Abstract
Background
Numerous studies have investigated anatomic factors for anterior cruciate ligament (ACL) injuries, such as posterior tibial slope (PTS) and notch width index (NWI). However, anterior tibial spine fracture (ATSF) as a specific pattern of ACL injury, a bony avulsion of the ACL from its insertion on the intercondylar spine of the tibia, has rarely been explored for its anatomical risk factors. Identifying anatomic parameters of the knee associated with ATSF is important for understanding injury mechanisms and prevention.
Methods
Patients who underwent surgery for ATSF between January 2010 and December 2021 were retrospectively reviewed, and 38 patients were included in the study group. Thirty-eight patients who suffered from isolated meniscal tear without other pathologic findings were matched in a 1:1 fashion by age, sex and BMI to the study group. The lateral posterior tibial slope (LPTS), medial posterior tibial slope (MPTS), medial tibial depth, lateral tibial height, lateral femoral condyle ratio (LFCR) and NWI were measured and compared between the ATSF and control groups. Binary logistic regressions identified independent predictors of ATSF. Receiver operator characteristic (ROC) curves were performed to compare the diagnostic performance and determine the cutoff values of associated parameters.
Results
The LPTS, LFCR and MPTS were significantly larger in the knees in the ATSF group than in the control group (P = 0.001, P = 0.012 and P = 0.005, respectively). The NWI was significantly smaller in the knees in the ATSF group than in the control group (P = 0.005). According to the results of logistic regression analysis, the LPTS, LFCR and NWI were independently associated with ATSF. The LPTS was the strongest predictor variable, and the ROC analysis revealed 63.2% sensitivity and 76.3% specificity (area under the curve, 0.731; 95% CI 0.619–0.844) for values above 6.9.
Conclusion
The LPTS, LFCR and NWI were found to be associated with the ATSF; in particular, LPTS could provide the most accurate predictive performance. The findings of this study may aid clinicians in identifying people at risk for ATSF and taking individualized preventive measures. However, further investigation regarding the pattern and biomechanical mechanisms of this injury is required.
Similar content being viewed by others
Introduction
Anterior tibial spine fractures (ATSFs) or tibial eminence fractures are avulsion fractures of the anterior cruciate ligament (ACL) from its insertion on the tibial intercondylar eminence. ATSFs are rare, with an incidence between 3.0 and 3.5 per 100,000 individuals in the general population [1, 2]. These fractures commonly occur in children and adolescents, predominantly between the ages of 8–14 [3,4,5]. However, the recent literature suggested that the prevalence of ATSFs in adults is higher than previously reported [6, 7]. The etiology of fractures is various; for pediatric patients, it may be due in part to skeletal immaturity, weak knee muscle tissue and increased ligament elasticity, while for adults, it is mostly due to traffic accidents. The most typical mechanism of injury is knee hyperextension with a valgus or rotational force [8], often resulting from a fall from a bicycle. But these fractures are increasingly common in noncontact injuries in sports, such as skiing and soccer [9].
According to the Meyers and McKeever classification system [10], ATSFs can be classified into 4 types. Type I injury is a minimally displaced fragment. Type II injury involves superior displacement of the anterior bony fragment, while type III and IV injuries involve complete separation of the fragment from the tibia. Type IV injury includes comminution of a displaced avulsion fracture. The different treatment options for ATSFs are full of hot debate, but there is a paucity of literature on the anatomical risk factors for these fractures.
Samora et al. [11] included 25 pediatric patients to evaluate risk factors for ATSF and found no significant differences in posterior tibial slope (PTS) and notch width index (NWI) compared to controls. A previous study by Messner et al. [12] explored the relationship between posterior tibial slope (PTS) and pediatric ATSFs. They found that only male patients undergoing surgical fixation of ATSF had an increased PTS compared with controls. However, their study population was pediatric patients, and their measurement of PTS was performed by plain radiographs, limiting their ability to distinguish the subtle differences between the medial and lateral compartments. In addition to PTS, we want to further explore morphological risk factors for ATSFs of the tibial plateau and femoral condyle. Various osseous morphologic risk factors associated with ACL injuries have been identified in the literature, such as shallower medial tibial depth [13], increased lateral tibial plateau slope [14, 15] and intercondylar notch stenosis [16]. Therefore, the purpose of this study was to determine which anatomic parameters are independently associated with ATSF in adults (1) and the diagnostic values of the individual anatomic parameters (2). It was hypothesized that patients with ATSF would exhibit elevated PTS compared to uninjured controls on MRI measurements.
Methods
Patients and study design
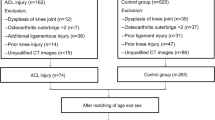
Before the research was started, this retrospective comparative study received institutional review board approval. Patients diagnosed with ATSF from January 2010 to December 2021 at our hospital were identified according to the hospital Electronic Medical Record System. The requirement for written informed consent was waived since this was a retrospective study, it could not cause any adverse effects for included patients, and the patient data were reported anonymously. A total of 173 patients who were diagnosed with ATSF from 2010 to 2021 were screened for eligibility by medical records (Fig. 1). Patients were excluded from the study group if they met one of the following criteria: (1) age < 18 years (because of the small number of patients), (2) concomitant ligamentous injury, (3) combined fractures of the patella or fractures that have an impact on measurements of the femur and tibia, (4) patellofemoral dysplasia/instability, (5) prior history of knee surgery on the affected limb, (6) osteoarthritis Outterbridge > Grade II, (7) significant osteoporosis. The included patients with ATSF were classified as Meyers-McKeever on radiographs or MRI [17]. During the same period, A total of 38 age-, sex- and BMI–matched patients who suffered isolated meniscus injuries were selected as the control group. Participants had any pathological condition that could affect the anatomical morphology of the knee joint, such as a discoid meniscus, were excluded from the control group. Participants with previous surgery or fractures in the lower extremity were also excluded.
Data extraction
Patient characteristics, including age at the time of surgery, sex and BMI, were obtained from the medical records. To ensure the accuracy of the results, all the measurements were performed using good-quality MRI before surgical intervention. MRI was performed using a 1.5-T MR scanner (Magnetom Avanto, Siemens AG, Germany) with an imaging protocol composed of T1-weighted turbo spin echo (T1W-TSE) and T2-weighted fat-suppressed turbo spin echo (T2W-TSE-FS) sequences. All measurements were performed by 2 independent orthopedic surgeons using a blinded method and were repeated twice by each reviewer within an interval of 1 month to determine intraobserver and interobserver reliability. The mean of the 4 measurements was used for the corresponding final results for each patient.
The lateral and medial posterior tibial slopes (LPTS and MPTS, respectively) were measured according to the method described by Hudek et al. [18]. First, to determine the anatomical axis of the tibia, the central sagittal slice of the tibia was selected, and 2 circles were placed on the proximal tibia. The proximal circle was matched with the anterior, posterior and proximal cortical borders, while the distal circle was matched with the anterior and posterior cortices. The line connecting the centers of the two circles was the anatomic axis of the tibia (Fig. 2A). The angle between the perpendicular line of the anatomical axis of the tibia and the tangent line of the lateral and medial tibial plateau is the LPTS and MPTS, respectively. This method has been reported as the most reproducible for measuring lateral tibial slope and is independent of the length of proximal tibia [19]. To determine the most predictive bony morphological risk factors, the following parameters were also measured according to the original description: medial tibial depth (MTD) [20], lateral tibial height (LTH) [21], notch width index (NWI) [22] and lateral femoral condyle ratio (LFCR) [23, 24] (Fig. 2).
MRI images measurements performed according to the original description. A The anatomic axis of the tibia is determined as the line connecting the centers of 2 circles. B Lateral posterior tibial slope is composed of the vertical line of the anatomic axis of the tibia and the tangent to the lateral tibial plateau. C Medial posterior tibial slope is composed of the vertical line of the anatomic axis of the tibia and the tangent to the medial tibial plateau. D Medial tibial depth. E Lateral tibial height. F Notch width index. G The long axis of the femoral shaft. H Lateral femoral condyle ratio was defined as b/(a + b) × 100%
Statistical analysis
The data were checked for normal distribution by the Kolmogorov‒Smirnov test. The differences of continuous variables between the two groups were analyzed by the Student’s t test or Mann‒Whitney U test according to the normality test. Binary logistic regressions were calculated to identify the significant independent predictors of ATSF. Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were constructed to evaluate the diagnostic accuracy of different parameters with the cutoffs calculated. The ideal predictive cutoff point with the highest sensitivity and specificity was determined based on the Youden index. The intraclass correlation coefficients (ICCs) were calculated to determine the intraobserver and interobserver reliability and classified as good (≥ 0.75), fair (0.50–0.74) and poor (< 0.50). Statistical analyses were performed using SPSS software (version 26; IBM). A P value < 0.05 was considered significant for all analyses.
Results
A total of 38 patients who were diagnosed with ATSFs by surgery or MRI and met the inclusion criteria were included in the ATSF group. For the control cohort, 38 age-, sex- and BMI-matched participants with isolated meniscal tear were included to match the cases. The mean age of all participants was 33.64 ± 9.07 years (33.08 ± 8.53 years for the ATSF group vs. 34.21 ± 9.67 years for the control group), and the mean BMI of all participants was 23.36 ± 3.65 (22.63 ± 3.11 vs. 24.08 ± 4.04, respectively). There were no significant differences between the 2 groups in demographic characteristics (Table 1).
The MRI measurements are presented in Table 2. The intraobserver and interobserver reliabilities of measurements were categorized as good with the minimum ICCs of 0.864 and 0.851, respectively (Table 3). There were significant differences between the ATSF group and the control group regarding the following parameters: LPTS (P = 0.001), MPTS (P = 0.005), NWI (P = 0.005) and LFCR (P = 0.012). However, no significant differences were observed between groups for MTD, LTH, LPTS-MPTS and LPTS/MPTS (Fig. 3).
Box-and-whisker plots showed distribution of the measurement variables between the ATSF group and the control group. ATSF anterior tibial spine fracture. *P < 0.05. LFCR lateral femoral condyle ratio, LPTS lateral posterior tibial slope, MPTS medial posterior tibial slope, MTD medial tibial depth, LTH lateral tibial height, NWI notch width index
The binary logistic regression results for the LPTS, MPTS, NWI and LFCR are shown in Table 4. From the table, the LPTS (OR = 1.326, 95% CI = 1.026–1.713, P = 0.031), NWI (OR = 3.1194E−14, 95% CI = 1.2831E−23–7.60E−05, P = 0.005) and LFCR (OR = 1.295, 95% CI = 1.063–1.578, P = 0.010) were found to be independently associated with ATSF.
The ROC curves were conducted for three significant independent predictors for ATSFs to compare their diagnostic performance (Fig. 4). The most accurate predictor was the LPTS, which had a sensitivity of 63.2% and specificity of 76.3% for predicting ATSFs at an optimal cutoff of 6.9 (Table 5).
Discussion
The most important finding of this study was that LPTS, MPTS, NWI and LFCR were significantly different between patients with and those without ATSFs. However, only the LPTS, NWI and LFCR were found to be independently associated with ATSFs. The LPTS had 63.2% sensitivity and 76.3% specificity at a cutoff value of 6.9, providing the best predictive performance.
There is a paucity of research comparing the injury mechanisms of ATSFs and ACL tears. Previous investigations have shown that the demographic distribution and activity associated with ATSFs differ from those of ACL tears. Leie et al. demonstrated that skiing accounted for 56% of ATSFs, followed by soccer (22%) and rugby (16%) [25], whereas ACL tears were most prevalent in girls' soccer, followed by boys' football and basketball [26, 27]. These findings suggest that the mechanisms of these two injuries are subtly distinct. A biomechanical analysis of primate specimens has indicated that the viscoelastic properties of bone and ligaments are influenced by the rate of loading applied to the specimens [28]. Avulsion fractures are more likely to occur in cases with slower loading rates, while injuries to the intrasubstance portion of the ACL are more likely to be seen in cases with faster loading rates. In children and adolescents, discrepancies in the degree of tibial eminence ossification can also give rise to differences in injury patterns between ligament rupture and avulsion fractures.
Numerous studies have reported that increased PTS is an independent risk factor for ACL injury [13, 29, 30]. Increased PTS has an adverse effect on knee kinematics and kinetics, exerting more strain on ACL and consequently leading to stretching and tearing of ACL [31]. A recent biomechanical study found that increased PTS significantly increased tension on the ACL, and even at a 2.5° increase in PTS angle, knee joint instability and larger loading on the medial meniscus were found on the ACL-deficient knee [32]. Two studies reported the relationship between PTS and ATSF in pediatric populations, but they neither found a statistically significant association of PTS with ATSF when comparing cases with controls [11, 12]. But after stratifying by sex, Messner et al. [12] found that male patients who undergo surgical fixation of ATSF tend to have increased PTS as compared with controls. Differences with our current study in imaging modality and study populations may account for the unique findings. One of the concerns with their measurements using plain radiographs was that it was not easy to discern subtle differences between the compartments due to the superimposed medial and lateral tibial plateau, limiting their ability to analyze medial and lateral PTS, respectively [33]. Articular cartilage represents the functional point of tibiofemoral articulation, and MRI can accurately assess the 3D geometry of the cartilage surface, while plain radiographs cannot [34]. Our current study found that as the LPTS increases, for every additional degree, the risk of ATSF increases by 32.6%. And according to the results of the logistic regression analysis, MPTS was not an independent risk factor for ATSF. Although many studies have evaluated the effect of medial and lateral PTS on ACL injury, there are some conflicting findings, and LPTS measured on MRI is the most consistently reported risk factor [13, 30, 35, 36]. Our findings are in line with previous studies showing that LPTS is a more significant risk factor for ACL injury than MPTS [37,38,39]. Previous biomechanical literature also suggested that an increased LPTS has a greater impact on anterior tibial translation than MPTS, which creates a net internal rotation that increases the strain on the ACL [40, 41].
The association between the femoral intercondylar notch shape and dimensions and ACL injury has attracted extensive interest. In particular, NWI based on MRI measurements has widely been shown to be an important risk factor for ACL injury [42, 43]. Kocher et al. [44] evaluated skeletally immature patients with ATSF compared to matched ACL injury. They found that the ACL group had narrower notch indices than the ATSF group (0.230 vs. 0.253; P = 0.020). This may partially explain the different injury patterns of ATSF and ACL injury in the skeletally immature knee. Although we identified the decreased NWI as an independent risk factor for ATSF, the logistic regression results suggest that the OR value for NWI is too small to further indicate its clinical significance. The current study demonstrated that the LFCR—a novel anatomical risk factor for ACL injuries, is also correlated with ATSFs. This association may arise from the influence of the distal femoral morphology on knee kinematics. An enhanced depth of the posterior femoral condyle could induce changes in tibiofemoral interactions, ultimately leading to modifications in gait and loading mechanics, which may increase the susceptibility to ATSFs [45, 46]. Further investigations involving additional cohorts and laboratory biomechanical studies are warranted to elucidate the distinct mechanisms of ACL tears and ATSFs.
Although these anatomic risk factors are largely unmodifiable, a growing body of data highlights the role of critical anatomical parameters that can help clinicians take preventive measures to avoid injuries. Numerous studies have identified the morphological risk factors for ACL injury of the knee joint. People with a high risk of ACL injury can benefit from modifiable interventions, including landing biomechanics, neuromuscular training, balance training and improvements in playing surfaces and footwear [47]. A meta-analysis performed by Huang et al. [48] demonstrated significant protective effects of ACL injury prevention programs and reduced injury rates by 53%. Currently, although MRI is not routinely performed for screening, preoperative MRI for ATSF patients is necessary to identify other combined injuries, such as meniscus and ligament injuries, and is cost-effective. Identifying high-risk individuals through preoperative MRI allows us to individualize treatment and rehabilitation programs to improve postoperative outcomes and avoid reinjury, such as refracture and ACL injury. For individuals at high risk of ATSFs, such as skiers, it is postulated that training athletes to jump, land and cut in a biomechanical position and using appropriate athletic equipment may potentially diminish the incidence of ATSFs [9, 49]. Future research will also be warranted to determine if more conservative rehabilitation programs and return-to-sport protocols are necessary for people at high risk of ATSFs.
Limited studies have investigated morphological risk factors associated with ATSFs. To the best of our knowledge, this is the first study to confirm the relationship between ATSFs and anatomic parameters, including LPTS, MPTS, asymmetry of the medial and lateral slopes, MTD, LTH, NWI and LFCR. Our results showed that the increased LPTS and LFCR and decreased NWI are associated with an increased risk of ATSF. However, this study has certain limitations. First, due to the small number of pediatric patients, we included only adult patients, limiting the generalization of our findings to pediatric populations. Nevertheless, as the two groups exhibit different ATSF mechanisms, forthcoming studies should consider addressing them separately. Second, we screened in the hospital system based on surgical records. Patients with ATSF who did not undergo surgery were not included, especially those with Meyers and McKeever classification type I fractures. Third, we included patients with only meniscal injury in the control group. Although this method has been widely adopted by previous studies [24, 50, 51], the inclusion of this population may introduce some bias.
Conclusion
Increased LPTS and LFCR and decreased NWI are significant risk factors for the incidence of ATSF in adults. All three parameters provide good predictive performance, but LPTS is the strongest predictor. These findings may contribute to the clinician identifying risk factors for ATSF and developing related preventive strategies. Future studies with a larger population are needed to further understand the anatomical risk factors and injury mechanisms of ATSF.
Availability of data and materials
All data generated or analyzed during this study are included in this article. The data are available from the corresponding author upon reasonable request.
Abbreviations
- ACL:
-
Anterior cruciate ligament
- ATSF:
-
Anterior tibial spine fracture
- PTS:
-
Posterior tibial slope
- LPTS:
-
Lateral posterior tibial slope
- MPTS:
-
Medial posterior tibial slope
- MTD:
-
Medial tibial depth
- LTH:
-
Lateral tibial height
- NWI:
-
Notch width index
- ROC:
-
Receiver operator characteristic
References
Skak SV, Jensen TT, Poulsen TD, Stürup J. Epidemiology of knee injuries in children. Acta Orthop Scand. 1987;58:78–81.
Prasad N, Aoyama JT, Ganley TJ, Ellis HB Jr, Mistovich RJ, Yen YM, Fabricant PD, Green DW, Cruz AI Jr, McKay S, et al. A comparison of nonoperative and operative treatment of type 2 tibial spine fractures. Orthop J Sports Med. 2021;9:2325967120975410.
Kim JR, Song JH, Lee JH, Lee SY, Yoo WH. Cartilaginous avulsion fracture of the tibial spine in a 5-year-old girl. Skelet Radiol. 2008;37:343–5.
Kocher MS, Foreman ES, Micheli LJ. Laxity and functional outcome after arthroscopic reduction and internal fixation of displaced tibial spine fractures in children. Arthroscopy. 2003;19:1085–90.
Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2014;42:1743–50.
Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop Relat Res. 2005;434:207–12.
Hayes JM, Masear VR. Avulsion fracture of the tibial eminence associated with severe medial ligamentous injury in an adolescent. A case report and literature review. Am J Sports Med. 1984;12:330–3.
Lubowitz JH, Elson WS, Guttmann D. Part II: arthroscopic treatment of tibial plateau fractures: intercondylar eminence avulsion fractures. Arthroscopy. 2005;21:86–92.
Albertson B, Beynnon B, Endres N, Johnson R. Incidence of anterior tibial spine fracture among skiers does not differ with age. Knee Surg Sports Traumatol Arthrosc. 2022;30:2291–7.
Meyers MH, McKeever FM. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1970;52:1677–84.
Samora W, Beran MC, Parikh SN. Intercondylar roof inclination angle: is it a risk factor for ACL tears or tibial spine fractures? J Pediatr Orthop. 2016;36:e71-74.
Messner MK, McGee AS, Elphingstone JW, Schartung DF, Frazier MB, Schick S, Brabston EW, Momaya AM. The relationship between posterior tibial slope and pediatric tibial eminence fractures. Am J Sports Med. 2023;51:32–7.
Hashemi J, Chandrashekar N, Mansouri H, Gill B, Slauterbeck JR, Schutt RC Jr, Dabezies E, Beynnon BD. Shallow medial tibial plateau and steep medial and lateral tibial slopes: new risk factors for anterior cruciate ligament injuries. Am J Sports Med. 2010;38:54–62.
Stijak L, Herzog RF, Schai P. Is there an influence of the tibial slope of the lateral condyle on the ACL lesion? A case–control study. Knee Surg Sports Traumatol Arthrosc. 2008;16:112–7.
Rahnemai-Azar AA, Abebe ES, Johnson P, Labrum J, Fu FH, Irrgang JJ, Samuelsson K, Musahl V. Increased lateral tibial slope predicts high-grade rotatory knee laxity pre-operatively in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25:1170–6.
Everhart JS, Flanigan DC, Simon RA, Chaudhari AM. Association of noncontact anterior cruciate ligament injury with presence and thickness of a bony ridge on the anteromedial aspect of the femoral intercondylar notch. Am J Sports Med. 2010;38:1667–73.
Meyers MH, Mc KF. Fracture of the intercondylar eminence of the tibia. J Bone Joint Surg Am. 1959;41-a:209–20 (discussion 220–202).
Hudek R, Schmutz S, Regenfelder F, Fuchs B, Koch PP. Novel measurement technique of the tibial slope on conventional MRI. Clin Orthop Relat Res. 2009;467:2066–72.
Lipps DB, Wilson AM, Ashton-Miller JA, Wojtys EM. Evaluation of different methods for measuring lateral tibial slope using magnetic resonance imaging. Am J Sports Med. 2012;40:2731–6.
Hashemi J, Chandrashekar N, Gill B, Beynnon BD, Slauterbeck JR, Schutt RC Jr, Mansouri H, Dabezies E. The geometry of the tibial plateau and its influence on the biomechanics of the tibiofemoral joint. J Bone Joint Surg Am. 2008;90:2724–34.
Kujala UM, Nelimarkka O, Koskinen SK. Relationship between the pivot shift and the configuration of the lateral tibial plateau. Arch Orthop Trauma Surg. 1992;111:228–9.
Souryal TO, Freeman TR. Intercondylar notch size and anterior cruciate ligament injuries in athletes. A prospective study. Am J Sports Med. 1993;21:535–9.
Pfeiffer TR, Burnham JM, Hughes JD, Kanakamedala AC, Herbst E, Popchak A, Shafizadeh S, Irrgang JJ, Debski RE, Musahl V. An increased lateral femoral condyle ratio is a risk factor for anterior cruciate ligament injury. J Bone Joint Surg Am. 2018;100:857–64.
He M, Li J. Increased lateral femoral condyle ratio measured by MRI is associated with higher risk of noncontact anterior cruciate ligament injury. BMC Musculoskelet Disord. 2022;23:190.
Leie M, Heath E, Shumborski S, Salmon L, Roe J, Pinczewski L. Midterm outcomes of arthroscopic reduction and internal fixation of anterior cruciate ligament tibial eminence avulsion fractures with K-wire fixation. Arthroscopy. 2019;35:1533–44.
Joseph AM, Collins CL, Henke NM, Yard EE, Fields SK, Comstock RD. A multisport epidemiologic comparison of anterior cruciate ligament injuries in high school athletics. J Athl Train. 2013;48:810–7.
Gornitzky AL, Lott A, Yellin JL, Fabricant PD, Lawrence JT, Ganley TJ. Sport-specific yearly risk and incidence of anterior cruciate ligament tears in high school athletes: a systematic review and meta-analysis. Am J Sports Med. 2016;44:2716–23.
Noyes FR, Butler DL, Grood ES, Zernicke RF, Hefzy MS. Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am. 1984;66:344–52.
Hohmann E, Bryant A, Reaburn P, Tetsworth K. Is there a correlation between posterior tibial slope and non-contact anterior cruciate ligament injuries? Knee Surg Sports Traumatol Arthrosc. 2011;19(Suppl 1):S109-114.
Hohmann E, Tetsworth K, Glatt V, Ngcelwane M, Keough N. Medial and lateral posterior tibial slope are independent risk factors for noncontact ACL injury in both men and women. Orthop J Sports Med. 2021;9:23259671211015940.
Shelburne KB, Kim HJ, Sterett WI, Pandy MG. Effect of posterior tibial slope on knee biomechanics during functional activity. J Orthop Res. 2011;29:223–31.
Shu L, Abe N, Li S, Sugita N. Importance of posterior tibial slope in joint kinematics with an anterior cruciate ligament-deficient knee. Bone Joint Res. 2022;11:739–50.
Amerinatanzi A, Summers RK, Ahmadi K, Goel VK, Hewett TE, Nyman E. Automated measurement of patient-specific tibial slopes from MRI. Bioengineering (Basel). 2017;4:69.
Dare DM, Fabricant PD, McCarthy MM, Rebolledo BJ, Green DW, Cordasco FA, Jones KJ. Increased lateral tibial slope is a risk factor for pediatric anterior cruciate ligament injury: an MRI-based case–control study of 152 patients. Am J Sports Med. 2015;43:1632–9.
Simon RA, Everhart JS, Nagaraja HN, Chaudhari AM. A case-control study of anterior cruciate ligament volume, tibial plateau slopes and intercondylar notch dimensions in ACL-injured knees. J Biomech. 2010;43:1702–7.
Wahl CJ, Westermann RW, Blaisdell GY, Cizik AM. An association of lateral knee sagittal anatomic factors with non-contact ACL injury: sex or geometry? J Bone Joint Surg Am. 2012;94:217–26.
Dean RS, DePhillipo NN, LaPrade RF. Posterior tibial slope in patients with torn ACL reconstruction grafts compared with primary tear or native ACL: a systematic review and meta-analysis. Orthop J Sports Med. 2022;10:23259671221079380.
Webb JM, Salmon LJ, Leclerc E, Pinczewski LA, Roe JP. Posterior tibial slope and further anterior cruciate ligament injuries in the anterior cruciate ligament-reconstructed patient. Am J Sports Med. 2013;41:2800–4.
Shen L, Jin ZG, Dong QR, Li LB. Anatomical risk factors of anterior cruciate ligament injury. Chin Med J (Engl). 2018;131:2960–7.
Fleming BC, Renstrom PA, Beynnon BD, Engstrom B, Peura GD, Badger GJ, Johnson RJ. The effect of weightbearing and external loading on anterior cruciate ligament strain. J Biomech. 2001;34:163–70.
Bernhardson AS, DePhillipo NN, Aman ZS, Kennedy MI, Dornan GJ, LaPrade RF. Decreased posterior tibial slope does not affect postoperative posterior knee laxity after double-bundle posterior cruciate ligament reconstruction. Am J Sports Med. 2019;47:318–23.
Li H, Zeng C, Wang Y, Wei J, Yang T, Cui Y, Xie D, Liu H, Lei GH. Association between magnetic resonance imaging-measured intercondylar notch dimensions and anterior cruciate ligament injury: a meta-analysis. Arthroscopy. 2018;34:889–900.
Micicoi G, Jacquet C, Khakha R, LiArno S, Faizan A, Seil R, Kocaoglu B, Cerciello S, Martz P, Ollivier M. Femoral and tibial bony risk factors for anterior cruciate ligament injuries are present in more than 50% of healthy individuals. Am J Sports Med. 2021;49:3816–24.
Kocher MS, Mandiga R, Klingele K, Bley L, Micheli LJ. Anterior cruciate ligament injury versus tibial spine fracture in the skeletally immature knee: a comparison of skeletal maturation and notch width index. J Pediatr Orthop. 2004;24:185–8.
Eckhoff DG, Bach JM, Spitzer VM, Reinig KD, Bagur MM, Baldini TH, Flannery NM. Three-dimensional mechanics, kinematics, and morphology of the knee viewed in virtual reality. J Bone Joint Surg Am. 2005;87(Suppl 2):71–80.
Eckhoff DG, Bach JM, Spitzer VM, Reinig KD, Bagur MM, Baldini TH, Rubinstein D, Humphries S. Three-dimensional morphology and kinematics of the distal part of the femur viewed in virtual reality. Part II. J Bone Joint Surg Am. 2003;85-A(Suppl 4):97–104.
Sturnick DR, Vacek PM, DeSarno MJ, Gardner-Morse MG, Tourville TW, Slauterbeck JR, Johnson RJ, Shultz SJ, Beynnon BD. Combined anatomic factors predicting risk of anterior cruciate ligament injury for males and females. Am J Sports Med. 2015;43:839–47.
Huang YL, Jung J, Mulligan CMS, Oh J, Norcross MF. A majority of anterior cruciate ligament injuries can be prevented by injury prevention programs: a systematic review of randomized controlled trials and cluster-randomized controlled trials with meta-analysis. Am J Sports Med. 2020;48:1505–15.
Mandelbaum BR, Silvers HJ, Watanabe DS, Knarr JF, Thomas SD, Griffin LY, Kirkendall DT, Garrett W Jr. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33:1003–10.
Li K, Zheng X, Li J, Seeley RA, Marot V, Murgier J, Liang X, Huang W, Cavaignac E. Increased lateral femoral condyle ratio is associated with greater risk of ALC injury in non-contact anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2021;29:3077–84.
Hodel S, Kabelitz M, Tondelli T, Vlachopoulos L, Sutter R, Fucentese SF. Introducing the lateral femoral condyle index as a risk factor for anterior cruciate ligament injury. Am J Sports Med. 2019;47:2420–6.
Acknowledgements
None.
Funding
The study received no fundings.
Author information
Authors and Affiliations
Contributions
WF and LZ conceived, designed and planned the study. QX and RY conducted the database searching and data extraction. WF and YH analyzed the data. WF was responsible to explain the results. LZ wrote the manuscript while WF and YH revised it. And all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Before the research was started, this retrospective comparative study received institutional review board approval (approval no. 2023-348). The requirement for written informed consent was waived since this was a retrospective study.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, L., Xia, Q., Yang, R. et al. Anatomical factors associated with the development of anterior tibial spine fractures based on MRI measurements. J Orthop Surg Res 18, 357 (2023). https://doi.org/10.1186/s13018-023-03836-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-03836-z