Abstract
Background
Neurodegeneration due to cerebral folate transport deficiency is a rare autosomal recessive disorder caused by biallelic pathogenic variants in FOLR1. Onset typically occurs in late infancy and is characterized by psychomotor regression, epilepsy, and a hypomyelinating leukodystrophy on magnetic resonance imaging. If left untreated, progressive neurodegeneration occurs. However, early treatment with folinic acid has been shown to stabilize or reverse neurological features. Approximately thirty patients have been described worldwide. Here, we report the first two cases with genetically proven cerebral folate transport deficiency from South-Eastern Europe, describe the effect of oral folinic acid therapy on clinical and neuroradiological features and review the literature.
Results
Two siblings presented in childhood with clinical and radiological findings consistent with a hypomyelinating leukodystrophy. Exome sequencing revealed a novel homozygous pathogenic variant in FOLR1 (c.465_466delinsTG; p.W156G), confirming the diagnosis of neurodegeneration due to cerebral folate transport deficiency. Folinic acid treatment was promptly initiated in both patients. The younger sibling was treated early in disease course at 2 years of age, and demonstrated complete recovery in clinical and MRI features. The older sibling, who was 8 years of age at the time of diagnosis and treatment, demonstrated partial but substantial improvements.
Conclusion
We present the first account in the literature that early treatment initiation with oral folinic acid alone can result in complete neurological recovery of both clinical and radiological abnormalities in neurodegeneration due to cerebral folate deficiency. Moreover, through the report of these patients along with review of the literature, we provide information about the natural history of the disease with comparison of treatment effects at different stages of disease progression. This report also reinforces the importance of universal access to genetic testing to ensure prompt diagnoses for treatable disorders.
Similar content being viewed by others
Background
Neurodegeneration due to cerebral folate transport deficiency (OMIM #613068), first described in 2009, is caused by biallelic pathogenic variants in FOLR1 [1]. FOLR1 (OMIM *136430) encodes for the folate receptor-alpha (FOLRα), which is abundantly expressed in the choroid plexus and considered the main folate transporter of 5-methyltetrahydrofolate (MTHF) across the blood–brain barrier. FOLRα is the only transporter responsible for cerebral folate supply via exosome-mediated delivery of MTHF from the CSF to the brain parenchyma [1,2,3].
Biallelic hypomorphic pathogenic variants in FOLR1 cause FOLRα deficiency, impairing cerebral folate transport and supply, leading to isolated cerebral folate deficiency and progressive neurodegeneration [1,2,3]. This disorder typically starts to manifest in late infancy with psychomotor regression, ataxia, and refractory epilepsy, with brain magnetic resonance imaging (MRI) demonstrating a hypomyelinating leukodystrophy [1, 2].
The late-infantile onset and absence of embryonic malformations in this disorder suggest preserved expression of folate receptor-beta (FOLRβ) in fetal choroid cells, which compensates for the lack of FOLRα function [1, 2, 4]. However, downregulation of FOLRβ expression is thought to occur in the human choroid plexus from 4 to 6 postnatal months onwards, which may explain the onset of the disease only in late infancy [1, 3, 4].
The pathophysiological mechanisms by which MTHF deficiency causes neurological disease are still under investigation. The prevailing hypothesis links the lack of MTHF to impaired myelin formation through cerebral methylation processes, which results in a deficiency of phosphatidylcholine, sphingomyelin, and other methylated membrane phospholipids crucial for myelin formation and stability [1]. Another recent hypothesis posits that folates are important for oligodendrocyte maturation, survival, and thus for the myelination during CNS development [5].
Here, we report siblings with hypomyelination and neurodegeneration for whom exome sequencing revealed a homozygous novel pathogenic variant in FOLR1. We also present an in-depth report before and during folinic acid treatment, with clinical and MRI evolution, as well as a review of the previously published cases.
Methods
Ethics approval and research consent
This research was approved by the Institutional Review Boards of Clinic for Child Neurology and Psychiatry University of Belgrade (IRB number 1-48/3-2016) and the McGill University Health Center and Montreal Children’s Hospital Research Ethic Boards (11-105-PED and 2019-4972), and conducted following the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from the patients’ parents/legal guardians.
Genetic analysis
Exome sequencing was performed using genomic DNA extracted from whole blood following standard protocols. DNA was prepared using the TruSeq library prep and samples were enriched using the IDT xGenv2 exome research panel supplemented with custom mitochondrial probes and sequenced to a minimum of 7 Gb for a mean of 80 × average coverage or greater on an Illumina NovaSeq 6000 (2 × 150 paired end reads). Bidirectional sequences were assembled, aligned to reference gene sequences based on human genome build GRCh37/UCSC hg19, and analyzed using the custom-developed software RUNES and VIKING [6, 7]. Variants were filtered to 1% minor allele frequency and prioritized using the American College of Medical Genetics and Genomics (ACMG) guidelines [8], including phenotypic assessment with OMIM disease associations.
Medical record and MRI review
We retrospectively reviewed medical records and evaluated MRI studies conducted serially over 10 years for Patient 1 and over 4 years for Patient 2.
Further, we assessed the data from all published patients with biallelic pathogenic variants in FOLR1, considering their clinical features, neuroimaging results, genetic findings, treatment regimen, and response to treatment. This was completed by reviewing all biomedical literature available in the PubMed Medline database between September 2009-December 2022, using the following MeSH terms: FOLR1 gene, cerebral folate deficiency, hypomyelination, leukodystrophy, folinic acid.
Serum folate measurements
Serum folate levels were measured using Abbott Architect i4000Sr test equipment and Abbott Architect Folate reagent using the Chemiluminescent Microparticle Immunoassay (CMIA) method.
Results
The patients described in this study are siblings, including a boy currently aged 12 years (Patient 1), and his younger sister currently aged 6 years (Patient 2). They were born to non-consanguineous unaffected parents of Serbian origin, with a family history negative for neurological disorders. Both patients were referred to our department for additional investigations at the ages of 8 years (Patient 1, for epileptic encephalopathy) and 2 years (Patient 2, for mild cerebellar features).
Pre-treatment clinical findings
The older male sibling (Patient 1) had uneventful early psychomotor development until 18 months of age, when he gradually developed an ataxic gait and speech regression. At 4 years of age, he started having epileptic seizures, which were treatment-resistant, occurred daily, and of multiple different types (tonic, focal with impaired awareness, atonic, and tonic–clonic). Various combinations of ten standard antiepileptic drugs (AEDs) were tried without success. At the time, the patient was being treated in a department without resources for access to detailed metabolic investigations or genetic sequencing, resulting in his cause of illness remaining unknown. Cerebellar ataxia and hypotonia progressed, and at 6.5 years of age, he lost the ability to walk and sit without support, with poor head control. His expressive language consisted of up to five meaningful words and he showed autistic behavioral changes with outbursts of anger and poor social contact. No other abnormalities were found on physical examination. The severity of his seizures increased, frequently leading to status epilepticus. EEG showed diffuse disturbance in cerebral activity with slow background activity and multifocal epileptiform discharges. The patient’s neurological motor, cognitive, and language function progressively worsened, leading to dependency for all activities of daily living and severe neurological impairment at 7.5 years of age. His examination at the time was characterized by a complete loss of speech and social interactions, as well as significant cerebellar signs (i.e., truncal and limb ataxia), axial hypotonia, and mild pyramidal and bulbar signs.
The younger female sibling (Patient 2) had normal psychomotor development. At the age of 22 months, she started manifesting mild intention tremor in the upper limbs and mild truncal ataxia. She did not exhibit seizures or any other neurological abnormalities.
Pre-treatment brain MRI
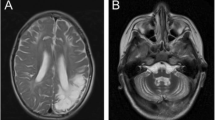
In Patient 1, brain MRI at the age of 7 years (Fig. 1A3–E3) showed diffuse supratentorial hypomyelination, with relative preservation of myelination in the internal capsule, the splenium and body of the corpus callosum (Fig. 1B3, C3, D3), with thinning of the corpus callosum (Fig. 1A3). Cerebellar white matter was also hypomyelinated (Fig. 1E3). There was cerebral and marked cerebellar atrophy (Fig. 1A3–E3). When compared with MRIs obtained at age 5 years (Fig. 1A2–E2) and 2 years (Fig. 1A1–E1), the degree of hypomyelination was stable, but progression of cerebral and cerebellar atrophy was evident. These findings were consistent with a hypomyelinating leukodystrophy. In Patient 2, the first brain MRI at age 2 years revealed insufficient cerebral and cerebellar myelination for age, with a pattern similar to Patient 1, but with milder thinning of the corpus callosum and without cerebellar atrophy (Fig. 2A1–E1). Brain magnetic resonance spectroscopy (MRS) showed decreased white matter choline in both patients.
Brain MRI of Patient 1 from age 2 to 12 years. T2-weighted images are shown at 2 years (column 1: A1–E1), 5 years (column 2: A2–E2), 7 years (column 3: A3–E3), 10 years (column 4: A4–E4) and 12 years (column 5: A5–E5). Sagittal (panel A) images show mild to moderate thinning of the corpus callosum (white arrowhead), as well as mild cerebellar atrophy (white arrow). In panels B1–3, C1–3, and D1–3, severe lack of myelin deposition, together with progressive cerebral atrophy are appreciated. In panels B4–5, C4–5, and D4–5, improvement in myelination is seen, but incomplete myelination is still present at age 12 years. Of note, brain volume has improved at ages 10 and 12 years (B4–5, C4–5, and D4–5). In panel E, insufficient myelin deposition is seen in E1-2 in both the pons (white double-lined arrow) and cerebellum (white dashed arrow), with improvement in the pons at age 7 years (E3–5, white double-lined arrow) and significant improvement in the cerebellum at ages 10 and 12 years (E4–5, white double-lined arrows). Progressive cerebellar atrophy is also seen between ages 2 and 7 years (E1–3), with improvement in subsequent MRIs done at ages 10 and 12 years (E4–5)
Brain MRI of Patient 2 from age 2 to 6 years. T2-weighted images are shown at 2 years (column 1: A1–E1), 3 years (column 2: A2–E2), 4 years (column 3: A3–E3) and 6 years (column 4: A4–E4). Sagittal (panel A) images show mild thinning of the corpus callosum (white arrowhead) but otherwise normal midline structures. In panels B, C, D and E, delayed myelination is appreciated, with complete myelination achieved only at age 6 years (B4, C4, D4 and E4)
Clinical laboratory measurements
For both patients, routine blood analyses and urinalyses, including blood/urine metabolic screening (lactate, pyruvate, amino-acids, organic acids, very-long-chain fatty acids) and vitamin B12 and homocysteine concentrations in serum, were normal. Of note, serum folate concentration values were also within normal range in both patients, measured at 17.8 ngr/ml in Patient 1, and 15.3 ngr/ml in Patient 2 (normal range: 3.1–20.5 ngr/ml).
Lumbar punctures to measure CSF neurotransmitters were not performed due to lack of parental approval and resources in Serbia. Therefore, the most efficient and the least invasive method to investigate the genetic diagnosis was to promptly perform exome sequencing using patient DNA extracted from whole blood.
Genetic analysis
Using exome sequencing, in both siblings we identified a homozygous novel pathogenic variant in FOLR1: c.465_466delinsTG; p.W156G (NM_016725.3), which we assessed for pathogenicity using the ACMG guidelines and classifications. Using Sanger sequencing, we validated the presence of the variant in both patients and confirmed the parents to be heterozygous carriers (PP1). This specific variant has not been reported in large population databases (gnomAD; https://gnomad.broadinstitute.org/) (PM2), and a missense variant causing the same protein change is reported in heterozygous form in only 3 individuals, with no homozygous individuals reported (minor allele frequency = 0.00001061). This variant is reported by in silico softwares to be pathogenic and is present in a conserved amino acid region (PP3). Additionally, this specific indel variant has not been reported in the literature, however a missense variant at the same position leading to the same protein change (c.466 T > G; p.W156G) has been reported in two affected siblings in a compound heteozygous form [9], and one affected female in a homozygous form [10] (Table 1) (PS1). Therefore, the genetic diagnosis for both patients was confirmed, with the opportunity to treat this disease with folinic acid.
Treatment and response to therapy
Treatment with folinic acid was initiated immediately after obtaining the genetic results, (i.e., at 8 years of age in Patient 1 and at 2 years of age in Patient 2) and the response to therapy was monitored over 4 years. Specifically, the patients were treated with levofolinic acid, the L-isomer of folinic acid. Notably, folinic acid can also be prescribed as a mixture of both the biologically active L-isomer and the inactive D-isomer, however, reports show that a better outcome may be associated with the use of only the L-folinic acid compound [11].
In Patient 1, the initial dose of oral folinic acid of 2 mg/kg/day did not lead to notable improvements, and therefore within a month, the dose was increased to 5 mg/kg/day, which resulted in a dramatic improvement of neurological features. The patient’s bulbar symptoms disappeared, weakness and ataxia began to subside, and over the next 8 months, he gradually began to walk independently, while his speech comprised 4–5 meaningful words. His dose of oral folinic acid was then slowly increased to 8 mg/kg/day. The severity and frequency of seizures decreased from dozens per day to 0–3 brief atonic and focal seizures with impaired awareness, and the antiepileptic therapy was reduced to two AEDs which are presumed to have a minimal anti-folate effect (levetiracetam and lamotrigine). Any attempt to further modify/reduce antiepileptic therapy would result in the aggravation of seizures. During folinic acid treatment, his serum folate concentration remained within the normal range, with values measured at 17.1 ngr/ml (normal 3.1–20.5 ngr/ml) at 12 years of age.
On the latest neurological examination at age 12 years, the patient presented with cerebellar signs, while bulbar and pyramidal signs were completely resolved. Cerebellar ataxia and hypotonia appeared milder, and he could walk and perform simple motor tasks independently. His behavioral abnormalities subsided, however, no significant improvement in expressive language was observed. Follow-up brain MRI at 10 years of age showed progression of both supra- and infratentorial myelination (Fig. 1A4–E4), with a further improved myelination on the latest MRI at age 12 years (Fig. 1A5–E5). Brain MRS also improved, with normalization of the white matter choline peaks for age.
In Patient 2, neurological signs completely resolved after 3 months of treatment with 2 mg/kg/day of oral folinic acid. The patient has since been symptom-free and developing normally. Follow-up brain MRI at 3 years of age showed amelioration of the abnormal cerebral and cerebellar white matter signal, but without complete normalization of myelination (Fig. 2A2–E2). Folinic acid oral dose was then gradually increased to 7 mg/kg/day. Brain MRI at 4 years of age showed further improvement (Fig. 2A3–E3), and at 6 years of age myelination appeared normal (Fig. 2A4–E4). Her latest neurological examination at 6 years of age was normal. Her levels of serum folate also remained within the normal range, with the latest value at 6 years of age measuring 15.8 ngr/ml (normal range: 3,1–20.5 ngr/ml).
Literature review
Our review of the pre-treatment clinical and brain MRI findings among 31 reported FOLR1-related patients (Table 1) revealed no notable genotype–phenotype correlations.
The age of the disease onset among the reported patients ranged from 3 months [2] to 3 years and 2 months [12], but in most cases, onset was between 1 year and 2.5 years of life. The commencement of folinic acid treatment ranged from ages 12 months [13] to 33 years [14]. Likewise, the time interval between onset of symptoms and folinic acid treatment initiation among patients ranged from almost immediately in two patients [1, 13] to a delay of more than 31 years in the oldest reported patient [14]. On average, the delay in therapy was 2–10 years.
The earliest reported symptoms were psychomotor regression and cerebellar ataxia. Epileptic seizures usually appeared afterwards, rarely before 18 months of age, and were of different types. They were not documented in three reported patients [1, 13, 15], while all the other patients manifested various combinations of myoclonic, atonic, tonic–clonic, tonic, absence seizures, epileptic spasms [10, 13], and/or focal seizures with and without impaired awareness. The most common were myoclonic seizures, observed in all but four patients [2, 13, 16, 17]. The seizures were commonly described as drug-resistant, of high frequency, and frequently evolving to status epilepticus.
Cerebellar signs were described in all patients and were typically accompanied by other neurological signs. Extrapyramidal motor signs were also present in 10 patients [1, 2, 12, 13, 16, 18, 20, 21]. Four patients had accompanied bulbar signs [14, 18], and 13 patients had accompanied pyramidal signs [1, 2, 9, 10, 18, 19]. Autistic behavioral features were observed in 18 patients [1, 2, 12, 13, 17, 19,20,21,22]. Congenital microcephaly was described in one patient [2], while acquired microcephaly was noted in five patients [2, 17, 21]. Head circumference was normal in all other patients.
The majority of patients had supratentorial hypomyelination of various degrees, with or without cerebellar atrophy [1, 2, 10, 12, 14, 18,19,20,21]. Cerebellar atrophy was absent in seven patients [12, 13, 15, 16, 22]. Four patients also had basal ganglia calcifications [9, 16, 21, 23], one patient had accompanied bilateral temporal cortical laminar necrosis and ulegyria [10], while one other patient had white matter encephalomalacia [17]. Two patients had no myelin abnormalities but cerebellar atrophy with or without cerebral calcifications [2, 9], and one had cerebral cortical atrophy only [22]. Delayed myelination with or without cerebellar atrophy was seen in four patients [2, 13]. Apart from the two patients described in this study, infratentorial hypomyelination was described only in one patient [23], while thinning of the corpus callosum was reported in one other [2]. MRS values before treatment showed low white matter choline and/or inositol in all patients except for five, which were normal [2, 15, 19].
The effect of folinic acid treatment has been associated with various clinical and radiological outcomes (Table 1). Regarding folinic acid administration, the recommendation is to give 2–10 mg/kg/day orally, with the suggestion to change the route of administration to intravenous or intrathecal if the response is suboptimal [24]. However, the dose of folinic acid and route of administration vary in different reports from 1.7 mg/kg/day orally [19] to the combination of 8.9 mg/kg/day orally with 500 mg/week/intravenously [23] (Table 1). Incomplete amelioration was accomplished in all but four patients [2, 18], regardless of the route of folinic acid administration. It should be noted that the lack of response to treatment in these patients was suggested to result from POLG1 mutations additionally found in one patient [2], and a long delay in diagnosis (13–15 years) in the other three patients [18]. Interestingly, in the two oldest reported patients who had a delay in diagnosis of 27 years and 31 years respectively, administration of oral folinic acid at 2 mg/kg/day resulted in a marked reduction in the frequency of seizures, permitting a reduction of antiepileptic therapy and improvement of quality of life [14]. The best treatment results were observed in children who were diagnosed and treated early [1, 2, 12]. In addition to Patient 2 from the current study, complete clinical recovery was only accomplished in one other patient for whom folinic acid therapy was started immediately after the symptom onset in the second year of life [1]. Complete recovery of both clinical and radiological features, such as seen in Patient 2 of this study, has never been documented.
Discussion
The siblings we describe here provide strong support for the effectiveness and importance of folinic acid treatment initiation at a very early age in patients with pathogenic variants in FOLR1 and neurodegeneration due to cerebral folate deficiency. The younger sibling (Patient 2) is the first reported patient with neurodegeneration due to cerebral folate deficiency who demonstrated complete recovery of both clinical features and brain MRI abnormalities following oral folinic acid treatment started just after symptom onset.
Contrarily, the 6-year delay in diagnosis of Patient 1 can explain his incomplete clinical recovery. It is important to note that the main cause for the delay in diagnosis was the inability to provide timely access to metabolic and genetic testing, as the patients were treated in a department without the necessary resources to perform this testing on a clinical basis. Therefore, genetic analyses were only performed later on a research basis, and by the time of genetic diagnosis, his neurological impairments had more substantially progressed. In Patient 2, genetic analysis was performed near the beginning of symptom onset at 2 years of age, and the immediate initiation of folinic acid therapy led to the complete resolution of both clinical and MRI abnormalities.
Additionally, the prolonged exposure of Patient 1 to a plethora of antiepileptic drugs for intractable epilepsy prior to establishing the correct diagnosis may have resulted in negative effects on his disease course. Indeed, some AEDs may have a harmful anti-folate effect, including valproate, phenobarbital, primidone, phenytoin, carbamazepine, oxcarbazepine, topiramate, gabapentin, and pregabalin [25, 26]. However, specific AEDs such as lamotrigine, levetiracetam, clobazam, and clonazepam have not demonstrated notable interactions with cerebral folates [26].
Based on the published literature, psychomotor regression with cerebellar ataxia starting in the second to third year of life, along with refractory epilepsy with mostly myoclonic seizures and radiological findings of cerebral hypomyelination with or without cerebellar atrophy should raise suspicion of this disease. Other clinical signs such as autistic behavioral features, deceleration of head growth, frequent occurrence of status epilepticus, and radiological findings of brain calcifications can be seen as well and do not exclude the possibility of this disorder/diagnosis. Furthermore, due to the phenotypic variability associated with this disease and other inherited neurological diseases, all individuals with drug-resistant epilepsy should undergo genetic testing.
Prior to our report, a complete resolution of neurologic symptoms was only accomplished in one patient, however, their MRI features did not fully resolve, with the preexisting cerebellar atrophy remaining despite the therapy [1, 2]. This patient was treated with both oral and intravenous folinic acid, including 5 mg/kg/day orally and 100 mg/week intravenously [2].
Brain MRI features improved in both of our patients with oral folinic acid therapy. Specifically, white matter T2-signal hyperintensity started to decrease and the volume of both supra- and infratentorial structures began to increase after treatment for two years in Patient 1, and one year in Patient 2. After four years of treatment, the brain MRI findings in Patient 1 did not yet normalize, but continued to improve. Moreover, brain MRS revealed normalization of the white matter choline peaks for age, in line with the reactivation of the myelination process [27]. The 5-year evolution of Patient 1’s brain MRI features prior to treatment is the longest reported for this disease, showing progressive cerebral and cerebellar atrophy together with a pattern of hypomyelination. In Patient 2, the level of myelination completely recovered after four years of treatment, demonstrating the first complete radiological recovery from this disease.
The route of folinic acid administration may be of importance when treating neurodegeneration due to cerebral folate deficiency, given that FOLRα is necessary for the transfer of MTHF from the CSF to the brain parenchyma [2, 3]. In the absence of FOLRα, other transporters such as the reduced-folate-carrier and proton-coupled-transporter may transport MTHF across the blood–brain barrier, but due to their very low MTHF affinity, there is a need for high plasma MTHF concentrations (i.e., high folinic acid doses) [2, 3]. However, the further delivery of exosomes with MTHF from CSF into the brain parenchyma likely exclusively depends on FOLRα, which could explain the lack of complete recovery despite the application of high doses of folinic acid and despite the normalization of MTHF concentration in CSF [2, 3, 12]. Since even high doses of folinic acid are typically unable to fully overcome the lack of FOLRα, new therapeutic strategies are needed. These may include the application of FOLRα + exosomes into the CSF as proposed by Grapp et al. [3]. With recent advances in gene therapy development, this avenue is certainly also very appealing.
Although both siblings harbored the same homozygous pathogenic variant in FOLR1, they exhibited phenotypic variability as the older sibling demonstrated an earlier disease onset with a more severe disease course. It has been proposed that even in the presence of the same FOLR1 pathogenic variants, the variable phenotypic severity may reflect the individual variability in the timing of fetal FOLRβ inactivation, different potency of FOLRβ functioning, variable residual FOLRα functions, or variable capacity of alternative folate transport mechanisms [2]. It may also reflect the different hypothesized processes through which folates contribute to myelin formation [2, 5]. Peripheral nerves were intact in our patients, while peripheral neuropathy was found in three reported patients, further suggesting a link between FOLRα and Schwann cells homeostasis [2, 10]. Regardless of the specific pathogenic mechanisms, folinic acid is the only disease-modifying therapy for the clinical and radiological manifestations of cerebral folate deficiency, and these cases demonstrate the importance of early treatment for the amelioration of disease features.
Conclusions
We report a novel pathogenic variant in FOLR1 in two Serbian siblings with clinical and brain MRI presentations consistent with neurodegeneration due to cerebral folate transport deficiency, along with the response to treatment and long-term follow-up, therefore contributing to the literature delineating the natural history of the disease. The youngest sibling is the first patient reported for whom complete recovery of both clinical and brain radiological abnormalities was achieved with oral folinic acid treatment, suggesting that early oral therapy may be sufficient to treat this condition compared to other more invasive routes of administration.
Furthermore, in patients with genetically undiagnosed hypomyelination, FOLR1 should be investigated promptly and included in all leukodystrophy panels to ensure early treatment with folinic acid and optimize clinical outcomes. Finally, these cases highlight the importance of universal access to genetic testing, to ensure that treatable conditions are promptly diagnosed and treatment initiated early to optimize clinical outcomes.
Availability of data and materials
Anonymized data supporting the findings of this study not published within this article will be made available from the corresponding author, upon reasonable request.
Abbreviations
- FOLR1 :
-
Folate receptor-alpha gene
- FOLRα:
-
Folate receptor-alpha
- FOLRβ:
-
Folate receptor-beta
- MTHF:
-
5-Methyltetrahydrofolate
- CSF:
-
Cerebrospinal fluid
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Magnetic resonance spectroscopy
- AED:
-
Antiepileptic drug
References
Steinfeld R, Grapp M, Kraetzner R, Dreha-Kulaczewski S, Helms G, Dechent P, et al. Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am J Hum Genet. 2009;85(3):354–63. https://doi.org/10.1016/j.ajhg.2009.08.005.
Grapp M, Just IA, Linnankivi T, Wolf P, Lücke T, Häusler M, et al. Molecular characterization of folate receptor 1 mutations delineates cerebral folate transport deficiency. Brain. 2012;135(Pt 7):2022–31. https://doi.org/10.1093/brain/aws122.
Grapp M, Wrede A, Schweizer M, Hüwel S, Galla HJ, Snaidero N, et al. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun. 2013;4:2123. https://doi.org/10.1038/ncomms3123.
Ramaekers VT, Segers K, Sequeira JM, Koenig M, Van Maldergem L, Bours V, et al. Genetic assessment and folate receptor autoantibodies in infantile-onset cerebral folate deficiency (CFD) syndrome. Mol Genet Metab. 2018;124(1):87–93. https://doi.org/10.1016/j.ymgme.2018.03.001.
Weng Q, Wang J, Wang J, Tan B, Wang J, Wang H, et al. Folate metabolism regulates oligodendrocyte survival and differentiation by modulating AMPKα activity. Sci Rep. 2017;7(1):1705. https://doi.org/10.1038/s41598-017-01732-1.
Soden SE, Saunders CJ, Willig LK, Farrow EG, Smith LD, Petrikin JE, et al. Effectiveness of exome and genome sequencing guided by acuity of illness for diagnosis of neurodevelopmental disorders. Sci Transl Med. 2014;6(265):265ra168. https://doi.org/10.1126/scitranslmed.3010076.
Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012;4(154):154ra135. https://doi.org/10.1126/scitranslmed.3004041.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17(5):405–24. https://doi.org/10.1038/gim.2015.30.
Ohba C, Osaka H, Iai M, Yamashita S, Suzuki Y, Aida N, et al. Diagnostic utility of whole exome sequencing in patients showing cerebellar and/or vermis atrophy in childhood. Neurogenetics. 2013;14(3–4):225–32. https://doi.org/10.1007/s10048-013-0375-8.
Kobayashi Y, Tohyama J, Akiyama T, Magara S, Kawashima H, Akasaka N, et al. Severe leukoencephalopathy with cortical involvement and peripheral neuropathy due to FOLR1 deficiency. Brain Dev. 2017;39(3):266–70. https://doi.org/10.1016/j.braindev.2016.09.011.
Hyland K, Shoffner J, Heales SJ. Cerebral folate deficiency. J Inherit Metab Dis. 2010;33(5):563–70. https://doi.org/10.1007/s10545-010-9159-6.
Delmelle F, Thöny B, Clapuyt P, Blau N, Nassogne MC. Neurological improvement following intravenous high-dose folinic acid for cerebral folate transporter deficiency caused by FOLR-1 mutation. Eur J Paediatr Neurol. 2016;20(5):709–13. https://doi.org/10.1016/j.ejpn.2016.05.021.
Papadopoulou MT, Dalpa E, Portokalas M, Katsanika I, Tirothoulaki K, Spilioti M, et al. Cerebral folate deficiency in two siblings caused by biallelic variants including a novel mutation of FOLR1 gene: intrafamilial heterogeneity following early treatment and the role of ketogenic diet. JIMD Rep. 2021;60(1):3–9. https://doi.org/10.1002/jmd2.12206.
Ferreira P, Luco SM, Sawyer SL, Davila J, Boycott KM, Dyment DA. Late diagnosis of cerebral folate deficiency: fewer seizures with folinic acid in adult siblings. Neurol Genet. 2015;2(1):e38. https://doi.org/10.1212/NXG.0000000000000038.
Tabassum S, AlAsmari A, AlSaman AA. Widening the phenotypic spectrum - Non epileptic presentation of folate transporter deficiency. J Clin Neurosci. 2019;59:341–4. https://doi.org/10.1016/j.jocn.2018.10.075.
Karin I, Borggraefe I, Catarino CB, Kuhm C, Hoertnagel K, Biskup S, et al. Folinic acid therapy in cerebral folate deficiency: marked improvement in an adult patient. J Neurol. 2017;264(3):578–82. https://doi.org/10.1007/s00415-016-8387-6.
Zhang C, Deng X, Wen Y, He F, Yin F, Peng J. First case report of cerebral folate deficiency caused by a novel mutation of FOLR1 gene in a Chinese patient. BMC Med Genet. 2020;21(1):235. https://doi.org/10.1186/s12881-020-01162-3.
Brunetti S, Malerba L, Giordano L, Parrini E, Guerrini R, Palumbo G, et al. Cerebral folate transporter deficiency syndrome in three siblings: Why genetic testing for developmental and epileptic encephalopathies should be performed early and include the FOLR1 gene. Am J Med Genet A. 2021;185(8):2526–31. https://doi.org/10.1002/ajmg.a.62345.
Al-Baradie RS, Chaudhary MW. Diagnosis and management of cerebral folate deficiency. A form of folinic acid-responsive seizures. Neurosciences. 2014;19(4):312–6.
Pérez-Dueñas B, Toma C, Ormazábal A, Muchart J, Sanmartí F, Bombau G, et al. Progressive ataxia and myoclonic epilepsy in a patient with a homozygous mutation in the FOLR1 gene. J Inherit Metab Dis. 2010;33(6):795–802. https://doi.org/10.1007/s10545-010-9196-1.
Toelle SP, Wille D, Schmitt B, Scheer I, Thöny B, Plecko B. Sensory stimulus-sensitive drop attacks and basal ganglia calcification: new findings in a patient with FOLR1 deficiency. Epileptic Disord. 2014;16(1):88–92. https://doi.org/10.1684/epd.2014.0629.
Kanmaz S, Simsek E, Yilmaz S, Durmaz A, Serin HM, Gokben S. Cerebral folate transporter deficiency: a potentially treatable neurometabolic disorder. Acta Neurol Belg. 2021. https://doi.org/10.1007/s13760-021-01700-7.
Mafi S, Laroche-Raynaud C, Chazelas P, Lia AS, Derouault P, Sturtz F, et al. Pharmacoresistant epilepsy in childhood: think of the cerebral folate deficiency, a treatable disease. Brain Sci. 2020;10(11):762. https://doi.org/10.3390/brainsci10110762.
Plecko B, Steinfeld R. Disorders of vitamin metabolism. In: Swaiman KF, Ashwal S, Ferriero DM, editors. Swaiman’s Pediatric Neurology. Principles and Practice. 6th edition. Philadelphia: Mosby Elsevier; 2017: e943.
Opladen T, Blau N, Ramaekers VT. Effect of antiepileptic drugs and reactive oxygen species on folate receptor 1 (FOLR1)-dependent 5-methyltetrahydrofolate transport. Mol Genet Metab. 2010;101(1):48–54. https://doi.org/10.1016/j.ymgme.2010.05.006.
Linnebank M, Moskau S, Semmler A, Widman G, Stoffel-Wagner B, Weller M, et al. Antiepileptic drugs interact with folate and vitamin B12 serum levels. Ann Neurol. 2011;69(2):352–9. https://doi.org/10.1002/ana.22229.
van der Knaap MS, Pouwels PJW. Magnetic resonance spectroscopy: basic principles and application in white matter disorders. In: van der Knaap MS, Valk J, editors. Magnetic resonance of myelination and myelin disorders. 3rd ed. Springer-Verlag: Berlin Heidelberg; 2005. p. 859–88.
Acknowledgements
The authors wish to thank the patients and their family for their participation in this study.
Funding
This study was funded by grants from the Canadian Institute of Health Research (377869, 426534). G.B. has received a Clinical Research Scholar Junior 1 award from the Fonds de Recherche du Quebec–Santé (FRQS) (2012–2016), the New Investigator Salary Award from the Canadian Institutes of Health Research (2017–2022) and a Clinical Research Scholar Senior award from the FRQS (2022–2025). S.P. has been supported by the FRQS Doctoral Scholarship, the Fondation du Grand défi Pierre Lavoie Doctoral Scholarship, the McGill Faculty of Medicine F.S.B. Miller Fellowship, and the Research Institute of the McGill University Health Centre Desjardins Studentship in Child Health Research. The work by T.P. and I.T. was made possible by the generous gifts to Children’s Mercy Research Institute and Genomic Answers for Kids program at Children’s Mercy Kansas City. T.P. holds the Dee Lyons/Missouri Endowed Chair in Pediatric Genomic Medicine.
Author information
Authors and Affiliations
Contributions
AP, GB, RS conceived the study and participated in its design and coordination; AP, GB, RS, TP, SP, IT, TR, SG, JO, LTT critically revised the manuscript for important intellectual content; AP, SP made the review of the literature, and wrote the first draft which was completed and implemented by GB, RS, TP, IT, TR, SG, JO, LTT; AP, TR performed the clinical examinations of the patients; AP, TR, SG, JO, GB, RS, SP performed the acquisition and interpretation of radiological data; GB, IT, TP, SP, LTT performed genetic studies and analyzed the results. All authors read the final manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is conducted following the 1964 Declaration of Helsinki and its later amendments, with approval of the Institutional Review Boards of Clinic for Child Neurology and Psychiatry University of Belgrade (IRB number 1-48/3-2016) and the McGill University Health Center and Montreal Children’s Hospital Research Ethic Boards (11-105-PED and 2019-4972).
Consent for publication
Written informed consents to participate in the research study and written informed consents for publication were obtained from the parents of the affected patients.
Competing interests
G.B. is/was a consultant for Passage Bio Inc (2020-2022) and Ionis (2019). She is/was a site investigator for the Alexander’s disease trial of Ionis (2021-present), Metachromatic leukodystrophy of Shire/Takeda (2020–2021), Krabbe and GM1 gene therapy trials of Passage Bio (2021-present), GM1 natural history study sponsored by the University of Pennsylvania with funding from Passage Bio (2021-present) and Adrenoleukodystrophy/Hematopoietic stem cell transplantation natural history study of Bluebird Bio (2019), a site sub-investigator for the MPS II gene therapy trial of Regenxbio (2021-present) and the MPS II clinical trial of Denali (2022-present). She has received unrestricted educational grants from Takeda (2021–2022). She serves on the scientific advisory board of the Pelizaeus-Merzbacher Foundation, the Yaya Foundation Scientific and Clinical Advisory Council and is the Chair of the Medical and Scientific Advisory Board of the United Leukodystrophy Foundation. She is a member of the Vanishing White Matter Consortium, the MLC Consortium, the H-ABC Clinical Advisory Board and the Chair of the POLR3-related (4H) Leukodystrophy Consortium. She is on the editorial boards of Neurology Genetics, Frontiers in Neurology—Neurogenetics, and Journal of Medical Genetics. L.T.T. currently manages sponsored clinical trials at the site level for Ionis Pharmaceuticals (Alexander disease clinical trial 2021-present), Passage Bio (Krabbe disease and GM1 gangliosidosis clinical trials, 2021-present), and Teva Pharmaceuticals (chronic and episodic migraine clinical trials, 2022-present). Several other sponsored clinical trials are in various stages of the start-up phase. He also manages a GM1 gangliosidosis natural history study sponsored by the University of Pennsylvania with funding from Passage Bio. The other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Potic, A., Perrier, S., Radovic, T. et al. Hypomyelination caused by a novel homozygous pathogenic variant in FOLR1: complete clinical and radiological recovery with oral folinic acid therapy and review of the literature. Orphanet J Rare Dis 18, 187 (2023). https://doi.org/10.1186/s13023-023-02802-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-023-02802-6