Abstract
Background
The clinical effect of copper accumulation on the heart of patients suffering from Wilson’s disease (WD) is not completely understood. We aimed to determine if patients with WD show signs of cardiac involvement, structural heart disease or autonomic dysfunction. In this prospective trial, we studied 61 patients (mean age 44.3 ± 15.2 years, 51% males) with WD and compared them to 61 age- and gender-matched healthy controls. All subjects underwent clinical examination, blood tests, echocardiography and 24 h electrocardiographic (ECG) recording.
Results
Left- and right ventricular systolic function did not differ significantly between WD patients and controls. However, 5 of the 61 patients had a reduced left ventricular ejection fraction (LVEF). Furthermore, diastolic dysfunction was more prevalent in WD patients (9 of 61 vs. 0 of 61, p = 0.001).
The severity of WD based on the Unified Wilson’s Disease Rating Scale was significantly correlated to NT-pro BNP (r = 0.34, P = 0.013). Patients with an exacerbation of WD in medical history had higher troponin levels compared to those without (11.3 ± 4.7 vs 4.6 ± 1.2).
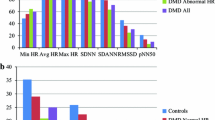
The autonomic function assessed by triangular index (TI) and SDNN-index was significantly reduced in WD patients compared to controls in most in almost every age category (p-value TI and SDNN: age 20–29, p < 0.001 and 0.05; age 30–39, p < 0.01 and not significant (ns); age 40–49, p < 0,01 and 0.001; age 50–59, p = ns and < 0.001, age 60–70, p < 0.05 and ns).
Conclusion
Our data demonstrate that cardiac involvement and autonomic dysfunction in WD is possible, however the underlying cause is still not known. We suggest that patients with signs and symptoms of structural heart disease should be examined by a cardiologist in addition to the interdisciplinary treatment team of WD.
Similar content being viewed by others
Background
Wilson’s disease (WD) is an inherited autosomal recessive disorder resulting from abnormal copper metabolism. It was first described by Kinnier Wilson in 1912. WD leads to reduced copper excretion and causes an excessive deposition of the copper into different organs, including the liver, the central nervous system, the cornea, the kidney and joints [1]. WD is rare,with an averaged incidence in the world of 1:30.000 inhabitants that may reaches significantly higher peaks in some isolated geographic areas [2]. However, relatively little is known about the effects of copper accumulation on the heart. Kaduk et al. described a case of cardiomyopathy in WD. Myocardial copper accumulation was revealed by electron microscope [3]. Furthermore, Factor et al. could demonstrate increased copper values in the myocardium in an autopsy study in 5 of 9 patients suffering from WD [4].
It was recently reported that WD patients have a significantly elevated risk of both atrial fibrillation and heart failure [5], but signs of structural heart disease have not yet been found. Some smaller WD studies, focusing on electrocardiographic changes and standard echocardiographic parameters, showed a mild, non-pathological increase in the thickness of the interventricular septum and the posterior wall [6]. For that reason, cardiac involvement in WD has generally been recognized as benign, but there have been reports in single cases of cardiac-related deaths due to lethal arrhythmias and unknown dilated cardiomyopathy [7]. In a recently published paper, a subtle pre-clinical cardiovascular involvement in WD was described since adolescence, in terms of an abnormal blood pressure response after 3 min of upright position as well as lower ankle-brachial index compared to healthy counterparts [8].
Prospective trials evaluating the underlying cause of arrhythmias and heart failure in WD are still missing. The aim of this study was to comprehensively investigate the signs of cardiac involvement in WD.
Methods
The cardiac manifestation WD study is a prospective clinical and imaging study. From 2016 to 2017, 61 patients suffering from WD were consecutively included in the study. The WD ambulatory clinic at University Hospital Dresden of Technische Universität Dresden, Germany, recruited 34 patients. The remaining 27 patients were recruited from five different neurology departments from all over Germany. The diagnosis of WD was reviewed according to the scoring system provided by the 8th International Meeting on WD and Menkes disease [9].
We selected 61 age- and sex-matched controls without a familial relationship to the patients from our study database. Controls were free from cardiac disease based on clinical examination, cardiac echocardiography, and cardiac magnetic resonance imaging (CMRI). This study was approved by the local ethics committee, and written informed consent was obtained from all participants and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Evaluation of WD severity
The quantification of clinical symptoms was performed in consensus, using the 55-item Unified Wilson’s Disease Rating Scale (UWDRS) [10]. The UWDRS consists of a neurological, a psychiatric, and a hepatic subscale, each one differentiating in anamnestic information from the last two to four weeks and present clinical data. Based on the present clinical status, UWDRS subscores were calculated for different WD typical symptom complexes: dystonia (sum of UWDRS items 11A, 22, 23, 25A, and 26A; range 0–28), dysarthria (item 10; range 0–4), parkinsonism (items 11B, 12, 14–16, 20, 24, 25C, 26C; range 0–76), ataxia (items 25B, 26B; range 0–8), and tremor (items 12, 13, 18, 21; range 0–44).
Laboratory examination
A list of all serum values is found in Table 2 of the supplemental data.
24 h-ECG
While wearing an ambulatory electrocardiograph (ECG, CardioMem® CM 3000, Getemed) for 24 h, patients continued their daily activities and the following parameters were measured from 24 h recordings: mean heart rate, maximal heart rate, minimal heart rate, count of supraventricular and ventricular premature contractions (PSVC, PVC), and disturbance of rhythm (arrhythmia, tachycardia, bradycardia). In our study, the time domain heart rate variability measurements were the mean of the standard deviations of all normal sinus RR intervals for all 5-min segments (SDNN index) and triangular index (TI).
Echocardiography
Conventional echocardiographic studies using standard views and echo techniques, as recommended by the American Society of Echocardiography, were performed for all patients by experienced investigators using an iE33 Echocardiography System (Philips, NL) [11, 12]. From four-chamber and two-chamber views, left ventricular end-diastolic and end-systolic volumes and ejection fraction (LVEF) were measured by the modified Simpson disk method. Additionally, for measurement of the right ventricular (RV) function, we used the tricuspid annular plane systolic excursion (TAPSE), which is an excellent surrogate parameter for the RV systolic function [13]. Left ventricular (LV) diastolic function was assessed by Doppler analysis.
Statistical analysis
SPSS 20.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analyses. The results are presented as mean and standard deviation, as median with lower and upper quartiles (25th and 75th percentiles), or as percentages. For group comparisons, independent samples t-test and Wilcoxon signed-rank test were used. Correlations were evaluated with the Pearson’s correlation coefficient. 24 h-ECG recording were not available from controls. For statistical comparison with healthy individuals we used the data from Bonnemeier et al [14].
Results
The mean age for the WD patients and the control patients was 44.3 years (31 men, 30 women) and 45.7 years (31 men, 30 women), respectively. An overview of the demographic data and clinical characteristics of the 61 examined WD patients and controls is presented in Table 1. WD specific data, including duration of illness, phenotype, incidence of exacerbation, liver fibrosis grading, liver transplantation, and medical treatment, are displayed in Additional file 1: Table S1.
Echocardiography
The echocardiography data are shown in Table 2. There were no differences between the groups with respect to LVEF (p = 0.382). Five of 61 patients had a reduced left ventricular ejection fraction (LVEF) < 50%. In patients with depressed LVEF, coronary heart disease was excluded by cardiac computed tomography angiography.
Systolic right ventricular (RV) function, assessed by tricuspid annular plane systolic excursion (TAPSE) (p = 0.15), didn’t significantly differ between both groups. Pulmonary hypertension was not detectable in any of the WD patients. However, nine of 61 patients had a diastolic dysfunction grade I, which is significantly more frequent than in controls (p = 0.001). Mild valve regurgitation was a common finding in patients with WD, however no statistical significance (p = 0.26) to controls was obvious. None of the patients showed valve stenosis.
Laboratory examinations
Laboratory data are shown in the Additional file 1: Table S2.
UWDRS and exacerbation
We could detect no correlation between UWDRS and LVEF or TAPSE. However, a moderate significant correlation between UWDRS and NT-Pro BNP was obvious (Additional file 1: Table S3).
Eighteen patients had an acute exacerbation within the disease process. Four of them had elevated troponin levels. None of the patients without an exacerbation showed elevated cardiac biomarkers. Patients with an exacerbation of WD in medical history had higher troponin levels compared to those without (11.3 ± 4.7 vs 4.6 ± 1.2, p < 0.001).
At the time of cardiac imaging, one of these patients suffered from an acute exacerbation with elevated cardiac troponin. After successful de-coppering troponin levels decreased significantly (from 46 ng/L to 16 ng/L).
24 h electrocardiographic recording
The majority of WD patients showed a sinus rhythm (56 patients, 91.8%). Three patients had an ectopic atrial rhythm; one patient had atrial flutter; and in one patient a high-grade atrioventricular block was detectable, which required permanent pacemaker implantation. Premature supraventricular and ventricular ectopic contractions were a common finding in WD patients (50 patients with PSVC, 30 patients with PVC). On average, 226.7 PSVC (range 0;3979) and 216.3 PVC (range 0;6727) were seen, but we could not detect any potentially fast malignant arrhythmias.
Autonomic function
The autonomic function assessed by triangular index (TI) and SDNN-index (SDNNi) is age dependent as described by Bonnemeier et al. which we refer to concerning our normal values [14]. Therefore, Online Additional file 1: Figure S1 and S2 show a comparison of autonomic function parameters on the basis of age. With the exception of patients and controls in the 50–59 year old group, TI was significantly reduced in WD patients. In addition, SDNNi showed significantly lower values in the 40–49 and 50–59 years old age categories.
Discussion
To date, this is the largest prospective clinical and imaging study evaluating a cardiac manifestation of patients suffering from WD. We found that signs of structural heart disease with reduced LVEF were obvious in 5 of the 61 patients.
Furthermore, patients who are severely affected by WD have higher NT-pro BNP levels and patients with an exacerbation in medical history have higher troponin than those without.
WD is an inherited autosomal recessive disease, characterized by an inability of the
liver to excrete copper in the bile, resulting in excessive copper deposition in the body, primarily in the liver and the brain [1]. Copper toxicity to the liver has been demonstrated in several models. The generally cited mechanism of pathology development in WD involves oxidative damage due to copper overload and intramitochondrial membrane crosslinking that culminates in mitochondrial destruction and liver failure [15, 16].
There is still no clear evidence of how and to what extent excessive copper accumulations leads to cardiac dysfunction. Cardiac involvement in WD was first described by Kuan [7] in 1987. He followed-up with a cohort of 53 WD patients. During that period, one patient died due to cardiac heart failure and another one due to repeated ventricular fibrillations. Several studies [5, 17, 18] have reported a broad spectrum of electrocardiographic findings without any clear, unified cardiac diagnosis. A mildly increased QRS in WD patients (92 ms versus 87.5 ms in controls), a prolongation of P-wave dispersion, and a higher risk for atrial fibrillation were reported. In our study, we used 24 h Holter monitoring to detect rhythm disturbances. The majority of our patients had supraventricular ectopic beats (84%) and about half of the WD patients had premature ventricular contractions, but the clinical relevance of these findings is difficult to evaluate. Premature ventricular contractions are common and have been described in 40–69% of healthy low-risk individuals as detected by 24 h ambulatory ECG recordings [19, 20]. Traditionally, PVC have been thought to be relatively benign in the absence of structural heart disease, but they also represent increased risk of ventricular dysfunction, sudden death, and all-cause mortality [21,22,23]. The presence of fibrotic tissue is considered to be the basis for ventricular arrhythmias, which can be also detected in WD patients [24]. In an autopsy study, Factor et al. [4] found evidence of interstitial and replacement fibrosis and focal inflammatory cell inflammation to a variable degree in all of the nine cases studied. This might explain why PVC burden in our cohort was increased compared to the aforementioned studies. Therefore, we conclude that premature ventricular contractions are a sign of copper toxicity to the heart.
Mildly increased heart rate in Wilson’s disease was found in several studies, but the underlying mechanisms remained unknown [25]. One possible explanation might be a decreased HRV, which was observed in our WD patients. Depressed HRV was confirmed to be a clear predictor of heart failure and over- all mortality in several studies [26, 27]. However, the clinical implication remains unclear. The underlying mechanism between decreased HRV in the cited studies and WD patients might be different. Autonomic dysfunction in heart failure patients is mainly due to small fiber dysfunction of the peripheral nervous system [28]. Wilson’s disease mainly involves the central nervous system rather than the peripheral nervous system [29]. The brain structures commonly involved in Wilson’s disease contain important autonomic centers. In a study by Chu et al., the sympathetic central conduction time was prolonged in the Wilson’s disease patients, which favors a central mechanism [30].
A recently published longitudinal cohort study revealed that patients suffering from WD exhibited a statistically significant 55% increased risk of heart failure (HF) [5]. Because the study design lacked cardiac imaging, the authors could only speculate about an underlying cause. Before this current study describing 61 patients, the literature regarding cardiac imaging of WD has been restricted to a total of 42 in adults. Hlubocka et al. [6] showed that patients with WD had a mildly increased thickness of the interventricular septum and the posterior wall in comparison to healthy subjects, but without a difference of LV mass.
It remains unclear how WD patients develop heart failure, as recently shown by Grandis et al. [5]
In our cohort 5 of the 61 patients had a mildly reduced LVEF. Whether this is due to copper toxicity is still a matter of speculation. The mechanism might be a result of multiple issues. A correlation of the LVEF to the severity of illness assessed by the UWDRS or WD exacerbation in medical history was not obvious. However, patients with an exacerbation in medical history had higher troponin than those without, which might point to a copper related toxicity and inflammation to the myocardium. This hypothesis is supported by the fact, that in one patient suffering from acute exacerbation adequate medical therapy and successful de-coppering was able to decrease elevated troponin levels and thus reduce the effect of copper-overload inflammation.
According to previous mentioned data of Grandis et al., patients with WD who developed HF had a mean age of 66 ± 18 years old. In comparison, the mean age of our cohort was 46 years, indicating that the detected structural changes may become clinically relevant much later.
Limitations
Some limitations of our study should be acknowledged. The majority of our cohort, mostly stabilized symptomatic patients or asymptomatic subjects, are treated for WD. We hypothesize that this is the reason we only detected minor cardiac manifestations of WD. The cardiac involvement could be more obvious in an untreated cohort. Second, it would be favorable to perform cardiac magnetic resonance imaging in WD patients, especially in those patients with reduced systolic function. This way, subclinical minor changes, e.g. fibrosis or inflammatory response due to copper deposition, could be detected.
Conclusion
As previous data and our study demonstrate, WD has a cardiac involvement. Although heart and vessels are not the main target of WD, being liver and brain those mainly involved, the detection of this unique population, potentially predisposed to cardiovascular accidents suggests to enhance strategies of primary prevention. Therefore, we suggest that patients with signs and symptoms of structural heart disease should undergo further cardiac examination in addition to the interdisciplinary treatment team of WD.
Abbreviations
- CHD:
-
Coronary heart disease
- CMRI:
-
Cardiac magnetic resonance imaging
- ECG:
-
Electrocardiography
- EDD:
-
End
- EDD:
-
End-diastolic diameter
- EF:
-
Ejection fraction
- ESD:
-
End-systolic diameter
- HF:
-
Heart failure
- IVST:
-
Interventricular septal thickness
- LV:
-
Left ventricle
- PSVC :
-
Premature supraventricular ventricular contractions
- PVC :
-
Premature ventricular contractions
- PWT:
-
Posterior wall thickness
- RV:
-
Right ventricle
- SD:
-
Standard deviation
- SDNN:
-
Standard deviation of the normal to normal intervals
- TAPSE:
-
Tricuspid annular systolic excursion
- TI:
-
Triangular index
- UWDRS:
-
Unified Wilson’s Disease Rating Scale
- WD :
-
Wilson’s disease
References
Rosencrantz R, Schilsky M. Wilson disease: pathogenesis and clinical considerations in diagnosis and treatment. Semin Liver Dis. 2011;31(3):245–59.
Gialluisi A, Incollu S, Pippucci T, Lepori MB, Zappu A, Loudianos G, et al. The homozygosity index (HI) approach reveals high allele frequency for Wilson disease in the Sardinian population. Eur J Hum Genet. 2013;21(11):1308–11.
Kaduk B, Metze K, Schmidt PF, Brandt G. Secondary athrocytotic cardiomyopathy--heart damage due to Wilson's disease. Virchows Arch A Pathol Anat Histol. 1980;387(1):67–80.
Factor SM, Cho S, Sternlieb I, Scheinberg IH, Goldfischer S. The cardiomyopathy of Wilson's disease. Myocardial alterations in nine cases. Virchows Arch A Pathol Anat Histol. 1982;397(3):301–11.
Grandis DJ, Nah G, Whitman IR, Vittinghoff E, Dewland TA, Olgin JE, et al. Wilson’s disease and cardiac myopathy. Am J Cardiol. 2017;120(11):2056–60.
Hlubocka Z, Marecek Z, Linhart A, Kejkova E, Pospisilova L, Martasek P, et al. Cardiac involvement in Wilson disease. J Inherit Metab Dis. 2002;25(4):269–77.
Kuan P. Cardiac Wilson's disease. Chest. 1987;91(4):579–83.
Kouvelas G, PP B, Pisano V, Demelia L, Nurchi A, Mercuro G. Premature vascular deterioration in young patients affected by Wilson’s disease: a pilot study. J Pediatr Neonat Individual Med. 2017;6(2):e060201.
Ferenci P, Caca K, Loudianos G, Mieli-Vergani G, Tanner S, Sternlieb I, et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 2003;23(3):139–42.
Leinweber B, Möller JC, Scherag A, Reuner U, Günther P, Lang CJ, et al. Evaluation of the unified Wilson's disease rating scale (UWDRS) in German patients with treated Wilson's disease. Mov Disord. 2008;23(1):54–62.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63.
Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA, Echocardiography DQTFotNaSCotASo. Recommendations for quantification of Doppler echocardiography: a report from the Doppler quantification task force of the nomenclature and standards Committee of the American Society of echocardiography. J Am Soc Echocardiogr. 2002;15(2):167–84.
Speiser U, Hirschberger M, Pilz G, Heer T, Sievers B, Strasser RH, et al. Tricuspid annular plane systolic excursion assessed using MRI for semi-quantification of right ventricular ejection fraction. Br J Radiol. 2012;85(1017):e716–21.
Bonnemeier H, Richardt G, Potratz J, Wiegand UK, Brandes A, Kluge N, et al. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variability. J Cardiovasc Electrophysiol. 2003;14(8):791–9.
Burkhead JL, Gray LW, Lutsenko S. Systems biology approach to Wilson's disease. Biometals. 2011;24(3):455–66.
Zischka H, Lichtmannegger J, Schmitt S, Jagemann N, Schulz S, Wartini D, et al. Liver mitochondrial membrane crosslinking and destruction in a rat model of Wilson disease. J Clin Invest. 2011;121(4):1508–18.
Arat N, Kacar S, Golbasi Z, Akdogan M, Sokmen Y, Kuran S, et al. P wave dispersion is prolonged in patients with Wilson's disease. World J Gastroenterol. 2008;14(8):1252–6.
Meenakshi-Sundaram S, Sinha S, Rao M, Prashanth LK, Arunodaya GR, Rao S, et al. Cardiac involvement in Wilson’s disease--an electrocardiographic observation. J Assoc Physicians India. 2004;52:294–6.
von Rotz M, Aeschbacher S, Bossard M, Schoen T, Blum S, Schneider S, et al. Risk factors for premature ventricular contractions in young and healthy adults. Heart. 2017;103(9):702–7.
Kostis JB, McCrone K, Moreyra AE, Gotzoyannis S, Aglitz NM, Natarajan N, et al. Premature ventricular complexes in the absence of identifiable heart disease. Circulation. 1981;63(6):1351–6.
Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, et al. Ventricular Ectopy as a predictor of heart failure and death. J Am Coll Cardiol. 2015;66(2):101–9.
Ataklte F, Erqou S, Laukkanen J, Kaptoge S. Meta-analysis of ventricular premature complexes and their relation to cardiac mortality in general populations. Am J Cardiol. 2013;112(8):1263–70.
Lee V, Hemingway H, Harb R, Crake T, Lambiase P. The prognostic significance of premature ventricular complexes in adults without clinically apparent heart disease: a meta-analysis and systematic review. Heart. 2012;98(17):1290–8.
Oebel S, Dinov B, Arya A, Hilbert S, Sommer P, Bollmann A, et al. ECG morphology of premature ventricular contractions predicts the presence of myocardial fibrotic substrate on cardiac magnetic resonance imaging in patients undergoing ablation. J Cardiovasc Electrophysiol. 2017;28(11):1316–23.
Netto AB, Sinha S, Taly AB, Panda S, Rao S. Sleep in Wilson’s disease: a polysomnography-based study. Neurol India. 2010;58(6):933–8.
Galinier M, Pathak A, Fourcade J, Androdias C, Curnier D, Varnous S, et al. Depressed low frequency power of heart rate variability as an independent predictor of sudden death in chronic heart failure. Eur Heart J. 2000;21(6):475–82.
La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107(4):565–70.
Vinik AI, Erbas T, Casellini CM. Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J Diabetes Investig. 2013;4(1):4–18.
Jung KH, Ahn TB, Jeon BS. Wilson disease with an initial manifestation of polyneuropathy. Arch Neurol. 2005;62(10):1628–31.
Chu EC, Chu NS, Huang CC. Autonomic involvement in Wilson’s disease: a study of sympathetic skin response and RR interval variation. J Neurol Sci. 1997;149(2):131–7.
Acknowledgements
“Not applicable”.
Funding
“Not applicable”.
Availability of data and materials
All data generated and analyzed during this study are included in this published article and its supplementary information files.
Author information
Authors and Affiliations
Contributions
SQ: manuscript preparation, data analysis, statistical analysis. UR: neurological examination, proof reading manuscript. MW: patients recruitement, echocardiography, neurologiocal examination. CH: data collection, echocardiography. FMH: manuscript preparation, echocardiography, patients recruitement. KMS: echocardiography, data collection, CM: statistical analysis, cardiac examination, 24 h -ECG analysis. KI: statistical analysis, cardiac examination, 24 h -ECG analysis. AL: manuscript review. US: manuscript review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the local ethics committee of the Technische University of Dresden (Ethics approval from 20.10.2015, EK 408092015), and written informed consent was obtained from all participants and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Consent for publication
“Not applicable”.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1 Clinical characteristics of the WD patients. Table S2 Laboratory characteristics of the studied WD patients. Table S3 Association between UWDRS and clinical characteristics. Figure S1. Comparison of SDNN-Index of WD patients and controls. *** P < 0.001. Figure S2. Comparison of Triangular Index of WD patients and controls. *** P < 0.001, ** P = 0.005, * P = 0.05. (ZIP 45 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Quick, S., Reuner, U., Weidauer, M. et al. Cardiac and autonomic function in patients with Wilson’s disease. Orphanet J Rare Dis 14, 22 (2019). https://doi.org/10.1186/s13023-019-1007-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-019-1007-7