Abstract
Background
Early chest tube removal should be considered to enhance recovery after surgery. The current study aimed to provide a predictive algorithm for air leak episodes (ALE) and to create a knowledge base for early chest tube removal.
Methods
This retrospective study enrolled patients who underwent thoracoscopic anatomical pulmonary resections in our unit. We defined ALE as any airflow ≥ 10 mL/min recorded in the follow-up charts based on the digital thoracic drainage device. Multivariate regression analysis was used to control for preoperative and intraoperative confounding factors. The ALE prediction algorithm was constructed by combining an additive ALE risk-scoring system using the coefficients of the significant predictive factors with the intraoperative water-sealing test.
Results
In 485 consecutive thoracoscopic major pulmonary resections, ALE developed in 209 (43%) patients. Statistically significant ALE-associated preoperative factors included male sex, lower body mass index, radiologically evident emphysema, lobectomy, and upper lobe surgery. Significant ALE-associated intraoperative factors were incomplete fissure and pleural adhesion. The ALE risk scoring demonstrated an average area under the receiver operating characteristic curve of 0.72 in the fivefold cross-validation test. The ALE prediction algorithm correctly predicted ALE-absent patients at a negative predictive value of 80%.
Conclusions
The algorithm may promote the optimization of the chest tube-dwelling duration by identifying potential ALE-absent patients for accelerated tube removal.
Similar content being viewed by others
Background
The placement of chest drainage tubes after pulmonary resections has a negative impact on postoperative chest pain and ventilatory function [1]. Surgeons should consider removing the chest tubes as early as possible to encourage early mobilization, reduce opioid analgesic requirements, and prevent potential complications to enhance recovery after surgery [2]. Hence, tube removal on the day of surgery is ideal. A few institutes have reported the safety and benefits of early drain removal procedures in selected patients who underwent anatomical lung resection [3, 4]. However, most surgeons still prefer conservative drainage management and keep the chest tubes in place, at least until the day after the lobectomy or segmentectomy. This tendency is largely due to their concerns regarding potential risks, such as postoperative pulmonary fistula development.
Prolonged air leak (PAL) lasting 5–7 days after surgery is a common complication of pulmonary resections. To date, many studies have described the frequency of PAL occurrence and its associated risk factors, including smoking, limited pulmonary function, comorbidities, lower body mass index (BMI), upper lobe surgery, and the presence of pleural adhesion [5]. Accumulated data associated with PAL has provided evidence to reasonably predict its development [6] with an accuracy of a certain degree [7] and appropriately manage this complication [8, 9].
Meanwhile, little attention has been given to air leak episodes (ALE), defined as any air leak event after lung surgery. Although ALE is not a postoperative complication, we believe that the prediction of ALE development would provide valuable information and help further support the concept of accelerated drain removal. Thus, the present study aimed to provide an ALE prediction algorithm to discern candidates for chest tube removal immediately after surgery. First, we revealed the frequency and patterns of ALE development associated with thoracoscopic lobectomy and segmentectomy. Quantitative air leakage information was obtained from the digital thoracic drainage system to detect latent ALE development. Subsequently, we constructed the ALE prediction algorithm consisting of two evaluation steps: the pre-resection assessment based on the ALE risk-scoring system and the post-resection assessment based on the routine intraoperative water-sealing test.
Methods
Study patients
Consecutive patients who underwent thoracoscopic (video-assisted thoracic surgery [VATS] or robot-assisted thoracic surgery [RATS]) anatomical pulmonary resections, including lobectomy and segmentectomy, from January 1, 2015, through December 31, 2021, in our institute were enrolled in this retrospective study (Fig. 1A). Exclusion criteria were bilateral lung surgery (n = 1), extended lung resection beyond the scope of standard lobectomy or segmentectomy (n = 1 for combined lobectomy and segmentectomy), postoperative tracheal intubation management (n = 1), and the use of a conventional 3-bottle chest drainage device (n = 1). Patients with incomplete clinical information were excluded from the following analysis (n = 9). This study was approved by the institutional review board of St. Luke’s International Hospital (21-R177). The requirement for written informed consent was waived, and the opt-out method was used.
Data collection
Preoperative factors collected from electronic medical records included baseline clinical features, serum albumin concentration, preoperative pulmonary function test results, and the radiological diameter of lung tumors (including ground-glass sections) recorded before the initiation of the study. Additionally, surgical information, including the affected lobe and procedure, and intraoperative findings (e.g., adhesion, incomplete fissure, and intraoperative water-sealing test results) were extracted from the surgical records. In addition, all serial records of volumetric air leak flow, which had been measured by the digital thoracic drainage device (Thopaz®, Medela, Inc., Baar, Switzerland) and documented at each medical check-up timepoint, were gathered to identify ALE occurrence.
Operative procedures
Thoracoscopic surgery in this study refers to thoracoscopic lung resection performed through a maximum of an 8 cm (typically 4–5 cm) utility incision plus two to four (typically two) smaller accessory ports. Interlobar and intersegmental lines were resected solely using staplers to minimize air leaks. This principle was also applied to the division of lung parenchyma near the hilum in segmentectomy; these procedures did not usually involve electro-sections along intersegmental veins unless electro-sections were required from the technical or oncological viewpoints. Intersegmental lines were identified primarily by the bronchoscopic multi-spot dye-marking technique (Virtual Assisted Lung Mapping [VALMAP] [10]) for invisible lesions.
The water sealing test was manually performed by gradually increasing the intra-tracheal pressure up to 25 cm H2O. The test result was positive when continuous air bubbles were detected at any leak point, including the bronchiole fistula, lung parenchyma lacerations, and stapler lines. Fibrin glue mist was sprayed on the dissected lung surface when substantial bubbling was evident during the water submersion test, severe emphysema was present, or surgeons anticipated air leaks.
Postoperative management
The digital thoracic drainage device (Thopaz®) was used for postoperative chest drainage. The “regulated” Thopaz® suction pressure was typically set to − 8 cm H2O, which is considered equivalent to physiological pressure. The chest tubes were removed when the air leak was ≤ 20 mL/min at − 8 cm H2O for 8 h. This removal criterion was set according to a previous study [11] and has been adopted throughout the study period in our department. The indication for pleurodesis was continuous, non-declining air leaks lasting more than three days. Blood patch procedures were first applied one to three times. If the air leaks did not cease or decline, we used OK-432. When pleurodesis was ineffective, reoperation was performed. There were no cases where endobronchial embolization was performed.
Outcomes
The primary outcome was the incidence of ALE. We defined ALE as any airflow ≥ 10 mL/min recorded in the follow-up charts. The details of the chart recording are described below. The secondary outcome was the incidence of PAL, defined as an air leak lasting ≥ 5 days after surgery.
ALE measurement
Well-trained nurses in the thoracic surgery department recorded flow volume trends observed in 2–5 min periods during routine medical check-ups based on the measurements of the digital thoracic drainage device. Leak volume checks were typically done on arrival at the ward, every 1 h for the first 4 h post-surgery, every 2 h until the morning of postoperative day (POD) 1, and every 6 h after that with patients in a supine position.
Statistical analysis
Univariate and multivariate logistic regression analyses were used to estimate the odds ratio (OR) of the incidence of ALE. Welch’s t-test, Mann–Whitney U-test, and Fisher’s exact test were performed to assess the null assumption of equality of continuous variables, time variables, and the independence of categorical variables, respectively. Statistical significance was set at an alpha level of 0.05.
The ALE prediction algorithm was composed of two steps: (1) Pre-resection assessment with an ALE scoring system and (2) post-resection assessment with a water-sealing test (Fig. 1B). (1) The ALE scoring was formulated using the coefficients of the significant predictive factors in the multivariate regression analysis. First, continuous variables were discretized at the threshold obtained by approximating the “top left” value of the best ALE-predictive ability in the univariate receiver operating characteristic (ROC) curve plot (e.g., BMI < 22 vs. BMI ≥ 22). Second, the coefficients of the multivariate regression model were rounded to a 0.5-increment number to obtain the corresponding scores. Those scores were summed up to calculate the total ALE risk score; then, a threshold was set to the “top left” value of the best diagnostic ability on the ROC curve. (2) The ALE prediction algorithm was then constructed by integrating the ALE risk-scoring system and water-sealing test results. Analyses were performed using the R statistical software (R Foundation for Statistical Computing, Vienna, Austria. Version 3.6.3). All codes used above are available via https://github.com/Kuniyo-Sueyoshi/PoAL.
Results
A total of 485 patients (primarily male, 56%) with a mean age of 66 years (standard deviation [SD], 11) were included in the analysis (Fig. 1A, Table 1). Primary lung carcinoma (92%) with a relatively small radiological gross diameter (2.0 cm on average) was the predominant diagnosis in this cohort. The proportion of surgical procedures performed (50% for segmentectomy vs. 50% for lobectomy), and the proportion of the surgical lobe location (58% for the upper lobe vs. 42% for the other lobes), were well balanced. Thirty-three patients (6.8%) required pleurodesis to manage their postoperative pleural fistula (Table 2). PAL (> 5 days) developed in 13 patients (2.7%). The chest drain was removed on the 1.7th POD on average (median 1, IQR 1) across all cases. The mean length of hospital stay was 5.0 days (median 4, IQR 2). The median interval between each leak flow record was 135.0 min (1st and 3rd IQR, [88.7, 196.5]).
Two hundred and nine patients (43%) developed ALE at any point after recovery from anesthesia, while the other 276 (57%) demonstrated no air leak up to chest tube removal (Table 1). ALE occurred from the middle of the time course in some cases. For example, more than 25% of the ALE-present patients developed ALE at 6 h or later for the first time (Fig. 2A). Meanwhile, ALE duration varied among patients; 52% of the ALE-present patients had any air leakage after 20 h in the postoperative course. (Fig. 2B). In a univariate analysis, patients who developed ALE were older, more likely to be male, had a lower BMI, a history of tobacco use, emphysema evident on computed tomography findings, and a larger tumor size (Table 1). Patients in the ALE-present group predominantly underwent lobectomy and upper lobe surgery. Intraoperatively, adhesions and incomplete fissures were likely to be observed. No significant differences were noted in the percent predicted forced expiratory volume in one second, diabetes mellitus prevalence, history of intravascular diseases including cardiovascular and cerebral vascular disease, preoperative serum albumin concentration, surgeons, and surgery year-trends between the two groups. Multivariate logistic regression analysis demonstrated that significant preoperative factors associated with the development of ALE were male sex (OR 2.2, CI 1.3–3.6), lower BMI (OR 1.2, CI 1.1–1.3), radiologically evident emphysema (OR 2.0, CI 1.1–3.6), lobectomy (OR 2.9, CI 1.8–4.6), and upper lobe surgery (OR 1.7, CI 1.1–2.6) (Table 3). Significant intraoperative factors associated with ALE development were adhesion (OR 2.9, CI 1.4–6.7) and incomplete fissure (OR 1.9, CI 1.3–3.0).
Patterns of ALE development. A Timepoints of ALE development documented for the first time after surgery in ALE-present cases. B Timepoints of ALE cessation after surgery in ALE-present cases. Vertical dashed lines denote the 1st, 2nd, and 3rd quantile positions of the histograms of cases (A) and cumulative curve of cases (B)
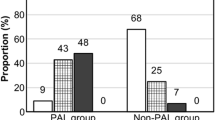
To explore the negative association of segmentectomy with ALE development (Table 3), which was somewhat counterintuitive, we further stratified the cohort by the results of the post-resection water sealing test (Additional file 1: Fig. S1A). The leak cessation rate, defined as the proportion of ALE-absent cases in the water-bubble test-positive subgroup, was significantly higher after segmentectomy than after lobectomy. Likewise, the leak commencement rate, the proportion of ALE-present cases in the water-bubble test-negative subgroup, was significantly higher after lobectomy (Additional file 1: Fig. S1B). Polyglycolic acid sheets were predominantly used in segmentectomy (p < 0.05), but there was no significant imbalance of surgeons, year trend, or fibrin glue between the two procedures.
To construct an easy-to-use algorithm for ALE prediction, we first calculated an ALE risk score based on the coefficients of the multivariate regression model (Fig. 3A). The ALE risk scoring system exhibited a mean area under the curve of 0.72 (SD 0.06) in a five-fold cross-validation test, which was stable concerning the random partitioning procedures into training and test sub-cohorts (Fig. 3B). Subsequently, we assembled the representative scoring system and water-sealing test results to construct an ALE prediction algorithm (Fig. 3C). The algorithm categorized 166 patients into the ALE-low-risk group, a candidate cohort for early drain removal. Of them, 132 patients were actual ALE-absent cases (a negative predictive value of 80%, Fig. 3D) with a mean drain removal of 1.1 days (Median 1, IQR 0) (Table 4). The remaining 34 patients, incorrectly categorized into the ALE-low-risk group, did not develop PAL nor require pleurodesis procedures (Table 4). In addition, the maximum leak flow rate was 10–20 mL/min in 67% and ALE ceased within 4 h in nearly 40% of this false-negative group (Fig. 3E).
ALE prediction. A A representative ALE scoring model developed in a fivefold cross-validation method. B ROC curve of the ALE risk scoring system (left) and a box plot showing its performance in fivefold cross-validation (right). C ALE prediction algorithm and patient flow. D The confusion matrix of the ALE prediction algorithm illustrated in (C). A dagger † denotes the true negative cohort, while double daggers ‡ denotes the false negative cohort. E Maximum leak flow (top) and leak duration time (bottom) in the false negative (FN) cohort†. AUC, area under the curve. ALE risk score, the total score of the ALE scoring model (A). npv, negative predictive value
Discussion
Literature has suggested a positive impact of early chest drain removal on patient experience, analgesic requirement and use, and postoperative physical function [1, 12]. Hence, there is an increasing interest in minimal drainage management to enhance recovery after surgery [13, 14]. A few institutes follow workflows to remove chest tubes on the day of lung surgery by repeatedly checking whether any pleural fistula exits during or after surgery [3, 4]. However, air leaks can occur in varied patterns: intermittent, variable [15], and delayed (Fig. 1). This uncertainty hampers, at least in part, surgeons’ pursuit of accelerated chest tube removal.
In the present study, we integrated a pre-resection scoring system and water-sealing test results to enhance the prediction value. The algorithm correctly predicted 132 patients as ALE-absent with a true negative rate of 80%, who could have their chest tubes removed immediately after surgery from the viewpoint of air leak management. Here, we adopted the stringent criteria of ALE, any airflow ≥ 10 mL/min, to minimize the potential risks of thoracic tube re-insertion in case the algorithm incorrectly predicted ALE-absent patients. A clinically valid threshold for chest tube removal is reported to be more pliable, ranging from 0 to 50 mL/min over 4–8 h [16]. In this regard, it would be notable that the maximum leak flow rate was 10–20 mL/min in 67% and that ALE ceased within 4 h in nearly 40% of our 34 false negative cases (Fig. 3E), suggesting the margin of safety in our algorithm is practically larger than the false negative rate of 20%. Therefore, it may be safe and reasonable to withdraw the chest tube on the day, particularly for patients who undergo surgery in the morning, if they meet the criteria. However, the validity of the algorithm should be evaluated in prospective studies in the future.
There is substantial discordance between water-sealing test results and ALE occurrence (Table 2). This discrepancy may be partly attributed to cough-associated air leaks that start immediately after coughing at extubation, leading to pleural laceration caused by rapidly increased intratracheal pressure. The cough-related ALE may be suppressed by removing trigger stimuli, such as gentle recovery from anesthesia or the use of supraglottic airway devices at extubation [17]. Meanwhile, approximately 25% of air leaks started 6 h or more after surgery (Fig. 1). This cohort needs to be correctly predicted as potential air leak-positive cases. It is also important not to miss air bubbles during the water-submersion test, as inflamed lungs narrow the thoracoscope view, leading to inadequate examination for ALE.
Several ALE-associated factors, including male sex, lower BMI, emphysema, and upper lobe surgery, were noted in this study (Table 3). Those are well-known PAL-related risk factors reported in previous studies [18,19,20], in line with the ALE’s definition that includes PAL. Interestingly, segmentectomy was one of the negative predictive factors for ALE events (Table 3). The higher ALE cessation rate and lower ALE commencement rate (Additional file 1: Fig. S1B) also support the robustness of the predictive value. This is somewhat counterintuitive, considering that more lung parenchyma resection is needed in procedures involving a segmentectomy rather than a lobectomy. However, varied outcomes have been reported regarding PAL incidence associated with those procedures in propensity-matching studies [21, 22] and safety analysis of a randomized control study [23]. A possible advantage of segmentectomy, if any, is the remnant lung volume in the thoracic cavity. The remaining lobes could be placed against the remaining segments, which may patch over minor pleural lacerations or fix the lung position, preventing weak points of the pleura from being torn by tension, as discussed by Berg et al. [22]. This “remnant lung” hypothesis is concordant with the lower leak incidence in the right middle lobectomy than other lobe lobectomies [24, 25], in the points of the longer parenchymal resection lines, larger volume of remaining lungs, and relative position of the resected lung. In addition, our procedure to use staplers for intersegmental line divisions instead of electrocautery needs to be considered to discuss the surgical procedure factor (see the “Method” section). However, it should be noted that the causality between surgical procedures and ALE was not tested in this study.
There were several limitations to our study design. First, the volume of air leaks was not recorded continuously but intermittently during medical check-ups. Also, inter-observer variations in the airflow records exist due to the different observation durations. A thorough ALE assessment can be conducted using continuous leak data digitally recorded in Thopaz®’s memory. Second, selection bias, owing to the retrospective nature, should be considered. We incorporated the major confounders into the regression models, but we acknowledge that some covariates, such as surgeon's preference, probably remain unadjusted. For example, we omitted the fibrin glue factor from our model because unassessed co-founders, such as surgeons' anticipation of air leaks, probably overwhelmed the fibrin’s ability as sealants, leading to the odds ratio over one (Table 2). Third, drained fluid volume was not assessed, which could impede chest tube removal. Finally, the validation of our ALE prediction algorithm was not sufficient. Although the scoring system exhibited stability in the five-fold cross-validation test (internal validation), we need to plan prospective studies to put the concept of accelerated tube removal into practice.
Conclusions
We investigated the development of ALE. Our ALE prediction algorithm performed well, although it is yet to be verified using prospectively accumulated data. Accumulating quantitative data of ALE would promote the construction of theoretical frameworks to help surgeons select cases suitable for early tube removal.
Availability of data and materials
The data underlying this article will be shared on reasonable request to the corresponding author with appropriate anonymization. All codes used for analysis are available via https://github.com/Kuniyo-Sueyoshi/PoAL.
Abbreviations
- ALE:
-
Air leak episode
- PAL:
-
Prolonged air leak
- ERAS:
-
Enhanced recovery after surgery
- VATS:
-
Video-assisted thoracic surgery
- RATS:
-
Robot-assisted thoracic surgery
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the ROC curve
- POD:
-
Postoperative day
- FEV1.0 :
-
The percent predicted forced expiratory volume in one second
- BMI:
-
Body mass index
- DM:
-
Diabetes mellitus
- IVD:
-
Intravascular diseases
References
Refai M, Brunelli A, Salati M, Xiumè F, Pompili C, Sabbatini A. The impact of chest tube removal on pain and pulmonary function after pulmonary resection†. Eur J Cardiothorac Surg. 2012;41(4):820–3.
Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, Gonzalez M, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 2019;55(1):91–115.
Murakami J, Ueda K, Tanaka T, Kobayashi T, Kunihiro Y, Hamano K. The validation of a no-drain policy after thoracoscopic major lung resection. Ann Thorac Surg. 2017;104(3):1005–11.
Pfeuty K, Lenot B. Early postoperative day 0 chest tube removal using a digital drainage device protocol after thoracoscopic major pulmonary resection. Interact Cardiovasc Thorac Surg. 2020;31(5):657–63.
Rivera C, Bernard A, Falcoz PE, Thomas P, Schmidt A, Bénard S, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg. 2011;92(3):1062–8.
Gonzalez M, Karenovics W, Bédat B, Forster C, Sauvain MO, Triponez F, et al. Performance of prolonged air leak scoring systems in patients undergoing video-assisted thoracoscopic surgery segmentectomy. Eur J Cardiothorac Surg. 2022;62(3):ezac100.
Zaraca F, Pipitone M, Feil B, Perkmann R, Bertolaccini L, Curcio C, et al. Predicting a prolonged air leak after video-assisted thoracic surgery, is it really possible? Semin Thorac Cardiovasc Surg. 2021;33(2):581–92.
Zaraca F, Brunelli A, Pipitone MD, Abdellateef A, Abu Akar F, Augustin F, et al. A Delphi Consensus report from the “Prolonged Air Leak: a Survey” study group on prevention and management of postoperative air leaks after minimally invasive anatomical resections. Eur J Cardiothorac Surg. 2022;62(3):ezac211.
Dezube AR, Dolan DP, Mazzola E, Kucukak S, De Leon LE, Bueno R, et al. Risk factors for prolonged air leak and need for intervention following lung resection. Interact Cardiovasc Thorac Surg. 2021;34(2):212–8.
Sato M, Kobayashi M, Kojima F, Tanaka F, Yanagiya M, Kosaka S, et al. Effect of virtual-assisted lung mapping in acquisition of surgical margins in sublobar lung resection. J Thorac Cardiovasc Surg. 2018;156(4):1691-1701.e5.
Pompili C, Detterbeck F, Papagiannopoulos K, Sihoe A, Vachlas K, Maxfield MW, et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg. 2014;98(2):490–6.
Ueda K, Haruki T, Murakami J, Tanaka T, Hayashi M, Hamano K. No drain after thoracoscopic major lung resection for cancer helps preserve the physical function. Ann Thorac Surg. 2019;108(2):399–404.
Abdul Khader A, Pons A, Palmares A, Booth S, Proli C, De Sousa P, et al. Are chest drains routinely required after thoracic surgery? A drainology study of on-table chest-drain removals. JTCVS Open [Internet]. 2023 Jun 2 [cited 2023 Dec 6]; Available from: https://www.sciencedirect.com/science/article/pii/S2666273623001237
Scarci M, Gkikas A, Patrini D, Minervini F, Cerfolio RJ. Editorial: early chest drain removal following lung resection. Front Surg. 2023;31(10):1185334.
Shintani Y, Funaki S, Ose N, Kawamura T, Kanzaki R, Minami M, et al. Air leak pattern shown by digital chest drainage system predict prolonged air leakage after pulmonary resection for patients with lung cancer. J Thorac Dis. 2018;10(6):3714–21.
Zhou J, Lyu M, Chen N, Wang Z, Hai Y, Hao J, et al. Digital chest drainage is better than traditional chest drainage following pulmonary surgery: a meta-analysis. Eur J Cardiothorac Surg. 2018;54(4):635–43.
Ishibashi H, Wakejima R, Kurihara Y, Seto K, Baba S, Asakawa A, et al. Extubation by supraglottic airway after lobectomy prevents cough-associated air leaks and prolonged coughing or sore throat. Eur J Cardiothorac Surg. 2023;63(5):ezad158.
Pompili C, Falcoz PE, Salati M, Szanto Z, Brunelli A. A risk score to predict the incidence of prolonged air leak after video-assisted thoracoscopic lobectomy: an analysis from the European Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg. 2017;153(4):957–65.
Elsayed H, McShane J, Shackcloth M. Air leaks following pulmonary resection for lung cancer: is it a patient or surgeon related problem? Ann R Coll Surg Engl. 2012;94(6):422–7.
Orsini B, Baste JM, Gossot D, Berthet JP, Assouad J, Dahan M, et al. Index of prolonged air leak score validation in case of video-assisted thoracoscopic surgery anatomical lung resection: results of a nationwide study based on the French national thoracic database. EPITHOR Eur J Cardiothorac Surg. 2015;48(4):608–11.
Hwang Y, Kang CH, Kim HS, Jeon JH, Park IK, Kim YT. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study†. Eur J Cardiothorac Surg. 2015;48(2):273–8.
Berg E, Madelaine L, Baste JM, Dahan M, Thomas P, Falcoz PE, et al. Interest of anatomical segmentectomy over lobectomy for lung cancer: a nationwide study. J Thorac Dis. 2021;13(6):3587–96.
Suzuki K, Saji H, Aokage K, Watanabe S, Okada M, Mizusawa J, et al. Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J Thorac Cardiovasc Surg. 2019;158(3):895–907.
Yoon J, Hyun K, Sung SW. Using continuous flow data to predict the course of air leaks after lung lobectomy. J Chest Surg. 2023;56(3):179–85.
Ponholzer F, Ng C, Maier H, Lucciarini P, Öfner D, Augustin F. Risk factors, complications and costs of prolonged air leak after video-assisted thoracoscopic surgery for primary lung cancer. J Thorac Dis. 2023;15(2):866–77.
Craig SR, Walker WS. A proposed anatomical classification of the pulmonary fissures. J R Coll Surg Edinb. 1997;42(4):233–4.
Acknowledgements
Not applicable.
Funding
The authors received no financial support for this article’s research, authorship, or publication.
Author information
Authors and Affiliations
Contributions
MM, FK, and TB conceived of the study. FK and TB prepared resources and supervised the study process. KS, MM, and KO curated data. MM and KS performed data analysis. KS prepared the manuscript. All authors contributed to discussion and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional review board of St. Luke's International Hospital (21-R177, March 20, 2022). The requirement for written informed consent was waived, and the opt-out method was used.
Consent for publication
The requirement for consent for publication was waived, and the opt-out method was used.
Competing interests
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Supplementary Figure 1
. Leak conversion rate following segmentectomy and lobectomy. A Air bubble findings in intraoperative water sealing test and following ALE states. The color bands width represents case numbers. B Leak cessation rate represents the proportion of ALE-absent cases in the water-sealing test-positive cohort. Leak commencement rate represents the proportion of ALE-present cases in the water-sealing test-negative cohort. Fisher's exact test was applied.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sueyoshi, K., Merlini, M., Otsubo, K. et al. Zero-leak prediction during major lung resection aiming for minimal chest drainage duration: a retrospective analysis. J Cardiothorac Surg 19, 120 (2024). https://doi.org/10.1186/s13019-024-02620-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-024-02620-2