Abstract
Metabolic rewiring offers novel therapeutic opportunities in cancer. Until recently, there was scant information regarding soft tissue sarcomas, due to their heterogeneous tissue origin, histological definition and underlying genetic history. Novel large-scale genomic and metabolomics approaches are now helping stratify their physiopathology. In this review, we show how various genetic alterations skew activation pathways and orient metabolic rewiring in sarcomas. We provide an update on the contribution of newly described mechanisms of metabolic regulation. We underscore mechanisms that are relevant to sarcomagenesis or shared with other cancers. We then discuss how diverse metabolic landscapes condition the tumor microenvironment, anti-sarcoma immune responses and prognosis. Finally, we review current attempts to control sarcoma growth using metabolite-targeting drugs.
Similar content being viewed by others
Background
Sarcomas encompass a wide variety of tumors, with more than 170 subtypes, according to the last WHO classification. They originate from the neoplastic transformation of mesenchymal cells in connective tissues [1, 2]: 87% arise from soft tissue and 13% from bone [3, 4]. Soft tissue sarcoma (STS) presents as an indolent or aggressive disease, often only diagnosed at an advanced and/or metastatic stages. Current sarcoma classification relies on histopathology that may lead to errors in up to a quarter of cases [5]. In terms of prevalence, they represent less than 1% of adult cancers, but up to one fifth of pediatric solid malignant cancers [3]. Surgery is the standard of care for patients supplemented with chemotherapy or radiotherapy [6]. Targeted therapies remain limited to tumors with well-defined oncogenic drivers [1, 2]. Clinical trials targeting immune checkpoints show low response rates, with few responsive histotypes. Finally, biomarkers or tertiary lymphoid structures may be be predictive tools for 10% of patients [7]. Consequently, improving sarcoma typing and treatment requires the use of large-scale “omics” tools to identify the oncogenic drivers, often resulting from multiple genetic alterations in adult STS. These can include translocations, mutations or amplifications/deletions that cripple major growth and differentiation pathways [8,9,10,11,12].
Given the limits of current treatments, exploiting drugs targeting metabolic pathways may pave the way to effective therapy for these largely incurable diseases.
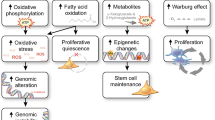
Aggressive tumors must survive in a reorganized, stressful and metabolically competitive microenvironment. This necessary adaptation exploits tumor heterogeneity and cell networks in the tumor microenvironment. Furthermore, within a given cell, plasticity depends on interconnections between various metabolic pathways to adapt growth to the available metabolites. A major trait often amplified in these tumors is the use of aerobic glycolysis, known as the Warburg effect [13], that optimizes tumor cell growth through provision of building blocks to increase biomass [14]. Since Warburg’s discovery, a debate has existed about the persistence of mitochondrial activity in glycolytic tumors and its potential to be a drug target [15]. Despite the central role of mitochondria not only in cell energetics, homeostasis and stress sensing [16] but also reactive oxygen species (ROS) production [17] their contribution to oncogenic transformation is still debated. In some STS, germline mutations affecting mitochondrial enzymes lead to the accumulation of oncometabolites that induce a pseudo-hypoxic response and alter epigenetic marks and differentiation [18]. In the tumor microenvironment, glycolytic and oxidizing cells may compete or cooperate for an optimal use and exchange of energetic metabolites. This network involves immune cells that adapt their metabolism to exert their functions in this competitive environment [19]. The purpose of this review is to link recent findings on STS genetics to the alterations of intracellular pathways affecting their tumor metabolic landscapes. Although not necessarily specific to STS, they may represent novel therapeutic opportunities.
Unsupervised omics and single cell-based analyses highlight metabolic signatures in cancer
The development of more integrated technologies with increased sensitivity and/or resolution has helped to unravel tumor genomic and metabolic complexity in situ and to bridge the gap between mouse models and patients. Several recent studies documented the power of integrated genomic or metabolomic strategies to decipher tumors complexity. An article from The Cancer Genome Atlas (TCGA) Research Network [8] combined genetic, epigenetic and transcriptomic analyses and proposed a novel classification of STS subtypes with complex genomes. In their analysis of the number and nature of copy number variations (CNVs), they identified three dominant profiles from leiomyosarcoma (LMS), myxofibrosarcoma (MFS), undifferentiated pleomorphic sarcoma (UPS) to dedifferentiated liposarcoma (DDLPS) displaying the highest level of genomic alterations. In addition to these modifications, the nature of epigenetics marks, activating pathways or immune signatures add further prognostic value. Another article exploited TCGA data to describe the relative contribution of 114 metabolic pathways to cancer progression [20]. This analysis showed that master metabolic transcriptional regulators behave as genetic drivers explaining the metabolic profiles displayed by various tumors compared to normal tissues, and help predict responsiveness to metabolism-targeting drugs. For example, alterations of specific transcriptional regulators explain the defect in polyamine biosynthesis in prostate cancer. Similarly, distinct pathways enriched in breast cancer allow the discrimination of aggressive tumors from those associated with a good prognostic. Based on this finding, metformin, a mitochondrial complex 1 inhibitor, has been proposed as a potential adjunct therapy against basal breast cancer cells, due to its unique deregulation of the Tricarboxylic Acid (TCA) cycle. In STS, this analysis highlighted the enrichment in the pentose and glucuronate interconversion (PGI) pathway, also amplified in the Yang Huang syndrome described in the context of traditional Chinese pharmacology [21]. The PGI pathway relies on UDP-glucuronosyltransferase (UGT) enzymes that catalyze the binding of d-glucuronic acid to toxic substances or endogenous compounds such as bilirubin via glycosidic bonds, contributing to the detoxification of lipophilic compounds or glucuronides.
Exploration of the TCGA database allows one to identify more discrete signatures displayed by major STS subtypes versus other types of cancers. As shown in Fig. 1, all cancer types display abnormalities in cell cycle regulation. Most carcinomas show an enrichment in oncogenic pathways, glycolytic signatures and alterations of energetic, nucleotide, amino acid or macromolecule pathways. When considering STS as a whole, RAS, PI3K and HIPPO pathways light up, as in [8], coupled to a dominant glycolytic/OXPHOS signature. More discrete signals confirm the enhancement of the PGI pathway in STS, although this trend is not detectable when considering individually the STS subtypes. Our analysis also indicates that distinct signatures preferentially match with STS subtypes, with UPS featuring an enrichment in PPAR/fatty acids and glycine/serine/threonine pathways, whereas LMS display an enhanced OXPHOS signature. Similarly, differences in oncogenic pathway usage are apparent but it is difficult to relate these pathways to the metabolic bias in tumors.
Analysis of the TCGA transcriptomic database. Dotplots showing functional enrichment for co-expression modules found in various cancer types and predominant sarcoma subtypes. Htseq raw counts were retrieved from TCGA using GDCquery [22] and VST-normalized [23]. For each dataset, the unsigned co-expression network was produced using WGCNA with automatic pick for soft-thresholding powers. Genes in each module were queried for functional enrichment against Reactome Pathway Database [24] using clusterProfiler [25]. p values were adjusted using Benjamini–Hochberg procedure. For each dataset-pathway pair, the p value corresponds to the lowest one from all the co-expression modules. A subset of the significant (q-value < 0.05) pathways was manually annotated into functional groups for display in the figure. Dots highlight significant pathway-dataset pairs
The improvement of chromatographic and mass spectrometry (MS) analyses such as Ultra High Performance Liquid Chromatography Q-Exactive MS (UHPLC-QE MS) has allowed time to be saved in sample separation while preserving the detection capacity of a large spectrum of metabolites. In osteosarcoma (OS), studies combining state-of-the-art transcriptomic and metabolomics approaches highlighted the amplification of nucleotide and amino acid (namely alanine, aspartate, glutamate, arginine, proline, methionine) pathways, glycolysis and the pentose phosphate shunt [26, 27]. Spatially-correlated analysis, mass spectrometry imaging (MALDI-MSI) can further reveal how biomolecular ions are distributed on tissue sections, linking their molecular identification to their spatial distribution. Results from two studies [28, 29] comparing STS subtypes showed that the overexpression of acyl-CoA-binding protein and stearyl-CoA desaturase (directly involved in the processing of fatty acids) as well as MIF1, galectin 1, thioredoxin could help distinguish LMS from MFS, and predict their prognostic. To identify tumor-associated metabolites in situ, airflow-assisted desorption electrospray ionization mass spectrometry imaging (AFADESI-MSI) was used on tissues from 256 esophageal cancer (ESCA) patients [30]. This analysis unraveled the dysregulation of several metabolic pathways affecting proline, glutamine, histidine, uridine, fatty acid and polyamine homeostasis. Among others, it identified pyrroline-5-carboxylate reductase 2 (PYCR2) and ornithine decarboxylase (ODC), rate-limiting enzymes in proline and polyamine biosynthesis, respectively, as markers of tumor proliferation. Table 1 summarizes biomarkers and metabolites linked to alterations in the activation of metabolic pathways in STS. In the future, these explorations will benefit from single-cell strategies evaluating the metabolic status of tumor versus surrounding cells. In this context, the development of novel flow cytometry-based methods to assess metabolic activity such as Met-Flow [31] or SCENITH [32] have already proven their potential to assay the metabolic status of circulating or tumor-infiltrating immunocytes.
Here, we will review and update the mechanisms that link complex oncogenic stimuli associated with various STS to metabolic alterations.
Oncogenic drivers upstream of growth pathways rewire metabolism in sarcomas
Physiologically, growth factor receptors (GFR) trigger the RAS/MAPK and PI3K/AKT/mTOR pathways and activate transcriptional regulators such as JUN/FOS/EGR1 that drive cell division (Fig. 2A). This process is temporally regulated by the co-engagement of restriction points controlled by tumor suppressor genes (TSGs) (Fig. 2B) [73]. In cancer cells, prolonged exposure to oncogenic signals strongly stimulates ERK-dependent EGR1 activation, bypassing cell cycle regulation and provoking PI3K activation that antagonizes p53-dependent tumor suppression [74] (Fig. 2B). In p53-mutated cancers, the temporal regulation of MEK/MYC/PI3K is dysfunctional and this allows cancer cells exposed to transient growth signals to proliferate, in a context of increased genomic instability. These pathways have been shown to be involved in sarcomagenesis in both human and rodent models (Fig. 2A), downstream of oncogenic GFRs or receptors involved in tissue organization and trophicity. We will highlight how various activation pathways engage these metabolic programs to sustain STS cell growth and rely alternatively on various carbon sources such as glucose, amino acids or lipids (Fig. 3). We also provide an update on current clinical trials exploiting metabolic interference in Table 2.
Oncogenic and tumor suppressor pathways altered in STS. (A) This figure highlights mutations that alter regulations of PI3K/AKT/mTOR and MAP kinase pathways in sarcoma. Colored triangles associate sarcoma subtypes (listed on the bottom right corner) with the corresponding genes alterations, either expression or loss, on the scheme. Expression or regulations of tumor suppressor genes is altered (p53, PTEN) concomitantly with increased expression of oncogenes driving malignant transformation (increase Anabolism, Warburg effect). (B) Panel B focuses on cell cycle alterations at the level of the p53 and RB1 tumor suppressor genes notably
Metabolic consequences of STS-associated molecular alterations. This scheme integrates sarcoma genetic alterations affecting tumor suppressor genes (green background) or oncogenes (black background) in the tumor metabolic network. These alterations enhance enzymatic reactions in favor of anabolic pathways by increasing the glycolytic flux (pink) and branched pathways, notably nucleotide (yellow), fatty acids (orange) and DNA/RNA synthesis at the cost of dampens mitochondrial function and TCA cycle proper functioning
Overactivated MAP and PI3 kinase pathways drive a Warburg effect
Both mutations and oncogene-driven overexpression of GFRs contribute to STS development. Gain-of-function mutations of the GFR KIT or PDGF-Rα drive gastrointestinal stromal tumor (GIST) progression [93]. In the translocation-associated Ewing’s sarcoma (EWS), myxoid liposarcomas (MLS) or alveolar rhabdomyosarcoma (ARMS), the fusion proteins EF, FUS-DDIT3 or PAX3-FOXO1, respectively, enhance IGF1R expression, a major driver of RAS/AKT/mTOR activation [37]. Hyperactivation of the RAS pathway is predictive of a high risk of disease recurrence and impaired overall survival in 30% undifferentiated pleomorphic sarcoma (UPS), a common adult STS [33, 34]. Similarly, the loss of the phosphatase PTEN induces growth-factor independent PI3K/AKT activation that sustains autonomous nutrient uptake in some LMS or MPNST [94]. Accordingly, sarcoma incidence increases in hereditary neurofibromatosis patients with carrying deletions of the RAS negative regulator genes NF1 or NF2 [49, 95]. To investigate the mechanisms of tumor progression in a mouse model, whole-exome sequencing was performed on STS induced by either KrasG12D activation/p53 deletion, 3-methylcholanthrene (MCA) or ionizing radiation [96]. Whereas CNVs were very frequent in radiation-induced STS, MCA-induced tumors showed a high mutational burden, combined with high genomic instability in the absence of p53. Candidate oncogenic drivers affecting MAPK signaling were identified either as mutations of Kras, Nf1 and Hippo effectors (Fat1/4), or as amplification of Kras and Myc in p53 deficient mice, or Met and Yap1 in radiation-induced STS. Mutations in the RAS pathway influence the prognosis of human STS such as DDLPS or pediatric embryonic rhabdomyosarcoma (ERMS) [97]. In the latter, the overactivation of p38 MAPK induces high levels of reactive oxygen species (ROS) that increase the mutation rate [97] and may sensitize tumors to therapies enhancing oxidative stress [98, 99].
RAS- or PI3K/AKT-driven activation increases the expression of glucose importers (GLUT) and of the upstream ATP-consuming glycolytic enzymes hexokinase (HK) and phosphofructokinase (PFK), recently shown to control the glycolytic flow quantitatively [100]. The oncogenic KRAS4A isoform and to lesser extent other RAS isoforms were shown to interact with HK1 on mitochondria (Fig. 3), preventing its allosteric inhibition by glucose-6-phosphate (G6P), thereby enhancing glycolysis [101]. Hypoxia or mutations affecting the RAS pathway modulated the activity of PKM2 or PGK1, two ATP-generating enzymes in the last steps of glycolysis, thus providing them with non-metabolic pro-oncogenic functions [102]. Thus, an increase in the proportion of PKM2 dimers, lacking pyruvate kinase activity, drives PKM2 nuclear translocation where it participates in STAT3 phosphorylation and mek5 gene transcription, driving cell growth [103]. Another study showed that activated ERK phosphorylates PGK1, promoting its association with PIN1 and its translocation into the mitochondria. There, it phosphorylates and activates pyruvate dehydrogenase kinase 1 (PDK1), an inhibitor of pyruvate dehydrogenase (PDH), the checkpoint of pyruvate entry in the TCA cycle [104] (Fig. 3). Globally, these RAS-driven effects reinforce glycolysis over mitochondrial respiration and favor glucose- and glutamine-dependent anabolism as shown in a pancreatic ductal adenocarcinoma (PDAC) model [105, 106]. Several clinical trials are currently based on drugs inhibiting PI3K, AKT, mTOR and ERK signaling in STS (Fig. 4 and Table 2).
Integrated view of cues and pathways amenable to pharmacological modulation in STS. This diagram places the different existing therapies in sarcoma according to their therapeutic targets. Panel (A) stratify therapeutic option according to main cellular pathways and table B index the current clinical trial and biomarker available in sarcoma disease
An altered HIPPO pathway induces aerobic glycolysis in STS
Several sarcoma histiotypes re-express genes involved in developmental pathways [107,108,109,110], such as HIPPO that controls organ size. Its engagement in intercellular adhesion complexes activates the MST and LATS kinases that phosphorylate the transcriptional factors YAP1 and TAZ, promoting their degradation. In contrast, upon nuclear translocation, YAP/TAZ cooperate with mitogenic effectors and boost proliferation (Fig. 2A). HIPPO interferes with the RAS and PI3K pathways that control cell death induction [111], acting as a tumor suppressor pathway [112]. Through cross-inhibition, MST and AKT differentially regulate the expression level of pro-apoptotic effectors (NOXA, FASL, BIM, TRAIL). In addition, ERK induces the expression of anti-apoptotic effectors (BCL2, BCL-XL, IAP, MCL1) partly via YAP/TAZ activation. Furthermore, in a PDAC model, YAP1 amplification can bypass the need for oncogenic KRAS activation [113]. The amount of translocated YAP/TAZ determines their co-activating potential for TEAD transcriptions factors and thereby the balance of proliferation versus cell death [40].
Loss of MST/LATS or YAP overexpression is pro-tumoral in mice [114, 115], and this pathway is often affected in MCA- or radiation-induced STS [96] and UPS [8]. In transgenic models, altering HIPPO effectors alone or in combination with other deficits augmented STS frequency [41]. An increased YAP/TAZ nuclear staining is predictive of poor survival in UPS, DDLPS and ERMS [42,43,44,45, 116]. Two studies investigated how fusion gene products mediate sarcomagenesis through alteration of the HIPPO pathway in mice. One study explored MLS that account for 5–10% STS, among which 90% tumors depend on the product of the FUS:DDIT3 translocation [44]. The authors performed a large-scale RNA interference screen and identified YAP1 as a non-redundant oncogenic driver. In MLS cell lines, FUS:DDIT3 led to a two to threefold increase in expression and co-transcriptional activity of YAP1. Co-immunoprecipitation and immunofluorescence studies revealed a physical interaction of YAP1 with FUS:DDIT3 in the nucleus. Pharmacological inhibition of YAP1 activity inhibited the growth of MLS xenografts. The other study, based on a new transgenic model, showed that doxycycline (DOX)-induced expression of YAP1 in the myogenic MYOD1 cell lineage provoked the development of ERMS through the transformation of activated satellite cells [45]. Retrieval of DOX released a YAP1-dependent differentiation block and reduced tumor formation. Transcriptional profiling of the tumors revealed that YAP1 induces pro-oncogenic effector genes and represses terminal differentiation of myoblasts. In line with these observations, an independent study found no evidence that mutant RAS isoforms were responsible for YAP overexpression in myoblasts [116]. In ARMS, the translocation product PAX3-FOXO1 suppresses HIPPO signaling through overexpression of RASSF4, which inhibits the MST1 kinase. Similarly, this effect is linked to PAX3-FOXO1 co-localization with YAP1 in the nuclei of cancer cells. This chimeric transcription factor cooperates with YAP1/TEAD to induce downstream effectors that trigger IGFs and NF-κB activation, and repress senescence and apoptosis in mesenchymal cells [40, 44]. Therefore, mutations of HIPPO effectors can be oncogenic in STS.
The HIPPO pathway restricts tissue growth and is connected to nutrient cues [117, 118]. Upon glucose starvation, AMPK- and LATS-kinases phosphorylate YAP resulting in its degradation [119]. Furthermore, Wang et al. showed that the phosphorylation level of YAP1 at position S61 is regulated by AMPK, itself recruited to YAP protein complexes in the cytosol of glucose-deprived cells. Addition of glucose was associated with a decrease in YAP phosphorylation and its nuclear translocation, where it interacted with TEAD transcriptional regulators to induce glycolytic genes. This glucose-sensing pathway via YAP and TAZ was required for the full deployment of glucose growth-promoting activity in breast cancer. In addition, glycolysis was required to sustain YAP/TAZ tumorigenic properties [120]. Mechanistically, phosphofructokinase (PFK1) bound to and co-activated the YAP/TAZ transcriptional cofactors TEADs (Fig. 3). In some cancers, the loss of NF2, an upstream negative regulator of HIPPO signaling, simultaneously unleashed YAP/TAZ and SMAD2/3 activation leading indirectly to the induction of aerobic glycolysis via derepression of GLUT, HK2, LDH and MCT genes [121]. Interestingly, NF2 mutations might contribute to the maintenance of rare aggressive sarcomas [122]. This hypothesis was tested in a kidney cancer cell model bearing NF2 mutations [123]. There, DOX-induced expression of shRNA downregulating YAP/TAZ expression provoked the regression of tumors in vivo. YAP/TAZ-depletion induced a substantial decrease in EGFR and AKT phosphorylation, associated with a reduction in glucose uptake, and a switch to glutamine anaplerosis that boosted mitochondrial respiration and ROS production. Under conditions of glucose or glutamine withdrawal, this metabolic shift favored cell death. Whereas restoration of AKT signaling by expression of a constitutively active form of AKT rescued cell proliferation, it did not prevent starvation-induced death. In vivo, YAP/TAZlow tumors survived due to the engagement of a compensatory lysosome-mediated activation of MAPK signaling. By combining YAP/TAZ and MEK inhibition, tumor growth durably regressed. YAP can also induce aerobic glycolysis through a direct interaction with HIF1α in a hypoxic environment [124, 125]. Therefore, the proglycolytic effect of YAP/TAZ engagement depends on their participation in various nuclear transcriptional complexes. In a muscle-derived UPS model, a combination of epigenetic modulators suppressed YAP1 activity and reduced sarcomagenesis through regulation of metabolism. In this case, YAP1 nuclear translocation was associated with a poor prognosis [126] and its inactivation by epigenetic modulators allowed the restoration of a clock gene-mediated unfolded protein response and muscle differentiation. It also promoted a switch toward lipid catabolism and autophagy, limiting YAP-driven UPS cell growth. The YAP/TAZ inhibitor Verteporfin is currently being tested in Ewing’s sarcoma (EWS) (Fig. 4 and Table 2).
Glutamine and arginine metabolic pathways contribute to STS growth
In a UPS mouse model harboring Kras mutations and p53 deletion in the muscle [59], tumors developed in the hindlimb and metastasized in the lung, as in the human disease. Additional deletion of HIF-2α or its binding partner aryl hydrocarbon receptor nuclear translocator (ARNT) enhanced tumor development. Use of an unbiased pan metabolomics strategy combining LC–MS and stable isotope metabolite tracing revealed a reliance on glutaminolysis for tumors, unlike muscle cells. Accordingly, glutaminase (GLS) inhibitors blocked UPS tumor growth in vivo and are the object of clinical trials in humans (Fig. 4 and Table 2). Mechanistically, GLS hydrolyses glutamine to glutamate, which is then dehydrogenated to alpha-ketoglutarate (αKG) by glutamate dehydrogenase GLUD or an aminotransferase (such as PSAT), boosting mitochondrial anaplerosis [127]. In this UPS model, tracing using C13- or N15-labeled glutamine demonstrated that glutamine is a carbon donor for the TCA cycle and a nitrogen donor for aspartate production from oxaloacetate. Aspartate is a crucial carbon source for purine and pyrimidine synthesis and sustains cell growth [128, 129]. Aspartate is also required for the conversion of citrullin into arginine through the activity of the rate-limiting argininosuccinate synthase 1 (ASS1) that initiates the urea cycle. This reaction generates arginine and contributes to the clearance of nitrogenous wastes. ASS1 deficiency has been observed in various cancers, including MFS, due to epigenetic silencing of its promoter [61]. Reexpression of ASS1 inhibited tumor growth and metastases. To investigate how arginine auxotrophy induced by ASS1 deficiency contributed to the progression of tumors, another study used a pegylated arginine deiminase (ADI-PEG20) to deplete arginine pharmacologically [89]. A short-term treatment by ADI-PEG20 applied to LMS cell lines, immediately induced cell proliferation arrest and autophagy. Upon prolonged therapy, cell lines became resistant to ADI-PEG20 due to the reexpression of ASS1 that regenerated arginine. A metabolomic profiling of treated cell lines revealed a reduction in PKM2 levels. In addition, U13C glucose tracing studies showed that carbons were shifted away from lactate and citrate production, and reoriented toward serine/glycine synthesis. Analysis of metabolic requirements for growth showed a reduced reliance on glucose and a reinforcement in OXPHOS and glutaminolysis, as an alternative source of TCA cycle intermediates via anaplerosis. In STS, a clinical trial using ADI-PEG20 in combination with Gemcitabine and Docetaxel has been launched (Fig. 4 and Table 2). Similarly, targeting glutamine metabolism through GLS inhibition could provoke the lethality of ASS1-deficient cancers and is currently being evaluated in GIST and NF1-mutated cancers (Fig. 4 and Table 2).
Complex metabolic rewiring in Kaposi’s sarcoma
Kaposi’s sarcoma (KS) is caused by a lytic oncogenic herpes virus (KSHV/HHV8), infecting endothelial cell precursors in immunosuppressed individuals. Infection, which is necessary but not sufficient for the growth of KS lesions, leads to the development of a vascular neoplasm associated with cytokine dysregulation driven by the virally encoded G protein coupled receptor (vGPCR). In lytically infected cells, vGPCR induced Rac1/NOX-dependent production of ROS that activated the redox sensitive STAT3 and HIF pathways [130]. Infected cells had increased lactate production and decreased mitochondrial respiration, a phenotype in part attributable to HIF1α activation [131]. Indeed, aerobic glycolysis favored by PKM2 induction sustains the maintenance of KS cells [132] (Fig. 3). Infected cells also exert a paracrine effect on neighboring endothelial cells. Indeed, PKM2 acts as a coactivator of HIF1α reinforcing the production of angiogenic cytokines [131]. Among them, PDGFRA, found phosphorylated in KS-biopsies [133], plays a major oncogenic role. HIF1α also participates in the reactivation of latently infected cells [134]. Upon transformation by KHSV, however, endothelial cells depended on glutamine for proliferation. KHSV also provoked an increase in ASS1 expression, in part through the action of KHSV-encoded miRNAs [62], leading to increased arginine production. Knockdown of ASS1 inhibited cell proliferation and iNOS-dependent, arginine-derived NO production. Treatment of KS cells with a NO donor-activated STAT3 without affecting ROS cell levels. A recent article questioned the relevance of these metabolic changes by comparing 2D versus 3D cultures of KHSV-infected cells [135]. An unbiased metabolomics analysis revealed significant changes in the levels of various non-essential amino acids in 3D cultures. GST-pull down studies showed that the viral K1 protein physically interacted with and activated the pyrroline-5-carboxylate reductase PYCR leading to increased proline production. This phenotype, abrogated by PYCR depletion, promoted 3D spheroid culture and tumorigenesis in nude mice. These results highlight the complex metabolic rewiring that occurs during infection and transformation by KHSV but also the need for appropriate in vitro culture systems to evaluate metabolic adaptation.
One carbon metabolism is overactive in aggressive STS
In Ewing’s sarcoma (EWS), the fusion protein resulting from a single translocation event between the regulatory domain of EWS and the DNA-binding domain of FLI1 behaves as a chimeric transcription factor called EF that enhances IGFR1 activation (Fig. 2A). Transcriptomic studies [60, 64] identified EF as an upstream regulator of PHGDH, PSAT, PSPH and SHMT1/2 genes involved in serine-glycine biosynthesis as well as SLC1A4/5 glutamine transporter genes (Fig. 3). Accordingly, knockdown of EF reduced the proportion of glucose-derived 3-phosphoglycerate reoriented toward serine and glycine synthesis; EWS cell lines were highly dependent on glutamine for growth and survival. Earlier work using a metabolomics approach with isotope labeling had already shown that a large proportion of glycolytic carbon was diverted into serine and glycine metabolism in melanoma; this was due to the amplification of the PHGDH gene [136], also elevated in high-risk EWS patients [100]. Serine or glycine can provide one carbon to tetrahydrofolate initiating the folate cycle. The enhancement of one-carbon metabolism, considered as an integrator of nutrient status [97], boosts the interconnected folate and methionine cycles leading to enhanced NADPH and nucleotide synthesis. NADPH regulates ROS-dependent death and methyl transfer contributes to epigenetic modifications. Knockdown of PHGDH recapitulated the effect of anti-metabolite chemotherapies and had a major effect on cell growth and epigenetic control. Two studies investigated the sensitizing effect of methionine restriction on chemo- or radio resistant models of RAS-driven colorectal cancer and STS, respectively [137, 138]. In the FSF KrasG12D/+; Tp53−/− STS mouse model, tumor development was triggered by the intramuscular injection of an adenovirus carrying the FlpO recombinase. In these aggressive tumors, only the combination of diet and radiation delayed tumor growth. By combining tumor metabolomics and metabolite tracing with a time-course analysis of data, alterations were observed for nucleotide and redox metabolisms. Interestingly, the consequences of methionine restriction could be detected at the metabolic level when applied to healthy individuals. These results indicated that a targeted dietary manipulation could improve tumor response to therapies.
Linking Wnt signaling alterations to metabolic rewiring
Physiologically, the canonical Wnt pathway participates to the maintenance of stem cell pools and cell fate, in part via the nuclear translocation of β-catenin, leading to its interaction with TCF/LEF transcription factors [139]. In a model of osteoblast differentiation, Wnt3a signaling induces aerobic glycolysis by increasing the level of glycolytic effectors (HK2, LDHA, PDK1, GLUT1). This process requires LRP5-mediated mTORC2/AKT activation but not β-catenin [140]. Other studies showed that Wnt engagement also reduced nuclear acetyl coenzyme A (AcCoA) levels and consequently impaired osteoblastic gene expression [141]. In contrast, in mature osteoblasts, Wnt-LRP5 boosted fatty acid oxidation and was required for bone mass increase [142]. In osteosarcoma (OS), Wnt participates in bone remodeling, maintenance of stem cell niches and EMT in collaboration with TGF-β and BMP signaling (reviewed in [143, 144]). In EWS, synovial sarcoma (SS), OS, and to a lesser extent in LMS [143, 145], a high level of Wnt activation, scored by the nuclear localization of β-catenin or LEF-1, is associated with a poor clinical outcome [46]. In OS, deletion of Wnt-related genes has been reported [146]. In some primary OS, the loss of the tumor suppressor RASSF1A enhanced Wnt activation through the AKT/GSK-3-Wnt/β-catenin pathway [147]. Other studies indicated that MEG3, a long non-coding RNA downregulated in OS, controlled the expression of several tumor suppressor genes and oncogenes including P53, RB, MYC and TGF-β; it also negatively regulated the expression of microRNA-184 (miR-184) and down-stream effectors of the Wnt/β-catenin pathway including β-catenin, TCF4 and c-MYC [148]. Therefore, downregulation of MEG3 attenuated its tumor suppressive effect and partly resulted in the upregulation of Wnt signaling. In this model, its impact on cell metabolism relied on mTORC1-mediated activation of the S6 kinase pathway and protein synthesis. In EWS, Wnt activation was also essential for the acquisition of a metastatic phenotype and controlled a proangiogenic switch via the secretion of specific extracellular matrix (ECM) proteins called angiomatrix in a TGF-β-dependent context [47]. However, overexpression of sFRP2, a secreted Wnt antagonist, promoted osteosarcoma invasion and metastatic potential [149]. Therefore, Wnt participates at various stages of STS progression.
Loss of tumor suppressors affects several metabolic pathways
Tumor suppressors interrupt cell cycle and growth in a stressed environment, in part by regulating access to trophic pathways. Patients and mice carrying a hereditary defect in p53 (Li Fraumeni syndrome) or Rb1 (retinoblastoma) show a predisposition to sarcomas [49, 150]. In low/medium grade STS such as well-differentiated liposarcoma (WDLPS), the initial oncogenic event is the amplification of the p53 inhibitor MDM2. In DDLPS, MDM2 amplification synergizes with alterations affecting genes that regulate growth such as CDK4 and FRS2 [4], or that are required for adipocyte differentiation such as JUN, DDIT3, PTPRQ, YAP1 or CEBPA, or with alterations of DNA methylation. More generally, in STS with complex genomes (LMS, UPS, MFS, LPS, MPNST), the accumulation of frequent somatic copy number alterations (SCNAs) and/or focal mutations of TSGs leads to the deregulation of the PI3K/AKT/mTOR axis, mitosis and chromosomal maintenance [48]. As in most cancers, the timing of occurrence of p53 mutations affects tumor progression and prognosis [8, 151,152,153]. Similarly, in mice, the combined loss of p53 [154] or CDKN2A (Ink4/Arf) [66] TSGs with oncogenic RAS lead to the development of undifferentiated STS. Conditional mutations in KRAS and p53 in muscle were sufficient to provoke high-grade STS with myofibroblastic differentiation [155].
As highlighted in Fig. 2A, p53 and AKT exert a negative feedback loop on each other, through PTEN and MDM2 regulation, respectively. Also, p53 indirectly counteracts AKT-dependent downstream effects on growth, apoptosis or metabolism [156, 157]. The tumor suppressive function of p53 depends on its role as transcription factor inducing cell cycle arrest or apoptosis via the CDKN1A (p21), or PUMA and NOXA effectors, respectively (Fig. 2A). However, in their absence, tumor suppression persists suggesting that additional mechanisms [158], including those with an impact on metabolism [156, 159] are also important (Fig. 3). Indeed, GLUT gene transcription is enhanced in STS-bearing p53 mutations [160, 161]. p53 is anti-glycolytic partly through the induction of the expression of TIGAR and PARK2 regulators [162, 163]. TIGAR dephosphorylates fructose biphosphate (FBP) into fructose-6-phosphate (F6P), shifting glucose-6-phosphate (G6P) back toward the pentose phosphate pathway (PPP). Furthermore, the cytosolic form of p53 interacts with and inhibits G6P-dehydrogenase (G6PDH) by preventing the formation of the active dimer, therefore inhibiting PPP-dependent redox control and anabolism [164] (Fig. 3). Since TIGAR expression is not strictly p53-dependent, the resulting p53 effect may be difficult to predict with regards the engagement of the PPP, but it globally interferes with glycolysis. In STS, deep deletions or more frequently amplifications of TIGAR have been documented and high TIGAR expression correlates with a better outcome [50]. PARK2/Parkin is an E3 ubiquitin ligase regulating the degradation of mitochondrial proteins. It cooperates with the mitochondrial serine/threonine kinase PINK1 and contributes to mitochondrial fitness [163, 165]. p53-mediated mitochondrial homeostasis also involves the quality control of mitochondrial DNA (mtDNA) and the expression of cell death regulators [166]. Finally, p53 induces the expression of pyruvate decarboxylase (PDC) that regenerates mitochondrial oxaloacetate and reinitiates the TCA cycle, and that of isocitrate dehydrogenase 1 (IDH1) that converts cytosolic citrate into α-ketoglutarate. In a KRAS driven-PDAC model, p53-dependent accumulation of cytosolic α-ketoglutarate activates aKGDD enzymes that regulate 5-hydroxymethylcytosine-producing TET enzymes, allowing tumor cell differentiation and growth control [167] (Fig. 3).
Metabolic fluxes and mitochondrial fitness in STS
Metabolite fluxes between organelles regulate the efficiency of various metabolic pathways in cells, but also contribute to the plasticity of metabolic adaptation.
Metabolic imbalances in STS
Through their evolution, most tumors tend to acquire metabolic features including an increase in nucleotide synthesis [168]. Investigations using PET-FDG uptake in STS patients confirmed the strong glycolytic bias documented in metastasized and poor prognosis STS [169, 170] such as ARMS [171] or ES [172]. However, these studies also revealed the considerable heterogeneity within a given tumor and between different tumor types, suggesting that the Warburg phenotype might be unstable and amenable to pharmacologic control [173]. Whereas the level of oxidative phosphorylation (OXPHOS) varies between tumors (Fig. 1), there is a general correlation between reduced mitochondrial activity, an epithelial-to-mesenchymal transition (EMT) gene signature and a poor prognosis [168]. Some STS tend to exhibit high levels of mitochondrial respiration compared to carcinomas (Fig. 1) [55, 174]. In vitro analysis of OS and RMS cell lines showed differences in the reliance on glycolysis versus respiration of tumors, with ARMS being in general less oxidative than OS or ERMS [175]. The equilibrium between glycolysis and mitochondrial respiration can be affected by various oncogenic alterations and/or metabolic requirements. Accordingly, the receptor tyrosine kinase Her4/ErbB4, an EGFR family member, is upregulated in several cancers including OS [176]. Exploration of xenograft models using untargeted metabolomics and 18F-FDG microPET/CT scan approaches showed that Her4 overexpression boosted glycolysis, glutaminolysis and OXPHOS in tumors. This hypermetabolic phenotype contributed to sustained growth and ATP production while conferring chemoresistance, as also shown in PDAC [177].
The crosstalk between metabolic pathways can also be altered in cancer as discussed in the following examples. Firstly, the upstream reaction committing glucose to glycolysis is catalyzed by phosphofructokinase-1 (PFK-1), itself allosterically inhibited by high ATP levels [178] (Fig. 3). Cancer cells express various PFK isoenzymes [179] such as the bifunctional 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB) that produces F2,6-BP, thereby overriding ATP-dependent inhibition of PFK1 [180, 181] (Fig. 3). Reciprocally, an activation of a F1,6-biphosphatase such as FBP1 enhances the gluconeogenic flow and restrains glycolysis [174]. The FBP2 isoform is frequently lost in STS including LPS, FS, LMS and UPS and lower FBP2 mRNA levels correlated with poor survival in LPS [55]. In the latter study, increasing FBP2 expression impaired sarcoma cell growth, through glycolysis inhibition and induction of mitochondrial biogenesis. The latter effect was due to FBP2 nuclear translocation where, independently of its enzymatic activity, it interacted with and inhibited c-Myc-driven transcriptional activation of TFAM, an inducer of mitochondrial biogenesis [182].
Secondly, the maintenance of glycolytic flow requires the regeneration of NAD+ which originates from cytosolic lactate dehydrogenase (LDH) activity and from the malate aspartate shuttle between mitochondria and cytosol [183] (Fig. 2). By regulating NAD+ levels, mitochondrial activity limits glycolysis and consequently the Warburg effect [184]. This suggests that the persistence of mitochondrial activity can be beneficial to tumors. In addition, an LDH activity has been identified in the mitochondria where it catalyzes the aerobic oxidation of lactate into pyruvate. It is thought to contribute to the maintenance or enhancement of OXPHOS in glycolytic cells [185, 186]. Pyruvate oxidation in the mitochondria depends on PDH activity, itself inhibited by PDK. The inhibition of PDK by dichloroacetate (DCA) shifts metabolism from glycolysis to glucose oxidation and boosts ROS production as well as mitochondria-dependent apoptosis in tumors [187]. This effect is exploited in EWS and other tumors where DCA synergizes with apoptosis-inducing drugs such as cisplatin. Manipulating ROS levels appears to be a promising therapeutic approach [188]. Indeed, scavenging mitochondrial ROS (mtROS) induces p53, reduces the cell transforming potential of oncogenic RAS and in some fibrosarcoma (FS) and RMS model cell lines suppresses tumor growth [17, 189].
Thirdly, the level of mitochondrial activity depends on the availability of coenzyme A (CoA) and the acetylated form, AcCoA. CoA synthesis requires the intracellular phosphorylation of pantothenate (or vitamin B5) by pantothenate kinases [190]. Reciprocally, pantothenate derives from the recycling of food-derived or cellular CoA through an extracellular degradative process involving the vanin pantetheinases [191, 192]. Interestingly, a high vanin1 (VNN1) level correlates with a better prognosis in STS patients [66]. Lack of Vnn1 in CDKN2A deficient mice enhanced the proportion of fibrosarcomas compared to that of other cancers. In RAS-driven mouse STS lines, Vnn1 exerted an anti-Warburg effect by enhancing CoA levels and mitochondrial activity to the detriment of glycolysis, and by maintaining cell differentiation.
Mitochondrial abnormalities disrupt the TCA cycle
Mitochondrial biogenesis depends on the transcriptional coactivator PGC1α [193]. This process regulates the transition from myoblast growth to differentiation and requires a switch from the classical to the alternative NF-kB activation pathway. The latter controls PGC1α transcription [194], in cooperation with MyoD [195]. In RMS and OS models, an alteration in this switch leads to the induction of the pro-glycolytic HK2 isoform through the persistent activation of the classical NF-kB pathway [196] (Fig. 3). This might also contribute to the incomplete mitochondrial biogenesis observed in a rat RMS model featuring a deficiency in respiratory potential and poor mtROS control, thereby enhancing tumorigenesis [197].
Mutations in TCA enzymes SDH [198] and FH [95], found in STS [57], are frequent in wild-type GIST without KIT or PDGFRA mutations [152]. They provoke an interruption of the TCA cycle, uncoupled from ATP production. Consequently, excess succinate diffuses in the cytoplasm where it inhibits aKGDD enzymes involved in the regulation of epigenetic modifications, DNA repair [199] or HIF degradation, rewiring cells toward glycolysis [200]. In a mouse ovarian cancer model, targeted knockdown of Sdhb resulted in enhanced proliferation and lead to a hypermethylated epigenome promoting EMT [198]. Using metabolic tracing and SeaHorse analysis, the authors documented an increased reliance on glutamine for cell survival and a reduced mitochondrial reserve capacity, rendering cells highly sensitive to the complex I inhibitor metformin.
Mutations in IDH1 and 2 lead to the production of 2-hydroxyglutarate [201], an inducer of HIF1α stabilization. HIF1a expression and hypoxia are associated with poor survival of sarcoma patients [68,69,70, 202–204]. Hypoxia regulates apoptosis resistance, cancer stemness, metastatic properties in RMS [71, 205] and is involved in ES, GIST and LPS progression [69, 70, 203, 204]. Uncoupling of electron transport chain (ETC) complexes from ATP production does not impede anaplerotic mitochondrial uptake of glutamine, transformed into glutamate via the activity of GLS to feed the reverse metabolic flow toward citrate production and anabolism [206] (Fig. 3). Accordingly, the growth of STS subtypes overexpressing GLS is sensitive to glutamine depletion in vitro and glutaminase inhibition in vivo [59].
Some components of the ETC are encoded by mtDNA. Therefore, alterations in mtDNA may lead to respiratory defects. In OS, insufficient or altered mtDNA is associated with stressed mitochondria and enhanced tumor invasiveness [207]. In an OS cell line, ethidium bromide induced-mtDNA depletion provoked a deficiency in cytochrome oxidase and OXPHOS, leading to enhanced glycolysis and EMT [208, 209]. Furthermore, mitochondrial dysfunction and loss of transmembrane potential provoked high cytosolic Ca2+ levels, triggering calcineurin-dependent mitochondria-to-nucleus retrograde signaling that resulted in AKT activation and glycolysis [210]. In a tunable model of mitochondrial dysfunction using cytoplasmic hybrids [57], impairment of respiration lead to NADH accumulation and cytosolic recycling into NAD+ by the malate deshydrogenase pathway. NAD+ boosted glycolysis and ATP-dependent cell migration. This suggests that glycolysis-derived ATP might be preferentially used during cell migration [211]. In conclusion, there is a high intra- and inter-tumor heterogeneity in mitochondrial activity, which can be enhanced or lost, depending on the tumor context.
Tumor metabolome impacts STS progression
Metastasis
Aerobic glycolysis induced by oncogenic or hypoxic signaling provokes changes in the tumor metabolome. Lactate excretion, hypoxia-associated hypercapnia and acidification of the extracellular milieu accelerate the degradation of the extracellular matrix and facilitate metastasis [212, 213]. Reciprocally, as shown in STS [214, 215], cancer-associated fibroblasts can produce lactate and 3-hydroxybutyrate that boost cell growth, metastasis and angiogenesis when administered to tumor-bearing mice [216–218]. Lactate uptake by tumors feeds their oxidative metabolism [212, 216, 219] and requires the importer MCT1, a marker of mitochondrial activity and stemness in cancer and a target gene of the fusion protein ASPSCR1/TFE3 in alveolar soft part sarcoma (ASPS) [220]. The persistence of mitochondrial activity can enhance metabolic plasticity [221], mtROS-driven anoikis, metastasis [222, 223] or resistance to therapy as shown for LPS [224]. Metabolic plasticity, required during EMT transition [225], is still incompletely documented in poorly polarized and migration-prone mesenchymal tumor cells such as sarcoma cells [226]. Indeed, hybrid epithelial/mesenchymal (E/M) phenotypes or switching from E- to N-cadherin and vimentin expression contribute to aggressiveness, metastatic properties and drug resistance [226–229]. In addition, fusion protein events and translocations, frequent in childhood STS, can regulate epithelial differentiation [230, 231]. EMT is induced by cytokines such as FGFs, PDGF, TGF-β that enhance glycolysis and TCA activity [232]. TGF-β signaling synergizes with the AKT and NF-kB pathways, both potent drivers of glycolysis [233], but also antagonizes PDK4, thereby allowing pyruvate entry into the TCA. YB-1, an enhancer of HIF1α translation, is overexpressed in high-risk human sarcomas and promotes EMT and metastasis [234]. Hypoxia regulates the expression of several intracellular collagen-modifying enzymes, particularly OGDH enzymes that hydroxylate proline and lysine residues, contributing to the quality of collagen folding and the stiffness of the tissue, and thereby affecting cell migration [235]. In a UPS model, HIF1α enhances the expression of the intracellular enzyme procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (PLOD2). Loss or overexpression of PLOD2 abrogates or restores, respectively, the metastatic potential of HIF1α-deficient tumors and human sarcomas show elevated HIF1α and PLOD2 expression in metastatic primary lesions [236]. Finally, HIF can enhance ECM degradation through the induction of various metalloproteases such as MMP or PLAUR, facilitating invasiveness.
Immunoreactivity
Several features including the level of infiltration of cytotoxic CD8+T cells or of myeloid cells, the expression of markers of immune-stimulation or -depression and the localization of these cells within the tumor, emerge as landmarks of tumor immunogenicity [8, 237–240]. STS display low mutational burden as compared to other cancer types and are generally considered to be poorly immunogenic and poorly responsive to immune checkpoint blockade [8, 241]. Synovial sarcoma, soft tissue and undifferentiated LMS are the three subtypes with the lowest CNV [8, 237] and display reduced immune infiltration, virtually devoid of lymphocytes [242–245]. In contrast, STS with several SCNAs, nucleotide and chromosome instabilities, such as undifferentiated LPS, MPNST [246–248], AS and GIST [243] and OS [244] can present high levels of lymphoid infiltration including CD8+ T cells. Consequently, STS display a wide range of immunophenotypes [238, 249, 250]. The metabolic rewiring imposed by tumors generates a situation of competition for essential energetic resources. This concerns glucose, vitamins and essential amino acids (serine, leucine, methionine, etc.) leading to impairment of immune cell functions and memory [19, 251, 252]. The exchange of fatty acids is required for the survival of immunosuppressive myeloid cells [253] or Tregs [254], particularly under conditions of activation of the PI3K/AKT/mTOR axis that boosts lipogenesis [255]. Other metabolites such as extracellular nucleotides released upon cell death can induce immunosuppression via various mechanisms [256–258]. Altogether, metabolic disturbances imposed by tumor cells directly contributes to the reorganization of the microenvironment but an exhaustive analysis of the immune landscape in STS is still lacking. Techniques such as Met-Flow [31] or SCENITH [32] should help dissecting the metabolic status of immunocytes.
Conclusions
Unraveling the complexity of sarcoma genetics has benefited from the development and improvement of multi-omics strategies. The phenotypic and molecular description of genomic alterations can now be complemented with the identification of prognostic mechanistic signatures in patients. The heterogeneity and scarcity of STS originally limited the description of their metabolic landscapes but PET-FDG analysis has contributed to their staging, prognostication and evaluation of their response to therapy. Several cell-autonomous pathways or environmental factors influence the degree of conversion toward aerobic glycolysis, justifying the use of drugs that antagonize these processes. Nevertheless, the classic distinctions between glycolytic and oxidative tumors must be carefully reconsidered; hybrid phenotypes may confer more adaptable behaviors to STS cells. Several important metabolic pathways associated with STS progression such as those of one carbon and arginine metabolisms, or the PGI pathway might provide novel therapeutic options in combination with conventional therapies. In addition, the development of novel animal models or 2D/3D culture systems has highlighted the metabolic plasticity of these tumors that may impact their energetic resources. However, these adaptations may have a price, rendering tumor cells more sensitive to combined therapies.
Oncogenic signals can lead to the expression of isoforms of glycolytic enzymes that display new functions tilting the balance between glycolysis and mitochondrial activity. Similarly, p53 can also act as a regulator of G6PDH activity, impacting biosynthetic pathways. Some metabolites such as α-ketoglutarate have emerged as key effectors of p53 action, whereas others behave as oncometabolites leading to alterations of genome integrity, metastatic behavior and therapeutic response. The balance between glycolysis, glutaminolysis and OXPHOS depends on the respective availability of key metabolites, such as amino acids, NAD+/NADH, lactate or VitB5, that regulate STS progression or differentiation. Metabolomic studies have already shown that novel metabolite signatures will complement conventional biomarkers and help stratifying prognosis and therapeutic options. The stability of the glycolytic phenotype also depends on mitochondrial activity. Alterations of mitochondrial fitness observed in STS upon alterations of mtDNA or TCA enzymes aggravate the prognostic of tumors or can affect their chemoresistance. An unstable tumor metabolome has tumor-intrinsic or extrinsic effects causing it to be pro-metastatic or immunosuppressive. Therefore, the combination of drugs targeting different metabolic pathways should impact both tumor and immune cells in a concerted manner to reinvigorate anti-tumor immunity while tilting the balance toward cell differentiation over growth.
Availability of data and materials
Data used for the bioinformatics analysis come from the publically available TCGA database. The code to reproduce the analysis is available at GitHub (https://github.com/guillaumecharbonnier/mw-miallot2021).
Abbreviations
- 3HB:
-
3‐Hydroxybutyrate
- AcCoA:
-
Acetyl coenzyme A
- AIDS:
-
Acquired immunodeficiency syndrome
- aKGDD:
-
Alpha-ketoglutarate dioxygenase
- ARMS:
-
Alveolar rhabdomyosarcoma
- AS:
-
Angiosarcoma
- ASPS:
-
Alveolar soft part sarcoma
- ATF6:
-
Activating transcription factor 6
- ATRX:
-
α-Thalassemia/mental retardation syndrome x-linked
- BCL2:
-
B-cell cell/lymphoma 2
- BCL-XL:
-
B-cell lymphoma-extra large
- BIM:
-
BCL-2-interacting mediator of cell death
- BLCA:
-
Bladder urothelial carcinoma
- BMP:
-
Bone morphogenetic proteins
- BRCA:
-
Breast invasive carcinoma
- CAF:
-
Cancer-associated fibroblast
- CDK4:
-
Cyclin-dependent kinase 4
- CDKN1A = p21:
-
Cyclin-dependent kinase inhibitor 1a
- CDKN2A = p16:
-
Cyclin-dependent kinase inhibitor 2a
- CEBPA:
-
CCAAT/enhancer-binding protein alpha
- CNV:
-
Copy number variations
- CS:
-
Canine sarcoma
- DCA:
-
Dichloroacetate
- DDIT3:
-
DNA damage-inducible transcript 3
- DDLPS:
-
Dedifferentiated liposarcoma
- DNMT1:
-
DNA methyltransferase 1
- ECM:
-
Extracellular matrix
- EF:
-
EWS/FLI1 fusion protein
- EGR1:
-
Early growth response protein 1
- EMT:
-
Epithelial to mesenchymal transition
- ERMS:
-
Embryonal rhabdomyosarcoma
- ESCA:
-
Esophageal carcinoma
- ETC:
-
Electron transport chain
- EWS:
-
Ewing sarcoma
- F2,6BP:
-
Fructose-2,6-bisphosphate
- FASL:
-
Fas ligand
- FBP:
-
Fructose-biphosphate
- FGFs:
-
Fibroblast growth factors
- FLI1:
-
Friend leukemia integration 1 transcription factor
- FRS2:
-
Fibroblast growth factor receptor substrate 2
- FS:
-
Fibrosarcomas
- FUS/DDIT3:
-
Fused in sarcoma (FUS) to DNA damage inducible transcript 3 (DDIT3)
- G6P:
-
Glucose-6-phosphate
- GFR:
-
Growth factor receptor
- GIST:
-
Gastrointestinal stromal tumor
- GLUT:
-
Glucose transporter
- GSK:
-
Glycogen synthase kinase
- GTPase:
-
Guanosine triphosphate hydrolase
- HHV8:
-
Human herpes virus 8
- HIF:
-
Hypoxia inducible factor
- HIPPO:
-
Hippopotamus (according to the tissue overgrowth)
- HK:
-
Hexokinase
- IAP:
-
Inhibitors of apoptosis proteins
- IDH1:
-
Isocitrate dehydrogenase 1
- IGF1:
-
Insulin-like growth factor 1
- IGF1R:
-
Insulin-like growth factor 1 receptor
- KRAS:
-
Kirsten rat sarcoma viral
- KS:
-
Kaposi sarcoma
- KSHV:
-
Kaposi sarcoma-associated herpesvirus
- LDH:
-
Lactate deshydrogenase
- LEF-1:
-
Lymphoid enhancer-binding factor 1
- LIHC:
-
Liver hepatocellular carcinoma
- LMS:
-
Leiomyosarcoma
- lncRNA:
-
Long non-coding RNA
- LPR5:
-
Low-density lipoprotein receptor-related protein 5
- LPS:
-
Liposarcoma
- LUAD:
-
Lung adenocarcinoma
- LUSC:
-
Lung squamous cell carcinoma
- MAPK:
-
Mitogen-activated protein kinase
- MCA:
-
Methylcholanthrene
- MCL1:
-
Myeloid leukemia cell differentiation protein
- MCT:
-
Monocarboxylate transporter
- MDH:
-
Malate dehydrogenase
- MDM2:
-
Mouse double minute 2 homolog
- MEG3:
-
Maternally expressed 3
- MEK:
-
Mitogen-activated protein kinase
- MESO:
-
Mesothelioma
- MFS:
-
Myofibrosarcoma
- MFH:
-
Malignant fibrous histiocytoma (equivalent to UPS)
- miR:
-
Micro RNA
- mitROS:
-
Mitochondrial reactive oxygen species
- MLS:
-
Myxoid liposarcoma
- MMP:
-
Matrix metallopeptidases
- MPNST:
-
Malignant peripheral nerve sheath tumors
- MSC :
-
Mesenchymal stem cells
- mtDNA :
-
Mitochondrial DNA
- mTOR :
-
Mammalian target of rapamycin
- mTORC1 :
-
Mammalian target of rapamycin complex 1
- MyoD :
-
Myoblast determination protein 1
- NAD :
-
Nicotinamide adenine dinucleotide
- NADPH :
-
Nicotinamide adenine dinucleotide phosphate
- ncRNA :
-
Non-coding RNA
- NF :
-
Neurofibromin
- NFκB :
-
Nuclear factor kappa-light-chain-enhancer of activated b
- NOX :
-
NADPH oxidase
- NOXA :
-
Phorbol-12-myristate-13-acetate-induced protein 1
- OAA :
-
Oxaloacetate
- OS :
-
Osteosarcoma
- OVCA :
-
Ovarian serous cystadenocarcinoma
- OXPHOS :
-
Oxidative phosphorylation
- PARK2 :
-
Phosphatidic acid-regulated protein kinase
- PAAD :
-
Pancreatic adenocarcinoma
- PDAC :
-
Pancreatic ductal adenocarcinoma
- PDC :
-
Pyruvate decarboxylase
- PDGF :
-
Platelet-derived growth factor receptors
- PDHK :
-
Pyruvate dehydrogenase kinase
- PDK :
-
3-Phosphoinositide-dependent protein kinase
- PERK :
-
Protein kinase r-like endoplasmic reticulum kinase
- PET-FDG :
-
Fluorine-18-fluorodeoxyglucose positron emission tomography
- PFK :
-
Phosphofructokinase
- PFKFB :
-
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase
- PGC1a :
-
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PGK1 :
-
Phosphoglycerate kinase 1
- PHGDH :
-
3-Phosphoglycerate dehydrogenase
- PI3K :
-
Phosphatidylinositol-3-kinase
- PKM2 :
-
Pyruvate kinase M2
- PLAUR :
-
Plasminogen activator, urokinase receptor
- PLOD2 :
-
Procollagen-lysine,2-oxoglutarate 5-dioxygenase 2
- PPP :
-
Pentose phosphate pathway
- PSAT :
-
Phosphohydroxythreonine aminotransferase
- PSPH :
-
Phosphoserine phosphatase
- PTEN :
-
Phosphatase and tensin homolog
- PTPRQ :
-
Protein tyrosine phosphatase receptor type q
- RAC1:
-
Phosphoserine phosphatase
- RAS :
-
Rat sarcoma oncogene
- RASSF14 :
-
Ras-association domain family 1 isoform a
- Rb1 :
-
Retinoblastoma protein
- RMS :
-
Rhabdomyosarcoma
- ROS :
-
Reactive oxygen species
- RREB1 :
-
Ras-responsive element binding protein 1
- S6K :
-
S6 kinase
- SCNAs :
-
Somatic copy-number alterations
- SHMT1/2 :
-
Serine hydroxymethyltransferase 1 and 2
- SMARCB1 :
-
SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily b, member 1
- SS :
-
Synovial sarcoma
- STAD :
-
Stomach adenocarcinoma
- STS :
-
Soft tissue sarcoma
- SV :
-
Structural variation
- TA :
-
Transit amplifying
- TAZ :
-
Transcriptional coactivator with PDZ-binding motif
- TCA :
-
Tricarboxylic acid cycle
- TCF :
-
T-cell factor
- TEAD :
-
Tea domain family member 1
- TERT :
-
Telomerase reverse transcriptase
- TF :
-
Transcription factor
- TFAM :
-
Transcription factor a mitochondrial
- TGF-β :
-
Transforming growth factor beta
- TIGAR :
-
p53-induced glycolysis regulatory phosphatase
- P53 :
-
Tumor protein p53
- TRAIL :
-
TNF-related apoptosis-inducing ligand
- TSC1/2 :
-
Tuberous sclerosis complex 1/2
- TSG :
-
Tumor suppressor gene
- UPR :
-
Unfolded protein response
- UPS :
-
Undifferentiated pleomorphic sarcoma
- VEGF :
-
Vascular endothelial growth factor
- vGPCR :
-
Viral encoded g protein-coupled receptor
- VHL :
-
Von Hippel–Lindau tumor suppressor
- VNN1 :
-
Vanin-1
- WDLS :
-
Well-differentiated liposarcoma
- WNT :
-
Wingless/integrated
- YAP1 :
-
Yes-associated protein
- YB-1 :
-
Y-box binding protein 1
References
Lye KL, Nordin N, Vidyadaran S, Thilakavathy K. Mesenchymal stem cells: from stem cells to sarcomas. Cell Biol Int. 2016;40:610–8.
Xiao W, Mohseny AB, Hogendoorn PCW, Cleton-Jansen A-M. Mesenchymal stem cell transformation and sarcoma genesis. Clin Sarcoma Res. 2013;3:10.
Burningham Z, Hashibe M, Spector L, Schiffman JD. The epidemiology of sarcoma. Clin Sarcoma Res. 2012;2:14.
Italiano A, Di Mauro I, Rapp J, Pierron G, Auger N, Alberti L, et al. Clinical effect of molecular methods in sarcoma diagnosis (GENSARC): a prospective, multicentre, observational study. Lancet Oncol. 2016;17:532–8.
Skubitz KM, Skubitz AP, Xu WW, Luo X, Lagarde P, Coindre J-M, et al. Gene expression identifies heterogeneity of metastatic behavior among high-grade non-translocation associated soft tissue sarcomas. J Transl Med. 2014;12:176.
Grimer R, Judson I, Peake D, Seddon B. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:1–15.
Thanindratarn P, Dean DC, Nelson SD, Hornicek FJ, Duan Z. Advances in immune checkpoint inhibitors for bone sarcoma therapy. J Bone Oncol. 2019;15:100221.
Abeshouse A, Adebamowo C, Adebamowo SN, Akbani R, Akeredolu T, Ally A, et al. Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell. 2017;171:950–965.e28.
Guillou L, Aurias A. Soft tissue sarcomas with complex genomic profiles. Virchows Arch. 2010;456:201–17.
Penel N, Coindre J-M, Giraud A, Terrier P, Ranchere-Vince D, Collin F, et al. Presentation and outcome of frequent and rare sarcoma histologic subtypes: a study of 10,262 patients with localized visceral/soft tissue sarcoma managed in reference centers. Cancer. 2018;124:1179–87.
Taylor BS, Barretina J, Maki RG, Antonescu CR, Singer S, Ladanyi M. Advances in sarcoma genomics and new therapeutic targets. Nat Rev Cancer. 2011;11:541–57.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–37.
Sinkala M, Mulder N, Patrick MD. Metabolic gene alterations impact the clinical aggressiveness and drug responses of 32 human cancers. Commun Biol. 2019;2:1–14.
Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G. Metabolic targets for cancer therapy. Nat Rev Drug Discov. 2013;12:829–46.
Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012;13:780–8.
Ralph SJ, Rodríguez-Enríquez S, Neuzil J, Saavedra E, Moreno-Sánchez R. The causes of cancer revisited: “Mitochondrial malignancy” and ROS-induced oncogenic transformation—why mitochondria are targets for cancer therapy. Mol Asp Med. 2010;31:145–70.
Sciacovelli M, Schmidt C, Maher ER, Frezza C. Metabolic drivers in hereditary cancer syndromes. Annu Rev Cancer Biol. 2020;4:77–97.
Klein Geltink RI, Kyle RL, Pearce EL. Unraveling the complex interplay between T cell metabolism and function. Annu Rev Immunol. 2018;36:461–88.
Rosario SR, Long MD, Affronti HC, Rowsam AM, Eng KH, Smiraglia DJ. Pan-cancer analysis of transcriptional metabolic dysregulation using The Cancer Genome Atlas. Nat Commun. 2018;9:5330.
Liu X, Zhang A, Fang H, Li M, Song Q, Su J, et al. Serum metabolomics strategy for understanding the therapeutic effects of Yin-Chen-Hao-Tang against Yanghuang syndrome. RSC Adv. 2018;8:7403–13.
Colaprico A, Silva TC, Olsen C, Garofano L, Cava C, Garolini D, et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44:e71–e71.
Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106.
Jassal B, Matthews L, Viteri G, Gong C, Lorente P, Fabregat A, et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020;48:D498-503.
Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS J Integr Biol. 2012;16:284–7.
Chen J, Sun M, Hua Y, Cai Z. Prognostic significance of serum lactate dehydrogenase level in osteosarcoma: a meta-analysis. J Cancer Res Clin Oncol. 2014;140:1205–10.
Zhong Z, Mao S, Lin H, Li H, Lin J, Lin J-M. Alteration of intracellular metabolome in osteosarcoma stem cells revealed by liquid chromatography–tandem mass spectrometry. Talanta. 2019;204:6–12.
Lou S, Balluff B, de Graaff MA, Cleven AHG, Bruijn IB, Bovée JVMG, et al. High-grade sarcoma diagnosis and prognosis: biomarker discovery by mass spectrometry imaging. Proteomics. 2016;16:1802–13.
Takahashi A, Nakayama R, Ishibashi N, Doi A, Ichinohe R, Ikuyo Y, et al. Analysis of gene expression profiles of soft tissue sarcoma using a combination of knowledge-based filtering with integration of multiple statistics. PLoS ONE. 2014;9:e106801.
Sun C, Li T, Song X, Huang L, Zang Q, Xu J, et al. Spatially resolved metabolomics to discover tumor-associated metabolic alterations. PNAS. 2019;116:52–7.
Ahl PJ, Hopkins RA, Xiang WW, Au B, Kaliaperumal N, Fairhurst A-M, et al. Met-Flow, a strategy for single-cell metabolic analysis highlights dynamic changes in immune subpopulations. Commun Biol. 2020;3:1–15.
Argüello RJ, Combes AJ, Char R, Gigan J-P, Baaziz AI, Bousiquot E, et al. SCENITH: a flow cytometry-based method to functionally profile energy metabolism with single-cell resolution. Cell Metab. 2020;32:1063-1075.e7.
Serrano C, Romagosa C, Hernández-Losa J, Simonetti S, Valverde C, Moliné T, et al. RAS/MAPK pathway hyperactivation determines poor prognosis in undifferentiated pleomorphic sarcomas. Cancer. 2016;122:99–107.
Dodd RD. Emerging targets in sarcoma: rising to the challenge of RAS signaling in undifferentiated pleomorphic sarcoma: RAS/MAPK levels in UPS. Cancer. 2016;122:17–9.
Mora J, Rodríguez E, de Torres C, Cardesa T, Ríos J, Hernández T, et al. Activated growth signaling pathway expression in Ewing sarcoma and clinical outcome. Pediatr Blood Cancer. 2012;58:532–8.
Ahmed AA, Sherman AK, Pawel BR. Expression of therapeutic targets in Ewing sarcoma family tumors. Hum Pathol. 2012;43:1077–83.
Noh B-J, Jung W-W, Kim H-S, Park Y-K. Pathogenetic implications of early growth response 1 in Ewing sarcoma. Pathology. 2019;51:605–9.
Machado I, López-Guerrero JA, Scotlandi K, Picci P, Llombart-Bosch A. Immunohistochemical analysis and prognostic significance of PD-L1, PD-1, and CD8+ tumor-infiltrating lymphocytes in Ewing’s sarcoma family of tumors (ESFT). Virchows Arch. 2018;472:815–24.
Glorie N, Baert T. Circulating Protein biomarkers to differentiate uterine sarcomas from leiomyomas. Anticancer Res. 2019;39:3981–9.
Regina C, Hettmer S. Myxoid liposarcoma: it’s a Hippo’s world. EMBO Mol Med. 2019;11(5):e10470. https://doi.org/10.15252/emmm.201910470.
Mohamed AD, Tremblay AM, Murray GI, Wackerhage H. The Hippo signal transduction pathway in soft tissue sarcomas. Biochim Biophys Acta (BBA) Rev Cancer. 2015;1856:121–9.
Crose LES, Galindo KA, Kephart JG, Chen C, Fitamant J, Bardeesy N, et al. Alveolar rhabdomyosarcoma-associated PAX3-FOXO1 promotes tumorigenesis via Hippo pathway suppression. J Clin Investig. 2014;124:285–96.
Fullenkamp CA, Hall SL, Jaber OI, Pakalniskis BL, Savage EC, Savage JM, et al. TAZ and YAP are frequently activated oncoproteins in sarcomas. Oncotarget. 2016;7:30094–108.
Trautmann M, Cheng Y, Jensen P, Azoitei N, Brunner I, Hüllein J, et al. Requirement for YAP1 signaling in myxoid liposarcoma. EMBO Mol Med. 2019;11:e9889.
Tremblay AM, Missiaglia E, Galli GG, Hettmer S, Urcia R, Carrara M, et al. The Hippo transducer YAP1 transforms activated satellite cells and is a potent effector of embryonal rhabdomyosarcoma formation. Cancer Cell. 2014;26:273–87.
Pedersen EA, Menon R, Bailey KM, Thomas DG, Van Noord RA, Tran J, et al. Activation of Wnt/-catenin in Ewing sarcoma cells antagonizes EWS/ETS function and promotes phenotypic transition to more metastatic cell states. Can Res. 2016;76:5040–53.
Hawkins AG, Pedersen EA, Treichel S, Temprine K, Sperring C, Read JA, et al. Wnt/β-catenin-activated Ewing sarcoma cells promote the angiogenic switch. JCI Insight. 2020;5:e135188.
Chibon F, Lagarde P, Salas S, Pérot G, Brouste V, Tirode F, et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nat Med. 2010;16:781–7.
Ballinger ML, Goode DL, Ray-Coquard I, James PA, Mitchell G, Niedermayr E, et al. Monogenic and polygenic determinants of sarcoma risk: an international genetic study. Lancet Oncol. 2016;17:1261–71.
Fang P, de Souza C, Minn K, Chien J. Genome-scale CRISPR knockout screen identifies TIGAR as a modifier of PARP inhibitor sensitivity. Commun Biol. 2019;2:16.
van Maldegem AM, Hogendoorn PC, Hassan AB. The clinical use of biomarkers as prognostic factors in Ewing sarcoma. Clin Sarcoma Res. 2012;2:7.
de Necochea-Campion R, Zuckerman LM, Mirshahidi HR, Khosrowpour S, Chen C-S, Mirshahidi S. Metastatic biomarkers in synovial sarcoma. Biomark Res. 2017;5:1–8.
Benz MR, Dry SM, Eilber FC, Allen-Auerbach MS, Tap WD, Elashoff D, et al. Correlation between glycolytic phenotype and tumor grade in soft-tissue sarcomas by 18F-FDG PET. J Nucl Med. 2010;51:1174–81.
Chen H, Chen Y, Liu H, Que Y, Zhang X, Zheng F. Integrated expression profiles analysis reveals correlations between the IL-33/ST2 axis and CD8+ T cells, regulatory T cells, and myeloid-derived suppressor cells in soft tissue sarcoma. Front Immunol. 2018;9:1179.
Huangyang P, Li F, Lee P, Nissim I, Weljie AM, Mancuso A, et al. Fructose-1,6-bisphosphatase 2 inhibits sarcoma progression by restraining mitochondrial biogenesis. Cell Metab. 2020;31:174-188.e7.
Pillozzi S, Bernini A, Palchetti I, Crociani O, Antonuzzo L, Campanacci D, et al. Soft tissue sarcoma: an insight on biomarkers at molecular metabolic and cellular level. Cancers. 2021;13:3044.
Gaude E, Schmidt C, Gammage PA, Dugourd A, Blacker T, Chew SP, et al. NADH shuttling couples cytosolic reductive carboxylation of glutamine with glycolysis in cells with mitochondrial dysfunction. Mol Cell. 2018;69:581-593.e7.
Zhang B, Yang L, Wang X, Fu D. Identification of a survival-related signature for sarcoma patients through integrated transcriptomic and proteomic profiling analyses. Gene. 2021;764:145105.
Lee P, Malik D, Perkons N, Huangyang P, Khare S, Rhoades S, et al. Targeting glutamine metabolism slows soft tissue sarcoma growth. Nat Commun. 2020;11:498.
Sen N, Cross AM, Lorenzi PL, Khan J, Gryder BE, Kim S, et al. EWS-FLI1 reprograms the metabolism of Ewing sarcoma cells via positive regulation of glutamine import and serine-glycine biosynthesis. Mol Carcinog. 2018;57:1342–57.
Huang H-Y, Wu W-R, Wang Y-H, Wang J-W, Fang F-M, Tsai J-W, et al. ASS1 as a novel tumor suppressor gene in myxofibrosarcoma: aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin Cancer Res. 2013;19:2861–72.
Li T, Zhu Y, Cheng F, Lu C, Jung JU, Gao S-J. Oncogenic Kaposi’s sarcoma-associated herpesvirus upregulates argininosuccinate synthase 1, a rate-limiting enzyme of the citrulline-nitric oxide cycle, to activate the STAT3 pathway and promote growth transformation. J Virol. 2019;93:e01599-e1618.
Choi YM, Yeo HK, Park YW, Lee JY. Structural analysis of thymidylate synthase from Kaposi’s sarcoma-associated herpesvirus with the anticancer drug raltitrexed. PLoS ONE. 2016;11:e0168019.
Tanner JM, Bensard C, Wei P, Krah NM, Schell JC, Gardiner J, et al. EWS/FLI is a master regulator of metabolic reprogramming in Ewing sarcoma. Mol Cancer Res. 2017;15:1517–30.
Mutz CN, Schwentner R, Kauer MO, Katschnig AM, Kromp F, Aryee DNT, et al. EWS-FLI1 impairs aryl hydrocarbon receptor activation by blocking tryptophan breakdown via the kynurenine pathway. FEBS Lett. 2016;590:2063–75.
Giessner C, Millet V, Mostert KJ, Gensollen T, Vu Manh T-P, Garibal M, et al. Vnn1 pantetheinase limits the Warburg effect and sarcoma growth by rescuing mitochondrial activity. Life Sci Alliance. 2018;1:e201800073.
Lou S, Balluff B, Cleven AHG, Bovée JVMG, McDonnell LA. Prognostic metabolite biomarkers for soft tissue sarcomas discovered by mass spectrometry imaging. J Am Soc Mass Spectrom. 2017;28:376–83.
Li Y, Zhang W, Li S, Tu C. Prognosis value of Hypoxia-inducible factor-1α expression in patients with bone and soft tissue sarcoma: a meta-analysis. Springerplus. 2016;5:1–10.
Aryee DNT, Niedan S, Kauer M, Schwentner R, Bennani-Baiti IM, Ban J, et al. Hypoxia modulates EWS-FLI1 transcriptional signature and enhances the malignant properties of Ewing’s sarcoma cells in vitro. Can Res. 2010;70:4015–23.
Jham BC, Ma T, Hu J, Chaisuparat R, Friedman ER, Pandolfi PP, et al. Amplification of the angiogenic signal through the activation of the TSC/mTOR/HIF axis by the KSHV vGPCR in Kaposi’s sarcoma. PLoS ONE. 2011;6:e19103.
Kilic M, Kasperczyk H, Fulda S, Debatin K-M. Role of hypoxia inducible factor-1 alpha in modulation of apoptosis resistance. Oncogene. 2007;26:2027–38.
Merry E. Predictive and prognostic transcriptomic biomarkers in soft tissue sarcomas. npj Precis Oncol. 2021;5:1–8.
Zwang Y, Oren M, Yarden Y. Consistency test of the cell cycle: roles for p53 and EGR1. Can Res. 2012;72:1051–4.
Jones SM, Kazlauskas A. Growth-factor-dependent mitogenesis requires two distinct phases of signalling. Nat Cell Biol. 2001;3:165–72.
Liu H, Nazmun N, Hassan S, Liu X, Yang J. BRAF mutation and its inhibitors in sarcoma treatment. Cancer Med. 2020;9:4881–96.
Janku F, Hong DS, Fu S, Piha-Paul SA, Naing A, Falchook GS, et al. Assessing PIK3CA and PTEN in early-phase trials with PI3K/AKT/mTOR inhibitors. Cell Rep. 2014;6:377–87.
García-Valverde A, Rosell J, Serna G, Valverde C, Carles J, Nuciforo P, et al. Preclinical activity of PI3K inhibitor copanlisib in gastrointestinal stromal tumor. Mol Cancer Ther. 2020;19:1289–97.
Damodaran S, Zhao F, Deming DA, Mitchell EP, Wright JJ, Doyle LA, et al. Phase II study of copanlisib in patients with tumors with PIK3CA mutations (PTEN loss allowed): NCI MATCH EAY131-Z1F. JCO Wolters Kluwer. 2020;38:3506–3506.
Pollack SM, Ingham M, Spraker MB, Schwartz GK. Emerging targeted and immune-based therapies in sarcoma. JCO. 2018;36:125–35.
Babichev Y, Kabaroff L, Datti A, Uehling D, Isaac M, Al-awar R, et al. PI3K/AKT/mTOR inhibition in combination with doxorubicin is an effective therapy for leiomyosarcoma. J Transl Med. 2016;14:1–12.
Desai C, Thomason J, Kohlmeyer JL, Reisetter AC, Ahirwar P, Jahanseir K, et al. Prognostic and therapeutic value of the Hippo pathway, RABL6A, and p53-MDM2 axes in sarcomas. Oncotarget Impact J. 2021;12:740–55.
Rytlewski JD, Scalora N, Garcia K, Tanas M, Toor F, Miller B, et al. Photodynamic therapy using Hippo pathway inhibitor verteporfin: a potential dual mechanistic approach in treatment of soft tissue sarcomas. Cancers (Basel). 2021;13:675.
Isfort I, Cyra M, Elges S, Kailayangiri S, Altvater B, Rossig C, et al. SS18-SSX-dependent YAP/TAZ signaling in synovial sarcoma. Clin Cancer Res. 2019;25:3718–31.
Bierbaumer L, Katschnig AM, Radic-Sarikas B, Kauer MO, Petro JA, Högler S, et al. YAP/TAZ inhibition reduces metastatic potential of Ewing sarcoma cells. Oncogenesis. 2021;10:1–13.
Tap WD, Villalobos VM, Cote GM, Burris H, Janku F, Mir O, et al. Phase I study of the mutant IDH1 inhibitor ivosidenib: safety and clinical activity in patients with advanced chondrosarcoma. J Clin Oncol. 2020;38:1693–701.
Lee D, Omofoye OA, Karnati T, Graff JP, Shahlaie K. Intracranial myeloid sarcoma presentation in distant acute myeloid leukemia remission. J Clin Neurosci. 2021;89:158–60.
Cojocaru E, Wilding C, Engelman B, Huang P, Jones RL. Is the IDH mutation a good target for chondrosarcoma treatment? Curr Mol Biol Rep. 2020;6:1–9.
Bean GR, Kremer JC, Prudner BC, Schenone AD, Yao J-C, Schultze MB, et al. A metabolic synthetic lethal strategy with arginine deprivation and chloroquine leads to cell death in ASS1-deficient sarcomas. Cell Death Dis. 2016;7:e2406–e2406.
Kremer JC, Prudner BC, Lange SES, Bean GR, Schultze MB, Brashears CB, et al. Arginine deprivation inhibits the Warburg effect and upregulates glutamine anaplerosis and serine biosynthesis in ASS1-deficient cancers. Cell Rep. 2017;18:991–1004.
Rooke M, Coupland LA, Truong T, Blackburn AC. Dichloroacetate is an effective treatment for sarcoma models in vitro and in vivo. Cancer Metab. 2014;2:P9.
Vasileva E, Warren M, Triche TJ, Amatruda JF. Dysregulated heparan sulfate proteoglycan metabolism promotes Ewing sarcoma tumor growth. bioRxiv. 2021. https://doi.org/10.1101/2021.05.25.445683.
Travelli C, Consonni FM, Sangaletti S, Storto M, Morlacchi S, Grolla AA, et al. Nicotinamide phosphoribosyltransferase acts as a metabolic gate for mobilization of myeloid-derived suppressor cells. Cancer Res. 2019;79:1938–51.
Hirota S. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80.
Goncalves MD, Hopkins BD, Cantley LC. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N Engl J Med. 2018;379:2052.
Farid M, Ngeow J. Sarcomas associated with genetic cancer predisposition syndromes: a review. Oncologist. 2016;21:1002–13.
Lee C-L, Mowery YM, Daniel AR, Zhang D, Sibley AB, Delaney JR, et al. Mutational landscape in genetically engineered, carcinogen-induced, and radiation-induced mouse sarcoma. JCI Insight. 2019;4:e128698.
Chen X, Stewart E, Shelat AA, Qu C, Bahrami A, Hatley M, et al. Targeting oxidative stress in embryonal rhabdomyosarcoma. Cancer Cell. 2013;24:710–24.
Pal A, Chiu HY, Taneja R. Genetics, epigenetics and redox homeostasis in rhabdomyosarcoma: emerging targets and therapeutics. Redox Biol. 2019;25:101124.
Zhang M, Linardic CM, Kirsch DG. RAS and ROS in rhabdomyosarcoma. Cancer Cell. 2013;24:689–91.
Tanner LB, Goglia AG, Wei MH, Sehgal T, Parsons LR, Park JO, et al. Four key steps control glycolytic flux in mammalian cells. Cell Syst. 2018;7:49-62.e8.
Amendola CR, Mahaffey JP, Parker SJ, Ahearn IM, Chen W-C, Zhou M, et al. KRAS4A directly regulates hexokinase 1. Nature. 2019;576:482–6.
Xu D, Shao F, Bian X, Meng Y, Liang T, Lu Z. The evolving landscape of noncanonical functions of metabolic enzymes in cancer and other pathologies. Cell Metab. 2021;33:33–50.
Gao X, Wang H, Yang JJ, Liu X, Liu Z-R. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell. 2012;45:598–609.
Li X, Jiang Y, Meisenhelder J, Yang W, Hawke DH, Zheng Y, et al. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol Cell. 2016;61:705–19.
Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–5.
Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–70.
Genadry KC, Pietrobono S, Rota R, Linardic CM. Soft tissue sarcoma cancer stem cells: an overview. Front Oncol. 2018;8:475.
Hatina J, Kripnerova M, Houfkova K, Pesta M, Kuncova J, Sana J, et al. Sarcoma stem cell heterogeneity. In: Birbrair A, editor., et al., Stem cells heterogeneity—novel concepts. Cham: Springer International Publishing; 2019. p. 95–118.
Kelleher FC, O’Sullivan H. FOXM1 in sarcoma: role in cell cycle, pluripotency genes and stem cell pathways. Oncotarget. 2016;7:42792–804.
Martins-Neves SR, Corver WE, Paiva-Oliveira DI, van den Akker BEWM, Briaire-de-Bruijn IH, Bovée JVMG, et al. Osteosarcoma stem cells have active Wnt/β-catenin and overexpress SOX2 and KLF4. J Cell Physiol. 2016;231:876–86.
Vališ K, Novák P. Targeting ERK-Hippo interplay in cancer therapy. Int J Mol Sci. 2020;21:3236.
Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–57.
Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, et al. Yap1 Activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–97.
St John MA, Tao W, Fei X, Fukumoto R, Carcangiu ML, Brownstein DG, et al. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet. 1999;21:182–6.
Zhou D, Conrad C, Xia F, Park J-S, Payer B, Yin Y, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress the development of hepatocellular carcinoma through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–38.
Mohamed AD, Shah N, Hettmer S, Vargesson N, Wackerhage H. Analysis of the relationship between the KRAS G12V oncogene and the Hippo effector YAP1 in embryonal rhabdomyosarcoma. Sci Rep. 2018;8:15674.
Koo JH, Guan K-L. Interplay between YAP/TAZ and Metabolism. Cell Metab. 2018;28:196–206.
Santinon G, Pocaterra A, Dupont S. Control of YAP/TAZ activity by metabolic and nutrient-sensing pathways. Trends Cell Biol. 2016;26:289–99.
Wang W, Xiao Z-D, Li X, Aziz KE, Gan B, Johnson RL, et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–9.
Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M, et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 2015;34:1349–70.
Mota MSV, Jackson WP, Bailey SK, Vayalil P, Landar A, Rostas JW, et al. Deficiency of tumor suppressor Merlin facilitates metabolic adaptation by co-operative engagement of SMAD-Hippo signaling in breast cancer. Carcinogenesis. 2018;39:1165–75.
Agresta L, Salloum R, Hummel TR, Ratner N, Mangano FT, Fuller C, et al. Malignant peripheral nerve sheath tumor: transformation in a patient with neurofibromatosis type 2. Pediatr Blood Cancer. 2019;66:e27520.
White SM, Avantaggiati ML, Nemazanyy I, Di Poto C, Yang Y, Pende M, et al. YAP/TAZ inhibition induces metabolic and signaling rewiring resulting in targetable vulnerabilities in NF2-deficient tumor cells. Dev Cell. 2019;49:425-443.e9.
Chen R, Zhu S, Fan X-G, Wang H, Lotze MT, Zeh HJ, et al. High mobility group protein B1 controls liver cancer initiation through yes-associated protein-dependent aerobic glycolysis. Hepatology. 2018;67:1823–41.
Zhang X, Li Y, Ma Y, Yang L, Wang T, Meng X, et al. Yes-associated protein (YAP) binds to HIF-1α and sustains HIF-1α protein stability to promote hepatocellular carcinoma cell glycolysis under hypoxic stress. J Exp Clin Cancer Res. 2018;37:216.
Rivera-Reyes A, Ye S, Marino GE, Egolf S, Ciotti GE, Chor S, et al. YAP1 enhances NF-κB-dependent and independent effects on clock-mediated unfolded protein responses and autophagy in sarcoma. Cell Death Dis. 2018;9:1108.
Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619–34.
Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell. 2015;162:540–51.
Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell. 2015;162:552–63.
Ma Q, Cavallin LE, Leung HJ, Chiozzini C, Goldschmidt-Clermont PJ, Mesri EA. A role for virally induced reactive oxygen species in Kaposi’s sarcoma herpesvirus tumorigenesis. Antioxid Redox Signal. 2013;18:80–90.
Ma T, Patel H, Babapoor-Farrokhran S, Franklin R, Semenza GL, Sodhi A, et al. KSHV induces aerobic glycolysis and angiogenesis through HIF-1-dependent upregulation of pyruvate kinase 2 in Kaposi’s sarcoma. Angiogenesis. 2015;18:477–88.
Delgado T, Carroll PA, Punjabi AS, Margineantu D, Hockenbery DM, Lagunoff M. Induction of the Warburg effect by Kaposi’s sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc Natl Acad Sci. 2010;107:10696–701.
Cavallin LE, Ma Q, Naipauer J, Gupta S, Kurian M, Locatelli P, et al. KSHV-induced ligand mediated activation of PDGF receptor-alpha drives Kaposi’s sarcomagenesis. PLoS Pathog. 2018;14:e1007175.
Veeranna RP, Haque M, Davis DA, Yang M, Yarchoan R. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen induction by hypoxia and hypoxia-inducible factors. J Virol. 2012;86:1097–108.
Choi UY, Lee JJ, Park A, Zhu W, Lee H-R, Choi YJ, et al. Oncogenic human herpesvirus hijacks proline metabolism for tumorigenesis. PNAS. 2020;117:8083–93.
Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–74.
Bose S, Allen AE, Locasale JW. The molecular link from diet to cancer cell metabolism. Mol Cell. 2020;78:1034–44.
Gao X, Sanderson SM, Dai Z, Reid MA, Cooper DE, Lu M, et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature. 2019;572:397–401.
Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–99.
Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab. 2013;17:745–55.
Karner CM, Esen E, Chen J, Hsu F-F, Turk J, Long F. Wnt protein signaling reduces nuclear acetyl-CoA levels to suppress gene expression during osteoblast differentiation. J Biol Chem. 2016;291:13028–39.
Frey JL, Li Z, Ellis JM, Zhang Q, Farber CR, Aja S, et al. Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol Cell Biol. 2015;35:1979–91.
Danieau G, Morice S, Rédini F, Verrecchia F, Royer BL. New insights about the Wnt/β-catenin signaling pathway in primary bone tumors and their microenvironment: a promising target to develop therapeutic strategies? IJMS. 2019;20:3751.
Fuxe J, Vincent T, Garcia de Herreros A. Transcriptional crosstalk between TGFβ and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexes. Cell Cycle. 2010;9:2363–74.
Briski LM, Thomas DG, Patel RM, Lawlor ER, Chugh R, McHugh JB, et al. Canonical Wnt/β-catenin signaling activation in soft-tissue sarcomas: a comparative study of synovial sarcoma and leiomyosarcoma. Rare Tumors. 2018;10:203636131881343.
Du X, Yang J, Yang D, Tian W, Zhu Z. The genetic basis for inactivation of Wnt pathway in human osteosarcoma. BMC Cancer. 2014;14:450.
Wang W-G, Chen S-J, He J-S, Li J-S, Zang X-F. The tumor suppressive role of RASSF1A in osteosarcoma through the Wnt signaling pathway. Tumor Biol. 2016;37:8869–77.
Li Y, Zhang S, Zhang C, Wang M. LncRNA MEG3 inhibits the inflammatory response of ankylosing spondylitis by targeting miR-146a. Mol Cell Biochem. 2020;466:17–24.
Techavichit P, Gao Y, Kurenbekova L, Shuck R, Donehower LA, Yustein JT. Secreted Frizzled-Related Protein 2 (sFRP2) promotes osteosarcoma invasion and metastatic potential. BMC Cancer. 2016;16:869.
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7.
Barretina J, Taylor BS, Banerji S, Ramos AH, Lagos-Quintana M, DeCarolis PL, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42:715–21.
Blay J-Y, Ray-Coquard I. Evolving biological understanding and treatment of sarcomas. Nat Rev Clin Oncol. 2017;14:78–80.
Bui NQ. A clinico-genomic analysis of soft tissue sarcoma patients reveals CDKN2A deletion as a biomarker for poor prognosis. Clin Sarcoma Res. 2019;9:11.
Mito JK, Riedel RF, Dodd L, Lahat G, Lazar AJ, Dodd RD, et al. Cross species genomic analysis identifies a mouse model as undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma. PLoS ONE. 2009;4:e8075.
Kirsch DG, Dinulescu DM, Miller JB, Grimm J, Santiago PM, Young NP, et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat Med. 2007;13:992–7.
Liu J, Zhang C, Hu W, Feng Z. Tumor suppressor p53 and its mutants in cancer metabolism. Cancer Lett. 2015;356:197–203.
Simabuco FM, Morale MG, Pavan ICB, Morelli AP, Silva FR, Tamura RE. p53 and metabolism: from mechanism to therapeutics. Oncotarget. 2018;9:23780–823.
Valente LJ, Gray DHD, Michalak EM, Pinon-Hofbauer J, Egle A, Scott CL, et al. p53 efficiently suppresses tumor development in the complete absence of its cell-cycle inhibitory and proapoptotic effectors p21, Puma, and Noxa. Cell Rep. 2013;3:1339–45.
Vousden KH, Ryan KM. p53 and metabolism. Nat Rev Cancer. 2009;9:691–700.
Ahrens WA, Ridenour RV, Caron BL, Miller DV, Folpe AL. GLUT-1 expression in mesenchymal tumors: an immunohistochemical study of 247 soft tissue and bone neoplasms. Hum Pathol. 2008;39:1519–26.
Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 2004;64:2627–33.
Bensaad K, Tsuruta A, Selak MA, Vidal MNC, Nakano K, Bartrons R, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–20.
Zhang C, Lin M, Wu R, Wang X, Yang B, Levine AJ, et al. Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proc Natl Acad Sci. 2011;108:16259–64.
Jiang P, Du W, Wang X, Mancuso A, Gao X, Wu M, et al. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol. 2011;13:15.
Erpapazoglou Z, Corti O. The endoplasmic reticulum/mitochondria interface: a subcellular platform for the orchestration of the functions of the PINK1–Parkin pathway? Biochem Soc Trans. 2015;43:297–301.
Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol. 2015;16:393–405.
Morris JP, Yashinskie JJ, Koche R, Chandwani R, Tian S, Chen C-C, et al. α-Ketoglutarate links p53 to cell fate during tumour suppression. Nature. 2019;573:595–9.
Gaude E, Frezza C. Tissue-specific and convergent metabolic transformation of cancer correlates with metastatic potential and patient survival. Nat Commun. 2016;7:13041.
Benz MR, Tchekmedyian N, Eilber FC, Federman N, Czernin J, Tap WD. Utilization of positron emission tomography in the management of patients with sarcoma. Curr Opin Oncol. 2009;21:345–51.
Wagner LM, Kremer N, Gelfand MJ, Sharp SE, Turpin BK, Nagarajan R, et al. Detection of lymph node metastases in pediatric and adolescent/young adult sarcoma: sentinel lymph node biopsy versus fludeoxyglucose positron emission tomography imaging—a prospective trial: sentinel lymph node biopsy versus PET in sarcoma. Cancer. 2017;123:155–60.
Issaq SH, Teicher BA, Monks A. Bioenergetic properties of human sarcoma cells help define sensitivity to metabolic inhibitors. Cell Cycle. 2014;13:1152–61.
Dasgupta A, Trucco M, Rainusso N, Bernardi RJ, Shuck R, Kurenbekova L, et al. Metabolic modulation of Ewing sarcoma cells inhibits tumor growth and stem cell properties. Oncotarget. 2017;8:77292–308.
Mao L, Dauchy RT, Blask DE, Dauchy EM, Slakey LM, Brimer S, et al. Melatonin suppression of aerobic glycolysis (Warburg effect), survival signalling and metastasis in human leiomyosarcoma. J Pineal Res. 2016;60:167–77.
Li B, Qiu B, Lee DSM, Walton ZE, Ochocki JD, Mathew LK, et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513:251–5.
Issaq SH, Mendoza A, Fox SD, Helman LJ. Glutamine synthetase is necessary for sarcoma adaptation to glutamine deprivation and tumor growth. Oncogenesis. 2019;8:20.
Han J, Zhang Y, Xu J, Zhang T, Wang H, Wang Z, et al. Her4 promotes cancer metabolic reprogramming via the c-Myc-dependent signaling axis. Cancer Lett. 2021;496:57–71.
Masoud R, Reyes-Castellanos G, Lac S, Garcia J, Dou S, Shintu L, et al. Targeting mitochondrial complex i overcomes chemoresistance in high OXPHOS pancreatic cancer. Cell Rep Med. 2020;1:100143.
Mor I, Cheung EC, Vousden KH. Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb Symp Quant Biol. 2011;76:211–6.
Moreno-Sánchez R, Marín-Hernández A, Gallardo-Pérez JC, Quezada H, Encalada R, Rodríguez-Enríquez S, et al. Phosphofructokinase type 1 kinetics, isoform expression, and gene polymorphisms in cancer cells. J Cell Biochem. 2012;113:1692–703.
Cabrera R, Baez M, Pereira HM. Kinetic and structural analysis of the allosteric ATP inhibition S. J Biol Chem. 2011;286:11.
Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol. 2009;86:174–9.
Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23:459–66.
Borst P. The malate–aspartate shuttle (Borst cycle): how it started and developed into a major metabolic pathway. IUBMB Life. 2020;72:2241–59.
Dai Z, Shestov AA, Lai L, Locasale JW. A flux balance of glucose metabolism clarifies the requirements of the Warburg effect. Biophys J. 2016;111:1088–100.
Altinok O, Poggio JL, Stein DE, Bowne WB, Shieh AC, Snyder NW, et al. Malate–aspartate shuttle promotes l-lactate oxidation in mitochondria. J Cell Physiol. 2020;235:2569–81.
Young A, Oldford C, Mailloux RJ. Lactate dehydrogenase supports lactate oxidation in mitochondria isolated from different mouse tissues. Redox Biol. 2020;28:101339.
Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51.
Ippolito L, Giannoni E, Chiarugi P, Parri M. Mitochondrial redox hubs as promising targets for anticancer therapy. Front Oncol. 2020;10:256.
Titova E, Shagieva G, Ivanova O, Domnina L, Domninskaya M, Strelkova O, et al. Mitochondria-targeted antioxidant SkQ1 suppresses fibrosarcoma and rhabdomyosarcoma tumour cell growth. Cell Cycle. 2018;17:1797–811.
Leonardi R, Zhang Y, Rock C, Jackowski S. Coenzyme A: back in action. Prog Lipid Res. 2005;44:125–53.
Granjeaud S, Naquet P, Galland F. An ESTs description of the new Vanin gene family conserved from fly to human. Immunogenetics. 1999;49:964–72.
Naquet P, Kerr EW, Vickers SD, Leonardi R. Regulation of coenzyme A levels by degradation: the ‘Ins and Outs.’ Prog Lipid Res. 2020;78:101028.
Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta (BBA) Gene Struct Expr. 2002;1576:1–14.
Bakkar N, Wang J, Ladner KJ, Wang H, Dahlman JM, Carathers M, et al. IKK/NF-κB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol. 2008;180:787–802.
Shintaku J, Peterson JM, Talbert EE, Gu J-M, Ladner KJ, Williams DR, et al. MyoD regulates skeletal muscle oxidative metabolism cooperatively with alternative NF-κ B. Cell Rep. 2016;17:514–26.
Londhe P, Yu PY, Ijiri Y, Ladner KJ, Fenger JM, London C, et al. Classical NF-κB metabolically reprograms sarcoma cells through regulation of hexokinase 2. Front Oncol. 2018;8:104.
Jahnke VE, Sabido O, Defour A, Castells J, Lefai E, Roussel D, et al. Evidence for mitochondrial respiratory deficiency in rat rhabdomyosarcoma cells. PLoS ONE. 2010;5:e8637.
Aspuria P-JP, Lunt SY, Väremo L, Vergnes L, Gozo M, Beach JA, et al. Succinate dehydrogenase inhibition leads to epithelial–mesenchymal transition and reprogrammed carbon metabolism. Cancer Metab. 2014;2:21.
Sulkowski PL, Oeck S, Dow J, Economos NG, Mirfakhraie L, Liu Y, et al. Oncometabolites suppress DNA repair by disrupting local chromatin signalling. Nature. 2020;582:586–91.
Salminen A, Kauppinen A, Kaarniranta K. 2-Oxoglutarate-dependent dioxygenases are sensors of energy metabolism, oxygen availability, and iron homeostasis: potential role in the regulation of aging process. Cell Mol Life Sci. 2015;72:3897–914.
Pollock RE, Randall RL, O’Sullivan B. Sarcoma oncology: a multidisciplinary approach. New York: PMPH USA; 2019.
Hoffmann A-C, Danenberg KD, Taubert H, Danenberg PV, Wuerl P. A three-gene signature for outcome in soft tissue sarcoma. Clin Cancer Res. 2009;15:5191–8.
Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865–78.
Sadri N, Zhang P. Hypoxia-inducible factors: mediators of cancer progression; prognostic and therapeutic targets in soft tissue sarcomas. Cancers. 2013;5:320–33.
Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008;26:1818–30.
Bott AJ, Maimouni S, Zong W-X. The pleiotropic effects of glutamine metabolism in cancer. Cancers (Basel). 2019;11:770.
Jackson M, Serada N, Sheehan M, Srinivasan S, Mason N, Guha M, et al. Mitochondrial genome and functional defects in osteosarcoma are associated with their aggressive phenotype. PLoS ONE. 2018;13:e0209489.
Srinivasan S, Guha M, Dong DW, Whelan KA, Ruthel G, Uchikado Y, et al. Disruption of cytochrome c oxidase function induces the Warburg effect and metabolic reprogramming. Oncogene. 2016;35:1585–95.
Srinivasan S, Guha M, Kashina A, Avadhani NG. Mitochondrial dysfunction and mitochondrial dynamics—the cancer connection. Biochim Biophys Acta (BBA) Bioenerg. 2017;1858:602–14.
Guha M, Srinivasan S, Ruthel G, Kashina AK, Carstens RP, Mendoza A, et al. Mitochondrial retrograde signaling induces epithelial–mesenchymal transition and generates breast cancer stem cells. Oncogene. 2014;33:5238–50.
Yizhak K, Le Dévédec SE, Rogkoti VM, Baenke F, Boer VC, Frezza C, et al. A computational study of the Warburg effect identifies metabolic targets inhibiting cancer migration. Mol Syst Biol. 2014;10:744.
Gouirand V, Guillaumond F, Vasseur S. Influence of the tumor microenvironment on cancer cells metabolic reprogramming. Front Oncol. 2018;8:117.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98.
Bittner JG, Wilson M, Shah MB, Albo D, Feig BW, Wang TN. Fibroblast-conditioned media promote human sarcoma cell invasion. Surgery. 2009;145:42–7.
Bonuccelli G, Avnet S, Grisendi G, Salerno M, Granchi D, Dominici M, et al. Role of mesenchymal stem cells in osteosarcoma and metabolic reprogramming of tumor cells. Oncotarget. 2014;5:7575–88.
Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, et al. Ketones and lactate “fuel” tumor growth and metastasis. Cell Cycle. 2014;9:9.
Dai L, Qin Z, Defee M, Toole BP, Kirkwood KL, Parsons C. Kaposi sarcoma-associated herpesvirus (KSHV) induces a functional tumor-associated phenotype for oral fibroblasts. Cancer Lett. 2012;318:214–20.
De Saedeleer CJ, Copetti T, Porporato PE, Verrax J, Feron O, Sonveaux P. Lactate activates HIF-1 in oxidative but not in Warburg-phenotype human tumor cells. PLoS ONE. 2012;7:e46571.
Sotgia F, Martinez-Outschoorn UE, Lisanti MP. The reverse Warburg effect in osteosarcoma. Oncotarget. 2014;5:7982.
Goodwin ML, Jin H, Straessler K, Smith-Fry K, Zhu J-F, Monument MJ, et al. Modeling alveolar soft part sarcomagenesis in the mouse: a role for lactate in the tumor microenvironment. Cancer Cell. 2014;26:851–62.
Porporato PE. Mitochondrial metabolism and cancer. Cell Res. 2018;28:16.
Danhier P, Bański P, Payen VL, Grasso D, Ippolito L, Sonveaux P, et al. Cancer metabolism in space and time: beyond the Warburg effect. Biochim Biophys Acta (BBA) Bioenerg. 2017;1858:556–72.
Porporato PE. Metabolic changes associated with tumor metastasis, part 2: mitochondria, lipid and amino acid metabolism. Cell Mol Life Sci. 2016;73:1349–63.
Harati K, Daigeler A, Hirsch T, Jacobsen F, Behr B, Wallner C, et al. Tumor-associated fibroblasts promote the proliferation and decrease the doxorubicin sensitivity of liposarcoma cells. Int J Mol Med. 2016;37:1535–41.
Bellairs R, Van Peteghem M-C. Gastrulation: is it analogous to malignant invasion. Am Zool. 1984;24:563–70.
Sannino G, Marchetto A, Kirchner T, Grünewald TGP. Epithelial-to-mesenchymal and mesenchymal-to-epithelial transition in mesenchymal tumors: a paradox in sarcomas? Cancer Res. 2017;77:4556–61.
Chaklader M, Pan A, Law A, Chattopadhayay S, Chatterjee R, Law S. Differential remodeling of cadherins and intermediate cytoskeletal filaments influence microenvironment of solid and ascitic sarcoma. Mol Cell Biochem. 2013;382:293–306.
Kahlert UD, Joseph JV, Kruyt FAE. EMT- and MET-related processes in nonepithelial tumors: importance for disease progression, prognosis, and therapeutic opportunities. Mol Oncol. 2017;11:860–77.
Tian W, Wang G, Yang J, Pan Y, Ma Y. Prognostic role of E-cadherin and Vimentin expression in various subtypes of soft tissue leiomyosarcomas. Med Oncol. 2013;30:401.
Saito T. The SYT-SSX fusion protein and histological epithelial differentiation in synovial sarcoma: relationship with extracellular matrix remodeling. Int J Clin Exp Pathol. 2013;6:2272.
Thuault S, Hayashi S, Lagirand-Cantaloube J, Plutoni C, Comunale F, Delattre O, et al. P-cadherin is a direct PAX3–FOXO1A target involved in alveolar rhabdomyosarcoma aggressiveness. Oncogene. 2013;32:1876–87.
Hua W, ten Dijke P, Kostidis S, Giera M, Hornsveld M. TGFβ-induced metabolic reprogramming during epithelial-to-mesenchymal transition in cancer. Cell Mol Life Sci. 2020;77:2103–23.
Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9:317–24.
El-Naggar AM, Veinotte CJ, Cheng H, Grunewald TGP, Negri GL, Somasekharan SP, et al. Translational activation of HIF1α by YB-1 promotes sarcoma metastasis. Cancer Cell. 2015;27:682–97.
Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–9.
Eisinger-Mathason TSK, Zhang M, Qiu Q, Skuli N, Nakazawa MS, Karakasheva T, et al. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 2013;3:1190–205.
Petitprez F, Meylan M, de Reyniès A, Sautès-Fridman C, Fridman WH. The tumor microenvironment in the response to immune checkpoint blockade therapies. Front Immunol. 2020;11:784.
Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang T-H, et al. The immune landscape of cancer. Immunity. 2018;48:812-830.e14.
Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34.
Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52:17–35.
Fletcher CDM. The evolving classification of soft tissue tumours—an update based on the new 2013 WHO classification. Histopathology. 2014;64:2–11.
Cohen JE, Eleyan F, Zick A, Peretz T, Katz D. Intratumoral immune-biomarkers and mismatch repair status in leiyomyosarcoma -potential predictive markers for adjuvant treatment: a pilot study. Oncotarget. 2018;9:30847–54.
D’Angelo SP, Tap WD, Schwartz GK, Carvajal RD. Sarcoma immunotherapy: past approaches and future directions. Sarcoma. 2014;2014:1–13.
van Erp AEM, Versleijen-Jonkers YMH, Hillebrandt-Roeffen MHS, van Houdt L, Gorris MAJ, van Dam LS, et al. Expression and clinical association of programmed cell death-1, programmed death-ligand-1 and CD8+ lymphocytes in primary sarcomas is subtype dependent. Oncotarget. 2017;8:71371–84.
Wedekind MF, Wagner LM, Cripe TP. Immunotherapy for osteosarcoma: where do we go from here? Pediatr Blood Cancer. 2018;65:e27227.
Dancsok AR, Setsu N, Gao D, Blay J-Y, Thomas D, Maki RG, et al. Expression of lymphocyte immunoregulatory biomarkers in bone and soft-tissue sarcomas. Mod Pathol. 2019;32:1772–85.
Feng X, Pleasance E, Zhao EY, Ng T, Grewal JK, Mohammad N, et al. Therapeutic implication of genomic landscape of adult metastatic sarcoma. JCO Precis Oncol. 2019;3:1–25.
Keung EZ, Tsai J-W, Ali AM, Cormier JN, Bishop AJ, Guadagnolo BA, et al. Analysis of the immune infiltrate in undifferentiated pleomorphic sarcoma of the extremity and trunk in response to radiotherapy: rationale for combination neoadjuvant immune checkpoint inhibition and radiotherapy. Oncoimmunology. 2017;7:e1385689.
Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19:307–25.
Varn FS, Wang Y, Mullins DW, Fiering S, Cheng C. Systematic pan-cancer analysis reveals immune cell interactions in the tumor microenvironment. Cancer Res. 2017;77:1271–82.
Locasale JW. Serine, glycine and the one-carbon cycle: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572–83.
Hayward SL, Scharer CD, Cartwright EK, Takamura S, Li Z-RT, Boss JM, et al. Environmental cues regulate epigenetic reprogramming of airway-resident memory CD8+ T cells. Nat Immunol. 2020;21:309–20.
Yan D, Adeshakin AO, Xu M, Afolabi LO, Zhang G, Chen YH, et al. Lipid metabolic pathways confer the immunosuppressive function of myeloid-derived suppressor cells in tumor. Front Immunol. 2019;10:1399.
Weinberg SE, Singer BD, Steinert EM, Martinez CA, Mehta MM, Martínez-Reyes I, et al. Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature. 2019;565:495–9.
Kumagai S, Togashi Y, Sakai C, Kawazoe A, Kawazu M, Ueno T, et al. An oncogenic alteration creates a microenvironment that promotes tumor progression by conferring a metabolic advantage to regulatory T cells. Immunity. 2020;53:187-203.e8.
Choi SYC, Collins CC, Gout PW, Wang Y. Cancer-generated lactic acid: a regulatory, immunosuppressive metabolite? J Pathol. 2013;230:350–5.
Naquet P, Giessner C, Galland F. Metabolic adaptation of tissues to stress releases metabolites influencing innate immunity. Curr Opin Immunol. 2016;38:30–8.
Wang H, Franco F, Tsui Y-C, Xie X, Trefny MP, Zappasodi R, et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat Immunol. 2020;21:23.
Acknowledgements
We wish to thank Alice Carrier, Romain Roncagalli, Rafael Arguello and Jean Erland Ricci for their critical reading of the manuscript and Jonathan Ewbank for revising English expression. Guillaume Charbonnier performed the bioinformatics analysis of the TCGA database.
Funding
Richard Miallot is a recipient of an INCA doctoral fellowship (PLBIO19-015). Philippe Naquet is supported by institutional funding from CNRS, INSERM, and AMU, as well as grants from Fondation pour la Recherche Médicale (DEQ20140329532), ARC (PJA 20181208002) and INCA (PLBIO19-015). Jean Yves Blay is supported by funds from NetSARC, LYRIC (INCA-DGOS 4664), LYon Recherche Innovation contre le CANcer, European Clinical trials in Rare Sarcomas (FP7-278742), and European network for Rare Adult solid Cancer.
Author information
Authors and Affiliations
Contributions
RM and PN drafted the manuscript and prepared the figures. RM, VM, FG collected the references and participated in the discussion. JYB and PN designed the review. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Miallot, R., Galland, F., Millet, V. et al. Metabolic landscapes in sarcomas. J Hematol Oncol 14, 114 (2021). https://doi.org/10.1186/s13045-021-01125-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-021-01125-y