Abstract
Immunotherapy for cancer is a rapidly developing treatment that modifies the immune system and enhances the antitumor immune response. B7-H3 (CD276), a member of the B7 family that plays an immunoregulatory role in the T cell response, has been highlighted as a novel potential target for cancer immunotherapy. B7-H3 has been shown to play an inhibitory role in T cell activation and proliferation, participate in tumor immune evasion and influence both the immune response and tumor behavior through different signaling pathways. B7-H3 expression has been found to be aberrantly upregulated in many different cancer types, and an association between B7-H3 expression and poor prognosis has been established. Immunotherapy targeting B7-H3 through different approaches has been developing rapidly, and many ongoing clinical trials are exploring the safety and efficacy profiles of these therapies in cancer. In this review, we summarize the emerging research on the function and underlying pathways of B7-H3, the expression and roles of B7-H3 in different cancer types, and the advances in B7-H3-targeted therapy. Considering different tumor microenvironment characteristics and results from preclinical models to clinical practice, the research indicates that B7-H3 is a promising target for future immunotherapy, which might eventually contribute to an improvement in cancer immunotherapy that will benefit patients.
Similar content being viewed by others
Importance of this study
-
1.
We comprehensively reviewed the literature concerning B7-H3, from the biological features of B7-H3 to the roles of B7-H3 in the TME and malignant tumor behaviors, and discuss newly emerging evidence.
-
2.
We interpreted the relationship between B7-H3 and multiple TME characteristics and summarized the signaling pathways involved in tumorigenesis and the therapeutic approaches developed on the basis of previous studies, fueling the application of targeting B7-H3 from bench to bedside.
-
3.
We comprehensively reviewed the distribution, expression and function of potential receptors that were recently discovered using high-throughput methods.
-
4.
We reviewed the research progress on B7-H3 in different solid cancer types, pointing out the research lacking in the field and conflicting evidence that needs further verification.
-
5.
We summarized rapidly developing clinical trials targeting B7-H3 and other clinical applications of B7-H3.
Introduction
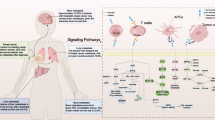
Immune checkpoints are a group of cell surface proteins that provide either activating or inhibitory signals to control the initiation, duration and magnitude of the immune response [1]. These inhibitory immune checkpoints usually function as a brake to prevent T cell death, reduce damage in healthy tissue and maintain self-tolerance and homeostasis [2], while in cancer, these checkpoints contribute to the ability of cancer cells to evade immune destruction, which is often cited as a “hallmark of cancer,” providing therapeutic targets for rapidly developing immune-oncological drugs, such as immune-activating PD-1 monoclonal antibodies [3]. The efficacy of antibodies targeting immune checkpoints has been verified in several clinical trials, mostly targeting CTLA-4 and PD-1/PD-L1 [4,5,6]. The great potential of these immune checkpoint inhibitor (ICI) therapies in both preclinical models and clinical trials has greatly sparked scientific interest and made immune checkpoint inhibitors a rapidly developing field, with ICIs alone or in combination being evaluated in 5683 active clinical trials in 2021 [7]. Figure 1 summarizes the commonly used checkpoints of potential translational value. Nevertheless, many patients are still unresponsive to the available ICI therapies, indicating a need to explore the underlying mechanism and other potential targets [8].
Current immune checkpoint receptors and their respective ligands. Many immune checkpoints expressed on the surface of T cells, such as PD-1, CTLA-4, LAG-3, TIGIT, VISTA, and TIM-3, bind to their respective ligands on APCs and/or tumor cells, eliciting positive and/or negative activity in the T cell response. TIM-3 also participates in associated signaling through PtdSer, HMGB-1 and Gal-9 in dying tumor cells. Notably, checkpoints such as PD-L1, CD80, CD226, and VISTA (B7-H5) are expressed on both T cells and APC/tumor cells. B7-H3 is also expressed on the surface of both T cells and APC/tumor cells, but its receptors have not been clearly elucidated, which has engendered great enthusiasm in cancer immunology investigators. In this article, we identify TLT-2, IL20RA, and PLA2R1 as three potential receptors for B7-H3. “+” in green indicates the immunostimulatory (positive) signal, and “−” in red indicates the immunosuppressive (negative) signal. PtdSer, phosphatidylserine; HMGB-1, high-mobility group protein B1; Gal-9, galectin-9
B7 family members have been identified as a group of immune regulatory ligands that modulate T lymphocyte activation and differentiation and exhibit a marked interaction with the CD28 superfamily. They are expressed extensively in adaptive and innate immune cells as well as in various cancer tissues, contributing to cancer immune evasion capacity [9]. In addition to the well-studied B7-H1 (PD-L1), the B7 family consists of ten members in total: B7-1 (CD80), B7-2 (CD86), B7-DC (PD-L2), B7-H2 (CD275), B7-H3 (CD276), B7-H4, B7-H5, B7-H6, and B7-H7 (HHLA2) [10]. As a member of the B7 family, B7-H3 has gained great attention in the last decade since its discovery in 2001 [11]. B7-H3 has shown a seemingly contradictory role in T cell activation, while the nature of the B7-H3 receptor has not been clearly elucidated. It has been demonstrated that B7-H3 contributes to tumorigenesis, metastasis and malignant behaviors through various mechanisms, and an association between B7-H3 expression and poor prognosis has been established. To further extend our knowledge of cancer immunotherapy and fuel clinical research targeting B7-H3, an up-to-date and comprehensive review is needed as the literature regarding B7-H3 is rapidly accumulating. Herein, in this review, we summarize the latest research on the function and underlying pathways of B7-H3, the expression and roles of B7-H3 in different cancer types, and the advances in B7-H3 immunotherapy in clinical trials.
Structure of B7-H3
The human B7-H3 gene locates in 15q24.1 and has 12 exons encoding 316 amino acids, it is a type 1 transmembrane glycoprotein with two isoforms: 2IgB7-H3 (B7-H3 VC) and 4IgB7-H3 (B7-H3b or B7-H3 VCVC) [11, 12]. The 2IgB7-H3 structure comprises single extracellular V- and C-like Ig domains, a transmembrane region and a 45-aa cytoplasmic tail, which was described in an early study using nucleic acid sequence analysis in a human dendritic cell (DC)-derived cDNA library [11]. The presence of the 4IgB7-H3 isoform with two identical pairs of IgV-like and IgC-like domains was later verified in humans [13]. In humans, 4IgB7-H3 is the major isoform expressed on immunocytes as well as on malignant cells [14]. The murine B7-H3 gene locates in chromosome 9, it has a structure similar to that of human 2IgB7-H3, with 93% amino acid similarity [13]. The predicted molecular weight of 2IgB7-H3 is ~ 70 kDa based on the amino acid sequence, while B7-H3 was detected as an ~ 110 kDa glycoprotein via western blotting in human breast cancer samples [15]. The crystal structure of murine B7-H3 has been reported, suggesting that the FG loop of the IgV domain plays a critical role in its inhibitory function [16]. In addition to the transmembrane form, soluble B7-H3 (sB7-H3) has been detected in normal human serum [17]. sB7-H3 is produced by alternative splicing from the fourth intron of B7-H3 [18] or matrix metallopeptidase (MMP) [17], and the sB7-H3 serum level has been correlated with prognosis in various malignancies [19, 20].
Receptor of B7-H3
The identity of the receptor of B7-H3 is controversial and has not been verified. The unknown nature of the B7-H3 receptor has become the biggest hurdle to understanding the biology of B7-H3, yet the available data for the B7-H3 receptor are still conflicting and scarce, although great efforts have been devoted to solving this issue. Figure 2 summarizes the structure, biological function and interaction with B7-H3 and putative B7-H3 receptors.
Structures, distributions, interactions and biological functions of B7-H3 and putative receptors. Three proteins have been identified as potential B7-H3 receptors, including TLT-2 (A), IL20RA (B) and PLA2R1 (C). TLT-2 is widely expressed on the surface of myeloid, B and T cells, and its function in specific cell types has been separately studied. TLT-2 plays a proinflammatory role in CD8+ T cells, neutrophils and microglia while reducing the Th1 immune response and blocking Th1 differentiation when activated on monocytes. The effect of B7-H3 binding to TLT-2 on CD8+ T cells is controversial, and the functional interaction between B7-H3 and TLT-2 in other cell types remains unknown (A). Little is known about the specific cell types that express IL20RA and PLA2R1 and their cell type-specific functions. IL20RA activation enhances breast cancer cell stemness and establishes an immunosuppressive TME via the JAK1/STAT3 signaling pathway, while modulation of the TME via the JAK1/STAT3 pathway through IL20RA and IL20RB is still disputed, requiring more robust and direct evidence (B). PLA2R1 has been indicated as a tumor-suppressive regulator that induces breast cancer cell apoptosis and inhibits transformation to renal cell carcinoma (C). Considering the diverse roles of B7-H3 in the TME, other unknown receptors must be reported continuously. Human B7-H3 gene locates in 15q24.1 and has 12 exons encoding 316 amino acids; the structure of B7-H3 (4IgB7-H3 here, the major isoform in humanity) comprises two identical pairs of extracellular lgV-like and IgC-like domains, a transmembrane region and a 45-aa cytoplasmic tail. Seven B7-H family members (B7-H1 to B7-H7) and their receptors expressed on T cells are also displayed, where B7-H3 binds to TLT-2, IL20RA, PLA2R1 and other interesting as yet unknown receptors. “+” in green indicates the immunostimulatory (positive) signal, and “−” in red indicates the immunosuppressive (negative) signal (D). TME, tumor microenvironment
TLT-2
Triggering receptor expressed on myeloid cells (TREM)-like transcript 2 (TLT-2, TREML2) was the first identified and most well-studied B7-H3 receptor candidate. TLT-2 is extensively expressed in neutrophils, macrophages, the B lymphoid lineage [21], microglia [22], CD8+ T cells and activated CD4+ T cells [23, 24]. Although the crystal structure of TLT-2 has not been solved, researchers have speculated that, similar to other members of the TREM family, TLT-2 is a single transmembrane protein in the immunoglobulin superfamily that contains a putative SH3 binding motif [25]. The function of TLT-2 has been widely studied in various components of the innate and adaptive immune systems. Ligation of TLT-2 by a monoclonal antibody activates neutrophils to induce reactive oxygen species production, degranulation and chemotaxis, especially in response to G protein-coupled receptor signaling [26] and TLT-2, whose expression in neutrophils is stimulated by inflammatory mediators, is predominantly localized in intracellular vesicles in neutrophils, potentially modulates the exocytosis process [27]. In microglia, TLT-2 promotes the expression of proinflammatory cytokines, antagonizing the anti-inflammatory role of TREM2 [28]. Using proteomics analysis and TLT-2 knockdown, Xu and colleagues revealed that TLT-2 activates NF-κB signaling by inhibiting IκBα to promote the expression of granzyme B (GZMB) and enhance the immune function and proliferation ability of CD8+ T cells [29]. However, in contrast to its overall proinflammatory effect on other cell types, TLT-2 expressed in monocytes promotes interleukin-6 (IL-6) expression via the Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) pathway, blocks Th1 differentiation and hinders the immune response to tuberculosis [30]. These studies suggest that TLT-2 is an important modulator of both the innate and adaptive immune systems. The multifaceted and seemingly contradictory function of TLT-2 as a putative B7-H3 receptor that is present in different cell types may potentially explain the contradictory role of B7-H3 in the immune response.
Using flow cytometry, Hashiguchi et al. found that the murine B7-H3 fusion protein B7-H3Ig specifically binds to TLT-2. The relative affinity between B7-H3Ig and TLT-2 was estimated to be comparable to the affinity between PD-L1 and PD-1 in the same model, and the B7-H3/TLT-2 interaction was shown to functionally enhance T cell responses and IL-2 and IFN-γ production [23], consistent with the first discovered role of B7-H3 [11]. The binding and flow cytometry analyses provided direct evidence for B7-H3 binding to TLT-2, although the difference between the B7-H3Ig fusion protein and endogenous membrane-bound B7-H3 and the difference between high TLT-2 expression in transfected cells and endogenous TLT-2 expression should not be ignored. In vivo validation was conducted in a murine contact hypersensitivity model [23] and tumor model [24], where both anti-B7-H3 and anti-TLT-2 monoclonal antibodies attenuated the inflammatory response. Unfortunately, the validation study still did not exclude the possibility that B7-H3 and TLT-2 stimulate the T cell response through different pathways. Later, Fang et al. found that sB7-H3 induces the chemotaxis of myeloid-derived suppressor cells (MDSCs) in vitro, which might be attenuated by blocking TLT-2 [31]. Together, these studies provide direct and indirect evidence for the hypothesis that TLT-2 is the receptor for B7-H3.
However, in another study by Leitner et al., no evidence was found to support the interaction between TLT-2 and B7-H3 in either a murine model or in human cells, in which B7-H3 also potently and consistently downregulated human T cell responses [32]. The authors performed an in-depth study that considered the ligand concentration, B7-H3 isoforms, types of fusion proteins and differences between murine and human genes. Although the analysis of the binding between murine B7-H3Ig and TLT-2 was very similar to the study by Hashiguchi et al. [23], differences in the cellular background, transfection efficiency and fusion protein construct might explain the discrepancy in the conclusions. Using a similar design to examine another cell line, CHO cells, Yan et al. did not detect any interaction between TLT-2 and B7-H3 [33]. Further studies provide other forms of evidence opposing the binding between these two molecules. The crystal structure of B7-H3 did not support the binding of TLT-2 and B7-H3 [16], and the unsynchronized expression of TLT-2 and B7-H3 might also serve as a negative indicator [29]. The binding of TLT-2 and B7-H3 is still disputed, further validation of the binding is needed, and studies exploring the functional interaction between B7-H3 and TLT-2 expressed in innate immune cells might also substantially improve our understanding of their interaction.
IL20RA
With a new interactome platform using high-throughput data, Husain et al. found that interleukin-20 receptor subunit α (IL20RA) was the first target of B7-H3 binding [34]. The platform provided valuable information, as it detected the interaction between membrane proteins, consistent with the normal function of B7-H3. In the receptor library, all the single-pass transmembrane proteins in the public database were included to provide a comprehensive interactome landscape [34], while a previous study only screened proteins homologous to the CD28 family [23, 32]. The binding with IL20RA was further verified by Cao et al., who performed another extracellular vesicle-based high-throughput interactome platform [35], but the binding still requires further functional validation from either in vitro or in vivo studies.
IL20RA was first identified as a subunit of the binding complex that forms a heterodimer with interleukin-20 receptor subunit β (IL20RB) to bind IL20 [36]. IL20RA is a transmembrane glycoprotein containing two tandem β-sandwich domains in the extracellular domain and an intracellular domain that functions with its counterpart in the heterodimer, whose structure belongs to the type II interleukin receptor family [37]. The expression of IL20RA was enriched in many types of tissues, particularly in skin, lung and testis [36], but its expression was not detected in circulating immune cells, including monocytes, T cells, B cells and NK cells [38]. Further research on the IL20RA expression pattern, especially in the TME, is still lacking. As reviewed by Rutz et al., IL20RA transduces signals from IL19, IL20 and IL24 with IL20RB, activates JAK1/STAT3 pathways and modulates inflammation, angiogenesis, metabolism and epithelial remodeling, in which the signals from interleukin exert both tumor-promoting and tumor-suppressing effects [39]. The significance of IL20RA as a biomarker in cancer has been addressed [40], and overexpression of IL20RA alone promotes cancer stemness via the transcription factor SOX2 and induces an immunosuppressive TME by increasing PD-L1 expression [41]. Similarly, IL20RA activation might profoundly influence tumorigenesis, although the functional consequence of B7-H3 binding to IL20RA remains to be explored. As the available evidence suggests low expression of IL20RA in immune cells, the B7-H3-IL20RA interaction might indirectly modulate inflammation and the immune response through stromal and tumor cells, as shown in the study by Ungaro et al. that IL20RA promotes chronic inflammation via the lymphatic endothelium [42].
PLA2R1
In addition to IL20RA, Cao and colleagues also detected phospholipase A2 receptor 1 (PLA2R1) as another high-affinity binding protein among all the single-pass transmembrane proteins with their extracellular vesicle-based interactome platform [35]. PLA2R1 belongs to the mannose receptor family, which is composed of a short cytoplasmic tail, a transmembrane domain, a tandem C-type lectin domain with 8 repeats, a fibronectin type II domain and a cysteine-rich terminal domain [43]. The expression pattern of PLA2R1 in normal cells has not been fully elucidated, but the decreased expression of PLA2R1 in different malignancies has been reviewed [44]. PLA2R1 has been shown to function as a tumor suppressor by inducing cellular senescence via the increased production of reactive oxygen species in mitochondria, suppression of PARP1 expression and activation of the p53 pathway in PLA2R1-overexpressing cells [45, 46], and it inhibited cell transformation into tumor cells via downstream estrogen-related receptor α1 and JAK2 signaling [47, 48]. On the other hand, the binding of secreted phospholipase A2, the known ligand for PLA2R1, increases the survival of mast cells [49] and promotes the migration of fibrosarcoma cells [50]. The mechanism by which the binding of B7-H3 to PLA2R1 modulates its antitumor function remains to be elucidated in the future.
The identity of the B7-H3 receptor is still uncertain, although three candidates, TLT-2, IL20RA and PLA2R1, have been proposed. The available evidence for these receptors does not fully explain the complicated effects of B7-H3 on malignant behaviors and the TME. As the B7 family member B7-1 binds to CD28, CTLA-4, PD-L1 and NGFR [51], B7-H6 interacts with KIR3DL3 to stimulate or TMIGD2 to inhibit the immune response [52]. The finding that B7-H3 has multiple binding partners must not be ignored, especially when considering the opposing roles of B7-H3. Recent interactome studies have expanded our scope of screening all the single-pass transmembrane proteins and identified two potential B7-H3 receptors that are not homologous to CD28. However, the evidence for the two recently discovered partners is far from sufficient, and further functional validation of IL20RA or PLA2R1 binding would be of great value. Unknown receptors with a structure beyond a single-pass transmembrane protein might also be discovered.
B7-H3 and malignant behaviors
B7-H3 in cancer proliferation
Unrestricted proliferation bypassing the cell cycle checkpoint is found in almost all tumor types and is a driving force of tumorigenesis [53]. Downregulation of B7-H3 reduced the proliferation of colorectal cancer (CRC) cell lines, and several key cell cycle-related proteins, including cyclin D1 and CDK4, were also dramatically decreased [54]. Recent studies have shown similar results in which B7-H3 overexpression significantly facilitated cell multiplication and migration in CRC cells [55]. B7-H3 silencing also reduced proliferation, invasion and migration in the A549 lung adenocarcinoma cell line [56]. In mouse spermatogonial stem cells (SSCs), B7-H3 was found to promote mouse SSC proliferation and cell cycle progression in a CCK-8 assay in which mouse SSCs were incubated with different concentrations of B7-H3 [57]. The proliferation promotion was inhibited by the PI3K inhibitor LY294002, indicating that B7-H3 promotes proliferation by activating the PI3K signaling pathway. Liu et al. found that B7-H3 binds to major vault protein (MVP) and activates MEK through the interaction between B-RAF and MEK in breast cancer stem cells, demonstrating a novel B7-H3/MVP/MEK signaling axis by which B7-H3 can promote cancer proliferation [58]. Both endogenous B7-H3 expression and exogenous B7-H3 stimulation promote cancer cell proliferation through various mechanisms.
B7-H3 in deregulating cancer metabolism
Aberrant metabolism, including increased aerobic glycolysis and anabolic pathways, is a major hallmark of cancer that can fuel the tumorigenic process by providing energy, building blocks and redox potential [59]. Lim et al. confirmed in vivo and in vitro that B7-H3 overexpression promoted glucose intake and lactate production, contributing to aberrant glycolysis [60]. They further revealed that B7-H3 stabilized hypoxia inducible factor-1 (HIF-1α) through the transcription factor Nrf2 and its target genes SOD1, SOD2 and PRX3 and activated downstream glycolytic enzymes to exert a hyperglycolytic role. In ovarian cancer cell lines, B7-H3 knockout resulted in a decreased level of glycolysis and reduced the expression of lactate dehydrogenase A (LDHA), phosphoglycerate kinase 1 (PGK1) and HIF-1α, which suggests that B7-H3 promotes glycolysis [61]. Zuo et al. found that B7-H3 directly interacts with the rate-limiting glycolytic enzyme ENO1 and alters its activity in HeLa cells; moreover, B7-H3 silencing reduced the production of ATP, lactate, c-Myc and LDHA, indicating that B7-H3 alters metabolism by affecting the activity of ENO1 and the c-Myc-LDHA axis [62]. Li et al. explored the metabolism-reprogramming mechanism of B7-H3 in oral squamous carcinoma cells and demonstrated that B7-H3 upregulates the expression of HIF-1α, GlUT1 and PFKFB3 downstream through the PI3K/Akt/mTOR pathway to enhance glycolysis [63]. Shi et al. found that hexokinase 2 (HK2) was the key mediator of glucose metabolism regulation. They demonstrated that treating cells with HK2 inhibitors could reverse the B7-H3-induced increase in aerobic glycolysis, which suggested a novel underlying mechanism [64]. These studies strongly support the involvement of B7-H3 in the dysregulation of cancer cell metabolism and its contribution to tumorigenesis.
B7-H3 in cancer invasion
B7-H3 has been reported to promote cancer cell migration and invasion in various types of cancer [55, 65, 66]. In glioma cells overexpressing B7-H3, Zhong et al. demonstrated that the JAK2/STAT3 signaling pathway was activated and that B7-H3-induced glioma progression was suppressed by a JAK2/STAT3 inhibitor. They further revealed that B7-H3 induces glioma invasion through the JAK2/STAT3/Slug/MMP-2/-9 pathway and is involved in epithelial‑mesenchymal transition (EMT) [67]. EMT is a key step in cancer metastasis. Yu et al. demonstrated that the downregulation of B7-H3 may inhibit EMT in lung adenocarcinoma cells [56]. In hepatocyte carcinoma, B7-H3 was found to promote EMT via the JAK2/STAT3/Slug pathway [68], while Liao et al. found another mechanism through which B7-H3 might promote EMT. They discovered that B7-H3 upregulated the expression of SIRT1 via the PI3K/AKT pathway in a non-small cell lung cancer (NSCLC) cell line and further promoted the expression of E-cadherin and EMT [69]. In clear cell renal cell carcinoma (CCRCC), B7-H3 was found to promote the EMT process in CCRCC cells by activating the PI3K/AKT and p38/ERK mitogen‐activated protein kinase (MAPK) signaling pathways, which are mediated by fibronectin [70]. Although mechanistically different, these studies demonstrate that B7-H3 greatly influences tumor invasion and metastasis.
B7-H3 in cancer anti-apoptosis activity
B7-H3 can also promote tumor progression by inhibiting cancer cell apoptosis. In ovarian cancer cell lines, an evaluation of Annexin V-stained cells using flow cytometry showed that B7-H3 silencing promoted apoptosis mainly in the early stage, and subsequent western blotting results showed a decrease in the expression of the anti-apoptotic proteins Bcl-2 and Bcl-xl, as well as an increase in the levels of the proapoptotic proteins Bax, caspase-8 and cleaved caspase-8 [71]. Silencing of B7-H3 was also found to enhance apoptosis in cervical cancer cell lines [72]. The levels of phosphorylated JAK2 and STAT3 were increased in B7-H3-overexpressing cells, while treatment with the JAK2 inhibitor AG490 decreased the expression of related proteins in the JAK2/STAT3 pathway, and apoptosis was subsequently enhanced, indicating that B7-H3 exerts its anti-apoptotic effect through the JAK2/STAT3 pathway [71].
B7-H3 in cancer therapy resistance
B7-H3 has been identified as promoting resistance to conventional cancer therapies in different types of cancer. It has been discovered that knockdown of B7-H3 increases the sensitivity of melanoma cells to the chemotherapeutic agents dacarbazine and cisplatin, which are small-molecule inhibitors targeting the MAPK and AKT/mTOR pathways [73, 74], and increases gemcitabine sensitivity in pancreatic carcinoma [75] and everolimus sensitivity in triple-negative breast cancer (TNBC) [76]. Altered glucose metabolism and increased apoptosis were shown to contribute to B7-H3-mediated chemotherapy resistance, which provides further evidence for the effect of B7-H3 on dysregulating metabolism and its anti-apoptotic role discussed previously [75, 76]. Notably, B7-H3 increased the radioresistance of gastric cancer cells by inhibiting baseline cell autophagy, apoptosis and DNA double-strand break repair [77]. Based on these studies, B7-H3 decreases the sensitivity of tumor cells to a series of chemotherapy agents and radiation and thus is a valuable target to augment the effect of conventional cancer therapy.
B7-H3 in cancer stem cells
Cancer stem cells (CSCs) are a small subpopulation of cancer cells with “stemness” properties, and CSCs are widely accepted to promote metastasis, radioresistance, chemoresistance and cancer recurrence [78]. Significantly higher B7-H3 expression in CSCs than in the nonstem cell population have been observed in breast cancer, prostate cancer and head and neck squamous cell carcinoma (HNSCC) [58, 79, 80]. As demonstrated by Liu et al., after transfection with exogenous B7-H3, several breast cancer cell lines with B7-H3 overexpression dramatically enriched their CSC population, which was marked by CD24 and CD44, while B7-H3 knockdown led to the opposite results, and these effects were mediated via the B7-H3/MVP/MEK pathway [58]. B7-H3 has also been found to serve as an enrichment surface marker for Bmi1+ HNSCC CSCs, and anti-B7-H3 antibodies eliminated the CSC population and inhibited tumor growth in a CD8+ T-cell-dependent manner [80]. ALDH+ CD44+ prostate CSCs showed increased expression of B7-H3 after radiotherapy, and the expression difference between CSCs and bulk prostate cancer cells indicates that B7-H3 targeting immunotherapy is a promising combination alternative for prostate cancer therapy [79]. The association between stemness and B7-H3 expression provides support from another perspective for B7-H3-based antitumor therapy.
B7-H3 and other cancer hallmarks
In a recent review, Hanahan et al. proposed several additional cancer hallmark traits, including phenotypic plasticity, senescent cells and epigenetic reprogramming [81]. There have been publications describing the involvement of B7-H3 in these malignant traits. B7-H3 unlocked phenotypic plasticity by blocking differentiation in alveolar rhabdomyosarcoma, where B7-H3 overexpression in alveolar rhabdomyosarcoma cell lines induced a myogenic differentiation block and a more invasive phenotype, while B7-H3 knockdown exerted the opposite effect [82]. B7-H3 also inhibits cellular senescence induced by doxorubicin (DOX) in CRC cell lines, possibly through the AKT/TM4SF1/SIRT1 pathway [83]. The epigenetic regulation of B7-H3 expression has been widely investigated. The expression level of B7-H3 was found by Wang et al. to be potentially regulated by the microRNA-29 family and B7-H3 promoter methylation [84]. In addition to microRNA-29, more than 10 microRNAs have been demonstrated to regulate the expression of B7-H3 and influence tumor behaviors, as reviewed by Feng et al. [9]. Moreover, N6-methyladenosine (m6A) RNA modification of B7-H3 mRNA was found to be significantly downregulated in CRC tissues compared with normal tissues, which further participated in immune escape, indicating epigenetic reprogramming’s role in B7-H3 function [85]. Altogether, B7-H3 is involved in tumor proliferation, metabolism, and invasion and a series of malignant behaviors, which confirms that B7-H3 is a valuable research object to further elucidate tumor biology and a therapeutic target to block tumor progression.
B7-H3 in the tumor microenvironment
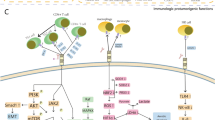
The tumor microenvironment (TME) dynamically modulates tumor progression and greatly influences the outcomes of cancer immunotherapy. The TME typically comprises immune cells, including tumor-infiltrating lymphocytes (TILs), tumor-associated macrophages (TAMs), dendritic cells (DCs) and natural killer (NK) cells; stromal cells; extracellular matrix (ECM) and secreted molecules, including cytokines, chemokines and exosomes; and blood and lymphatic vascular networks [86]. Accumulating evidence has been found to support the idea that B7-H3 modulates the immune response by influencing different TME characteristics. Figure 3 critically summarizes the interactions of B7-H3 with immune cells and related pathways to facilitate their functions.
Interactions of B7-H3 with immune cells and related pathways facilitate B7-H3 function in the microenvironment. The top panel exhibits interactions with immune cells. B7-H3 was originally identified for its effect on promoting the growth of CD4+ T cells and inhibiting the growth of CD8+ T cells. Activated CD4+ T cells induce IFN-γ production and promote the production of IL-12, while IL-2, IL-10, IL-13 and IFN-γ production are suppressed in CD8+ T cells. B7-H3 also negatively regulates the release of IFN-γ and T cell proliferation in B7-H3-deficient mice. B7-H3 suppresses Th1- and Th2-mediated responses, activity and Treg accumulation. IFN-γ and IFN-5 production and Th1-mediated hypersensitivity are inhibited. However, the release of IL-2 and IL-10 is promoted from Th2 cells. B7-H3 enhances M2 macrophage polarization and the release of cytolytic factors from monocytes, which still requires stronger evidence. The cytolytic function of NK cells is curbed. The bottom panel presents distinct pathways to facilitate B7-H3 function. In the TME and related signaling pathways, the roles of B7-H3 are associated with tumor growth, migration, invasion, metastasis and other processes mediated by the PI3K/AKT/mTOR, JAK2/STAT3 and NF-κB signaling pathways and cell metabolism through the TCA cycle. Overall, B7-H3 regulates tumor cell invasion, migration, apoptosis, metabolism and drug response/resistance through classic pathways; B7-H3 also interacts with many types of immune cells in the microenvironment to influence the immune response. TME, tumor microenvironment; TCA cycle, tricarboxylic acid cycle
B7-H3 and TME immune cells
When discovered, B7-H3 was originally found to be an immune costimulator [11], where B7-H3-Ig induced the proliferation of CD4+ and CD8+ T cells, increased the secretion of interferon γ and enhanced cytotoxic T cell activity. The costimulatory role of B7-H3 was subsequently supported by several studies in different models, including cancer, autoimmune diseases and allografts [23, 87, 88]. However, in the past decade, most studies in the oncology field have indicated that B7-H3 plays an inhibitory role in the TME. The discrepancy in immunomodulatory roles is most evident between autoimmune diseases and cancer models. In contrast to the predominant immunoinhibitory data reported in tumor studies, B7-H3 plays a proinflammatory role in autoimmune disease models, with some controversies [89,90,91]. Several factors might contribute to the discrepancy in the immunomodulatory roles of B7-H3. The first is the disease-specific expression pattern of B7-H3. Aberrantly upregulated expression of B7-H3 has been detected in tumor cells, immune cells and a series of stromal cells in malignancies with different levels and distributions, while the expression of B7-H3 in synoviocytes, osteoblasts, endothelial cells and other cells was noted in an autoimmune disease model [92]. This difference in the expression pattern between disease models may reshape the immunomodulatory capacity of B7-H3. B7-H3 is expressed in not only adaptive immune cells but also cancer-associated fibroblasts, neutrophils, and the endothelium [93,94,95], where it may shape the immunosuppressive TME. The distribution and functions of these stromal cells vary substantially in different models, which might dominate the function of the most well-studied T cell-mediated immunoregulatory axis. The second is the multiple downstream pathways activated by different binding partners. As discussed in the receptor section, B7-H6 interacts with TMIGD2 to stimulate the immune response or with KIR3DL3 to inhibit the immune response [52], and B7 family members with multiple binding partners are not rare. The abundance of inhibitory B7-H3 receptors in certain TMEs compared with other models might at least partially explain the discrepancy. Despite the dispute in the overall conclusion, the immunomodulatory roles of B7-H3 mediated by interactions with different cellular components of the TME have been widely investigated.
The correlation between the function and differentiation of T helper cells and B7-H3 has mainly been explored in the setting of autoimmune diseases, with some controversy. Suh et al. found that B7-H3-deficient mice exhibited accelerated progression of experimental autoimmune encephalomyelitis [96] and produced higher concentrations of DNA autoantibodies [91]. In addition to the immunoinhibitory role of B7-H3, Suh et al. found that T helper cells in B7-H3-deficient mice preferentially differentiate into Th1 cells rather than Th2 cells, i.e., B7-H3 negatively regulates Th1 differentiation preferentially. On the other hand, in a model of allergic conjunctivitis, Fukushima et al. reported that B7-H3 negatively regulated both Th2 immune responses and Th1 immune responses [90]. Conflicting results were reported by Luo et al., who analyzed the roles of B7-H3 in regulating the Th1, Th2 and Th17 subsets in an autoimmune disease model, and the results suggested that B7-H3 has a costimulatory function for Th1/Th17 cells but a coinhibitory function in Th2 responses [89]. The seemingly contradictory effect of B7-H3 on these CD4+ T helper cells might result from the analysis of different disease settings and B7-H3 targeting approaches. Possible failure to block B7-H3 with antibodies due to the uncertainty of its receptor and possible cross-reaction with other B7 family members may hinder the discovery. The conclusion might also be different in the context of tumors; thus, further analysis of T helper cell differentiation in tumors might be helpful.
T lymphocytes are the major component of antitumor immunity, and a correlation between T cells inhibition and B7-H3 expression has been established in several cancer models. An early study revealed that B7-H3 inhibited T cell activity by downregulating the NF-κB, NFAT and AP-1 signaling pathways and that blocking B7-H3 enhanced T cell activation in murine models [97]. Recently, it was reported that blocking B7-H3 resulted in dramatically increased CD8+ T cell infiltration and subsequent tumor inhibition in HNSCC [80]. This inhibition behaves in a CD8+ T cell-dependent manner, with increased infiltration of NK cells and GZMB+ cells, which mediate the apoptosis of squamous cancer cells. In triple-negative breast cancer, NanoString results for tumor samples revealed that B7-H3 was overexpressed in samples from the low TIL group [98]. In ovarian cancer, B7-H3 was shown to be highly expressed in both tumor cells and TILs, and B7-H3 expressed in tumor cells has been shown to play the main role in immunity inhibition [99]. B7-H3 deficiency in a murine model significantly downregulated other coinhibitory molecules, including PD-1, and increased the production of the proliferation markers Ki-67, IFN-γ, TNF-α and granzyme B in CD8+ T cells, which indicated a role of B7-H3 in CD8+ T cell exhaustion. In the same model, CD4+ T cells and NK cells were found to shift into an active IFN-γ- and TNF-α-producing state in the TME [99]. It was also demonstrated in a murine NSCLC model that B7-H3 blockade led to an increased number and functional recovery of infiltrated CD8+ T cells [96]. The expression of B7-H3 was found to be critically correlated with nonresponsiveness to anti-PD-1 immunotherapy in patient-derived NSCLC samples, and dual blockade of PD-L1 and B7-H3 in a murine model revealed an enhanced antitumor effect, which highlights B7-H3 as a promising anti-PD-1 combination option.
Regulatory T (Treg) cells have been demonstrated to confer immune tolerance and are involved in cancer immune evasion [100]. It was established in an in vivo model that Treg cells affect DCs in situ, decrease MHC-II-peptide formation in DCs and induce the expression of IL-10 and B7-H3, subsequently rendering DCs immunosuppressive [101]. Reduced infiltration of Treg cells both in absolute number and in ratio was observed in a B7-H3-deficient model [99], and a significant positive correlation between the number of FOXP3+ Treg cells and B7-H3 expression has been identified in human NSCLC tissues [102], which indicates a possible immunosuppressive mechanism of B7-H3 mediated by the recruitment of Treg cells. Although B7-H3 expression was again found to be negatively related to CD8+ TILs and overall survival, no significant correlation was observed between B7-H3 expression and Foxp3+ Treg cells in a prostate cancer model [103]. Similarly, no significant correlation between B7-H3 expression and Treg cells was identified in breast cancer [104], indicating possible variations in TME characteristics in different TME settings, and more extensive investigation is needed.
TAMs are highly plastic cells that serve a multitude of functions and are one of the key components of the TME [105]. The activation states of TAMs are generally categorized into two types: M1 classically activated macrophages, which promote inflammation and serve as costimulatory molecules to enhance the T cell response, and M2 alternatively activated macrophages, which play a critical role in immune modulation and tumor progression [105]. In triple-negative breast cancer, B7-H3 was also found to be highly expressed in TAMs, and these B7-H3-high TAMs played great prometastatic and immunosuppressive roles through intriguing ECM reconstruction and tumor angiogenesis, eventually reducing T cell infiltration in the tumor microenvironment [106]. In murine ovarian cancer models, B7-H3 knockout tumor cells showed a reduced number of M2 macrophages and increased IFN-γ+ CD8+ T cell infiltration. CCL-2 production was found to be upregulated by B7-H3, potentially via the STAT3 pathway, and a downstream CCR inhibitor partly eliminated the effect of B7-H3 knockout on M2 macrophages, indicating that the B7-H3-CCL2-CCR2 axis modulates TAM function [107]. It was also found that B7-H3 upregulated by lncRNA NEAT1 promotes M2 macrophage polarization via the JAK2-STAT3 pathway in multiple myeloma [108], showing that TAMs are important mediators of the immune-inhibitory function of B7-H3.
Neutrophils are a prominent component of the innate immune system and are often found in the TME. In human gastric cancer, it was found that tumor-derived GM-CSF induces the proliferation of neutrophils and stimulates B7-H3 expression in neutrophils via the JAK2-STAT3 signaling pathway. B7-H3-high neutrophils, which are often found in the gastric cancer TME, are correlated with a poor prognosis and tumor progression in human gastric cancer [94]. DCs were also found to be correlated with B7-H3. In NSCLC tumor samples, DCs were found to highly express B7-H3, with reduced IL-12 secretion and T cell activation capacity [109], in accordance with a previous study in which bone marrow-derived DCs with high B7-H3 expression appeared to be highly immune-inhibitory [101].
The intense investigation into the mechanism by which B7-H3 influences immune cell function has connected B7-H3 expression to both adaptive and innate immune systems, in addition to the extensively studied immune checkpoint-mediated change in T cell function. The complex interactions between B7-H3 and multiple immune cells account for the complex immunoregulatory roles of B7-H3, whereas the immunoregulatory roles mainly depend on the specific TME cellular composition. Although B7-H3 was mainly identified as an inhibitory immunoregulator in cancers, its functions vary in different diseases and even in different types of cancer. The conclusions must be interpreted with caution when translating the result into other models. A cancer-type-specific analysis would be more informative.
B7-H3 and vascular network
Aberrant angiogenesis is an important hallmark of cancer because it allows for the delivery of oxygen, nutrients, and growth factors and is even the route for tumor metastasis [110]. B7-H3 expression has been found in tumor-associated endothelial cells. Seaman et al. demonstrated that B7-H3 overexpression was often found in tumor endothelial cells, while normal angiogenic tissues were uniformly negative for this marker [111]. Using B7-H3 knockdown in human umbilical vein endothelial cells (HUVECs) and in vitro and in vivo Matrigel models, Lai et al. suggested that B7-H3 in HUVECs enhanced VEGF secretion and subsequently increased cell proliferation, migration and tube formation [95]. However, it was shown that in late endothelial progenitor cells (LEPCs), which are circulating vascular repair cells with abundant expression of B7-H3 on the cell surface, B7-H3 knockdown promotes endothelial cell differentiation and angiogenesis but inhibits proliferation and migration, indicating a complex role of B7-H3 in LEPCs [112]. In a CRC model, it was demonstrated that the NF-κB pathway has a major effect on B7-H3-induced VEGF-A expression in CRC cells [113]. In medulloblastoma (MB) cells, through F-actin visualization and angiogenesis tube formation assays, B7-H3-overexpressing MB cells were found to significantly promote the angiogenic ability of co-cultured HUVECs, which can be attenuated by miR-29, and in vivo chick chorioallantoic membrane angiogenesis assays demonstrated similar results [114]. Furthermore, they showed that B7-H3 overexpression upregulated a series of proangiogenic molecules, including IL-6, IL-1, VEGF-D and VEGFR2, and a significant correlation between MMP-9 levels and sB7-H3 levels was identified, suggesting that B7-H3 promotes angiogenesis by upregulating MMP-9 [114]. MMP-2 and B7-H3 have been shown to be correlatively upregulated in several tumor types, and in melanoma, B7-H3 silencing significantly reduced MMP-2 protein expression, as reviewed by Zhou et al. [115], indicating that MMP-2 is involved in B7-H3-mediated angiogenesis. In hepatocellular carcinoma (HCC), B7-H3 knockdown upregulated E-cadherin expression but inhibited AKT phosphorylation, VE-cadherin expression and MMP2/9 activation in HCC cell lines, suggesting a PI3K/AKT/MMP pathway for B7-H3-mediated MMP activation [116]. The expression of B7-H3 in both tumor cells and endothelial cells, as well as their crosstalk, fuels the process of ECM reconstruction and aberrant angiogenesis by inducing cytokine and MMP secretion.
B7-H3 and other TME characteristics
Among all the stromal cells that populate the tumor microenvironment, cancer-associated fibroblasts (CAFs) are the most abundant and function in cell–cell contact, releasing numerous regulatory factors and remodeling the extracellular matrix [117]. Zhang et al. explored the relationship between B7-H3 and CAF function and revealed that B7-H3 knockdown in CAFs significantly inhibited cell proliferation, increased apoptosis, inhibited cell cycle progression and decreased the expression of hepatocyte growth factor protein and stromal cell-derived factor-1 protein, indicating that B7-H3 has a strong anti-apoptotic effect on CAFs [93]. They also found that B7-H3+ CAFs promoted renal cell carcinoma growth and metastasis both in vivo and in vitro, possibly through the AKT signaling pathway. In a gastric adenocarcinoma model, α-SMA and B7-H3 expression was detected in fibroblasts, and a positive correlation between their expression levels was found in stromal cells [118]. B7-H3 knockdown in gastric adenocarcinoma-derived CAFs caused decreased IL-6, CXCL12, FGF1 and VEGF expression and inhibited the migration ability of CAFs [118]. These results revealed that B7-H3 expression in CAFs increases their viability and secretory capacity to in turn promote the growth and metastasis of tumors, suggesting another B7-H3-mediated pro-tumorigenic interaction from B7-H3+ CAFs. It has also been widely demonstrated that B7-H3 modulates cytokine secretion in various TME cells, including T cells, endothelial cells and CAFs [11, 99, 114, 118], and B7-H3 is involved in ECM remodeling through the activation of MMP2/MMP9 [115, 119]. Overall, B7-H3 greatly shapes the TME in different ways. The main associations between B7-H3 and CAFs, tumor cells and other TME cells (i.e., immune cells, stromal cells, pericytes and mesenchymal stromal cells (MSCs)) are depicted in Fig. 4.
Main associations between B7-H3 and CAFs, tumor cells and other TME cells. Activated B7-H3 increases the proliferation, progression and migration of CAFs and inhibits the apoptosis of CAFs. Inhibiting B7-H3 in gastric cancer decreases the expression of IL-6, CXCL12, FGF1 and VEGF and suppresses the migration of CAFs. B7-H3 activation also promotes tumor cell growth and enhances tumor cell metastasis through AKT pathways. B7-H3 modulates cytokine secretion in many types of TME cells, including T cells, endothelial cells and CAFs, and B7-H3 helps to remodel the ECM by activating MMP2/MMP9. TME, tumor microenvironment; CAFs, cancer-associated fibroblasts; MSCs, mesenchymal stromal cells; ECM, extracellular matrix
B7-H3 in different malignancies
Immunohistochemical staining targeting B7-H3 in multiple normal tissues only revealed weak cytoplasmic staining in the salivary gland, gastric epithelium and adrenal gland [120], while B7-H3 has been found to be highly expressed in various cancer cells, as reviewed by Zhou et al. [121]. B7-H3 has been extensively studied in different cancer types, elucidating the expression level of B7-H3, its correlation with prognosis, and the possible underlying mechanism through which B7-H3 influences tumor progression. Here, we review the roles of B7-H3 in specific cancer types, and the main roles of B7-H3 are presented in Fig. 5.
Roles of B7-H3 in several specific cancer types. B7-H3 has various roles in brain tumors, lung cancer, breast cancer, melanoma, liver cancer, gastric cancer, colorectal cancer, cervical cancer and prostate cancer by activating different mechanisms. B7-H3 is negatively associated with the prognosis of glioma and ERG-negative prostate cancer and serves as a promising immunotherapy target in brain tumors, lung cancer and melanoma. The boxes show the available characteristics and function of B7-H3 in cancers. Most characteristics are based on evidence from preclinical models, while specific characteristics highlighted in bold are based on evidence from human (clinical) studies/trials. DIPG, diffuse intrinsic pontine glioma; medulloblastoma, pediatric medulloblastoma; ATRTs, atypical teratoid/rhabdoid tumors; GBM, glioblastoma; CAR-T, chimeric antigen receptor-T cells; NSCLC, non-small cell lung cancer; EMT, epithelial–mesenchymal transformation; SCLC, small-cell lung cancer; MDSCs, myeloid-derived suppressor cells; Tregs, regulatory T cells; TME, tumor microenvironment
B7-H3 in lung cancer
B7-H3 has attracted strong interest in the field of lung cancer. B7-H3 expression was found in 510 out of 634 patients with NSCLC, and a high expression level was demonstrated to have a negative impact on prognosis [122]. On the other hand, in small cell lung cancer (SCLC), B7-H3 showed no significant correlation with clinicopathologic variables or TIL markers, although it was expressed in 64.9% of SCLC cases [123], while the contradictory conclusion that B7-H3 was a negative predictor in SCLC was suggested by Qiu et al. [124]. B7-H3 might promote NSCLC proliferation, EMT and metastasis via the PI3K/AKT pathway, as previously mentioned [56, 69]. It was also reported that downregulated B7-H3 can reduce lipid synthesis via the SREBP-1/FASN signaling pathway in lung cancer [125]. T cells expressing B7-H3-specific chimeric antigen receptors (CARs) and bispecific killer cell engager (BiKE)-redirected NK cells have shown significant antitumor activity in a preclinical model [126], and combined targeting of B7-H3 and PD-1 has resulted in promising response rates in clinical trials [127].
B7-H3 in CRC
B7-H3 expression was detected in 50.8% of the primary CRC samples in a large cohort [128], and elevated B7-H3 expression was related to advanced overall stages, decreased disease-free survival and increased CD45RO T cell infiltration. In addition, Zhang et al. noted that higher B7-H3 expression was related to more lymph node involvement and poor tumor differentiation [129]. In CRC cells, it has been demonstrated that B7-H3 promotes tumor angiogenesis through the NF-κB pathway [113], and Meng et al. discovered that B7-H3 increases the expression of intracellular TNF-α, which modulates the inflammatory response and promotes tumor growth by inducing cell survival [130]. The anti-apoptotic role of B7-H3 exerted in a JAK2/STAT3-dependent manner was also verified in CRC cells [131]. In addition to different underlying mechanisms modulating tumorigenesis, correlations between B7-H3 and resistance to conventional cancer therapy have been extensively explored in CRC. B7-H3 has been shown to enhance chemoresistance to oxaliplatin (L-OHP) or 5-fluorouracil (5-FU) via the B7-H3-STAT3-HK2 axis [64] or in a STAT3-CDC25A-dependent manner [132]. B7-H3 expression was also found to be elevated after irradiation during radiotherapy for CRC, thus promoting cell viability and radioresistance via the B7-H3/KIF15/ERK axis [133]. Taken together, these studies thoroughly investigated the mechanism of B7-H3-mediated tumorigenesis, especially in CRC, and laid a solid foundation for ongoing trials evaluating B7-H3-targeted therapies for CRC.
B7-H3 in breast cancer
Through transcriptome analysis, matched RNA and protein expression comparison and verification in 198 breast cancer samples, Kim et al. found that B7-H3 expression was significantly higher in tumor samples and highly expressed in 73.6% of the cases [134]. Although B7-H3 expression was negatively correlated with T cell infiltration and more frequently present in certain molecular subtypes, including TNBC, no significant relationship between overall survival (OS) and progression-free survival (PFS) was found in the study [134]. In contrast, Fang et al. reported that high B7-H3 expression was correlated with a poor prognosis, with a 56.8% B7-H3+ rate in 74 cases [135], and a B7-H3 association with the extent of regional nodal metastasis was also reported [136]. In a breast cancer cell line, B7-H3 expression was found to reduce the proliferation of CD4+ and CD8+ T cells and inhibit IFN-γ release via mTOR signaling [137], and modulating the TME through macrophages was another possible mechanism, as previously mentioned [106]. B7-H3 also regulates stem cell enrichment and promotes chemoresistance and aberrant glycolysis in breast cancer [58, 76]. In addition to a potential therapeutic target, B7-H3 also serves as a molecular ultrasound imaging target that can be used in image-contrasting microbubbles and has been applied in preclinical mammography [138]. The future applications of B7-H3 in breast cancer are promising and broad.
B7-H3 in prostate cancer
Prostate cancer is the second most common cancer type in men [139]. Nunes et al. revealed a correlation between B7-H3 expression and worse outcome, especially the recurrence rate, in prostate cancer in two different cohorts, and a strong correlation between B7-H3 and androgen receptor (AR) protein expression was also revealed, although the B7-H3 expression rate was only 15% and 38% in the two cohorts [140]. In a large-scale immunohistochemistry analysis, B7-H3 immunostaining was positive in 47.0% of more than 17,000 prostate cancer cases, and B7-H3 appeared to be a negative prognostic factor, especially in the ERG-negative subgroup [141]. B7-H3 might promote prostate cancer progression through the accumulation of MDSCs [142], while a spontaneous prostate cancer model in mice revealed a contradictory costimulatory role of B7-H3 in inhibiting Treg cells [143]. B7-H3-targeting CAR T cell therapy has been assessed in murine prostate cancer stem cells and demonstrated a potent antitumor effect [79]. Both antibody-based and CAR-T therapies targeting B7-H3 in prostate cancer are being evaluated in clinical trials, which will be discussed further.
B7-H3 in melanoma
Accumulating evidence has shown that melanoma responds well to immunotherapy, possibly due to the high immunogenicity of melanoma [144]. B7-H3 expression levels were revealed to be elevated in melanoma specimens, and higher expression was associated with advanced stages [145]. In vitro, B7-H3 enhanced cell migration and invasion via p-STAT3, although no significant effect on proliferation was observed in this study [145]. However, a later study demonstrated that B7-H3 overexpression enhanced proliferation and glycolytic capacity in melanoma cells, with reduced sensitivity toward dacarbazine, a MAPK- and AKT/mTOR-targeting small-molecule inhibitor [74]. Furthermore, Tekle et al. specifically elucidated a nonimmunological role of B7-H3: in melanoma cells, the expression levels of MMP-2, Stat3 and IL-8 were positively correlated with B7-H3 expression, while tissue inhibitor of metalloproteinase (TIMP)-1 and 2 showed the opposite results, indicating a prometastatic role of B7-H3 [146]. CD3+ T cell and B7-H3 bispecific antibodies and B7-H3-targeting CAR-T therapy have shown potent antimelanoma activity when investigated using in vivo and in vitro models [147, 148], validating the great potential of B7-H3 in melanoma immunotherapy. Nevertheless, mechanistic studies and preclinical success have not yet been translated into a clinical benefit, and a poor response rate was observed in patients with melanoma who were treated with anti-B7-H3 and anti-PD-1 antibodies [127]. Clinical evaluations of multiple modalities in a larger cohort are still ongoing.
B7-H3 in gastric cancer
Multiple studies have shown that B7-H3 is widely present in gastric cancer and is associated with pathological features and prognosis. Wu et al. found that 58.8% of 102 gastric cancer tissues were B7-H3 positive and revealed a correlation between higher B7-H3 expression in cancer tissues and better overall survival, decreased tumor infiltration depth and more differentiated histological features, suggesting that B7-H3 is a positive indicator for gastric cancer prognosis [149]. In contrast, in stomach cancer cell lines and xenograft models, it was revealed that B7-H3 knockdown significantly inhibited cancer invasion and metastasis capacity [150]. Mechanistically, B7-H3+ neutrophils have been found in gastric cancer tissues, which increases tumor progression and is a negative predictive marker of reduced survival [94], and a more restricted CD8+ T cell location was noted in B7-H3-high gastric cancer samples, indicating a potential immunosuppressive role of B7-H3 in gastric cancer, although no significant survival difference was observed between the B7-H3-high and B7-H3-low groups in the study [151]. Evidence for the role of B7-H3 in gastric cancer is limited and conflicting, and more studies, particularly in patients with gastric cancer, are warranted.
B7-H3 in liver cancer
The clinical significance of B7-H3 has been investigated in HCC. B7-H3 expression was found in 225 out of 240 HCC patients, and a correlation between high B7-H3 expression and poor survival and increased recurrence was confirmed in two independent cohorts [152]. Validation of the HCC cell line in vitro revealed that B7-H3 expression promoted cell proliferation, invasion and migration and suppressed the proliferation and IFN-γ secretion of infiltrating T cells [152, 153]. It has been demonstrated that B7-H3 promotes EMT and HCC invasion via the JAK2/STAT3/slug pathway [68], and it was discovered that B7-H3 induces M2 polarization of TAMs and contributes to an immunoinhibitory TME in a STAT3-dependent manner [154]. The diagnostic potential of serum sB7-H3 in early-stage hepatocellular carcinoma has also been investigated, and a promising result was found [155].
B7-H3 in cervical cancer
B7-H3 expression was found in 62.8% of 673 cervical carcinomas or adenocarcinomas, and shorter disease-specific survival was found in the B7-H3-expressing group [156]. The increase in the secretion of IL-10 and TGF-β1 via the JAK2-STAT3 pathway activated by B7-H3 has been suggested as an underlying tumor-promoting mechanism [157], and AKT/mTOR might also be involved in tumorigenesis, as it was inhibited in SiHa cervical cancer cells by B7-H3 overexpression [158]. Although the significance of B7-H3 expression and its pro-tumorigenic mechanism has been investigated, implementation of B7-H3 immunotherapy even in preclinical cervical cancer models is not currently available.
B7-H3 in brain tumors
B7-H3 has been reported to be extensively expressed in diffuse intrinsic pontine glioma [159], pediatric medulloblastoma [160], atypical teratoid/rhabdoid tumors (ATRTs) [161], recurrent glioblastoma [162] and gliomas [84]. Based on gene ontology analysis in a public database, B7-H3 was found to be involved in Toll-like receptor signaling and T cell receptor signaling, affect the mitotic cycle, immune response and cell proliferation, and serve as an unfavorable prognostic marker in glioma [84]. In GBM, the two different isoforms of B7-H3 appeared to function differently, in which 4IgB7-H3 expression was restricted in GBM cells and can serve as a target for GBM-targeting therapy, whereas 2IgB7-H3 expression was higher in GBM recurrences and increased resistance to temozolomide [162]. Chimeric antigen receptor (CAR) T cells targeting B7-H3 have shown potent antitumor activity in xenograft murine models of ATRTs, glioma and glioblastoma [161, 163, 164]. B7-H3 provides a promising target therapy in brain tumors and is being intensively evaluated in clinical trials. CAR-T therapy and radionucleotide-based antibody therapy showed preclinical benefits. Issues related to delivery, toxicity and sustainability of B7-H3-targeted therapeutics in the central nervous system remain to be addressed in clinical trials [165]. Table 1 further summarizes the role of B7-H3 in other cancer types not mentioned above [99, 166,167,168,169,170,171,172].
Among different tumor types, B7-H3 is generally discovered to induce an inhibitory TME and malignant traits, and it represents an unfavorable prognostic marker, while controversies remain in a variety of tumor types, including SCLC, gastric cancer, and prostate cancer. Theoretically, cancer types with firm and substantial support from preclinical models, a high B7-H3 expression rate, and a “hot” immune landscape, such as NSCLC and melanomas, seem to have greater potential to respond to B7-H3 immunotherapy [173]. Currently available results from early-phase clinical trials have shown the potential of B7-H3 immunotherapy mainly in treating NSCLC and brain tumors, with efficacy in multiple cancer types still being evaluated. Additional results obtained from clinical trials will validate the preclinical conclusions and broaden our understanding of B7-H3-targeted therapy.
Immunotherapy targeting B7-H3
Although the receptor for B7-H3 has not been identified and the pro-tumorigenic role of B7-H3 has not been fully elucidated, success has been achieved in suppressing tumor growth by targeting B7-H3 as an inhibitory immune checkpoint in preclinical models and has greatly kindled the enthusiasm for clinical translation. Treatments targeting B7-H3 with different modalities are being intensely evaluated in clinical trials. Current immunotherapies strategies targeting B7-H3 are diagrammed in Fig. 6. Ongoing clinical trials targeting B7-H3 are summarized in Table 2, while the currently available clinical outcomes of these approaches are presented in detail in Table 3.
Immunology and future clinical immunotherapy of B7-H3. In T cells, OX40 liganded by OX40 L and the CD4/TCR-MHC II-antigen peptide complex elicit the following signals with a number of well-established proinflammatory mediators, such as PI3K, AKT, NFκB and ERK. Through the PI3K/AKT pathway, many downstream signatures are activated, including NFκB, IL-2 production, mTOR activation and Bcl-xl activation. Then, activated NFκB stimulates the release of cytokines and chemokines. The activation of the TGFβ receptor can inhibit the maturation of miR-21 and enhance PDCD4 levels. The translation of the anti-inflammatory cytokine IL-10 is suppressed in this signal, and the level is downregulated. TGFβ1 can participate in the adhesion, migration and invasion of renal cell carcinoma (RCC) cells. Clinical immunotherapy targeting B7-H3 includes blockade of B7-H3 monoclonal antibodies (mAbs), although the ligand is unclear; B7-H3-specific antibody‒drug conjugates (ADCs); B7-H3-specific antibody-dependent cell-mediated cytotoxicity (ADCC); B7-H3 and CD3 bispecific antibodies; engineered chimeric antigen receptor T cells (CAR-T cells); radionucleotides-induced radioimmunotherapy; other combined therapies (combined with PD-L1, PD-L2, etc.)
Targeting B7-H3 with an ADC
Antibody–drug conjugates (ADCs), which consist of a humanized antibody to target tumors, a potent cytotoxic payload and a linker to connect them, are a novel approach for cancer therapy [174]. MGC018, a developing ADC with a duocarmycin payload, has shown promising antitumor activity in preclinical models of breast, ovarian, prostate, lung cancer, head and neck cancer as well as melanoma, with bystander killing effect to eradicate tumors heterogeneously expressing B7-H3 [175]. MGC018 in six advanced solid tumors is being evaluated in a phase I/II clinical trial (NCT03729596); dose escalation study found a generally acceptable safety profile with two dose-limiting toxicities: one grade 4 neutropenia and one grade 3 fatigue [176]. Eighty patients were enrolled for cohort expansion, 87.7% of the patients encountered at least 1 adverse event, among which neutropenia, fatigue, palmar-plantar erythron dysesthesia and headache were seen > 10% of the patients. While further evaluation is still on the way, prostate-specific antigen (PSA) decline and tumor regression has been observed in prostate cancer patients [177].
DS-7300a is another B7-H3 targeting ADC which contains a DNA topoisomerase I inhibitor payload DXd and exerts potent antitumor activities in preclinical models [178]. The safety and efficacy of DS-7300a are being investigated in NCT04145622, and recently released interim results showed DS-7300a was well tolerated in heavily treated advanced tumor patients, and the objective response was observed in 30 out of 91 evaluable patients [179]. The early success of DS-7300a has greatly motivated the researchers and another trial specifically analyzing DS-7300a's efficacy in SCLC has been launched recently (NCT05280470).
Targeting B7-H3 via ADCC
Antibody-dependent cellular cytotoxicity (ADCC) relies on the interaction between the Fc portion of an antibody and immune cells to eradicate targets [180]. MGA271 (enoblituzumab), which is a humanized IgG1 B7-H3 targeting antibody developed by Loo et al., contains a five amino acid change at its humanized Fc site for increased activation affinity and showed potent antitumor activity in renal cell carcinoma and bladder cancer xenograft models [181]. Thus, MGA271 is being extensively evaluated in clinical trials (NCT02982941, NCT02923180, NCT02381314, NCT04630769, NCT04634825 and NCT02475213). Interim data from NCT01391143 showed an acceptable safety profile of MGA271 in patients with B7-H3+ tumors, where patients experienced disease stabilization or tumor shrinkage across several tumor types [182]. In a phase II single-arm trial evaluating the neoadjuvant use of MGA271 (NCT02923180), 32 patients with prostate cancer were enrolled and received neoadjuvant MGA271 50 days prior to prostatectomy. Twelve percent of the enrolled patients experienced grade 3/4 adverse events, post-treatment PSA declines (> 10%) were observed in 31% of the patients and PSA0 at 1-year post-op was seen in 66% of the patients. Gleason grade group changes were significantly associated with MGA271 treatment compared to matched historical controls. Pathologic and immunologic evaluation of the prostate revealed upregulation of CD8+ T cells, PD-1/PD-L1 expression, and immune activation. In general terms, MGA271 showed an acceptable safety profile, promising immune-stimulating activity and crosstalk with other immune checkpoints [183]. Results from a phase I/II trial analyzing MGA271 in combination with PD-1-targeted therapy in patients with advanced solid tumors have been published (NCT02475213). One hundred and sixteen of 133 patients experienced treatment-related adverse events and 38 patients were ≥ grade 3. The efficacy of the combination therapy was limited, with an objective response observed in 6 of 18 patients with HNSCC and in 5 of 14 patients with NSCLC who did not receive previous ICI treatment. Only 1 of 17 patients with urothelial cancer and 1 of 13 patients with melanoma showed an objective response, and patients with previous ICI treatment had a poorer prognosis [127]. Unfortunately, a phase II trial analyzing the combination of MGA271 with anti-PD-1 antibody or PD-1xLAG3 bispecific antibody in head and neck cancer (NCT04634825) has just been closed due to seven observed fatalities associated with hemorrhagic events. A more comprehensive evaluation of MGA271 is ongoing in multiple trials, and the further outcome is worth expecting.
Although DS-5573a showed potent ADCC activity in the breast adenocarcinoma xenograft model [184], the only clinical trial evaluating it has been terminated without any released results due to business decisions (NCT02192567). Omburtamab (8H9) is a murine IgG1 monoclonal antibody which was identified to bind 4IgB7-H3 [185]. A humanized version of omburtamab was found to bind to the FG loop of B7-H3 and exhibit potent ADCC activity in neuroblastoma cells when co-cultured with peripheral blood mononuclear cells [186]. Further clinical evaluation of omburtamab’s ADCC activity is not available now, but omburtamab has been the most widely used carrier for radioimmunoconjugates which will be discussed later.
Targeting B7-H3 with a bispecific antibody
Bispecific antibodies can recognize two different antigens simultaneously to induce synergistic and emergent antitumor activity via various mechanisms including targeting the receptors or engaging immune cells [187]. The structure of bispecific antibodies can be summarized as two or more antibody fragments held together by a linker with or without Fc domains for the fragments to attach to [188]. Multiple forms of B7-H3 targeting bispecific antibodies have been developed preclinically, including CD3/B7-H3 bispecific T cell engagers [189], CD16/B7-H3 bispecific killer cell engager [126], PD-1/B7-H3 bispecific antibody [190] and 4-1BB/B7-H3 bispecific antibody [191]. All these four kinds of agents showed antitumor capacities against tumor cell lines in vitro, while CD3/B7-H3, CD16/B7-H3 and 4-1BB/B7-H3 suppressed tumors in murine xenograft models. A B7-H3 Tri-Specific antibody containing an anti-CD16 fragment, an IL-15 moiety and an anti-B7-H3 scFv has also been developed by Valerra et al. and showed antitumor effects against various tumors in vitro and in a xenograft model [192]. MGD009 is a bispecific T cell engager which simultaneously targets CD3 on T cells and B7-H3. It is currently the only B7-H3 targeting bispecific antibody under clinical evaluation, investigating its synergistic effect with anti-PD-1 therapy in NCT03406949 with no results released yet.
Targeting B7-H3 via CAR-T therapy
Chimeric antigen receptor T cell (CAR-T) therapy utilizes T cells that have been redirected against the tumor antigen after the engineered expression of CARs to eradicate tumors [193]. CAR-T therapy targeting B7-H3 has shown great potential in a series of studies in preclinical models of multiple cancer types [161, 163, 168, 194, 195], accompanied by a boom in clinical trials confirming the efficacy of B7-H3-targeting CAR-T therapy. Case reports of patients with recurrent anaplastic meningioma, glioblastoma and relapsed basal cell carcinoma who were treated with B7-H3-targeted CAR-T cells have revealed good tolerance and reduced tumor growth [196,197,198]. A phase I, open-label clinical trial evaluating B7-H3-specific CAR-T cells has just released its early result in ASCO meeting [199]. Sixteen patients with relapsed or refractory non-CNS tumors have been enrolled in two groups and received 0.5 × 106 CAR-T/kg or 1 × 106 CAR-T cells/kg. No dose-limiting toxicity was observed in the first infusion, and maximum circulating CAR-T expansion on the first infusion was 4.98 cells/uL with median persistence of 28 days. Stable disease was observed in 3 of the 9 infused subjects. One subject experienced dramatical CAR-T expansion and transient grade 4 liver enzyme elevation after the second infusion and partial metabolomic response on FDG-PET was observed 28 days later. The biological effective dose has been determined as 1 × 106 CAR-T cells/kg in this trial; still the comparison between two arms needs further enrollment.
Targeting B7-H3 via radioimmunotherapy
Radioimmunotherapy labels tumor-targeting antibodies with radionucleotides and inhibits tumors through radiation-induced cytotoxicity [200]. Omburtamab is the most frequently used carrier in radioimmunoconjugates as aforementioned. Radioactive iodine labeled Omburtamab has been developed and evaluated in a human rhabdomyosarcoma xenograft model in 2005 [201], where 131I-Omburtamab showed specific binding with tumor cell lines and antitumor effects in rhabdomyosarcoma xenograft. Currently, 131I-Omburtamab and 124I-Omburtamab are the only radioimmunotherapy agents with available clinical evaluation results. In a phase I trial evaluating intrathecal administration of 131I-Omburtamab in recurrent metastatic CNS neuroblastoma (NCT00089245), Kramer et al. found that among 80 patients receiving 131I-Omburtamab in combination with conventional therapy, 45 (56%) patients remained alive when data were last updated, 45% of patients survive more than 36 months and 29% more than 60 months. The survival data are promising as historical median overall survival time of neuroblastoma in the same institution is 6.6 months. The adverse events in the trial appeared to be manageable, self-limited fever, nausea, headache and transient serum transaminase elevation were observed [202, 203]. Retrospective analysis of the same cohort revealed that intraventricular administration of 131I-Omburtamab did not increase the risk of radionecrosis, further confirming the safety of the intervention [204]. In the same institution, a retrospective review of 23 recurrent rhabdomyosarcoma patients also showed prolonged survival of patients receiving intraventricular 131I-Omburtamab [205]. The implementation of 131I-Omburtamab has also been evaluated in peritoneal tumors (NCT01099644), where intraperitoneal administration of 131I-Omburtamab was well tolerated [206]. 124I-Omburtamab is another developed radioimmunotherapy agent whose safety and effect in diffuse intrinsic pontine gliomas (DIPG) has been evaluated in phase I clinical trial (NCT01502917). Among the 46 DIPG patients enrolled and treated, 10 patients experienced grade 3 adverse effects which were mainly nervous system disorder. The median overall survival across the cohort was 14.8 months, about 3–4 months longer than historical control data from other trials [207, 208]. Other B7-H3-targeting radioimmunotherapy agents, including 212Pb-376.96 [209] and 131I-4H7 [210], have also demonstrated promising potential in preclinical models but clinical evaluation is lacking. Further evaluation of 131I-Omburtamab and 177Lu-DTPA-omburtamab is ongoing, with results yet to be released. Currently, radioimmunotherapy agents targeting B7-H3 were mainly analyzed in CNS and peritoneal tumors, possibly because compartmental administration to reduce systematic exposure is feasible in these tumor types. The management of radio-toxicity remains a great hurdle to overcome when trying to adopt B7-H3 targeting radioimmunotherapy in other solid tumors.
Although all of these approaches are supported by preclinical models, preliminary results of clinical safety and efficacy are available only for ADCC-based MGA271, B7-H3 CAR-T and some radionucleotide-bound antibodies. The number of trials evaluating B7-H3-targeted therapy has increased in recent years, and evidence is accumulating until a comprehensive comparison between different approaches can be made. Substantially supported by promising preclinical results from various cancer models, CAR-T-based B7-H3-targeted therapy is currently the most extensively investigated approach, with 17 phase I/II trials confirming its safety and efficacy. The results from these trials, which might provide novel alternatives and clinical benefits for patients with cancer, are expected.
B7-H3 in tumor imaging
In addition to serving as a prognostic marker and immunotherapy target, as mentioned above, B7-H3 has also shown clinical application potential in tumor imaging. B7-H3 has been validated as a molecular ultrasound imaging target in breast cancer. In mammography, molecular imaging with ultrasound contrast agents can provide accurate and sensitive imaging signals noninvasively, where microbubbles functionalized with B7-H3-targeted affibody [138] or B7-H3-targeted antibody [211] demonstrated great potential as molecular-targeting contrast agents. In hB7-H3-expressing tumors, microbubbles conjugated to the B7-H3-targeted affibody (MBABY-B7-H3) produced higher imaging signals than nontargeted microbubbles, while in normal mammary tissues and B7-H3-blocking tumors, MBABY-B7-H3 revealed a significantly reduced signal [138], validating the diagnostic value of B7-H3 in breast cancer imaging. With a similar approach, B7-H3-targeted ultrasound imaging was found to be capable of distinguishing metastatic sentinel lymph nodes from nonmetastatic sentinel lymph nodes in a murine breast cancer model [212], which further confirms the potential to evaluate the tumor burden of B7-H3 and indicates imaging value beyond breast cancer of B7-H3. Spectroscopic photoacoustic imaging is another targeted approach that provides sensitive imaging signals based on thermoelastic expansion after laser absorption and subsequent ultrasonic wave emission [213]. By conjugating B7-H3-targeted antibody or affibody to indocyanine green (ICG), a photoacoustic and fluorescence agent, researchers are now able to detect breast cancer [214], evaluate tumor grade [215] and even guide intraoperative resection [216]. Zirconium-89 (89Zr)-labeled anti-B7-H3 monoclonal antibody DS-5573a is another validated B7-H3 targeting imaging approach, where PET/MRI evaluation demonstrated promising in vivo biodistribution and stability of 89Zr-DS-5573a and revealed specific and prolonged targeting of B7-H3-positive tumors [217], further demonstrating the potential of B7-H3 in either target imaging or target immunotherapy, although mainly in the field of breast cancer, B7-H3 has shown its potential in tumor imaging.
Conclusions
The nature of the B7-H3 receptor remains unknown, which hinders the comprehensive understanding of the role of B7-H3 in the TME and the development of B7-H3-based immunotherapy, warranting further efforts to elucidate the biological characteristics. Nevertheless, the multifaceted role of B7-H3 in the TME has been extensively explored, and B7-H3 has been found to induce malignant behaviors and promote tumor progression through complicated pathways. The role of B7-H3 has been evaluated in tumor cells, T cells, DCs, NK cells, CAFs, neutrophils and endothelial cells in the TME, indicating that B7-H3 is a vital modulator in the TME and a valuable immunotherapy target. Extensive expression of B7-H3 has been reported in a variety of cancer types, and correlation with poor prognosis is also widely established, with the notion that the expression of B7-H3 is heterogenous and that B7-H3 in B7-H3 low-expressing or metastatic cancer needs additional investigation. As dozens of preclinical studies and early-stage trials are ongoing, B7-H3 applications in breast ultrasound imaging and as a serum marker for diagnosis and prognosis prediction have also been identified. Therefore, targeting B7-H3 might provide a novel and promising option for cancer therapy.
Availability of data and materials
All data and materials used are available from the corresponding author upon reasonable request.
Abbreviations
- 5-FU:
-
5-Fluorouracil
- 89Zr-:
-
Zirconium-89
- ADC:
-
Antibody‒drug conjugates
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- PKB, Akt:
-
Protein kinase B
- ALDH:
-
Aldehyde dehydrogenase
- AML:
-
Acute myeloid leukemia
- AP-1:
-
Activating protein 1
- AR:
-
Androgen receptor
- ATP:
-
Adenosine triphosphate
- ATRT:
-
Atypical teratoid/rhabdoid tumor
- Bax:
-
BCL2-associated X
- DTIC:
-
Dacarbazine
- Bcl-2:
-
B cell lymphoma-2
- Bcl-xl:
-
B cell lymphoma-xl
- BiKE:
-
Bispecific killer cell engager
- Bmi1:
-
B lymphoma Mo-MLV insertion region 1
- CAF:
-
Cancer-associated fibroblast
- CAR:
-
Chimeric antigen receptor
- CCK-8:
-
Cell counting kit-8
- CCL2:
-
CC-chemokine ligand 2
- CCR2:
-
CC-chemokine receptor 2
- CCRCC:
-
Clear cell renal cell carcinoma
- CDC25A:
-
Cell division cycle 25A
- CDK4:
-
Cyclin-dependent kinase 4
- cDNA:
-
Complementary DNA
- CRC:
-
Colorectal cancer
- CSCs:
-
Cancer stem cells
- CTLA-4:
-
Cytotoxic T lymphocyte antigen 4
- CXCL12:
-
CXC motif chemokine 12
- DIPG:
-
Diffuse intrinsic pontine glioma
- PSA:
-
Prostate-specific antigen
- DC:
-
Dendritic cells
- DOX:
-
Doxorubicin
- ECM:
-
Extracellular matrix
- EMT:
-
Epithelial–mesenchymal transition
- ENO1:
-
Enolase 1
- ERG:
-
E-twenty-six related gene
- ERK:
-
Extracellular regulated protein kinases
- FASN:
-
Fatty acid synthase
- FGF1:
-
Fibroblast growth factor 1
- FOXP3:
-
Forkhead transcription factor protein 3
- GBM:
-
Glioblastoma
- GlUT1:
-
Glucose transporter 1
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- GZMB:
-
Granzyme B
- HCC:
-
Hepatocellular carcinoma
- HHLA2:
-
HERV-H LTR-associated protein 2
- HIF-1α:
-
Hypoxia-inducible factor-1
- HK2:
-
Hexokinase 2
- HNSCC:
-
Head and neck squamous cell carcinoma
- HUVEC:
-
Human umbilical vein endothelial cell
- ICG:
-
Indocyanine green
- ICI:
-
Immune checkpoint inhibitor
- IL:
-
Interleukin
- IFN:
-
Interferon
- IL20RA:
-
Interleukin-20 receptor subunit α
- IL20RB:
-
Interleukin-20 receptor subunit β
- JAK2:
-
Janus kinase 2
- KIF15:
-
Kinesin family member 15
- LDHA:
-
Lactate dehydrogenase A
- LEPC:
-
Late endothelial progenitor cell
- LncRNA:
-
Long noncoding RNA
- L-OHP:
-
Oxaliplatin
- m6A:
-
N6-methylation of adenosine
- MAPK:
-
Mitogen‐activated protein kinase
- MB:
-
Medulloblastoma
- MCC:
-
Merkel cell carcinoma
- MDSC:
-
Myeloid-derived suppressor cell
- MEK:
-
Mitogen-activated protein kinase
- MHC:
-
Major histocompatibility complex
- MMP:
-
Matrix metalloproteinase
- mTOR:
-
Mammalian target of rapamycin
- MVP:
-
Major vault protein
- Neat1:
-
Nuclear-enriched abundant transcript 1
- NFAT:
-
Nuclear factor of activated T cells
- NF-κB:
-
Nuclear factor-kappa B
- NK:
-
Natural killer
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed cell death ligand 1
- PD-L2:
-
Programmed cell death ligand 2
- PFKFB3:
-
6-Phosphofructo -2-kinase/fructose-2,6-bisphosphatase 3
- PGK1:
-
Phosphoglycerate kinase 1
- PLA2R1:
-
Phospholipase A2 receptor 1
- PI3K:
-
Phosphatidylinositol 3 kinase
- PRX3:
-
Peroxiredoxin 3
- sB7-H3:
-
Soluble B7-H3
- SIRT1:
-
Sirtuin 1
- SOD1:
-
Superoxide dismutase 1
- SOD2:
-
Superoxide dismutase 2
- SREBP-1:
-
Sterol response element binding proteins-1
- SSCs:
-
Spermatogonial stem cells
- STAT3:
-
Signal transducer and activator of transcription 3
- TAM:
-
Tumor-associated macrophages
- TCGA:
-
The Cancer Genome Atlas
- TILs:
-
Tumor-infiltrating lymphocytes
- TIMP:
-
Tissue inhibitor of metalloproteinase 1
- TLT-2, TREML2:
-
Triggering receptor expressed on myeloid cells-like transcript 2
- TM4SM1:
-
Transmembrane 4 superfamily member 1
- TME:
-
Tumor microenvironment
- TNBC:
-
Triple-negative breast cancer
- TNF-α:
-
Tumor necrosis factor-alpha
- Treg cells:
-
Regulatory T cells
- VEGF:
-
Vascular endothelial growth factor
- VEGF-A:
-
Vascular endothelial growth factor A
- VEGF-D:
-
Vascular endothelial growth factor D
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
- α-SMA:
-
Alpha-smooth muscle actin
References
Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet (London, England). 2021;398(10304):1002–14.
Kubli SP, Berger T, Araujo DV, Siu LL, Mak TW. Beyond immune checkpoint blockade: emerging immunological strategies. Nat Rev Drug Discov. 2021;20(12):899–919.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–26.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, Oyervides Juarez VM, Hsieh JJ, Basso U, Shah AY, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–41.
PD-1/PD-L1 landscape. https://www.cancerresearch.org/scientists/immunooncology-landscape/pd-1-pd-l1-landscape#landscape. Accessed 31 May 2022.
Andrews LP, Yano H, Vignali DAA. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat Immunol. 2019;20(11):1425–34.
Feng R, Chen Y, Liu Y, Zhou Q, Zhang W. The role of B7-H3 in tumors and its potential in clinical application. Int Immunopharmacol. 2021;101(Pt B):108153.
Liu S, Liang J, Liu Z, Zhang C, Wang Y, Watson AH, Zhou C, Zhang F, Wu K, Zhang F, et al. The role of CD276 in cancers. Front Oncol. 2021;11(2234-943X):654684.
Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-γ production. Nat Immunol. 2001;2(3):269–74.
Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, Zlabinger G, Pickl WF, Stockl J, Knapp W. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol (Baltimore, MD: 1950). 2004;172(4):2352–9.
Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C. Characterization of mouse and human B7-H3 genes. J Immunol (Baltimore, MD: 1950). 2002;168(12):6294–7.
Zhou YH, Chen YJ, Ma ZY, Xu L, Wang Q, Zhang GB, Xie F, Ge Y, Wang XF, Zhang XG. 4IgB7-H3 is the major isoform expressed on immunocytes as well as malignant cells. Tissue Antigens. 2007;70(2):96–104.
Huang Y, Zhang HL, Li ZL, Du T, Chen YH, Wang Y, Ni HH, Zhang KM, Mai J, Hu BX, et al. FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nat Commun. 2021;12(1):2672.
Vigdorovich V, Ramagopal Udupi A, Lázár-Molnár E, Sylvestre E, Lee Jun S, Hofmeyer Kimberly A, Zang X, Nathenson Stanley G, Almo SC. Structure and T cell inhibition properties of B7 family member, B7-H3. Structure. 2013;21(5):707–17.
Zhang GB, Hou JQ, Shi JF, Yu GH, Lu BF, Zhang XG. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123(4):538–46.
Chen W, Liu P, Wang Y, Nie W, Li Z, Xu W, Li F, Zhou Z, Zhao M, Liu H. Characterization of a soluble B7-H3 (sB7-H3) spliced from the intron and analysis of sB7-H3 in the sera of patients with hepatocellular carcinoma. PLoS ONE. 2013;8(10):e76965.
Huang L, Zhou Y, Sun Q, Cao L, Zhang X. Evaluation of the role of soluble B7-H3 in association with membrane B7-H3 expression in gastric adenocarcinoma. Cancer Biomark. 2022;33(1):123–9.
Kovaleva OV, Belova TP, Korotkova EA, Kushlinskii DN, Gratchev AN, Petrikova NA, Kudlay DA, Kushlinskii NE. Soluble B7-H3 in ovarian cancer and its predictive value. Bull Exp Biol Med. 2021;171(4):472–4.
King RG, Herrin BR, Justement LB. Trem-like transcript 2 is expressed on cells of the myeloid/granuloid and B lymphoid lineage and is up-regulated in response to inflammation. J Immunol. 2006;176(10):6012–21.
Wang SY, Fu XX, Duan R, Wei B, Cao HM, Yan E, Chen SY, Zhang YD, Jiang T. The Alzheimer’s disease-associated gene TREML2 modulates inflammation by regulating microglia polarization and NLRP3 inflammasome activation. Neural Regen Res. 2023;18(2):434–8.
Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci. 2008;105(30):10495–500.
Kobori H, Hashiguchi M, Piao JH, Kato M, Ritprajak P, Azuma M. Enhancement of effector CD8+T-cell function by tumour-associated B7-H3 and modulation of its counter-receptor triggering receptor expressed on myeloid cell-like transcript 2 at tumour sites. Immunology. 2010;130(3):363–73.
Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7(12):1266–73.
Halpert MM, Thomas KA, King RG, Justement LB. TLT2 potentiates neutrophil antibacterial activity and chemotaxis in response to G protein-coupled receptor-mediated signaling. J Immunol (Baltimore, MD: 1950). 2011;187(5):2346–55.
Thomas KA, King RG, Sestero CM, Justement LB. TREM-like transcript 2 is stored in human neutrophil primary granules and is up-regulated in response to inflammatory mediators. J Leukocyte Biol. 2016;100(1):177–84.
Zheng H, Liu CC, Atagi Y, Chen XF, Jia L, Yang L, He W, Zhang X, Kang SS, Rosenberry TL, et al. Opposing roles of the triggering receptor expressed on myeloid cells 2 and triggering receptor expressed on myeloid cells-like transcript 2 in microglia activation. Neurobiol Aging. 2016;42(1558–1497):132–41.
Xu JC, Gao F, Liu YA, Zhang XL, Chen H, Zhu XY, Song HF, Qian F, Li M, Yang C, et al. Myeloid cell-like transcript 2 is related to liver inflammation and the pathogenesis of hepatitis B via the involvement of CD8(+)T cell activation. Clin Exp Med. 2019;19(1):93–104.
Li J, Cao C, Xiang Y, Hong Z, He D, Zhong H, Liu Y, Wu Y, Zheng X, Yin H, et al. TLT2 suppresses Th1 response by promoting IL-6 production in monocyte through JAK/STAT3 signal pathway in tuberculosis. Front Immunol. 2020;11(1664–3224):2031.
Fang C, Rinke AE, Wang J, Flaherty KR, Phan SH, Liu T. B7H3 expression and significance in idiopathic pulmonary fibrosis. J Pathol. 2022;256(3):310–20.
Leitner J, Klauser C, Pickl WF, Stockl J, Majdic O, Bardet AF, Kreil DP, Dong C, Yamazaki T, Zlabinger G, et al. B7-H3 is a potent inhibitor of human T-cell activation: no evidence for B7-H3 and TREML2 interaction. Eur J Immunol. 2009;39(7):1754–64.
Yan R, Yang S, Gu A, Zhan F, He C, Qin C, Zhang X, Feng P. Murine B7-H3 is a co-stimulatory molecule for T cell activation. Monoclon Antib Immunodiagn Immunother. 2013;32(2167–9436):395–8.
Husain B, Ramani SR, Chiang E, Lehoux I, Paduchuri S, Arena TA, Patel A, Wilson B, Chan P, Franke Y, et al. A platform for extracellular interactome discovery identifies novel functional binding partners for the immune receptors B7-H3/CD276 and PVR/CD155. Mol Cell Proteom. 2019;18(11):2310–23.
Cao S, Peterson SM, Muller S, Reichelt M, McRoberts Amador C, Martinez-Martin N. A membrane protein display platform for receptor interactome discovery. Proc Natl Acad Sci. 2021;118(39):e2025451118.
Blumberg H, Conklin D, Xu WF, Grossmann A, Brender T, Carollo S, Eagan M, Foster D, Haldeman BA, Hammond A, et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104(1):9–19.
Logsdon NJ, Deshpande A, Harris BD, Rajashankar KR, Walter MR. Structural basis for receptor sharing and activation by interleukin-20 receptor-2 (IL-20R2) binding cytokines. Proc Natl Acad Sci. 2012;109(31):12704–9.
Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol (Baltimore, MD: 1950). 2002;168(11):5397–402.
Rutz S, Wang X, Ouyang I. The IL-20 subfamily of cytokines—from host defence to tissue homeostasis. Nat Rev Immunol. 2014;14(12):783–95.
Liu R, Yin H, Sun X, Liu S, Wang A, Wu Y, Yuan Y, Gong Y, Xing C. Interleukin 20 receptor A expression in colorectal cancer and its clinical significance. PeerJ. 2021;9(2167–8359):e12467.
Gao W, Wen H, Liang L, Dong X, Du R, Zhou W, Zhang X, Zhang C, Xiang R, Li N. IL20RA signaling enhances stemness and promotes the formation of an immunosuppressive microenvironment in breast cancer. Theranostics. 2021;11(6):2564–80.
Ungaro F, Garlatti V, Massimino L, Spinelli A, Carvello M, Sacchi M, Spano S, Colasante G, Valassina N, Vetrano S, et al. mTOR-dependent stimulation of IL20RA orchestrates immune cell trafficking through lymphatic endothelium in patients with Crohn’s disease. Cells. 2019;8(8):924.
Bernard D, Vindrieux D. PLA2R1. Expression and function in cancer. Biochim Biophys Acta. 2014;1846(1):40–4.
Sukocheva O, Menschikowski M, Hagelgans A, Yarla NS, Siegert G, Reddanna P, Bishayee A. Current insights into functions of phospholipase A2 receptor in normal and cancer cells: more questions than answers. Semin Cancer Biol. 2019;56:116–27.
Augert A, Payre C, de Launoit Y, Gil J, Lambeau G, Bernard D. The M-type receptor PLA2R regulates senescence through the p53 pathway. Embo Rep. 2009;10(3):271–7.
Huna A, Griveau A, Vindrieux D, Jaber S, Flaman JM, Goehrig D, Azzi L, Medard JJ, Djebali S, Hernandez-Vargas H, et al. PLA2R1 promotes DNA damage and inhibits spontaneous tumor formation during aging. Cell Death Dis. 2021;12(2):190.
Griveau A, Devailly G, Eberst L, Navaratnam N, Le Calve B, Ferrand M, Faull P, Augert A, Dante R, Vanacker JM, et al. The PLA2R1-JAK2 pathway upregulates ERRalpha and its mitochondrial program to exert tumor-suppressive action. Oncogene. 2016;35(38):5033–42.
Vindrieux D, Augert A, Girard CA, Gitenay D, Lallet-Daher H, Wiel C, Le Calve B, Gras B, Ferrand M, Verbeke S, et al. PLA2R1 mediates tumor suppression by activating JAK2. Cancer Res. 2013;73(20):6334–45.
Fonteh AN, Marion CR, Barham BJ, Edens MB, Atsumi G, Samet JM, High KP, Chilton FH. Enhancement of mast cell survival. A novel function of some secretory phospholipase A(2) isotypes. J Immunol. 2001;167(8):4161–71.
Gorovetz M, Schwob O, Krimsky M, Yedgar S, Reich R. MMP production in human fibrosarcoma cells and their invasiveness are regulated by group IB secretory phospholipase A2 receptor-mediated activation of cytosolic phospholipase A2. Front Biosci. 2008;13(1093–9946):1917–25.
Verschueren E, Husain B, Yuen K, Sun Y, Paduchuri S, Senbabaoglu Y, Lehoux I, Arena TA, Wilson B, Lianoglou S, et al. The immunoglobulin superfamily receptome defines cancer-relevant networks associated with clinical outcome. Cell. 2020;182(2):329-44 e19.
Li Y, Lv C, Yu Y, Wu B, Zhang Y, Lang Q, Liang Z, Zhong C, Shi Y, Han S et al. KIR3DL3-HHLA2 and TMIGD2-HHLA2 pathways: the dual role of HHLA2 in immune responses and its potential therapeutic approach for cancer immunotherapy. J Adv Res. 2022;S2090-1232(22)00167-9.
Suski JM, Braun M, Strmiska V, Sicinski P. Targeting cell-cycle machinery in cancer. Cancer Cell. 2021;39(6):759–78.
Wang SX, Mou JG, Cui LS, Wang XG, Zhang ZQ. Astragaloside IV inhibits cell proliferation of colorectal cancer cell lines through down-regulation of B7-H3. Biomed Pharmacother. 2018;102(1950–6007):1037–44.
Hu X, Xu M, Hu Y, Li N, Zhou L. B7-H3, negatively regulated by miR-128, promotes colorectal cancer cell proliferation and migration. Cell Biochem Biophys. 2021;79(2):397–405.
Yu TT, Zhang T, Lu X, Wang RZ. B7-H3 promotes metastasis, proliferation, and epithelial-mesenchymal transition in lung adenocarcinoma. Onco Targets Ther. 2018;11(1178–6930):4693–700.
Wei X, Li K, Zhang G, Huang Y, Lv J, Li M, Zhao L, Fan C, Pu J, Hou J, et al. B7-H3 promoted proliferation of mouse spermatogonial stem cells via the PI3K signaling pathway. Oncotarget. 2018;9(2):1542–52.
Liu ZX, Zhang WL, Phillips JB, Arora R, McClellan S, Li JF, Kim JH, Sobol RW, Tan M. Immunoregulatory protein B7-H3 regulates cancer stem cell enrichment and drug resistance through MVP-mediated MEK activation. Oncogene. 2019;38(1):88–102.
Park JH, Pyun WY, Park HW. Cancer metabolism: phenotype, signaling and therapeutic targets. Cells. 2020;9(10):2308.
Lim S, Liu H, Madeira da Silva L, Arora R, Liu Z, Phillips JB, Schmitt DC, Vu T, McClellan S, Lin Y, et al. Immunoregulatory protein B7-H3 reprograms glucose metabolism in cancer cells by ROS-mediated stabilization of HIF1alpha. Cancer Res. 2016;76(8):2231–342.
Deng M, Wu D, Zhang Y, Jin Z, Miao J. MiR-29c downregulates tumor-expressed B7-H3 to mediate the antitumor NK-cell functions in ovarian cancer. Gynecol Oncol. 2021;162(1):190–9.
Zuo J, Wang B, Long M, Gao Z, Zhang Z, Wang H, Wang X, Li R, Dong K, Zhang H. The type 1 transmembrane glycoprotein B7-H3 interacts with the glycolytic enzyme ENO1 to promote malignancy and glycolysis in HeLa cells. FEBS Lett. 2018;592(14):2476–88.
Li Z, Liu J, Que L, Tang X. The immunoregulatory protein B7-H3 promotes aerobic glycolysis in oral squamous carcinoma via PI3K/Akt/mTOR pathway. J Cancer. 2019;10(23):5770–84.
Shi T, Ma Y, Cao L, Zhan S, Xu Y, Fu F, Liu C, Zhang G, Wang Z, Wang R, et al. B7-H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis. 2019;10(4):308.
Li Y, Yang X, Wu Y, Zhao K, Ye Z, Zhu J, Xu X, Zhao X, Xing C. B7-H3 promotes gastric cancer cell migration and invasion. Oncotarget. 2017;8(42):71725–35.
Fan TF, Deng WW, Bu LL, Wu TF, Zhang WF, Sun ZJ. B7-H3 regulates migration and invasion in salivary gland adenoid cystic carcinoma via the JAK2/STAT3 signaling pathway. Am J Transl Res. 2017;9(3):1369–80.
Zhong CH, Tao B, Chen YT, Guo ZC, Yang XB, Peng LL, Xia XG, Chen LG. B7-H3 regulates glioma growth and cell invasion through a JAK2/STAT3/Slug-dependent signaling pathway. Oncotargets Ther. 2020;13(1178–6930):2215–24.
Kang FB, Wang L, Jia HC, Li D, Li HJ, Zhang YG, Sun DX. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15(1475–2867):45.
Liao H, Ding M, Zhou N, Yang Y, Chen L. B7-H3 promotes the epithelial-mesenchymal transition of NSCLC by targeting SIRT1 through the PI3K/AKT pathway. Mol Med Rep. 2022;25(1791–3004):79.
Xie JJ, Sun MY, Zhang DZ, Chen CY, Lin SM, Zhang GB. Fibronectin enhances tumor metastasis through B7-H3 in clear cell renal cell carcinoma. FEBS Open Bio. 2021;11(11):2977–87.
Zhang JJ, Liu L, Han S, Li YH, Qian QH, Zhang QQ, Zhang H, Yang ZY, Zhang YZ. B7-H3 is related to tumor progression in ovarian cancer. Oncol Rep. 2017;38(4):2426–34.
Han S, Shi X, Liu L, Zong L, Zhang J, Chen Q, Qian Q, Chen L, Wang Y, Jin J, et al. Roles of B7-H3 in cervical cancer and its prognostic value. J Cancer. 2018;9(15):2612–24.
Flem-Karlsen K, Tekle C, Oyjord T, Florenes VA, Maelandsmo GM, Fodstad O, Nunes-Xavier CE. p38 MAPK activation through B7-H3-mediated DUSP10 repression promotes chemoresistance. Sci Rep UK. 2019;9(2045–2322):5839.
Flem-Karlsen K, Tekle C, Andersson Y, Flatmark K, Fodstad O, Nunes-Xavier CE. Immunoregulatory protein B7-H3 promotes growth and decreases sensitivity to therapy in metastatic melanoma cells. Pigment Cell Melanoma Res. 2017;30(5):467–76.
Zhao X, Zhang GB, Gan WJ, Xiong F, Li Z, Zhao H, Zhu DM, Zhang B, Zhang XG, Li DC. Silencing of B7-H3 increases gemcitabine sensitivity by promoting apoptosis in pancreatic carcinoma. Oncol Lett. 2013;5(3):805–12.
Nunes-Xavier CE, Karlsen KF, Tekle C, Pedersen C, Oyjord T, Hongisto V, Nesland JM, Tan M, Sahlberg KK, Fodstad O. Decreased expression of B7-H3 reduces the glycolytic capacity and sensitizes breast cancer cells to AKT/mTOR inhibitors. Oncotarget. 2016;7(6):6891–901.
Li YC, Yang XD, Yao PG, Shen WQ, Wu Y, Ye ZY, Zhao K, Chen HQ, Cao JP, Xing CG. B7-H3 increases the radioresistance of gastric cancer cells through regulating baseline levels of cell autophagy. Am J Transl Res. 2019;11(7):4438.
Prager BC, Xie Q, Bao S, Rich JN. Cancer stem cells: the architects of the tumor ecosystem. Cell Stem Cell. 2019;24(1):41–53.
Zhang Y, He L, Sadagopan A, Ma T, Dotti G, Wang Y, Zheng H, Gao X, Wang D, DeLeo AB, et al. Targeting radiation-resistant prostate cancer stem cells by B7-H3 CAR T cells. Mol Cancer Ther. 2021;20(3):577–88.
Wang C, Li Y, Jia L, Kim JK, Li J, Deng P, Zhang W, Krebsbach PH, Wang CY. CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance. Cell Stem Cell. 2021;28(9):1597-1613 e7.
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46.
Kanayama T, Miyachi M, Sugimoto Y, Yagyu S, Kikuchi K, Tsuchiya K, Iehara T, Hosoi H. Reduced B7-H3 expression by PAX3-FOXO1 knockdown inhibits cellular motility and promotes myogenic differentiation in alveolar rhabdomyosarcoma. Sci Rep. 2021;11(1):18802.
Wang RQ, Sun LQ, Xia SH, Wu HY, Ma YC, Zhan SH, Zhang GB, Zhang XG, Shi TG, Chen WC. B7-H3 suppresses doxorubicin-induced senescence-like growth arrest in colorectal cancer through the AKT/TM4SF1/SIRT1 pathway. Cell Death Dis. 2021;12(5):453.
Wang Z, Wang Z, Zhang C, Liu X, Li G, Liu S, Sun L, Liang J, Hu H, Liu Y, et al. Genetic and clinical characterization of B7-H3 (CD276) expression and epigenetic regulation in diffuse brain glioma. Cancer Sci. 2018;109(9):2697–705.
Zhou Y, Zhou H, Shi J, Guan A, Zhu Y, Hou Z, Li R. Decreased m6A modification of CD34/CD276(B7-H3) leads to immune escape in colon cancer. Front Cell Dev Biol. 2021;9(2296-634X):715674.
Bejarano L, Jordao MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021;11(4):933–59.
Lupu CM, Eisenbach C, Kuefner MA, Schmidt J, Lupu AD, Stremmel W, Encke J. An orthotopic colon cancer model for studying the B7-H3 antitumor effect in vivo. J Gastrointest Surg. 2006;10(5):635–45.
Wang L, Fraser CC, Kikly K, Wells AD, Han R, Coyle AJ, Chen L, Hancock WW. B7-H3 promotes acute and chronic allograft rejection. Eur J Immunol. 2005;35(2):428–38.
Luo L, Zhu G, Xu H, Yao S, Zhou G, Zhu Y, Tamada K, Huang L, Flies AD, Broadwater M, et al. B7-H3 promotes pathogenesis of autoimmune disease and inflammation by regulating the activity of different T cell subsets. PLoS ONE. 2015;10(6):e0130126.
Fukushima A, Sumi T, Fukuda K, Kumagai N, Nishida T, Yamazaki T, Akiba H, Okumura K, Yagita H, Ueno H. B7-H3 regulates the development of experimental allergic conjunctivitis in mice. Immunol Lett. 2007;113(1):52–7.
Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4(9):899–906.
Chen Y, Guan SY, Deng J, Yang H, Xu W, Xu S, Shao M, Gao X, Xu S, Shuai Z, et al. B7-H3: a promising therapeutic target for autoimmune diseases. Cell Immunol. 2020;352(1090–2163):104077.
Zhang S, Zhou C, Zhang D, Huang Z, Zhang G. The anti-apoptotic effect on cancer-associated fibroblasts of B7-H3 molecule enhancing the cell invasion and metastasis in renal cancer. Onco Targets Ther. 2019;12(1178–6930):4119–27.
Li Z-Y, Wang J-T, Chen G, Shan Z-G, Wang T-T, Shen Y, Chen J, Yan Z-B, Peng L-S, Mao F-Y, et al. Expression, regulation and clinical significance of B7-H3 on neutrophils in human gastric cancer. Clin Immunol. 2021;227:108753.
Lai H, Sun Z, Yang J, Wu P, Guo Y, Sun J. B7-H3 modulates endothelial cell angiogenesis through the VEGF cytokine. Immunol Res. 2019;67(2):202–11.
Yonesaka K, Haratani K, Takamura S, Sakai H, Kato R, Takegawa N, Takahama T, Tanaka K, Hayashi H, Takeda M, et al. B7-H3 negatively modulates CTL-mediated cancer immunity. Clin Cancer Res. 2018;24(11):2653–64.
Prasad DVR, Nguyen T, Li ZX, Yang Y, Duong J, Wang Y, Dong C. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173(4):2500–6.
Quintana A, Peg V, Prat A, Moline T, Villacampa G, Pare L, Galvan P, Dientsmann R, Schmid P, Curigliano G, et al. Immune analysis of lymph nodes in relation to the presence or absence of tumor infiltrating lymphocytes in triple-negative breast cancer. Eur J Cancer. 2021;148(1879–0852):134–45.
Cai D, Li J, Liu D, Hong S, Qiao Q, Sun Q, Li P, Lyu N, Sun T, Xie S, et al. Tumor-expressed B7-H3 mediates the inhibition of antitumor T-cell functions in ovarian cancer insensitive to PD-1 blockade therapy. Cell Mol Immunol. 2020;17(3):227–36.
Raffin C, Vo LT, Bluestone JA. T-reg cell-based therapies: challenges and perspectives. Nat Rev Immunol. 2020;20(3):158–72.
Mahnke K, Ring S, Johnson TS, Schallenberg S, Schonfeld K, Storn V, Bedke T, Enk AH. Induction of immunosuppressive functions of dendritic cells in vivo by CD4+CD25+ regulatory T cells: role of B7-H3 expression and antigen presentation. Eur J Immunol. 2007;37(8):2117–26.
Jin YJ, Zhang P, Li J, Zhao JQ, Liu CY, Yang F, Yang D, Gao AQ, Lin WL, Ma XX, et al. B7-H3 in combination with regulatory T cell is associated with tumor progression in primary human non-small cell lung cancer. Int J Clin Exp Patho. 2015;8(11):13987–95.
Zhou Q, Li K, Lai Y, Yao K, Wang Q, Zhan X, Peng S, Cai W, Yao W, Zang X, et al. B7 score and T cell infiltration stratify immune status in prostate cancer. J Immunother Cancer. 2021;9(2051–1426):e002455.
Maeda N, Yoshimura K, Yamamoto S, Kuramasu A, Inoue M, Suzuki N, Watanabe Y, Maeda Y, Kamei R, Tsunedomi R, et al. Expression of B7-H3, a potential factor of tumor immune evasion in combination with the number of regulatory T cells, affects against recurrence-free survival in breast cancer patients. Ann Surg Oncol. 2014;21 Suppl 4(1534–4681):S546–54.
Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res. 2021;81(5):1201–8.
Cheng N, Bei YC, Song Y, Zhang WJ, Xu LZ, Zhang WL, Yang NF, Bai XX, Shu YX, Shen PP. B7-H3 augments the pro-angiogenic function of tumor-associated macrophages and acts as a novel adjuvant target for triple-negative breast cancer therapy. Biochem Pharmacol. 2021;183(1873–2968):114298.
Miyamoto T, Murakami R, Hamanishi J, Tanigaki K, Hosoe Y, Mise N, Takamatsu S, Mise Y, Ukita M, Taki M, et al. B7-H3 suppresses antitumor immunity via the CCL2-CCR2-M2 macrophage axis and contributes to ovarian cancer progression. Cancer Immunol Res. 2022;10(1):56–69.
Gao Y, Fang P, Li W-J, Zhang J, Wang G-P, Jiang D-F, Chen F-P. LncRNA NEAT1 sponges miR-214 to regulate M2 macrophage polarization by regulation of B7-H3 in multiple myeloma. Mol Immunol. 2020;117:20–8.
Schneider T, Hoffmann H, Dienemann H, Schnabel PA, Enk AH, Ring S, Mahnke K. Non-small cell lung cancer induces an immunosuppressive phenotype of dendritic cells in tumor microenvironment by upregulating B7-H3. J Thorac Oncol. 2011;6(7):1162–8.
Al-Ostoot FH, Salah S, Khamees HA, Khanum SA. Tumor angiogenesis: current challenges and therapeutic opportunities. Cancer Treat Res Commun. 2021;28(2468–2942):100422.
Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, Croix BS. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11(6):539–54.
Son Y, Kwon SM, Cho JY. CD276 (B7-H3) maintains proliferation and regulates differentiation in angiogenic function in late endothelial progenitor cells. Stem Cells. 2019;37(3):382–94.
Wang R, Ma Y, Zhan S, Zhang G, Cao L, Zhang X, Shi TA-O, Chen W. B7-H3 promotes colorectal cancer angiogenesis through activating the NF-κB pathway to induce VEGFA expression. Cell Death Dis. 2020;11(2041–4889):55.
Purvis IJ, Avilala J, Guda MR, Venkataraman S, Vibhakar R, Tsung AJ, Velpula KK, Asuthkar S. Role of MYC-miR-29-B7-H3 in medulloblastoma growth and angiogenesis. J Clin Med. 2019;8(2077+0383):1158.
Zhou X, Ouyang S, Li J, Huang X, Ai X, Zeng Y, Lv Y, Cai M. The novel non-immunological role and underlying mechanisms of B7-H3 in tumorigenesis. J Cell Physiol. 2019;234(12):21785–95.
Cheng R, Wang B, Cai XR, Chen ZS, Du Q, Zhou LY, Ye JM, Chen YL. CD276 promotes vasculogenic mimicry formation in hepatocellular carcinoma via the PI3K/AKT/MMPs pathway. Onco Targets Ther. 2020;13(1178–6930):11485–98.
Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18(2):99–115.
Zhan S, Liu Z, Zhang M, Guo T, Quan Q, Huang L, Guo L, Cao L, Zhang X. Overexpression of B7-H3 in α-SMA-positive fibroblasts is associated with cancer progression and survival in gastric adenocarcinomas. Front Oncol. 2020;9(2234-943X):1466.
Xu LH, Ding XM, Tan H, Qian JJ. Correlation between B7-H3 expression and matrix metalloproteinases 2 expression in pancreatic cancer. Cancer Cell Int. 2013;13(1475–2867):81.
Kontos F, Michelakos T, Kurokawa T, Sadagopan A, Schwab JH, Ferrone CR, Ferrone S. B7-H3: an attractive target for antibody-based immunotherapy. Clin Cancer Res. 2021;27(5):1227–35.
Zhou WT, Jin WL. B7-H3/CD276: an emerging cancer immunotherapy. Front Immunol. 2021;12(1664–3224):701006.
Altan M, Pelekanou V, Schalper KA, Toki M, Gaule P, Syrigos K, Herbst RS, Rimm DL. B7-H3 expression in NSCLC and its association with B7-H4, PD-L1 and tumor-infiltrating lymphocytes. Clin Cancer Res. 2017;23(17):5202–9.
Carvajal-Hausdorf D, Altan M, Velcheti V, Gettinger SN, Herbst RS, Rimm DL, Schalper KA. Expression and clinical significance of PD-L1, B7-H3, B7-H4 and TILs in human small cell lung cancer (SCLC). J Immunother Cancer. 2019;7(1):65.
Qiu MJ, Xia Q, Chen YB, Fang XF, Li QT, Zhu LS, Jiang X, Xiong ZF, Yang SL. The expression of three negative co-stimulatory B7 family molecules in small cell lung cancer and their effect on prognosis. Front Oncol. 2021;11:600238.
Luo D, Xiao HW, Dong JL, Li Y, Feng GX, Cui M, Fan SJ. B7-H3 regulates lipid metabolism of lung cancer through SREBP1-mediated expression of FASN. Biochem Biophys Res Commun. 2017;482(4):1246–51.
Liu J, Yang S, Cao B, Zhou G, Zhang F, Wang Y, Wang R, Zhu L, Meng Y, Hu C, et al. Targeting B7-H3 via chimeric antigen receptor T cells and bispecific killer cell engagers augments antitumor response of cytotoxic lymphocytes. J Hematol Oncol. 2021;14(1):21.
Aggarwal C, Prawira A, Antonia S, Rahma O, Tolcher A, Cohen RB, Lou Y, Hauke R, Vogelzang N, D PZ, et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial. J Immunother Cancer. 2022;10(4):e004424.
Lu Z, Zhao ZX, Cheng P, Huang F, Guan X, Zhang MG, Chen HP, Liu Z, Jiang Z, Zheng ZX, et al. B7-H3 immune checkpoint expression is a poor prognostic factor in colorectal carcinoma. Mod Pathol. 2020;33(11):2330–40.
Zhang W, Acuna-Villaorduna A, Kuan K, Gupta S, Hu S, Ohaegbulam K, Albanese J, Kaumaya M, Levy R, Hwang RR, et al. B7-H3 and PD-L1 expression are prognostic biomarkers in a multi-racial cohort of patients with colorectal cancer. Clin Colorectal Cancer. 2021;20(2):161–9.
Meng F, Yang M, Chen Y, Chen W, Wang W. miR-34a induces immunosuppression in colorectal carcinoma through modulating a SIRT1/NF-κB/B7-H3/TNF-α axis. Cancer Immunol Immunother. 2021;70(8):2247–59.
Zhang T, Jiang B, Zou ST, Liu F, Hua D. Overexpression of B7-H3 augments anti-apoptosis of colorectal cancer cells by Jak2-STAT3. World J Gastroenterol. 2015;21(6):1804–13.
Ma Y, Wang R, Lu H, Li X, Zhang G, Fu F, Cao L, Zhan S, Wang Z, Deng Z, et al. B7-H3 promotes the cell cycle-mediated chemoresistance of colorectal cancer cells by regulating CDC25A. J Cancer. 2020;11(1837–9664):2158–70.
Ma Y, Zhan S, Lu H, Wang R, Xu Y, Zhang G, Cao L, Shi T, Zhang X, Chen W. B7-H3 regulates KIF15-activated ERK1/2 pathway and contributes to radioresistance in colorectal cancer. Cell Death Dis. 2020;11(10):824.
Kim NI, Park MH, Kweon SS, Lee JS. B7-H3 and B7-H4 expression in breast cancer and their association with clinicopathological variables and T cell infiltration. Pathobiology. 2020;87(3):179–92.
Cong F, Yu H, Gao X. Expression of CD24 and B7-H3 in breast cancer and the clinical significance. Oncol Lett. 2017;14(6):7185–90.
Arigami T, Narita N, Mizuno R, Nguyen L, Ye X, Chung A, Giuliano AE, Hoon DS. B7-H3 ligand expression by primary breast cancer and associated with regional nodal metastasis. Ann Surg. 2010;252(6):1044–51.
Shao L, Yu Q, Xia R, Zhang J, Gu S, Yu D, Zhuang Z. B7-H3 on breast cancer cell MCF7 inhibits IFN-γ release from tumour-infiltrating T cells. Pathol Res Pract. 2021;224(1618–0631):153461.
Bam R, Lown PS, Stern LA, Sharma K, Wilson KE, Bean GR, Lutz AM, Paulmurugan R, Hackel BJ, Dahl J, et al. Efficacy of affibody-based ultrasound molecular imaging of vascular B7-H3 for breast cancer detection. Clin Cancer Res. 2020;26(9):2140–50.
Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10(2):63–89.
Nunes-Xavier CE, Kildal W, Kleppe A, Danielsen HE, Waehre H, Llarena R, Maelandsmo GM, Fodstad O, Pulido R, Lopez JI. Immune checkpoint B7-H3 protein expression is associated with poor outcome and androgen receptor status in prostate cancer. Prostate. 2021;81(12):838–48.
Bonk S, Tasdelen P, Kluth M, Hube-Magg C, Makrypidi-Fraune G, Moller K, Hoflmayer D, Rico SD, Buscheck F, Minner S, et al. High B7-H3 expression is linked to increased risk of prostate cancer progression. Pathol Int. 2020;70(10):733–42.
Zhou Y, Zhang G, Zhang W, Wei X, Hou J, Huang Y. B7-H3 promotes prostate cancer progression in mice by antagonizing myeloid-derived suppressor cell apoptosis. Technol Cancer Res Treat. 2020;19(1533–0338):1533033820971649.
Kreymborg K, Haak S, Murali R, Wei J, Waitz R, Gasteiger G, Savage PA, van den Brink MR, Allison JP. Ablation of B7-H3 but not B7-H4 results in highly increased tumor burden in a murine model of spontaneous prostate cancer. Cancer Immunol Res. 2015;3(2326–6074):849–54.
Ralli M, Botticelli A, Visconti IC, Angeletti D, Fiore M, Marchetti P, Lambiase A, de Vincentiis M, Greco A. Immunotherapy in the treatment of metastatic melanoma: current knowledge and future directions. J Immunol Res. 2020;2020(2314–7156):9235638.
Wang J, Chong KK, Nakamura Y, Nguyen L, Huang SK, Kuo C, Zhang W, Yu H, Morton DL, Hoon DS. B7-H3 associated with tumor progression and epigenetic regulatory activity in cutaneous melanoma. J Investig Dermatol. 2013;133(8):2050–8.
Tekle C, Nygren MK, Chen YW, Dybsjord I, Nesland JM, Maelandsmo GM, Fodstad O. B7-H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis-associated genes. Int J Cancer. 2012;130(10):2282–90.
Ma J, Shang T, Ma P, Sun X, Zhao J, Sun X, Zhang M. Bispecific anti-CD3 x anti-B7-H3 antibody mediates T cell cytotoxic ability to human melanoma in vitro and in vivo. Mol Ther Oncolytics. 2020;37(1573–0646):1036–43.
Zhang Z, Jiang C, Liu Z, Yang M, Tang X, Wang Y, Zheng M, Huang J, Zhong K, Zhao S, et al. B7-H3-targeted CAR-T cells exhibit potent antitumor effects on hematologic and solid tumors. Mol Ther Oncolytics. 2020;17(2372–7705):180–9.
Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, Zhao JM, Zhang GB, Zhang XG. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol. 2006;12(3):457–9.
Dai W, Shen G, Qiu J, Zhao X, Gao Q. Aberrant expression of B7-H3 in gastric adenocarcinoma promotes cancer cell metastasis. Oncol Rep. 2014;32(5):2086–92.
Ulase D, Behrens HM, Krüger SA-O, Zeissig S, Röcken CA-O. Gastric carcinomas with stromal B7-H3 expression have lower intratumoural CD8+ T cell density. Int J Mol Sci. 2021;22(1422–0067):2129.
Sun TW, Gao Q, Qiu SJ, Zhou J, Wang XY, Yi Y, Shi JY, Xu YF, Shi YH, Song K, et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol Immunother. 2012;61(11):2171–82.
Wang FF, Wang GY, Liu TS, Yu GH, Zhang GB, Luan XY. B7-H3 was highly expressed in human primary hepatocellular carcinoma and promoted tumor progression. Cancer Investig. 2014;32(6):262–71.
Kang FB, Wang L, Li D, Zhang YG, Sun DX. Hepatocellular carcinomas promote tumor-associated macrophage M2-polarization via increased B7-H3 expression. Oncol Rep. 2015;33(1):274–82.
Zhao L, Xie C, Liu D, Li T, Zhang Y, Wan C. Early detection of hepatocellular carcinoma in patients with hepatocirrhosis by soluble B7-H3. J Gastrointest Surg. 2017;21(5):807–12.
Zong LA-O, Gu YA-O, Zhou Y, Kong Y, Mo S, Yu SA-O, Xiang YA-O, Chen JA-O. Expression of B7 family checkpoint proteins in cervical cancer. Mod Pathol. 2021;35(1530–0285):786–93.
Han S, Wang Y, Shi X, Zong L, Liu L, Zhang J, Qian Q, Jin J, Ma Y, Cui B, et al. Negative roles of B7-H3 and B7-H4 in the microenvironment of cervical cancer. Exp Cell Res. 2018;371(1):222–30.
Yang X, Feng KX, Li H, Wang L, Xia H. MicroRNA-199a inhibits cell proliferation, migration, and invasion and activates AKT/mTOR signaling pathway by targeting B7-H3 in cervical cancer. Technol Cancer Res Treat. 2020;19(1533–0338):1533033820942245.
Zhou ZP, Luther N, Ibrahim GM, Hawkins C, Vibhakar R, Handler MH, Souweidane MM. B7-H3, a potential therapeutic target, is expressed in diffuse intrinsic pontine glioma. J Neuro-Oncol. 2013;111(3):257–64.
Li S, Poolen GC, van Vliet LC, Schipper JG, Broekhuizen R, Monnikhof M, Van Hecke W, Vermeulen JF, Bovenschen NA-O. Pediatric medulloblastoma express immune checkpoint B7-H3. Clin Transl Oncol. 2022;24(1699–3055):1204–8.
Theruvath J, Sotillo E, Mount CW, Graef CM, Delaidelli A, Heitzeneder S, Labanieh L, Dhingra S, Leruste A, Majzner RG, et al. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat Med. 2020;26(5):712–9.
Digregorio M, Coppieters N, Lombard A, Lumapat PN, Scholtes F, Rogister B. The expression of B7-H3 isoforms in newly diagnosed glioblastoma and recurrence and their functional role. Acta Neuropathol Commun. 2021;9(1):59.
Haydar DA-O, Houke H, Chiang J, Yi Z, Odé Z, Caldwell K, Zhu X, Mercer KS, Stripay JL, Shaw TI, et al. Cell-surface antigen profiling of pediatric brain tumors: B7-H3 is consistently expressed and can be targeted via local or systemic CAR T-cell delivery. Neuro Oncol. 2021;23(1523–5866):999–1011.
Nehama D, Di Ianni N, Musio S, Du H, Patane M, Pollo B, Finocchiaro G, Park JJH, Dunn DE, Edwards DS, et al. B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. EBioMedicine. 2019;47(2352–3964):33–43.
Bourdeaut F. Are B7-H3 CAR-T cells the future universal treatment for pediatric brain tumors? Neuro Oncol. 2021;23(6):872–3.
Chen L, Chen J, Xu B, Wang Q, Zhou W, Zhang G, Sun J, Shi L, Pei H, Wu C, et al. B7-H3 expression associates with tumor invasion and patient’s poor survival in human esophageal cancer. Am J Transl Res. 2015;7(12):2646–60.
Aung PP, Parra ER, Barua S, Sui D, Ning J, Mino B, Ledesma DA, Curry JL, Nagarajan P, Torres-Cabala CA, et al. B7-H3 expression in merkel cell carcinoma-associated endothelial cells correlates with locally aggressive primary tumor features and increased vascular density. Clin Cancer Res. 2019;25(11):3455–67.
Lichtman EI, Du H, Shou P, Song F, Suzuki K, Ahn S, Li G, Ferrone S, Su L, Savoldo B, et al. Preclinical evaluation of B7-H3-specific chimeric antigen receptor T cells for the treatment of acute myeloid leukemia. Clin Cancer Res. 2021;27(11):3141–53.
Si S, Wang L, Cao H, Xu Y, Zhan Q. Co-deficiency of B7-H3 and B7-H4 identifies high CD8 + T cell infiltration and better prognosis in pancreatic cancer. BMC Cancer. 2022;22(1):211.
Koyama Y, Morikawa T, Miyama Y, Miyakawa J, Kawai T, Kume H, Sawabe M, Ushiku T. B7-H3 expression in upper tract urothelial carcinoma associates with adverse clinicopathological features and poor survival. Pathol Res Pract. 2020;216(12):153219.
Li YX, Cai Q, Shen XM, Chen XT, Guan Z. Overexpression of B7-H3 is associated with poor prognosis in laryngeal cancer. Front Oncol. 2021;11(2234-943X):759528.
Long C, Li GW, Zhang CY, Jiang T, Li YJ, Duan X, Zhong G. B7-H3 as a target for CAR-T cell therapy in skull base chordoma. Front Oncol. 2021;11(2234-943X):659662.
Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197–218.
Hafeez U, Parakh SAO, Gan HK, Scott AM. Antibody-drug conjugates for cancer therapy. Molecules. 2020;25(1420–3049):4764.
Scribner JA, Brown JG, Son T, Chiechi M, Li P, Sharma S, Li H, De Costa A, Li Y, Chen Y, et al. Preclinical development of MGC018, a duocarmycin-based antibody-drug conjugate targeting B7-H3 for solid cancer. Mol Cancer Ther. 2020;19(11):2235–44.
Jang S, Powderly JD, Spira AI, Bakkacha O, Loo D, Bohac GC, Sharma M. Phase 1 dose escalation study of MGC018, an anti-B7-H3 antibody-drug conjugate (ADC), in patients with advanced solid tumors. J Clin Oncol. 2021; 39(15_suppl):2631-.
Shenderov E, Mallesara GHG, Wysocki PJ, Xu W, Ramlau R, Weickhardt AJ, Zolnierek J, Spira A, Joshua AM, Powderly J, et al. MGC018, an anti-B7-H3 antibody-drug conjugate (ADC), in patients with advanced solid tumors: preliminary results of phase I cohort expansion. Ann Oncol. 2021;32(suppl_5):S657–S246.
Yamato M, Hasegawa J, Maejima TA-O, Hattori C, Kumagai KA-OX, Watanabe A, Nishiya Y, Shibutani T, Aida T, Hayakawa I, et al. DS-7300a, a DNA topoisomerase I inhibitor, DXd-based antibody-drug conjugate targeting B7-H3 exerts potent antitumor activities in preclinical models. Mol Cancer Ther. 2022;21(1538–8514):635–46.
Doi T, Patel M, Falchook GS, Koyama T, Friedman CF, Piha-Paul S, Gutierrez M, Abdul-Karim R, Awad M, Adkins DR, Takahashi S, Kadowaki S, Cheng B, Ikeda N, Laadem A, Yoshizuka N, Qian M, Dosunmu O, Arkenau H, Johnson ML. DS-7300 (B7-H3 DXd antibody-drug conjugate [ADC]) shows durable antitumor activity in advanced solid tumors: extended follow-up of a phase I/II study. Ann Oncol. 2022;33(suppl_7):S197–224.
Kohrt HE, Houot R, Marabelle A, Cho HJ, Osman K, Goldstein M, Levy R, Brody J. Combination strategies to enhance antitumor ADCC. Immunotheraphy. 2012;4(5):511–27.
Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, Burke S, Ciccarone V, Li H, Yang Y, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res. 2012;18(14):3834–45.
Powderly J, Cote G, Flaherty K, Szmulewitz RZ, Ribas A, Weber J, Loo D, Baughman J, Chen F, Moore P, et al. Interim results of an ongoing Phase I, dose escalation study of MGA271 (Fc-optimized humanized anti-B7-H3 monoclonal antibody) in patients with refractory B7-H3-expressing neoplasms or neoplasms whose vasculature expresses B7-H3. J Immunother Cancer. 2015;3(Suppl 2):O8.
Shenderov E, De Marzo AM, Lotan TL, Wang H, Lim SJ, Allaf ME, Moore PA, Chen F, Sorg K, White AM, et al. Targeting B7-H3 in prostate cancer: phase 2 trial in localized prostate cancer using the anti-B7-H3 antibody enoblituzumab, with biomarker correlatives. J Clin Oncol. 2022;40(16_suppl):5015.
Nagase-Zembutsu A, Hirotani K, Yamato M, Yamaguchi J, Takata T, Yoshida M, Fukuchi K, Yazawa M, Takahashi S, Agatsuma T. Development of DS-5573a: a novel afucosylated mAb directed at B7-H3 with potent antitumor activity. Cancer Sci. 2016;107(5):674–81.
Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69(15):6275–81.
Ahmed M, Cheng M, Zhao Q, Goldgur Y, Cheal SM, Guo H-F, Larson SM, Cheung N-KV. Humanized affinity-matured monoclonal antibody 8H9 has potent antitumor activity and binds to FG loop of tumor antigen B7-H3*. J Biol Chem. 2015;290(50):30018–29.
Liu C, Zhang G, Xiang K, Kim Y, Lavoie RR, Lucien F, Wen TA-OX. Targeting the immune checkpoint B7-H3 for next-generation cancer immunotherapy. Cancer Immunol Immunother. 2022;71(7):1549–67.
Shim H. Bispecific antibodies and antibody–drug conjugates for cancer therapy: technological considerations. Biomolecules. 2020;10(3):360.
Feng Y, Xie K, Yin Y, Li B, Pi C, Xu X, Huang T, Zhang J, Wang B, Gu H, et al. A novel anti-B7-H3 x anti-CD3 bispecific antibody with potent antitumor activity. Life (Basel). 2022;12(2):157.
Xu Y, Xiao Y, Luo C, Liu Q, Wei A, Yang Y, Zhao L, Wang Y. Blocking PD-1/PD-L1 by an ADCC enhanced anti-B7-H3/PD-1 fusion protein engages immune activation and cytotoxicity. Int Immunopharmacol. 2020;84(1878–1705):106584.
You G, Lee Y, Kang YW, Park HW, Park K, Kim H, Kim YM, Kim S, Kim JH, Moon D et al. B7-H3x4-1BB bispecific antibody augments antitumor immunity by enhancing terminally differentiated CD8(+) tumor-infiltrating lymphocytes. Sci Adv. 2021;7(3):eaax3160.
Vallera DA, Ferrone S, Kodal B, Hinderlie P, Bendzick L, Ettestad B, Hallstrom C, Zorko NA, Rao A, Fujioka N, et al. NK-cell-mediated targeting of various solid tumors using a B7-H3 tri-specific killer engager in vitro and in vivo. Cancers. 2020;12(9):2659.
Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. “Off-the-shelf” allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19(3):185–99.
Majzner RG, Theruvath JL, Nellan A, Heitzeneder S, Cui Y, Mount CW, Rietberg SP, Linde MH, Xu P, Rota C, et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. 2019;25(8):2560–74.
Du H, Hirabayashi K, Ahn S, Kren NP, Montgomery SA, Wang X, Tiruthani K, Mirlekar B, Michaud D, Greene K, et al. Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell. 2019;35(2):221-237 e8.
Tang X, Liu F, Liu Z, Cao Y, Zhang Z, Wang Y, Huang J, Fan S, Zhao S, Chen Y, et al. Bioactivity and safety of B7-H3-targeted chimeric antigen receptor T cells against anaplastic meningioma. Clin Transl Immunol. 2020;9(6):e1137.
Tang X, Wang Y, Huang J, Zhang Z, Liu F, Xu J, Guo G, Wang W, Tong A, Zhou L. Administration of B7-H3 targeted chimeric antigen receptor-T cells induce regression of glioblastoma. Signal Transduct Target Ther. 2021;6(1):125.
Hu G, Liang Y, Li G, Ding W, Luo M. B7H3 CAR-T therapy in relation to tumor growth in skin tumor. J Clin Oncol. 2022;40(16_suppl):e21502-e.
Pinto NR, Albert CM, Taylor M, Wilson A, Rawlings-Rhea S, Huang W, Seidel K, Narayanaswany P, Wu V, Brown C, et al. STRIVE-02: a first-in-human phase 1 trial of systemic B7H3 CAR T cells for children and young adults with relapsed/refractory solid tumors. J Clin Oncol. 2022;40(16_suppl):10011.
Leaman Alcibar O, Candini D, Lopez-Campos F, Albert Antequera M, Morillo Macias V, Conde AJ, Rodriguez Perez A, Hervas Moron A, Contreras Martinez J, Ferrer Albiach C, et al. Time for radioimmunotherapy: an overview to bring improvements in clinical practice. Clin Transl Oncol. 2019;21(8):992–1004.
Modak S, Guo HF, Humm JL, Smith-Jones PM, Larson SM, Cheung NKV. Radioimmunotargeting of human rhabdomyosarcoma using monoclonal antibody 8H9. Cancer Biother Radiopharm. 2005;20(5):534–46.
Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, Humm JL, Xu H, Wolden SL, Souweidane MM, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neuro-Oncol. 2010;97(3):409–18.
Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Tomlinson U, Wolden SL, Zanzonico P, John HL, Haque S, Souweidane MM, et al. A curative approach to central nervous system metastases of neuroblastoma. J Clin Oncol. 2017;35(15_suppl):10545.
Kramer K, Pandit-Taskar N, Zanzonico P, Wolden SL, Humm JL, DeSelm C, Souweidane MM, Lewis JS, Cheung N-KV. Low incidence of radionecrosis in children treated with conventional radiation therapy and intrathecal radioimmunotherapy. J Neuro-Oncol. 2015;123(2):245–9.
De B, Kinnaman MD, Wexler LH, Kramer K, Wolden SL. Central nervous system relapse of rhabdomyosarcoma. Pediatr Blood Cancer. 2018;65(1):e26710.
Modak S, Zanzonico P, Grkovski M, Slotkin EK, Carrasquillo JA, Lyashchenko SK, Lewis JS, Cheung IY, Heaton T, LaQuaglia MP, et al. B7H3-directed intraperitoneal radioimmunotherapy with radioiodinated omburtamab for desmoplastic small round cell tumor and other peritoneal tumors: results of a phase I study. J Clin Oncol. 2020;38(36):4283–91.
Souweidane MM, Kramer K, Pandit-Taskar N, Haque S, Zanzonico P, Carrasquillo JA, Lyashchenko SK, Thakur SB, Khakoo Y, Donzelli M, et al. Phase 1 dose-escalation trial using convection-enhanced delivery of radiolabeled monoclonal antibody for diffuse intrinsic pontine glioma following external radiation therapy. J Clin Oncol. 2021;39(15_suppl):2010.
Souweidane MM, Kramer K, Pandit-Taskar N, Zhou Z, Haque S, Zanzonico P, Carrasquillo JA, Lyashchenko SK, Thakur SB, Donzelli M, et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018;19(8):1040–50.
Kasten BB, Gangrade A, Kim H, Fan J, Ferrone S, Ferrone CR, Zinn KR, Buchsbaum DJ. (212)Pb-labeled B7-H3-targeting antibody for pancreatic cancer therapy in mouse models. Nucl Med Biol. 2018;58(1872–9614):67.
Wang G, Wu Z, Wang Y, Li X, Zhang G, Hou J. Therapy to target renal cell carcinoma using 131I-labeled B7-H3 monoclonal antibody. Oncotarget. 2016;7(1949–2553):24888–98.
Bachawal SV, Jensen KC, Wilson KE, Tian L, Lutz AM, Willmann JK. Breast cancer detection by B7-H3-targeted ultrasound molecular imaging. Cancer Res. 2015;75(12):2501–9.
Zheng F, Li P, Bachawal SV, Wang H, Li C, Yuan W, Huang B, Paulmurugan R. Assessment of metastatic and reactive sentinel lymph nodes with B7-H3-targeted ultrasound molecular imaging: a longitudinal study in mouse models. Mol Imaging Biol. 2020;22(4):1003–11.
Wilson KE, Bachawal SV, Abou-Elkacem L, Jensen K, Machtaler S, Tian L, Willmann JK. Spectroscopic photoacoustic molecular imaging of breast cancer using a B7-H3-targeted ICG contrast agent. Theranostics. 2017;7(6):1463–76.
Bam R, Laffey M, Nottberg K, Lown PS, Hackel BJ, Wilson KE. Affibody-indocyanine green based contrast agent for photoacoustic and fluorescence molecular imaging of B7-H3 expression in breast cancer. Bioconjug Chem. 2019;30(6):1677–89.
Bachawal S, Bean GR, Krings G, Wilson KE. Evaluation of ductal carcinoma in situ grade via triple-modal molecular imaging of B7-H3 expression. NPJ Breast Cancer. 2020;6(2374–4677):14.
Wilson KE, Bachawal SV, Willmann JK. Intraoperative resection guidance with photoacoustic and fluorescence molecular imaging using an anti-B7-H3 antibody-indocyanine green dual contrast agent. Clin Cancer Res. 2018;24(15):3572–82.
Burvenich IJG, Parakh S, Lee FT, Guo N, Liu Z, Gan HK, Rigopoulos A, O’Keefe GJ, Gong SJ, Goh YW, et al. Molecular imaging of T cell co-regulator factor B7-H3 with (89)Zr-DS-5573a. Theranostics. 2018;8(15):4199–209.
Acknowledgements
The authors thank the findings revealed by the primary authors on B7-H3 immunotherapy and immunology. The authors acknowledge support from the Beijing Municipal Natural Science Foundation.
Funding
This work is supported by the National Natural Science Foundation of China (82151302), Beijing Municipal Natural Science Foundation (7202150, 19JCZDJC64200(Z)), and the Tsinghua University-Peking Union Medical College Hospital Initiative Scientific Research Program (2019ZLH101).
Author information
Authors and Affiliations
Contributions
All authors designed and conducted this review. ZB and LH conceived the review. LH and ZB wrote the manuscript. XY, WY, WY and SY designed the figures and tables. XH, QT, WY and MW revised the manuscript. All the authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors give consent for the publication of the manuscript.
Competing interests
All of the authors declare that they have no competing interests or conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, B., Li, H., Xia, Y. et al. Immune checkpoint of B7-H3 in cancer: from immunology to clinical immunotherapy. J Hematol Oncol 15, 153 (2022). https://doi.org/10.1186/s13045-022-01364-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-022-01364-7