Abstract
Background
Cervical cancer is associated with high‐risk human papillomavirus (HR-HPV) infection in the world. We aimed to evaluate the status of HPV infection among women in Guangzhou, China.
Methods
The study recruited 28,643 female patients from the Guangzhou Women and Children’s Medical Center for HPV genotype testing between 2019 and 2021.
Results
5668 patients were infected with HPV, resulting in an overall infection prevalence of 19.78%. The prevalence of HR-HPV was recorded at 13.94% (both single-infections and multi-infections), probably high-risk HPV/possibly carcinogenic (pHR-HPV) as 3.51%; and low-risk HPV (LR-HPV) as 3.56%. The most common HR-HPV genotype detected was HPV-52 with an infection rate of 4.99%, followed by HPV 58 (2.18%), 16 (2.12%), 51 (1.61%), 39 (1.19%), 56 (1.09%), 59 (0.85%), 18 (0.72%), 33 (0.61%), 31 (0.53%), 35 (0.20%), 45 (0.17%). Among LR-HPV genotypes, HPV-42 was the most common (1.08%), followed by 44 (0.77%), 81 (0.68%), 6 (0.48%), 43 (0.40%), 11 (0.23%) and 83 (0.07%). The prevalence of infection among different genotypes in pHR-HPV was: 68 (1.29%), 53 (1.21%), 66 (0.77%), 82 (0.25%), 73 (0.16%). Additionally, the prevalence of single genotype HPV infection exceeded that of multiple HPV infections except HPV-59.
Conclusion
Our findings imply that HPV genotype infections in Guangzhou demonstrate a regional and age-related distribution. Therefore, these data can provide a substantial foundation for further epidemiologic analysis to control and prevent HPV infections in Guangzhou.
Similar content being viewed by others
Introduction
Cervical cancer (CC) stands as the fourth most common cancer and a leading cause of cancer-related deaths among women worldwide, accounting for an estimated 604,000 new cases and 342,000 deaths in 2020 [1]. Owing to its large population, China accounts for 11.9% of global CC deaths [2]. Numerous studies have shown a strong association between several types of human papillomavirus (HPV) and cervical precancerous lesions [3]. It is recognized that CC caused are attributed to high-risk HPV types (HR-HPV) in all body regions [4]. Twelve HR-HPVs have been identified as cancer-causing and classified as Group 1 carcinogens in the International Agency for Research on Cancer monograph. In addition, HPV infection is implicated in the majority of CC [5], head and neck [6], anal and vulvar cancers, as well as other cancer types.
Over 200 types of HPV have been identified, with these genotypes categorized based on their risk for causing CC into: HR-HPV, low-risk HPV (LR-HPV), and probably high-risk HPV/possibly carcinogenic (IARC Groups 2A and 2B, respectively), hereafter referred to as pHR-HPV [7, 8]. HR-HPV types encompass HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 58, 56, and 59 [4]. The LR-HPV types comprise HPV-6, 11, 28, 32, 40, 42, 43, 44, 54, 55, 57, 61, 62, 71, 72, 74, 81, 83, 84, 86, 87, and 89 etc. [8]. Lastly, the probably high-risk types encompass HPV-68, while the possibly carcinogenic types include HPV-26, 53, 66, 67, 70, 73, and 82 [4, 9].
Several studies have affirmed that persistent HR-HPV infection is the primary risk factor for CC [10]. In addition, different HPV genotypes exhibit distinct oncogenic capabilities, and certain types of HR-HPV prevail in specific geographic regions [11]. In North America (Canada and the United States), HPV-16 is the most common genotype found in low-grade squamous intraepithelial lesions (LSILs), accounting for 26.3% of a study sample of 2425 patients with an average age of 47.8 (± 12.9) years [12]. On the other hand, Patients from Africa aged 33.9 years (± 11.4 years) infected with HPV-16 were twice as many as those from southern Europe aged 56.5 years (± 14.3 years) [12]. Additionally, HPV prevalence in China was 84.37% in a meta-analysis of 2950 cervical intraepithelial neoplasia (CIN)1 patients and 5393 CIN2/3 patients, but the distribution of HPV types varies across regions [13]. In western China (Tibet Autonomous Region, Chongqing, Guizhou, Shaanxi), HPV-52, 16, 58, and 53 were the most commonly detected HR-HPV types, with the highest HPV detection rate observed in the 36–50 age group [14]. In Northeast China, the predominant HPV types identified in a survey of 110,927 women aged 18–80 years were HPV-16, 58, 52, 33, 53, and 18 [15]. In Beijing, a survey of 46,365 sexually active women between 2017 and 2020 found that the most frequently detected HR-HPV types were HPV-52, 58, 16, 51, 66, and 59 [16]. Several regions in central China, such as Zhengzhou (healthy women aged 25–64 years) [17], Wuhan (patients including physical examination, infertility and vaginitis, and the subjects were aged between 16 and 83 years) [18], and Sichuan (healthy women aged 15–94 years), had the high prevalence of HPV-52 infection [19]. Therefore, it is useful to understand the distribution of HPV types in the population of a region to guide the application of HPV vaccine and CC screening strategies in prevention efforts.
This study focused on the epidemiology of HPV infection in Guangzhou in the past 3 years from January 2019 to December 2021. It is hoped that the study results will provide effective strategies for HPV prevention in Guangzhou and promote HPV-targeted vaccination by the local government.
Materials and methods
Study population
The study involved 28,643 women who underwent annual routine gynecological examinations at the outpatient gynecological clinic of Guangzhou Women and Children’s Medical Center between January 2019 and December 2021. Patients’ etiologies consisted of cervical cytological abnormalities like atypical squamous cells and cervical intraepithelial neoplasia (CIN) 1/2/3 also detected during histological examination. It also includes patients with genital warts, physical infertility, vaginitis, cervicitis.
Ethical standards
The clinical protocol of this study was approved by the Clinical Research Committee of Guangzhou Women and Children’s Medical Center. All participants signed a written informed consent form saying that all procedures will be performed according to the standard. The authors assert that all research procedures comply with ethical standards set by relevant national and institutional human experimental committees, as well as the 1975 Helsinki Declaration, updated by the World Medical Association in 2008.
Specimen collection and management
On the day of sample collection, participants were required not to be menstruating, not to have received any pelvic or vaginal treatments, and to abstain from sexual activity in the previous 24 h. To obtain the cervical sample, a cotton swab was utilized to delicately remove excess secretions from the cervical orifice. The cytobrush was inserted into the cervical opening at a depth of 1–1.5 cm until the outermost bristles of the brush touched the ectocervix, and gently rotated five times to collect a sufficient number of exfoliated cells. The samples were placed in the preservation fluid bottle for the specimen and stirred several times to remove as many cells as possible. The sample is preserved in a solution maintained at − 80 °C until it is ready for extraction and genotyping of HPV DNA. The collected specimens can be kept at room temperature for no more than 1 week and at 4 °C for no more than 2 weeks. HPV sample genotyping is completed within 1 week.
Test method
Genomic DNA was extracted from collected samples using a DNA extraction kit (TIANGEN, DP304, Beijing, China) according to the manufacturer’s instructions. All DNA samples were amplified using Cobas Z 480 Real-Time PCR and its corresponding reagents (HybriBio Ltd, HBRT-H13C, Chaozhou, China). HPV genotyping was performed using the HybriMax HPV Gene Array Assay Kit (HybriBio Ltd, C230503A, Chaozhou, China) according to the manufacturer’s instructions. Positive and negative controls were included in each PCR assay to ensure accuracy and check for possible contaminations. This method was utilized to distinguish 23 HPV types, including 16-HR-HPV types. The infection rates among individuals who tested positive for HR-HPV were as follows: 52, 58, 16, 51, 39, 56, 59, 18, 33, 31, 35, 45. Prevalence of LR-HPV infections were as follows: 42, 44, 81, 6, 43, 11 and 83. The prevalence of pHR-HPV-positive infections were 68, 53, 66, 82, 73. In the final step of the process, the different genotypes are identified by adding the Nitro Blue Tetrazolium/5-Bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP) solution to show results, where clearly visible indigo dots indicate HPV positivity. The placement of the HPV genotype probe on the microarray chip determines the outcome, with multiple marks indicating the presence of co-infection or multiple HPV infections.
Data analysis
All data was analyzed using the SPSS 16.0 software package. Participants’ age and other measurements were expressed as mean ± standard deviation (SD). Chi-square tests were used to compare HPV prevalence in each group. The comparison of data between different ages was considered statistically significant at P < 0.0017.
Results
The overall prevalence of HPV infection
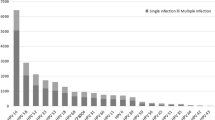
Out of the 28,643 participants, their ages ranged from 16 to 83 years (with an average age of 36.69 ± 8.72), and they were divided into eight groups, each spanning 5 years. The main reasons for HPV testing in these participants included Cervical intraepithelial neoplasia (CIN)1/2/3 (14.78%), biopsy pathology with genital warts (0.03%), physiologic infertility (17.37%), vaginitis (30.61%), cervicitis (37.21%) (Additional file 1: Table S1). The largest number of HPV infections in CIN 1/2/3, physiological infertility, vaginitis and cervicitis were predominantly in the 31–35 age (Additional file 1: Table S2). In addition, there were more HR-HPV infections in each disease than in the pHR-HPV and LR-HPV groups (Additional file 1: Table S3). The results showed that 3992 (13.94%) were HR-HPV, 1021 (3.56%) were LR-HPV, and 1005 (3.51%) were pHR-HPV, including co-infection. The number of positive HR-HPV infections was higher than that of LR-HPV infections. The infection rates for HR-HPV were as follows: 52 (4.99%), 58 (2.18%), 16 (2.12%), 51 (1.61%), 39 (1.19%), 56 (1.09%), 59 (0.85%), 18 (0.72%), 33 (0.61%), 31 (0.53%), 35 (0.20%), 45 (0.17%). Prevalence of LR-HPV infections were as follows: 42 (1.08%), 44 (0.77%), 81 (0.68%), 6 (0.48%), 43 (0.40%), 11 (0.23%) and 83 (0.07%). The prevalence of pHR-HPV-positive infections were 68 (1.29%), 53 (1.21%), 66 (0.77%), 82 (0.25%), 73 (0.16%) (Fig. 1 and Additional file 1: Table S4).
Single and multiple infections with different HPV genotypes in HPV-positive individuals. Co-infections include multiple HPV genotypes, such as HR-HPV with HR-HPV, HR-HPV with LR-HPV, pHR-HPV and LR-HPV, etc. Among the 5668 patients identified with HPV infection (including single and multiple infections), there were 3992 cases of HR-HPV infections, 1021 cases involving LR-HPV, and 1005 cases of pHR-HPV infections. The types of HR-HPV infections were: HPV-52, 58, 16, 51, 39, 56, 59, 18, 33, 31, 35 and 45. The numbers and percentages of these infections were 1,432 (35.87%), 625 (15.66%), 608 (15.23%), 462 (11.57%), 342 (8.57%), 314 (7.87%), 244 (6.11%), 207 (5.19%), 177 (4.43%), 153 (3.83%), 60 (1.50%) and 49 (1.23%), respectively (Fig. 2A and Additional file 1: Table S5). The types of pHR-HPV infections were: 68, 53, 66, 82 and 73. The numbers and percentages of these infections were: 370 (36.82%), 349 (34.73%), 221 (21.99%), 73 (7.26%), 48 (4.78%) (Fig. 2B and Additional file 1: Table S5). The different types of LR-HPV positive infections identified were HPV-42, 44, 81, 6, 43, 11, and 83. The numbers and percentages of these infections were: 312 (30.56%), 222 (21.74%), 195 (19.10%), 138 (13.52%), 116 (11.36%), 70 (6.86%) and 21 (2.06%) of cases, respectively (Fig. 2C and Additional file 1: Table S5). Analysis of data on the proportion of different HPV types in the patient group that were positive for the virus revealed HPV-52 as the most prevalent subtype. Following HPV-52 were the HR-HPV types 58, 16, 51. Among the LR-HPV types, HPV-42 was the most common, followed by HPV-44, 81, and 6 (Fig. 2C and Additional file 1: Table S5).
Relative prevalence of different genotypes by carcinogenic risk. A Prevalence of each of the HR-HPV genotypes compared to total HR-HPV infections. B Prevalence of each of the pHR-HPV genotypes compared to total pHR-HPV infections. C Prevalence of each of the LR-HPV genotypes compared to total LR-HPV infections
Age distribution features of HPV infection
Upon data analysis, we discovered that the average age of individuals with HPV in Guangzhou was 37.05 ± 10.02 years. We divided the patients into eight age groups, and the infection rates for these groups were as follows: 24.02% (482/2,007) for those ≤ 25 years old, 19.31% (1109/5742) for individuals aged 26–30 years, 18.96% (1343/7082) for those aged 31–35 years, 18.74% (989/5278) for individuals aged 36–40 years, 19.08% (603/3160) for those aged 41–45 years, 18.46% (459/2487) for individuals aged 46–50 years, 20.81% (304/1461) for those aged 51–55 years, and 26.58% (379/1426) for those ≥ 56 years old (Additional file 1: Table S6). In comparison to the ≤ 25 years age group, the ≥ 56 years age group showed no significant difference, but exhibited a higher HPV infection rate than all other age groups, including the 26–30 years, 31–35 years, 36–40 years, 41–45 years, 46–50 years, and 51–55 years groups (Additional file 1: Table S7). The above findings indicate that individuals in the ≥ 56 years age group had a higher HPV-52 infection rate (P < 0.0017) compared to other age groups, except for the ≤ 25 years age group (P = 0.234) and the 51–55 years age group (P = 0.026) (Fig. 4, and Additional file 1: Tables S8 and S9).
Based on an analysis of trends in the age group affected by HPV infections, both the total infection rate and HR-HPV declined after peaking at age 25 or younger, and continued to decline until age 45. However, after age 46, there was an increase in the prevalence rate (Fig. 3). Between the ages of 46 and 56, the overall infection rate continued to increase, along with the presence of HR-HPV infections (Fig. 3). The prevalence of LR-HPV infection declined after age 25 and remained relatively low until age 55. However, prevalence then began to rise again after age 55 (Fig. 3).
After studying the occurrence of HR-HPV, LR-HPV, and overall HPV infection in women of different age groups, we noted a decrease in the prevalence of HPV infection in middle-aged women. However, the prevalence of LR-HPV infection showed no significant change across the age groups we observed (Fig. 3 and Additional file 1: Table S5). The prevalence of the five most common HR-HPV genotypes in each age group indicates that the prevalence of HPV-52 infection increases with age from 31 to 56 (Fig. 4). Furthermore, the prevalence of HPV-52 infection was higher in the 51–55 age group than in any other age group, except for the ≥ 56 age group and the ≤ 25 age group (Additional file 1: Table S9).
Comparison of the number and genotype of HPV single and multiple infections
The analysis of infection numbers for different HR-HPV genotypes showed that the number of single infections was significantly higher than that of multiple genotypes, except for HPV 59 (Fig. 5A, Table 1 and Additional file 1: Table S10). For pHR-HPV, the number of monogenic infections, including 68, 53, 66, and 73, was also significantly higher than multiple infections except for HPV-82 (Fig. 5B, Table 1 and Additional file 1: Table S10). As for LR-HPV, the number of single infections, including 42, 44, 81, 43, 6, 11, and 83, was significantly higher compared to multi-genotype infections (Fig. 5C, Table 1 and Additional file 1: Table S10).
Four HR-HPV genotypes exhibit a wide distribution of prevalence in less economically developed regions of China
As the HPV genotypes distribution in China is so peculiar, we summarized the epidemiological distribution of the four common HR-HPV genotypes (HPV-16, 18, 52, 58) in China by referring to the studies of HPV prevalence in all regions of China over the past 10 years (Additional file 1: Table S11). The results showed that the prevalence of HPV-16 genotypes was higher in Gansu (28.59%), Liaoning (26.20%), and Qinghai (22.21%) than in other provinces and cities (Fig. 6A); and the prevalence of HPV-18 genotypes was higher in Macau (8.90%), Anhui (8.26%), Inner Mongolia (7.76%), Gansu (7.77%), and Liaoning (7.50%) provinces (Fig. 6B). The prevalence of HPV-52 genotype was higher in Tibet (24.80%), Sichuan (21.05%), Jiangsu (20.93%), Hainan (20.40%), Anhui (20.07%), and Liaoning (19.40%) than in other provinces (Fig. 6C). In addition, the prevalence of HPV-58 genotype was higher in Gansu (17.01%), Sichuan (15.14%), Tibet (14.50%), Jiangsu (14.43%), Liaoning (13.80%), and Qinghai (13.74%) than in other regions (Fig. 6D). These indicate that the prevalence of these four HR-HPV is mainly in some economically underdeveloped regions of China. Comparison of this investigation of ours shows that Guangzhou, as the center city of Guangdong province, is one of the more economically developed cities in China, the prevalence of these four HPV genotypes is lower, and close to the same as the results of other Guangdong Province studies.
Prevalence distribution of four HR-HPV genotypes in China. A Distribution of HPV-16 genotype infection rates in different regions of China. B Distribution of HPV-18 genotype infection rates in different regions of China. C Distribution of HPV-52 genotype infection rates in different regions of China. D Distribution of HPV-58 genotype infection rates in different regions of China. Darker colours indicate higher infection rates
Discussion
According to the study, data show that 5668 individuals in Guangzhou were diagnosed with HPV in the past 3 years, resulting in an overall infection rate of 19.78%. The predominant genotype for HR-HPV infection was HPV-52, followed by HPV 58, 16, 51, 39, and 56. For LR-HPV, HPV-42 was the most common genotype, followed by HPV-44, 81, and 6. We also conducted an analysis of the age groups among the infected patients. The highest prevalence was observed in patients aged 56 years and older, with the second-highest prevalence recorded among patients under 25 years of age. This finding raises concerns as it suggests a significant infection rate in both these age groups. Women ages ≥ 56 have higher rates of HPV infection, which may be due to their immune system being less efficient at eliminating HPV infection, as well as their sex hormone status or vaginal epithelial functioning [20]. This data could serve as a foundation for further epidemiological analysis of HPV infection control and prevention in the region.
Persistent HR-HPV infection has a strong association with the development of CC in women [21]. Although HPV vaccines have been available in many countries since 2007, there has been no vaccine development that considers the distribution and prevalence of different HPV genotypes [21]. The July 2020 WHO report and subsequent studies reported that global coverage of HPV immunization stands at just 15% [22,23,24]. It is estimated that cumulative HPV vaccine coverage for women aged 9–45 in China between 2018 and 2020 is only 2.24% [25]. Therefore, there is still a long way to go to ensure universal access to HPV vaccines.
In this study, the age of participants was primarily concentrated between 16 and 83 years, with a mean age of HPV-infected patients at 37.05 ± 10.02 years. Numerous studies have demonstrated a strong association between HPV infection and sexual activity [21]. Furthermore, previous analyses have indicated that HPV infection can be acquired relatively soon after the onset of sexual activity. Therefore, it is of utmost importance to engage in HPV vaccination prior to initiating sexual activity in order to mitigate the risk of HPV infection to some extent.
Data from the current study showed that HPV infection was mainly concentrated in the under-25 and over-56 age groups, with the highest prevalence of HPV-52 in the under-25 and over-56 age groups (Fig. 4), at 6.33% and 7.36%, respectively. The total HPV infection rates, HR-HPV infection rates, and LR-HPV infection rates in the over-56 age group (respectively 26.58%, 18.72% and 4.91%) were the highest among all age groups, which may be due to the effects of menopause or ovarian function decline.
We conducted a comparison between two studies on the prevalence of HPV genotypes in Guangzhou. The first study, conducted in 2011, involved a sample of 250 healthy women aged 20–63 years [26]. This study, conducted at the Obstetrics and Gynecology Clinic of the Second Affiliated Hospital of Sun Yat-sen University in Guangzhou from January 2007 to April 2008, revealed that HPV-52 (2.62%) was the most common high-risk type among the Guangzhou population. The highest prevalence of HPV infection was observed in the age group of 20–29 years. The second study, conducted in 2022 [27], sampled a total of 6480 patients from the gynecology outpatient clinic of the Guangzhou Women and Children’s Medical Center. This study, carried out from August 2020 to September 2021, included healthy individuals as well as those with other cervical diseases. The results showed that HPV-52 infection was the most prevalent at 5%, followed by HPV-16 (2.3%), HPV-58 (1.8%), HPV-39 (1.6%), and HPV-51 (1.5%). The highest prevalence of HPV infection was found in the age group ≤ 24 years. Our own study, covering a period of nearly 3 years from January 2019 to December 2021, utilized a sample from the Guangzhou Women and Children’s Medical Center. Our findings also indicated the highest prevalence of HPV-52 at 4.99%. In comparison to the previous two studies, our results were similar but had a higher rate due to the larger sample size and wider range of study subjects. The prevalence rates of HPV-58 (2.18%) and HPV-51 (1.61%) were higher than those of HPV-16 (2.12%) and HPV-39 (1.19%), respectively. However, overall, HR-HPV infections were still predominantly dominated by these genotypes, and the rates were not significantly different.
HPV vaccination for women of the right age is an important measure to effectively prevent and control HPV infection and thus reduce the incidence of cervical cancer. From 2016 until May 2022, five HPV vaccines have been approved for registration in China, including three imported HPV vaccines and two domestic vaccines. They are: bivalent vaccine from Vantage Cang hai Biotech (China) and GlaxoSmithKline, Merck Sharp & Dohme quadrivalent vaccine and nine-valent vaccine. As we all know, bivalent vaccine mainly targets HPV-16, 18 genotypes; quadrivalent vaccine mainly prevents HPV-6, 11, 16, 18 genotypes; and nine-valent vaccine is mainly used to prevent HPV-6, 11, 16, 18, 31, 33, 45, 52, 58 genotypes. From 2018 to 2020, the number of HPV vaccinations rose from 3.417 million in 2018 to 12.279 million doses in 2020, according to data from China’s routine vaccine statement. However, the overall relative HPV vaccination rate is still at a low level. This is likely one of the reasons why HPV-16, 18 and other genotypes remain the main prevalent genotypes in China so far.
In addition, our study shows that HPV-52, 58, 16, 51 may be the key prevalent genotypes in Guangzhou. Among the vaccines currently approved for marketing in China, only the nine-valent vaccine contains these four genotypes. However, the HPV nine-valent vaccine in Guangzhou is currently available in limited number of injections due to its limited quantity and high price. This may be an important reason for the prevalence of HPV-52 genotypes in Guangzhou.
In response to the WHO’s call for global action to eliminate cervical cancer announced in 2018, China has actively strengthened the scientific publicity of HPV vaccination and promoted the pilot work of HPV vaccine. It has initiated the implementation of free domestic bivalent vaccination for girls of the right age across the province in Guangdong, Hainan, and Fujian provinces, incorporating this work into a project of the provincial government to do practical things for the people in 2022. Pilot cities such as Jinan, Xiamen, Wuxi, and Ordos have introduced a policy of free HPV vaccination for school-age girls. Chengdu has given a flat-rate subsidy for HPV vaccination for school-age girls, among others. It is hoped that the implementation of these policies will effectively reduce the number of new cases of cervical cancer in China in the future.
In conclusion, the study only analyzed the prevalence of various genotypes of HPV infection among local women for almost 3 years. Data from this study were limited. We did not perform pathological or cytological analyses of the participants’ cervixes. In addition, only patients admitted to our hospital were statistically analyzed, and some patients were included based on their reason for coming to the outpatient center, suggesting that these results may not be the representation of the general female population in Guangzhou and that the study data were limited and representation of the results of this study.
Availability of data and materials
All needed data are available in manuscript and Additional file 1.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Guo M, Xu J, Du J. Trends in cervical cancer mortality in China from 1989 to 2018: an age-period-cohort study and joinpoint analysis. BMC Public Health. 2021;21:1329. https://doi.org/10.1186/s12889-021-11401-8.
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. https://doi.org/10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F.
Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, et al. A review of human carcinogens–part B: biological agents. Lancet Oncol. 2009;10:321–2. https://doi.org/10.1016/s1470-2045(09)70096-8.
Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. https://doi.org/10.1016/S0140-6736(07)61416-0.
Chen X, Loo JX, Shi X, Xiong W, Guo Y, Ke H, Yang M, Jiang Y, Xia S, Zhao M, et al. E6 protein expressed by high-risk HPV activates super-enhancers of the EGFR and c-MET oncogenes by destabilizing the histone demethylase KDM5C. Cancer Res. 2018;78:1418–30. https://doi.org/10.1158/0008-5472.CAN-17-2118.
Humans IWGotEoCRt. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100:1–441.
Boumba LM, Qmichou Z, Mouallif M, Attaleb M, El Mzibri M, Hilali L, Donatien M, Ennaji MM. Human papillomavirus genotypes distribution by cervical cytologic status among women attending the General Hospital of Loandjili, Pointe-Noire, Southwest Congo (Brazzaville). J Med Virol. 2015;87:1769–76. https://doi.org/10.1002/jmv.24221.
Halec G, Alemany L, Lloveras B, Schmitt M, Alejo M, Bosch FX, Tous S, Klaustermeier JE, Guimera N, Grabe N, et al. Pathogenic role of the eight probably/possibly carcinogenic HPV types 26, 53, 66, 67, 68, 70, 73 and 82 in cervical cancer. J Pathol. 2014;234:441–51. https://doi.org/10.1002/path.4405.
Rahangdale L, Mungo C, O’Connor S, Chibwesha CJ, Brewer NT. Human papillomavirus vaccination and cervical cancer risk. BMJ. 2022;379:e070115. https://doi.org/10.1136/bmj-2022-070115.
de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. https://doi.org/10.1016/S1470-2045(10)70230-8.
Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomark Prev. 2005;14:1157–64. https://doi.org/10.1158/1055-9965.EPI-04-0812.
Zhang J, Cheng K, Wang Z. Prevalence and distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia in China: a meta-analysis. Arch Gynecol Obstet. 2020;302:1329–37. https://doi.org/10.1007/s00404-020-05787-w.
Chen L, Dong Y, Li J, Zhao J, Wang D, Xu L, Wu Y, Liu H, Lu J, Yao Z, et al. The genomic distribution map of human papillomavirus in Western China. Epidemiol Infect. 2021;149:e135. https://doi.org/10.1017/S0950268821001175.
Zhang H, Zhang S. Prevalence and genotype distribution of human papillomavirus infection among female outpatients in Northeast China: a population-based survey of 110,927 women. Arch Gynecol Obstet. 2022. https://doi.org/10.1007/s00404-022-06653-7.
Zhu X, Wang Y, Lv Z, Su J. Prevalence and genotype distribution of high-risk HPV infection among women in Beijing, China. J Med Virol. 2021;93:5103–9. https://doi.org/10.1002/jmv.27013.
Liu J, Ma S, Qin C, Zheng S, Chen Z, Huang Y, Xiong J, Huo Y. Prevalence and genotype distribution of human papillomavirus in Zhengzhou, China, in 2016. Arch Virol. 2020;165:731–6. https://doi.org/10.1007/s00705-019-04515-3.
Xiang F, Guan Q, Liu X, Xiao H, Xia Q, Liu X, Sun H, Song X, Zhong Y, Yuan CH, et al. Distribution characteristics of different human papillomavirus genotypes in women in Wuhan, China. J Clin Lab Anal. 2018;32:e22581. https://doi.org/10.1002/jcla.22581.
Li B, Wang H, Yang D, Ma J. Prevalence and distribution of cervical human papillomavirus genotypes in women with cytological results from Sichuan province, China. J Med Virol. 2019;91:139–45. https://doi.org/10.1002/jmv.25255.
Castle PE, Schiffman M, Herrero R, Hildesheim A, Rodriguez AC, Bratti MC, Sherman ME, Wacholder S, Tarone R, Burk RD. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1808–16. https://doi.org/10.1086/428779.
de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, Bosch FX. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9. https://doi.org/10.1016/S1473-3099(07)70158-5.
Spayne J, Hesketh T. Estimate of global human papillomavirus vaccination coverage: analysis of country-level indicators. BMJ Open. 2021;11:e052016. https://doi.org/10.1136/bmjopen-2021-052016.
Bruni L, Saura-Lazaro A, Montoliu A, Brotons M, Alemany L, Diallo MS, Afsar OZ, LaMontagne DS, Mosina L, Contreras M, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. 2021;144:106399. https://doi.org/10.1016/j.ypmed.2020.106399.
Benard E, Drolet M, Laprise JF, Jit M, Prem K, Boily MC, Brisson M. Potential benefit of extended dose schedules of human papillomavirus vaccination in the context of scarce resources and COVID-19 disruptions in low-income and middle-income countries: a mathematical modelling analysis. Lancet Glob Health. 2023;11:e48–58. https://doi.org/10.1016/S2214-109X(22)00475-2.
宋祎凡, 刘晓雪, 尹遵栋, 余文周, 曹雷, 曹玲生, 叶家楷, 李力, 吴静. 2018–2020年中国9–45岁女性人乳头瘤病毒疫苗估算接种率. 中国疫苗和免疫. 2021:027.
Liu SS, Chan KY, Leung RC, Chan KK, Tam KF, Luk MH, Lo SS, Fong DY, Cheung AN, Lin ZQ, et al. Prevalence and risk factors of human papillomavirus (HPV) infection in southern Chinese women—a population-based study. PLoS ONE. 2011;6:e19244. https://doi.org/10.1371/journal.pone.0019244.
张志勤. 广州地区女性HPV感染状况及其与宫颈病变的相关性研究.. In: 广州医科大学. 2022.
Acknowledgements
None.
Funding
This work was supported by the Science and Technology Program of Guangzhou, China (Grant numbers: 202102020057, 202201011522), Guangzhou Basic Research Program Jointly Funded by Municipal Schools and Colleges (Institutes) (202201020645), Guangzhou Women and Children's Medical Center Clinical Doctoral Research Fund (2020BS023) and Pediatric Institute Foundation of Guangzhou Women and Children’s Medical Center (No. GWCMC2020-4-010).
Author information
Authors and Affiliations
Contributions
The study was designed by FG, YL and SL. FG collaborated in the studies search, data extraction, YL and SL helped in double checking. SL and KZ collaborated in the manuscript writing, LY, JW and NB helped in revision. All the authors commented on the drafts of the manuscript and approved the final version of the article. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Approval for the study obtained from the local institutional ethics committee and informed consent obtained from all study participants.
Consent for publication
Not applicable.
Competing of interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table 1. HPV prevalence in different disease. Table 2. The number and rate of HPV infections in different age groups for different diseases respectively. Table 3. Number of HR-HPV, pHR-HPV and LR-HPV infections in different diseases. Table 4. Number and rate of infection for different HPV genotypes, respectively. Table 5. Number of cases and prevalence of each genotype in HRHPV, LR-HPV and pHR-HPV. Table 6. Prevalence of HPV infection at different ages. Table 7. Comparison of the prevalence of HPV infection in the >=56 age group with the prevalence of infection in the other groups, respectively. Table 8. Prevalence of HPV-52, HPV-58, HPV-16, HPV-51, and HPV-68 in different age groups, respectively. Table 9. Comparison of infection rates in different age groups separately and in the age group greater than or equal to 56 years old. Table 10. Ratio of single and multiple infections for different HPV genotypes. Table 11. Prevalence of HPV-16, 52, 18 and 58 infections in provinces, municipalities and autonomous regions of China, respectively.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, S., Zhang, K., Yang, L. et al. Distribution patterns of human papillomavirus genotypes among women in Guangzhou, China. Infect Agents Cancer 18, 67 (2023). https://doi.org/10.1186/s13027-023-00541-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13027-023-00541-8