Abstract
Women with HSIL typically undergo conization/LEEP to remove cervical lesions, but the risk of HSIL lesions returning after surgical treatment remains higher than in the general population. HPV vaccination is essential to prevent cervical cancer. However, the effect of prophylactic HPV vaccination on reducing the risk of recurrent cervical lesions after surgical treatment remains unclear. This review aims to analyze and summarize the latest literature on the role of prophylactic HPV vaccine in reducing the recurrence of cervical lesions after surgery in patients with HSIL, and to review and update the history, efficacy, effectiveness and safety of HPV vaccine, focusing on the current status of global HPV vaccine implementation and obstacles.
Similar content being viewed by others
Introduction
The majority (> 95%) of cervical cancers are associated with persistent infection with high-risk human papillomavirus (HPV) [1]. In 2020, the World Health Organization reported that there were more than 600,000 new cases and 340,000 deaths worldwide every year, and more than 85% of the cases and deaths occurred in low- and middle-income countries [2, 3]. There were 110,000 new cases of cervical cancer in China, accounting for 18% of the new cases in the world [4]. Despite advances in cervical cancer screening and prevention in recent years, cervical cancer remains one of the most common gynecological tumors worldwide [5, 6]. More than 100 countries worldwide now include HPV vaccination in their routine prevention programs [7,8,9]. HPV vaccination is the lowest-cost public health measure against cervical cancer and a key measure to prevent invasive cervical cancer [10]. There are currently three types of vaccines available (bivalent, quadvalent, and ninvalent), all of which target at least two of the most carcinogenic virus genotypes (HPV 16, 18) [11, 12]. HPV vaccine has the best effect before first sexual intercourse. To ensure the effectiveness of the vaccine, women aged 11–12 should receive routine preventive vaccination [13].
There are two grades of squamous intraepithelial lesions in the 5th edition of WHO Classification of Tumors of female reproductive organs: low-grade squamous intraepithelial lesion (LSIL), including ervical intraepithelial neoplasia(CIN) 1, high-grade squamous intraepithelial lesions (HSIL), including ervical intraepithelial neoplasia2, 3 (CIN2, CIN 3). The primary objective of the cervical screening program is the early detection and treatment of precancerous lesions, namely low-grade squamous intraepithelial lesions (LSIL) and high-grade squamous intraepithelial lesions (HSIL). Women with high-grade squamous intraepithelial lesions(HSIL) typically undergo cervical conization to remove cervical lesions and prevent progression [14]. Although eliminating high-risk human papillomavirus (HPV) is not a treatment goal, many such women have achieved elimination of infection [15, 16]. While the effectiveness of resection surgery has been clearly demonstrated, the overall risk of recurrence of cervical lesions after cervical intraepithelial neoplasia(CIN) 2 + surgery is approximately 10–14%, and the risk of recurrence of CIN 3 + and CIN 2 + after 5 years is 6% and 16.5% respectively [17]. The women had a higher risk than the general population of developing invasive cervical cancer years after treatment [18]. There is growing evidence that HPV vaccination in women who have been treated with HSIL may reduce the recurrence rate of cervical lesions. There is increasing evidence that vaccination with the HPV vaccine in women with HSIL who have undergone conization may reduce the recurrence rate of cervical lesions [19,20,21,22,23,24]. The rationale behind the efficacy of the HPV vaccine as an adjunct remains unclear. So far, several hypotheses have been proposed to explain how women who are already infected might benefit from HPV vaccination. It has been hypothesized that HPV vaccination stimulates local antibodies that increase the immune response, blocking the entry of the virus into uninfected cells in the basal layer and preventing disease recurrence [24]. It has also been hypothesized that surgical treatment may reduce local inflammatory responses, induce higher intensity and longer-lasting local cellular immunity, and restore HPV naive microenvironment. The HPV vaccine is theoretically effective in preventing persistent and recurrent HPV infection, but the potential role of prophylactic HPV vaccination as adjunctive therapy following surgical removal has not been demonstrated [25,26,27,28,29,30]. It is clinically important to explore whether post-treatment HPV vaccination is associated with a reduced risk of CIN. Therefore, The aim of this paper is to analyze and summarize the latest literature on how prophylactic HPV vaccination can help reduce the risk of recurrence after CIN surgery, and to review and update the history, efficacy, effectiveness, and safety of HPV vaccines, with a focus on the current status of global implementation of HPV vaccines and the obstacles they face.
HPV and cervical carcinogenesis
HPV is a small, non-encapsulated, double-stranded DNA virus that can infect mucosal and skin epithelial cells. The DNA genome of HPV encodes approximately eight open reading frames (ORFs). ORF is divided into three functional areas, including early area (E), late area (L), and non-coding part or long control area (LCR). The E region gene encodes proteins E1–E7 necessary for viral replication and is involved in the pathogenicity of the virus. The L region genes encode the capsid proteins L1 and L2 required for virion assembly, and the long control region (LCR) regulates gene expression and replication [31]. Most genital HPV infections are acquired through sexual contact, through which HPV can cause minor cervical damage. Subsequently, HPV penetrates into basal layer cells mediated by heparin sulfate proteoglycans and integrins (α6, β1, and β4) in basal epithelial cells [32, 33]. When basal cells are infected with HPV, heparin sulfate proteoglycans and integrins divide, remaining in the basal layer and retaining their ability to divide, acting as reservoirs for viral replication. When the HPV genome is transferred to the nucleus, the expression of the early HPV genes E1 and E2 is activated. E1 is an ATPase helicase. E1 and E2 are involved in viral replication and transcriptional regulation. E2 maintains the dissociation of the HPV genome through interactions with other cytokines. E2 also regulates the transcription of the cancer proteins E6 and E7. The E1 and E2 complexes interact with the ori locus in the long control region, which is thought to be the starting point for HPV DNA replication [34, 35]. E2 protein recruits E1 protein (a viral DNA helicase) to the binding site of the origin of viral replication and activates viral DNA replication [36, 37]. To ensure that cervical cells continue to grow and divide, the early HPV genes E5, E6 and E7 are expressed. E6 and E7 are small proteins with approximately 150 and 100 amino acids, respectively. They disrupt cell cycle checkpoint control by degrading cell cycle regulators and inhibiting CDK inhibitors (p21, p27), thereby evading host immune responses. E6 and E7 can promote the immortalization of cancer cells by activating the hTERT promoter through a C-myc-dependent mechanism [38, 39]. The expression of E4, L1 and L2 is activated after differentiation of basal cells. The viral genome then encodes the synthesis of L1 and L2 capsid proteins, which assemble into mature virions that are secreted from epithelial cells and allow the infection to spread [40]. During viral DNA replication, each cell has at least 1000 copies of the virus, which increases the expression of L1 and L2 capsid proteins and the assembly of infectious viruses [41].
Most women infected with HPV rely on their own immunity to clear the virus within a few years, and approximately 10–20% of women continue to be infected [41]. The persistence of HPV infection is due to the following mechanisms: (1) Malignant transformation caused by down-regulation of cell cycle control mediated by HPV cancer proteins and genetic damage. (2) High-risk papillomavirus develops multiple immune escape mechanisms to promote its invasion and prevent its recognition by host immune cells. Persistent HPV infection can lead to cervical dysplasia, CIN and even invasive cervical cancer [40, 42].
Prophylactic vaccines
The prophylactic HPV vaccine is effective in reducing the incidence of HPV infection and HPV-related diseases, and there are currently three licensed vaccines available [43]. The bivalent vaccine (Cervarix), approved in 2007, targets two types of HPV16 and HPV18, which are potentially carcinogenic. The quadrivalent vaccine (Gardasil), approved in 2006, targets HPV16 and 18, as well as low-risk types of HPV11 and 6 that cause genital warts. The nonavalent vaccines (Gardasil 9) against HPV6, 11, 16, 18, 31, 33, 45, 52, 58was approved in 2016. HPV vaccines are made from purified L1 proteins using recombinant DNA technology. L1 protein self-assembles to form HPV-specific shells (Virus-like particles; VLP), which is structurally similar to HPV but does not contain any active products or DNA, and is therefore considered non-carcinogenic and non-infectious, and less harmful to humans than vaccines made from attenuated HPV genomes [44]. After the prophylactic HPV vaccine is introduced into the human body, the antigen portion of the vaccine VLP binds to antigen presenting cells (APC) and then presents the antigen to T cells, which have a variety of functions and can differentiate into several T cell lineages, including cytotoxic T cells, T helper cells or memory T cells. T helper cells in turn stimulate naive B cells to become plasma or memory B cells [45]. Long-lived plasma cells (LLPCS) produce and secrete antigen-specific antibodies after vaccination, thus allowing circulating antibodies to persist and also aid in quick recall when encountering HPV antigens again [46]. As a result, LLPC, memory B cells, and T cells prevent true HPV infection by inducing and maintaining high levels of neutralizing antibodies. However, neutralizing antibodies against HPV does not control or eliminate existing HPV infection and/or transformed cells [47].
Therapeutic vaccines
Therapeutic vaccines differ from preventive vaccines in that they rely on T-cell-mediated immune responses (specifically CD8 + T cells) that target the elimination of infected HPV and lesions rather than the production of neutralizing antibodies. Therapeutic HPV vaccines are facilitated by antigen-presenting cells (APCs), such as dendritic cells (DC). DC present HPV antigens via major histocompatibility class I (MHC-I) and class II (MHC-II) molecules, respectively activating HPV antigen-specific CD8 + and CD4 + T cells, targeting the killing of infected and/or transformed cells. E6 and E7 are not only near ideal targets for cervical cancer immunotherapy, but also the best targets for the development of therapeutic HPV vaccines. E6 and E7 are expressed in precancerous and invasive lesions and participate in cell cycle arrest, while they are not expressed in healthy cells. They are necessary to initiate and sustain HPV-related malignancies [47, 48]. Thus, therapeutic HPV vaccines against E6 and E7 are safe and can circumvent immune tolerance to autoantigens [47]. Most therapeutic vaccines currently in development include E6 and E7, where the DNA sequence encoding the E6 and E7 fusion proteins is inserted into the vector and mutations are introduced into the regions responsible for the interaction of E6 with p53 and E7 with pRB to reduce their carcinogenic ability. Various research teams are trying to develop a safe and effective therapeutic vaccine, and several HPV therapeutic vaccines designed to enhance the response of CD4 + and CD8 + T cells have been studied, including genetic vaccines (such as DNA/RNA/virus/bacteria), as well as protein-based, peptide-based or dendritic cell-based vaccines [49]. Numerous clinical trials have shown that some vaccines have great potential in treating precancerous lesions of uterine cervix. However, research on therapeutic vaccines against precancerous lesions has been slow, and no therapeutic vaccine has been approved for HPV infection and cervical precancerous lesions. Vaccine therapy has not yet been included in clinical guidelines, possibly due to the fact that vaccine development requires a greater human, material, financial and time investment, as well as many difficulties in terms of safety, immunogenicity, HPV polymorphism and mutagenicity [50,51,52]. In the future, further understanding of the molecular biology of tumors, further clinical trials, and the development of more effective therapeutic vaccines are needed.
Prophylactic vaccines can be used as therapeutic vaccines
Continuous infection with high-risk HPV is the primary and only modifiable factor leading to the development of cervical lesions into HSIL and cervical cancer. Therefore, it has been proposed to vaccinate patients undergoing HSIL surgery with the preventive HPV vaccine. To date, no therapeutic HPV vaccine has been approved for HSIL. Attempting to vaccinate patients with advanced cervical lesions with the preventative HPV vaccine may be another simple and effective way to reduce recurrent HSIL and prevent cervical cancer. Del Pino et al. reported that 153 (57.7%) women in HSIL who underwent cervical conization received vaccination and 112 (42.3%) women refused vaccination. The incidence of persistent/recurrent HSIL was lower in vaccinated women compared to unvaccinated women [14]. Casajuana-Pérez A et al. reported that 563 HSIL patients underwent the cervical conization. The incidence of persistent/recurrent HSIL after surgery was 4.3% in the vaccinated group and 9.8% in the non-vaccinated group (HR: 0.43, 95% Confidence interval 0.22–0.84, p = 0.014). At follow-up 6 months after surgery, the persistence/recurrence rates were found to be very low in both groups, 1.1% in the vaccinated group and 1.5% in the non-vaccinated group (p > 0.05) [53]. A meta-analysis by Di Donato V showed that the recurrence rate of CIN1 + in Cervical Dysplasia who received the HPV vaccine during perioperative period was significantly lower than in those who did not receive the vaccine (OR 0.51; 95% CI 0.31–0.83; p = 0.006). The surgery reduced the overall risk of new or persistent CIN 2 + by 65 percent.The pooled estimated OR was 0.35 (95% CI 0.21–0.56; p < 0.0001) [54].
However, vaccination does not eliminate HPV that already exists in women [55,56,57], and there is still no conclusive evidence of the effectiveness of vaccination in patients after conization. In an analysis of a randomized controlled trial, it was found that no efficacy of the vaccine against cervical precancerous lesions was observed in patients who had cervical reactions after vaccination, and future randomized controlled trials are needed to further demonstrate this [58].
Mechanism of preventive vaccines in preventing the recurrence of cervical lesions after cervical dissection
Vaccination can be used as a primary preventive measure against new HPV infections in previously unvaccinated patients, but no large-scale, multidisciplinary clinical trial has investigated the efficacy of the HPV vaccine for secondary prevention in patients with active HPV-related disease. The mechanism of action of the HPV vaccine in patients with pre-existing cervical lesions is not fully understood, and several hypotheses have been proposed so far to explain the exact protective mechanism of HPV vaccination in infected individuals. (1) The HPV vaccine induces the production of neutralizing antibodies against HPV capsid protein L1 virus particles. Neutralizing antibodies bind to newly acquired virus particles or virus particles produced by infected cells to inhibit infection of new cells by residual viruses, prevent HPV from entering host cells, and reduce the spread of existing infections. These vaccines do not effectively clear pre-existing infections because viral antigens are not expressed on the surface of infected cells and therefore cannot be targeted by antibodies after vaccination [59, 60]. (2) Cross protection against other HPV types [61, 62]. (3) Persistent HPV infection appears to be associated with changes in the local microenvironment and elevated levels of proinflammatory cytokines [63, 64]. From an immunological point of view, the presence of some inflammatory response in the local cervix after surgical treatment of cervical lesions is similar to that seen in patients without HPV infection. On the one hand, the vaccine has a protective effect against HPV infection in this localized microenvironment. On the other hand, the anti-inflammatory microenvironment is not conducive to sustained HPV infection, and surgical intervention to remove lesions from sustained HPV infection may be a good prerequisite for post-operative vaccine intervention [25, 26]. The HPV vaccine stimulates cell-mediated immunity, which may also play a role in preventing recurrent infections [65]. Therefore, HPV vaccination can protect women after cervical removal.
Immunogenicity of prophylactic vaccines
Immunogenicity is the ability to generate an immune response and can be measured by antibody formation or cell-mediated immunity through cytotoxic T lymphocytes [66]. Long-term protection after HPV vaccination is important to prevent people from contracting HPV for life. Studies have shown that all HPV vaccines are highly immunogenic, with specific neutralizing antibodies against HPV antigens generated by HPV vaccination being 10–100 times higher than those produced by natural infection [67,68,69]. More than 98 percent of those vaccinated developed antibodies within a month of completing the vaccine, and they appear to provide protection for at least 10 years [70]. However, the level of antibodies produced depends on several factors:
Sex and age of the individual and type of vaccine received
Several studies have shown that 9- and 15-year-old women have higher specific antibody titers than 16- and 26-year-old women who have been vaccinated [71,72,73,74,75]. A long-term assessment of the immunogenicity of the HPV16/HPV18 vaccine in serum from women aged 15–55 years showed high levels of seropositive antibodies against the HPV16 vaccine in all age groups 10 years after initial vaccination. In contrast, the positive rate of anti-HPV18 antibodies was higher in the 15–25 age group (99.2%) than in the 26–45 age group (93.7%) and the 46–55 age group (83.8%) [76]. However, in all study groups, anti-HPV16 and anti-HPV18 antibodies were higher than antibody titers produced by natural infection, and were predicted to persist above natural infection levels more than 30 years after vaccination [77]. As a result, young women between the ages of 15 and 26 are the primary target population for HPV vaccination [78].
Adjuvant use and total vaccination doses
The addition of adjuvants improves the strength and persistence of the immune response. The bivalent vaccine contains the proprietary adjuvant ASO4, which consists of 500 mg of aluminum hydroxide and 50 mg of the Toll-like receptor 4 agonist, 3-O-desacyl-4 'phospholipid A, as an additional immune stimulant. 225 mg amorphous aluminum hydroxyphosphate sulfate (AAHS) as an adjuvant for tetravalent vaccines. Both vaccines have been reported to show effective safety and immunogenicity. However, bivalent vaccines yield higher immunogenicity against HPV infection than tetravalent vaccines [79,80,81].
Active ingredients of vaccines
The concentration of each L1 VLPs and the antigen to adjuvant ratio are important differences between different prophylactic HPV vaccines, including Gardasil and Ceravix. Clinical studies have shown that the immunogenicity of three different doses of the preventative vaccine against HPV infection and cervical cancer is similar. However, levels of anti-PV16 and anti-PV18 antibodies were significantly reduced after vaccination with Gardasil compared to Cervarix [82]. Gardasil has twice the HPV16 L1 VLP concentration and the same HPV18 L1 VLP concentration as Cervarix. Gardasil-9 contains twice as much HPV18 L1 VLP, more than 50% as much HPV16 antigen, and twice as much Gardasil adjuvant. Therefore, in terms of vaccine immunogenicity, we should prioritize 9-valent vaccines, followed by 2-valent vaccines over 4-valent vaccines.
Vaccination dose
Two doses of HPV vaccine are more protective than one, but different studies have not found statistically significant differences between two and three doses [81, 81, 83].
These results suggest that boosting vaccine doses and using appropriate adjuvants can improve the immune response and increase levels of neutralizing antibodies.
Prophylactic HPV vaccines provide cross-over immune protection
The prophylactic HPV vaccine type elicits a specific immune response, and the structure of the L1 gene is similar to that of the non-vaccinated HPV type, resulting in long-term cross-reactive immunogenicity against the HPV type not included in the vaccine. Previous studies reported cross protection against HPV31 and HPV45 types after vaccination with a bivalent (HPV16/HPV18) vaccine. In addition, the cross-reactive immunogenicity of the quadrivalent HPV vaccine against HPV45 has been detected [84]. FUTURE I and II trials evaluated the cross-protection of the quadrivalent vaccine against 10 other HPV subtypes (32, 34, 36, 40, 45, 51, 52, 56, 58, 59). Vaccinating a portion of the infected population with the quadrivalent vaccine reduced the incidence of infection for up to six months, and CIN1 was associated with other non-vaccine-causing HPV types [85]. The PATRICIA and Costa Rica trials evaluated the cross protection of bivalent vaccines against persistent infection of non vaccine type HPV and CIN2 + , and found that the vaccines had higher efficacy against HPV-31, HPV-33 (two subtypes closely related to HPV-16), HPV-45 (closely related to HPV-18), and HPV-51 related infections and CIN2 + [86, 87]. Cervarix ® long-term follow-up data from the trial indicate that the cross-protection effect lasts at least 11 years [88, 89].These findings suggest that HPV vaccination may protect against new infections by providing cross-protection against non-vaccinated HPV types as well as other strains of HPV not previously exposed.
Time for HPV vaccination: pre-surgery or post-surgery?
Post-operative HPV vaccination in women with HSIL can reduce the recurrence rate of lesions [19, 90]. However, the optimal timing for HPV vaccination has yet to be determined. Some studies have suggested that it may be more beneficial to get vaccinated before treatment. A recent follow-up by Sand FL in Denmark of more than 17000 women undergoing resection for HSIL found that women vaccinated before conization (between 0 and 3 months) had a lower absolute risk of developing HSIL compared to women who were not vaccinated, and women vaccinated after conization (0–12 months) had a similar risk of developing HSIL compared to women who were not vaccinated [91]. This is consistent with the results of Henere et al. [92]. There is growing evidence that pre-vaccination may improve patient outcomes [91]. Vaccination before conization ensures that the cervicovaginal area at the time of removal has sufficient anti-HPV neutralizing antibodies (removing most of the infected cells) to prevent re-infection of basal layer cells [93]. However, results from different studies were inconsistent. Saftlas et al. demonstrated that TNF-α in patients treated with LEEP was immediately reduced to levels similar to those in untreated controls [25]. Since surgical treatment induces changes in the microenvironment of the inflammatory tissue similar to those seen in patients without HPV infection, this may be a good prerequisite for post-operative vaccine intervention. A recent meta-analysis involving 11 studies and 21,310 patients showed that the HPV vaccine reduced the risk of relapse as an adjunct to CIN therapy. In seven of these studies [54], the first dose of vaccination was administered within the first month after surgery. Two studies reported that the first dose was administered between 3 months before and 12 months after surgery, while the other two studies did not specify the exact timing of the first dose [58, 91, 94, 95]. Due to the lack of standardization in the timing of HPV vaccination, it was not possible to compare the rates of CIN recurrence in women vaccinated before and after surgery. According to most studies, vaccination was given either before or shortly after LEEP/conization (up to 1 month after LEEP). Therefore, vaccination before or after surgery is recommended as soon as possible. Delaying vaccination may not prevent re-infection in women at risk of infection. In the future, we need to conduct further prospective studies and standardize the timing of vaccine administration to determine the optimal and most appropriate timing of vaccination to benefit more women and reduce the health care burden.
Effect of HPV vaccination on women with persistent HPV infection following treatment
Continuous HPV infection after treatment, regardless of residual HSIL/CIN2-3, is the most significant identified risk factor for cervical cancer [96]. Another risk factor is the acquisition of a new HPV infection, as women who already have HSIL/CIN2-3 are at increased risk of developing new HPV-related lesions [97]. This is related to the persistence of lifestyle risk factors for HPV infection, which can persist for a lifetime, and these women are more sensitive to the persistence of HPV [98, 99].
Does the HPV vaccine benefit women who remain infected after treatment? Although the HPV vaccine stimulates local antibodies in the cervix that prevent the virus from entering the basal layer of the cervix, the effectiveness of prophylactic HPV vaccination in preventing widespread HPV infection has not been proven [28, 30, 100]. Some studies have suggested that the HPV vaccine may have some benefits in women with prevalent HPV infection [19]. Del Pino et al. [15] reported that women with persistent LSIL/HPV infection and even HSIL after the first coning also tended to have a lower HSIL persistence/recurrence rate by the end of follow-up after vaccination, but the difference was not statistically significant. A recent randomized controlled trial of 312 women with persistent SIL after conservative treatment of the cervix reported that HPV vaccination resulted in a more than 50% reduction in women with persistent LSIL or HSIL lesions [101].
Dose and method of HPV vaccination
WHO initially recommended a three-dose regimen for HPV vaccination in 2009. A three-dose regimen is typical for infant vaccines based on inactivated proteins [93].
The second dose is given 1–2 months after the first dose, and the third dose is given 6 months after the first dose. The first two doses stimulate the production of immunological memory B cells in bone marrow, so they are called "initial dose". The second dose had an increased affinity for the antigen, resulting in a larger antibody response than the first dose. Due to this "affinity maturation" process, high affinity B lymphocytes are produced and differentiated into B memory cells that can quickly respond to antigens and produce NAbs [102]. Schiller and Lowy [103] showed that affinity maturation does not require multiple repeated doses. Because VLP is similar in shape to actual infection, sustained plasma cell production under reduced dose conditions ensures a strong and sustained immune response [102]. The Costa Rican vaccine trial team found that one, two and three doses of the bivalent HPV vaccine were equally protective against persistent HPV-16/HPV-18 infection over a four-year period and for up to a decade [104, 105]. However, the single dose vaccine was found to have a lower but stable antibody response [80]. In the CVT and PATRICIA trials, researchers demonstrated that the protective effect of a single dose of bivalent HPV vaccine persisted after seven years of follow-up. There were no cases of HPV infection during the entire follow-up period in the single dose group compared to the control group, which had an infection rate of 6.6 percent [105,106,107]. Another trial of the vaccine in 17,729 girls resulted in robust and sustained immune responses against HPV-16 and HPV-18 after a single dose. However, antibody conversion rates were significantly lower than in patients who received two and three doses of the vaccine [108]. The above studies suggest that a single dose of bivalent HPV vaccine may confer adequate protection [105,106,107,108,109].
Reducing vaccination doses due to financial and infrastructure constraints has significant public health implications, alleviating financial and logistical barriers to the introduction of HPV vaccination programmes. Single dose regimens will significantly reduce supply and storage issues and improve compliance. In recent years, multiple studies have evaluated the efficacy of shortening vaccination time in adolescents [110,111,112,113,114]. There is also tentative evidence that a two-dose regimen may also be appropriate for adult women [113, 114].
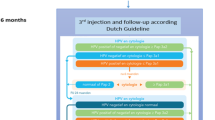
As a result, in 2014 the European Medical Association recommended only two doses of HPV vaccine for adults, [114, 115]. In 2016, the Advisory Committee on Immunization Practices announced that only two doses of the vaccine would be required for people younger than 15. The UK Joint Committee on Vaccination and Immunisation (JCVI) recently recommended routine use of the single-dose HPV vaccine in adolescent and MSM vaccination programmes. However, it is recommended to use a three-dose plan for vaccination of women aged 15–45 (at 0, 1–2, and 6 months) [83, 116]. Patients with weakened immune function should follow a three-dose regimen, regardless of gender and age at the time of vaccination [117]. Researchers are still exploring how and when to administer the vaccine to maximize its effectiveness. In essence, vaccines can be given multiple times by intramuscular (IM) or subcutaneous (SC) injections after a fixed time interval to stimulate the immune system and produce antigen-specific antibodies [118]. The skin is the first line of defense and is made up of large numbers of immune cells. For example, Langerhans cells in the epidermis and dendritic cells in the dermis can block the entry of pathogens and effectively absorb antigens to activate the immune system [119]. In a preclinical study, sublingual administration of the HPV16 L1 protein vaccine to a mouse model showed significant production of mucosal secretory IgA and serum IgG compared to other delivery methods, including intranasal, vaginal and percutaneous delivery [120]. Alternatively, it would be ideal to dehydrate the vaccine to make freeze-dried powder, transport it in powder form, reinsert it, then inject it in powder form or administer it intranasally [121].
Side effects of HPV vaccination
According to the WHO Global Advisory Committee on Vaccine Safety (GACVS), HPV vaccines are classified as extremely safe [122]. The most common adverse reactions include swelling, pain, and redness at the injection site, but are usually short-lived and reversible. These reactions may be related to inflammation associated with VLP. Although it can immediately awaken the human immune system, its applications are limited [123, 124]. The systemic reactions of HPV vaccine include dizziness, headache, myalgia, fatigue, fever, vomiting, nausea, and diarrhea [123], with fatigue and headache being the most common (50–60%) [125]. Few other reactions were observed after vaccination [126]. While several case reports have described diagnosis of primary ovarian dysfunction following vaccination with the tetravalent HPV vaccine, there is no evidence to date to support a causal relationship [127]. The safety of vaccines in the progression of autoimmune diseases, venous thromboembolism, and neurological disorders has been studied in a variety of contexts, but no association has been found between HPV vaccines and these diseases [128]. In vaccine clinical trials, there was no difference in the rate of serious adverse events between the vaccine and control groups [126, 128]. National surveillance data following the introduction of the bivalent vaccine (Cervarix) and the quadrivalent vaccine (Gardasil) have shown lower rates of serious adverse events [128]. Most of the available surveillance data on the nonavalent vaccine (Gardasil 9) is from the United States and suggests that the nonavalent vaccine has a similar safety profile to the quadrivalent vaccine [129]. There have been no reported deaths from HPV vaccination [126, 129, 130].
HPV vaccination in men
HPV is transmitted between men and women through sexual intercourse, and female patients may be re-infected by a man after the HPV has subsided. Sexual intercourse with condoms can reduce the risk of transmission, and the most appropriate method is to vaccinate both men and women against HPV to reduce the risk of infection [131]. The HPV vaccine was publicly funded in Canada in 2017 for men ages 9–26 and included in a school-based vaccination program. In 2019, HPV vaccination is recommended as routine for all U.S. men and women ages 9–26. However, since the introduction of the vaccine in men, adherence has been surprisingly low [132]. According to a 2017 systematic review, HPV vaccine adherence among men was only 47%, well below the Canadian government's target of > 85% [132]. Similarly, in the United States, about 44% of teenage boys (ages 13–17) are vaccinated [133], while among male college students, 53% say they have been vaccinated [134]. Other studies have shown that HPV vaccine completion rates among men (including a series of three HPV vaccines administered over a period of 6 months at the time) were only 14% [135]. Laserson AK et al. summarized the latest literature on HPV vaccination in college-age men and pointed out that the lack of understanding of the use of HPV vaccine and the lack of perceived risk and susceptibility to HPV infection were the main reasons for the low hpv vaccination rate in men. Sexual health education and advocacy campaigns through HPV sexual health education for men are key to addressing this issue. There is little literature on HPV vaccination in men to prevent the recurrence of cervical lesions in women, and large prospective studies are needed in the future to demonstrate this [136].
The status and difficulties of HPV vaccination
Since the HPV vaccine was first approved in the United States in June 2006, a large amount of real-world data has proven that HPV vaccination is safe and effective in preventing and treating HPV infection and related diseases [129, 137, 138]. However, many low-income and middle-income countries have been unable to introduce HPV vaccines into their national immunization programmes due to financial and infrastructure constraints. Global coverage of the HPV vaccine as of 2020 remains low, with only 12% of women receiving two doses [139]. As of March 2022, 60% of WHO member countries have included the HPV vaccine in their national immunization schedules. HPV vaccination has been introduced in 114 of 145 upper-middle-income countries (78.6%), compared with only 20 of 80 low-and middle-income countries (37.5%) that have introduced national HPV vaccination [140, 141].
HPV vaccination rates have an important impact on cervical lesions and cervical cancer rates. Thus, addressing barriers to vaccination is key to achieving the full benefits of vaccines. Currently, there are still multiple barriers to HPV vaccination programs, including the following:
High vaccine costs
The cost of vaccines is a significant barrier, especially in low- and middle-income countries. Recommendations to address this are to add HPV vaccination to the childhood immunization schedule and/or in combination with other vaccines, but this approach is not sustainable without external support [142].
Cold-chain requirement
Another huge obstacle to HPV vaccination in low- and middle-income countries is the cold chain preservation of HPV vaccines, which requires high costs for preservation and transportation. Cold chain supply alone accounts for 80 percent of the cost of the vaccine. One solution to this problem is to dehydrate the vaccine ingredients and freeze them in powder form for storage and transportation at higher temperatures [143]. Another approach is that thermally stabilized capsomer preparations will also allow for a greater degree of temperature fluctuations, thus reducing transport costs [144]. Unfortunately, none of these agents are currently available. In addition, a single dose regimen can reduce costs and supply and storage issues. Global vaccination rates are likely to increase as vaccine stability improves and vaccination costs decrease.
Vaccine hesitancy
In most low- and middle-income countries, the public is unaware of HPV-related diseases and national vaccination programmes [145]. Due to the diversity of cultural/religious beliefs, many baselessly associate HPV vaccine use with sexual promiscuity [142]. A cross-sectional survey of parents completed in 2017–2018 found that safety concerns were the most common reason parents gave for not vaccinating their adolescent children [146]. The solution is a combination of sex education and government-driven health policies that help increase public acceptance of vaccines and address parents' concerns through appropriate community education campaigns [147,148,149].
Covid-19 pandemic
Globally, the COVID-19 pandemic in 2019 and the closure of schools and suspension of routine immunization schedules between 2019 and 2022 disrupted health systems around the world. Twenty-five million children missed the 2021 vaccination, a 30 percent increase from 2019. Many schemes are still recovering from the effects of the pandemic [142].
A shortage of HPV vaccines, lack of insurance coverage [150], the need to provide accurate information about the safety and effectiveness of HPV vaccines, and the need to make the vaccines available to health care providers have hampered HPV vaccination worldwide [151].
Conclusion
There is increasing evidence that HPV vaccination reduces the risk of recurrent cervical lesions in women with surgically treated HSIL, and prospective studies are needed to assess the effect of the prophylactic vaccine on recurrent cervical lesions.
The best time for perioperative HPV vaccination is before or shortly after LEEP/ conization (up to 1 month after LEEP). Vaccination as soon as possible can better prevent reinfection in women at risk for HPV infection.The HPV vaccine is highly safe with few side effects and excellent immunogenicity. A single-dose administration regimen showed significant protection in susceptible populations, but its cross-over protection was limited. Existing HPV vaccines do not prevent all types of HPV, eliminate previous HPV infections, and treat related cervical diseases. As a result, significant numbers of people remain infected with HPV despite receiving the vaccine. In addition, multiple barriers to HPV vaccination currently keep global coverage low, particularly in low- and middle-income countries. There is an unmet need for therapeutic HPV vaccination, and HPV-associated malignancies will remain high for many years to come.
With our continuous in-depth understanding of the specific pathological mechanism of HPV and the molecular biological mechanism of tumors, the development of HPV therapeutic vaccines for various types of HPV immunogenicity, cheaper, less cold chain tolerance can be expected, and the goal of improving the coverage rate and success rate of the global HPV vaccine and eliminating cervical cancer will be achieved.
Availability of data and matarials
All data grnerated or analyzed during this study are included in this published article.
References
Okunade KS. human papillomavirus and cervical cancer. J Obstet Gynaecol. 2020;40:602–8.
World Health Organization. Estimated Number of Incident Cases and Deaths Worldwide, Females, All Ages
Denny L, Prendiville W. Cancer of the cervix: early detection and cost-effective solutions. Int J Gynaecol Obstet. 2015;131:S28–32.
Lin S, Gao K, Gu S, You L, Qian S, Tang M, Wang J, Chen K, Jin M. Worldwide trends in cervical cancer incidence and mortality, with predictions for the next 15 years. Cancer. 2021;127:4030–9.
Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, Bray F. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–203.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069.
Dostalek L, Åvall-Lundqvist E, Creutzberg CL, Kurdiani D, Ponce J, Dostalkova I, Cibula D. ESGO survey on current practice in the management of cervical cancer. Int J Gynecol Cancer. 2018;28:1226–31.
Koh W-J, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, Chon HS, Chu C, Clark R, Cohn D, et al. Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2019;17:64–84.
Sankaranarayanan R. HPV vaccination: the most pragmatic cervical cancer primary prevention strategy. Int J Gynaecol Obstet. 2015;131(Suppl. 1):S33–5.
Radley D, Saah A, Stanley M. Persistent infection with human papillomavirus 16 or 18 is strongly linked with high-grade cervical disease. Hum Vaccines Immunother. 2016;12:768–72.
Pils S, Joura EA. From the monovalent to the nine-valent HPV vaccine. Clin Microbiol Infect. 2015;21:827–33.
Huh WK, Joura EA, Giuliano AR, Iversen O-E, de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN, Hirschberg AL, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet. 2017;390:2143–59.
Martin-Hirsch PP, Paraskevaidis E, Bryant A, Dickinson HO, Keep SL. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2010;6:CD001318.
Del Pino M, Martí C, Torras I, Henere C, Munmany M, Marimon L, Saco A, Torné A, Ordi J. HPV vaccination as adjuvant to conization in women with cervical intraepithelial neoplasia: a study under real-life conditions. Vaccines. 2020;8:245.
Rodriguez-Manfredi A, Alonso I, del Pino M, Fusté P, Torné A, Ordi J. Predictors of absence of cervical intraepithelial neoplasia in the conization specimen. Gynecol Oncol. 2013;128:271–6.
Kocken M, Helmerhorst TJM, Berkhof J, Louwers JA, Nobbenhuis MAE, Bais AG, Hogewoning CJ, Zaal A, Verheijen RHM, Snijders PJF, et al. Risk of recurrent high-grade cervical intraepithelial neoplasia after successful treatment: a long-term multi-cohort study. Lancet Oncol. 2011;12:441–50.
Strander B, Hallgren J, Sparen P. Effect of ageing on cervical or vaginal cancer in Swedish women previously treated for cervical intraepithelial neoplasia grade 3: population based cohort study of long term incidence and mortality. BMJ. 2014;348:f7361.
Ghelardi A, Parazzini F, Martella F, Pieralli A. SPERANZA project: HPV vaccination after treatment for CIN2+. Gynecol Oncol. 2018;151:229–34.
Velentzis LS, Brotherton JML, Canfell K. Recurrent disease after treatment for cervical pre-cancer: determining whether prophylactic HPVvaccination could play a role in prevention of secondary lesions. Climacteric. 2019;22:596–602.
Bartels HC, Postle J, Rogers AC, Brennan D. Prophylactic human papillomavirus vaccination to prevent recurrence of cervical intraepithelial neoplasia: a meta-analysis. Int J Gynecol Cancer. 2020;30:777–82.
Jentschke M, Kampers J, Becker J, Sibbertsen P, Hillemanns P. Prophylactic HPV vaccination after conization: a systematic review and meta-analysis. Vaccine. 2020;38:6402–9.
Lichter K, Krause D, Xu J, Tsai SHL, Hage C, Weston E, Eke A, Levinson K. Adjuvant human papillomavirus vaccine to reduce recurrent cervical dysplasia in unvaccinated women: a systematic review and meta-analysis. Obstet Gynecol. 2020;135:1070–83.
Kang WD, Choi HS, Kim SM. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2-3)? Gynecol Oncol. 2013;130:264–8.
Saftlas AF, Spracklen CN, Ryckman KK, Stockdale CK, Penrose K, Ault K, Rubenstein LM, Pinto LA. Influence of a loop electrosurgical excision procedure (LEEP) on levels of cytokines in cervical secretions. J Reprod Immunol. 2015;109:74–83.
Frazer IH. Interaction of human papillomaviruses with the host immune system: a well evolved relationship. Virology. 2009;384:410–4.
Scott ME, Shvetsov YB, Thompson PJ, Hernandez BY, Zhu X, Wilkens LR, Killeen J, Vo DD, Moscicki A, Goodman M. Cervical cytokines and clearance of incident human papillomavirus infection: Hawaii HPV cohort study: mucosal cytokines and cervical HPV clearance. Int J Cancer. 2013;133:1187–96.
Joura EA, Garland SM, Paavonen J, Ferris DG, Perez G, Ault KA, Huh WK, Sings HL, James MK, Haupt RM. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ. 2012;344:e1401.
Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54:891–8.
Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–23.
Brianti P, Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80–5.
Joyce JG, Tung JS, Przysiecki CT, Cook JC, Lehman ED, Sands JA, Jansen KU, Keller PM. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J Biol Chem. 1999;274:5810–22.
Evander M, Frazer IH, Payne E, Qi YM, Hengst K, McMillan NA. Identification of the alpha6 integrin as a candidate receptor for papillomaviruses. J Virol. 1997;71:2449–56.
McBride AA. Replication and partitioning of papillomavirus genomes. Adv Virus Res. 2008;72:155–205.
Bergvall M, Melendy T, Archambault J. The E1 proteins. Virology. 2013;445:35–56.
Stubenrauch F, Laimins LA. Human papillomavirus life cycle: active and latent phases. Semin Cancer Biol. 1999;9:379–86.
Howie HL, Katzenellenbogen RA, Galloway DA. Papillomavirus E6 proteins. Virology. 2009;384:324–34.
Liu X, Disbrow GL, Yuan H, Tomaic V, Schlegel R. Myc and human papillomavirus type 16 E7 genes cooperate to immortalize human keratinocytes. J Virol. 2007;81:12689–95.
Zhang Y, Dakic A, Chen R, Dai Y, Schlegel R, Liu X. Direct HPV E6/Myc interactions induce histone modifications, pol II phosphorylation, and hTERT promoter activation. Oncotarget. 2017;8:96323–39.
Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. 2013;382:889–99.
Huber J, Mueller A, Sailer M, Regidor P-A. Human papillomavirus persistence or clearance after infection in reproductive age. What is the status? Review of the literature and new data of a vaginal gel containing silicate dioxide, citric acid, and selenite. Womens Health. 2021;17:17455065211020702.
Wang R, Pan W, Jin L, Huang W, Li Y, Wu D, Gao C, Ma D, Liao S. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88–102.
Kombe Kombe AJ, Li B, Zahid A, Mengist HM, Bounda GA, Zhou Y, et al. Epidemiology and burden of human papillomavirus and related diseases, molecular pathogenesis, and vaccine evaluation. Front Public Health. 2021;8:552028.
Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller J. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992;89(24):12180–4.
Stanley M. Potential mechanisms for HPV vaccine-induced long-term protection. Gynecol Oncol. 2010;118(1):S2–7.
Siegrist C-A. Vaccine immunology. Vaccines. 2008;5:17–36.
Yang A, Farmer E, Wu TC, Hung CF. Perspectives for therapeutic HPV vaccine development. J Biomed Sci. 2016;23:75.
Chabeda A, Yanez RJR, Lamprecht R, Meyers AE, Rybicki EP, Hitzeroth II. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018;5:46–58.
Garbuglia AR, Lapa D, Sias C, Capobianchi MR, Porto PD. The use of both therapeutic and prophylactic vaccines in the therapy of papillomavirus disease. Front Immunol. 2020;11:1–14.
World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, 2nd Ed: Use of mRNA Tests for Human Papillomavirus (HPV). Accessed 15 Feb 2022.
Wang Y, Zhang H, Yu J, Li C, Lu Y. Advances of human papillomavirus therapeutic vaccine. J Int Oncol. 2017;44(7):526–30.
Hua C, Sun S, Cheng H, Han R. Advance in human papillomavirus major capsid protein L1-based vaccines. Chin J Microbiol Immunol. 2019;39(10):788–93.
Casajuana-Pérez A, Ramírez-Mena M, Ruipérez-Pacheco E, et al. Effectiveness of prophylactic human papillomavirus vaccine in the prevention of recurrence in women conized for HSIL/CIN 2–3: the VENUS study. Vaccines (Basel). 2022;10(2):288.
Di Donato V, Caruso G, Petrillo M, et al. Adjuvant HPV vaccination to prevent recurrent cervical dysplasia after surgical treatment: a meta-analysis. Vaccines (Basel). 2021;9(5):410.
Szarewski A, Poppe WA, Skinner SR, Wheeler CM, Paavonen J, Naud P, et al. Efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in women aged 15–25 years with and without serological evidence of previous exposure to HPV-16/18. Int J Cancer. 2012;131:106–16.
Giuliano AR, Joura EA, Garland SM, Huh WK, Iversen OE, Kjaer SK, et al. Nine-valent HPV vaccine efficacy against related diseases and definitive therapy: comparison with historic placebo population. Gynecol Oncol. 2019;154:110–7.
Scherer EM, Smith RA, Gallego DF, Carter JJ, Wipf GC, Hoyos M, et al. A single human papillomavirus vaccine dose improves B cell memory in previously infected subjects. EBioMedicine. 2016;10:55–64.
Hildesheim A, Gonzalez P, Kreimer AR, Wacholder S, Schussler J, Rodriguez AC, et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am J Obstet Gynecol. 2016;215:212.e1-212.e15.
Mariz FC, Bender N, Anantharaman D, Basu P, Bhatla N, Pillai MR, Prabhu PR, Sankaranarayanan R, Eriksson T, Pawlita M, et al. Peak neutralizing and cross-neutralizing antibody levels to human papillomavirus types 6/16/18/31/33/45/52/58 induced by bivalent and quadrivalent HPV vaccines. NPJ Vaccines. 2020;5:14.
Athanasiou A, Bowden S, Paraskevaidi M, Fotopoulou C, Martin-Hirsch P, Paraskevaidis E, Kyrgiou M. HPV vaccination and cancer prevention. Best Pract Res Clin Obstet Gynaecol. 2020;65:109–24.
Ault KA. Human papillomavirus vaccines and the potential for cross-protection between related HPV types. Gynecol Oncol. 2007;107:S31–3.
de Vincenzo R, Ricci C, Conte C, Scambia G. HPV vaccine cross-protection: highlights on additional clinical benefit. Gynecol Oncol. 2013;130:642–51.
Paradkar PH, Joshi JV, Mertia PN, Agashe SV, Vaidya R. A role of cytokines in genesis, progression and prognosis of cervical cancer. Asian Pac J Cancer Prev. 2014;15:3851–64.
Song SH, Lee JK, Lee NW, Saw HS, Kang JS, Lee KW. Interferon-gamma (IFN-gamma): a possible prognostic marker for clearance of high-risk human papillomavirus (HPV). Gynecol Oncol. 2008;108:543–8.
Zurek M-M, Toft L, Kube T, Richter R, Ostergaard L, Søgaard OS, Tolstrup M, Kaufmann AM. Cellular immunogenicity of human papillomavirus vaccines Cervarix and Gardasil in adults with HIV infection. Hum Vaccines Immunother. 2018;14:909–16.
Turner TB, Huh WK. HPV vaccines: translating immunogenicity into efficacy. Hum Vaccines Immunother. 2016;12:1403–5.
Schauner S, Lyon C. Bivalent HPV recombinant vaccine (cervarix) for the prevention of cervical cancer. Am Fam Physician. 2010;82:1541–2.
Szarewski A. HPV vaccine: cervarix. Expert Opin Biol Ther. 2010;10:477–87.
Isidean SD, Tota EJ, Gagnon AJ, Franco EL. Human papillomavirus vaccines: key factors in planning cost-effective vaccination programs. Expert Rev Vaccines. 2014;14:119–33.
Roden RBS, Stern PL. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer. 2018;18:240–54.
Reisinger KS, Block SL, Lazcano-Ponce E, Samakoses R, Esser MT, Erick J, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J. 2007;26(3):201–9.
Romanowski B, Naud PS, Roteli-Martins CM, De NSC, Teixeira JC, Aoki F, et al. sustained efficacy and immunogenicity of the Human Papillomavirus (HPV)-16/18 AS04-Adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet (Lond Engl). 2009;374(9706):1975–85.
Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Heal. 2007;40(6):564–71.
Vesikari T, Brodszki N, Van Damme P, Diez-Domingo J, Icardi G, Petersen LK, et al. A randomized, double-blind, phase III Study of the immunogenicity and safety of a 9-valent human papillomavirus L1 virus-like particle vaccine (V503) versus Gardasil® in 9–15-year-old girls. Pediatr Infect Dis J. 2015;34(9):992–8.
Garland SM, Cheung T-H, McNeill S, Petersen LK, Romaguera J, Vazquez-Narvaez J, et al. Safety and immunogenicity of a 9-valent HPV vaccine in females 12–26 years of age who previously received the quadrivalent HPV vaccine. Vaccine. 2015;33(48):6855–64.
GlaxoSmithKline Vaccine HPV-007 Study Group, Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374:1975–85.
Schwarz TF, Galaj A, Spaczynski M, et al. Ten-year immune persistence and safety of the HPV-16/18 AS 04-adjuvanted vaccine in females vaccinated at 15–55 years of age. Cancer Med. 2017;6(11):2723–31.
Arbyn M, Xu L. Efficacy and safety of prophylactic HPV vaccines. A cochrane review of randomized trials. Expert Rev Vaccines. 2018;17(12):1085–91.
Harper DM. Currently approved prophylactic HPV vaccines. Expert Rev Vaccines. 2009;8(12):1663–79.
Safaeian M, Porras C, Pan Y, Kreimer A, Schiller JT, Gonzalez P, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the costa rica vaccine trial. Cancer Prev Res. 2013;6(11):1242–50.
Villa LL, Ault KA, Giuliano AR, Costa RLR, Petta CA, Andrade RP, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine. 2006;24(27–28):5571–83.
Leung TF, Liu AP-Y, Lim FS, Thollot F, Oh HML, Lee BW, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and 4vhpv vaccine administered according to two-or three-dose schedules in girls aged 9–14 years: results to month 36 from a randomized trial. Vaccine. 2018;36(1):98–106.
Dobson SRM, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309(17):1793–802.
Malagón T, Drolet M, Boily M-C, Franco EL, Jit M, Brisson J, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(10):781–9.
Wheeler CM, Kjaer SK, Sigurdsson K, Iversen O-E, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, García P, et al. The impact of quadrivalent human papillomavirus (HPV. Types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J Infect Dis. 2009;199:936–44.
Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmerón J, Chow SN, Apter D, Kitchener H, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–10.
Herrero R, Wacholder S, Rodríguez AC, Solomon D, González P, Kreimer AR, Porras C, Schussler J, Jiménez S, Sherman ME, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste. Costa Rica Cancer Discov. 2011;1:408–19.
Tsang SH, Sampson JN, Schussler J, Porras C, Wagner S, Boland J, Cortes B, Lowy DR, Schiller JT, Schiffman M, et al. Durability of cross-protection by different schedules of the bivalent HPV vaccine: the CVT trial. JNCI. 2020;112:1030–7.
Schwarz TF, Huang L-M, Valencia A, Panzer F, Chiu C-H, Decreux A, Poncelet S, Karkada N, Folschweiller N, Lin L, et al. A ten-year study of immunogenicity and safety of the AS04-HPV-16/18 vaccine in adolescent girls aged 10–14 years. Hum Vaccines Immunother. 2019;15:1970–9.
Garland SM, Paavonen J, Jaisamrarn U, Naud P, Salmerón J, Chow S, Apter D, Castellsagué X, Teixeira JC, Skinner SR, et al. Prior human papillomavirus-16/18 AS04-adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: post-hoc analysis from a randomized controlled trial. Int J Cancer. 2016;139:2812–26.
Sand FL, Kjaer SK, Frederiksen K, Dehlendorff C. Risk of cervical intraepithelial neoplasia grade 2 or worse after conization in relation to HPV vaccination status. Int J Cancer. 2020;147:641–7.
Henere C, Torné A, Llupià A, et al. HPV vaccination in women with cervical intraepithelial neoplasia undergoing excisional treatment: insights into unsolved questions. Vaccines (Basel). 2022;10(6):887.
Tjalma WAA, van Heerden J, van den Wyngaert T. If prophylactic HPV vaccination is considered in a woman with CIN2+, what is the value and should it be given before or after the surgical treatment? Eur J Obstet Gynecol Reprod Biol. 2022;269:98–101.
Ortega-Quiñonero P, Remezal-Solano M, Carazo-Díaz MC, Prieto-Merino D, Urbano-Reyes MI, de Guadiana-Romualdo LG, Martínez-Cendán JP. Impact of the human papillomavirus vaccination on patients who underwent conization for high-grade cervical intraepithelial neoplasia. Eur J Gynaecol Oncol. 2019;40:402–7.
Bogani G, Raspagliesi F, Sopracordevole F, Ciavattini A, Ghelardi A, Simoncini T, Petrillo M, Plotti F, Lopez S, Casarin J, et al. Assessing the long-term role of vaccination against HPV after loop electrosurgical excision procedure (LEEP): a propensity-score matched comparison. Vaccines. 2020;8:717.
Roberts JM, Jin F, Poynten IM, Law C, Templeton DJ, Thurloe JK, Garland SM, Grulich AE, Farnsworth A, Hillman RJ. Histological outcomes of anal high-grade cytopredictions. Cancer Cytopathol. 2018;126:136–44.
Rebolj M, Helmerhorst T, Habbema D, Looman C, Boer R, van Rosmalen J, van Ballegooijen M. Risk of cervical cancer after completed post-treatment follow-up of cervical intraepithelial neoplasia: population based cohort study. BMJ. 2012;345:e6855.
Ebisch RM, Rutten DW, Hout J, Melchers WJ, Massuger LF, Bulten J, Bekkers RL, Siebers AG. Long-lasting increased risk of human papillomavirus–related carcinomas and premalignancies after cervical intraepithelial neoplasia grade 3: a population-based cohort study. J Clin Oncol. 2017;35:2542–50.
Strander B, Andersson-Ellström A, Milsom I, Sparén P. Long term risk of invasive cancer after treatment for cervical intraepithelial neoplasia grade 3: population based cohort study. Br Med J. 2007;335:1077–80.
Villa LL, Kjaer SK, Muñoz N. The FUTURE II study group quadrivalent vaccine against human papillomavirus to prevent hgh-grade cervical lesions. N Engl J Med. 2010;356:1991–2002.
Karimi-Zarchi M, Allahqoli L, Nehmati A, Kashi AM, Taghipour-Zahir S, Alkatout I. Can the prophylactic quadrivalent HPV vaccine be used as a therapeutic agent in women with CIN? A randomized trial. BMC Public Health. 2020;20:274.
Bergman H, Buckley BS, Villanueva G, Petkovic J, Garritty C, Lutje V, Riveros-Balta AX, Low N, Henschke N. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males. Cochrane Database Syst Rev. 2019;11:CD013479.
Schiller J, Lowy D. Explanations for the high potency of HPV prophylactic vaccines. Vaccine. 2018;36:4768–73.
Porras C, Tsang SH, Herrero R, Guillén D, Darragh TM, Stoler MH, Hildesheim A, Wagner S, Boland J, Lowy DR, et al. Efficacy of the bivalent HPV vaccine against HPV 16/18-associated precancer: long-term follow-up results from the Costa Rica Vaccine Trial. Lancet Oncol. 2020;21:1643–52.
Kreimer AR, Herrero R, Sampson JN, Porras C, Lowy DR, Schiller JT, Schiffman M, Rodriguez AC, Chanock S, Jimenez S, et al. Evidence for single-dose protection by the bivalent HPV vaccine—review of the Costa Rica HPV vaccine trial and future research studies. Vaccine. 2018;36:4774–82.
Kreimer AR, Sampson JN, Porras C, Schiller JT, Kemp T, Herrero R, Wagner S, Boland J, Schussler J, Lowy DR, et al. Evaluation of durability of a single dose of the bivalent HPV vaccine: the CVT trial. J Natl Cancer Inst. 2020;112:1038–46.
Tota EJ, Struyf F, Hildesheim A, Gonzalez P, Ryser M, Herrero R, Schussler J, Karkada N, Rodriguez AC, Folschweiller N, et al. Efficacy of AS04-adjuvanted vaccine against human papillomavirus (HPV) types 16 and 18 in clearing incident HPV infections: pooled analysis of data from the costa rica vaccine trial and the PATRICIA study. J Infect Dis. 2021;223:1576–81.
Sankaranarayanan R, Prabhu PR, Pawlita M, Gheit T, Bhatla N, Muwonge R, Nene BM, Esmy PO, Joshi S, Poli URR, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. 2015;17:67–77.
Porras C, Sampson JN, Herrero R, Gail MH, Cortés B, Hildesheim A, Cyr J, Romero B, Schiller JT, Montero C, et al. Rationale and design of a double-blind randomized non-inferiority clinical trial to evaluate one or two doses of vaccine against human papillomavirus including an epidemiologic survey to estimate vaccine efficacy: the Costa Rica ESCUDDO trial. Vaccine. 2022;40:76–88.
Kavanagh K, Pollock KGJ, Potts A, Love J, Cuschieri K, Cubie H, Robertson C, Donaghy M. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer. 2014;110:2804–11.
Brotherton JML, Budd A, Rompotis C, Bartlett N, Malloy MJ, Andersen RL, Coulter KAR, Couvee PW, Steel N, Ward GH, et al. Is one dose of human papillomavirus vaccine as effective as three? A national cohort analysis. Papillomavirus Res. 2019;8:100177.
Basu P, Bhatla N, Ngoma T, Sankaranarayanan R. Less than 3 doses of the HPV vaccine—review of efficacy against virological and disease end points. Hum Vaccines Immunother. 2016;12:1394–402.
Crowe E, Pandeya N, Brotherton JML, Dobson AJ, Kisely S, Lambert SB, Whiteman DC. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: Case-control study nested within a population based screening programme in Australia. BMJ. 2014;348:g1458.
Gertig DM, Brotherton JM, Budd AC, Drennan K, Chappell G, Saville AM. Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study. BMC Med. 2013;11:227.
Toh ZQ, Licciardi PV, Fong J, et al. Reduced dose human papillomavirus vaccination: an update of the current state-of-the-art. Vaccine. 2015;33(39):5042–50.
Donken R, Knol MJ, Bogaards JA, et al. Inconclusive evidence for non-inferior immunogenicity of two- compared with three-dose HPV immunization schedules in preadolescent girls: a systematic review and meta-analysis. J Infect. 2015;71(1):61–73.
Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405–8.
Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human papillomavirus vaccination: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2014;63:1–30.
Zhang L, Wang W, Wang S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev Vaccines. 2015;14(11):1509–23.
Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26(26):3197–208.
Constantino J, Gomes C, Falcão A, Cruz MT, Neves BM. Antitumor dendritic cell-based vaccines: lessons from 20 years of clinical trials and future perspectives. Transl Res. 2016;168:74–95.
Wang J, Peng Y, Xu H, Cui Z, Williams RO. The COVID-19 vaccine race: challenges and opportunities in vaccine formulation. AAPS PharmSciTech. 2020;21:225.
Global Advisory Committee on Vaccine Safety (2017) 30 November–1 December 2016, Weekly Epidemiological Record, World Health Organization
Gonçalves AK, Cobucci RN, Rodrigues HM, Melo AG, Giraldo PC. Safety, tolerability and side effects of human papillomavirus vaccines: a systematic quantitative review. Braz J Infect Dis. 2014;18(6):651–9.
Kurugöl Z, Erensoy S, Akşit S, Egemen A, Bilgiç A, et al. Low-dose intradermal administration of recombinant hepatitis b vaccine in children: 5-year follow-up study. Vaccine. 2001;19(28–29):3936–9.
Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–70.
Phillips A, Patel C, Pillsbury A, Brotherton J, Macartney K. Safety of human papillomavirus vaccines: an updated review. Drug Saf. 2018;41(4):329–46.
Pattyn J, Keer SV, Tjalma W, Matheeussen V, Damme PV, Vorsters A. Infection and vaccine-induced HPV-specific antibodies in cervicovaginal secretions. A review of the literature. Papillomavirus Res. 2019;8:100185.
Costa APF, Cobucci RNO, Da Silva JM, Lima PHDC, Giraldo PC, Gonçalves A. Safety of human papillomavirus 9-valent vaccine: a meta-analysis of randomized trials. J Immunol Res. 2017;2017:3736201.
Shimabukuro TT, Su JR, Marquez PL, Mba-Jonas A, Arana JE, Cano MV. Safety of the 9-valent human papillomavirus vaccine. Pediatrics. 2019;144:e20191791.
Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401–11.
Bird Y, Obidiya O, Mahmood R, et al. Human papillomavirus vaccination uptake in canada: a systematic review and meta-analysis. Int J Prev Med. 2017;8:71. https://doi.org/10.4103/ijpvm.IJPVM4917.
Kaiser Family Foundation. The HPV vaccine: Access and use in the USA. Retrieved from https://www.kff.org/womens-health-policy/fact-sheet/the-hpv-vaccine-access-and-use-in-the-u-s/.
American College Health Association. (2019) American College Health Association-National College Health Assessment II: Undergraduate Student Reference Group Data Report Spring 2019. American College Health Association.
Johnson KL, Lin MY, Cabral H, Kazis LE, Katz IT. Variation in human papillomavirus vaccine uptake and acceptability between female and male adolescents and their caregivers. J Commun Health. 2017;42(3):522–532. https://doi.org/10.1007/s10900-016-0284-5.
Laserson AK, Oliffe JL, Krist J, et al. HPV vaccine and college-age men: a scoping review. Am J Mens Health. 2020;14(6):1557988320973826.
Phillips A, Patel C, Pillsbury A, Brotherton J, Macartney K. Safety of human papillomavirus vaccines: an updated review. Drug Saf. 2018;41:329–46.
Gee J, Weinbaum C, Sukumaran L, Markowitz LE. Quadrivalent HPV vaccine safety review and safety monitoring plans for nine-valent HPV vaccine in the United States. Hum Vaccines Immunother. 2016;12:1406–17.
Human Papillomavirus (HPV) Vaccination Coverage. Accessed on 24 May 2022. Available online https://immunizationdata.who.int/pages/coverage/hpv.html?CODE=Global&ANTIGEN=PRHPVC_F&YEAR=.
WHO HPV Vaccine Global Market Study April 2022. Accessed on 24 Apr 2022. Available online https://www.who.int/publications/m/item/who-hpv-vaccine-global-market-study-april-2022.
Global HPV Vaccine Introduction Overview. Accessed on 24 May 2022. Available online https://path.azureedge.net/media/documents/Global_Vaccine_Intro_Overview_Slides_Final_PATHwebsite_MAR_2022_qT92Wwh.pdf.
Toh ZQ, Russell FM, Garland SM, Mulholland EK, Patton G, Licciardi PV. Human papillomavirus vaccination after COVID-19. JNCI Cancer Spectr. 2021;5:pkab011.
Hassett KJ, Meinerz NM, Semmelmann F, Cousins MC, Garcea RL, Randolph TW. Development of a highly thermostable, adjuvanted human papillomavirus vaccine. Eur J Pharm Biopharm. 2015;94:220–8.
Rosalik K, Tarney C, Han J. Human papilloma virus vaccination. Viruses. 2021;13:1091.
Ladner J, Besson M-H, Audureau E, Rodrigues M, Saba J. Experiences and lessons learned from 29 HPV vaccination programs implemented in 19 low and middle-income countries, 2009–2014. BMC Health Serv Res. 2016;16(1):575.
Sonawane K, Zhu Y, Montealegre JR, Lairson DR, Bauer C, McGee LU, Giuliano AR, Deshmukh AA. Parental intent to initiate and complete the human papillomavirus vaccine series in the USA: a nationwide, cross-sectional survey. Lancet Public Health. 2020;5:e484–92.
Newman AP, Logie CH, Doukas N, Asakura K. HPV vaccine acceptability among men: a systematic review and meta-analysis. Sex Transm Infect. 2013;89:568–74.
Taumberger N, Joura AE, Arbyn M, Kyrgiou M, Sehouli J, Gultekin M. Myths and fake messages about human Papillomavirus (HPV) vaccination: answers from the ESGO Prevention Committee. Int J Gynecol Cancer. 2022;32:1316–20.
Bruni L, Saura-Lázaro A, Montoliu A, Brotons M, Alemany L, Diallo MS, Afsar OZ, LaMontagne DS, Mosina L, Contreras M, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. 2021;144:106399.
Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics. 2011;128:830–9.
de Oliveira CM, Fregnani JHTG, Villa LL. HPV vaccine: updates and highlights. Acta Cytol. 2019;63(2):159–68.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors listed have contributed to the writing and review of the manuscript. Literature review, paper design and manuscript writing: LH. Data collection: BZ.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Han, L., Zhang, B. Can prophylactic HPV vaccination reduce the recurrence of cervical lesions after surgery? Review and prospect. Infect Agents Cancer 18, 66 (2023). https://doi.org/10.1186/s13027-023-00547-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13027-023-00547-2