Abstract
Longitudinal sampling of tumor tissue from patients with solid cancers, aside from melanoma and a few other cases, is often unfeasible, and thus may not capture the plasticity of interactions between the tumor and immune system under selective pressure of a given therapy. Peripheral blood analyses provide salient information about the human peripheral immunome while offering technical and practical advantages over traditional tumor biopsies, and should be utilized where possible alongside interrogation of the tumor. Some common blood-based biomarkers used to study the immune response include immune cell subsets, circulating tumor DNA, and protein analytes such as cytokines. With the recent explosion of immune checkpoint inhibitors (ICI) as a modality of treatment in multiple cancer types, soluble immune checkpoints have become a relevant area of investigation for peripheral immune-based biomarkers. However, the exact functions of soluble immune checkpoints and their roles in cancer for the most part remain unclear. This review discusses current literature on the production, function, and expression of nine soluble immune checkpoints – sPD-L1, sPD-1, sCTLA4, sCD80, sTIM3, sLAG3, sB7-H3, sBTLA, and sHVEM – in patients with solid tumors, and explores their role as biomarkers of response to ICI as well as to conventional therapies (chemotherapy, radiotherapy, targeted therapy, and surgery) in cancer patients.
Similar content being viewed by others

Introduction
Peripheral blood analyses provide salient information about the human peripheral immunome while offering technical and practical advantages over tumor biopsies [1,2,3,4,5]. Tumor biopsies have been traditionally analyzed for protein expression and/or tumor mutational burden (TMB) to identify biomarkers of treatment response [1]. However, tumor specimens often do not account for intratumoral heterogeneity or heterogeneity between the primary tumor and metastases, and are difficult to obtain at multiple time points to assess changes over time [1, 2, 4]. Practically, tumor biopsies are expensive and can cause both treatment delays and the potential risk of adverse events [2]. On the other hand, obtaining blood from patients is non-invasive, low-risk, and can be performed repeatedly over multiple time points [1,2,3,4,5]. In recent years, an effort has been made to develop blood-based biomarkers in cancer patients to study the systemic effects of a given therapy on the immune system; when possible, these studies should be used to complement methods that directly interrogate the tumor and tumor microenvironment. Some common blood-based biomarkers include immune cell subsets, the neutrophil-to-lymphocyte ratio (NLR), circulating tumor DNA, and protein analytes such as cytokines [1,2,3,4,5]. With the introduction of immune checkpoint inhibitors (ICI) as a widespread modality of cancer treatment, soluble immune checkpoints have become a relevant area of investigation for potentially identifying peripheral immune-based biomarkers.
Immune checkpoints are stimulatory or inhibitory signaling molecules that regulate T cell response upon antigen presentation [6,7,8,9,10]. The ligand is often found on the antigen-presenting cell (APC), while its corresponding receptor is typically located on the T cell [9]. Stimulatory molecules include CD137, CD137L, OX40, OX40L, CD28, CD86, CD80, inducible T cell co-stimulator (ICOS), B7-related protein 1 (B7RP1), CD27, and CD70, while inhibitory molecules include programmed cell death protein 1 (PD-1), programmed cell death-ligand 1 (PD-L1), programmed cell death-ligand 2 (PD-L2), cytotoxic T-lymphocyte-associated antigen 4 (CTLA4), CD86, CD80, T cell immunoglobulin and mucin domain-containing protein 3 (TIM3), galectin 9 (GAL9), lymphocyte activation gene 3 (LAG3), B and T lymphocyte attenuator (BTLA), herpesvirus entry mediator (HVEM), T cell immunoglobulin and ITIM domain (TIGIT), B7-H3, and B7-H4 (Fig. 1A) [9, 11]. Blocking inhibitory immune checkpoints can enable T cell activation upon recognition of a tumor antigen, thus harnessing the power of the immune system to destroy cancer cells [6,7,8,9]. The CTLA4 inhibitor ipilimumab was the first ICI to obtain FDA approval in 2011 [9, 12]. Since then, anti-PD-L1, anti-PD-1, and anti-CTLA4 antibodies have become widespread as cancer therapeutic agents and are utilized across multiple cancer types [6]. Many studies are currently investigating inhibitors of other inhibitory immune checkpoints, such as LAG3, TIM3, and TIGIT, and agonists of stimulatory immune checkpoints, such as CD137, OX40 and ICOS [6,7,8, 11, 13]. Despite the promise and widespread use of ICI, many patients are resistant to this modality of treatment [14, 15]. The mechanisms of resistance are unclear, and it is unknown which patients will derive clinical benefit [14, 15]. It is therefore imperative to identify biomarkers that can serve as predictors of clinical response in patients with solid tumors receiving ICI.
Membrane-bound (A) and soluble (B) immune checkpoints. Soluble immune checkpoints are produced through cleavage of the membrane-bound immune checkpoint proteins and/or alternative splicing of mRNA. ICOS, inducible T cell co-stimulator; B7RP1, B7-related protein 1; CTLA4, cytotoxic T-lymphocyte-associated antigen 4; PD-1, programmed cell death protein 1; PD-L1, programmed cell death-ligand 1; PD-L2, programmed cell death-ligand 2; TIM3, T cell immunoglobulin and mucin domain-containing protein 3; GAL9, galectin 9; LAG3, lymphocyte activation gene 3; MHC, major histocompatibility complex; BTLA, B and T lymphocyte attenuator; HVEM, herpesvirus entry mediator; TIGIT, T cell immunoglobulin and ITIM domain
Immune checkpoints can exist in two forms – membrane-bound and soluble [10, 16, 17]. Membrane-bound immune checkpoints are found on both cell membranes and exosomal membranes [10, 18, 19], while soluble immune checkpoints are produced through the alternative splicing of mRNA or the cleavage of membrane-bound immune checkpoint proteins (Fig. 1B) [10, 16, 17]. Soluble immune checkpoints have been observed in bodily fluids such as blood (plasma and serum) [10, 16, 20,21,22], urine [21], cerebrospinal fluid [20, 22], and peritoneal fluid [23, 24], and their levels can be measured quantitatively through methods such as ELISAs or multiplexed assays [10, 16, 17]. However, the exact functions of soluble immune checkpoints and their roles in normal biology and diseased states such as cancer remain unclear [10, 16, 17].
This review will discuss nine soluble immune checkpoints measured in plasma or serum and summarize what is known in terms of their production, function, expression, and association with tumor stage in patients with solid tumors other than melanoma. The potential role of these soluble immune checkpoints to serve as biomarkers of clinical response in patients with cancer, both prior to and during treatment with ICI or conventional therapies (e.g., chemotherapy, radiotherapy, targeted therapy, surgery, and combinations of these treatment modalities), will be discussed. To our knowledge, this is the first review to comprehensively compare the biomarker potential of multiple soluble immune checkpoints, at time points both before and during therapy, in patients with solid tumors other than melanoma receiving different treatment modalities.
Levels of soluble immune checkpoints as indicators of clinical response to ICI
Several studies have reported that baseline, or pre-treatment, levels of soluble immune checkpoints can indicate clinical response to ICI. In addition, circulating levels of soluble immune checkpoints can change upon treatment with ICI, and in some cases, the magnitude of this change associates with clinical response.
sPD-L1
Structure and function of membrane-bound PD-L1
Programmed cell death-ligand 1 (PD-L1) is expressed on APCs (such as macrophages and dendritic cells), T cells, and tumor cells [25,26,27,28]. It is a transmembrane glycoprotein that consists of immunoglobulin C-like (IgC) and immunoglobulin variable-type (IgV-type) extracellular domains, a transmembrane domain, and a cytoplasmic domain [25, 28]. The binding of PD-L1 to PD-1 induces the intracellular phosphorylation of PD-1; phosphorylated PD-1 recruits Src homology region 2 domain-containing phosphatase-1/2 (SHP-1/2), which downregulates downstream pathways such as the PI3K-AKT-mTOR and RAS-MEK-ERK pathways [29,30,31,32]. The downregulation of these pathways suppresses T cell growth, survival, and proliferation [32,33,34]; thus, the activation of the PD-1/PD-L1 pathway is immunosuppressive.
Production and function of sPD-L1
Soluble PD-L1 (sPD-L1) is the most well-studied soluble immune checkpoint. It is produced through two different mechanisms – alternative splicing of PD-L1 mRNA [35, 36] or cleavage of the membrane-bound PD-L1 protein [37,38,39,40]. sPD-L1 has been found to be immunosuppressive, inhibiting T cell secretion of interferon gamma (IFN-γ) [35, 36] and interleukin 2 (IL-2) [36] and inducing the apoptosis of CD4+ [41, 42] and CD8+ [38, 42] T cells. Like its membrane counterpart, sPD-L1 can bind to PD-1 [36, 40, 43]. sPD-L1 can also bind to anti-PD-L1 monoclonal antibodies, thus potentially inducing resistance to anti-PD-L1 therapy [43].
sPD-L1 expression in cancer patient plasma/serum and association with tumor stage
Most studies have found sPD-L1 to be elevated in cancer patients compared to healthy donors, with higher levels observed in patients with non-small cell lung cancer (NSCLC) [44,45,46,47], small cell lung cancer [48], gastric cancer [49, 50], hepatocellular carcinoma [51,52,53], colorectal cancer [54], nasopharyngeal carcinoma [55], differentiated thyroid carcinoma [56], glioma [22], basal cell carcinoma [57], renal cell carcinoma [58], prostate cancer [59], and ovarian cancer [23, 60, 61]. However, some studies have reported no difference in the levels of sPD-L1 between healthy donors and patients with a variety of tumors including NSCLC [62], glioma [20], esophageal cancer [63], triple-negative breast cancer [64], and bladder cancer [21], and a few studies have described lower levels of sPD-L1 in patients with nasopharyngeal carcinoma [65], early breast cancer [66, 67], gastric carcinoma [68], hepatocellular carcinoma [69], and clear cell renal cell carcinoma [70] compared to healthy donors.
Among cancer patients, most studies (n = 17) have found higher sPD-L1 levels to be associated with a higher histological tumor grade and/or more advanced disease stage. sPD-L1 is associated with a higher histological tumor grade/stage of cancer in patients with gastric cancer [50], hepatocellular carcinoma [69, 71], glioma [20, 22], nasopharyngeal carcinoma [55], triple-negative breast cancer [64], renal cell carcinoma [72], and clear cell renal cell carcinoma [41]. Other studies have also reported a correlation between elevated sPD-L1 and more advanced disease. For example, higher sPD-L1 correlated with larger tumor size and greater venous invasion in patients with hepatocellular carcinoma [71], and with a larger tumor size and the presence of cervical lymph node metastasis in patients with differentiated thyroid carcinoma [56]. In patients with clear cell renal cell carcinoma, higher sPD-L1 was associated with larger tumors and increased tumor necrosis [41], and the presence of metastatic disease [70]. Higher sPD-L1 also correlated with the presence of metastasis in patients with renal cell carcinoma [72] and with the presence of liver metastasis in patients with NSCLC [73]. Elevated sPD-L1 also associated with the presence of muscle invasive disease and metastasis in patients with bladder cancer [21], lymph node metastasis in patients with colorectal cancer [54], higher Gleason scores in prostate cancer [59], less differentiated tumors and increased invasion and metastasis in renal cell carcinoma patients [58], and a greater residual tumor burden in patients with ovarian cancer [60]. Only a small number of reports (n = 4) have shown no association between sPD-L1 and tumor stage (in epithelial ovarian cancer [60], gastric cancer [74], hepatocellular carcinoma [51], and lung cancer [75]). Many variables exist among these studies, such as the number of patients evaluated, the material measured (serum or plasma), the assay used (ELISA or multiplex assay), and a high degree of variation in the cutoff values of sPD-L1 that were used to stratify patients into “high” vs “low” groups. Differences in any of these variables could explain why a few studies found conflicting results; however, these studies on sPD-L1 collectively suggest that levels are elevated in cancer patients compared to healthy donors, and that elevated levels are associated with a higher histological tumor grade and/or more advanced cancer stage. The prognostic value of sPD-L1 as an indicator of clinical response to ICI and conventional therapies will be addressed in subsequent sections.
Baseline sPD-L1 as an indicator of clinical response to ICI
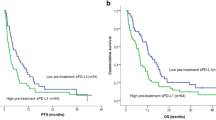
Eight studies involving 1,067 patients with solid tumors have reported that elevated levels of sPD-L1 at baseline are statistically associated with poor clinical response to ICI (Table 1). This is seen in patients treated with anti-PD-1 (e.g., nivolumab and pembrolizumab), anti-CTLA4 (e.g., ipilimumab), and anti-PD-L1 (e.g., durvalumab and atezolizumab) therapies, along with those treated with a combination of multiple ICI. Specifically, among NSCLC patients treated with anti-PD-1, higher baseline sPD-L1 levels correlated with a worse response rate and a shorter time to treatment failure (median: 1.48 vs 5.36 months) [76]. In this population, higher baseline sPD-L1 levels also correlated with shorter progression-free survival (PFS) [77] and overall survival (OS) [76]. A similar study in NSCLC patients treated with anti-PD-1 or anti-PD-L1 monotherapy found that elevated baseline sPD-L1 correlated with both shorter PFS (median: 76 vs 132 days, p = 0.019, Fig. 2A) and OS (median: 115 vs 444 days, p < 0.001, Fig. 2B) [78]. Elevated baseline sPD-L1 also associated with shorter PFS (median: 1.7 vs 2.1 months) and OS (median: 4.1 vs 8.9 months) in gastric cancer patients treated with anti-PD-1 [79], and with shorter OS (median: 24.6 vs > 40 months) and lower objective response rate (ORR) in metastatic renal cell carcinoma patients treated with nivolumab [80]. In another study of NSCLC patients treated with pembrolizumab or nivolumab, high baseline sPD-L1 associated with shorter PFS (median: 57 vs 177 days) and OS (median: 182 vs > 1000 days) [73]. In the same study, high baseline sPD-L1 also associated with a lower disease control rate, defined as the percent of patients with complete response (CR), partial response (PR), or stable disease (SD) (37% vs 57%) [73]. High baseline sPD-L1 also associated with shorter OS in urothelial cancer patients treated with atezolizumab or pembrolizumab [81]. Finally, Oh et al. showed that elevated baseline sPD-L1 correlated with shorter PFS (median: 2.9 vs 6.3 months) and OS (median: 7.4 vs 13.3 months) and a lower disease control rate (58% vs 79%) in patients with a variety of cancers who were treated with nivolumab, pembrolizumab, ipilimumab, durvalumab, atezolizumab or combination therapy [82].

Copyright © 2023, Himuro et al., under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature. Panel (C) modified from Gorgulho, Int J Cancer, 2021 [83]. © 2021 Gorgulho et al. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC. Creative Commons CC-BY-NC license
Elevated baseline levels of soluble immune checkpoints correlate with worse response to immune checkpoint therapy. Kaplan–Meier curves showing (A) progression-free survival (PFS) and (B) overall survival (OS) in NSCLC patients treated with anti-PD-1 or anti-PD-L1 monotherapy based on pre-treatment plasma sPD-L1 levels. Pre-treatment levels of sBTLA associated with OS of patients with advanced cancers treated with anti-PD-1 or the combination of anti-PD-1 plus anti-CTLA4 or other immune checkpoint inhibitors (C). Panels (A) and (B) modified from Himuro, Cancer Immunol Immunother, 2023 [78]. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC. Creative Commons CC-BY-NC license
While most studies have shown that elevated baseline sPD-L1 is indicative of a worse response to ICI (Table 1), a few (n = 3) have found baseline sPD-L1 to have a negligible prognostic value, while two others have reported high levels to positively associate with patient outcome. Castello et al. found that baseline sPD-L1 levels did not impact PFS or OS in NSCLC patients treated with nivolumab, pembrolizumab or a combination of nivolumab and ipilimumab [84], while Ando et al. observed that baseline sPD-L1 was not correlated with OS in patients with NSCLC, gastric cancer, or bladder cancer who received nivolumab or pembrolizumab [85]. Among NSCLC patients treated with nivolumab, there were no differences in baseline sPD-L1 levels between responders and non-responders [86]. Incorvaia et al. showed that high baseline sPD-L1 correlated with longer PFS (median: 19 vs 9 months) in clear cell renal cell carcinoma patients treated with nivolumab [87], while Zhao et al. observed that high baseline sPD-L1 correlated with a better response to anti-PD-1 or anti-PD-L1 monotherapy in patients with a variety of cancers [88]. As detailed above, numerous investigators have evaluated the association between baseline levels of sPD-L1 and association with patient outcome following ICI. While there are some conflicting findings, which may be impacted by heterogeneity of the patient populations evaluated, the type of material and assays utilized to measure sPD-L1, and variation in the cut points used in which to stratify patients into groups, the vast majority of these studies indicate that lower levels of sPD-L1, prior to initiating ICI, can identify patients with a variety of cancers with improved clinical responses following ICI.
Post-treatment levels of sPD-L1 after ICI as an indicator of clinical response
Plasma and serum levels of soluble immune checkpoints can change upon treatment with ICI, and in some cases, the magnitude of this change associates with clinical response. Three studies reported that sPD-L1 increased upon ICI therapy [81, 84, 89], while two observed sPD-L1 to remain constant upon ICI treatment (Table S1) [77, 90]. In terms of clinical outcome, either a decrease or less of an increase in sPD-L1 upon treatment with ICI is correlated with improved clinical responses, with four studies (n = 204 patients) reporting similar findings (Table 2). In patients with NSCLC, gastric cancer, or bladder cancer treated with nivolumab or pembrolizumab, a greater decrease in sPD-L1 after four cycles of treatment correlated with a greater decrease in tumor size [85]. Similarly, in NSCLC patients treated with nivolumab, an increase in sPD-L1 after 2 months of treatment correlated with a lower ORR (17% in patients with increases vs 68% in patients with decreases or stable levels) and shorter PFS (median: 1.8 vs 6.5 months, Fig. 3A), and OS (median: 5.4 months vs not reached as of 20 months, Fig. 3B) [86]. In that study, high levels of sPD-L1 after 2 months of treatment also associated with poor response, with non-responders having higher levels of sPD-L1 than responders (median: 67.64 vs 32.94 pg/mL) [86]. In another study, patients with NSCLC who responded to nivolumab treatment had lower levels of sPD-L1 3 months after treatment compared to non-responders (Fig. 3C), and low sPD-L1 was correlated with longer PFS (Fig. 3D) [90]. NSCLC patients treated with pembrolizumab or nivolumab who had high levels of sPD-L1 after 6 weeks of treatment exhibited a shorter PFS (median: 64 vs 239 days) than patients with low levels of sPD-L1 at this timepoint [78]. In the same study, patients with high levels of sPD-L1 after 6 weeks of treatment exhibited shorter OS (median: 118 vs 653 days) than patients with levels below this threshold [78]. These studies collectively show that a reduction or stabilization in sPD-L1 levels after ICI associate with improved clinical outcomes.
Post-treatment levels of soluble immune checkpoints after immune checkpoint therapy associate with patient response. The change in circulating levels of sPD-L1 at the first tumor evaluation (2 months after the initiation of nivolumab treatment) compared to baseline associated with progression-free survival (PFS) (A) and overall survival (OS) (B) in advanced NSCLC patients. Post treatment levels of the soluble immune checkpoints sTIM3, sBTLA4, sHVEM, sCTLA4, sPD-L1 and sPD-1 were higher after treatment with nivolumab in non-responding (NR) vs responding (R) patients with NSCLC (C). In this study, post treatment levels of sPD-L1, sTIM3, and sBTLA4 also associated with PFS (D). Panels (A and B) from Constantini, Oncoimmunology April 20, 2018 [86]. Reprinted by permission of the publisher Taylor & Francis Ltd., http://www.tandfonline.com. Panels (C and D) modified from Zizzari, J Pers Med 2020 [90]. Copyright © 2020 by Zizzari et al. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)
sPD-1
Structure and function of membrane-bound PD-1
Programmed cell death protein 1 (PD-1) is expressed on T cells, B cells, and myeloid cells [34, 91,92,93]. It is a transmembrane glycoprotein that consists of an IgV-type extracellular domain, a stalk, a transmembrane domain, and a cytoplasmic domain [94,95,96]. The cytoplasmic domain contains two tyrosines, one of which forms an immunoreceptor tyrosine-based inhibitory motif (ITIM) while the other forms an immunoreceptor tyrosine-based switch motif (ITSM) [29,30,31]. The binding of PD-L1 to PD-1 induces the phosphorylation of the cytoplasmic ITSM on PD-1; a phosphorylated ITSM recruits SHP-1/2, which downregulates downstream pathways such as the PI3K-AKT-mTOR and RAS-MEK-ERK pathways [29,30,31,32]. The downregulation of these pathways suppresses T cell growth, survival, and proliferation [32,33,34]; thus, the activation of the PD-1/PD-L1 pathway is immunosuppressive.
Production and function of sPD-1
Soluble PD-1 (sPD-1) is produced through alternative splicing of PD-1 mRNA [97] and is not nearly as well-studied as sPD-L1. Functional studies of sPD-1 have been performed both in vitro and in mice, with most of these studies, in contrast to membrane-bound PD-1, reporting sPD-1 to exhibit pro-inflammatory and anti-tumor effects; sPD-1 activated T lymphocytes [98,99,100,101], upregulated the expression of IFN-γ and tumor necrosis factor alpha (TNF-α) [98], and reduced expression of IL-10 [98]. These changes correlated with increased tumor cell lysis [100] and reduced tumor growth in several murine cancer models [98,99,100, 102]. However, one study reported that sPD-1 inhibited CD4+ T cell activation in the presence of dendritic cells [103]. In addition, sPD-1 can bind to PD-L1 and PD-L2 [102], but whether this interaction is immunosuppressive or immune-activating requires further study.
sPD-1 expression in cancer patient plasma/serum and association with tumor stage
Most studies have found sPD-1 to be elevated in cancer patients compared to healthy donors, with elevated levels reported in patients with NSCLC [47, 104], esophageal cancer [63], differentiated thyroid carcinoma [56], hepatocellular carcinoma [105], nasopharyngeal carcinoma [55], basal cell carcinoma [57], triple-negative breast cancer [64], prostate cancer [59], and ovarian cancer [23]. One study reported no difference between the levels of sPD-1 in healthy donors and early breast cancer patients [67], while a few others reported lower levels of sPD-1 in patients with nasopharyngeal carcinoma [65], gastric cancer [106], and gastric carcinoma [68].
Among cancer patients, higher sPD-1 levels in most reports (n = 5) are associated with more advanced cancers. Higher levels of sPD-1 associate with a higher histological tumor grade/stage of cancer in patients with triple-negative breast cancer [64], ovarian cancer [23], and gastric cancer [106]. Similarly, higher sPD-1 correlated with increased tumor invasion in patients with renal cell carcinoma [58] and with a larger tumor size and the presence of cervical lymph node metastasis in patients with differentiated thyroid carcinoma [56]; however, sPD-1 did not correlate with Gleason scores in patients with prostate cancer [59]. Only one study in patients with nasopharyngeal carcinoma found that lower levels of sPD-1 associated with more advanced stages of disease [65]. Thus, while there are some conflicting reports, the majority of studies on sPD-1 suggest that levels are elevated in cancer patients compared to healthy donors, and that higher levels are most typically associated with a more advanced cancer phenotype.
Baseline sPD-1 as an indicator of clinical response to ICI
Elevated levels of sPD-1 prior to initiation of ICI have been shown in two studies (with a total of 490 patients) to statistically correlate with a poor clinical response to therapy (Table S2). High baseline sPD-1 was correlated with shorter PFS in NSCLC patients treated with nivolumab [77] and with shorter OS (median: 5.7 vs 8.5 months) in gastric cancer patients treated with nivolumab [79]. In contrast, a single study found that high baseline sPD-1 correlated with longer PFS (median: 20.7 vs 6.9 months) and overall response in clear cell renal cell carcinoma patients treated with nivolumab [87]. Additional studies are needed to elucidate the role of baseline levels of sPD-L1 as an indicator of patient response to ICI.
Post-treatment levels of sPD-1 after ICI as an indicator of clinical response
Studies have reported conflicting findings regarding changes in sPD-1 upon ICI therapy, with some studies reporting an increase [107], decrease [90] or no change upon treatment (Table S1) [77]. Three studies, however, have shown that either a decline, or less of an increase in sPD-1 upon treatment with ICI associates with improved response to therapy (Table S3). Clear cell renal cell carcinoma patients responding to nivolumab experienced a decrease in sPD-1 (from a median of 13.25 to 1.23 ng/mL) after two cycles of treatment [87]; however, in that study, changes in sPD-1 after nivolumab treatment were not investigated in non-responders. In a different study in patients with NSCLC, responders experienced a decrease in sPD-1 after 3 months of nivolumab treatment, while sPD-1 levels remained constant in non-responding patients [90]. The authors also showed that responders had lower absolute levels of sPD-1 3 months after treatment than non-responders (p < 0.05, Fig. 3C) [90]. Among patients with NSCLC, gastric cancer, or bladder cancer treated with nivolumab or pembrolizumab, sPD-1 levels were typically higher after the second cycle of treatment than at baseline, and a greater rate of increase between the second and fourth cycle of treatment correlated with an increase in tumor size [107]. While these studies collectively suggest that a decrease, or lower levels of sPD-1 after ICI associate with improved clinical outcomes, two other studies have reported conflicting findings (Table S3). Tiako Meyo et al. found that increased (> 30%) or stable levels of sPD-1 after two cycles of nivolumab in NSCLC patients correlated with both longer PFS (median: 121 vs 50 days) and OS (median: 450 vs 153 days) [77]. In addition, Himuro et al. showed that NSCLC patients treated with pembrolizumab or nivolumab who had high levels of sPD-1 after 6 weeks of treatment exhibited a longer OS (median: 821 vs 183 days) than patients with levels below this threshold [78]. These studies demonstrate that changes or post-treatment levels in sPD-1 do not consistently associate with patient outcomes following ICI and suggest that further evaluations of this soluble checkpoint are needed.
sCTLA4
Structure and function of membrane-bound CTLA4
Cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) is expressed on T cells [108,109,110]. It is a transmembrane glycoprotein that consists of an IgV-type extracellular domain, a transmembrane domain, and a cytoplasmic domain [111,112,113]. The binding of CTLA4 to CD80 (or to CD86) suppresses T cell growth, survival, and proliferation [109, 110, 114]. However, the intracellular signaling mechanisms of the CTLA4/CD80-CD86 pathway remain unclear. The cytoplasmic domain of CTLA4 has been shown to interact with protein phosphatase 2A (PP2A), SHP-2, and PI3K, interactions which may affect downstream pathways such as the PI3K-AKT-mTOR and RAS-MEK-ERK pathways [31, 115,116,117,118,119].
Production and function of sCTLA4
Soluble CTLA4 (sCTLA4) is produced through alternative splicing of CTLA4 mRNA [120, 121]. Like its membrane counterpart, sCTLA4 has been found to have immunosuppressive functions, with the addition of recombinant sCTLA4 to human peripheral blood mononuclear cells (PBMCs) in vitro reducing CD8+ and CD4+ T cell proliferation and inhibiting the secretion of IFN-γ, IL-17A, and IL-10 [122]. In this study, antibody blockade of sCTLA4 increased effector cytokine secretion to partially reverse this immunosuppression [122]. Another group similarly showed that antibody blockade of sCTLA4 induced T cell proliferation and cytokine (IFN-γ and IL-17) secretion by human PBMCs in vitro [123]. sCTLA4 can bind to CD80 and CD86, and this interaction was found to inhibit a mixed lymphocyte reaction [121]. This report also suggested that binding of sCTLA4 to CD80 and CD86 could compete with and inhibit the binding of CD80 and CD86 to the co-stimulatory CD28 molecule [121].
sCTLA4 expression in cancer patient plasma/serum and association with tumor stage
Several studies have found sCTLA4 to be elevated in cancer patients compared to healthy donors, with higher levels reported in patients with NSCLC [104], nasopharyngeal carcinoma [55], basal cell carcinoma [57], breast cancer [124], and ovarian cancer [23]. Only a few studies have reported lower levels of sCTLA4 in cancer patients (early breast cancer [66, 67], nasopharyngeal carcinoma [65], and clear cell renal cell carcinoma [125]) than in healthy donors. A single study, in patients with hepatocellular carcinoma with chronic hepatitis C infection, found higher concentrations of sCTLA4 to correlate with a later TNM stage and a larger tumor size [126]. Overall, most studies suggest that sCTLA4 is elevated in cancer patients compared to healthy donors; however, further work is needed to understand its association with cancer stage.
Baseline and post-treatment levels of sCTLA4 as an indicator of clinical response to ICI
Only one study has reported on the association between levels of sCTLA4 prior to ICI in solid tumors other than melanoma and clinical response (Table S2). Increased levels of sCTLA4 prior to treatment correlated with shorter OS (median: 5.3 vs 7.9 months) in gastric cancer patients treated with nivolumab [79]. Zizzari et al. showed that the levels of sCTLA4 were not significantly changed in NSCLC patients upon treatment with nivolumab (Table S1) [90]. The same study observed that patients with NSCLC who responded to nivolumab had lower levels of sCTLA4 3 months after treatment than non-responders (Table S3, Fig. 3C) [90].
sCD80
Structure and function of membrane-bound CD80
CD80 is expressed on dendritic cells, macrophages, B cells, and tumor cells [127,128,129,130]. It is a transmembrane glycoprotein that consists of IgC and IgV-type extracellular domains, a transmembrane domain, and a cytoplasmic domain [129, 130]. The binding of CTLA4 to CD80 (or to CD86) suppresses T cell growth, survival, and proliferation [109, 110, 114]. However, the intracellular signaling mechanisms of the CTLA4/CD80-CD86 pathway remain unclear. The cytoplasmic domain of CTLA4 has been shown to interact with PP2A, SHP-2, and PI3K, interactions which may affect downstream pathways such as the PI3K-AKT-mTOR and RAS-MEK-ERK pathways [31, 115,116,117,118,119]. In addition to CTLA4, CD80 (and CD86) can bind to CD28; this is an immunostimulatory interaction that induces T cell proliferation [110, 131].
Production and function of sCD80
Soluble CD80 (sCD80) is produced through alternative splicing of CD80 mRNA [132, 133], and studies regarding its function are conflicting. Kakoulidou et al. showed that recombinant sCD80 inhibited both a mixed lymphocyte reaction and anti-CD3-induced T cell proliferation of human PBMCs [132]. However, multiple other studies, both in vitro and in vivo, have found sCD80 to have anti-tumor effects. In vitro, Haile et al. demonstrated that sCD80 bound to PD-L1, inhibiting the PD-L1-PD-1 immunosuppressive pathway and restoring T cell secretion of IFN-γ [134]. Concurrently, sCD80 induced the co-stimulation of CD28 [134, 135]. In vivo, sCD80 reduced tumor growth and induced the recruitment of tumor-infiltrating lymphocytes (TILs) in a murine model of colon carcinoma [136]. Similarly, Sturmhoefel et al. showed that sCD80 reduced tumor growth and increased OS in mice both when given as an independent treatment and as a vaccine adjuvant [137]. That study found the anti-tumor response of sCD80 to be dependent on CD8+ T cells but independent of CD4+ T cells and IFN-γ [137].
sCD80 expression in cancer patient plasma/serum and association with tumor stage
Studies reporting on differences in the level of sCD80 between cancer patients and healthy donors are conflicting, with higher levels observed in patients with NSCLC [104] and nasopharyngeal carcinoma [55], but lower levels in a different study of patients with nasopharyngeal carcinoma [65], and in patients with early breast cancer [66]. In addition, similar levels have been reported between healthy donors and patients with soft tissue sarcoma and benign tumors [138], or early breast cancer [67]. To date, the association between sCD80 levels and tumor stage has been reported in only two studies; higher sCD80 levels correlated with increased tumor invasion in patients with NSCLC [45] and with increased invasion and less tumor differentiation in patients with renal cell carcinoma [58]. Thus, while sCD80 levels do not consistently differ between healthy donors and cancer patients, higher levels within cancer patients appear to associate with a more advanced cancer stage.
Baseline and post-treatment levels of sCD80 after ICI as an indicator of clinical response
No studies to date have evaluated the association between baseline levels or changes in levels of sCD80 after ICI and clinical response. Only one study has reported on changes in sCD80 levels after ICI, with Zizzari et al. showing that the levels were not significantly changed in NSCLC patients upon treatment with nivolumab (Table S1) [90].
sTIM3
Structure and function of membrane-bound TIM3
T cell immunoglobulin and mucin domain-containing protein 3 (TIM3) is expressed on T cells [139]. It is a transmembrane glycoprotein that consists of IgV-type and mucin-like extracellular domains, a transmembrane domain, and a cytoplasmic domain [139]. TIM3 has several binding partners, including GAL9 [140, 141]. The binding of TIM3 to GAL9 induces the phosphorylation of at least one of the cytoplasmic tyrosines on TIM3 [142]. Further intracellular signaling is unclear, but the TIM3/GAL9 pathway induces T cell death and MDSC expansion [140, 143, 144].
Production and function of sTIM3
Soluble TIM3 (sTIM3) is produced through both alternative splicing of TIM3 mRNA [145, 146] and cleavage of the membrane-bound TIM3 protein [147]. Few studies have investigated the function of sTIM3, and findings are conflicting. Sabatos et al. reported that sTIM3-Ig bound to the same ligands as membrane-bound TIM3, and that mice treated with a fusion protein of sTIM3 (sTim-3-Ig) had hyperproliferation of Th1 cells and increased release of Th1 cytokines IL-2 and IFN-γ [145]. The authors hypothesized that the normal interaction between TIM3 and TIM3 ligands is immunosuppressive, and the binding of sTIM-3-Ig to TIM3 ligands can block this inhibitory response, thus promoting anti-tumor effects [145]. In contrast, Geng et al. found sTIM3 to have immunosuppressive functions in both in vitro and in vivo studies [146]. In vitro, sTIM3 inhibited T cell proliferation in response to antigen-specific stimulation and anti-CD3/anti-CD28 costimulation, and inhibited the production of IL-2 and IFN-γ. Similarly, in murine models, sTIM3 reduced anti-tumor cytotoxic T lymphocyte activity, the number of TILs, and the expression of Th1 cytokines IL-2, IFN-γ, and TNF-β [146]. In these studies, sTIM3 also increased tumor growth in a murine model of hepatocarcinoma [146].
sTIM3 expression in cancer patient plasma/serum and association with tumor stage
Most studies that have evaluated sTIM3 levels have found them to be higher in cancer patients compared to healthy donors, with increased levels observed specifically in patients with NSCLC [104], hepatocellular carcinoma [148], oral squamous cell carcinoma [149], differentiated thyroid carcinoma [56], basal cell carcinoma [57], gastric cancer [150], and osteosarcoma [151]. Only two studies have reported lower levels of sTIM3, both in patients with early breast cancer, compared to those of healthy donors [66, 67], while two others found no difference in the levels between healthy donors and patients with nasopharyngeal carcinoma [55, 65].
Among cancer patients, higher sTIM3 has consistently been reported (in seven different studies) to associate with a higher histological tumor grade/stage of cancer. This is true in NSCLC [104], hepatocellular carcinoma [152], oral squamous cell carcinoma [149], gastric cancer [150], clear cell renal cell carcinoma [153], and osteosarcoma [151]. Higher sTIM3 also correlated with a larger tumor size and the presence of distant metastases in patients with osteosarcoma [151], and with larger tumor size, later TNM stage, and the presence of cervical lymph node metastases in patients with differentiated thyroid carcinoma [56]. Overall, while sTIM3 may not consistently differ in level between healthy donors and cancer patients, higher levels within patients with cancer consistently associate with more advanced disease.
Baseline and post-treatment levels of sTIM3 after ICI as an indicator of clinical response
One study found that elevated baseline sTIM3 correlated with a better response to anti-PD-1 or anti-PD-L1 monotherapy in patients with a variety of cancers (Table S2) [88]. Zizzari et al. showed that the levels of sTIM3 were not significantly changed in NSCLC patients upon treatment with nivolumab (Table S1) [90]; however, the same study observed that patients with NSCLC who responded to nivolumab had lower levels of sTIM3 3 months after treatment than non-responders (Table S3, Fig. 3C), and low concentrations of sTIM3 were correlated with longer PFS (Fig. 3D) [90]. Additional cohorts of patients are needed to confirm the relevance of pre- and post-treatment levels of sTIM3 as an indicator of response to ICI.
sLAG3
Structure and function of membrane-bound LAG3
Lymphocyte activation gene 3 (LAG3) is expressed on T cells and NK cells [154, 155]. It is a transmembrane glycoprotein that consists of four immunoglobulin superfamily extracellular domains, a transmembrane domain, and a cytoplasmic domain [154]. The cytoplasmic domain contains a “KIEELE” motif, the lysine of which (K468) is thought to be important for intracellular signaling [156]. Though the exact intracellular signaling mechanisms remain unclear, the binding of membrane-bound LAG3 to MHC class II molecules induces the suppression of T cell function and proliferation [155,156,157,158,159,160].
Production and function of sLAG3
Soluble LAG3 (sLAG3) is produced through cleavage of the membrane-bound LAG3 protein [161, 162], and several studies, in contrast to membrane-bound LAG3, have shown sLAG3 to have immunostimulatory functions [163,164,165,166,167,168,169,170,171,172]. sLAG3 binds to MHC class II molecules [168, 169, 172] and induces dendritic cell maturation [166, 168,169,170, 172], increases production of IL-12, TNF-α, and IFN-γ [163, 166,167,168,169], and induces T cell proliferation [163, 164, 167, 168]. In murine models, sLAG3 reduced tumor growth [163, 165, 171] and increased the duration of OS [163]. One study also showed that high levels of sLAG3 in gastric cancer patients correlated with increased immune activation, as evidenced by higher levels of IL-12 and IFN-γ [163]. Other studies have reported that the cleavage of membrane LAG3 is required for T cell proliferation [161], and the induction of an anti-tumor response upon anti-PD-1 treatment [173]. However, one study suggested that sLAG3 itself does not cause this immunostimulatory effect but is instead an inert byproduct of LAG3 cleavage [161].
sLAG3 expression in cancer patient plasma/serum and association with tumor stage
Most studies evaluating levels of sLAG3 have found this checkpoint to be elevated in cancer patients compared to healthy donors, with higher levels described in patients with NSCLC [104], hepatocellular carcinoma [51], nasopharyngeal carcinoma [55, 65], differentiated thyroid carcinoma [56], pancreatic ductal adenocarcinoma [174], and basal cell carcinoma [57]. To date, only a single study reported no difference in sLAG3 levels between healthy donors and patients with early breast cancer [67], while one other study reported lower levels in patients with gastric cancer compared to healthy donors [163]. Among cancer patients, sLAG3 has been reported in three studies to associate with more advanced disease, with higher levels associating with an advanced cancer stage in hepatocellular carcinoma [51] and clear cell renal cell carcinoma [153], and the presence of cervical lymph node metastasis in patients with differentiated thyroid carcinoma [56]. Only one report has found that lower sLAG3 levels were associated with a more advanced stage in patients with NSCLC [175]. Overall, higher levels of sLAG3 are observed in cancer patients than healthy individuals, and higher levels associate with a more advanced cancer phenotype; however, further studies are warranted.
Baseline and post-treatment levels of sLAG3 after ICI as an indicator of clinical response
A single study reported that elevated baseline sLAG3 was correlated with shorter PFS and OS in head and neck squamous cell carcinoma patients treated with chemotherapy or nivolumab (Table S2) [176]. Another study, by Zizzari et al., showed that the levels of sLAG3 increased in NSCLC patients upon treatment with nivolumab (Table S1) [90]. The same study also reported that responders experienced no change in sLAG3 while non-responders experienced an increase in this soluble immune checkpoint after six cycles of nivolumab treatment (Table S3) [90]. Additional studies are needed to elucidate the role of sLAG3 as an indicator of clinical response to ICI.
sB7-H3
Structure and function of membrane-bound B7-H3
B7-H3 is expressed on APCs (such as dendritic cells and monocytes), T cells, B cells, NK cells, and tumor cells [177, 178]. It is a transmembrane glycoprotein that consists of IgC and IgV-type extracellular domains, a transmembrane domain, and a cytoplasmic domain [177]. A second isoform, called 4Ig-B7-H3, contains two pairs of IgC-IgV extracellular domains as opposed to simply one pair [178,179,180]. Both isoforms of B7-H3 (2Ig-B7-H3 and 4Ig-B7-H3) are present in humans, but 4-Ig-B7-H3 is predominant [179, 180]. Little is known about B7-H3 intracellular signaling – the binding partner of B7-H3 is unknown, and B7-H3 signaling has been shown to have both immunosuppressive and immunostimulatory effects [177, 178, 180,181,182,183,184].
Production and function of sB7-H3
Soluble B7-H3 (sB7-H3) is produced through both alternative splicing of B7-H3 mRNA [185] and cleavage of the membrane-bound B7-H3 protein [186, 187]. Only two studies have evaluated the function of sB7-H3, with both reporting immunosuppressive functions. One group found that sB7-H3 inhibited T cell proliferation and cytokine production (IL-2 and IFN-γ) in vitro [185], while another found that sB7-H3 increased TLR4 expression, which in turn activated NF-kB signaling and then induced IL-8 and VEGF expression [188]. As a result, sB7-H3 induced the migration and invasion of pancreatic cancer cells in vitro and led to increased lung metastasis in a murine model of pancreatic cancer [188].
sB7-H3 expression in cancer patient plasma/serum and association with tumor stage
Seven different studies have reported sB7-H3 to be elevated in cancer patients compared to healthy donors; elevated levels have been observed in patients with NSCLC [189], colorectal carcinoma [187], gastric adenocarcinoma [190], hepatocellular carcinoma [185, 191], non-muscle-invasive bladder cancer [192], and osteosarcoma [193]. Only a single report found lower levels of sB7-H3 in patients with clear cell renal cell carcinoma compared to those of healthy donors [125], while another observed no difference in levels between healthy donors and cancer patients with glioma [20].
Among cancer patients, multiple studies (n = 6) have consistently shown that higher sB7-H3 levels associated with a higher histological tumor grade/stage of cancer. This has been demonstrated in patients with NSCLC [189], gastric adenocarcinoma [190], hepatocellular carcinoma [191], glioma [20], ovarian cancer [194], and osteosarcoma [193]. Higher sB7-H3 also correlated with larger tumor size, nodal metastasis, and the presence of distant metastasis in patients with NSCLC [189], greater metastasis and less tumor differentiation in patients with osteosarcoma [193], and with larger tumor size, greater vascular invasion, and less tumor differentiation in patients with hepatocellular carcinoma [191]. Overall, higher levels of sB7-H3 are observed in patients with cancer compared to healthy controls, and higher levels of sB7-H3 associate with more advanced disease. No studies to date have evaluated the association between baseline levels or changes in levels of sB7-H3 after ICI as an indicator of clinical response.
sBTLA
Structure and function of membrane-bound BTLA
B and T lymphocyte attenuator (BTLA) is expressed on T cells, B cells, and APCs (including dendritic cells and macrophages) [195, 196]. It is a transmembrane glycoprotein that consists of an IgV-type extracellular domain, a transmembrane domain, and a cytoplasmic domain [195]. The cytoplasmic domain contains three tyrosines, two of which form ITIMs [195]. BTLA intracellular signaling is very similar to that of PD-1. The binding of BTLA to HVEM induces the phosphorylation of both cytoplasmic ITIMs on BTLA; as a result, BTLA recruits SHP-1/2 [197,198,199]. The targets of SHP-1/2 after recruitment to BTLA are unknown [200], but the BTLA/HVEM signaling pathway induces the suppression of T cell activation and proliferation [195, 196, 198, 199, 201].
Production and function of sBTLA
Soluble BTLA (sBTLA) is produced through alternative splicing of BTLA mRNA [202]. One study evaluated the function of sBTLA and, in contrast to membrane-bound BTLA, reported it to have pro-inflammatory and anti-tumor effects. Han et al. demonstrated that sBTLA can bind to HVEM, and reduced IL-10 and TGF-β expression in a murine model of cervical cancer, but did not sufficiently eliminate the tumor [203]. The authors identified that the combination of sBTLA and an HSP70 vaccine significantly improved the anti-tumor immune response, with combination treatment increasing expression of IL-2, IFN-γ, and CD8+ TILs, and reducing expression of IL-10, TGF-β, and Foxp3 [203]. This study demonstrates that by binding to HVEM, sBTLA can inhibit the immunosuppressive BTLA-HVEM interaction and in turn exert anti-tumor effects.
sBTLA expression in cancer patient plasma/serum and association with tumor stage
Five studies have evaluated the expression of sBTLA in cancer patients, with most reporting elevated levels compared to healthy donors. sBTLA was elevated in patients with nasopharyngeal carcinoma [55] and pancreatic ductal adenocarcinoma [174], as well as in patients with a variety of other cancers (including NSCLC, urogenital tract cancer, gastrointestinal cancer, and head and neck cancer) compared to healthy donors [83]. To date, a single study reported no difference in sBTLA between patients with early breast cancer and healthy controls [67], while another described lower levels in patients with nasopharyngeal carcinoma than healthy donors [65]. Notably, no studies have reported on the association between circulating levels of sBTLA and cancer stage.
Baseline and post-treatment levels of sBTLA after ICI as an indicator of clinical response
Gorgulho et al. showed that elevated levels of sBTLA at baseline correlated with shorter OS (median: 138 vs 526 days) in patients with a variety of cancers (n = 84) treated with nivolumab, pembrolizumab, combination treatment (nivolumab and ipilimumab) or other ICI (Table S2, Fig. 2C) [83]. This same study also showed that levels of sBTLA remained constant in patients with a variety of cancers who were treated with anti-PD-1, combination therapy (of anti-PD-1 plus anti-CTLA4) or other ICI (Table S1) [83]. Zizzari et al. similarly reported that the levels of sBTLA were not significantly changed in NSCLC patients upon treatment with nivolumab (Table S1) [90]. Zizzari et al. also observed that patients with NSCLC who responded to nivolumab had lower levels of sBTLA 3 months after treatment than non-responders (Table S3, Fig. 3C), and low concentrations of sBTLA were correlated with longer PFS (Fig. 3D) [90]. Similarly, Gorgulho et al. demonstrated that low levels of sBTLA post therapy associated with improved OS at both an early (p = 0.018) and late (p = 0.009) time point in patients with a variety of cancers who were treated with anti-PD-1, combination therapy (of anti-PD-1 plus anti-CTLA4) or other ICI (Table S3) [83]. Thus, low levels of sBTLA, both before and after ICI, are associated with improved clinical response. Further studies in additional patient populations treated with ICI are needed to confirm these findings.
sHVEM
Structure and function of membrane-bound HVEM
Herpesvirus entry mediator (HVEM) is expressed on T cells, B cells, NK cells, monocytes, dendritic cells, and tumor cells [204,205,206]. It is a transmembrane glycoprotein that consists of an extracellular domain with four cysteine-rich domains, a transmembrane domain, and a cytoplasmic domain [204, 207]. The binding of BTLA to HVEM induces the phosphorylation of both cytoplasmic ITIMs on BTLA; as a result, BTLA recruits SHP-1/2 [197,198,199]. The targets of SHP-1/2 after recruitment to BTLA are unknown [200], but the BTLA/HVEM signaling pathway induces the suppression of T cell activation and proliferation [195, 196, 198, 199, 201]. In addition to BTLA, HVEM can also bind to other molecules, such as LIGHT; these interactions can have immunostimulatory effects [208,209,210].
Production and function of sHVEM
There are few studies reporting on the production and function of soluble HVEM (sHVEM). sHVEM is thought to be produced through cleavage of the membrane-bound HVEM protein [211]; however, the exact role of sHVEM is unclear. HVEM has multiple binding partners, including BTLA and LIGHT, with the HVEM-BTLA interaction serving as immunosuppressive, and the HVEM-LIGHT interaction immune-stimulatory [199, 212, 213]. sHVEM binds with higher affinity to LIGHT than BTLA [213, 214]; thus, two groups have hypothesized that sHVEM, by binding to LIGHT and interfering with the HVEM-LIGHT immune-stimulatory pathways, primarily has immunosuppressive effects [215, 216].
sHVEM expression in cancer patient plasma/serum and association with tumor stage
Six studies have evaluated sHVEM level in cancer patients as compared to healthy donors, with four reporting higher levels in cancer patients (with gastric cancer [211, 216], hepatocellular carcinoma [215], and nasopharyngeal carcinoma [55]), and two reporting lower levels in cancer patients (with nasopharyngeal carcinoma [65] and early breast cancer [67]). The association between sHVEM and cancer stage has only been evaluated in a single study; here, hepatocellular carcinoma patients with higher sHVEM levels had a more advanced stage of cancer [215]. Overall, most studies suggest that sHVEM is elevated in cancer patients compared to healthy donors; however, further work is needed to understand its association with tumor stage.
Baseline and post-treatment levels of sHVEM after ICI as an indicator of clinical response
No studies have evaluated the association between baseline levels of sHVEM and clinical response to ICI. One study, by Zizzari et al., showed that the levels of sHVEM were not significantly changed in NSCLC patients upon treatment with nivolumab (Table S1) [90]. However, the same study also reported that patients who responded to nivolumab had lower levels of sHVEM 3 months after treatment than non-responders (Table S3, Fig. 3C) [90]. Further work is needed to explore the role of sHVEM before and after ICI as an indicator of clinical response.
Baseline soluble immune checkpoints as indicators of clinical response to conventional therapies
Since the levels of soluble immune checkpoints may reflect the immune status of patients, it makes sense that the magnitude of these analytes could correlate with response to ICI, both before and during treatment. However, a large body of literature also indicates that the levels of soluble immune checkpoints, both before and during treatment, correlate with clinical response to non-immunotherapies, or conventional therapies; this may have important implications for combination studies in which ICI is administered in combination with conventional therapies. This section will summarize and discuss studies that have reported that baseline, or pre-treatment, levels of soluble immune checkpoints can indicate clinical response to conventional therapies. For the purposes of this review, conventional therapies refer to non-immunotherapies, and include chemotherapy, targeted drug therapy, radiotherapy, surgery, or combinations of these modalities.
sPD-L1
Elevated baseline sPD-L1 is associated with a poor clinical response to conventional therapies, with 29 different studies involving 3,200 cancer patients reporting a negative association with response rates, PFS or OS (Table 3). Specifically, among cancer patients treated with chemotherapy, higher baseline sPD-L1 levels correlated with shorter OS in those patients with NSCLC [46], gastric cancer [217, 218], pancreatic cancer [219, 220], urothelial cancer [81], and upper tract urothelial carcinoma [89]. As an example, in advanced gastric cancer patients receiving systemic chemotherapy, those with low sPD-L1 prior to therapy had both an improved OS (median: 8.9 months vs 14.6 months, p = 0.012, Fig. 4A) and PFS (median: 4.7 months vs 7.5 months, p = 0.025, Fig. 4B) compared to patients with higher baseline sPD-L1 [83]. In that study, baseline levels of sPD-L1 also associated with best overall response (BOR), with patients developing SD or PR having significantly lower levels of sPD-L1 prior to therapy than those patients developing progressive disease (PD) (p = 0.039, Fig. 4C) [83]. Similarly, patients with upper tract urothelial carcinoma with elevated sPD-L1 levels prior to therapy had a shorter duration of OS (median: ~ 10 months vs not reached at ~ 70 months, p = 0.006) following treatment with chemotherapy compared to patients with lower baseline levels (Fig. 4D) [89]. In small cell lung cancer patients receiving chemotherapy consisting of cisplatin-etoposide, elevated levels of sPD-L1 prior to therapy correlated with poor response to therapy and increased rates of death [48].

Copyright © 2023, Shin et al. Open access license Creative Commons CC BY. Panel (D) modified from Szeles, Biomedicines 2022 [89]. © 2022 by Szeles et al. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/)
Elevated baseline levels of sPD-L1 correlate with worse response to conventional therapies. Advanced gastric cancer patients treated with chemotherapy were stratified by overall survival (OS) (A) and progression-free survival (PFS) (B) based on baseline levels of sPD-L1. In this study, levels of sPD-L1 prior to therapy were also significantly lower in those patients developing partial response (PR) or stable disease (SD) after chemotherapy compared to patients developing progressive disease (C). Analyses in A and B were performed using the Kaplan–Meier method and the log rank test and in C with an unpaired t-test. D Upper tract urothelial carcinoma patients treated with chemotherapy were stratified by overall survival based on baseline levels of sPD-L1 ≥ vs < 96.1 pg/mL. PD, progressive disease. Panels (A-C) modified from Shin, Sci Rep 2023 [218].
In patients receiving targeted drug therapy, high baseline sPD-L1 associated with shorter PFS in patients with metastatic gastrointestinal stromal tumors [221] and clear cell renal cell carcinoma [222]. Among cancer patients treated with radiotherapy, elevated baseline sPD-L1 correlated with shorter OS in patients with NSCLC [223] and hepatocellular carcinoma [71]. High baseline sPD-L1 was also correlated with worse distant metastasis-free survival (74.0% vs 87.5% rate at 4 years) after radiotherapy or chemo-radiotherapy in nasopharyngeal carcinoma patients [55].
Many studies have also found a negative association between elevated pre-treatment sPD-L1 and response of cancer patients to surgery (Table 3). Higher baseline sPD-L1 correlated with shorter OS after surgical resection in patients with gastric cancer [49, 224], colorectal cancer [226, 227], hepatocellular carcinoma [225], renal cell carcinoma [72], soft tissue sarcoma [229], and upper tract urothelial carcinoma [89]. Elevated sPD-L1 prior to surgery was also associated with worse disease-free survival (DFS) in patients with gastric cancer [49], colorectal cancer [226], head and neck cancer [228], and hepatocellular carcinoma [225] and with worse relapse-free survival in patients with gastric cancer [74] and colorectal cancer with liver metastasis [227]. Elevated pre-treatment sPD-L1 levels were also associated with lower metastasis-free survival (42.4% vs 88.4% rate at 5 years) following surgery in patients with soft tissue sarcoma [229]. Among patients with hepatitis B virus (HBV)-related hepatocellular carcinoma, elevated baseline sPD-L1 correlated with shorter OS and DFS following treatment with surgery or thermal (radiofrequency or microwave) ablation [52]. High baseline sPD-L1 was also correlated with shorter OS and PFS in ovarian cancer patients receiving surgery or neoadjuvant chemotherapy [23] and with shorter OS in hepatocellular carcinoma patients treated with resection, local ablation, sorafenib or liver transplantation [69].
There are also several studies reporting on the association between baseline levels of sPD-L1 and response to combination treatment with conventional therapies (Table 3). In all cases, elevated baseline sPD-L1 correlated with worse clinical outcomes. Among renal cell carcinoma patients, high pre-treatment sPD-L1 correlated with shorter OS after treatment with (1) surgery, (2) surgery and first-line treatment with sunitinib, or (3) surgery and second-line treatment with axitinib [58]. In patients with clear cell renal cell carcinoma, high baseline sPD-L1 correlated with worse 5-year OS rate after surgery in non-metastatic patients and a combination of nephrectomy and systemic therapy in metastatic patients [70]. Among epithelial ovarian cancer patients, high baseline sPD-L1 correlated with reduced PFS and OS 5 years after treatment with surgery and chemotherapy [60]. Elevated baseline sPD-L1 also correlated with shorter PFS (median: 24 vs 40 months) after combination treatment with surgery and chemotherapy in patients with high-grade serous ovarian cancer [230].
While most studies show that elevated baseline sPD-L1 is associated with a worse clinical response to a variety of conventional therapies, a few studies (n = 8) have reported baseline sPD-L1 to have either a negligible [51, 62, 222, 231,232,233] or positive [47, 234] prognostic value. Baseline sPD-L1 levels were not significantly associated with clinical outcomes in pancreatic cancer patients treated with chemotherapy [231], NSCLC patients receiving chemotherapy or targeted drug therapy [62] or treated with surgery [233], hepatocellular carcinoma patients treated with radiotherapy [232] or transarterial chemoembolization (TACE) [51], and metastatic clear cell renal cell carcinoma patients receiving bevacizumab [222]. sPD-L1 levels at baseline were positively associated with clinical outcome in NSCLC patients after surgery [47] and hepatocellular carcinoma patients following hepatic resection or liver transplantation [234]. Taken all together, most studies demonstrate that elevated circulating sPD-L1 prior to treatment with chemotherapy, radiotherapy, targeted drug therapy or surgery is associated with a poor clinical response to these conventional therapies.
sPD-1
While there are certainly fewer studies focusing on circulating levels of sPD-1 than sPD-L1, six different reports involving 299 cancer patients have shown that high levels of sPD-1 prior to conventional therapy correlated with a shorter PFS or OS following treatment (Table 4). Specifically among patients treated with chemotherapy, higher baseline sPD-1 levels were correlated with shorter OS (median: 3.4 vs 20.0 months) in patients with pancreatic adenocarcinoma (Fig. 5A) [219]. In addition, in patients receiving targeted drug therapy, high baseline sPD-1 associated with shorter PFS in patients with metastatic gastrointestinal stromal tumors [221] and clear cell renal cell carcinoma [222]. In patients with ovarian cancer, elevated baseline sPD-1 associated with a shorter 5-year OS rate (median: 37 vs 49 months) in patients treated with surgery [24], and a shorter duration of PFS in patients treated with surgery or neoadjuvant chemotherapy [23]. Furthermore, among patients with high-grade serous ovarian cancer, elevated baseline sPD-1 correlated with shorter PFS (median: 24 vs 30 months) following combination treatment with surgery and chemotherapy [230].

Reproduced with permission from Springer Nature, https://www.springernature.com/gp
Elevated baseline levels of soluble immune checkpoints correlate with worse response to conventional therapies. Kaplan-Meier analysis of overall survival (OS) in patients with pancreatic ductal adenocarcinoma treated with chemotherapy stratified by baseline levels of sPD-1 and sBTLA (A) in a learning cohort (left) and validation cohort (right). Levels of sCTLA4 prior to therapy in colorectal cancer patients treated with surgery associated with both OS (B) and disease-free survival (C). Panel (A) modified from Bian, OncoImmunology, 2019 [219]. Reprinted by permission of the publisher Taylor & Francis Ltd., http://www.tandfonline.com. Panels (B and C) modified from Omura, Cancer Immunol Immunother 2020 [226].
Four studies involving 198 patients have found baseline sPD-1 to have a negligible [63, 222, 231, 232] prognostic value in cancer patients treated with conventional therapies. Specifically, baseline levels of sPD-1 did not associate with clinical outcomes in esophageal cancer patients receiving chemotherapy (with or without radiation or surgical resection) [63], pancreatic cancer patients treated with chemotherapy [231], metastatic clear cell renal cell carcinoma receiving bevacizumab [222], and hepatocellular carcinoma patients treated with radiotherapy [232]. Conversely, a single study found that high baseline sPD-1 correlated with longer DFS and OS in hepatocellular carcinoma patients after surgical resection [225]. Collectively, these studies demonstrate that high sPD-1 levels at baseline, in some cases but not others, associate with poor clinical outcomes following treatment with conventional therapies. These discrepant findings may be impacted by multiple factors, including the specific type, dose, and schedule of the conventional therapy evaluated.
sCTLA4
Three studies involving 409 patients with solid tumors have evaluated the association between baseline levels of sCTLA4 and response to conventional therapies, with all reporting a negative association with PFS (Table 4). In colorectal cancer patients treated with surgery, higher baseline sCTLA4 levels correlated with both shorter OS (Fig. 5B) and DFS (Fig. 5C) [226]. Elevated baseline sCTLA4 also correlated with a higher risk of biochemical recurrence and increased progression in prostate cancer patients after treatment with radical prostatectomy, radiotherapy or surveillance [235]. Finally, among patients with hepatocellular carcinoma and chronic hepatitis C, high baseline sCTLA4 correlated with earlier local recurrence and development of intrahepatic metastasis after treatment with radiofrequency ablation [126]. Collectively these studies demonstrate that elevated levels of sCTLA4 prior to therapy associate with poor outcomes in cancer patients treated with a variety of conventional therapies.
sCD80, sTIM3, sLAG3, sB7-H3, sBTLA and sHVEM
While the soluble immune checkpoints sCD80, sTIM3, sLAG3, sB7-H3, sBTLA, and sHVEM are far less studied than sPD-L1, sPD-1, and sCTLA4, elevated levels are generally associated with a poor clinical response to a variety of conventional therapies. A total of 12 different studies involving 1,672 patients have reported that high levels at baseline of at least one of these analytes (sCD80, sTIM3, sLAG3, sB7-H3, sBTLA, and sHVEM) are associated with poor clinical outcomes following conventional therapy (Table 4). Higher baseline sBTLA levels correlated with shorter OS in pancreatic adenocarcinoma patients receiving chemotherapy (median: 3.4 vs 17.4 months, Fig. 5A) [219], and in hepatocellular carcinoma patients after treatment with sorafenib, a targeted drug therapy (median: 8.4 vs 20.3 months) [237]. High baseline sLAG3 associated with poor ORR (lower frequencies of CR and PR), and shorter OS (median: 13.63 vs 34.43 months) in hepatocellular carcinoma patients treated with TACE [51]. In patients having had surgical resection, high baseline sCD80 associated with lower metastasis-free survival (44.0% vs 75.3% rate at 5 years) and shorter OS (65.0% vs 89.5% rate at 5-years) in soft tissue sarcoma patients [138], while high baseline sB7-H3 correlated with lower OS (median: 25.62 vs 47.75 months) in hepatocellular carcinoma patients [191]. High baseline sB7-H3 correlated with shorter recurrence-free survival (25.4% vs 60.2% rate at 3-years and 23.2% vs 51.9% rate at 5 years) and PFS (85.0% vs 95.0% rate at 3 years and 68.8% vs 91.7% rate at 5-years) in non-muscle-invasive bladder cancer patients treated with transurethral resection [192]. In addition, in prostate cancer patients treated with radical prostatectomy, radiotherapy or surveillance, high baseline levels of both sCD80 and sHVEM correlated with a higher risk of biochemical recurrence and increased progression, while high baseline sTIM3 and sBTLA correlated with increased aggressiveness [235]. In that study, high baseline sBTLA also correlated with an increased rate of disease progression. High baseline sLAG3 associated with both lower PFS and OS in advanced head and neck cancer patients after treatment with chemotherapy [176].
In clear cell renal cell carcinoma patients treated with surgery or a combination of surgery and chemotherapy, high baseline sTIM3 and sBTLA associated with reduced OS (Table 4) [153]. In addition, high pre-treatment sTIM3 correlated with lower OS in osteosarcoma patients after treatment with neoadjuvant chemotherapy, radical surgery, and chemotherapy [151]. Among patients with high-grade serous ovarian cancer, elevated baseline sBTLA correlated with shorter PFS (median: 24 vs 32 months) following surgery and chemotherapy [230]. Finally, high baseline sLAG3 was associated with tumor relapse and shorter relapse-free survival in lung cancer patients after treatment with surgical resection alone or in combination with either chemotherapy or radiotherapy [236]. Only two studies to date have found any of these soluble checkpoints at baseline to have either a negligible [152, 238] or positive [238] prognostic value.
Post-treatment levels of soluble immune checkpoints after conventional therapies as indicators of clinical response
sPD-L1
As observed with ICI, plasma and serum levels of soluble immune checkpoints can change upon treatment with conventional therapies and these changes can, in some cases, correlate with clinical response. Numerous studies (n = 22, involving 1,223 patients) have reported on the effect of conventional therapies, including chemotherapy, targeted drug therapy, radiotherapy, surgery, or combinations of these modalities, on the level of sPD-L1 (Table S4). The majority of these studies have found that sPD-L1 is increased in patients upon treatment [62, 65, 67, 71, 89, 232, 233, 237, 239,240,241,242,243]; however, others have reported that levels either remained constant [51, 62, 64, 68, 81, 89, 222, 243] or decreased [55, 223, 227, 244] following treatment with conventional therapies.
Many fewer studies (n = 8, involving 513 patients) have reported on the association between patient outcomes and levels or changes in the levels of sPD-L1 following treatment with conventional therapies (Table 5). In general, as was seen with ICI, a decrease or less of an increase in sPD-L1 upon treatment with conventional therapy is typically correlated with better response, while greater increases or higher levels are often associated with poor clinical responses. Pancreatic cancer patients who responded to chemotherapy experienced a reduction in sPD-L1 after three cycles of treatment; 48.3% of patients who experienced a decrease in sPD-L1 achieved CR or PR, compared to only 20.8% of patients with stable levels or increases in sPD-L1 [220]. Among triple-negative breast cancer patients receiving neoadjuvant chemotherapy, those who achieved CR or PR had reduced sPD-L1 after treatment, while non-responders (with either SD or progressive disease (PD)) had no change after treatment [64]. In addition, patients with locally advanced rectal cancer who responded to neoadjuvant chemoradiotherapy experienced decreases in sPD-L1 during the course of treatment with sPD-L1 that returned to baseline levels by the end of treatment; non-responders, in contrast, experienced no change in sPD-L1 throughout the course of treatment [244]. Metastatic renal cell carcinoma patients who were responsive to sunitinib or pazopanib (both targeted therapies) had lower levels of sPD-L1 after 3–4 months of treatment (56.25 pg/mL) than non-responders (146.5 pg/mL) [245]. Furthermore, locally advanced rectal cancer patients treated with neoadjuvant chemoradiotherapy experienced a correlation between high sPD-L1 after treatment and an increased presence of lymphovascular invasion [239], while high sPD-L1 after hepatic resection in colorectal cancer patients with liver metastasis was indicative of a higher early recurrence rate (52.9% vs 13.8%) and shorter relapse-free survival (median of 5.87 vs 15.54 years, p = 0.0041) [227]. Lastly, in patients with hepatocellular carcinoma, high sPD-L1 levels after radiotherapy identified patients with significantly shorter PFS (median: 13.25 vs 18.75 months, p = 0.028, Fig. 6A) and OS (median: 25.03 vs 36.33 months, p = 0.033, Fig. 6B) [232]. In this study, not only higher post treatment levels, but also a higher rate of increase in sPD-L1 after radiotherapy correlated with a worse response rate (lower frequency of CR and PR, Fig. 6C) and a shorter duration of PFS (p = 0.032, Fig. 6D) and OS (p = 0.045, Fig. 6E) [232]. Only one study, in hepatocellular carcinoma patients treated with sorafenib, found no association between changes in sPD-L1 and patient outcome [237]. Collectively, these studies indicate that an increase in sPD-L1 after conventional therapy associates with poor clinical response.
Post treatment levels of sPD-L1 after conventional therapy associate with patient response. Kaplan–Meier analyses of (A) progression-free survival (PFS) and (B) overall survival (OS) in hepatocellular carcinoma patients with sPD-L1 levels > vs < 14.60 pg/ml after treatment with radiotherapy. The degree of change in sPD-L1 after radiotherapy compared to baseline also associated with overall response rate (C), PFS (D), and OS (E). Panels (A-E) from Zhang, Transl Oncol, 2022 [232].
sPD-1
Multiple studies (n = 13, involving 641 patients) have also reported on the effects of conventional therapies on the level of sPD-1, with some reporting an increase [65, 68, 237, 240, 246], no change [64, 67, 222, 239, 241, 243] or a decrease [242, 245] after therapy (Table S5). Only four studies, involving 187 patients, have evaluated the association between levels or changes in the levels of sPD-1 after conventional therapy and patient outcome (Table S6). One study found that triple-negative breast cancer patients developing CR or PR had reduced sPD-1 after treatment with neoadjuvant chemotherapy, while non-responders (with SD or PD) had no change after treatment [64]. In contrast, an increase in sPD-1 after conventional treatment with erlotinib was correlated with longer PFS and OS in NSCLC patients [246], and lower levels of sPD-1 post-surgery were associated with worse rate of OS at 2 years (60% vs 93%) in patients with gastric carcinoma [68]. Finally, one study also reported that the change in sPD-1 following 2 weeks of sorafenib had no association with response to therapy in hepatocellular carcinoma patients [237]. These studies highlight that sPD-1 levels or changes in levels after conventional therapy do not consistently associate with patient outcomes, and further work is needed to understand its relevance in this setting.
sCTLA4
Eight studies comprising 446 patients with solid malignancies have reported on changes in levels of sCTLA4 following conventional treatments (Table S5). While most showed that sCTLA4 is increased [126, 237, 240, 242, 243, 247], several studies have found that sCTLA4 either did not change [65, 243] or was reduced [67] following treatment with various conventional therapies. Only four studies with a total of 249 patients have reported on the relationship between levels or changes in levels sCTLA4 following treatment with conventional therapies and clinical response, and with conflicting findings (Table S6). Metastatic renal cell carcinoma patients who were responsive to sunitinib or pazopanib had lower levels of sCTLA4 after 3–4 months of treatment (281.6 pg/mL) than non-responders (616.4 pg/mL) [245]. Similarly, an increase in sCTLA4 3 days after treatment with radiofrequency ablation was only observed in chronic hepatitis C-hepatocellular carcinoma patients who exhibited early recurrence, while levels in patients without early recurrence remained constant [126]. In contrast, high levels of sCTLA4 after radiotherapy, chemotherapy, and/or chemoradiotherapy correlated with improved outcomes including longer OS and PFS in patients with lung, esophageal, liver, ovarian or cervical cancer [247]. Finally, one study found that changes in sCTLA4 after 2 weeks of sorafenib treatment had no association with response to therapy in hepatocellular carcinoma patients [237]. These studies highlight that sCTLA4 levels or changes in levels after conventional therapy do not consistently associate with patient response and further studies are needed to evaluate this association.
sCD80, sTIM3, sLAG3, sBTLA and sHVEM
Eleven different studies (involving 405 patients) have also evaluated the levels of sCD80, sTIM3, sLAG3, sBTLA, or sHVEM following treatment with conventional therapies (Tables S7 and S8). In some cases, some of these analytes increased [65, 67, 152, 237, 240, 242, 243], while in other cases reductions [51, 56, 65, 67, 237, 242, 244, 245] or no changes [65, 240, 243] were noted. Few studies (n = 5 and involving 189 patients) have evaluated the association between levels and/or changes in levels of sCD80, sTIM3, sLAG3, sBTLA, and sHVEM after conventional therapy and clinical outcome (Table S6). Some studies found that lower levels of these analytes after starting conventional therapy associate with improved outcome. For example, locally advanced cervical cancer patients developing a CR following concurrent chemoradiotherapy had lower levels of sLAG3 after therapy than those with PR or SD [240]. Similarly, hepatocellular carcinoma patients who responded to TACE had lower sLAG3 levels 3 days after treatment than did non-responders [51]. Contrasting findings have also been reported; Tampaki et al. found that hepatocellular carcinoma patients with CR following TACE treatment had higher sTIM3 levels 1 week after treatment than those who went on to develop a PR (median: 534 vs 222 pg/mL) [152]. Furthermore, locally advanced rectal cancer patients who responded to neoadjuvant chemoradiotherapy showed decreased levels of sCD80 during treatment that increased to baseline after treatment ended; poor responders also had decreased levels of sCD80 during treatment; however, the level of sCD80 remained low in these patients following cessation of treatment [244]. Finally, one study in hepatocellular carcinoma patients found that changes in the levels of sCD80, sTIM3, sLAG3, sBTLA, and sHVEM following 2 weeks of sorafenib treatment had no association with response to therapy [237]. Overall, these studies, like those with sPD-1 and CTLA4, highlight that changes in sCD80, sTIM3, sLAG3, sBTLA, or sHVEM do not consistently associate with patient response following conventional therapy.
Conclusions and future directions
ICI have revolutionized cancer immunotherapy. The concept of manipulating the immune system to recognize and target tumor antigens is extremely promising and has led to improved clinical benefit across multiple tumor indications [6,7,8,9, 11]. However, many patients remain resistant to this modality of treatment [14, 15], prompting the need to identify relevant biomarkers to predict response. Blood-based biomarkers are appealing for this purpose, due to technical and practical advantages over traditional tumor biopsies [1,2,3]. It should be noted that peripheral immune analyses can complement analyses of tumor biopsies and should be paired together whenever practically possible. Soluble immune checkpoints seem likely to be relevant in patients treated with both ICI and conventional therapies.
All the soluble checkpoints discussed here can bind to the same ligands as their membrane-bound counterparts, thus having the potential to modulate cytokine secretion, T cell viability, and T cell proliferation. Overall, sPD-L1, sCTLA4, sB7-H3, and sHVEM have primarily immunosuppressive functions, while sLAG3 and sBTLA have immunostimulatory functions, and sPD-1, sCD80, and sTIM3 are not as well functionally defined. Most of these soluble immune checkpoints (with the exception of sCD80) are elevated in cancer patients compared to healthy donors, with higher levels correlating with a more advanced/aggressive stage of cancer. This information may have diagnostic implications, as elevated levels may indicate the presence and/or stage of disease, or at the very least provide rationale for further diagnostic testing. In addition, many studies reported a prognostic value of these soluble immune checkpoints as indicators of clinical response to both ICI and conventional therapies. Numerous studies have shown that elevated levels of sPD-L1 prior to treatment are associated with worse response to ICI, while the other soluble immune checkpoints discussed require further investigation to determine their correlation with response. Greater increases and/or elevated levels of sPD-L1 and sPD-1 after ICI are associated with poor clinical outcome, while the literature is scarce and more conflicting regarding this association for the other soluble immune checkpoints reviewed here.
Surprisingly, a large body of literature has observed correlations between levels of soluble immune checkpoints in cancer patients and response to conventional, non-immunotherapies. This may support an indirect role of the immune system in the clinical response of patients to conventional therapies. Most studies indicated that elevated levels of the soluble immune checkpoints reviewed (sPD-L1, sPD-1, sCTLA4, sCD80, sTIM3, sLAG3, sB7-H3, sBTLA, and sHVEM) prior to conventional therapies correlated with poor clinical response. Most of these analytes were also increased following conventional therapies. However, with the exception of sPD-L1, changes observed in soluble immune checkpoints following conventional therapies have an unclear prognostic value. With sPD-L1, greater increases and/or elevated levels after conventional treatments are associated with poor clinical response to conventional therapy. These findings support the continued investigation of soluble immune checkpoints as potential biomarkers of response and may aid in the development of combination therapies.
Several gaps remain regarding the role that soluble immune checkpoints play in modulating an anti-tumor response. First, the exact functions of certain soluble immune checkpoints remain unclear. sPD-L1, the most well-studied among the soluble immune checkpoints, clearly has immunosuppressive functions [35, 36, 38, 41,42,43]. However, functional studies of sPD-1, sCD80, and sTIM3 are conflicting and inconsistent. Next, studies have shown that these soluble immune checkpoints can bind to the same ligands as their membrane-bound counterparts. Further work is needed to understand whether this serves to enhance immune suppressive signaling (as appears to be the case for sPD-L1) or block immune suppressive signaling, as has been proposed for sTIM3. In addition, in some cases, the function of the soluble immune checkpoint does not appear to align with what is known about its prognostic value. For example, extensive studies have shown sLAG3 to have anti-tumor functions [163,164,165,166,167,168,169,170,171,172]. However, higher baseline levels and an increase in sLAG3 correlate with a worse clinical response following both ICI and conventional therapy. This same disconnect between function and association with clinical outcome is seen with sBTLA. One reason for the disconnect may be that the functional studies were mostly conducted in vitro and in mice, while prognostic studies were performed in humans. It is also unknown whether variations in the levels of soluble immune checkpoints are reflective of the tumor burden, underlying immune activity, immune response to therapy, or a combination of these aspects. Finally, very little is known regarding the regulation of the production of these soluble immune checkpoints. Few studies have compared the expression of membrane-bound immune checkpoints with their soluble counterparts. In the case of PD-L1, there was no correlation in patients with various solid tumors between membrane-bound tumor expression of PD-L1 and circulating sPD-L1 levels [49, 82, 85, 86, 225, 226, 229, 234]. Additional studies are needed to investigate possible associations between the expression of other membrane-bound immune checkpoints and their soluble counterparts. Studies should also be performed to investigate whether certain physiological conditions can regulate the cleavage of membrane-bound immune checkpoints to induce the soluble forms, and whether certain conditions may halt this production process.
In addition to these theoretical questions, there are also practical issues that must be considered to implement the use of soluble immune checkpoints as biomarkers of clinical response to therapy. Many variables exist among the studies reviewed, such as the material measured (serum or plasma), the assay used (ELISA or multiplex assay), variation in timepoints in which samples were collected with respect to a given treatment, and a high degree of variation in the cutoff values of soluble immune checkpoints that were used to stratify patients into “high” vs “low” groups. In addition to a range of values, the method of obtaining these cutoffs also varied, with some studies using the mean, median, or 25th percentile or 75th percentile, while others determined cutoffs through receiver operating characteristic (ROC) curves, maximally selected log rank statistics, or spline curve analyses. Ideally, the method of cutoff determination and sample measurement will be optimized and standardized before widespread use. It should be noted that while most studies used ELISAs to measure sPD-L1 and sPD-1, the other soluble immune checkpoints reviewed here (sCTLA4, sCD80, sTIM3, sLAG3, sB7-H3, sBTLA, and sHVEM) were typically quantified through multiplexed immunoassays that screened for a panel of analytes. The use of common platforms, well-established analytical methods, and prospective evaluation in large clinical studies with well-defined clinical endpoints will be necessary to advance the utility of soluble immune checkpoints as a biomarker to guide treatment decisions. In addition, controlled studies directly comparing the performance of soluble immune checkpoints with tissue-based biomarkers that are currently approved will be needed. While much work remains to be done, the studies reviewed here support the continued investigation of biomarkers in peripheral blood along with the analyses of tumor biopsies and suggest that there may be value in further exploring soluble immune checkpoints as potential biomarkers of clinical response, both in patients treated with ICI and conventional therapies.
Availability of data and materials
Not applicable.
Abbreviations
- ICI:
-
Immune checkpoint inhibitor
- TMB:
-
Tumor mutational burden
- NLR:
-
Neutrophil-to-lymphocyte ratio
- APC:
-
Antigen-presenting cell
- ICOS:
-
Inducible T cell co-stimulator
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed cell death-ligand 1
- PD-L2:
-
Programmed cell death-ligand 2
- CTLA4:
-
Cytotoxic T-lymphocyte-associated antigen 4
- TIM3:
-
T cell immunoglobulin and mucin domain-containing protein 3
- GAL9:
-
Galectin 9
- LAG3:
-
Lymphocyte activation gene 3
- BTLA:
-
B and T lymphocyte attenuator
- HVEM:
-
Herpesvirus entry mediator
- TIGIT:
-
T cell immunoglobulin and ITIM domain
- sPD-L1:
-
Soluble programmed cell death-ligand 1
- IFN-γ:
-
Interferon gamma
- IL-2:
-
Interleukin 2
- NSCLC:
-
Non-small cell lung cancer
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- ORR:
-
Objective response rate
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- sPD-1:
-
Soluble programmed cell death protein 1
- TNF-α:
-
Tumor necrosis factor alpha
- sCTLA4:
-
Soluble cytotoxic T-lymphocyte-associated antigen 4
- PBMC:
-
Peripheral blood mononuclear cell
- sCD80:
-
Soluble CD80
- TIL:
-
Tumor-infiltrating lymphocyte
- sTIM3:
-
Soluble T cell immunoglobulin and mucin domain-containing protein 3
- sLAG3:
-
Soluble lymphocyte activation gene 3
- sB7-H3:
-
Soluble B7-H3
- sBTLA:
-
Soluble B and T lymphocyte attenuator
- sHVEM:
-
Soluble herpesvirus entry mediator
- BOR:
-
Best overall response
- SCLC: :
-
Small cell lung cancer
- DFS:
-
Disease-free survival
- HBV:
-
Hepatitis B virus
- TACE:
-
Transarterial chemoembolization
- PD:
-
Progressive disease
- ROC:
-
Receiver operating characteristic
References
Li S, Zhang C, Pang G, Wang P. Emerging Blood-Based Biomarkers for Predicting Response to Checkpoint Immunotherapy in Non-Small-Cell Lung Cancer. Front Immunol. 2020, 11603157.
Nixon AB, Schalper KA, Jacobs I, Potluri S, Wang IM, Fleener C. Peripheral immune-based biomarkers in cancer immunotherapy: can we realize their predictive potential? J Immunother Cancer. 2019;7(1):325.
Brueckl WM, Ficker JH, Zeitler G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer. 2020;20(1):1185.
Wang T, Denman D, Bacot SM, Feldman GM. Challenges and the Evolving Landscape of Assessing Blood-Based PD-L1 Expression as a Biomarker for Anti-PD-(L)1 Immunotherapy. Biomedicines. 2022, 10(5).
Hofman P, Heeke S, Alix-Panabieres C, Pantel K. Liquid biopsy in the era of immuno-oncology: is it ready for prime-time use for cancer patients? Ann Oncol. 2019;30(9):1448–59.
Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8(9):1069–86.
Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61.
Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–14.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Joseph J, Rahmani B, Cole Y, Puttagunta N, Lin E, Khan ZK, et al. Can Soluble Immune Checkpoint Molecules on Exosomes Mediate Inflammation? J Neuroimmune Pharmacol. 2021.
Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11(1):39.
Ledford H. Melanoma drug wins US approval. Nature. 2011;471(7340):561.
Cai L, Li Y, Tan J, Xu L, Li Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J Hematol Oncol. 2023;16(1):101.
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168(4):707–23.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–5.
Gu D, Ao X, Yang Y, Chen Z, Xu X. Soluble immune checkpoints in cancer: production, function and biological significance. J Immunother Cancer. 2018;6(1):132.
Chakrabarti R, Kapse B, Mukherjee G. Soluble immune checkpoint molecules: Serum markers for cancer diagnosis and prognosis. Cancer Rep (Hoboken). 2019;2(4): e1160.
Daassi D, Mahoney KM, Freeman GJ. The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol. 2020;20(4):209–15.
Niu M, Liu Y, Yi M, Jiao D, Wu K. Biological Characteristics and Clinical Significance of Soluble PD-1/PD-L1 and Exosomal PD-L1 in Cancer. Front Immunol. 2022, 13827921.
Baral A, Ye HX, Jiang PC, Yao Y, Mao Y. B7–H3 and B7–H1 expression in cerebral spinal fluid and tumor tissue correlates with the malignancy grade of glioma patients. Oncol Lett. 2014;8(3):1195–201.
Vikerfors A, Davidsson S, Frey J, Jerlstrom T, Carlsson J. Soluble PD-L1 in Serum and Urine in Urinary Bladder Cancer Patients. Cancers (Basel). 2021;13(22):5841.
Liu S, Zhu Y, Zhang C, Meng X, Sun B, Zhang G, et al. The Clinical Significance of Soluble Programmed Cell Death-Ligand 1 (sPD-L1) in Patients With Gliomas. Front Oncol. 2020, 109.
Swiderska J, Kozlowski M, Nowak K, Rychlicka M, Branecka-Wozniak D, Kwiatkowski S, et al. Clinical Relevance of Soluble Forms of Immune Checkpoint Molecules sPD-1, sPD-L1, and sCTLA-4 in the Diagnosis and Prognosis of Ovarian Cancer. Diagnostics (Basel). 2022;12(1):189.
Pawlowska A, Suszczyk D, Tarkowski R, Paduch R, Kotarski J, Wertel I. Programmed Death-1 Receptor (PD-1) as a Potential Prognosis Biomarker for Ovarian Cancer Patients. Cancer Manag Res. 2020;12:9691–709.
Dong H, Zhu G, Tamada K, Chen L. B7–H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9.
Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170(3):1257–66.
Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169(10):5538–45.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34.
Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173(2):945–54.
Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98(24):13866–71.
Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–53.
Khan M, Zhao Z, Arooj S, Fu Y, Liao G. Soluble PD-1: Predictive, Prognostic, and Therapeutic Value for Cancer Immunotherapy. Front Immunol. 2020;11:587460.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2(2):116–26.
Mahoney KM, Shukla SA, Patsoukis N, Chaudhri A, Browne EP, Arazi A, et al. A secreted PD-L1 splice variant that covalently dimerizes and mediates immunosuppression. Cancer Immunol Immunother. 2019;68(3):421–32.
Hassounah NB, Malladi VS, Huang Y, Freeman SS, Beauchamp EM, Koyama S, et al. Identification and characterization of an alternative cancer-derived PD-L1 splice variant. Cancer Immunol Immunother. 2019;68(3):407–20.
Romero Y, Wise R, Zolkiewska A. Proteolytic processing of PD-L1 by ADAM proteases in breast cancer cells. Cancer Immunol Immunother. 2020;69(1):43–55.
Orme JJ, Jazieh KA, Xie T, Harrington S, Liu X, Ball M, et al. ADAM10 and ADAM17 cleave PD-L1 to mediate PD-(L)1 inhibitor resistance. Oncoimmunology. 2020;9(1):1744980.
Hira-Miyazawa M, Nakamura H, Hirai M, Kobayashi Y, Kitahara H, Bou-Gharios G, et al. Regulation of programmed-death ligand in the human head and neck squamous cell carcinoma microenvironment is mediated through matrix metalloproteinase-mediated proteolytic cleavage. Int J Oncol. 2018;52(2):379–88.
Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, et al. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine. 2011;56(2):231–8.
Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, et al. Identification of a soluble form of B7–H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17(7):1915–23.
Frigola X, Inman BA, Krco CJ, Liu X, Harrington SM, Bulur PA, et al. Soluble B7–H1: differences in production between dendritic cells and T cells. Immunol Lett. 2012;142(1–2):78–82.
Gong B, Kiyotani K, Sakata S, Nagano S, Kumehara S, Baba S, et al. Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non-small cell lung cancer. J Exp Med. 2019;216(4):982–1000.
Cheng S, Zheng J, Zhu J, Xie C, Zhang X, Han X, et al. PD-L1 gene polymorphism and high level of plasma soluble PD-L1 protein may be associated with non-small cell lung cancer. Int J Biol Markers. 2015;30(4):e364–8.
Wang Q, He Y, Li W, Xu X, Hu Q, Bian Z, et al. Soluble Immune Checkpoint-Related Proteins in Blood Are Associated With Invasion and Progression in Non-Small Cell Lung Cancer. Front Immunol. 2022, 13887916.
Zhang J, Gao J, Li Y, Nie J, Dai L, Hu W, et al. Circulating PD-L1 in NSCLC patients and the correlation between the level of PD-L1 expression and the clinical characteristics. Thorac Cancer. 2015;6(4):534–8.
He J, Pan Y, Guo Y, Li B, Tang Y. Study on the Expression Levels and Clinical Significance of PD-1 and PD-L1 in Plasma of NSCLC Patients. J Immunother. 2020;43(5):156–64.
Jin J, Si J, Liu Y, Wang H, Ni R, Wang J. Elevated serum soluble programmed cell death ligand 1 concentration as a potential marker for poor prognosis in small cell lung cancer patients with chemotherapy. Respir Res. 2018;19(1):197.
Shigemori T, Toiyama Y, Okugawa Y, Yamamoto A, Yin C, Narumi A, et al. Soluble PD-L1 Expression in Circulation as a Predictive Marker for Recurrence and Prognosis in Gastric Cancer: Direct Comparison of the Clinical Burden Between Tissue and Serum PD-L1 Expression. Ann Surg Oncol. 2019;26(3):876–83.
Imai Y, Chiba T, Kondo T, Kanzaki H, Kanayama K, Ao J, et al. Interferon-gamma induced PD-L1 expression and soluble PD-L1 production in gastric cancer. Oncol Lett. 2020;20(3):2161–8.
Guo M, Qi F, Rao Q, Sun J, Du X, Qi Z, et al. Serum LAG-3 Predicts Outcome and Treatment Response in Hepatocellular Carcinoma Patients With Transarterial Chemoembolization. Front Immunol. 2021;12:754961.
Han X, Gu YK, Li SL, Chen H, Chen MS, Cai QQ, et al. Pre-treatment serum levels of soluble programmed cell death-ligand 1 predict prognosis in patients with hepatitis B-related hepatocellular carcinoma. J Cancer Res Clin Oncol. 2019;145(2):303–12.
Chen L, Liu S, Adah D, Sun Q, Liang Z, Ho M, et al. Soluble programmed death ligand-1-induced immunosuppressive effects on chimeric antigen receptor-natural killer cells targeting Glypican-3 in hepatocellular carcinoma. Immunology. 2023;169(2):204–18.
Shao W, Xu Y, Lin S, Gao J, Gao J, Wang H. The potential of soluble programmed death-ligand 1 (sPD-L1) as a diagnosis marker for colorectal cancer. Front Oncol. 2022;12:988567.
Lu T, Chen Y, Li J, Guo Q, Lin W, Zheng Y, et al. High Soluble Programmed Death-Ligand 1 Predicts Poor Prognosis in Patients with Nasopharyngeal Carcinoma. Onco Targets Ther. 2020;13:1757–65.
Shao Y, Gui X, Wang Y, Sheng L, Sun D, Zeng Q, et al. Serum soluble immune checkpoint levels predict cervical lymph node metastasis of differentiated thyroid carcinoma patients. Cancer Med. 2023;12(17):17648–59.
Malinga NZ, Siwele SC, Steel HC, Kwofie LLI, Meyer PWA, Smit T, et al. Systemic levels of the soluble co-inhibitory immune checkpoints, CTLA-4, LAG-3, PD-1/PD-L1 and TIM-3 are markedly increased in basal cell carcinoma. Transl Oncol. 2022, 19101384.
Fukuda T, Kamai T, Masuda A, Nukui A, Abe H, Arai K, et al. Higher preoperative serum levels of PD-L1 and B7–H4 are associated with invasive and metastatic potential and predictable for poor response to VEGF-targeted therapy and unfavorable prognosis of renal cell carcinoma. Cancer Med. 2016;5(8):1810–20.
Katongole P, Sande OJ, Reynolds SJ, Joloba M, Kajumbula H, Kalungi S, et al. Soluble programmed death-ligand 1 (sPD-L1) is elevated in aggressive prostate cancer disease among african men. Oncol Ther. 2022;10(1):185–93.
Buderath P, Schwich E, Jensen C, Horn PA, Kimmig R, Kasimir-Bauer S, et al. Soluble Programmed Death Receptor Ligands sPD-L1 and sPD-L2 as Liquid Biopsy Markers for Prognosis and Platinum Response in Epithelial Ovarian Cancer. Front Oncol. 2019;9:1015.
Chatterjee J, Dai W, Aziz NHA, Teo PY, Wahba J, Phelps DL, et al. Clinical Use of Programmed Cell Death-1 and Its Ligand Expression as Discriminatory and Predictive Markers in Ovarian Cancer. Clin Cancer Res. 2017;23(13):3453–60.
Vecchiarelli S, Passiglia F, D’Incecco A, Gallo M, De Luca A, Rossi E, et al. Circulating programmed death ligand-1 (cPD-L1) in non-small-cell lung cancer (NSCLC). Oncotarget. 2018;9(25):17554–63.
Yoshida J, Ishikawa T, Doi T, Ota T, Yasuda T, Okayama T, et al. Clinical significance of soluble forms of immune checkpoint molecules in advanced esophageal cancer. Med Oncol. 2019;36(7):60.
Li Y, Cui X, Yang YJ, Chen QQ, Zhong L, Zhang T, et al. Serum sPD-1 and sPD-L1 as Biomarkers for Evaluating the Efficacy of Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Patients. Clin Breast Cancer. 2019;19(5):326–32.e1.
Ruan Y, Hu W, Li W, Lu H, Gu H, Zhang Y, et al. Analysis of Plasma EBV-DNA and Soluble Checkpoint Proteins in Nasopharyngeal Carcinoma Patients after Definitive Intensity-Modulated Radiotherapy. Biomed Res Int. 2019;2019:3939720.
Rapoport BL, Steel HC, Hlatshwayo N, Theron AJ, Meyer PWA, Nayler S, et al. Systemic Immune Dysregulation in Early Breast Cancer Is Associated With Decreased Plasma Levels of Both Soluble Co-Inhibitory and Co-Stimulatory Immune Checkpoint Molecules. Front Immunol. 2022;13:823842.
Rapoport BL, Steel HC, Benn CA, Nayler S, Smit T, Heyman L, et al. Dysregulation of systemic soluble immune checkpoints in early breast cancer is attenuated following administration of neoadjuvant chemotherapy and is associated with recovery of CD27, CD28, CD40, CD80, ICOS and GITR and substantially increased levels of PD-L1, LAG-3 and TIM-3. Front Oncol. 2023;13:1097309.
Wei H, Wu F, Mao Y, Zhang Y, Leng G, Wang J, et al. Measurement of soluble PD-1 and soluble PD-L1 as well as PD-L1 and PD-1 from perioperative patients with gastric carcinoma. Jpn J Clin Oncol. 2022;52(4):331–45.
Finkelmeier F, Canli O, Tal A, Pleli T, Trojan J, Schmidt M, et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer. 2016;59:152–9.
Larrinaga G, Solano-Iturri JD, Errarte P, Unda M, Loizaga-Iriarte A, Perez-Fernandez A, et al. Soluble PD-L1 Is an Independent Prognostic Factor in Clear Cell Renal Cell Carcinoma. Cancers (Basel). 2021;13(4):667.
Kim HJ, Park S, Kim KJ, Seong J. Clinical significance of soluble programmed cell death ligand-1 (sPD-L1) in hepatocellular carcinoma patients treated with radiotherapy. Radiother Oncol. 2018;129(1):130–5.
Davidsson S, Huotilainen S, Carlsson J, Sundqvist P. Soluble Levels of CD163, PD-L1, and IL-10 in Renal Cell Carcinoma Patients. Diagnostics (Basel). 2022;12(2):336.
Murakami S, Shibaki R, Matsumoto Y, Yoshida T, Goto Y, Kanda S, et al. Association between serum level soluble programmed cell death ligand 1 and prognosis in patients with non-small cell lung cancer treated with anti-PD-1 antibody. Thorac Cancer. 2020;11(12):3585–95.
Matsumoto Y, Sasaki T, Kano M, Shiraishi T, Suito H, Murakami K, et al. Soluble PD-L1 reflects cachexia status in patients with gastric cancer and is an independent prognostic marker for relapse-free survival after radical surgery. Mol Clin Oncol. 2023;18(5):39.
Okuma Y, Hosomi Y, Nakahara Y, Watanabe K, Sagawa Y, Homma S. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer. Lung Cancer. 2017, 1041–6.
Okuma Y, Wakui H, Utsumi H, Sagawa Y, Hosomi Y, Kuwano K, et al. Soluble Programmed Cell Death Ligand 1 as a Novel Biomarker for Nivolumab Therapy for Non-Small-cell Lung Cancer. Clin Lung Cancer. 2018;19(5):410–7.e1.
Tiako Meyo M, Jouinot A, Giroux-Leprieur E, Fabre E, Wislez M, Alifano M, et al. Predictive Value of Soluble PD-1, PD-L1, VEGFA, CD40 Ligand and CD44 for Nivolumab Therapy in Advanced Non-Small Cell Lung Cancer: A Case-Control Study. Cancers (Basel). 2020;12(2):473.
Himuro H, Nakahara Y, Igarashi Y, Kouro T, Higashijima N, Matsuo N, et al. Clinical roles of soluble PD-1 and PD-L1 in plasma of NSCLC patients treated with immune checkpoint inhibitors. Cancer Immunol Immunother. 2023.
Kawakami H, Sunakawa Y, Inoue E, Matoba R, Noda K, Sato T, et al. Soluble programmed cell death ligand 1 predicts prognosis for gastric cancer patients treated with nivolumab: Blood-based biomarker analysis for the DELIVER trial. Eur J Cancer. 2023, 18410–20.
Wakita N, Hinata N, Bando Y, Hara T, Terakawa T, Furukawa J, et al. Prognostic Value of Serum Soluble PD-L1 in Metastatic Renal Cell Carcinoma Patients Treated With Nivolumab. Anticancer Res. 2023;43(2):841–7.
Krafft U, Olah C, Reis H, Kesch C, Darr C, Grunwald V, et al. High Serum PD-L1 Levels Are Associated with Poor Survival in Urothelial Cancer Patients Treated with Chemotherapy and Immune Checkpoint Inhibitor Therapy. Cancers (Basel). 2021;13(11):2548.
Oh SY, Kim S, Keam B, Kim TM, Kim DW, Heo DS. Soluble PD-L1 is a predictive and prognostic biomarker in advanced cancer patients who receive immune checkpoint blockade treatment. Sci Rep. 2021;11(1):19712.
Gorgulho J, Roderburg C, Heymann F, Schulze-Hagen M, Beier F, Vucur M, et al. Serum levels of soluble B and T lymphocyte attenuator predict overall survival in patients undergoing immune checkpoint inhibitor therapy for solid malignancies. Int J Cancer. 2021;149(5):1189–98.
Castello A, Rossi S, Toschi L, Mansi L, Lopci E. Soluble PD-L1 in NSCLC Patients Treated with Checkpoint Inhibitors and Its Correlation with Metabolic Parameters. Cancers (Basel). 2020;12(6):1373.
Ando K, Hamada K, Watanabe M, Ohkuma R, Shida M, Onoue R, et al. Plasma Levels of Soluble PD-L1 Correlate With Tumor Regression in Patients With Lung and Gastric Cancer Treated With Immune Checkpoint Inhibitors. Anticancer Res. 2019;39(9):5195–201.
Costantini A, Julie C, Dumenil C, Helias-Rodzewicz Z, Tisserand J, Dumoulin J, et al. Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. Oncoimmunology. 2018;7(8): e1452581.
Incorvaia L, Fanale D, Badalamenti G, Porta C, Olive D, De Luca I, et al. Baseline plasma levels of soluble PD-1, PD-L1, and BTN3A1 predict response to nivolumab treatment in patients with metastatic renal cell carcinoma: a step toward a biomarker for therapeutic decisions. Oncoimmunology. 2020;9(1):1832348.
Zhao C, Wu L, Liang D, Chen H, Ji S, Zhang G, et al. Identification of immune checkpoint and cytokine signatures associated with the response to immune checkpoint blockade in gastrointestinal cancers. Cancer Immunol Immunother. 2021;70(9):2669–79.
Szeles A, Kovacs PT, Csizmarik A, Varadi M, Riesz P, Fazekas T, et al. High Pretreatment Serum PD-L1 Levels Are Associated with Muscle Invasion and Shorter Survival in Upper Tract Urothelial Carcinoma. Biomedicines. 2022;10(10):2560.
Zizzari IG, Di Filippo A, Scirocchi F, Di Pietro FR, Rahimi H, Ugolini A, et al. Soluble Immune Checkpoints, Gut Metabolites and Performance Status as Parameters of Response to Nivolumab Treatment in NSCLC Patients. J Pers Med. 2020;10(4):208.
Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8(5):765–72.
Nishimura H, Agata Y, Kawasaki A, Sato M, Imamura S, Minato N, et al. Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4-CD8-) thymocytes. Int Immunol. 1996;8(5):773–80.
Vibhakar R, Juan G, Traganos F, Darzynkiewicz Z, Finger LR. Activation-induced expression of human programmed death-1 gene in T-lymphocytes. Exp Cell Res. 1997;232(1):25–8.
Zhang X, Schwartz JC, Guo X, Bhatia S, Cao E, Lorenz M, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 2004;20(3):337–47.
Finger LR, Pu J, Wasserman R, Vibhakar R, Louie E, Hardy RR, et al. The human PD-1 gene: complete cDNA, genomic organization, and developmentally regulated expression in B cell progenitors. Gene. 1997;197(1–2):177–87.
Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med. 2016;375(18):1767–78.
Nielsen C, Ohm-Laursen L, Barington T, Husby S, Lillevang ST. Alternative splice variants of the human PD-1 gene. Cell Immunol. 2005;235(2):109–16.
He L, Zhang G, He Y, Zhu H, Zhang H, Feng Z. Blockade of B7–H1 with sPD-1 improves immunity against murine hepatocarcinoma. Anticancer Res. 2005;25(5):3309–13.
Shin SP, Seo HH, Shin JH, Park HB, Lim DP, Eom HS, et al. Adenovirus expressing both thymidine kinase and soluble PD1 enhances antitumor immunity by strengthening CD8 T-cell response. Mol Ther. 2013;21(3):688–95.
Elhag OA, Hu XJ, Wen-Ying Z, Li X, Yuan YZ, Deng LF, et al. Reconstructed adeno-associated virus with the extracellular domain of murine PD-1 induces antitumor immunity. Asian Pac J Cancer Prev. 2012;13(8):4031–6.
Song MY, Park SH, Nam HJ, Choi DH, Sung YC. Enhancement of vaccine-induced primary and memory CD8(+) T-cell responses by soluble PD-1. J Immunother. 2011;34(3):297–306.
He YF, Zhang GM, Wang XH, Zhang H, Yuan Y, Li D, et al. Blocking programmed death-1 ligand-PD-1 interactions by local gene therapy results in enhancement of antitumor effect of secondary lymphoid tissue chemokine. J Immunol. 2004;173(8):4919–28.
Kuipers H, Muskens F, Willart M, Hijdra D, van Assema FB, Coyle AJ, et al. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. Eur J Immunol. 2006;36(9):2472–82.
Peng Y, Zhang C, Rui Z, Tang W, Xu Y, Tao X, et al. A comprehensive profiling of soluble immune checkpoints from the sera of patients with non-small cell lung cancer. J Clin Lab Anal. 2022;36(2): e24224.
Cheng HY, Kang PJ, Chuang YH, Wang YH, Jan MC, Wu CF, et al. Circulating programmed death-1 as a marker for sustained high hepatitis B viral load and risk of hepatocellular carcinoma. PLoS ONE. 2014;9(11): e95870.
Lima CAC, da Silva LM, Dos Santos RL, Silva JPA, Forones NM, Martins MR, et al. Unbalanced expression of sICOS and sPD-1 correlates with tumor progression in gastric cancer. J Surg Oncol. 2022;126(1):144–9.
Ohkuma R, Ieguchi K, Watanabe M, Takayanagi D, Goshima T, Onoue R, et al. Increased Plasma Soluble PD-1 Concentration Correlates with Disease Progression in Patients with Cancer Treated with Anti-PD-1 Antibodies. Biomedicines. 2021;9(12):1929.
Linsley PS, Greene JL, Tan P, Bradshaw J, Ledbetter JA, Anasetti C, et al. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176(6):1595–604.
Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405–13.
Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459–65.
Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. A new member of the immunoglobulin superfamily–CTLA-4. Nature. 1987;328(6127):267–70.
Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174(3):561–9.
Harper K, Balzano C, Rouvier E, Mattei MG, Luciani MF, Golstein P. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol. 1991;147(3):1037–44.
Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183(6):2533–40.
Chuang E, Fisher TS, Morgan RW, Robbins MD, Duerr JM, Vander Heiden MG, et al. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 2000;13(3):313–22.
Miyatake S, Nakaseko C, Umemori H, Yamamoto T, Saito T. Src family tyrosine kinases associate with and phosphorylate CTLA-4 (CD152). Biochem Biophys Res Commun. 1998;249(2):444–8.
Hu H, Rudd CE, Schneider H. Src kinases Fyn and Lck facilitate the accumulation of phosphorylated CTLA-4 and its association with PI-3 kinase in intracellular compartments of T-cells. Biochem Biophys Res Commun. 2001;288(3):573–8.
Schneider H, Prasad KV, Shoelson SE, Rudd CE. CTLA-4 binding to the lipid kinase phosphatidylinositol 3-kinase in T cells. J Exp Med. 1995;181(1):351–5.
Kim GR, Choi JM. Current Understanding of Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) Signaling in T-Cell Biology and Disease Therapy. Mol Cells. 2022;45(8):513–21.
Magistrelli G, Jeannin P, Herbault N, Benoit De Coignac A, Gauchat JF, Bonnefoy JY, et al. A soluble form of CTLA-4 generated by alternative splicing is expressed by nonstimulated human T cells. Eur J Immunol. 1999;29(11):3596–602.
Oaks MK, Hallett KM, Penwell RT, Stauber EC, Warren SJ, Tector AJ. A native soluble form of CTLA-4. Cell Immunol. 2000;201(2):144–53.
Khanolkar RC, Zhang C, Al-Fatyan F, Lawson L, Depasquale I, Meredith FM, et al. TGFbeta2 Induces the Soluble Isoform of CTLA-4 - Implications for CTLA-4 Based Checkpoint Inhibitor Antibodies in Malignant Melanoma. Front Immunol. 2021, 12763877.
Ward FJ, Dahal LN, Wijesekera SK, Abdul-Jawad SK, Kaewarpai T, Xu H, et al. The soluble isoform of CTLA-4 as a regulator of T-cell responses. Eur J Immunol. 2013;43(5):1274–85.
Erfani N, Razmkhah M, Ghaderi A. Circulating soluble CTLA4 (sCTLA4) is elevated in patients with breast cancer. Cancer Invest. 2010;28(8):828–32.
Masuda A, Arai K, Nishihara D, Mizuno T, Yuki H, Kambara T, et al. Clinical significance of serum soluble T cell regulatory molecules in clear cell renal cell carcinoma. Biomed Res Int. 2014, 2014396064.
Teng W, Jeng WJ, Chen WT, Lin CC, Lin CY, Lin SM, et al. Soluble form of CTLA-4 is a good predictor for tumor recurrence after radiofrequency ablation in hepatocellular carcinoma patients. Cancer Med. 2022;11(20):3786–95.
Larsen CP, Ritchie SC, Pearson TC, Linsley PS, Lowry RP. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med. 1992;176(4):1215–20.
Razi-Wolf Z, Freeman GJ, Galvin F, Benacerraf B, Nadler L, Reiser H. Expression and function of the murine B7 antigen, the major costimulatory molecule expressed by peritoneal exudate cells. Proc Natl Acad Sci U S A. 1992;89(9):4210–4.
Freeman GJ, Freedman AS, Segil JM, Lee G, Whitman JF, Nadler LM. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol. 1989;143(8):2714–22.
Freeman GJ, Gray GS, Gimmi CD, Lombard DB, Zhou LJ, White M, et al. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med. 1991;174(3):625–31.
Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173(3):721–30.
Kakoulidou M, Giscombe R, Zhao X, Lefvert AK, Wang X. Human Soluble CD80 is generated by alternative splicing, and recombinant soluble CD80 binds to CD28 and CD152 influencing T-cell activation. Scand J Immunol. 2007;66(5):529–37.
Ikemizu S, Gilbert RJ, Fennelly JA, Collins AV, Harlos K, Jones EY, et al. Structure and dimerization of a soluble form of B7–1. Immunity. 2000;12(1):51–60.
Haile ST, Dalal SP, Clements V, Tamada K, Ostrand-Rosenberg S. Soluble CD80 restores T cell activation and overcomes tumor cell programmed death ligand 1-mediated immune suppression. J Immunol. 2013;191(5):2829–36.
Haile ST, Horn LA, Ostrand-Rosenberg S. A soluble form of CD80 enhances antitumor immunity by neutralizing programmed death ligand-1 and simultaneously providing costimulation. Cancer Immunol Res. 2014;2(7):610–5.
Horn LA, Long TM, Atkinson R, Clements V, Ostrand-Rosenberg S. Soluble CD80 Protein Delays Tumor Growth and Promotes Tumor-Infiltrating Lymphocytes. Cancer Immunol Res. 2018;6(1):59–68.
Sturmhoefel K, Lee K, Gray GS, Thomas J, Zollner R, O’Toole M, et al. Potent activity of soluble B7-IgG fusion proteins in therapy of established tumors and as vaccine adjuvant. Cancer Res. 1999;59(19):4964–72.
Matsuyama Y, Asanuma K, Yoshida K, Hagi T, Iino T, Nakamura T, et al. The role of soluble CD80 in patients with soft tissue tumors. J Orthop Surg Res. 2022;17(1):404.
Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–41.
Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–52.
Cao E, Zang X, Ramagopal UA, Mukhopadhaya A, Fedorov A, Fedorov E, et al. T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface. Immunity. 2007;26(3):311–21.
van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem Biophys Res Commun. 2006;351(2):571–6.
Kang CW, Dutta A, Chang LY, Mahalingam J, Lin YC, Chiang JM, et al. Apoptosis of tumor infiltrating effector TIM-3+CD8+ T cells in colon cancer. Sci Rep. 2015, 515659.
Dardalhon V, Anderson AC, Karman J, Apetoh L, Chandwaskar R, Lee DH, et al. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J Immunol. 2010;185(3):1383–92.
Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, et al. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4(11):1102–10.
Geng H, Zhang GM, Li D, Zhang H, Yuan Y, Zhu HG, et al. Soluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune response. J Immunol. 2006;176(3):1411–20.
Moller-Hackbarth K, Dewitz C, Schweigert O, Trad A, Garbers C, Rose-John S, et al. A disintegrin and metalloprotease (ADAM) 10 and ADAM17 are major sheddases of T cell immunoglobulin and mucin domain 3 (Tim-3). J Biol Chem. 2013;288(48):34529–44.
Li F, Li N, Sang J, Fan X, Deng H, Zhang X, et al. Highly elevated soluble Tim-3 levels correlate with increased hepatocellular carcinoma risk and poor survival of hepatocellular carcinoma patients in chronic hepatitis B virus infection. Cancer Manag Res. 2018, 10941–51.
Lu K, Ma H, Wang T, Huang Y, Ru M. The diagnostic value of soluble TIM-3 in oral squamous cell carcinoma. Future Oncol. 2022.
Chen L, Hong J, Hu R, Yu X, Chen X, Zheng S, et al. Clinical Value of Combined Detection of Serum sTim-3 and Pepsinogen for Gastric Cancer Diagnosis. Cancer Manag Res. 2021, 137759–69.
Ge W, Li J, Fan W, Xu D, Sun S. Tim-3 as a diagnostic and prognostic biomarker of osteosarcoma. Tumour Biol. 2017;39(7):1010428317715643.
Tampaki M, Ionas E, Hadziyannis E, Deutsch M, Malagari K, Koskinas J. Association of TIM-3 with BCLC Stage, Serum PD-L1 Detection, and Response to Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma. Cancers (Basel). 2020;12(1):212.
Wang Q, Zhang J, Tu H, Liang D, Chang DW, Ye Y, et al. Soluble immune checkpoint-related proteins as predictors of tumor recurrence, survival, and T cell phenotypes in clear cell renal cell carcinoma patients. J Immunother Cancer. 2019;7(1):334.
Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171(5):1393–405.
Baixeras E, Huard B, Miossec C, Jitsukawa S, Martin M, Hercend T, et al. haracterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176(2):327–37.
Workman CJ, Dugger KJ, Vignali DA. Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol. 2002;169(10):5392–5.
Huard B, Mastrangeli R, Prigent P, Bruniquel D, Donini S, El-Tayar N, et al. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc Natl Acad Sci U S A. 1997;94(11):5744–9.
Huard B, Prigent P, Pages F, Bruniquel D, Triebel F. T cell major histocompatibility complex class II molecules down-regulate CD4+ T cell clone responses following LAG-3 binding. Eur J Immunol. 1996;26(5):1180–6.
Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol. 1998;161(8):4058–65.
Macon-Lemaitre L, Triebel F. The negative regulatory function of the lymphocyte-activation gene-3 co-receptor (CD223) on human T cells. Immunology. 2005;115(2):170–8.
Li N, Wang Y, Forbes K, Vignali KM, Heale BS, Saftig P, et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J. 2007;26(2):494–504.
Li N, Workman CJ, Martin SM, Vignali DA. Biochemical analysis of the regulatory T cell protein lymphocyte activation gene-3 (LAG-3; CD223). J Immunol. 2004;173(11):6806–12.
Li N, Jilisihan B, Wang W, Tang Y, Keyoumu S. Soluble LAG3 acts as a potential prognostic marker of gastric cancer and its positive correlation with CD8+T cell frequency and secretion of IL-12 and INF-gamma in peripheral blood. Cancer Biomark. 2018;23(3):341–51.
Casati C, Camisaschi C, Rini F, Arienti F, Rivoltini L, Triebel F, et al. Soluble human LAG-3 molecule amplifies the in vitro generation of type 1 tumor-specific immunity. Cancer Res. 2006;66(8):4450–60.
Prigent P, El Mir S, Dreano M, Triebel F. Lymphocyte activation gene-3 induces tumor regression and antitumor immune responses. Eur J Immunol. 1999;29(12):3867–76.
Avice MN, Sarfati M, Triebel F, Delespesse G, Demeure CE. Lymphocyte activation gene-3, a MHC class II ligand expressed on activated T cells, stimulates TNF-alpha and IL-12 production by monocytes and dendritic cells. J Immunol. 1999;162(5):2748–53.
El Mir S, Triebel F. A soluble lymphocyte activation gene-3 molecule used as a vaccine adjuvant elicits greater humoral and cellular immune responses to both particulate and soluble antigens. J Immunol. 2000;164(11):5583–9.
Demeure CE, Wolfers J, Martin-Garcia N, Gaulard P, Triebel F. T Lymphocytes infiltrating various tumour types express the MHC class II ligand lymphocyte activation gene-3 (LAG-3): role of LAG-3/MHC class II interactions in cell-cell contacts. Eur J Cancer. 2001;37(13):1709–18.
Andreae S, Piras F, Burdin N, Triebel F. Maturation and activation of dendritic cells induced by lymphocyte activation gene-3 (CD223). J Immunol. 2002;168(8):3874–80.
Buisson S, Triebel F. MHC class II engagement by its ligand LAG-3 (CD223) leads to a distinct pattern of chemokine and chemokine receptor expression by human dendritic cells. Vaccine. 2003;21(9–10):862–8.
Cappello P, Triebel F, Iezzi M, Caorsi C, Quaglino E, Lollini PL, et al. LAG-3 enables DNA vaccination to persistently prevent mammary carcinogenesis in HER-2/neu transgenic BALB/c mice. Cancer Res. 2003;63(10):2518–25.
Andreae S, Buisson S, Triebel F. MHC class II signal transduction in human dendritic cells induced by a natural ligand, the LAG-3 protein (CD223). Blood. 2003;102(6):2130–7.
Andrews LP, Somasundaram A, Moskovitz JM, Szymczak-Workman AL, Liu C, Cillo AR, et al. Resistance to PD1 blockade in the absence of metalloprotease-mediated LAG3 shedding. Sci Immunol. 2020;5(49):eabc2728.
Pan S, Zhao W, Li Y, Ying Z, Luo Y, Wang Q, et al. Prediction of risk and overall survival of pancreatic cancer from blood soluble immune checkpoint-related proteins. Front Immunol. 2023, 141189161.
He Y, Wang Y, Zhao S, Zhao C, Zhou C, Hirsch FR. sLAG-3 in non-small-cell lung cancer patients’ serum. Onco Targets Ther. 2018;11:4781–4.
Botticelli A, Zizzari IG, Scagnoli S, Pomati G, Strigari L, Cirillo A, et al. The Role of Soluble LAG3 and Soluble Immune Checkpoints Profile in Advanced Head and Neck Cancer: A Pilot Study. J Pers Med. 2021;11(7):651.
Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. B7–H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2(3):269–74.
Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, et al. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol. 2004;172(4):2352–9.
Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C. Characterization of mouse and human B7–H3 genes. J Immunol. 2002;168(12):6294–7.
Ling V, Wu PW, Spaulding V, Kieleczawa J, Luxenberg D, Carreno BM, et al. Duplication of primate and rodent B7–H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics. 2003;82(3):365–77.
Luo L, Chapoval AI, Flies DB, Zhu G, Hirano F, Wang S, et al. B7–H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J Immunol. 2004;173(9):5445–50.
Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, et al. The B7 family member B7–H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4(9):899–906.
Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, et al. Murine B7–H3 is a negative regulator of T cells. J Immunol. 2004;173(4):2500–6.
Vigdorovich V, Ramagopal UA, Lazar-Molnar E, Sylvestre E, Lee JS, Hofmeyer KA, et al. Structure and T cell inhibition properties of B7 family member, B7–H3. Structure. 2013;21(5):707–17.
Chen W, Liu P, Wang Y, Nie W, Li Z, Xu W, et al. Characterization of a soluble B7–H3 (sB7-H3) spliced from the intron and analysis of sB7-H3 in the sera of patients with hepatocellular carcinoma. PLoS ONE. 2013;8(10): e76965.
Zhang G, Hou J, Shi J, Yu G, Lu B, Zhang X. Soluble CD276 (B7–H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123(4):538–46.
Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M, Tan Y, et al. Clinical significance and regulation of the costimulatory molecule B7–H3 in human colorectal carcinoma. Cancer Immunol Immunother. 2010;59(8):1163–71.
Xie C, Liu D, Chen Q, Yang C, Wang B, Wu H. Soluble B7-H3 promotes the invasion and metastasis of pancreatic carcinoma cells through the TLR4/NF-kappaB pathway. Sci Rep. 2016, 627528.
Zhang G, Xu Y, Lu X, Huang H, Zhou Y, Lu B, et al. Diagnosis value of serum B7–H3 expression in non-small cell lung cancer. Lung Cancer. 2009;66(2):245–9.
Huang L, Zhou Y, Sun Q, Cao L, Zhang X. Evaluation of the role of soluble B7–H3 in association with membrane B7–H3 expression in gastric adenocarcinoma. Cancer Biomark. 2022;33(1):123–9.
Zhao L, Xie C, Liu D, Li T, Zhang Y, Wan C. Early Detection of Hepatocellular Carcinoma in Patients with Hepatocirrhosis by Soluble B7–H3. J Gastrointest Surg. 2017;21(5):807–12.
Azuma T, Sato Y, Ohno T, Azuma M, Kume H. Serum soluble B7–H3 is a prognostic marker for patients with non-muscle-invasive bladder cancer. PLoS ONE. 2020;15(12): e0243379.
Wang L, Kang FB, Zhang GC, Wang J, Xie MF, Zhang YZ. Clinical significance of serum soluble B7–H3 in patients with osteosarcoma. Cancer Cell Int. 2018;18:115.
Kovaleva OV, Belova TP, Korotkova EA, Kushlinskii DN, Gratchev AN, Petrikova NA, et al. Soluble B7–H3 in Ovarian Cancer and Its Predictive Value. Bull Exp Biol Med. 2021;171(4):472–4.
Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4(7):670–9.
Han P, Goularte OD, Rufner K, Wilkinson B, Kaye J. An inhibitory Ig superfamily protein expressed by lymphocytes and APCs is also an early marker of thymocyte positive selection. J Immunol. 2004;172(10):5931–9.
Gavrieli M, Watanabe N, Loftin SK, Murphy TL, Murphy KM. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatases SHP-1 and SHP-2. Biochem Biophys Res Commun. 2003;312(4):1236–43.
Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6(1):90–8.
Gonzalez LC, Loyet KM, Calemine-Fenaux J, Chauhan V, Wranik B, Ouyang W, et al. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci U S A. 2005;102(4):1116–21.
Murphy KM, Nelson CA, Sedy JR. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol. 2006;6(9):671–81.
Krieg C, Han P, Stone R, Goularte OD, Kaye J. Functional analysis of B and T lymphocyte attenuator engagement on CD4+ and CD8+ T cells. J Immunol. 2005;175(10):6420–7.
Monaghan SF, Banerjee D, Chung CS, Lomas-Neira J, Cygan KJ, Rhine CL, et al. Changes in the process of alternative RNA splicing results in soluble B and T lymphocyte attenuator with biological and clinical implications in critical illness. Mol Med. 2018;24(1):32.
Han L, Wang W, Fang Y, Feng Z, Liao S, Li W, et al. Soluble B and T lymphocyte attenuator possesses antitumor effects and facilitates heat shock protein 70 vaccine-triggered antitumor immunity against a murine TC-1 cervical cancer model in vivo. J Immunol. 2009;183(12):7842–50.
Kwon BS, Tan KB, Ni J, Oh KO, Lee ZH, Kim KK, et al. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem. 1997;272(22):14272–6.
Harrop JA, Reddy M, Dede K, Brigham-Burke M, Lyn S, Tan KB, et al. Antibodies to TR2 (herpesvirus entry mediator), a new member of the TNF receptor superfamily, block T cell proliferation, expression of activation markers, and production of cytokines. J Immunol. 1998;161(4):1786–94.
Salio M, Cella M, Suter M, Lanzavecchia A. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol. 1999;29(10):3245–53.
Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87(3):427–36.
Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8(1):21–30.
Zhai Y, Guo R, Hsu TL, Yu GL, Ni J, Kwon BS, et al. LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest. 1998;102(6):1142–51.
Steinberg MW, Cheung TC, Ware CF. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunol Rev. 2011;244(1):169–87.
Heo SK, Ju SA, Kim GY, Park SM, Back SH, Park NH, et al. The presence of high level soluble herpes virus entry mediator in sera of gastric cancer patients. Exp Mol Med. 2012;44(2):149–58.
Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev. 2009;229(1):244–58.
Cheung TC, Humphreys IR, Potter KG, Norris PS, Shumway HM, Tran BR, et al. Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway. Proc Natl Acad Sci U S A. 2005;102(37):13218–23.
del Rio ML, Lucas CL, Buhler L, Rayat G, Rodriguez-Barbosa JI. HVEM/LIGHT/BTLA/CD160 cosignaling pathways as targets for immune regulation. J Leukoc Biol. 2010;87(2):223–35.
Zhao Q, Zhang GL, Zhu X, Su D, Huang ZL, Hu ZX, et al. The paradoxical changes of membrane and soluble herpes virus entry mediator in hepatocellular carcinoma patients. J Gastroenterol Hepatol. 2017;32(8):1520–4.
Azarafza M, Tehrani M, Valadan R, Maleki I. Mohammad Mehdi Ghaffari-Hamedani S, Ghanadan A, et al. Role of BTLA/HVEM network in development of gastric cancer. Hum Immunol. 2022;83(8–9):637–44.
Takahashi N, Iwasa S, Sasaki Y, Shoji H, Honma Y, Takashima A, et al. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol. 2016;142(8):1727–38.
Shin K, Kim J, Park SJ, Lee MA, Park JM, Choi MG, et al. Prognostic value of soluble PD-L1 and exosomal PD-L1 in advanced gastric cancer patients receiving systemic chemotherapy. Sci Rep. 2023;13(1):6952.
Bian B, Fanale D, Dusetti N, Roque J, Pastor S, Chretien AS, et al. Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As, BTN3A1 and BTLA in patients with pancreatic adenocarcinoma. Oncoimmunology. 2019;8(4): e1561120.
Park H, Bang JH, Nam AR, Eun Park J, Hua Jin M, Bang YJ, et al. Prognostic implications of soluble programmed death-ligand 1 and its dynamics during chemotherapy in unresectable pancreatic cancer. Sci Rep. 2019;9(1):11131.
Fanale D, Incorvaia L, Badalamenti G, De Luca I, Algeri L, Bonasera A, et al. Prognostic Role of Plasma PD-1, PD-L1, pan-BTN3As and BTN3A1 in Patients Affected by Metastatic Gastrointestinal Stromal Tumors: Can Immune Checkpoints Act as a Sentinel for Short-Term Survival? Cancers (Basel). 2021;13(9):2118.
Montemagno C, Hagege A, Borchiellini D, Thamphya B, Rastoin O, Ambrosetti D, et al. Soluble forms of PD-L1 and PD-1 as prognostic and predictive markers of sunitinib efficacy in patients with metastatic clear cell renal cell carcinoma. Oncoimmunology. 2020;9(1):1846901.
Zhao J, Zhang P, Wang J, Xi Q, Zhao X, Ji M, et al. Plasma levels of soluble programmed death ligand-1 may be associated with overall survival in nonsmall cell lung cancer patients receiving thoracic radiotherapy. Medicine (Baltimore). 2017;96(7): e6102.
Ito M, Oshima Y, Yajima S, Suzuki T, Nanami T, Shiratori F, et al. Is high serum programmed death ligand 1 level a risk factor for poor survival in patients with gastric cancer? Ann Gastroenterol Surg. 2018;2(4):313–8.
Chang B, Huang T, Wei H, Shen L, Zhu D, He W, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68(3):353–63.
Omura Y, Toiyama Y, Okugawa Y, Yin C, Shigemori T, Kusunoki K, et al. Prognostic impacts of tumoral expression and serum levels of PD-L1 and CTLA-4 in colorectal cancer patients. Cancer Immunol Immunother. 2020;69(12):2533–46.
Chen X, Du Z, Huang M, Wang D, Fong WP, Liang J, et al. Circulating PD-L1 is associated with T cell infiltration and predicts prognosis in patients with CRLM following hepatic resection. Cancer Immunol Immunother. 2022;71(3):661–74.
Molga-Magusiak M, Rzepakowska A, Zurek M, Kotula I, Demkow U, Niemczyk K. Prognostic and predictive role of soluble programmed death ligand-1 in head and neck cancer. Braz J Otorhinolaryngol. 2023;89(3):417–24.
Asanuma K, Nakamura T, Hayashi A, Okamoto T, Iino T, Asanuma Y, et al. Soluble programmed death-ligand 1 rather than PD-L1 on tumor cells effectively predicts metastasis and prognosis in soft tissue sarcomas. Sci Rep. 2020;10(1):9077.
Fanale D, Brando C, Corsini LR, Cutaia S, Di Donna MC, Randazzo U, et al. Low plasma PD-L1 levels, early tumor onset and absence of peritoneal carcinomatosis improve prognosis of women with advanced high-grade serous ovarian cancer. BMC Cancer. 2023;23(1):437.
Kruger S, Legenstein ML, Rosgen V, Haas M, Modest DP, Westphalen CB, et al. Serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) in advanced pancreatic cancer. Oncoimmunology. 2017;6(5): e1310358.
Zhang Y, Li Z, Chen Y, Yang P, Hu Y, Zeng Z, et al. Higher serum sPD-L1 levels after radiotherapy indicate poor outcome in hepatocellular carcinoma patients. Transl Oncol. 2022;26:101537.
Teramoto K, Igarashi T, Kataoka Y, Ishida M, Hanaoka J, Sumimoto H, et al. Prognostic impact of soluble PD-L1 derived from tumor-associated macrophages in non-small-cell lung cancer. Cancer Immunol Immunother. 2023;72(11):3755–64.
Sideras K, de Man RA, Harrington SM, Polak WG, Zhou G, Schutz HM, et al. Circulating levels of PD-L1 and Galectin-9 are associated with patient survival in surgically treated Hepatocellular Carcinoma independent of their intra-tumoral expression levels. Sci Rep. 2019;9(1):10677.
Wang Q, Ye Y, Yu H, Lin SH, Tu H, Liang D, et al. Immune checkpoint-related serum proteins and genetic variants predict outcomes of localized prostate cancer, a cohort study. Cancer Immunol Immunother. 2021;70(3):701–12.
Montalban-Hernandez K, Casalvilla-Duenas JC, Cruz-Castellanos P, Gutierrez-Sainz L, Lozano-Rodriguez R, Avendano-Ortiz J, et al. Identification of sSIGLEC5 and sLAG3 as New Relapse Predictors in Lung Cancer. Biomedicines. 2022;10(5):1047.
Dong MP, Enomoto M, Thuy LTT, Hai H, Hieu VN, Hoang DV, et al. Clinical significance of circulating soluble immune checkpoint proteins in sorafenib-treated patients with advanced hepatocellular carcinoma. Sci Rep. 2020;10(1):3392.
Triebel F, Hacene K, Pichon MF. A soluble lymphocyte activation gene-3 (sLAG-3) protein as a prognostic factor in human breast cancer expressing estrogen or progesterone receptors. Cancer Lett. 2006;235(1):147–53.
Tominaga T, Akiyoshi T, Yamamoto N, Taguchi S, Mori S, Nagasaki T, et al. Clinical significance of soluble programmed cell death-1 and soluble programmed cell death-ligand 1 in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. PLoS ONE. 2019;14(2): e0212978.
Liu C, Li X, Li A, Zou W, Huang R, Hu X, et al. Concurrent Chemoradiotherapy Increases the Levels of Soluble Immune Checkpoint Proteins in Patients with Locally Advanced Cervical Cancer. J Immunol Res. 2022, 20229621466.
Keber CU, Derigs M, Schultz C, Wegner M, Lingelbach S, Wischmann V, et al. Cellular and soluble immune checkpoint signaling forms PD-L1 and PD-1 in renal tumor tissue and in blood. Cancer Immunol Immunother. 2022;71(10):2381–9.
Donlon NE, Davern M, Sheppard AD, O'Connell F, Dunne MR, Hayes C, et al. The Impact of Esophageal Oncological Surgery on Perioperative Immune Function; Implications for Adjuvant Immune Checkpoint Inhibition. Front Immunol. 2022, 13823225.
Odagiri N, Hai H, Thuy LTT, Dong MP, Suoh M, Kotani K, et al. Early Change in the Plasma Levels of Circulating Soluble Immune Checkpoint Proteins in Patients with Unresectable Hepatocellular Carcinoma Treated by Lenvatinib or Transcatheter Arterial Chemoembolization. Cancers (Basel). 2020;12(8):2045.
Liu C, Wang P, Sun Y, Dou X, Hu X, Zou W, et al. Neoadjuvant Chemoradiotherapy Changes the Landscape of Soluble Immune Checkpoint Molecules in Patients With Locally Advanced Rectal Cancer. Front Oncol. 2022, 12756811.
Zizzari IG, Napoletano C, Di Filippo A, Botticelli A, Gelibter A, Calabro F, et al. Exploratory Pilot Study of Circulating Biomarkers in Metastatic Renal Cell Carcinoma. Cancers (Basel). 2020;12(9):2620.
Sorensen SF, Demuth C, Weber B, Sorensen BS, Meldgaard P. Increase in soluble PD-1 is associated with prolonged survival in patients with advanced EGFR-mutated non-small cell lung cancer treated with erlotinib. Lung Cancer. 2016;100:77–84.
Liu Q, Hu P, Deng G, Zhang J, Liang N, Xie J, et al. Soluble cytotoxic T-lymphocyte antigen 4: a favorable predictor in malignant tumors after therapy. Onco Targets Ther. 2017;10:2147–54.
Acknowledgements
The authors thank Debra Weingarten for her editorial assistance in the preparation of the manuscript.
Funding
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, and the National Cancer Institute Intramural Continuing Umbrella of Research Experiences (iCURE) Program.
Author information
Authors and Affiliations
Contributions
SCP, JS, and RND contributed to the conception of the manuscript. SCP, JS, and RND wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13046_2024_3074_MOESM1_ESM.pptx
Additional file 1: Table S1. Changes in soluble immune checkpoints upon treatment with immune checkpoint therapy. Table S2. Baseline levels of other soluble immune checkpoints as indicators of clinical response to immune checkpoint therapy. Table S3. Post-treatment levels of other soluble immune checkpoints after immune checkpoint therapy as indicators of clinical response. Table S4. Changes in sPD-L1 upon treatment with conventional therapies. Table S5. Changes in sPD-1 and sCTLA4 upon treatment with conventional therapies. Table S6. Post-treatment levels of other soluble immune checkpoints after conventional therapies as indicators of clinical response. Table S7. Changes in sCD80, sTIM3, and sLAG3 upon treatment with conventional therapies. Table S8. Changes in sBTLA and sHVEM upon treatment with conventional therapies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pitts, S.C., Schlom, J. & Donahue, R.N. Soluble immune checkpoints: implications for cancer prognosis and response to immune checkpoint therapy and conventional therapies. J Exp Clin Cancer Res 43, 155 (2024). https://doi.org/10.1186/s13046-024-03074-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-024-03074-z