Abstract
Backgrounds
The incidence of gastric cardiac cancer (GCC) has obviously increased recently with poor prognosis. It’s necessary to compare GCC prognosis with other gastric sites carcinoma and set up an effective prognostic model based on a neural network to predict the survival of GCC patients.
Methods
In the population-based cohort study, we first enrolled the clinical features from the Surveillance, Epidemiology and End Results (SEER) data (n = 31,397) as well as the public Chinese data from different hospitals (n = 1049). Then according to the diagnostic time, the SEER data were then divided into two cohorts, the train cohort (patients were diagnosed as GCC in 2010–2014, n = 4414) and the test cohort (diagnosed in 2015, n = 957). Age, sex, pathology, tumor, node, and metastasis (TNM) stage, tumor size, surgery or not, radiotherapy or not, chemotherapy or not and history of malignancy were chosen as the predictive clinical features. The train cohort was utilized to conduct the neural network-based prognostic predictive model which validated by itself and the test cohort. Area under the receiver operating characteristics curve (AUC) was used to evaluate model performance.
Results
The prognosis of GCC patients in SEER database was worse than that of non GCC (NGCC) patients, while it was not worse in the Chinese data. The total of 5371 patients were used to conduct the model, following inclusion and exclusion criteria. Neural network-based prognostic predictive model had a satisfactory performance for GCC overall survival (OS) prediction, which owned 0.7431 AUC in the train cohort (95% confidence intervals, CI, 0.7423–0.7439) and 0.7419 in the test cohort (95% CI, 0.7411–0.7428).
Conclusions
GCC patients indeed have different survival time compared with non GCC patients. And the neural network-based prognostic predictive tool developed in this study is a novel and promising software for the clinical outcome analysis of GCC patients.
Similar content being viewed by others
Background
Gastric cancer (GC), as the GLOBOCAN 2020 reported, is the fifth most common cancer and the fourth primary cause of tumor-related death globally with over 1.08 million new cases and nearly 0.77 million deaths (about one out of every 13 deaths died of gastric cancer) [1]. The incidence of males is more than twice that of females. Gastric cancer can be anatomically divided into two categories: gastric cardiac cancer (GCC) and other sites of GC (non-gastric cardiac cancer, NGCC). Generally speaking, the midpoint of the GCC is between 1 cm proximal and 2 cm distal from the gastroesophageal junction with endoscopic image acquisition of different parts of stomach [2] (Fig. 1). In the past half century, the incidence of NGCC has decreased, replaced by the high incidence of GCC worldwide [3, 4]. Due to the rapid progression and metastasis, the prognosis of GCC is poor, for 5-year overall survival (OS) rate is only about 9–25% [5,6,7]. Black and white ethnicity with GCC had a higher mortality rate than yellow ethnicity, and the eastern region had a better prognosis than the western region globally [8]. Given the prognosis of GCC was different from that of NGCC, it is necessary to explore this clinical issue further [9]. GCC doesn’t have specific symptoms at its early stages and lacks effective diagnostic techniques either, which might contribute to the increasing mortality rate [6, 10]. Studies have found that some clinical features were related to its poor prognosis. For example, it has been observed that tumor size and its anatomical location might be related to GCC outcome [11]. Therefore, it is encouraging and makes sense to predict the prognosis of GCC patients.
To help predict survival outcomes and make treatment decisions, the American Joint Committee on Cancer (AJCC) staging system has been developed and widely used to classify patients based on tumor, node, and metastasis (TNM) stage [12]. However, the AJCC staging system is still controversial to predict the prognosis of GCC patients who received comprehensive treatment [13]. In order to improve the accuracy of the survival estimations in GCC patients, a nomogram based on the traditional Cox proportional hazards (CPH) has been used to achieve that by some clinical researchers [14,15,16,17]. Nomogram is a graph that aggregates various predictive factors through multiple regression analysis, and can be used to intuitively predict patient outcomes, such as OS or cancer-specific survival rate (CSS). Nevertheless, these models had several limitations in time-to-event prediction for the clinical management [18]. CPH is conducted based on the linear hypotheses but the occurrence and development of tumors are influenced by many non-liner factors. So, it is not sufficient to perform linear hypotheses alone to explore the relationship between patients’ covariates (such as clinical and genetic characteristics) and the effectiveness of various treatment options in the real-world. Therefore, better models or methods are required for nonlinear variable analysis further.
With the rapid development of artificial intelligence (AI), AI is increasingly used in the various area. For example, Khan et al. [19] proposed a novel combination of optimized intelligent smart irrigation systems to improve the energy management performance of the system. Irshad et al. [20] developed a Heap Optimization Based Generalized Intelligent Neural Fuzzy Control (HO-GINFC) for estimating the cooling load of an air conditioning system with cold thermal storage. An artificial ecosystem optimization with Deep Learning Enabled Water Quality Prediction and Classification (AEODL-WQPC) model presented by Islam et al. [21] which was utilized to predict and categorize water quality level. Kumar et al. [22] established rooted elliptic curve cryptography with Vigenère cipher (RECC-VC) centered security amelioration on the IoMT to enhance security. Praveen et al. [23] found that the FastAI technology could be used with the ResNet-32 model to precisely identify breast ductal carcinoma. Vulli et al. [24] ascertained the fine-tuned DenseNet-169 had improved considerably histopathologic interpretation and diagnostic accuracy using the FastAI framework and the 1-cycle policy. Deep learning, also known as neural network, a research direction in the field of machine learning and AI, could be used to solve multifactor and nonlinear problems more appropriately [25]. Katzman et al. [26] developed a novel deep learning survival theory called DeepSurv, a multi-layer feed-forward network composed by an artificial neural network (ANN) and CPH, which could integrate the nonlinear risk function related to outcomes and was more flexible to deal with complex clinical factors in the real-world, so as to predict the result events. The authors and previous researchers have demonstrated that DeepSurv performed better than other linear prediction models like CPH and could be a useful tool in providing better treatment recommendations [27, 28].
Our study demonstrated GCC patients indeed have different survival time compared with NGCC patients. So we aimed to develop and validate a prognostic model for GCC patients by applying neural network survival theory DeepSurv, so that it might offer some references for doctors on clinical decision-making, after packaging it into a convenient windows desktop tool.
Methods
Research design and data sources
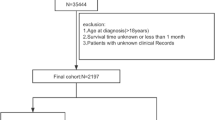
This retrospective cohort study used clinical data from American the Surveillance, Epidemiology, and End Results (SEER) (https://seer.cancer.gov/) database and China National Human Genetic Resources Sharing Service Platform (http://www.superchip.com.cn/technology/Default.aspx). First, we compared the prognosis of GC occurring different sites using the American data and the Chinese data, following these criteria: patients were diagnosed with GC pathologically; complete tumor site record and follow-up information especially survival status; the SEER 17 Registries database (2000–2019) was used this time. After finding GCC might have different prognosis with NGCC, we screened SEER data again, to conduct GCC neural network-based predictive tool, following these inclusion criteria: (1) SEER 17 Registries database (2000–2019), (2) Site and Morphology Site recode ICD-O-3/WHO 2008 was Stomach, (3) Behavior code ICD-O-3 was malignant, (4) Primary Site – labeled was Cardia, (5) detailed AJCC 7th edition TNM stage (patients diagnosed in 2010–2015), and following this exclusion criteria: data with missing values. As the Chinese data included many missing values on GCC patients’ therapy, they were not suitable to conduct the predictive tool. SEER data were then divided into two cohorts, the train cohort (patients diagnosed as GCC in 2010–2014) and the test cohort (diagnosed in 2015). The train cohort was utilized to conduct the neural network-based prognostic predictive model which validated by itself and the test cohort (Fig. 2). The principal study endpoint was OS. The follow-up cutoff date was December 31, 2019, according to the SEER research data description.
This study has been approved by the Ethics Committee of Beijing Chest Hospital affiliated to Capital Medical University (No. LW-2022–008).
Predictive variables and pre-processing
According to clinical experience, age, sex, pathology, T, N, M, stage, size of tumor, surgery or not, radiotherapy or not, chemotherapy or not and history of malignancy were the predictive clinical features. According to the recording rules of SEER, age greater than 100 years old remained registered as 100. And survival time less than 1 month was regarded as 1. Before modeling, numerical clinical features (age and tumor size) were standardized (minus the mean divided by the standard deviation), and categorical variables were converted to dummy variables. Test cohort was processed in terms of the train cohort (Supplement Table 1).
Neural network model training and packaging
To get more accurate prediction and avoid underfitting, we used batch normalization and batch training. Further, to avoid overfitting, early stopping callback and dropout layers were applied. The training curves were saved in Supplement Fig. 1.
Area under the receiver operating characteristics curve (AUC) was used to evaluate model performance. A better model usually scores an AUC closer to 1. As mentioned earlier, the model was trained using the train cohort, but evaluated with both the train and the test cohorts.
Finally, neural network-based prognostic predictive model for GCC was packaged into a tool (an executable program in Microsoft Windows 11 64bit).
Statistical analysis
All statistical analyses were completed with R software (https://www.r-project.org). Wilcoxon signed-rank test was used for numerical data of skewed distribution, and Chi-square test was performed on categorical data. The Kaplan–Meier curves and log rank test were used to compare the prognosis of different tumor sites of stomach. P value of two-sided smaller than 0.05 was considered statistically significant.
Results
Prognosis of gastric cancer varies by sites
We used the SEER data and the Chinese data to compare the prognosis of GC at the different sites. GCC might have different prognosis compared with other sites of GC. In SEER data, cancers in overlapping lesion of stomach had the worst prognosis, GCC the second worst and greater curvature of stomach had the best prognosis (N = 31,397, P < 0.0001). In China data, GC in overlapping lesion still had the worst survival, but pylorus cancer had the second worst prognosis and GCC had a moderate prognosis (N = 1049, P < 0.0001) (Fig. 3).
Patients’ demographic information
Following our inclusion and exclusion criteria, 5371 patients were included finally. There were 4414 patients in train cohort and 957 patients in test cohort. As Table 1 showed, patients from both train cohort and test cohort had similar clinical features in age, sex, pathology, TNM stage, tumor size, surgery ratio, chemotherapy ratio and history of malignancy. The median age of train cohort was 67 years, and that of test cohort was the same. Train cohort included 20.80% females and test cohort had 21.11% females. The most common pathology was adenocarcinoma, in both cohorts. Most patients were staged T3, N0, or M0 in both cohorts. In train cohort, most patients were diagnosed as IV, but in test cohort IIIA was the most common stage. The median of tumor size was both 40 mm in two cohorts. In train and test cohorts, most patients got surgery or chemotherapy. But as for radiotherapy, test cohort patients most received it, while train cohort patients most not. And most patients in both two cohorts did not have a history of malignancy.
Model performance and usage
Neural network-based prognostic predictive model for GCC owned 0.7431 AUC in train cohort (95%, confidence intervals, CI, 0.7423–0.7439) and 0.7419 in test cohort (95% CI, 0.7411–0.7428) (Table 2). This model had a satisfactory performance. We then packaged it into an EXE file. When clicking the Main.exe file after unzipping Supplement File 1 (the linkage: https://drive.google.com/file/d/11-1k1rkx5fLuwcFAQuSlmqhtmVRTOt3q/view?usp=share_link), we could run the neural network-based prognostic predictive tool for GCC (Fig. 4). After clinician and researcher inputted a patient’s demographic information and clicked Predict button, the tool would calculate the OS possibility of this patient and draw his survival curves automatically. The survival curves were shown in users’ web browser, and could be zoomed in or out interactively to show the OS for a specific month.
Discussion
GCC is a special malignant tumor located at the gastroesophageal junction (GEJ). The mucosa of gastric cardia is mainly composed of pure mucous and mixed mucoxyntic glands, with few parietal cells and scattered endocrine cells, but no chief cells [29]. It was reported that there were great differences from GCC with tumors of esophagus or distal stomach in epidemiological and biological behavior [6]. To date, its etiology is still unclear [30]. But some previous studies have shown that the prevalence of GCC was strongly correlated with aging, smoking, young women, Helicobacter pylori infection and Epstein-Barr virus (EBV) infection [31]. Meanwhile, there is no agreement on the accurate staging of GCC patients, though some studies have shown that GCC has a better prognosis than esophageal cancer when treated according to gastric cancer stages [32]. But some researchers did observe that the prognosis of GCC might be far worse than esophagus or other GC [33, 34]. So, to explore and compare the potential prognostic difference between GC and GCC, we used the Chinses data and SEER data to conduct survival analysis. As Kaplan–Meier curves illustrated, the prognosis of GCC patient in SEER database was worse than that of NGCC patients except for cancer from overlapping lesion of stomach, while it was not the second worst in the Chinese data, which was similar to previous studies [8, 35]. Some researchers reported that these differences might be related to the surgical method and the number of lymph node resection [36, 37], but more studies are still required. Thus, as far as we have found so far, it might need to treat GC and GCC differently.
To date, surgical resection has still been the most important treatment for early GCC patients [37]. For patients who were not suitable for gastrectomy, endoscopic submucosal dissection (ESD) resection could also achieve a good prognosis because of the low rate of lymph node metastasis in early GCC [38, 39]. Long et al. [10] found that the increased lymph node removal and chemoradiotherapy (CRT) contributed to improving the survival rate of GCC patients. And various other risk factors affecting the prognosis of these patients had been reported too, including sex, age, smoking, alcohol, histological type and TNM stage [40,41,42]. Therefore, it is necessary and feasible to develop a survival model to predict the prognosis of GCC patients based on clinical features. Previous studies have reported a few models to predict the survival of GCC patients [14,15,16,17]. For example, Shi et al. [14] demonstrated that a CPH-based nomogram’s consistency index (C-index) was 0.714 (95% CI, 0.705–0.723) and 0.734 (95% CI, 0.721–0.747) in training cohort and validation cohort, respectively when predicting GCC patients’ OS. Likewise, Chen et al. [15] built a CPH-based nomogram with only 0.590 (95% CI, 0.569–0.611) C-index in the training cohort and 0.569 (95% CI, 0.532–0.606) in the validation cohort when predicting GCC OS. Few parameters were incorporated in these studies, and the latter focused only on the prognosis of metastatic cancer, these might be the cause of the model's weak performance. Similarly, Liu et al. [16] created a CPH nomogram with 0.726 calibration index, and the model was established in one cohort only and not validated externally (Supplement Table 2). Obviously, all of these models behaved generally and had their own defects. They assumed that the risk of death was a simple linear combination of its covariates, which might be too idealistic in a real clinical world. Therefore, the prediction accuracy of these models has been limited, and developing a more reasonable survival prediction model which incorporates nonlinear factors has become an exploration direction to researchers.
As we know, deep learning models have been widely used in the diagnosis of endoscopic and histopathology of GC [7, 43,44,45,46], evaluation of tumor invasion depth and lymph node metastasis [47,48,49] and the prediction of treatment efficacy [50, 51]. These deep learning models have shown satisfactory performance in their respective fields. Excitingly, a novel deep learning theory called DeepSurv developed by Katzman et al. [26] in 2018, which combined deep learning with ANN and CPH, has achieved initial success in the survival prediction of some cancers. For example, She et al. [52] found that DeepSurv model was significantly better than the traditional AJCC TNM staging system in non-small-cell lung-cancer-specific survival (C-index = 0.739 vs 0.706). Huang et al. [53] demonstrated that the DeepSurv model was superior to the TNM staging model in predicting esophageal CSS with the internal test dataset (C-index = 0.753 vs 0.638) and external validation dataset (C-index = 0.687 vs 0.643). These suggested that the deep learning neural network model could be more widely used as a potential tool to assist clinicians with prognosis prediction. To our knowledge, there was no study using deep learning models to predict survival in patients with GCC.
In this study, the deep learning algorithm was used to analyze the large-scale GCC clinical data and conduct a neural network tool for the first time. The AUC was 0.7431 (95% CI, 0.7423–0.7439) for the train cohort and 0.7419 (95% CI, 0.7411–0.7428) for the test cohort when applying this prediction model. These results showed that this model might have more advantages than previous models in predicting the OS of GCC patients. Finally, we converted the model into a desktop tool to use conveniently, hoping it could offer some references for clinicians and researchers (Supplement File 1).
Limitations
Some characteristic information of GCC patients from SEER database was incomplete, such as surgical methods, chemotherapy types and tumor markers, which might be important to GC patients’ prognosis. And large-scale prospective multicenter data was needed for further verification.
Conclusions
GCC patients indeed have different survival time compared with non GCC patients. And the neural network-based prognostic predictive tool developed in this study is a novel and promising software for the clinical outcome analysis of GCC patients.
Abbreviations
- GC:
-
Gastric cancer
- GCC:
-
Gastric cardiac cancer
- NGCC:
-
Non-cardiac gastric cancer
- OS:
-
Overall survival
- AJCC:
-
American Joint Committee on Cancer
- TNM:
-
Tumor, node, and metastasis
- CPH:
-
Cox proportional hazards
- CSS:
-
Cancer-specific survival
- AI:
-
Artificial intelligence
- HO-GINFC:
-
Heap Optimization Based Generalized Intelligent Neural Fuzzy Control
- RECC-VC:
-
Rooted elliptic curve cryptography with Vigenère cipher
- ANN:
-
Artificial neural network
- SEER:
-
The Surveillance, Epidemiology, and End Results database
- AUC:
-
Area under the receiver operating characteristics curve
- CI:
-
Confidence intervals
- GEJ:
-
Gastroesophageal junction
- EBV:
-
Epstein-Barr virus
- ESD:
-
Endoscopic submucosal dissection
- CRT:
-
Chemoradiotherapy
- C-index:
-
Consistency index
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85(11):1457–9.
Sierra MS, Cueva P, Bravo LE, Forman D. Stomach cancer burden in Central and South America. Cancer Epidemiol. 2016;44(Suppl 1):S62–s73.
Wang Z, Graham DY, Khan A, Balakrishnan M, Abrams HR, El-Serag HB, et al. Incidence of gastric cancer in the USA during 1999 to 2013: a 50-state analysis. Int J Epidemiol. 2018;47(3):966–75.
Imamura Y, Watanabe M, Oki E, Morita M, Baba H. Esophagogastric junction adenocarcinoma shares characteristics with gastric adenocarcinoma: Literature review and retrospective multicenter cohort study. Ann Gastroenterol Surg. 2021;5(1):46–59.
Abdi E, Latifi-Navid S, Zahri S, Yazdanbod A, Pourfarzi F. Risk factors predisposing to cardia gastric adenocarcinoma: Insights and new perspectives. Cancer Med. 2019;8(13):6114–26.
Song Z, Zou S, Zhou W, Huang Y, Shao L, Yuan J, et al. Clinically applicable histopathological diagnosis system for gastric cancer detection using deep learning. Nat Commun. 2020;11(1):4294.
Zhao L, Niu P, Zhao D, Chen Y. Regional and racial disparity in proximal gastric cancer survival outcomes 1996–2016: results from SEER and China National Cancer Center database. Cancer Med. 2021;10(14):4923–38.
Petrelli F, Ghidini M, Barni S, Steccanella F, Sgroi G, Passalacqua R, et al. Prognostic role of primary tumor location in non-metastatic gastric cancer: a systematic review and meta-analysis of 50 studies. Ann Surg Oncol. 2017;24(9):2655–68.
Ze-Long Y, Guo-Hui M, Lin Z, Wei-Hong Y, Ke-Cheng Z, Yan-Wen J. Survival trends of patients with surgically resected gastric Cardia cancer from 1988 to 2015: a population-based study in the United States. Am J Clin Oncol. 2019;42(7):581–7.
Takeda FR, Ramos M, Pereira MA, Sallum RAA, Ribeiro Junior U, Nahas SC, et al. Tumor size predicts worse prognosis in esophagogastric junction adenocarcinoma. Updates Surg. 2022;74(6):1871–9.
Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH. Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(4):304–17.
Huang Q, Sun Q, Fan XS, Zhou D, Zou XP. Recent advances in proximal gastric carcinoma. J Dig Dis. 2016;17(7):421–32.
Shi X, Xu L, Ma B, Wang S. Development and validation of a nomogram to predict the prognosis of patients with gastric cardia cancer. Sci Rep. 2020;10(1):14143.
Chen K, Deng X, Yang Z, Yu D, Zhang X, Zhang J, et al. Survival nomogram for patients with metastatic siewert type II adenocarcinoma of the esophagogastric junction: a population-based study. Expert Rev Gastroenterol Hepatol. 2020;14(8):757–64.
Liu X, Jiang Q, Yue C, Wang Q. Clinicopathological characteristics and survival predictions for adenocarcinoma of the esophagogastric junction: a SEER population-based retrospective study. Int J Gen Med. 2021;14:10303–14.
Chen J, Xia YJ, Liu TY, Lai YH, Yu JS, Zhang TH, et al. Development and validation of a survival nomogram for patients with Siewert type II/III adenocarcinoma of the esophagogastric junction based on real-world data. BMC Cancer. 2021;21(1):532.
Randall RL, Cable MG. Nominal nomograms and marginal margins: what is the law of the line? Lancet Oncol. 2016;17(5):554–6.
Khan AI, Alsolami F, Alqurashi F, Abushark YB, Sarker IH. Novel energy management scheme in IoT enabled smart irrigation system using optimized intelligence methods. Eng Appl Artif Intell. 2022;114:104996.
Irshad K, Algarni S. Novel optimized hybrid neuro-fuzzy approach for analysis of cold thermal storage system-assisted air conditioning system performance. J Build Eng. 2023;65:105729.
Islam N, Irshad K. Artificial ecosystem optimization with deep learning enabled water quality prediction and classification model. Chemosphere. 2022;309:136615.
Kumar M, Kavita, Verma S, Kumar A, Ijaz MF, Rawat DB. ANAF-IoMT: a novel architectural framework for IoMT-enabled smart healthcare system by enhancing security based on RECC-VC. IEEE Trans Industr Inform. 2022;18(12):8936–43.
Praveen SP, Srinivasu PN, Shafi J, Wozniak M, Ijaz MF. ResNet-32 and FastAI for diagnoses of ductal carcinoma from 2D tissue slides. Sci Rep. 2022;12(1):20804.
Vulli A, Srinivasu PN, Sashank MSK, Shafi J, Choi J, Ijaz MF. Fine-tuned DenseNet-169 for breast cancer metastasis prediction using FastAI and 1-cycle policy. Sensors. 2022;22(8):2988.
Wainberg M, Merico D, Delong A, Frey BJ. Deep learning in biomedicine. Nat Biotechnol. 2018;36(9):829–38.
Katzman JL, Shaham U, Cloninger A, Bates J, Jiang T, Kluger Y. DeepSurv: personalized treatment recommender system using a Cox proportional hazards deep neural network. BMC Med Res Methodol. 2018;18(1):24.
Yan L, Gao N, Ai F, Zhao Y, Kang Y, Chen J, et al. Deep learning models for predicting the survival of patients with chondrosarcoma based on a surveillance, epidemiology, and end results analysis. Front Oncol. 2022;12:967758.
Byun SS, Heo TS, Choi JM, Jeong YS, Kim YS, Lee WK, et al. Deep learning based prediction of prognosis in nonmetastatic clear cell renal cell carcinoma. Sci Rep. 2021;11(1):1242.
Huang Q. Controversies of cardiac glands in the proximal stomach: a critical review. J Gastroenterol Hepatol. 2011;26(3):450–5.
Abnet CC. Asian Gastric Cardia Adenocarcinoma: a distinct and understudied cancer with etiologic similarities to both esophageal squamous cell carcinoma and noncardia gastric adenocarcinoma. J Natl Cancer Cent. 2021;1(2):44–6.
Huang Q, Read M, Gold JS, Zou XP. Unraveling the identity of gastric cardiac cancer. J Dig Dis. 2020;21(12):674–86.
Liu K, Feng F, Chen XZ, Zhou XY, Zhang JY, Chen XL, et al. Comparison between gastric and esophageal classification system among adenocarcinomas of esophagogastric junction according to AJCC 8th edition: a retrospective observational study from two high-volume institutions in China. Gastric Cancer. 2019;22(3):506–17.
Schlansky B, Sonnenberg A. Epidemiology of noncardia gastric adenocarcinoma in the United States. Am J Gastroenterol. 2011;106(11):1978–85.
Zhai Z, Zhu ZY, Cong XL, Han BL, Gao JL, Yin X, et al. Changing trends of clinicopathologic features and survival duration after surgery for gastric cancer in Northeast China. World J Gastrointest Oncol. 2020;12(10):1119–32.
Laszkowska M, Tramontano AC, Kim J, Camargo MC, Neugut AI, Abrams JA, et al. Racial and ethnic disparities in mortality from gastric and esophageal adenocarcinoma. Cancer Med. 2020;9(15):5678–86.
Blank S, Schmidt T, Heger P, Strowitzki MJ, Sisic L, Heger U, et al. Surgical strategies in true adenocarcinoma of the esophagogastric junction (AEG II): thoracoabdominal or abdominal approach? Gastric Cancer. 2018;21(2):303–14.
Chen Y, Zhao XK, Xu RH, Song X, Yang MM, Zhou FY, et al. Transthoracic, thoracoabdominal, and transabdominal surgical approaches for gastric cardia adenocarcinomas: a survival evaluation based on a cohort of 7103 patients. World J Surg Oncol. 2022;20(1):217.
Fan T, Sun Q, Cao S, Fan X, Huang Q, Zhang S, et al. Clinical outcomes of early gastric cardiac cancer treated with endoscopic submucosal dissection in patients with different indications. BMC Gastroenterol. 2021;21(1):119.
Cao S, Zou T, Sun Q, Liu T, Fan T, Yin Q, et al. Safety and long-term outcomes of early gastric cardiac cancer treated with endoscopic submucosal dissection in 499 Chinese patients. Therap Adv Gastroenterol. 2020;13:1756284820966929.
Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol. 2013;23(1):3–9.
Yu X, Hu F, Li C, Yao Q, Zhang H, Xue Y. Clinicopathologic characteristics and prognosis of proximal and distal gastric cancer. Onco Targets Ther. 2018;11:1037–44.
Gu J, Xie S, Wang S, Xue L, Zhou J, Li M, et al. Surveillance of premalignant gastric cardia lesions: a population-based prospective cohort study in China. Int J Cancer. 2021;149(9):1639–48.
Wang Z, Meng Q, Wang S, Li Z, Bai Y, Wang D. Deep learning-based endoscopic image recognition for detection of early gastric cancer: a Chinese perspective. Gastrointest Endosc. 2018;88(1):198–9.
Ling T, Wu L, Fu Y, Xu Q, An P, Zhang J, et al. A deep learning-based system for identifying differentiation status and delineating the margins of early gastric cancer in magnifying narrow-band imaging endoscopy. Endoscopy. 2021;53(5):469–77.
Ba W, Wang S, Shang M, Zhang Z, Wu H, Yu C, et al. Assessment of deep learning assistance for the pathological diagnosis of gastric cancer. Mod Pathol. 2022;35(9):1262–8.
Iizuka O, Kanavati F, Kato K, Rambeau M, Arihiro K, Tsuneki M. Deep learning models for histopathological classification of gastric and colonic epithelial tumours. Sci Rep. 2020;10(1):1504.
Dong D, Fang MJ, Tang L, Shan XH, Gao JB, Giganti F, et al. Deep learning radiomic nomogram can predict the number of lymph node metastasis in locally advanced gastric cancer: an international multicenter study. Ann Oncol. 2020;31(7):912–20.
Jin C, Jiang Y, Yu H, Wang W, Li B, Chen C, et al. Deep learning analysis of the primary tumour and the prediction of lymph node metastases in gastric cancer. Br J Surg. 2021;108(5):542–9.
Wu L, Wang J, He X, Zhu Y, Jiang X, Chen Y, et al. Deep learning system compared with expert endoscopists in predicting early gastric cancer and its invasion depth and differentiation status (with videos). Gastrointest Endosc. 2022;95(1):92–104.e3.
Jiang Y, Jin C, Yu H, Wu J, Chen C, Yuan Q, et al. Development and validation of a deep learning CT signature to predict survival and chemotherapy benefit in gastric cancer: a multicenter. Retrospective Study Ann Surg. 2021;274(6):e1153–61.
Fang M, Tian J, Dong D. Non-invasively predicting response to neoadjuvant chemotherapy in gastric cancer via deep learning radiomics. EClinicalMedicine. 2022;46:101380.
She Y, Jin Z, Wu J, Deng J, Zhang L, Su H, et al. Development and validation of a deep learning model for non-small cell lung cancer survival. JAMA Netw Open. 2020;3(6):e205842.
Huang C, Dai Y, Chen Q, Chen H, Lin Y, Wu J, et al. Development and validation of a deep learning model to predict survival of patients with esophageal cancer. Front Oncol. 2022;12:971190.
Funding
1. Three-Year Rolling Project from Beijing Chest Hospital (Jinghui Wang); 2. Beijing Natural Science Foundation (Yuqi He, No.7212107).
Author information
Authors and Affiliations
Contributions
Wei Li, MD and Minghang Zhang, MD, contributed equally to this study. Study design: Wei Li, Minghang Zhang, Yuanming Pan. Data acquisition: Wei Li, Yuanming Pan. Data collation: Wei Li, Siyu Cai, Liangliang Wu. Statistical analysis: Wei Li. Endoscopic interpretation: Chao Li, Guibin Yang, Yuqi He. Manuscript preparation: Wei Li, Minghang Zhang. Prediction tool contribution: Wei Li. Manuscript revision/review: Jinghui Wang, Yuanming Pan.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplement Figure 1.
The training curves of neural network-based prognostic predictive model for GCC.
Additional file 2: Supplement File 1.
The Neural network-based prognostic predictive tool for GCC patients.
Additional file 3: Supplement File 2.
The raw data of this study.
Additional file 4: Supplement Table 1.
The mean and standard deviation of numerical clinical features in train cohort.
Additional file 5: Supplement Table 2.
The models’ performance of previous research in overall survival.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, W., Zhang, M., Cai, S. et al. Neural network-based prognostic predictive tool for gastric cardiac cancer: the worldwide retrospective study. BioData Mining 16, 21 (2023). https://doi.org/10.1186/s13040-023-00335-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13040-023-00335-z