Abstract
Background
Wiskott–Aldrich syndrome (WAS) is a rare X-linked immunodeficiency disorder caused by abnormal expression of the WAS protein (WASp) due to mutations in the WAS gene, and is generally characterized by microthrombocytopenia, eczema, recurrent infections, and high susceptibility to autoimmune complications and hematological malignancies.
Results
Herein, we identified a novel WAS mutation (c.158 T > C) using next-generation sequencing in a Chinese pedigree with WAS. The expression of WASp in the patients and their families was detected by flow cytometry and western blot analysis. To explore the exon-splicing effect of intron mutations and the correlation between the genotype and clinical phenotype, four groups of wild-type (WT), exon mutant, intron mutant, and combined mutant recombinant plasmids were transfected into COS-7 cells in vitro. The proband showed dramatically decreased WASp expression, while the female carriers showed a slightly lower level of WASp. The expression of products in the mutant and WT recombinant plasmids was detected by real-time fluorescence quantitative polymerase chain reaction (PCR), which showed a significant reduction in the combined mutant group than in the WT, exon mutant, and intron mutant groups. The length of the expression products in the four groups showed no differences, each containing 360 base pairs. Sequence analysis confirmed that the c.158 T > C mutation appeared in the exon mutant and combined mutant groups, whereas the intron variant c.273 + 14C > T caused no other sequence changes.
Conclusion
This study confirmed that the intron mutation did not affect the splicing of exons and excluded the influence of the double mutations at the transcription level on the severe clinical manifestations in the cousin. This in vitro study provided new insights into the pathogenesis of intronic mutations in WAS.
Similar content being viewed by others
Background
Wiskott–Aldrich syndrome (WAS: OMIM 301,000) is a rare X-linked recessive immunodeficiency disease, that is usually characterized by the triad of eczema, recurrent infections and microthrombocytopenia leading to bleeding. Patients with severe WAS are susceptible to the development of autoimmune diseases, lymphoma or other malignancies. The severity of clinical manifestation is scored according to the degree of thrombocytopenia (score, 0.5–1), eczema and immunodeficiency (score, 2–4), and the presence of autoimmunity, malignancy or even death (score 5) [1, 2]. Generally, affected males show clinical manifestations at an early age, whereas females carrying the defective WAS gene are asymptomatic. The incidence of WAS is less than 1 in 100,000 live births [3]. Patients who show only thrombocytopenia initially are prone to be misdiagnosed as having immune thrombocytopenia (ITP), which may result in inappropriate treatment and delayed life-saving therapy [4]. Hematopoietic stem cell transplantation (HSCT) is the gold standard treatment for patients with WAS. The earlier HSCT for WAS patients is performed, the higher the survival rate [5, 6].
The WAS gene, which is located on Xp11.22–p11.23, is responsible for this disease. This gene consists of 12 exons that encode Wiskott–Aldrich syndrome protein (WASp), which contains 502 amino acid and participates in the regulation of actin cytoskeleton-dependent cellular processes, such as cell signaling, migration, immune synapse formation, and cytokine release [7, 8]. The WAS gene contains an Ena/VASP homology 1 (EVH1/WH1) domain, a basic region (BR), a GTPase-binding domain, a proline-rich region and the verprolin, cofilin and acidic region from the N terminus to the C terminus. Missense mutations, considered the most common mutations of WAS, mostly occur in exons 1–4 of the WAS-interacting protein (WIP) binding domain [9]. Splice-site mutations are commonly located in introns 6–10, whereas insertions and deletions occur across the WAS gene [10, 11]. The classic WAS is accompanied by entire loss-of-function mutations, while partial loss-of-function mutations result in X-linked thrombocytopenia (XLT) with decreased levels of WASp and gain-of-function mutations lead to the rare X-linked neutropenia (XLN) [12, 13].
Although the genotype–phenotype connection of WAS/XLT has not been fully clarified, several studies have revealed that the clinical phenotype of typical WAS or milder XLT is potentially influenced by the effects of these mutations and WASp expression [11, 14, 15]. Milder WAS symptoms are frequently associated with missense and splice-site mutations, whereas severe WAS symptoms are associated with nonsense and frameshift mutations [10, 16]. Moreover, patients with missense mutations that allow the expression of WASp present with mildly symptomatic XLT, showing a better prognosis [17].
Here, we report a novel mutation in WAS in a Chinese family (Fig. 1). The proband had typical features of WAS with a novel missense mutation in exon 2, and his cousin who presented with severe symptoms of thrombocytopenia, hemorrhage and recurrent infections was discovered to have a second-site mutation in intron 2. Therefore, this study explored whether the intron mutation affected exon splicing and evaluated the correlation between the second-site mutation and the aggravated phenotype in the cousin.
Results
WASp expression
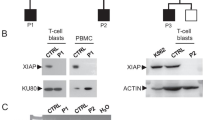
The intensity of WASp expression in peripheral blood mononuclear cells (PBMCs) isolated from the proband and his mother was 3.8% and 72.8%, respectively, as determined by flow cytometry (Fig. 2). The intensity of WASp expression in the PBMCs of the patient was significantly lower than in normal individual (96%). Western blot analysis showed that WASp expression in the proband was absent in comparison with that in his mother and normal control (Fig. 2). The intensity of WASp expression in patient 2’s mother and his sister was 77.68% and 64.58%, respectively, while that in the normal control was 95.11% (Fig. 3).
Expression of WASp in the proband and his mother using flow cytometry analysis on the above. A The proband (3.8%); B his mother (72.8%); C normal individual (96%); Expression of WASp in the proband and his mother performed by western blot analysis on the below. (a) the proband; (b) his mother; (c) normal control
Mutation analysis
Sequence analysis showed that the proband and his cousin had a hemizygous missense mutation (c.158 T > C) in exon 2 of the WAS gene, which was predicted to change leucine to proline (p.L53P) (Fig. 4a). The heterozygous mutation 158 T > C was also confirmed in the proband’s mother (Fig. 4b), whereas no mutation was detected in his father (Fig. 4c). In addition to 158 T > C, the cousin also had a second-site mutation c.273 + 14C > T (Fig. 5a); his mother showed the heterozygous mutations 158 T > C and c.273 + 14C > T (Fig. 5b), while his sister was also a WAS carrier with heterozygous mutations (Fig. 5c).
a Mutations in WAS gene of the younger cousin (c.158 T > C in exon 2 and c.273 + 14C > T in intron 2); b Mutations in WAS gene of the cousin's mother (heterozygous mutation of c.158 T > C in exon 2 and c.273 + 14C > T in intron 2); c Mutations in WAS gene of the cousin’s sister (heterozygous mutation of c.158 T > C in exon 2 and c.273 + 14C > T in intron 2)
Based on the consensus WAS sequence (ENST00000376701), online mutation analysis using Mutation Taster and PolyPhen-2 indicated that mutation 158 T > C in exon 2 may be a pathological factor. Mutation Taster indicated that the protein features might have been affected (http://www.mutationtaster.org/cgi-bin/MutationTaster/Mutation Taster69.cgi), while PolyPhen-2 predicted probable damage with a score of 1.00 (http://genetics.bwh.harvard.edu/ggi/pph2/7e5771b52fb6b1008d4a14d80a963e961bba4690/6293018.html).
Expression products of the recombinant mutant vectors in COS-7 cells
After enzymatic digestion with XhoI and BamHI, the polymerase chain reaction (PCR)-amplified gDNA showed two bands on agarose gel electrophoresis (Fig. 6): a 1849-bp band and a more than 4000-bp band, each of which corresponded to the length of the plasmid pET-01. We extracted these two bands and amplified them under the same PCR conditions. Direct sequencing confirmed that the short bands in the four groups were errorless for the target mutations (Fig. 6).
The restriction endonuclease analysis of four recombinant vectors in pET-01 on the left. The restriction enzymes are XhoI and BamHI. The expected size is 1849 bp and 4500 bp. The two bands show approximately 1800 bp and more than 4000 bp using electrophoresis. The gene sequencing map of the recombinant vectors on the right respectively represents wild-type, exon mutant, intron mutant and combined mutant group
The empty plasmid vector served as a negative control, and the relative transcript levels of the four groups were shown in Table 1. The combined mutant group showed significantly lower transcript levels than the wild-type (WT), exon mutant and intron mutant groups. However, the length of the expression products of four recombinant mutated vectors in COS-7 cells showed no differences. Meanwhile, sequence analysis confirmed the appearance of the c.158 T > C mutation in the exon mutant group and combined mutant group, whereas the intron variant c.273 + 14C > T caused no abnormal sequences variation in this part of WAS (Fig. 7). No differences were detected among the four cDNA fragments.
Discussion
WAS is a complex X-linked primary immunodeficiency disorder along with thrombocytopenia. Persistent thrombocytopenia with small platelets is a pathogenetic sign of WAS/XLT, presenting spontaneous or posttraumatic bleeding. The degree of bleeding varies from petechiae or ecchymosis to severe hematomas and even to life-threatening intracerebral or intestinal hemorrhages [1]. The patients in our study presented with varying degrees of bleeding, including petechiae, ecchymosis, and infrequent umbilical hemorrhage. In fact, continuous microthrombocytopenia can be attributed to accelerated destruction of defective platelets in the spleen combined with the co-action of antiplatelet autoantibodies [18]. However, due to the early onset of thrombocytopenia, the intermittent low platelet count can hamper accurate diagnosis of WAS; patients with WAS/XLT, especially XLT [19], are often misdiagnosed as showing ITP, and only 7% of these patients are ultimately diagnosed [20]. The proband and his cousin in the present case were both initially misdiagnosed with ITP at an early age. Although the platelet count improved temporarily after treatment with dexamethasone sodium phosphate and immunoglobulin, the patients later showed significantly decreased platelet count and progressed to more severe clinical manifestations, such as severe immune dysregulation with susceptibility to infections, eczema.
Based on the Human Gene Mutation Database (HGMD), there are more than 450 unique mutations across all 12 exons, including nonsense, missense, insertion, deletion, rearrangement, and splice-site mutation. In our report, mutation analysis identified a novel mutation of 158 T > C in the proband and his younger male cousin, which caused a change in leucine to proline at the 53rd amino acid. Missense mutations in exons 1, 2, and 3 of the WAS gene are the most common mutations in patients with milder phenotypic features, such as mild XLT [15]. However, both patients had typical WAS symptoms and were clinically assigned scores of 4 and 5 respectively. This missense mutation residing in the N-terminal WIP binding site can impair the affinity of WASp for WIP, thus leading to dissociation of the complex and degradation of WASp [21]. In addition, the mutation may lead to defective PLT production by megakaryocytes in the bone marrow, thereby affecting the number and volume of platelets [22].
More studies have identified various clinical phenotypes associated with diverse mutations in the WAS gene, indicating a close genotype–phenotype correlation with a certain predictability for clinical outcomes. The mutations 168C > T (T45M), 290C > N/291G > N (R86C/H/L) and IVS6 + 5 g > a consistently resulted in reduced WASp and relatively mild clinical manifestations whereas 665C > T (R211X), IVS8 + 1 g > n and IVS8 + 1 + 6del gtga predominantly represented the absence of WASp expression and a severe clinical phenotype; these six hotspot mutations accounted for approximately 25% of all mutations [11, 23]. Mutations adjacent to the 158 T > C in our case such as 151G > T (Val51Phe), 156G > C (Gln52His), and 162C > A (Tyr54Tem) contribute to the classical WAS phenotype [24,25,26]. Furthermore, mutations 155A > C (Gln52Pro) and 167C > T (Ala56Val) lead to the XLT phenotype and patients with 166G > A (Ala56Val) experienced intermittent XLT [23, 27, 28].
The absence of WASp or truncated proteins is typically associated with severe clinical symptoms in affected individuals. The decreased expression of WASp in the proband corresponded to his clinical manifestations. Meanwhile, the WASp expression in his mother, who was a heterozygous carrier, was only slightly reduced due to the defective WAS gene which might represent preferential X-chromosome inactivation (XCI) of the female WAS carrier that allowed survival of partially defective WAS cells [29].
Up to 11% of patients with WAS present with somatic mosaicism due to spontaneous reversion of the original mutation to normal or the appearance of second-site compensatory mutations, which partially restore the biological function of WASp [30]. Wada et al. [31] described a second-site mutation causing the deletion of 19 nucleotides from nucleotides 1299 to 1316 in two brothers with WAS, suggesting that the original mutation (1305insG) resulted in frameshift and abrogated protein expression, while the second-site mutation revised the adverse effects of the original mutation. Notably, a c.273 + 14C > T second-site mutation was detected in the younger male cousin in this study, who showed more severe clinical manifestations than the proband and died of fungal infection at 3 years of age. To date, splice-site mutations have been mostly shown to be distributed in intron 6–11 [15, 16] and are rarely reported in intron 2. Ariga et al. firstly performed a mutation in the acceptor site of intron 2 of the WAS gene (IVS2-1 g > a) and confirmed that the abnormal lack of exon 3 was due to the splicing defect [32]. In addition, IVS2 + 1 g > a and IVS2 + 1 g > t both resulted in the absence of WASp; however, the splicing effect was not determined [11]. Similarly, the other two mutations (IVS2 + 2t > c and IVS2-2a > c) were associated with predictive splicing abnormalities [25, 33].
Due to the unavailability of the cousin’s blood, we used a recombinant mutant vector in COS-7 cells in vitro to explore whether the second-site mutation in intron 2 caused the cousin’s more severe clinical manifestations compared to the proband. The results showed that the relative expression of the WAS gene, as determined by real-time fluorescence quantitative PCR (RT-qPCR), in the combined mutation was approximately 50% lower than that in the WT group (Table 2). However, the four groups showed no differences in the length and sequence of expression products, confirming that the intron variant c.273 + 14C > T had no effect on RNA splicing in this part of the WAS gene. The ratio of CD4 + and CD8 + T cells, which is closely related to opportunistic infections, was 0.3, indicating cellular immune dysfunction and a higher probability of opportunistic infection. Multiple opportunistic infections were most likely an important culprit for the rapid exacerbation of clinical symptoms and death of the younger cousin. Therefore, we speculated that the cousin had already suffered severe multiorgan damage or irreversible complications when recovery mutations occurred. Although revertant mutations could have resulted in partial immune or hematopoietic function reconstruction [34], the cousin died of severe infections that could not been reversed by single second-site mutation.
After excluding the splicing effect of the second-site mutation at the experimental molecular level, several factors may have aggravated the clinical manifestations in the cousin. First, some WAS patients may have already suffered severe multiorgan damage or irreversible complications, such as malignancies, before the occurrence of the recovery mutations [34]. Thus, although revertant mutations can facilitate partial immune or hematopoietic function reconstruction, they are not sufficient to maintain long-term survival of patients with WAS. In this regard, the cousin died of severe infections that could not be reversed by single second-site mutation. Second, age of onset and disease progression are also factors influencing the effects of second-site mutations. A retrospective analysis of 160 patients revealed that the risk of developing a clinical score of 5 was significantly higher during the first 2 years of life [2].
Gene therapy has emerged as an effective treatment option for WAS, and successful gene therapy procedures for WAS will increase the availability of a cure for patients with WAS and possibly reduce the potential side effects associated with mismatched allogeneic transplantation [35]. Nevertheless, HSCT remains the most reliable curative treatment, and shows excellent results for patients with WAS. Thus, prompt and correct diagnosis is of vital significance for patients with WAS to improve their quality of life and increase their survival rate.
Conclusions
In general, the results of this in vitro study supported the lack of splicing effects of the second-site mutation, thus indicating its weak function in the clinical phenotype. Genetic analysis and a review of family history are important for confirming the diagnosis of patients with WAS. In this regard, the results of effective research studies can be expected to substantially contribute to the diagnosis and treatment of WAS. More relevant studies are needed to identify the mechanism of phenotypic impact of second-site mutation in these cases.
Methods
Case presentation
Patient 1, a six-month-old boy, was initially admitted to our hospital in April 2014 with thrombocytopenia, lung infections, bleeding, and ecchymosis. The complete blood count showed the following findings: hemoglobin (Hb), 110 g/L (120–140 g/L), white blood cell (WBC), 6.74 × 109/L (3.5–9.5 × 109/L), platelet (PLT) count, 14 × 109/L (125–350 × 109/L); and mean platelet volume (MPV), 6.9 fl (7.4–11.0 fl). Owing to fever, thrombocytopenia and upper respiratory tract infection, the patient was diagnosed as ITP. After anti-infective therapy with dexamethasone sodium phosphate and γ-globulin, his PLT count rose to 164 × 109/L and respiratory tract infection was under control. The patient was then discharged from the hospital. Two months later, his PLT count decreased to 42 × 109/L, and he experienced recurrent infections, intermittent bleeding, and frequent eczema again. Blood examination showed leukocytosis of 22.82 × 109/L with hematophagocytes in the bone marrow aspirate. The liver function test indicated the following findings: alanine transaminase (ALT), 113.5 U/L (9.0–50.0 U/L), aspartate aminotransferase (AST), 2 U/L (15.0–40.0 U/L), gamma glutamyl transferase (γ-GT), 137 U/L (10.0–60.0 U/L), triglycerides (TG), 2.67 mmol/L (0.56–1.70 mmol/L); cholesterol (CHO), 6.16 mmol/L (3.11–5.20 mmol/L). The results of other laboratory examinations were presented in Table 2.
Patient 2, a younger male cousin of patient 1, presented with the same clinical symptoms at 3 months of age. The laboratory test showed the following results: WBC count, 11.5 × 109/L, Hb, 103 g/L and PLT count, 6 × 109/L. He suffered from thrombocytopenia, intermittent bleeding, and umbilical hemorrhage and was also diagnosed with ITP. The PLT count reached 94 × 109/L after treatment with γ-globulin, dexamethasone sodium phosphate and platelet transfusions. One month later, he was admitted to the hospital because of cough, expiratory dyspnea, diarrhea, and pulmonary hemorrhage. His WBC count was 16.1 × 109/L and PLT count was 44 × 109/L. He experienced frequent infections and died due to a fungal infection at three years of age.
Figure 1 shows the pedigree of the patients’ families. Their family history revealed that their mothers’ brother had presented with thrombocytopenia and died of infections at 3 years of age and that their mothers’ uncle was thrombocytopenic before death. No abnormalities were observed in the other family members. Therefore, the proband and his younger cousin were suspected to have WAS. On the basis of the medical and family history, they were assigned scores of 4 and 5, respectively, using a previously described scoring system [10, 14].
Patient samples
Informed consent was obtained from the patients’ parents, legal guardians and family members before enrollment in the study. This study was approved by the Research Ethics Committee of the Second Hospital of Hebei Medical University. Ethylenediaminetetraacetic acid (EDTA)-anticoagulated venous blood samples (2 ml) were extracted from patients, their parents and carriers for mutation analysis. Heparinized venous blood samples (5 ml) were obtained from patients and carriers in their families for WASp detection.
Flow cytometry and western blot analysis
PBMCs were isolated from the blood samples. Intracellular staining with an anti-WASp monoclonal antibody (mAb) was performed as described previously [36]. Cells were incubated with 0.25 mg/ml purified mouse anti-human WASp mAb (BD pharmingen, Franklin Lakes, NJ, USA) or 0.5 mg/ml isotype-matched control mouse IgG2a mAb (BioLegend, San Diego, CA, USA) and reacted with 1:100 diluted fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG2a (Affinity Biosciences, Jiangsu, China). The samples were analyzed on a BD FACSCanto II (Becton Dickinson, Franklin, Lakes, NJ, USA), using FlowJo V10 software (Becton Dickinson).
Western blotting of WASp and actin in PBMCs was performed using anti-WAS mAb (1:500; BD Pharmingen, Franklin Lakes, NJ, USA) and anti-β-actin control antibodies (1:3000; Servicebio, Wuhan, China), as described previously [37].
Mutation analysis
Total genomic DNA was extracted from PBMCs and amplified using PCR [38]. The PCR products were sequenced by the next-generation sequencing to screen for inherited platelet disorders and immunodeficiency diseases [39]. All PCR products were sequenced using NovaSeq 6000 Genetic Analyzer (Illumina, San Diego, CA, USA). Sanger sequencing was performed on the other family members for familial segregation [40]. The forward primer (5′-CTGTCATGAGGCAGGAAGGAC-3′) and reverse primer (5′-CATCTGGATGAGTCTTTGGTTCTG-3′) were designed using Primer Premier 5. Similarity analysis was performed using the NCBI BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/) to identify novel mutations. Sequences were aligned with WAS (NCBI reference sequence: NG_007877.1 and NM_000377.3) coding sequences.
Construction of mutant and wild-type plasmids and cell preparation
We constructed the expressing plasmid pET-01 containing the part-length gDNA of WAS, which included exons 1, 2, and 3 and introns 1 and 2 as the WT. The mutation in pET-01 was constructed by site-direct mutagenesis to generate an exon mutant group and the intron mutation in pET-01 was designed to generate an intron mutant group. Both mutations involved in pET-01 were constructed to generate a combined mutant group to verify the pathogenicity of compound mutations.
Recombinant mutant plasmid was constructed with the Transformer mutagenesis kit (Clonetech, Palo Alto, CA, USA). The sequence between the two unique restriction sites, XhoI and BamHI, of each construct was verified by sequencing. Empty Vector control and recombinant WAS containing the candidate mutation in the four groups were transiently expressed in COS-7 cells (SV40 transformed African green monkey kidney cells, ATCC CRL 1651). Transfection of eukaryotic COS-7 Cells with recombinant vector DNA was performed using the DEAE-dextran method [41].
cDNA synthesis was performed using the HiFiScript gDNA Removal cDNA Synthesis Kit (Cowin Biotech, Jiangsu, China). RT-qPCR was performed using CFX connect (Bio-Rad). PCR was performed with the following parameters: 1 cycle of 15 min at 95 °C; 40 cycles of 10 s at 95 °C, 30 s at 58 °C and 30 s at 72 °C. Ten mircoliters of PCR product were mixed with the sample buffer including gel dye markers and analyzed on an agarose gel.
Detection of the expression in mutant and wild-type recombinant plasmids in COS-7 cells
PCR products (20 μl of each PCR products including the amplified exons identified on the agarose gel) were digested with the restriction endonucleases XhoI and BamHI. Restricted DNA fragments were cloned into vectors. The inserted exons were amplified using the PCR primers 5′-CTCCAGGACCACGAGAACC-3′ and 5′-CCGTAAAGGCGGATGAAGTA-3′. The PCR products from the five groups were subjected to polyacrylamide gel electrophoresis (three replicates for each group). The cDNA products in the four groups were recovered and directly sequenced. The results were analyzed using Mygenostics software, and mutations were identified compared with the genomic gene of WAS (NG-007877.1) in GenBank's human genome database.
Availability of data and materials
Not applicable.
Abbreviations
- WAS:
-
Wiskott–Aldrich syndrome
- WASp:
-
Wiskott–Aldrich syndrome protein
- HSCT:
-
Hematopoietic stem cell transplantation
- ITP:
-
Immune thrombocytopenia
- PBMC:
-
Peripheral blood mononuclear cell
- PCR:
-
Polymerase chain reaction
- Hb:
-
Hemoglobin
- PLT:
-
Platelet
- WBC:
-
White blood cell
- MPV:
-
Mean platelet volume
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate aminotransferase
- γ-GT:
-
Gamma glutamyl transferase
- TG:
-
Triglycerides
- CHO:
-
Cholesterol
- EVH1/WH1:
-
Ena/VASP homology 1
- BR:
-
Basic region
- WIP:
-
WAS-interacting protein
- WT:
-
Wild-type
- XLT:
-
X-linked thrombocytopenia
- XLN:
-
X-linked neutropenia
- HGMD:
-
Human Gene Mutation Database
- NGS:
-
Next-generation sequencing
- IPD:
-
Inherited platelet disorders
- EDTA:
-
Ethylenediaminetetraacetic acid
References
Candotti F. Clinical manifestations and pathophysiological mechanisms of the Wiskott–Aldrich syndrome. J Clin Immunol. 2018;38(1):13–27.
Mahlaoui N, Pellier I, Mignot C, Jais JP, Bilhou-Nabéra C, Moshous D, et al. Characteristics and outcome of early-onset, severe forms of Wiskott–Aldrich syndrome. Blood. 2013;121(9):1510–6.
Baharin MF, Dhaliwal JS, Sarachandran SVV, Idris SZ, Yeoh SL. A rare case of Wiskott–Aldrich syndrome with normal platelet size: a case report. J Med Case Rep. 2016;10(1):188.
Karalexi MA, Tzanoudaki M, Fryganas A, Gkergki A, Spyropoulou D, Papadopoulou A, et al. Wiskott–Aldrich syndrome misdiagnosed as immune thrombocytopenic purpura: a case report. J Pediatr Hematol Oncol. 2018;40(3):240–2.
Kaneko R, Yamamoto S, Okamoto N, Akiyama K, Matsuno R, Toyama D, et al. Wiskott–Aldrich syndrome that was initially diagnosed as immune thrombocytopenic purpura secondary to a cytomegalovirus infection. SAGE. Open Med Case Rep. 2018;6:2050313X17753788.
Burroughs L, Petrovic A, Brazauskas R, Liu X, Griffith LM, Ochs HD, et al. Excellent outcomes following hematopoietic cell transplantation for Wiskott–Aldrich syndrome: A PIDTC report. Blood. 2020;135(23):2094–105.
Machesky LM, Insall RH. Scar1 and the related Wiskott–Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol. 1998;8(25):1347–56.
Rivers E, Thrasher AJ. Wiskott–Aldrich syndrome protein: Emerging mechanisms in immunity. Eur J Immunol. 2017;47(11):1857–66.
Massaad MJ, Ramesh N, Le Bras S, Giliani S, Notarangelo LD, Al-Herz W, et al. A peptide derived from the Wiskott–Aldrich syndrome (WAS) protein-interacting protein (WIP) restores WAS protein level and actin cytoskeleton reorganization in lymphocytes from patients with WAS mutations that disrupt WIP binding. J Allergy Clin Immunol. 2011;127(4):998–1005.
Imai K, Morio T, Zhu Y, Jin Y, Itoh S, Kajiwara M, et al. Clinical course of patients with WAS gene mutations. Blood. 2004;103(2):456–64.
Jin Y, Mazza C, Christie JR, Giliani S, Fiorini M, Mella P, et al. Mutations of the Wiskott–Aldrich Syndrome Protein (WASP): hotspots, effect on transcription, and translation and phenotype/genotype correlation. Blood. 2004;104(13):4010–9.
Blundell MP, Worth A, Bouma G, Thrasher AJ. The Wiskott–Aldrich syndrome: the actin cytoskeleton and immune cell function. Dis Markers. 2010;29(3–4):157–75.
Howard K, Hall CP, Al-Rahawan MM. Wiskott–Aldrich syndrome: description of a new gene mutation without immunodeficiency. J Pediatr Hematol Oncol. 2016;38(2):163.
Zhu Q, Watanabe C, Liu T, Hollenbaugh D, Blaese RM, Kanner SB, et al. Wiscott–Aldrich syndrome/X-linked thrombocytopenia: WAS gene mutations, protein expression, and phenotype. Blood. 1997;90(7):2680–9.
Ochs HD. Mutations of the Wiskott–Aldrich syndrome protein affect protein expression and dictate the clinical phenotypes. Immunol Res. 2009;44(1–3):84–8.
Albert MH, Notarangelo LD, Ochs HD. Clinical spectrum, pathophysiology and treatment of the Wiskott–Aldrich syndrome. Curr Opin Hematol. 2011;18(1):42–8.
Liu DW, Zhang ZY, Zhao Q, Jiang LP, Liu W, Tu WW, et al. Wiskott–Aldrich syndrome/X-linked thrombocytopenia in China: clinical characteristic and genotype-phenotype correlation. Pediatr Blood Cancer. 2015;62(9):1601–8.
Thrasher AJ, Burns SO. WAS: a key immunological multitasker. Nat Rev Immunol. 2010;10(3):182–92.
Medina SS, Siqueira LH, Colella MP, Yamaguti-Hayakawa GG, Duarte BKL, Dos Santos Vilela MM, et al. Intermittent low platelet counts hampering diagnosis of X-linked thrombocytopenia in children: report of two unrelated cases and a novel mutation in the gene coding for the Wiskott–Aldrich syndrome protein. BMC Pediatr. 2017;17(1):151.
Bryant N, Watts R. Thrombocytopenic syndromes masquerading as childhood immune thrombocytopenic purpura. Clin Pediatr. 2011;50(3):225–30.
Massaad MJ, Ramesh N, Geha RS. Wiskott–Aldrich syndrome: a comprehensive review. Ann N Y Acad Sci. 2013;1285:26–43.
Sereni L, Castiello MC, Villa A. Platelets in Wiskott–Aldrich syndrome: victims or executioners? J Leukoc Biol. 2018;103(3):577–90.
Liu C, Chen XY, Wu WQ, An WB, Chang LX, Lan Y, et al. Clinical features of Wiskott–Aldrich syndrome: an analysis of 13 cases. Zhong Guo Dang Dai Er Ke Za Zhi. 2019;21(5):463–7.
Ochfeld E, Grayer D, Sharma R, Schneiderman J, Giordano L, Makhija M. A novel mutation in WAS gene causing a phenotypic presentation of Wiskott–Aldrich syndrome: a case report. J Pediatr Hematol Oncol. 2021;43(2):e234–6.
El-Hakeh J, Rosenzweig S, Oleastro M, Basack N, Berozdnik L, Molina F, et al. Wiskott–Aldrich syndrome in Argentina: 17 unique, including nine novel, mutations. Hum Mutat. 2002;19(2):186–7.
Brooimans RA, van den Berg AJ, Tamminga RY, Revesz T, Wulffraat NM, Zegers BJ. Identification of six novel WASP gene mutations in patients suffering from Wiskott–Aldrich syndrome. Hum Mutat. 2000;15(4):386–7.
Villa A, Notarangelo L, Macchi P, Mantuano E, Cavagni G, Brugnoni D, et al. X-linked thrombocytopenia and Wiskott–Aldrich syndrome are allelic diseases with mutations in the WAS gene. Nat Genet. 1995;9(4):414–7.
Wada T, Itoh M, Maeba H, Toma T, Niida Y, Saikawa Y, et al. Intermittent X-linked thrombocytopenia with a novel WAS gene mutation. Pediatr Blood Cancer. 2014;61(4):746–8.
Shvetsova E, Sofronova A, Monajemi R, Gagalova K, Draisma HHM, White SJ, et al. Skewed X-inactivation is common in the general female population. Eur J Hum Genet. 2019;27(3):455–65.
Stewart DM, Candotti F, Nelson DL. The phenomenon of spontaneous genetic reversions in the Wiskott–Aldrich syndrome: a report of the workshop of the ESID Genetics Working Party at the XIIth Meeting of the European Society for Immunodeficiencies (ESID). Budapest, Hungary October 4–7, 2006. J Clin Immunol. 2007;27(6):634–9.
Wada T, Konno A, Schurman SH, Garabedian EK, Anderson SM, Kirby M, et al. Second-site mutation in the Wiskott–Aldrich syndrome (WAS) protein gene causes somatic mosaicism in two WAS siblings. J Clin Invest. 2003;111(9):1389–97.
Ariga T, Yamada M, Pudua FR, Sakiyama Y. Detection of a novel splice-site mutation that results in skipping exon 3 of the WAS gene in a patient with Wiskott–Aldrich syndrome. Biochem Biophys Acta. 1996;1317(3):158–60.
Wu J, Liu D, Tu W, Song W, Zhao X. T-cell receptor diversity is selectively skewed in T-cell populations of patients with Wiskott–Aldrich syndrome. J Allergy Clin Immunol. 2015;135(1):209–16.
Wada T. Revertant somatic mosaicism in primary immunodeficiency diseases. Nihon Rinsho Meneki Gakkai Kaishi. 2014;37(6):447–53.
Galy A, Roncarolo MG, Thrasher AJ. Development of lentiviral gene therapy for Wiskott–Aldrich syndrome. Expert Opin Biol Ther. 2008;8(2):181–90.
Zhang ZY, Xiao HQ, Jiang LP, Zhou Y, Zhao Q, Yu J, et al. Analysis of clinical and molecular characteristics of Wiskott–Aldrich syndrome in 24 patients from 23 unrelated Chinese families. Pediatr Allergy Immunol. 2010;21(3):522–32.
Watanabe Y, Sasahara Y, Ramesh N, Massaad MJ, Looi CY, Kumaki S, et al. T-cell receptor ligation causes Wiskott–Aldrich syndrome protein degradation and F-actin assembly downregulation. J Allergy Clin Immunol. 2013;132(3):648–55.
Park SK, Kim CS, Song DK, Kim JY, Choi IJ, Kim DK. A familial case of Wiskott–Aldrich syndrome with a hotspot mutation in exon 2 of the WAS Gene. J Korean Med Sci. 2007;22(6):998–1001.
Westbury SK, Mumford AD. Genomics of platelet disorders. Haemophilia. 2016;22(5):20–4.
Chan KW, Lee TL, Chung BH, Yang X, Lau YL. Identification of five novel WAS mutations in Chinese families with Wiskott–Aldrich syndrome. Hum Mutat. 2002;20(2):151–2.
Hilbert L, Gaucher C, de Romeuf C, Horellou MH, Vink T, et al. Leu 697–>Val mutation in mature von Willebrand factor is responsible for type IIB von Willebrand disease. Blood. 1994;83(6):1542–50.
Acknowledgements
We would like to thank all members of the study team, the patients and their families. We are grateful to Professor Kunishima Shinji from the department of Medical Technology in Gifu University of Medical Science for the given plasmid pET-01 and valuable advice.
Funding
This study is supported by Hebei Natural Science Foundation of China (H2020206349) and the Research Fund of the Second Hospital of Hebei Medical University of China (2h2019041).
Author information
Authors and Affiliations
Contributions
X.J. and X.H. drafted the manuscript. X.H. and X.J. contributed to the design and acquisition. X.G. and Y.S. acquired and analyzed the data. J.H. and F.M. gave final approval of the version to be published and offered professional guidance. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was carried out in accordance with the ethical standard of the Research Ethics Committee of the Second Hospital of Hebei Medical University. Informed consent was obtained from the patients’ parents, legal guardians and family members before enrollment in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ji, X., Hou, X., Guo, X. et al. Identification of a novel WAS mutation and the non-splicing effect of a second-site mutation in a Chinese pedigree with Wiskott–Aldrich syndrome. Orphanet J Rare Dis 17, 447 (2022). https://doi.org/10.1186/s13023-022-02589-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-022-02589-y