Abstract
Background
This study aimed to investigate the epidemiology of high-risk human papillomavirus (HPV) in the female population in Beijing, China, and identify the relationship between HPV genotypes and host factors.
Methods
HPV testing was performed on women aged 15–89 (mean age 38.0 ± 10.9 years) from Beijing in 2020. High-risk HPV genotyping real-time polymerase chain reaction was used to determine HPV genotypes. The overall prevalence, age-specific prevalence, genotype distribution, and the correlation between HPV genotypes and cervical cytology were analyzed.
Results
Among the 25,344 study participants, the single and double infection rates were 18.8% (4,777/25,344) and 4.2% (1,072/25,344), respectively. A total of 6,119 HPV-positive individuals were found to have 91.6% negative results for intraepithelial lesion or malignancy (NILM), 5.8% atypical squamous cells of undetermined significance (ASC-US), 0.9% low-grade squamous intraepithelial lesion (LSIL), and 1.7% high-grade squamous intraepithelial lesion (HSIL). In single HPV infections, the HPV16 genotype was highly associated with cervical cytology severity (χ2 trend = 172.487, P < 0.001). Additionally, HPV infection rates increased gradually with age, and statistical differences were observed across age groups (χ2 = 180.575; P < 0.001). High-risk HPV genotypes were highly prevalent in women below 25 years of age and those aged 55–59 years. Cluster analysis revealed that the 13 HPV genotypes could be roughly divided into two groups in a single infection; however, patterns of infection consistent with biological characteristics were not observed.
Conclusion
High-risk HPV was found in 24.1% of outpatients, with HPV52, HPV58, HPV16, HPV39, and HPV51 being the most common high-risk genotypes. Single high-risk HPV infection was predominant. HPV16, HPV39, HPV51, and HPV52 were associated with cervical lesion progression. HPV16 infection was especially worrying since it aggravates cervical lesions. Because the infection rates of the 13 HPV genotypes differed by age, the peak HPV infection rate should not guide vaccination, screening, and prevention programs. Instead, these initiatives should be tailored based on the regional HPV distribution characteristics. Moreover, it was determined that Beijing’s populace needed to receive treatment for HPV39 infection.
Similar content being viewed by others
Introduction
Cervical cancer is one of the most common cancers in women worldwide. In 2020, there were about 342,000 deaths and about 604,000 new cases identified worldwide, with a large share of these deaths taking place in low- and middle-income nations [1]. The persistence of human papillomavirus (HPV) infection is widely recognized as the most important causative factor in the development of cervical cancer [2].
The genome of HPV, a small, double-stranded circular DNA virus, is about 8 kb [3]. Over 200 HPV genotypes have been identified based on genomic differences, and most genotypes are harmless [4]. There is clear evidence linking the human papillomavirus (HPV) to cervical cancer: the incidence of cervical cancer is highly correlated with the frequency of high-risk HPV [5]. Thirteen HPV genotypes (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) that are designated high-risk HPV are essential factors for cervical tumorigenesis [6]. The five most common HPV types in women worldwide who are HPV-positive are HPV16, 18, 31, 58, and 52. However, the ranking of the prevalence of these types varies by region [7]. For instance, in Western Europe, HPV16, 18, 31, 35, and 33, and in North America, HPV16, 53, 18, 51, and 31 are the five most prevalent high-risk HPV strains [8]. HPV16, 52, and 58 have the highest infection rates in some areas of China [9,10,11,12].
The predominant HPV genotypes may cause different outcomes at different stages of the disease [13]. Negative for intraepithelial lesion or malignancy (NILM), atypical squamous cells of undetermined significance (ASC-US), low-grade squamous intraepithelial lesion (LSIL) and high-grade squamous intraepithelial lesion (HSIL) are the four categories used to categorize precancerous phases [14]. Among cases of HSIL, the prevalence of HPV16/18 is 52%. However, HPV 16, 18, and 45 are significantly under-represented, and other high-risk HPV types are significantly over-represented in HSIL compared with invasive cervical cancer, suggesting differences in type-specific risks for progression [15]. The most common HPV types in HSIL samples from China are HPV16, 58, 52, 18, and 33 [16].
Furthermore, age has a significant role in determining the risk of HPV infection [17]. In Western countries, HPV prevalence peaks only in women in their mid-twenties and then steadily declines as age increases [18]. However, the prevalence of high-risk HPV has two peaks in China: one at age 15–24 years and the other at age 35–49 years [19].
To investigate the state of high-risk HPV infection, the correlation between high-risk HPV genotypes and cervical lesion severity, and the genotype distribution in the area, 25,344 samples from female outpatients in Beijing, China, were gathered for this study in 2020. We aimed to investigate cervical cancer epidemiology, diagnosis, and vaccination in Beijing, China.
Materials and methods
Study population
The study’s data came from patients who visited Beijing Obstetrics and Gynecology Hospital, Capital Medical University’s gynecological outpatient department in 2020. After the exclusion of unqualified samples, the quantitative measurement and biopsy of high-risk HPV DNA from a total of 25,344 women was performed. The median age was 38.0 ± 10.9 years, and the age range was 15–89 years (Table 1). The study was approved by the ethics committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University, and written informed consent was acquired from the study participants.
Cervical specimen collection and high-risk HPV genotyping
Following instructions, women’s cervical exfoliated cell samples were obtained using cytobrushes, and the samples were utilized to extract genomic DNA. DNA was isolated using a nucleic acid extraction reagent (Shanghai ZJ Bio-Tech Co., Ltd., Shanghai, China). Then, a commercial HPV genotyping kit (Shanghai ZJ Bio-Tech Co., Ltd.) was used to detect 13 high-risk HPV types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) with use of TaqMan real-time fluorescent quantitative polymerase chain reaction. The kit’s instructions were strictly followed for every procedure.
ThinPrep cytologic test
Cervical cells were detected using the ThinPrep cytology test (TCT). Senior physicians assessed cytological pathology results according to the Bethesda System of Cervical Cytology, which classifies precancerous phases as NILM, ASC-US, LSIL, or HSIL. Histopathological diagnoses were made by a pathologist who was unaware of the HPV detection results.
Cluster analysis
We investigated the similarity of infection for 13 high-risk HPV types in abnormal cytology across age groups using cluster analysis. We considered that the classical K-means algorithm was suitable for this study. K-means used Manhattan distance to measure the distance between two observations. Hierarchical cluster analysis was conducted using a “hclust” function in R-Studio (Version 4.2.1).
Statistical analysis
All statistical analyses performed in this study were with R software version 4.2.1. Figures were created with GraphPad Prism version 9 (GraphPad Software, San Diego, CA, USA). A binomial 95% confidence interval (CI) was calculated for each calculation used to estimate the prevalence of HPV, with separate computations made for each genotype from single and multiple infections. These data were further stratified by age (< 20, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, and ≥ 60 years). Differences between groups were tested using the Pearson χ2 test depending on data type and distribution. Differences were considered statistically significant if P < 0.05.
Results
Prevalence of high-risk HPV genotypes in the cervical samples
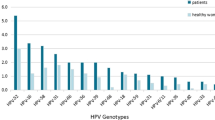
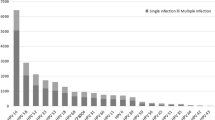
The high-risk HPV infection rate among the 25,344 patients was 24.1% (6,119/25,344), with 6,119 participants showing positive findings from high-risk HPV tests. Among the women who were HPV-positive, 4,777 were positive for a single HPV type (4,777/6,119 = 78.1%, 4,777/25,344 = 18.8%). Furthermore, 1,342 were positive for multiple types (1,342/6,119 = 21.9%, 1,342/25,344 = 5.3%), of which 1,072 were positive for two types (1,072/6,119 = 17.5%, 1,072/25,344 = 4.2%; Table 1). Among single HPV infections, the five most prevalent high-risk types were HPV52, 58, 16, 39, and 51, with prevalences of 3.7%, 3.0%, 2.8%, 1.8%, and 1.7%, respectively. HPV18 had a prevalence of 1.0% (246/25,344) and was ranked seventh. Among 1,342 individuals infected with multiple HPV types, the five most prevalent high-risk HPV types were HPV52, 39, 58, 16, and 51, with prevalences of 1.7%, 1.6%, 1.6%, 1.5%, and 1.0%, respectively. In addition, HPV68 ranked seventh in prevalence among multiple infections (0.8%; Table 2). The five high-risk types with the highest overall infection rates were HPV52 (1,385/25,344, 5.5%, 95% CI 5.2–5.7), 58 (1,161/25,344, 4.6%, 95% CI 4.3–4.8), 16 (1,099/25,344, 4.3%, 95% CI 4.1–4.6), 39 (865/25,344, 2.7%, 95% CI 3.2–3.6), and 51 (697/25,344 2.8%, 95% CI 2.6-3.0). Moreover, the five high-risk types with the lowest overall infection rates were HPV31 (280/25,344 1.1%, 95% CI 1.0-1.2), 68 (267/25,344, 1.1%, 95% CI 0.9–1.2), 33 (252/25,344, 1.0%, 95% CI 0.9–1.1), 35 (244/25,344, 1.0%, 95% CI 0.8–1.1), and 45 (124/25,344, 0.5%, 95% CI 0.4–0.6; Table 2).
Cervical cytological status of age groups and high‑risk genotypes
The prevalence of HPV in women with ASC-US was found to be 45.5% (357/785) in the cytologically abnormal samples, but in women with LSIL and HSIL, the rates were 78.3% (54/69) and 78.9% (105/133), respectively. The cervical cytological status of the 6,119 HPV-positive individuals was analyzed: 91.6% had NILM, 5.8% had ASC-US, 0.9% had LSIL, and 1.7% had HSIL, respectively (Table 3). The data were then divided by the following age groups: < 20, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, and ≥ 60 years. Among women with NILM, the 25-29-year age group had the highest rate (96.1%), whereas the < 20-year age group had the lowest rate (82.4%). The < 20-year age group (17.6%) had the highest rate among women with ASC-US. In women with LSIL and HSIL, the 35-39-year group and the ≥ 60-year age group had a high prevalence. The findings suggest that those who are HPV-positive may have a progressive increase in their risk of cervical carcinogenesis beyond the age of 35.
The relationship between high-risk HPV genotypes and abnormal cervical cytology
We examined the connection between TCT outcomes and high-risk HPV genotypes in HPV-positive people. The results showed that the association between single and multiple infections and abnormal cytology differed for each genotype. Among single infections, the top five genotypes among women with NILM were HPV52 (20.3%), 58 (16.5%), 16 (13.5%), 39 (10.0%), and 51 (9.4%). The most prevalent HPV types in HSIL were as follows: HPV16 (63.0%), 58 (7.4%), 33 (6.2%), 31 (4.9%), 52 (4.9%), and 18 (3.7%; Table 4). The overall single infection rate increased with cervical cytological status. The infection rate of HPV16 showed a gradual increase with disease progression, whereas the infection rates of HPV31, 33, and 45 were slightly higher in HSIL than in NILM samples. The infection rates of HPV18, 35, 39, 51, 52, 56, 58, 59, and 68 showed a decreasing trend. In the case of abnormal TCT, the infection rate of HPV16 (χ2 trend = 172.487, P < 0.001) increased with increasing cervical cytological severity, whereas the infection rates of HPV39 (χ2 trend = 8.569, P = 0.003), 51 (χ2 trend = 7.708, P = 0.005), and 52 (χ2 trend = 16.949, P < 0.001) showed the opposite trend (Table 4). An examination of the cervical cytological state and HPV genotype was not conducted due to the lack of data on multiple infections.
Two categories of HPV genotypes may be identified by cluster analysis based on the trend of high-risk HPV genotypes by cervical cytological status [20]. HPV16, 18, 31, 33, 35, 45, and 59 had similar infection trends, whereas HPV39, 51, 52, 56, 58, and 68 had similar infection trends (Fig. 1). The result is associated with the risk estimates of high-risk HPV genotypes in tumors.
Age-specific prevalence of HPV infection
The participants were divided into ten groups by age category: <20, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, and ≥ 60 years. The prevalence of high-risk HPV infection was significantly different across age groups (χ2 = 180.575; P < 0.001). In this study, the “two peaks” pattern was observed for the prevalence of HPV infection: the prevalence of overall HPV genotypes showed a first peak at age < 20 years (55.7%, 34/61) and a second peak at age 55–59 years (30.1%, 334/1,108; Table 5). We compiled the infection status of 13 high-risk HPV genotypes across 10 age groups in this investigation (Table 6). The age of the second peak varied among high-risk HPV genotypes; for HPV16 and HPV18, the second infection peak occurred after age 35 years, and for HPV31 and HPV51, it occurred at age 50–54 years. Despite this, the prevalence of all HPV genotypes increased once more to form a second peak at age 55–59 years.
In addition, we observed differing trends for HPV genotypes and age groups for single and multiple infections. We concentrated on the distribution of each HPV genotype in single infections solely because numerous factors affect multiple infections. Among the single infections, the first peak was observed in young women (15–25 years old), whereas the second peak was observed at different ages. HPV31, 33, 45, 51, 52, 56, and 68 had two infection peaks, and HPV16, 18, 35, 39, 58, and 59 had three or more infection peaks. Before the ages of 45 and 40, respectively, the infection rates for HPV33 and 58 rose with age. HPV16, 33, 45, and 56 had re-emerge peaks at 55–59 years of age; HPV18, 31, 35, 39, and 68 at 50–54 years of age; HPV51, 58, and 59 at 45–49 years of age; and HPV52 infection increased with age from 35 years of age (Fig. 2).
HPV genotypes may be separated into two groups depending on the age group infection rate, according to cluster analysis based on the trend of the percentage change of HPV genotypes among patients who were HPV-positive in each age group. HPV16, 18, 35, 45, 52, and 68 had similar infection trends, whereas HPV31, 33, 39, 51, 56, 58, and 59 had similar infection trends (Fig. 3). However, the infection trend of HPV genotypes in age groups was not significantly associated with biological characteristics. The correlates of infection trends were unclear.
Discussion
Cervical cancer is one of the most preventable cancers. The primary cause of HSIL in the cervix and cervical cancer is high-risk HPV persistent infection. In 2018, the World Health Organization called for global action to eliminate cervical cancer and proposed that 90% of girls aged 9–14 years receive the HPV vaccine [21]. In most nations, a complete strategy that includes HPV vaccination and HPV-based screening is economical [22]. However, regional disparities in cervical cancer screening have been caused by the varying strengths of local governments and differing patient participation rates. China launched a national public health program to curb cervical cancer in 2009 [23]. However, immunization and cervical cancer screening rates are still low [24].
The frequency of high-risk HPV genotypes among Beijing Obstetrics and Gynecology Hospital outpatients was 24.1% in this study, which is in line with earlier studies [25, 26]. The results supported that HPV52, 58, and 16 are the most prevalent HPV genotypes among women in Beijing. Previous studies have demonstrated that HPV52 and 58 are the two dominant HPV genotypes in East Asia and China [27]. This study’s high-risk HPV-type prevalence is consistent with earlier studies [9, 28, 29]. A single genotype typically causes high-risk HPV infection; however, multiple infections have gradually gained attention in recent years. Infection with multiple HPV types is reported in 20–45% of women worldwide who are infected with HPV [30, 31]. Regarding the distinction between the effects of a single high-risk HPV infection and numerous high-risk infections on cervical precancer and cancer, there is no conclusive evidence [9, 32, 33]. In this study, high-risk HPV infection was mainly caused by a single genotype (18.8%), whereas multiple infections accounted for 5.3% of cases. Furthermore, we estimated that 1.4% (355/25,344) of outpatients were infected with low-risk HPV types (HPV6 and 11).
Recent studies indicate that HSIL, a significant stage of cervical precancerous lesions, and cervical cancer may also be caused by other high-risk HPV strains. This study provides an in-depth analysis of the relationship between cervical cytology and HPV infection. We found that the relationship between cervical cytological state and HPV type varied depending on whether the infection was single or multiple. In single infection, HPV16 was the most common type among women with HSIL, followed by HPV58, 33, 31, 52, and 18. Furthermore, the prevalence of HPV16, 39, 51, and 52 infections was strongly correlated with the severity of cervical cytology. The proportion of HPV16 gradually increased with disease progression. On the other hand, as the illness advanced, the proportions of HPV39, 51, and 52 steadily decreased. These results were consistent with previous findings [10, 34]. In multiple infections, high-risk HPV was less likely to progress to HSIL than in single infections. This study observed no effect of multiple infections on abnormal cervical cytology.
In addition, the prevalence of overall high-risk HPV infections displayed a bimodal age distribution, with one peak at ≤ 25 years, a decline with age, and a second peak at 55–59 years of age. However, the infection rates of different HPV genotypes differed across age groups. For instance, HPV-16 and 18 peaked again between the ages of 35 and 39. Consequently, we suggest that women over 35 be mandated to undergo an annual HPV test. Despite similar infection trends for single and multiple infections, we only analyzed the shifts in infection curves for single HPV infections because the etiologies of multiple infections are more complex. We found that the peak age of each HPV strain varied.
Furthermore, the 13 high-risk HPV types classified as oncogenic based on epidemiologic and/or phylogenetic evidence are members of four species within the Alpha-papillomavirus genus. HPV 16, 31, 33, 35, 52, and 58 are the prototypes of the A9 species; HPV 18, 39, 45, 68, and 59 are the prototypes of the A7 species; HPV51 is the prototype of the A5 species; and HPV56 is the prototype of the A6 species. Our study categorized the 13 HPV types by cluster analysis and finally divided them into two groups: (1) HPV16, 18, 35, 45, 52, and 68, which had a similar age distribution, and (2) HPV31, 33, 39, 51, 56, 58, and 59, which had similar infection trends. Nevertheless, no noteworthy correlation was detected with epidemiology and systems biology classification techniques.
Ultimately, immunization is one of the most important strategies for lowering cervical cancer incidence. Vaccines should be effectively and rationally distributed by region according to HPV epidemiological characteristics. Cervical cancer vaccination is considered an important measure for the effective prevention of cervical precancer, cervical cancer, and acromegaly. Moreover, variations in HPV subtype infection are noted between regions and ethnic groups. Therefore, a thorough big sample survey may offer useful therapeutic value for vaccine development and vaccination to prevent cervical cancer in pertinent places. The sample in this study reflects the high-risk HPV infection status of the Beijing population. The imported nine-valent HPV vaccine has prevented the HPV subtypes, including HPV6, 11, 16, 18, 31, 33, 45, 52, and 58 in the Beijing population. Notably, however, HPV39 was not covered by the vaccine. As a result, the vaccine’s ability to prevent high-risk HPV in Beijing is still limited.
Conclusion
In conclusion, this study examined the frequency of high-risk HPV infection in females, the correlation between high-risk HPV genotypes and cervical lesion severity, and the association between high-risk HPV infection and age distribution features in Beijing, China in 2020. Our study will provide helpful information for screening and vaccinating cervical cancer in Beijing, China.
Data availability
The data was collected from Beijing Obstetrics and Gynecology Hospital, Capital Medical University. We thank them for their generous help.
Abbreviations
- HPV:
-
Human papillomavirus
- NILM:
-
Negative for intraepithelial lesion or malignancy
- ASC-US:
-
Atypical squamous cells of undetermined significance
- LISL:
-
Low-grade squamous intraepithelial lesion
- HSIL:
-
High-grade squamous intraepithelial lesion
- TCT:
-
ThinPrep cytology test
References
Sung H, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Ferenczy A, Franco E. Persistent human papillomavirus infection and cervical neoplasia. Lancet Oncol. 2002;3(1):11–6.
Shanmugasundaram S, You J. Targeting Persistent Human Papillomavirus infection. Viruses, 2017. 9(8).
Okunade KS. Human papillomavirus and cervical cancer. J Obstet Gynaecol. 2020;40(5):602–8.
Nobbenhuis MA, et al. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: a prospective study. Lancet. 1999;354(9172):20–5.
de Martel C, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–70.
de Sanjosé S, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7(7):453–9.
Taskin MH, et al. Genotype distribution and prevalence of high-risk human papillomavirus infection among women in Samsun Province of Turkey. Asian Pac J Cancer Prev. 2022;23(7):2477–82.
Li Y, et al. Cervical infection of Oncogenic Human Papillomavirus (HPV) types in Beijing, China. Biomed Environ Sci. 2016;29(10):734–41.
Chen Z, et al. Epidemiological study of HPV infection in 40,693 women in Putian: a population study based on screening for high-risk HPV infection. BMC Infect Dis. 2022;22(1):893.
Li Z, et al. Prevalence of HPV infection among 28,457 Chinese women in Yunnan Province, Southwest China. Sci Rep. 2016;6:21039.
Dai M, et al. Human papillomavirus infection in Shanxi Province, People’s Republic of China: a population-based study. Br J Cancer. 2006;95(1):96–101.
Kabuga AI, et al. Prevalence and type distribution of human papillomavirus recovered from the uterine cervix of Nigerian women: a systematic review and Meta-analysis. Asian Pac J Cancer Prev. 2020;21(10):2837–46.
Solomon D, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287(16):2114–9.
Smith JS, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121(3):621–32.
Li J, et al. Epidemiological features of human papillomavirus (HPV) infection among women living in Mainland China. Asian Pac J Cancer Prev. 2013;14(7):4015–23.
Adam E, et al. Papillomavirus detection: demographic and behavioral characteristics influencing the identification of cervical disease. Am J Obstet Gynecol. 2000;182(2):257–64.
Wheeler CM, et al. A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int J Cancer. 2013;132(1):198–207.
Zhao FH, et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: a pooled analysis of 17 population-based studies. Int J Cancer. 2012;131(12):2929–38.
Murtagh F, Legendre P. Ward’s hierarchical agglomerative clustering method: which Algorithms Implement Ward’s Criterion? J Classif. 2014;31(3):274–95.
Canfell K, et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395(10224):591–603.
Jit M, et al. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: a PRIME modelling study. Lancet Glob Health. 2014;2(7):e406–14.
Wen C. China’s plans to curb cervical cancer. Lancet Oncol. 2005;6(3):139–41.
Zou Z, et al. Domestic HPV vaccine price and economic returns for cervical cancer prevention in China: a cost-effectiveness analysis. Lancet Glob Health. 2020;8(10):e1335–44.
Wang X, et al. Prevalence and distribution of human papillomavirus genotypes among women attending gynecology clinics in northern Henan Province of China. Virol J. 2022;19(1):6.
Zhang C, et al. Distribution of human papillomavirus infection: a population-based study of cervical samples from Jiangsu Province. Virol J. 2019;16(1):67.
Bosch FX, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26:K1–16.
Luo LP, et al. Prevalence and genotype distribution of HPV infection among 214,715 women from Southern China, 2012–2018: baseline measures prior to mass HPV vaccination. BMC Infect Dis. 2021;21(1):328.
Wang J, et al. HPV genotype prevalence and distribution during 2009–2018 in Xinjiang, China: baseline surveys prior to mass HPV vaccination. BMC Womens Health. 2019;19(1):90.
Li M, et al. Prevalence characteristics of single and multiple HPV infections in women with cervical cancer and precancerous lesions in Beijing, China. J Med Virol. 2019;91(3):473–81.
Pista A, et al. Single and multiple human papillomavirus infections in cervical abnormalities in Portuguese women. Clin Microbiol Infect. 2011;17(6):941–6.
Kim M et al. Multiple human papilloma virus (HPV) infections are Associated with HSIL and Persistent HPV infection status in Korean patients. Viruses, 2021. 13(7).
Dickson EL, et al. Multiple-type human papillomavirus (HPV) infections: a cross-sectional analysis of the prevalence of specific types in 309,000 women referred for HPV testing at the time of cervical cytology. Int J Gynecol Cancer. 2013;23(7):1295–302.
Zhang J, Cheng K, Wang Z. Prevalence and distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia in China: a meta-analysis. Arch Gynecol Obstet. 2020;302(6):1329–37.
Acknowledgements
We thank those who contributed to the publication of the article. We thank those authors who published detailed data on the article.
Author information
Authors and Affiliations
Contributions
HTL and ZHL designed and supervised the study. JQZ and YL collected patient clinical information. HTL and HW detected the samples. JW analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study followed the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University.
Consent for publication
All of the patients signed an informed consent form.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Li, H., Zhang, J. et al. Epidemiology and genotypes analysis of human papillomavirus infection in Beijing, China. Virol J 21, 19 (2024). https://doi.org/10.1186/s12985-024-02292-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-024-02292-3