degrees of impaired ovarian function in women who have received chemotherapy [143,144,145].
OS damage has been identified as an important mechanism by which chemotherapy side effects occur [146]. Cisplatin is commonly used to treat solid tumors. Researchers gave rats an intraperitoneal injection of cisplatin and found that the expression levels of 8-OHdG and MDA increased significantly, while the levels of antioxidant enzymes SOD and GSH-Px decreased significantly in the ovaries [147]. At the same time, the ovarian cortex was significantly damaged or was even indistinguishable from the medulla [146]. Paclitaxel is often used in combination with carboplatin as a chemotherapy regimen for non-small cell lung cancer and ovarian cancer. Qin et al. found that paclitaxel intervention in mice resulted in a significant increase in the lipid peroxidation product 4-HNE and apoptosis in the ovary; this was accompanied by a reduced number of follicles, along with morphological abnormalities of follicles at all stages [140]. Methotrexate (MTX) is commonly used to treat tumors and autoimmune diseases. MTX has been shown to deplete NADPH and antioxidant enzymes and to induce mitochondrial dysfunction, DNA breaks and disruption of the spindle assembly, thereby affecting oocyte maturation [148]. MTX could also cause severe follicular degeneration and intra-ovarian hemorrhage and edema, ultimately destroying ovarian function [149].
Industrial pollution
With the rapid development of industrialization, many industrial by-products have been shown to affect ovarian function by increasing ROS levels. Bisphenol A (BPA) is an important material used in the synthesis of polycarbonate and epoxy resins. BPA was found to increase ROS levels in ovarian cells in a dose- and time-dependent manner, reduce mitochondrial membrane potential, and activate the JNK signaling pathway to accelerate granulosa cell apoptosis [150]. Fluorene 9-bisabolol (BHPF), as a substitute for BPA, also poses a risk to ovarian function. Following BHPF intervention, oocytes were found to exhibit increased levels of ROS and DNA damage, accompanied by mitochondrial dysfunction, polar body extrusion and disruption of the spindle assembly [151]. Nonylphenol (NP) is an important raw material for the fine chemical industry. Related studies have shown that NP can increase ROS levels in rat ovarian granulosa cells, further activating the AKT/AMPK/mTOR signaling pathway, triggering excessive cellular autophagy and ultimately reducing ovarian function [152]. Di-phthalate (DEHP) is a type of common plasticizer and has been found to increase intracellular β-galactosidase (β-gal) activity, activate the Bax/Bcl-2 signaling pathway and CASP3 expression, and inhibit steroid synthesis, ultimately leading to premature ovarian aging [153].
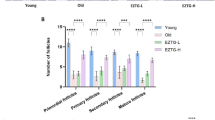
In conclusion, a variety of external factors can accelerate ovarian aging by increasing the OS state in the body. External factors include lifestyle choices such as smoking, high-sugar diets, and excessive psychological pressure; superovulation during ART, chemotherapy drugs and industrial environmental pollutants are also important factors, as summarized in Table 1. An increased OS state can cause cellular damage, including DNA damage, mitochondrial dysfunction and biomacromolecular damage. OS damage in cells can lead to the meiotic arrest of oocytes, the inhibition of granulosa cell proliferation, and the abnormal proliferation of interstitial cells, all of which can accelerate the ovarian aging process, as demonstrated in Figs. 1 and 2.
OS-related signaling pathways in ovarian aging
Excessive ROS can result in crosstalk between a series of signaling pathways and protein factors within the body. This section focuses on signaling pathways related to OS and ovarian function, including Nrf2, Sirtuins, MAPK, AKT, FoxO family and Klotho signaling pathways, as demonstrated in Fig. 3.
OS-related signaling pathways in ovarian aging. Excess levels of ROS promote the dissociation of the Keap1-Nrf2 complex and Nrf2 translocation into the nucleus to bind to AREs, thus promoting the expression of antioxidant enzymes. Sirt can deacetylate key proteins involved in the cellular stress response such as FoxO, and regulate both telomerase activity and mitochondrial function through PGC1α. The MAPK cascade signaling pathway is activated by ROS to deliver extracellular signals to the nucleus, promote apoptosis, inhibit proliferation and induce cell cycle arrest. AKT plays an important role in the regulation of cellular redox homeostasis, and phosphorylated AKT can regulate a variety of downstream proteins (Bad, mTOR, Cyclins and Nrf2) to further regulate cellular apoptosis, autophagy and proliferation. FoxO senses cellular OS status and acts as a transcription factor to regulate cell apoptosis and the expression of antioxidant enzymes. Klotho regulates cellular oxidative homeostasis through the PI3K/AKT pathway, and the HPO axis through the FGF-Klotho endocrine system
Nrf2 signaling pathway
Nuclear factor-E2-related factor 2 (Nrf2) is a transcription factor involved in regulating antioxidant responses to protect cellular functions [154]. Under normal conditions, Nrf2 binds to Kelch-like ECH-associated protein 1 (Keap1), leading to the ubiquitination and degradation of Nrf2. This facilitates only a basal level of antioxidant enzyme expression. However, in response to cellular OS, the Nrf2-Keap1 complex dissociates and Nrf2 is translocated to the nucleus, thus leading to the rapid accumulation of nuclear Nrf2. Nuclear Nrf2 binds genomic antioxidant response elements (AREs) to promote the gene expression of a series of target genes including antioxidant enzymes and detoxification factors, such as heme oxygenase-1 (HO-1), NAD(P)H: quinone-oxidoreductase (NQO1), and glutamate-cysteine ligase subunit catalysis (GCLC) [155]. Lead exposure in mice inhibited the Keap1/Nrf2 pathway and exacerbated ovarian OS damage, which inhibited oocyte maturation and fertilization [156]. Compared to wild-type mice, Nrf2−/− mice exhibited premature follicular activation and thus an age-dependent decline in ovarian function [157]. Loss of Nrf2 function also blocked microsomal epoxide hydrolase expression and reduced cellular antioxidant capacity, thereby increasing ovarian sensitivity to environmental pollutants [158, 159].
Related antioxidant treatment may also improve ovarian OS status by modulating the Nrf2 pathway. The oral administration of DMF intervention to mice was shown to upregulate ovarian tissue antioxidant enzyme and telomere protein expression, increase serum AMH level, slow down DNA damage accumulation, and protect the primordial follicular pool through the Nrf2 pathway [73]. In vitro experiments further revealed that the antioxidant epicatechin could protect granulosa cells from OS damage by activating the Nrf2 signaling pathway, upregulating NQO1, HO-1 and SOD expression [160]. Therefore, the Nrf2 signaling pathway is one of the most important defense mechanisms for cells to resist OS damage.
Sirt signaling pathway
Sirtuins belong to the class III nicotinamide adenine dinucleotide NAD + dependent deacetylase family which included seven subtypes; these proteins are involved in many physiological functions in cells [161]. Of these, Sirt1 is a key regulatory protein of cellular metabolism and OS and has been extensively studied in ovarian function [162].
Sirt1 can deacetylate key proteins involved in the cellular stress response, such as FoxO, causing the upregulation of antioxidant enzymes such as CAT and GSH-Px1 [163]. Previous research showed that consecutive superovulation could exacerbate OS damage in the ovary, increase granulosa cell apoptosis and decrease oocyte quality and primordial follicle number through the Sirt1/FoxO1 signaling pathway [136]. Sirt1 was shown to be involved in the first-line defense against ROS via the FoxO3a-MnSod axis in the germinal vesicle phase (GV) of immature mouse oocytes [164]. Sirt1 deletion in oocytes led to reduced oocyte quality, the inhibition of oocyte division, and increased OS damage during the embryonic period, ultimately causing negative effects on pregnancy outcome [165]. The knockdown of Sirt1 in granulosa cells was found to impair E2 synthesis and secretion and significantly reduce the expression of E2-related receptors (ESR, FSHR and AMHR2) [166]. In addition, Sirt1 mediates the activation of peroxisome proliferator-activated receptor gamma coactivator l-alpha (PGC1α), further promoting mitochondrial biogenesis and oxidative phosphorylation during primordial follicle activation [167]. Therefore, Sirt1 has an important role in the antioxidant protection of oocytes, granulosa cells and early embryos.
Other subtypes of the Sirtuins family also play a role in the protection of ovarian function. Sirt2 controlled histone H4K16 deacetylation and is therefore a key effector of oocyte meiosis [167]. Sirt3 can regulate the expression of aromatase and 17-hydroxysteroid dehydrogenase, and promote progesterone secretion [168]. Sirt6 has been shown to prevent OS-induced DNA damage and play an important role in stabilizing oocyte telomeres [79].
MAPK signaling pathway
The mitogen-activated protein kinase (MAPK) pathway is an important transmitter that mediates extracellular signals from the cell membrane surface to the nucleus [169]. The MAPK signaling pathway consists of MAP kinase kinase kinase (MKKK), MAP kinase kinase (MKK) and MAPK. Tertiary kinases are activated sequentially and participate in internal and external reactions to further regulate cell proliferation, differentiation, survival and death [170].
Extracellular regulated protein kinases (ERK), p38 MAPK and c-Jun N-terminal kinase (JNK) are all part of the MAPK family and have been extensively studied in ovarian aging [171]. Sun et al. demonstrated that CUMS could exacerbate ROS levels in mouse ovaries, inhibit granulosa cell proliferation and accelerate cellular senescence via the MAPK pathway [172]. The ERK pathway was shown to be involved in autophagy-related inhibition of cell proliferation and cellular senescence, while the JNK and p38 MAPK pathways have been shown to be associated with cell cycle arrest [172]. Apoptosis signal-regulating kinase 1 (ASK1) is a widely expressed redox-sensitive mitogen/threonine kinase. Upon cellular OS injury, ASK1 was activated and further activated JNK, thereby inducing downstream signaling [173]. Exposure to bisphenol AF can regulate the ROS-ASK1-JNK pathway, reduce mitochondrial membrane potential, and induce apoptosis in ovarian granulosa cells [174].
Antioxidant supplementation can reduce the damaging effects of ROS on the ovaries through the MAPK pathway. Melatonin can inhibit mROS production and increase antioxidant enzyme expression through the ERK pathway to further improve excessive autophagy induced G2/M cell cycle arrest [175]. NAC was shown to be able to attenuate OS damage in ovarian granulosa cells by advanced oxidation protein products (AOPPs) via the JNK/p38 MAPK-p21 pathway, ultimately improving follicular atresia [176].
AKT signaling pathway
Serine/threonine kinase AKT is the central survival mediator in cell signaling transmission and is activated by a variety of stimuli including ROS, growth factors and cytokines [177]. Phosphorylated AKT can regulate a variety of downstream proteins, such as Bad, mTOR and Cyclins, thus playing an important role in various biological processes such as apoptosis, autophagy and proliferation [178].
Bad is a Bcl-2 homology domain 3-related protein involved in apoptosis. AKT can regulate Bad activity by phosphorylating at Ser-136 [179]. Dephosphorylated Bad was able to form a pro-apoptotic complex with anti-apoptotic factors Bcl-2 or Bcl-xL, which activated pro-apoptotic members such as Bax or Bak, and jointly promoted cell apoptosis [180]. Studies have shown that H2O2 can inhibit the PI3K/AKT pathway, and induce the elevated expression of Bad, Bax and CASP9, further accelerating granulosa cell apoptosis [181]. Meanwhile, OS can also increase the expression of p53 up-regulated modulator of apoptosis (PUMA) through the PI3K/AKT pathway and induce apoptosis in another way [182]. mTOR is a serine/threonine protein kinase in the PI3K-related kinase (PIKK) family. AKT blocks the negative regulation of small G protein Rheb by TSC1/2 and indirectly activates mTOR complex 1 (mTORC1) which is involved in the critical regulation of cellular autophagy [183]. OS injury initiates autophagy by inhibiting the PI3K/AKT signaling pathway, promoting the dissociation between mTORC1 and ULK1, further phosphorylating autophagy-related proteins such as Atg13 [184]. It was found that H2O2 intervention in granulosa cells inhibited the PI3K/AKT/mTOR pathway and increased LC3-II/LC3-I and Beclin-1 expression [185]. Activation of AKT regulates the cell cycle by regulating the function of cell cycle proteins (cyclins). Intervention of isorhamnetin reduced cellular OS level, and increased the expression of Cyclin D, Cyclin E and Cyclin A by PI3K/AKT pathway, ultimately promoting granulosa cell proliferation [186].
FoxO signaling pathway
Forkhead box O (FoxO) is a family of transcription factors consisting of FoxO1, FoxO3, FoxO4 and FoxO6. OS can activate FoxO signaling pathway through phosphorylation, mono-ubiquitination and glycosylation [187]. Activated FoxO is transferred to the nucleus and regulates the expression of a series of downstream genes, further regulating apoptosis, autophagy and cell cycle arrest [188].
FoxO1 has been extensively studied in ovarian aging. It was confirmed that FoxO1 expression was most abundant in the granulosa cells of atretic follicles and was mainly localized in the nucleus [189]. Liu et al. found that 3-NP intraperitoneal injection significantly promoted FoxO1 transfer into the nucleus, upregulated the expression of PUMA, induced granulosa cell apoptosis and eventually led to follicular atresia [190]. In vitro experiments further showed that H2O2 activated the FoxO1 pathway and promoted the expression of downstream FasL, CASP3, and Bim, thus leading to apoptosis in the ovarian granulosa cells in a dose-dependent manner [191].
FoxO1 is also involved in the majority of the regulatory processes of FSH on granulosa cells [192]. FSH was previously shown to block post-translational modification of FoxO1 in granulosa cells and reduce FoxO1 expression, thus promoting cellular differentiation and follicle growth [189]. Furthermore, FSH inhibited the production of acetylated FoxO1 and its interaction with autophagy-related gene (Atg) protein through the PI3K/AKT pathway, thus reducing the level of cellular autophagy and ultimately inhibiting the death of ovarian granulosa cell [193].
FoxO3a is considered to be an ideal candidate gene for longevity and health [194]. The absence of FoxO3a led to the premature depletion of primordial follicles [195]. Cisplatin intervention significantly downregulated p-FoxO3a and antioxidant enzyme expression in mouse ovaries, impaired mitochondrial function, and ultimately accelerated apoptosis [196, 197]. A maternal high-fat diet could also reduce the number of primordial follicles in the ovaries of offspring via the FoxO3a pathway [198]. Conversely, oyster peptides upregulated the expression of FoxO3a and T-SOD, downregulated the expression of p53 and Bad, and attenuated D-galactose-induced premature ovarian decline [199].
Klotho signaling pathway
Klotho is known to play an important role in the inhibition of aging. High levels of Klotho expression can prolong human lifespan, while low expression levels can lead to accelerated aging and increase the risk of multisystem diseases [200]. Klotho is mainly present intracellularly in transmembrane and secreted forms [201]. The transmembrane form of Klotho is a full-length transcript encoding 1014 amino acids. Once the short transmembrane structural domain is removed, this fragment can be released into the circulation in a secreted form [202].
Membrane Klotho is a co-receptor for endocrine fibroblast growth factors (FGF) and is involved in the activation of FGF receptors. Numerous studies have shown that the FGF-Klotho endocrine system plays a key role in the development of reproductive disorders by regulating the HPO axis [203]. Klotho-deficient mice exhibited dysfunction of the HPO axis as evidenced by a decrease in FSH and LH, follicular arrest in proestrus, gonadal atrophy and infertility [204].
Secreted forms of Klotho are more abundant than membrane Klotho. As an endocrine regulator, Klotho is thought to inhibit OS and modulate ion channel activity to exert positive anti-aging effects [205]. Studies have demonstrated that reduced Klotho expression activates the PI3K/AKT pathway, downregulates intracellular FoxO3a expression, disrupts oxidative homeostasis and inhibits autophagy, thus accelerating apoptosis [206].
Additional antioxidant therapy that can improve ovarian aging
Melatonin
Melatonin is an indoleamine secreted by the pineal gland located in the third ventricle. With lipid-soluble and water-soluble properties, melatonin can pass through cell membranes easily and is thus present in the blood and body fluids abundantly [207]. It has been found that melatonin is present in high concentrations in human follicular fluid [208]. Melatonin has a good antioxidant effect by neutralizing free radicals and increasing the activity of antioxidant enzymes such as SOD and GSH-Px [209]. As an important endogenous antioxidant, its antioxidant properties are superior to conventional antioxidants, such as vitamin C and E [210].
Melatonin is widely used in ART [211]. In patients undergoing IVF-ET, exogenous melatonin supplementation significantly reduced 8-OhdG and HEL concentrations in follicular fluid [212]. Additional melatonin also increased follicular growth rate, improved oocyte quality, increased fertilization rate and the number of quality embryos, ultimately increasing pregnancy rate [213]. Melatonin has also been shown to protect luteal granulosa cells from OS damage and increase progesterone secretion [214]. Moreover, melatonin was shown to reduce excessive Ca2+ levels in immature human oocytes during in vitro maturation (IVM) and improved the maintenance of mitochondrial membrane potential, thereby avoiding further ROS production [215].
Numerous experimental studies have demonstrated the important role of melatonin in improving oocyte quality and pregnancy outcomes. Melatonin significantly reduced the production of mitochondria ROS (mROS) in rat ovaries, increased telomere length, improved oocyte quality and ultimately increased the litter size [216]. Melatonin enhances the repair of DSBs via the non-homologous end joining (NHEJ) pathway to protect oocytes from the accumulation of DNA damage [89]. Melatonin can also upregulate antioxidant enzyme expression by inducing demethylation of the promoter regions of SOD, GSH-Px4 and CAT, thereby enhancing the antioxidant capacity of cumulus cells [217]. Melatonin activates ErbB1 and ErbB4 gene expression to promote embryo implantation and blastocyst growth, and further protects embryos from OS [218].
Melatonin has been shown to protect the ovaries from chemotherapy-induced damage. Barberino et al. performed melatonin pretreatment in mice prior to exposure to cisplatin. The results showed that melatonin reduced ROS levels in the ovary, increased mitochondrial activity, inhibited CASP3 expression and alleviated oocyte retraction and lysis [219]. Similarly, Jang et al. demonstrated that melatonin reduced cisplatin-induced excessive activation of the primordial follicles by modulating the PTEN/AKT/FOXO3a pathway [220].
Vitamins
Vitamin C (VC), also known as ascorbic acid, is an excellent water-soluble natural antioxidant [221]. Extensive studies have demonstrated that VC can protect ovaries from harmful compounds such as NaF and As2O3 by upregulating the expression of SOD, CAT and GSH-Px and by reducing lipid peroxidation (LPO) [222]. Gai et al. found that VC also significantly ameliorated ambient aerosol fine particulate matter (PM2.5)-mediated ovarian damage, reduced the levels of oxidative products and inflammatory factors, decreased apoptosis and protected the mitochondrial ultrastructure [223]. In addition, VC could regulate cell proliferation and differentiation and steroid production, promote follicle growth and regulate endocrine secretion, and ultimately improve ovarian aging [224, 225].
Vitamin E (VE) is an important fat-soluble antioxidant [226]. A clinical study found that VE combined with selenium supplementation significantly improved AMH index, antral follicle count (AFC) and mean ovarian volume (MOV) in patients with occult POI [227]. Experimental studies have found that VE intervention can enhance the antioxidant capacity of ovarian tissue, regulate endocrine hormones, reverse follicular atresia, and restore the normal vascular distribution of ovarian tissue [228]. VE was also shown to be involved in the normal antioxidant function of GSH-Px1 as an essential cofactor [229]. In addition, VE can improve glucose uptake by follicular cells through the upregulation of glucose transporter-1 (GLUT-1) expression [230] and inhibit the abnormal proliferation of ovarian theca-interstitial cells [231], thereby protecting ovarian function.
Stem cell therapy
Stem cells are a class of multipotential cells that can self-replicate and can differentiate into cells with multiple functions under certain conditions [232]. According to their differentiation potential, stem cells can be classified into totipotent stem cells, pluripotent stem cells and unipotent stem cells [233]. Due to excellent self-replication and multi-directional differentiation ability, stem cell therapy has attracted much attention in ovarian aging over recent years [234].
Mesenchymal stem cells (MSCs) are one of the most widely studied pluripotent stem cells. MSCs derived from different sources such as bone marrow, fat and amniotic fluid have been found to play a key role in restoring ovarian function and reproductive potential [235]. In the ovary, stem cells migrate mainly to the hilum and medulla after transplantation, with a few migrating to the cortex [236]. The migration of stem cells can exert effects on cell OS, proliferation and apoptosis mainly via paracrine action, thus repairing damaged ovaries [237]. Research has shown that human placental mesenchymal stem cells (hPMSCs) secreted a large amount of epidermal growth factor (EGF) which further activated the Nrf2/HO-1 signaling pathway to promote granulosa cell proliferation and oocyte maturation [238, 239]. Human umbilical cord mesenchymal stem cells (hUCMSCs) have been shown to reduce the autophagy level of theca interstitial cells (TICs) through the AMPK/mTOR signaling pathway, attenuate ovarian cell apoptosis, and ultimately improve ovarian function in POI rats [240]. It has been demonstrated that transplanted MSCs are able to restore ovarian function, including reducing OS damage, and participating in follicular maturation [241]. These findings provide new insights into our understanding of stem cell therapy and provide new avenues for developing more effective anti-aging treatments.
Antioxidant monomers
Extensive research has demonstrated that a variety of monomers can protect ovarian function through antioxidant mechanisms. Tea polyphenols (TPS) is the general term for the polyphenols in tea leaves and it has been widely investigated for its important healthcare effects [242]. TPS has been shown to inhibit the surge of OS and relieved autophagic pressure in ovarian tissue caused by industrial plasticizers [243]. Grape seed proanthocyanidin extract (GSPE) is an excellent oxygen free radical scavenger [244]. GSPE exerts protective effects on both D-gal-induced ovarian hypofunction and natural ovarian aging. It can maintain the balance between cell proliferation and apoptosis, and reduce nuclear chromatin clumping in ovarian granulosa cells [245]. Resveratrol (Res) is a natural plant-derived phenol with excellent antioxidant activity [246]. Res has been found to attenuate the damage caused by cyclophosphamide on ovarian function, including reducing OS damage, inhibiting apoptosis and promoting ovarian stem cell repair [18]. In vitro studies have also revealed that Res can activate Nrf2 protein expression and reverse H2O2-induced OS damage in oogonial stem cells in a dose-dependent manner [18]. Curcumin is a diketone compound with excellent antioxidant and anti-inflammatory effects [247]. Curcumin intervention is shown to upregulate the expression of growth differentiation factor-9 (GDF-9) and bone morphogenetic protein 15 (BMP-15) and increase the number of follicles in mouse ovaries [248].
Traditional Chinese medicine
Traditional Chinese Medicine (TCM) has a long history in the treatment of ovarian aging. Several TCM therapies have been demonstrated to modulate ovarian aging by alleviating OS damage. Kuntai capsule is a proprietary Chinese medicine that is widely used to treat menopause syndrome [249]. Research has shown that Kuntai capsule can upregulate SOD2, reduce ovarian apoptosis and follicular atresia, and increase AMH level in the ovaries of superovulated mice [250]. Bu Shen Huo Xue Tang (BSHXT) is a TCM formula that is clinically used in the treatment of POI. Research has shown that BSHXT can upregulate the expression of antioxidant enzymes SOD, HO-1 and NQO1 by activating the Nrf2/Keap1 signaling pathway and increase the levels of AMH and E2 in POI mice [251]. Acupuncture is an important external TCM treatment that has been proven to treat multiple age-related diseases [252]. Transcutaneous electrical acupoint stimulation (TEAS) is a combination of acupoint stimulation and electrical stimulation. TEAS intervention has been shown to upregulate the expression of antioxidant enzymes and proliferating cell nuclear antigen (PCNA), reduce apoptosis, and inhibit the loss of primordial follicles in ovarian senescent mice [253].
In conclusion, additional antioxidant therapy can improve the redox imbalance in the body, as summarized in Table 2. Some of the existing antioxidants, such as melatonin and vitamin E, have been used clinically and have proven their excellent antioxidant efficacy. However, it should be noted that most of the current antioxidant monomer studies have been applied only in animal models. Moreover, the efficacy of stem cell therapy in antioxidant therapy is also gaining attention. Therefore, these therapies should be validated in clinical settings in the future.
Conclusion and future directions
The role and mechanisms of OS in ovarian aging have been extensively studied. Multiple factors, such as aging, smoking, and high sugar diets, can promote an OS state in the body; these factors could further accelerate ovarian aging via several key mechanisms, including apoptosis, increased inflammation and mitochondrial damage. The regulation of Nrf2, Sirt, MAPK, AKT and other OS signaling pathways play important roles in ovarian OS damage. Related antioxidants, such as melatonin and vitamin E, could improve OS status to restore ovarian function and therefore have potential clinical applications. There are still several aspects that need to be studied in the future: (1) multiple proteins and pathways are involved in the regulation of OS; the regulatory networks and key mechanisms of OS in ovarian aging still need to be studied in depth; (2) the majority of OS-related studies have focused on in vivo and in vitro experiments; further extension of these findings to clinical trials is needed to explore the safety and efficacy of antioxidant therapies; (3) antioxidant therapy has been initially proven to improve the quality of oocytes in vitro; further studies are now needed to determine whether it can improve pregnancy outcomes in patients undergoing ART.
Availability of data and materials
Not applicable.
Abbreviations
- 4HNE:
-
4-Hydroxynonenal
- AGEs:
-
Advanced glycation end products
- AKT:
-
Protein kinase B
- AMH:
-
Anti-Müllerian hormone
- ART:
-
Assisted reproductive technology
- Bax:
-
Bcl-2-associated x
- Bcl-2:
-
B-cell lymphoma-2
- CAT:
-
Catalase
- COH:
-
Controlled ovarian hyperstimulation
- DMF:
-
Dimethyl fumarate
- DSBs:
-
DNA double-strand breaks
- E2 :
-
Estradiol
- ERK:
-
Extracellular regulated protein kinases
- FoxO:
-
Forkhead box O
- FSH:
-
Follicle stimulating hormone
- GSH:
-
Glutathione
- GSH-Px:
-
Glutathione peroxidase
- HO-1:
-
Hemeoxy genase-1
- HPA:
-
Hypothalamic–pituitary–adrenal
- HPO:
-
Hypothalamic-pituitary-ovarian
- IVF-ET:
-
in vitro Fertilization-embryo transfer
- JNK:
-
C-Jun N-terminal kinase
- LH:
-
Luteinizing hormone
- LPO:
-
Lipid peroxidation
- MAPK:
-
Mitogen-activated protein kinase
- MDA:
-
Malondialdehyde
- MSCs:
-
Mesenchymal stem cells
- mtDNA:
-
Mitochondrial DNA
- mTOR:
-
Mammalian target of rapamycin
- NF-κB:
-
Nuclear factor-k-gene binding
- NLRP3:
-
Recombinant NLR family pyrin domain containing Protein 3
- NQO1:
-
NAD(P)H: nicotinamide adenine dinucleotide phosphate
- Nrf2:
-
Nuclear factor E2-related factor 2
- OS:
-
Oxidative stress
- PI3K:
-
Phosphatidylinositol 3-kinase
- POI:
-
Premature ovarian insufficiency
- ROS:
-
Reactive oxygen species
- Sirt:
-
Sirtuin
- SOD:
-
Superoxide dismutase
- TCM:
-
Traditional Chinese Medicine
References
Jadeja RN, Martin PM, Chen W. Mitochondrial Oxidative Stress and Energy Metabolism: Impact on Aging and Longevity. Oxid Med Cell Longev. 2021;2021:9789086.
Li Z, Cheng J, Huang L, Li W, Zhao Y, Lin W. Aging Diagnostic Probe for Research on Aging and Evaluation of Anti-aging Drug Efficacy. Anal Chem. 2021;93(41):13800–6.
Ansere VA, Ali-Mondal S, Sathiaseelan R, Garcia DN, Isola JVV, Henseb JD, et al. Cellular hallmarks of aging emerge in the ovary prior to primordial follicle depletion. Mech Ageing Dev. 2021;194:111425.
Tesarik J, Galán-Lázaro M, Mendoza-Tesarik R. Ovarian Aging: Molecular Mechanisms and Medical Management. Int J Mol Sci. 2021;22(3):1371.
Chon SJ, Umair Z, Yoon MS. Premature Ovarian Insufficiency: Past, Present, and Future. Front Cell Dev Biol. 2021;9:672890.
Fuloria S, Subramaniyan V, Karupiah S, Kumari U, Sathasivam K, Meenakshi DU, et al. Comprehensive Review of Methodology to Detect Reactive Oxygen Species (ROS) in Mammalian Species and Establish Its Relationship with Antioxidants and Cancer. Antioxidants (Basel). 2021;10(1):128.
Goud AP, Goud PT, Diamond MP, Gonik B, Abu-Soud HM. Reactive oxygen species and oocyte aging: role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radic Biol Med. 2008;44(7):1295–304.
Marques-Pinto A, Carvalho D. Human infertility: are endocrine disruptors to blame? Endocr Connect. 2013;2(3):R15-29.
Vatner SF, Zhang J, Oydanich M, Berkman T, Naftalovich R, Vatner DE. Healthful aging mediated by inhibition of oxidative stress. Ageing Res Rev. 2020;64:101194.
Agarwal A, Gupta S, Sekhon L, Shah R. Redox considerations in female reproductive function and assisted reproduction: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10(8):1375–403.
Behrman HR, Kodaman PH, Preston SL, Gao S. Oxidative stress and the ovary. J Soc Gynecol Investig. 2001;8(1 Suppl Proceedings):S40-2.
Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49.
Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A. 2011;108(4):1462–7.
Wang Z, Tamura K, Yoshie M, Tamura H, Imakawa K, Kogo H. Prostaglandin F2alpha-induced functional regression of the corpus luteum and apoptosis in rodents. J Pharmacol Sci. 2003;92(1):19–27.
von Mengden L, Klamt F, Smitz J. Redox Biology of Human Cumulus Cells: Basic Concepts, Impact on Oocyte Quality, and Potential Clinical Use. Antioxid Redox Signal. 2020;32(8):522–35.
Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA, Joshi NJ, et al. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81(4):973–6.
Shen L, Chen Y, Cheng J, Yuan S, Zhou S, Yan W, et al. CCL5 secreted by senescent theca-interstitial cells inhibits preantral follicular development via granulosa cellular apoptosis. J Cell Physiol. 2019;234(12):22554–64.
Wu M, Ma L, Xue L, Ye W, Lu Z, Li X, et al. Resveratrol alleviates chemotherapy-induced oogonial stem cell apoptosis and ovarian aging in mice. Aging (Albany NY). 2019;11(3):1030–44.
Yang X, Wang W, Zhang Y, Wang J, Huang F. Moxibustion improves ovary function by suppressing apoptosis events and upregulating antioxidant defenses in natural aging ovary. Life Sci. 2019;229:166–72.
Yang L, Chen Y, Liu Y, Xing Y, Miao C, Zhao Y, et al. The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front Pharmacol. 2020;11:617843.
Teixeira CP, Florencio-Silva R, Sasso GRS, Carbonel AAF, Simões RS, Simões MJ. Soy isoflavones protect against oxidative stress and diminish apoptosis in ovary of middle-aged female rats. Gynecol Endocrinol. 2019;35(7):586–90.
Jalouli M, Mofti A, Elnakady YA, Nahdi S, Feriani A, Alrezaki A, et al. Allethrin Promotes Apoptosis and Autophagy Associated with the Oxidative Stress-Related PI3K/AKT/mTOR Signaling Pathway in Developing Rat Ovaries. Int J Mol Sci. 2022;23(12):6397.
Wu J, Li J, Liu Y, Liao X, Wu D, Chen Y, et al. Tannic acid repair of zearalenone-induced damage by regulating the death receptor and mitochondrial apoptosis signaling pathway in mice. Environ Pollut. 2021;287:117557.
Kong L, Gao X, Zhu J, Cheng K, Tang M. Mechanisms involved in reproductive toxicity caused by nickel nanoparticle in female rats. Environ Toxicol. 2016;31(11):1674–83.
Xue R, Li S, Zou H, Ji D, Lv M, Zhou P, et al. Melatonin alleviates deoxynivalenol-induced apoptosis of human granulosa cells by reducing mutually accentuated FOXO1 and ER stress‡. Biol Reprod. 2021;105(2):554–66.
Kunitomi C, Harada M, Takahashi N, Azhary JMK, Kusamoto A, Nose E, et al. Activation of endoplasmic reticulum stress mediates oxidative stress-induced apoptosis of granulosa cells in ovaries affected by endometrioma. Mol Hum Reprod. 2020;26(1):40–52.
Palmerini MG, Nottola SA, Tunjung WA, Kadowaki A, Bianchi S, Cecconi S, et al. EGF-FSH supplementation reduces apoptosis of pig granulosa cells in co-culture with cumulus-oocyte complexes. Biochem Biophys Res Commun. 2016;481(1–2):159–64.
Jiang Y, Zhu D, Liu W, Qin Q, Fang Z, Pan Z. Hedgehog pathway inhibition causes primary follicle atresia and decreases female germline stem cell proliferation capacity or stemness. Stem Cell Res Ther. 2019;10(1):198.
Guan Y, Zhang W, Wang X, Cai P, Jia Q, Zhao W. Cell-free DNA induced apoptosis of granulosa cells by oxidative stress. Clin Chim Acta. 2017;473:213–7.
Chung HY, Kim DH, Lee EK, Chung KW, Chung S, Lee B, et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019;10(2):367–82.
Huang Y, Hu C, Ye H, Luo R, Fu X, Li X, et al. Inflamm-Aging: A New Mechanism Affecting Premature Ovarian Insufficiency. J Immunol Res. 2019;2019:8069898.
Sanverdi I, Kilicci C, Cogendez E, Abide Yayla C, Ozkaya E. Utility of complete blood count parameters to detect premature ovarian insufficiency in cases with oligomenorrhea/amenorrhea. J Clin Lab Anal. 2018;32(5):e22372.
Wang D, Weng Y, Zhang Y, Wang R, Wang T, Zhou J, et al. Exposure to hyperandrogen drives ovarian dysfunction and fibrosis by activating the NLRP3 inflammasome in mice. Sci Total Environ. 2020;745:141049.
Navarro-Pando JM, Alcocer-Gómez E, Castejón-Vega B, Navarro-Villarán E, Condés-Hervás M, Mundi-Roldan M, et al. Inhibition of the NLRP3 inflammasome prevents ovarian aging. Sci Adv. 2021;7(1):eabc7409.
Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22(13):1111–29.
Long Y, Liu X, Tan XZ, Jiang CX, Chen SW, Liang GN, et al. ROS-induced NLRP3 inflammasome priming and activation mediate PCB 118- induced pyroptosis in endothelial cells. Ecotoxicol Environ Saf. 2020;189:109937.
Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023.
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–67.
Timóteo-Ferreira F, Mendes S, Rocha NA, Matos L, Rodrigues AR, Almeida H, et al. Apocynin Dietary Supplementation Delays Mouse Ovarian Ageing. Oxid Med Cell Longev. 2019;2019:5316984.
Chei S, Oh HJ, Jang H, Lee K, Jin H, Choi Y, et al. Korean Red Ginseng Suppresses the Expression of Oxidative Stress Response and NLRP3 Inflammasome Genes in Aged C57BL/6 Mouse Ovaries. Foods. 2020;9(4):526.
He L, Long X, Yu N, Li Y, Liu X, Cheng X. Premature Ovarian Insufficiency (POI) Induced by Dynamic Intensity Modulated Radiation Therapy via P13K-AKT-FOXO3a in Rat Models. Biomed Res Int. 2021;2021:7273846.
Topcu A, Balik G, Atak M, Mercantepe T, Uydu HA, Tumkaya L. An investigation of the effects of metformin on ovarian ischemia-reperfusion injury in rats. Eur J Pharmacol. 2019;865:172790.
He L, Ling L, Wei T, Wang Y, Xiong Z. Ginsenoside Rg1 improves fertility and reduces ovarian pathological damages in premature ovarian failure model of mice. Exp Biol Med (Maywood). 2017;242(7):683–91.
Deniz E, Topcu A, Ozturk A, Ozturk SD, Arpa M, Atak M. The effects of vitamin B12 on the TLR-4/NF-κB signaling pathway in ovarian ischemia-reperfusion injury-related inflammation. Int Immunopharmacol. 2022;107:108676.
Elkady MA, Shalaby S, Fathi F, El-Mandouh S. Effects of quercetin and rosuvastatin each alone or in combination on cyclophosphamide-induced premature ovarian failure in female albino mice. Hum Exp Toxicol. 2019;38(11):1283–95.
Sastre J, Pallardó FV, García de la Asunción J, Viña J. Mitochondria, oxidative stress and aging. Free Radic Res. 2000;32(3):189–98.
Sharma P, Sampath H. Mitochondrial DNA Integrity: Role in Health and Disease. Cells. 2019;8(2):100.
Kasapoğlu I, Seli E. Mitochondrial Dysfunction and Ovarian Aging. Endocrinology. 2020;161(2):bqaa001.
Adhikari D, Lee IW, Yuen WS, Carroll J. Oocyte mitochondria-key regulators of oocyte function and potential therapeutic targets for improving fertility. Biol Reprod. 2022;106(2):366–77.
Chiang JL, Shukla P, Pagidas K, Ahmed NS, Karri S, Gunn DD, et al. Mitochondria in Ovarian Aging and Reproductive Longevity. Ageing Res Rev. 2020;63:101168.
Kirillova A, Smitz JEJ, Sukhikh GT, Mazunin I. The Role of Mitochondria in Oocyte Maturation. Cells. 2021;10(9):2484.
Jiang Z, Shen H. Mitochondria: emerging therapeutic strategies for oocyte rescue. Reprod Sci. 2022;29(3):711–22.
Yang Z, Wei ML, Dong XY. Effects of Yu Linzhu on ovarian function and oocyte mitochondria in natural aging mice. Aging (Albany NY). 2021;13(19):23328–37.
Zhang X, Wu XQ, Lu S, Guo YL, Ma X. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 2006;16(10):841–50.
Sonn SK, Song EJ, Seo S, Kim YY, Um JH, Yeo FJ, et al. Peroxiredoxin 3 deficiency induces cardiac hypertrophy and dysfunction by impaired mitochondrial quality control. Redox Biol. 2022;51:102275.
Sasaki H, Hamatani T, Kamijo S, Iwai M, Kobanawa M, Ogawa S, et al. Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front Endocrinol (Lausanne). 2019;10:811.
Alberico HC, Woods DC. Role of Granulosa Cells in the Aging Ovarian Landscape: A Focus on Mitochondrial and Metabolic Function. Front Physiol. 2021;12:800739.
Wang J, Wu J, Zhang Y, Zhang J, Xu W, Wu C, et al. Growth hormone protects against ovarian granulosa cell apoptosis: Alleviation oxidative stress and enhancement mitochondrial function. Reprod Biol. 2021;21(2):100504.
Hoque SAM, Umehara T, Kawai T, Shimada M. Adverse effect of superoxide-induced mitochondrial damage in granulosa cells on follicular development in mouse ovaries. Free Radic Biol Med. 2021;163:344–55.
Tanabe M, Tamura H, Taketani T, Okada M, Lee L, Tamura I, et al. Melatonin protects the integrity of granulosa cells by reducing oxidative stress in nuclei, mitochondria, and plasma membranes in mice. J Reprod Dev. 2015;61(1):35–41.
May-Panloup P, Boucret L, Chao de la Barca JM, Desquiret-Dumas V, Ferré-L’Hotellier V, Morinière C, et al. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update. 2016;22(6):725–43.
Chakravarti D, LaBella KA, DePinho RA. Telomeres: history, health, and hallmarks of aging. Cell. 2021;184(2):306–22.
Lansdorp PM. Telomeres, aging, and cancer: the big picture. Blood. 2022;139(6):813–21.
Xu X, Chen X, Zhang X, Liu Y, Wang Z, Wang P, et al. Impaired telomere length and telomerase activity in peripheral blood leukocytes and granulosa cells in patients with biochemical primary ovarian insufficiency. Hum Reprod. 2017;32(1):201–7.
Uysal F, Kosebent EG, Toru HS, Ozturk S. Decreased expression of TERT and telomeric proteins as human ovaries age may cause telomere shortening. J Assist Reprod Genet. 2021;38(2):429–41.
Kosebent EG, Ozturk S. Telomere associated gene expression as well as TERT protein level and telomerase activity are altered in the ovarian follicles of aged mice. Sci Rep. 2021;11(1):15569.
Cheng EH, Chen SU, Lee TH, Pai YP, Huang LS, Huang CC, et al. Evaluation of telomere length in cumulus cells as a potential biomarker of oocyte and embryo quality. Hum Reprod. 2013;28(4):929–36.
Erusalimsky JD. Oxidative stress, telomeres and cellular senescence: What non-drug interventions might break the link? Free Radic Biol Med. 2020;150:87–95.
Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452(7186):492–6.
He Y, Yang G, Sun L, Gao H, Yao F, Jin Z, et al. SIRT6 inhibits inflammatory response through regulation of NRF2 in vascular endothelial cells. Int Immunopharmacol. 2021;99:107926.
Liao CY, Kennedy BK. SIRT6, oxidative stress, and aging. Cell Res. 2016;26(2):143–4.
Ge J, Li C, Li C, Huang Z, Zeng J, Han L, et al. SIRT6 participates in the quality control of aged oocytes via modulating telomere function. Aging (Albany NY). 2019;11(7):1965–76.
Akino N, Wada-Hiraike O, Isono W, Terao H, Honjo H, Miyamoto Y, et al. Activation of Nrf2/Keap1 pathway by oral Dimethylfumarate administration alleviates oxidative stress and age-associated infertility might be delayed in the mouse ovary. Reprod Biol Endocrinol. 2019;17(1):23.
Liu J, Liu M, Ye X, Liu K, Huang J, Wang L, et al. Delay in oocyte aging in mice by the antioxidant N-acetyl-L-cysteine (NAC). Hum Reprod. 2012;27(5):1411–20.
Bruic M, Grujic-Milanovic J, Miloradovic Z, Jovovic D, Zivkovic L, Mihailovic-Stanojevic N, et al. DNA, protein and lipid oxidative damage in tissues of spontaneously hypertensive versus normotensive rats. Int J Biochem Cell Biol. 2021;141:106088.
Dahl JU, Gray MJ, Jakob U. Protein quality control under oxidative stress conditions. J Mol Biol. 2015;427(7):1549–63.
Pietraforte D, Paulicelli E, Patrono C, Gambardella L, Scorza G, Testa A, et al. Protein oxidative damage and redox imbalance induced by ionising radiation in CHO cells. Free Radic Res. 2018;52(4):465–79.
Elmorsy E, Al-Ghafari A, Aggour AM, Khan R, Amer S. The role of oxidative stress in antipsychotics induced ovarian toxicity. Toxicol In Vitro. 2017;44:190–5.
Sharma D, Sangha GK, Khera KS. Triazophos-induced oxidative stress and histomorphological changes in ovary of female Wistar rats. Pestic Biochem Physiol. 2015;117:9–18.
Liu B, Wang JL, Wang XM, Zhang C, Dai JG, Huang XM, et al. Reparative effects of lycium barbarum polysaccharide on mouse ovarian injuries induced by repeated superovulation. Theriogenology. 2020;145:115–25.
Bouet PE, Boueilh T, de la Barca JMC, Boucret L, Blanchard S, Ferré-L’Hotellier V, et al. The cytokine profile of follicular fluid changes during ovarian ageing. J Gynecol Obstet Hum Reprod. 2020;49(4):101704.
Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl. 2014;16(1):31–8.
Rynkowska A, Stępniak J, Karbownik-Lewińska M. Fenton Reaction-Induced Oxidative Damage to Membrane Lipids and Protective Effects of 17β-Estradiol in Porcine Ovary and Thyroid Homogenates. Int J Environ Res Public Health. 2020;17(18):6841.
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633–4.
Yoldemir T. Fertility in midlife women. Climacteric. 2016;19(3):240–6.
Silber SJ, Kato K, Aoyama N, Yabuuchi A, Skaletsky H, Fan Y, et al. Intrinsic fertility of human oocytes. Fertil Steril. 2017;107(5):1232–7.
Wang Q, Sun QY. Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reprod Fertil Dev. 2007;19(1):1–12.
Winship AL, Stringer JM, Liew SH, Hutt KJ. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum Reprod Update. 2018;24(2):119–34.
Leem J, Bai GY, Kim JS, Oh JS. Melatonin protects mouse oocytes from DNA damage by enhancing nonhomologous end-joining repair. J Pineal Res. 2019;67(4):e12603.
Maidarti M, Anderson RA, Telfer EE. Crosstalk between PTEN/PI3K/Akt Signalling and DNA Damage in the Oocyte: Implications for Primordial Follicle Activation, Oocyte Quality and Ageing. Cells. 2020;9(1):200.
Zhang D, Zhang X, Zeng M, Yuan J, Liu M, Yin Y, et al. Increased DNA damage and repair deficiency in granulosa cells are associated with ovarian aging in rhesus monkey. J Assist Reprod Genet. 2015;32(7):1069–78.
Turan V, Oktay K. BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum Reprod Update. 2020;26(1):43–57.
Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5(172):172ra21.
Oktay K, Turan V, Titus S, Stobezki R, Liu L. BRCA Mutations, DNA Repair Deficiency, and Ovarian Aging. Biol Reprod. 2015;93(3):67.
Titus S, Stobezki R, Oktay K. Impaired DNA Repair as a Mechanism for Oocyte Aging: Is It Epigenetically Determined? Semin Reprod Med. 2015;33(6):384–8.
Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet. 2015;47(11):1294–303.
Li Q, Cai M, Wang J, Gao Q, Guo X, Jia X, et al. Decreased ovarian function and autophagy gene methylation in aging rats. J Ovarian Res. 2020;13(1):12.
Ruder EH, Hartman TJ, Goldman MB. Impact of oxidative stress on female fertility. Curr Opin Obstet Gynecol. 2009;21(3):219–22.
Chelchowska M, Ambroszkiewicz J, Gajewska J, Laskowska-Klita T, Leibschang J. The effect of tobacco smoking during pregnancy on plasma oxidant and antioxidant status in mother and newborn. Eur J Obstet Gynecol Reprod Biol. 2011;155(2):132–6.
Kovacic P. Unifying mechanism for addiction and toxicity of abused drugs with application to dopamine and glutamate mediators: electron transfer and reactive oxygen species. Med Hypotheses. 2005;65(1):90–6.
Tiboni GM, Bucciarelli T, Giampietro F, Sulpizio M, Di Ilio C. Influence of cigarette smoking on vitamin E, vitamin A, beta-carotene and lycopene concentrations in human pre-ovulatory follicular fluid. Int J Immunopathol Pharmacol. 2004;17(3):389–93.
Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–83.
Ornoy A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reprod Toxicol. 2007;24(1):31–41.
Anderson K, Nisenblat V, Norman R. Lifestyle factors in people seeking infertility treatment - A review. Aust N Z J Obstet Gynaecol. 2010;50(1):8–20.
Camlin NJ, Sobinoff AP, Sutherland JM, Beckett EL, Jarnicki AG, Vanders RL, et al. Maternal Smoke Exposure Impairs the Long-Term Fertility of Female Offspring in a Murine Model. Biol Reprod. 2016;94(2):39.
Camlin NJ, Jarnicki AG, Vanders RL, Walters KA, Hansbro PM, McLaughlin EA, et al. Grandmaternal smoke exposure reduces female fertility in a murine model, with great-grandmaternal smoke exposure unlikely to have an effect. Hum Reprod. 2017;32(6):1270–81.
Gill V, Kumar V, Singh K, Kumar A, Kim JJ. Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules. 2019;9(12):888.
Li Y, Peng Y, Shen Y, Zhang Y, Liu L, Yang X. Dietary polyphenols: regulate the advanced glycation end products-RAGE axis and the microbiota-gut-brain axis to prevent neurodegenerative diseases. Crit Rev Food Sci Nutr. 2022;1–27. https://doi.org/10.1080/10408398.2022.2076064.
Khalid M, Petroianu G, Adem A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules. 2022;12(4):542.
Pinto RS, Minanni CA, de Araújo Lira AL, Passarelli M. Advanced Glycation End Products: A Sweet Flavor That Embitters Cardiovascular Disease. Int J Mol Sci. 2022;23(5):2404.
Sadowska J, Dudzińska W, Dziaduch I. Effects of different models of sucrose intake on the oxidative status of the uterus and ovary of rats. PLoS ONE. 2021;16(5):e0251789.
Akhter F, Chen D, Akhter A, Yan SF, Yan SS. Age-dependent accumulation of dicarbonyls and advanced glycation endproducts (AGEs) associates with mitochondrial stress. Free Radic Biol Med. 2021;164:429–38.
Ramasamy R, Yan SF, Schmidt AM. Advanced glycation endproducts: from precursors to RAGE: round and round we go. Amino Acids. 2012;42(4):1151–61.
Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, et al. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008;14(2):131–42.
Yin QQ, Dong CF, Dong SQ, Dong XL, Hong Y, Hou XY, et al. AGEs induce cell death via oxidative and endoplasmic reticulum stresses in both human SH-SY5Y neuroblastoma cells and rat cortical neurons. Cell Mol Neurobiol. 2012;32(8):1299–309.
Pertynska-Marczewska M, Diamanti-Kandarakis E. Aging ovary and the role for advanced glycation end products. Menopause. 2017;24(3):345–51.
Mouanness M, Merhi Z. Impact of Dietary Advanced Glycation End Products on Female Reproduction: Review of Potential Mechanistic Pathways. Nutrients. 2022;14(5):966.
Chaudhary GR, Yadav PK, Yadav AK, Tiwari M, Gupta A, Sharma A, et al. Necroptosis in stressed ovary. J Biomed Sci. 2019;26(1):11.
Zhang SY, Wang JZ, Li JJ, Wei DL, Sui HS, Zhang ZH, et al. Maternal restraint stress diminishes the developmental potential of oocytes. Biol Reprod. 2011;84(4):672–81.
Kala M, Nivsarkar M. Role of cortisol and superoxide dismutase in psychological stress induced anovulation. Gen Comp Endocrinol. 2016;225:117–24.
Premkumar KV, Chaube SK. RyR channel-mediated increase of cytosolic free calcium level signals cyclin B1 degradation during abortive spontaneous egg activation in rat. In Vitro Cell Dev Biol Anim. 2014;50(7):640–7.
Hojo T, Siemieniuch MJ, Lukasik K, Piotrowska-Tomala KK, Jonczyk AW, Okuda K, et al. Programmed necrosis - a new mechanism of steroidogenic luteal cell death and elimination during luteolysis in cows. Sci Rep. 2016;6:38211.
Tripathi A, Khatun S, Pandey AN, Mishra SK, Chaube R, Shrivastav TG, et al. Intracellular levels of hydrogen peroxide and nitric oxide in oocytes at various stages of meiotic cell cycle and apoptosis. Free Radic Res. 2009;43(3):287–94.
Tiwari M, Chaube S. Spontaneous exit from diplotene arrest associates with the increase of calcium and reactive oxygen species in rat oocytes cultured in vitro. React Oxygen Species. 2017;4(12):418–33.
Zhao X, Ma R, Zhang X, Wang B, Rong B, Jiang N, et al. Transcriptomic study of the mechanism by which the Kai Yu Zhong Yu recipe improves oocyte quality in a stressed mouse model. J Ethnopharmacol. 2021;278:114298.
Roth Z. Symposium review: Reduction in oocyte developmental competence by stress is associated with alterations in mitochondrial function. J Dairy Sci. 2018;101(4):3642–54.
Xu HX, Lin SX, Gong Y, Huo ZX, Zhao CY, Zhu HM, et al. Chaiyu-Dixian Formula Exerts Protective Effects on Ovarian Follicular Abnormal Development in Chronic Unpredictable Mild Stress (CUMS) Rat Model. Front Pharmacol. 2020;11:245.
Fu X, Zheng Q, Zhang N, Ding M, Pan X, Wang W, et al. CUMS Promotes the Development of Premature Ovarian Insufficiency Mediated by Nerve Growth Factor and Its Receptor in Rats. Biomed Res Int. 2020;2020:1946853.
Dorfman M, Arancibia S, Fiedler JL, Lara HE. Chronic intermittent cold stress activates ovarian sympathetic nerves and modifies ovarian follicular development in the rat. Biol Reprod. 2003;68(6):2038–43.
Li YW, Liang XW, Fang JH, Chen ZY. Application of ultrasound markers measured at different time points of COH cycle in the prediction of ovarian response for individualised ovulation induction. J Obstet Gynaecol. 2022;42(5):1467–73.
Sato EF, Kobuchi H, Edashige K, Takahashi M, Yoshioka T, Utsumi K, et al. Dynamic aspects of ovarian superoxide dismutase isozymes during the ovulatory process in the rat. FEBS Lett. 1992;303(2–3):121–5.
Pérez-Ruiz I, Meijide S, Ferrando M, Larreategui Z, Ruiz-Larrea MB, Ruiz-Sanz JI. Ovarian stimulated cycles reduce protection of follicular fluid against free radicals. Free Radic Biol Med. 2019;145:330–5.
Angelucci S, Ciavardelli D, Di Giuseppe F, Eleuterio E, Sulpizio M, Tiboni GM, et al. Proteome analysis of human follicular fluid. Biochim Biophys Acta. 2006;1764(11):1775–85.
Twigt J, Steegers-Theunissen RP, Bezstarosti K, Demmers JA. Proteomic analysis of the microenvironment of developing oocytes. Proteomics. 2012;12(9):1463–71.
Ma Y, Zhao Z, Hao G, Cui N, Fan Y, Cao Y, et al. Effects of multicycle gonadotropin-releasing hormone antagonist protocols on oxidative stress of follicular fluid and ovarian granulosa cells. Hum Cell. 2021;34(5):1324–34.
Nie X, Dai Y, Zheng Y, Bao D, Chen Q, Yin Y, et al. Establishment of a Mouse Model of Premature Ovarian Failure Using Consecutive Superovulation. Cell Physiol Biochem. 2018;51(5):2341–58.
Miyamoto K, Sato EF, Kasahara E, Jikumaru M, Hiramoto K, Tabata H, et al. Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free Radic Biol Med. 2010;49(4):674–81.
Zhang J, Lai Z, Shi L, Tian Y, Luo A, Xu Z, et al. Repeated superovulation increases the risk of osteoporosis and cardiovascular diseases by accelerating ovarian aging in mice. Aging (Albany NY). 2018;10(5):1089–102.
Isono W, Wada-Hiraike O, Kawamura Y, Fujii T, Osuga Y, Kurihara H. Administration of Oral Contraceptives Could Alleviate Age-Related Fertility Decline Possibly by Preventing Ovarian Damage in a Mouse Model. Reprod Sci. 2018;25(9):1413–23.
Qin Y, Iwase A, Murase T, Bayasula, Ishida C, Kato N, et al. Protective effects of mangafodipir against chemotherapy-induced ovarian damage in mice. Reprod Biol Endocrinol. 2018;16(1):106.
Liedtke C, Kiesel L. Chemotherapy-Induced Amenorrhea - An Update. Geburtshilfe Frauenheilkd. 2012;72(9):809–18.
Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53(4):727–39.
Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7(6):535–43.
Romito A, Bove S, Romito I, Zace D, Raimondo I, Fragomeni SM, et al. Ovarian Reserve after Chemotherapy in Breast Cancer: A Systematic Review and Meta-Analysis. J Pers Med. 2021;11(8):704.
Goldfarb SB, Turan V, Bedoschi G, Taylan E, Abdo N, Cigler T, et al. Impact of adjuvant chemotherapy or tamoxifen-alone on the ovarian reserve of young women with breast cancer. Breast Cancer Res Treat. 2021;185(1):165–73.
Meng X, Chen H, Wang G, Yu Y, Xie K. Hydrogen-rich saline attenuates chemotherapy-induced ovarian injury via regulation of oxidative stress. Exp Ther Med. 2015;10(6):2277–82.
Altuner D, Gulaboglu M, Yapca OE, Cetin N. The effect of mirtazapine on cisplatin-induced oxidative damage and infertility in rat ovaries. ScientificWorldJournal. 2013;2013:327240.
Hess JA, Khasawneh MK. Cancer metabolism and oxidative stress: Insights into carcinogenesis and chemotherapy via the non-dihydrofolate reductase effects of methotrexate. BBA Clin. 2015;3:152–61.
Ata N, Kulhan NG, Kulhan M, Turkler C, Kiremitli T, Kiremitli S, et al. The effect of resveratrol on oxidative ovary-damage induced by methotrexate in rats (Resveratrol oxidative ovary-damage). Gen Physiol Biophys. 2019;38(6):519–24.
Huang M, Huang M, Li X, Liu S, Fu L, Jiang X, et al. Bisphenol A induces apoptosis through GPER-dependent activation of the ROS/Ca(2+)-ASK1-JNK pathway in human granulosa cell line KGN. Ecotoxicol Environ Saf. 2021;208:111429.
Jia Z, Wang H, Feng Z, Zhang S, Wang L, Zhang J, et al. Fluorene-9-bisphenol exposure induces cytotoxicity in mouse oocytes and causes ovarian damage. Ecotoxicol Environ Saf. 2019;180:168–78.
Liu T, Di QN, Sun JH, Zhao M, Xu Q, Shen Y. Effects of nonylphenol induced oxidative stress on apoptosis and autophagy in rat ovarian granulosa cells. Chemosphere. 2020;261:127693.
Tripathi A, Pandey V, Sahu AN, Singh A, Dubey PK. Di-(2-ethylhexyl) phthalate (DEHP) inhibits steroidogenesis and induces mitochondria-ROS mediated apoptosis in rat ovarian granulosa cells. Toxicol Res (Camb). 2019;8(3):381–94.
Baird L, Yamamoto M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol Cell Biol. 2020;40(13):e00099-e120.
Madden SK, Itzhaki LS. Structural and mechanistic insights into the Keap1-Nrf2 system as a route to drug discovery. Biochim Biophys Acta Proteins Proteom. 2020;1868(7):140405.
Jiang X, Xing X, Zhang Y, Zhang C, Wu Y, Chen Y, et al. Lead exposure activates the Nrf2/Keap1 pathway, aggravates oxidative stress, and induces reproductive damage in female mice. Ecotoxicol Environ Saf. 2021;207:111231.
Zhang M, Yu X, Li D, Ma N, Wei Z, Ci X, et al. Nrf2 Signaling Pathway Mediates the Protective Effects of Daphnetin Against D-Galactose Induced-Premature Ovarian Failure. Front Pharmacol. 2022;13:810524.
Lim J, Ortiz L, Nakamura BN, Hoang YD, Banuelos J, Flores VN, et al. Effects of deletion of the transcription factor Nrf2 and benzo [a]pyrene treatment on ovarian follicles and ovarian surface epithelial cells in mice. Reprod Toxicol. 2015;58:24–32.
Hu X, Roberts JR, Apopa PL, Kan YW, Ma Q. Accelerated ovarian failure induced by 4-vinyl cyclohexene diepoxide in Nrf2 null mice. Mol Cell Biol. 2006;26(3):940–54.
Yan F, Zhao Q, Gao H, Wang X, Xu K, Wang Y, et al. Exploring the mechanism of (-)-Epicatechin on premature ovarian insufficiency based on network pharmacology and experimental evaluation. Biosci Rep. 2021;41(2):BSR20203955.
Alam F, Syed H, Amjad S, Baig M, Khan TA, Rehman R. Interplay between oxidative stress, SIRT1, reproductive and metabolic functions. Curr Res Physiol. 2021;4:119–24.
Tatone C, Di Emidio G, Vitti M, Di Carlo M, Santini S Jr, D’Alessandro AM, et al. Sirtuin Functions in Female Fertility: Possible Role in Oxidative Stress and Aging. Oxid Med Cell Longev. 2015;2015:659687.
Cheng Y, Takeuchi H, Sonobe Y, Jin S, Wang Y, Horiuchi H, et al. Sirtuin 1 attenuates oxidative stress via upregulation of superoxide dismutase 2 and catalase in astrocytes. J Neuroimmunol. 2014;269(1–2):38–43.
Di Emidio G, Falone S, Vitti M, D’Alessandro AM, Vento M, Di Pietro C, et al. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum Reprod. 2014;29(9):2006–17.
Iljas JD, Wei Z, Homer HA. Sirt1 sustains female fertility by slowing age-related decline in oocyte quality required for post-fertilization embryo development. Aging Cell. 2020;19(9):e13204.
Guo L, Liu X, Chen H, Wang W, Gu C, Li B. Decrease in ovarian reserve through the inhibition of SIRT1-mediated oxidative phosphorylation. Aging (Albany NY). 2022;14(5):2335–47.
Tatone C, Di Emidio G, Barbonetti A, Carta G, Luciano AM, Falone S, et al. Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility. Hum Reprod Update. 2018;24(3):267–89.
Fu H, Wada-Hiraike O, Hirano M, Kawamura Y, Sakurabashi A, Shirane A, et al. SIRT3 positively regulates the expression of folliculogenesis- and luteinization-related genes and progesterone secretion by manipulating oxidative stress in human luteinized granulosa cells. Endocrinology. 2014;155(8):3079–87.
Yue J, López JM. Understanding MAPK Signaling Pathways in Apoptosis. Int J Mol Sci. 2020;21(7):2346.
Jalmi SK, Sinha AK. ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Front Plant Sci. 2015;6:769.
Brown MD, Sacks DB. Compartmentalised MAPK pathways. Handb Exp Pharmacol. 2008;186:205–35.
Sun J, Guo Y, Fan Y, Wang Q, Zhang Q, Lai D. Decreased expression of IDH1 by chronic unpredictable stress suppresses proliferation and accelerates senescence of granulosa cells through ROS activated MAPK signaling pathways. Free Radic Biol Med. 2021;169:122–36.
Xu J, Yan B, Zhang L, Zhou L, Zhang J, Yu W, et al. Theabrownin Induces Apoptosis and Tumor Inhibition of Hepatocellular Carcinoma Huh7 Cells Through ASK1-JNK-c-Jun Pathway. Onco Targets Ther. 2020;13:8977–87.
Huang M, Li X, Jia S, Liu S, Fu L, Jiang X, et al. Bisphenol AF induces apoptosis via estrogen receptor beta (ERβ) and ROS-ASK1-JNK MAPK pathway in human granulosa cell line KGN. Environ Pollut. 2021;270:116051.
Xie QE, Wang MY, Cao ZP, Du X, Ji DM, Liang D, et al. Melatonin protects against excessive autophagy-induced mitochondrial and ovarian reserve function deficiency though ERK signaling pathway in Chinese hamster ovary (CHO) cells. Mitochondrion. 2021;61:44–53.
Zhou XY, Zhang J, Li Y, Chen YX, Wu XM, Li X, et al. Advanced Oxidation Protein Products Induce G1/G0-Phase Arrest in Ovarian Granulosa Cells via the ROS-JNK/p38 MAPK-p21 Pathway in Premature Ovarian Insufficiency. Oxid Med Cell Longev. 2021;2021:6634718.
Zhao Y, Hu X, Liu Y, Dong S, Wen Z, He W, et al. ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol Cancer. 2017;16(1):79.
Liang Y, Li J, Lin Q, Huang P, Zhang L, Wu W, et al. Research Progress on Signaling Pathway-Associated Oxidative Stress in Endothelial Cells. Oxid Med Cell Longev. 2017;2017:7156941.
Hayakawa J, Ohmichi M, Kurachi H, Kanda Y, Hisamoto K, Nishio Y, et al. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res. 2000;60(21):5988–94.
Michurina SV, Kolesnikov SI, Ishchenko IY, Bochkareva AL, Arkhipov SA. Effect of Melatonin on Expression of Apoptosis Regulator Proteins Bcl-2 and Bad in Ovarian Follicular Apparatus after High Temperature Exposure. Bull Exp Biol Med. 2021;170(5):598–603.
Yan J, Deng D, Wu Y, Wu K, Qu J, Li F. Catalpol protects rat ovarian granulosa cells against oxidative stress and apoptosis through modulating the PI3K/Akt/mTOR signaling pathway. Biosci Rep. 2020;40(4):BSR20194032.
Shi XY, Guan ZQ, Yu JN, Liu HL. Follicle Stimulating Hormone Inhibits the Expression of p53 Up-Regulated Modulator of Apoptosis Induced by Reactive Oxygen Species Through PI3K/Akt in Mouse Granulosa Cells. Physiol Res. 2020;69(4):687–94.
Gao D, Ma L, Xie Y, Xiao B, Xue S, Xiao W, et al. Electroacupuncture Promotes Autophagy by Regulating the AKT/mTOR Signaling Pathway in Temporal Lobe Epilepsy. Neurochem Res. 2022;47(8):2396–404.
Dodson M, Darley-Usmar V, Zhang J. Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic Biol Med. 2013;63:207–21.
Deng D, Yan J, Wu Y, Wu K, Li W. Morroniside suppresses hydrogen peroxide-stimulated autophagy and apoptosis in rat ovarian granulosa cells through the PI3K/AKT/mTOR pathway. Hum Exp Toxicol. 2021;40(4):577–86.
Li X, Chen H, Zhang Z, Xu D, Duan J, Li X, et al. Isorhamnetin Promotes Estrogen Biosynthesis and Proliferation in Porcine Granulosa Cells via the PI3K/Akt Signaling Pathway. J Agric Food Chem. 2021;69(23):6535–42.
Link W. Introduction to FOXO Biology. Methods Mol Biol. 2019;1890:1–9.
Murtaza G, Khan AK, Rashid R, Muneer S, Hasan SMF, Chen J. FOXO Transcriptional Factors and Long-Term Living. Oxid Med Cell Longev. 2017;2017:3494289.
Shi F, LaPolt PS. Relationship between FoxO1 protein levels and follicular development, atresia, and luteinization in the rat ovary. J Endocrinol. 2003;179(2):195–203.
Liu ZQ, Shen M, Wu WJ, Li BJ, Weng QN, Li M, et al. Expression of PUMA in Follicular Granulosa Cells Regulated by FoxO1 Activation During Oxidative Stress. Reprod Sci. 2015;22(6):696–705.
Shen M, Lin F, Zhang J, Tang Y, Chen WK, Liu H. Involvement of the up-regulated FoxO1 expression in follicular granulosa cell apoptosis induced by oxidative stress. J Biol Chem. 2012;287(31):25727–40.
Herndon MK, Law NC, Donaubauer EM, Kyriss B, Hunzicker-Dunn M. Forkhead box O member FOXO1 regulates the majority of follicle-stimulating hormone responsive genes in ovarian granulosa cells. Mol Cell Endocrinol. 2016;434:116–26.
Shen M, Jiang Y, Guan Z, Cao Y, Li L, Liu H, et al. Protective mechanism of FSH against oxidative damage in mouse ovarian granulosa cells by repressing autophagy. Autophagy. 2017;13(8):1364–85.
Pradhan R, Kumar R, Shekhar S, Rai N, Ambashtha A, Banerjee J, et al. Longevity and healthy ageing genes FOXO3A and SIRT3: Serum protein marker and new road map to burst oxidative stress by Withania somnifera. Exp Gerontol. 2017;95:9–15.
Thanatsis N, Kaponis A, Koika V, Georgopoulos NA, Decavalas GO. Reduced Foxo3a, FoxL2, and p27 mRNA expression in human ovarian tissue in premature ovarian insufficiency. Hormones (Athens). 2019;18(4):409–15.
Gouveia BB, Barberino RS, Dos Santos Silva RL, Lins T, da Silva Guimarães V, do Monte APO, et al. Involvement of PTEN and FOXO3a Proteins in the Protective Activity of Protocatechuic Acid Against Cisplatin-Induced Ovarian Toxicity in Mice. Reprod Sci. 2021;28(3):865–76.
Lins T, Gouveia BB, Barberino RS, Silva RLS, Monte APO, Pinto JGC, et al. Rutin prevents cisplatin-induced ovarian damage via antioxidant activity and regulation of PTEN and FOXO3a phosphorylation in mouse model. Reprod Toxicol. 2020;98:209–17.
Yan S, Wang F, Shi Q. The effect of maternal high-fat-diet mediated oxidative stress on ovarian function in mice offspring. Exp Ther Med. 2020;20(6):135.
Li Y, Qiu W, Zhang Z, Han X, Bu G, Meng F, et al. Oral oyster polypeptides protect ovary against d-galactose-induced premature ovarian failure in C57BL/6 mice. J Sci Food Agric. 2020;100(1):92–101.
Xu X, Hao Y, Zhong Q, Hang J, Zhao Y, Qiao J. Low KLOTHO level related to aging is associated with diminished ovarian reserve. Fertil Steril. 2020;114(6):1250–5.
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51.
Kim JH, Hwang KH, Park KS, Kong ID, Cha SK. Biological Role of Anti-aging Protein Klotho. J Lifestyle Med. 2015;5(1):1–6.
Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19(9):1147–52.
Toyama R, Fujimori T, Nabeshima Y, Itoh Y, Tsuji Y, Osamura RY, et al. Impaired regulation of gonadotropins leads to the atrophy of the female reproductive system in klotho-deficient mice. Endocrinology. 2006;147(1):120–9.
Di Bona D, Accardi G, Virruso C, Candore G, Caruso C. Association of Klotho polymorphisms with healthy aging: a systematic review and meta-analysis. Rejuvenation Res. 2014;17(2):212–6.
Xie T, Ye W, Liu J, Zhou L, Song Y. The Emerging Key Role of Klotho in the Hypothalamus-Pituitary-Ovarian Axis. Reprod Sci. 2021;28(2):322–31.
Amaral FGD, Cipolla-Neto J. A brief review about melatonin, a pineal hormone. Arch Endocrinol Metab. 2018;62(4):472–9.
Nakamura Y, Tamura H, Takayama H, Kato H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil Steril. 2003;80(4):1012–6.
Jiang Y, Shi H, Liu Y, Zhao S, Zhao H. Applications of Melatonin in Female Reproduction in the Context of Oxidative Stress. Oxid Med Cell Longev. 2021;2021:6668365.
Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71(16):2997–3025.
Tamura H, Jozaki M, Tanabe M, Shirafuta Y, Mihara Y, Shinagawa M, et al. Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging. Int J Mol Sci. 2020;21(3):1135.
Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44(3):280–7.
Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocr J. 2013;60(1):1–13.
Taketani T, Tamura H, Takasaki A, Lee L, Kizuka F, Tamura I, et al. Protective role of melatonin in progesterone production by human luteal cells. J Pineal Res. 2011;51(2):207–13.
Liu YJ, Ji DM, Liu ZB, Wang TJ, Xie FF, Zhang ZG, et al. Melatonin maintains mitochondrial membrane potential and decreases excessive intracellular Ca(2+) levels in immature human oocytes. Life Sci. 2019;235:116810.
Song C, Peng W, Yin S, Zhao J, Fu B, Zhang J, et al. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci Rep. 2016;6:35165.
Fang Y, Zhang J, Li Y, Guo X, Li J, Zhong R, et al. Melatonin-induced demethylation of antioxidant genes increases antioxidant capacity through RORα in cumulus cells of prepubertal lambs. Free Radic Biol Med. 2019;131:173–83.
Ivanov D, Mazzoccoli G, Anderson G, Linkova N, Dyatlova A, Mironova E, et al. Melatonin, Its Beneficial Effects on Embryogenesis from Mitigating Oxidative Stress to Regulating Gene Expression. Int J Mol Sci. 2021;22(11):5885.
Barberino RS, Menezes VG, Ribeiro A, Palheta RC Jr, Jiang X, Smitz JEJ, et al. Melatonin protects against cisplatin-induced ovarian damage in mice via the MT1 receptor and antioxidant activity. Biol Reprod. 2017;96(6):1244–55.
Jang H, Lee OH, Lee Y, Yoon H, Chang EM, Park M, et al. Melatonin prevents cisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO3a pathway activation in the mouse ovary. J Pineal Res. 2016;60(3):336–47.
He J, Xu W, Zheng X, Zhao B, Ni T, Yu P, et al. Vitamin C reduces vancomycin-related nephrotoxicity through the inhibition of oxidative stress, apoptosis, and inflammation in mice. Ann Transl Med. 2021;9(16):1319.
Jhala DD, Chinoy NJ, Rao MV. Mitigating effects of some antidotes on fluoride and arsenic induced free radical toxicity in mice ovary. Food Chem Toxicol. 2008;46(3):1138–42.
Gai HF, An JX, Qian XY, Wei YJ, Williams JP, Gao GL. Ovarian Damages Produced by Aerosolized Fine Particulate Matter (PM(2.5)) Pollution in Mice: Possible Protective Medications and Mechanisms. Chin Med J (Engl). 2017;130(12):1400–10.
Hossain MS, Dutta RK, Muralidhar K, Gupta RD. Decreased ascorbic acid biosynthesis in response to PMSG in the pre-pubertal female rat ovary. Res Vet Sci. 2020;131:15–20.
Abdollahifar MA, Azad N, Sajadi E, Shams Mofarahe Z, Zare F, Moradi A, et al. Vitamin C restores ovarian follicular reservation in a mouse model of aging. Anat Cell Biol. 2019;52(2):196–203.
Hassoun EA, Vodhanel J, Abushaban A. The modulatory effects of ellagic acid and vitamin E succinate on TCDD-induced oxidative stress in different brain regions of rats after subchronic exposure. J Biochem Mol Toxicol. 2004;18(4):196–203.
Safiyeh FD, Mojgan M, Parviz S, Sakineh MA, Behnaz SO. The effect of selenium and vitamin E supplementation on anti-Mullerian hormone and antral follicle count in infertile women with occult premature ovarian insufficiency: A randomized controlled clinical trial. Complement Ther Med. 2021;56:102533.
Sargazi Z, Reza Nikravesh M, Jalali M, Reza Sadeghnia H, Rahimi AF. The protective effect of vitamin E on rats’ ovarian follicles following an administration of diazinon: An experimental study. Int J Reprod Biomed. 2019;17(2):79–88.
Ceko MJ, Hummitzsch K, Hatzirodos N, Bonner WM, Aitken JB, Russell DL, et al. Correction: X-Ray fluorescence imaging and other analyses identify selenium and GPX1 as important in female reproductive function. Metallomics. 2015;7(1):188.
Molavi M, Razi M, Cheraghi H, Khorramjouy M, Ostadi A, Gholirad S. Protective effect of vitamin E on cypermethrin-induced follicular atresia in rat ovary: Evidence for energy dependent mechanism. Vet Res Forum. 2016;7(2):125–32.
Duleba AJ, Foyouzi N, Karaca M, Pehlivan T, Kwintkiewicz J, Behrman HR. Proliferation of ovarian theca-interstitial cells is modulated by antioxidants and oxidative stress. Hum Reprod. 2004;19(7):1519–24.
Vats A, Bielby RC, Tolley NS, Nerem R, Polak JM. Stem cells. Lancet. 2005;366(9485):592–602.
Lagarkova MA. Such Various Stem Cells. Biochemistry (Mosc). 2019;84(3):187–9.
Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl Med. 2017;6(12):2173–85.
Hong W, Wang B, Zhu Y, Wu J, Qiu L, Ling S, et al. Female germline stem cells: aging and anti-aging. J Ovarian Res. 2022;15(1):79.
Liu J, Zhang H, Zhang Y, Li N, Wen Y, Cao F, et al. Homing and restorative effects of bone marrow-derived mesenchymal stem cells on cisplatin injured ovaries in rats. Mol Cells. 2014;37(12):865–72.
Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells. 2019;8(5):467.
Yang S, Yang Y, Hao H, Du W, Pang Y, Zhao S, et al. Supplementation of EGF, IGF-1, and Connexin 37 in IVM Medium Significantly Improved the Maturation of Bovine Oocytes and Vitrification of Their IVF Blastocysts. Genes (Basel). 2022;13(5):805.
Ding C, Zou Q, Wu Y, Lu J, Qian C, Li H, et al. EGF released from human placental mesenchymal stem cells improves premature ovarian insufficiency via NRF2/HO-1 activation. Aging (Albany NY). 2020;12(3):2992–3009.
Lu X, Bao H, Cui L, Zhu W, Zhang L, Xu Z, et al. hUMSC transplantation restores ovarian function in POI rats by inhibiting autophagy of theca-interstitial cells via the AMPK/mTOR signaling pathway. Stem Cell Res Ther. 2020;11(1):268.
Seok J, Park H, Choi JH, Lim JY, Kim KG, Kim GJ. Placenta-Derived Mesenchymal Stem Cells Restore the Ovary Function in an Ovariectomized Rat Model via an Antioxidant Effect. Antioxidants (Basel). 2020;9(7):591.
Wang D, Zhang M, Wang T, Liu T, Guo Y, Granato D. Green tea polyphenols mitigate the plant lectins-induced liver inflammation and immunological reaction in C57BL/6 mice via NLRP3 and Nrf2 signaling pathways. Food Chem Toxicol. 2020;144:111576.
Yang S, Shao S, Huang B, Yang D, Zeng L, Gan Y, et al. Tea polyphenols alleviate tri-ortho-cresyl phosphate-induced autophagy of mouse ovarian granulosa cells. Environ Toxicol. 2020;35(4):478–86.
Wang Y, Chen F, Liang M, Chen S, Zhu Y, Zou Z, et al. Grape seed proanthocyanidin extract attenuates varicocele-induced testicular oxidative injury in rats by activating the Nrf2-antioxidant system. Mol Med Rep. 2018;17(1):1799–806.
Liu X, Lin X, Mi Y, Li J, Zhang C. Grape Seed Proanthocyanidin Extract Prevents Ovarian Aging by Inhibiting Oxidative Stress in the Hens. Oxid Med Cell Longev. 2018;2018:9390810.
Mehri F, Ranjbar A, Shirafkan N, Asl SS, Esfahani M. The protective effect of resveratrol on diazinon-induced oxidative stress and glucose hemostasis disorder in rats’ liver. J Biochem Mol Toxicol. 2022;36(7): e23063.
Aziz SG, Pourheydar B, Chodari L, Hamidifar F. Effect of exercise and curcumin on cardiomyocyte molecular mediators associated with oxidative stress and autophagy in aged male rats. Microvasc Res. 2022;143:104380.
Azami SH, Nazarian H, Abdollahifar MA, Eini F, Farsani MA, Novin MG. The antioxidant curcumin postpones ovarian aging in young and middle-aged mice. Reprod Fertil Dev. 2020;32(3):292–303.
Liu W, Nguyen TN, Tran Thi TV, Zhou S. Kuntai Capsule plus Hormone Therapy vs. Hormone Therapy Alone in Patients with Premature Ovarian Failure: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2019;2019:2085804.
Zhang J, Fang L, Shi L, Lai Z, Lu Z, Xiong J, et al. Protective effects and mechanisms investigation of Kuntai capsule on the ovarian function of a novel model with accelerated aging ovaries. J Ethnopharmacol. 2017;195:173–81.
Chen S, Lu Y, Chen Y, Xu J, Chen L, Zhao W, et al. The effect of Bu Shen Huo Xue Tang on autoimmune premature ovarian insufficiency via Modulation of the Nrf2/Keap1 signaling pathway in mice. J Ethnopharmacol. 2021;273:113996.
Huang KY, Liang S, Yu ML, Fu SP, Chen X, Lu SF. A systematic review and meta-analysis of acupuncture for improving learning and memory ability in animals. BMC Complement Altern Med. 2016;16(1):297.
Tan R, He Y, Zhang S, Pu D, Wu J. Effect of transcutaneous electrical acupoint stimulation on protecting against radiotherapy- induced ovarian damage in mice. J Ovarian Res. 2019;12(1):65.
Acknowledgements
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn/) for the expert linguistic services provided.
Funding
This study was supported by the National Natural Science Foundation of China (82074480).
Author information
Authors and Affiliations
Contributions
YF and ZQ contributed in conception and design of the manuscript. YF and LY contributed in data collection and manuscript drafting. ZZB, KXL and SC contributed in manuscript modification. LYF and SY oversaw the study. All authors read and approved the final manuscript. YF, ZQ and LY should be considered as joint first authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yan, F., Zhao, Q., Li, Y. et al. The role of oxidative stress in ovarian aging: a review. J Ovarian Res 15, 100 (2022). https://doi.org/10.1186/s13048-022-01032-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-022-01032-x