Abstract
Background
This systematic review investigated the efficacy and safety of surgical procedures augmented with cell therapies for chondral defects of the talus.
Methods
The present systematic review was conducted according to the 2020 PRISMA guidelines. PubMed, Google scholar, Embase, and Scopus databases were accessed in March 2022. All the clinical trials investigating surgical procedures for talar chondral defects augmented with cell therapies were accessed. The outcomes of interest were to investigate whether surgical procedures augmented with cell therapies promoted improvement in patients reported outcomes measures (PROMs) with a tolerable rate of complications.
Results
Data from 477 procedures were retrieved. At a mean follow-up of 34.8 ± 9.7 months, the Visual Analogic Scale (VAS) improved of 4.4/10 (P = 0.002) and the American Orthopaedic Foot and Ankle Score (AOFAS) of 31.1/100 (P = 0.0001) points. No improvement was found in Tegner score (P = 0.4). Few articles reported data on complications. At last follow-up, the rate of reoperation and failure were 0.06% and 0.03%, respectively. No graft delamination or hypertrophy was observed.
Conclusion
The current evidence suggests that cell therapies may be effective and safe to enhance surgical procedures for chondral defects of the talus. These results should be considered within the limitations of the present study. The current literature should be enriched with randomized controlled clinical trials with larger population size and longer follow-up.
Similar content being viewed by others
Introduction
Focal chondral defects of the talus are common in the young and active population [1, 2]. Given the limited healing potential of hyaline cartilage, these lesions are most likely unable to regenerate [3, 4]. If left untreated, patients may experience chronic instability, persistent pain, and early onset osteoarthritis [5,6,7]. Defects smaller than 0.5 cm2 can be managed arthroscopically with microfractures (MFx), and several procedures have been advocated for bigger defects [8,9,10,11,12]. Autologous chondrocyte implantation (ACI), osteochondral transplantation, Autologous Matrix-Induced Chondrogenesis (AMIC) have been advocated for chondral defects of the talus [13,14,15,16,17]. Although results are promising, the rate of failure of these procedures is between 1 and 10% [18,19,20,21,22,23]. Chondral procedures can be enhanced with cell therapies to augment the healing process, increase the regeneration potential, and reduce fibrosis [24,25,26,27,28]. Cell therapies to enhance chondral repair of the talus evolved in the past two decades. Several preclinical studies have been published, while clinical investigations are still limited. Surgical augmentation with mesenchymal stem cells (MSCs) for chondral repair developed recently [18, 29, 30]. These procedures are mainly based on MSCs or bone marrow aspirate concentrate (BMAC) transplantation [31,32,33]. Chondral procedures combined with stem cells therapies are obtained, processed, and delivered during a relatively simple one-step procedure [25,26,27, 34, 35]. The delivery cells rich in growth factors and chemokines to enhance cell migration and proliferation represent another commonly used type of cell therapy [36,37,38]. In this regard, the injection of platelet rich plasma (PRP) and/or its derivate growth factors, or simple peripheral blood injections also gained interest in the past decades [39,40,41,42,43]. The current literature reports several clinical investigations which evaluated the efficacy and safety of cell therapies augmentation for chondral defects of the talus. However, to the best of our knowledge, a comprehensive systematic review is lacking. We therefore updated the current available evidence and investigated the efficacy and safety of cell therapies augmentation for chondral defects of the talus.
Materials and methods
Search strategy
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checlist [44] and the guidelines form the Cochrane Handbook for Systematic Reviews of Interventions [45]. Two authors (F.M. and J.E.) independently performed the literature search in March 2022 accessing PubMed, Google scholar, Embase, and Scopus databases. The following keywords were used in combination using the Boolean operators AND/OR: (talus) AND (chondral defects AND focal) AND (management OR surgery OR therapy OR arthroscopy) AND (pain OR sports OR ACI OR autologous chondrocyte implantation OR matrix-induced OR periosteum OR membrane OR chondral OR collagen OR visual analogic scale OR PROMs OR patient reported outcome measures AND ((blood OR mesenchymal AND stem (cells OR cell) AND (concentrate OR application OR augmentation OR enhancement) AND bone marrow OR adipose OR peripheral blood)). No further filters, keywords, or limits were used for the databases search. No time constrains were used for the search. The same framework was used in each database. The two reviewers independently screened the resulting titles per hand. If titles matched the topic, the abstract was accessed. Titles which did not focus the main topic were excluded. The two reviewers independently screened the resulting abstract by hand. For those abstract which could potentially match the topic, the full text article was downloaded. The two reviewers independently evalauted the bibliographies of the full text articles. In a second phase, all the articles were listed aphabetically according to the surname of the first author and year of pubblication in Microsoft Excel (version MacOS 16.37, Microsoft Corporation, USA). Duplicates were excluded. Discrepancies were further evaluated, and debated by both reviewers and disagreements were solved by a third author (N.M.).
Inclusion criteria
All the published literature investigating procedures to address chondral defects of the talus augmented with cell therapies were accessed. Given to the authors’ language capabilities, articles in English, German, Italian, French and Spanish were eligible. Studies with level I to IV of evidence, according to Oxford Centre of Evidence-Based Medicine [46], were considered. Only clinical investigations which focused on chondral defects of the talus were eligible. Only clinical studies published in peer reviewed journals were considered. Studies reporting data on cell therapies augmentation for ACI, osteochondral allograft and autograft transplantation, and AMIC were eligible. Only articles reporting quantitative data under the outcomes of interest were considered for inclusion.
Exclusion criteria
Studies reporting data on patients who underwent chondral procedures in participants with advanced degenerative chondropathy were not eligible. In vitro, animal, and computational studies were not considered. Studies which augmented chondral procedures with less differentiated stem cells (e.g. totipotential, multipotential, humbelical) or fully committed cells (e.g. chondrocytes) were not considered. Studies reporting data on patient who underwent allogenic or xenogenic cells were not considered. Missing data under the outcomes of interest warranted the exclusion from this study.
Data extraction and outcomes of interest
Two authors (F.M. and J.E.) independently performed data extraction. Study generalities (author, year, journal, type of study, length of the follow-up) and patients baseline characteristics (number of procedures, mean BMI, mean age, mean length of the symptoms before surgery, percentage of women, and mean size of the defect) were collected. Data from the following patient reported outcome measures (PROMs) were collected at baseline and at last follow-up: Visual Analogic Scale (VAS), Tegner Activity Scale [47], and American Orthopaedic Foot and Ankle Score (AOFAS) [48]. The rate of graft hypertrophy or delamination was retrieved, as was the rate of failure and revision surgery. We investigated whether PROMs improved from baseline to the last follow-up, and reported the frequency of complications.
Methodology quality assessment
The methodological quality assessment was performed by one author (F.M.) following the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions [45]. The risk of bias graph tool of the Review Manager Software Version 5.3 (The Nordic Cochrane Collaboration, Copenhagen) was used. The following bias were considered: selection, detection, attrition, reporting, and other source of bias. The risk of selection bias analysed the random sequence generation and the allocation concealment. The risk of detection bias in the blinding procedure during the outcome assessment were analysed. The risk of attrition bias refers to incomplete outcome data, such as missing outcome data from attrition during study enrollment or analysis. The risk of reporting bias refers to the selective publication of results based on the their statistical or clinical relevance. If the authors indentified additional risk of bias, these were considered as “other bias”. The risk of bias tool evalautes each bias as low (green), high (red), or unclear (yellow). The risk associated to each bias is expressed as percentage. The quality of evidence of collective outcomes were evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system was used [49, 50].
Statistical analysis
The statistical analyses were performed by the main author (F.M.) using the IBM SPSS software (version 25). For descriptive statistics of continuous endpoint, mean and standard deviation was evaluated. For binary data (rate of failure, revision surgery, graft hypertrophy and delamination), the number of observations and the number of patients for each study were collected. To evaluate the improvement of PROMs from baseline to the last follow-up, the mean difference (MD) was calculated, with P values of t-test < 0.05 considered statistically significant.
Results
Search result
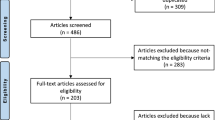
The literature search resulted in 1165 articles. Of these, 344 were duplicates. A further 806 studies were excluded: not clinical studies (N = 307), not focusing on talus (N = 279), study design (N = 101), evaluating other procedures rather than ACI, osteochondral allograft and autograft transplantation, or AMIC (N = 74), reporting data on patients with advanced degenerative chondropathy (N = 2), not published in peer reviewed journal (N = 19), using less differentiated stem cells, fully committed cells, allogenic or xenogenic cells (N = 21), language limitation (N = 3). A further eight studies were not included as they did not report quantitative data under the outcomes of interest. Finally, 7 articles were included in the present study. The results of the literature search are shown in Fig. 1.
Methodological quality assessment
The retrospective design in 43% (3 of 7) of the included studies increases the risk of selection bias which was scored as moderate. Moreover, most of the included studies did not adopt a blinding method, thus increasing the risk of detection bias. Overall, the missing outcome data from attrition during study enrollment or analysis was low, leading to a low to moderate attrition bias. The risk of reporting bias was moderate, and the risk of other biases moderate to high. Concluding, the overall quality of the methodological assessment was moderate (Figs. 2, 3).
Patient demographics
Data from 477 procedures were retrieved. The mean duration of symptoms before the index surgery was 6.6 ± 4.6 months. The mean follow-up was 34.8 ± 9.7 months. The mean age of the patients was 34.2 ± 6.7 years. 32% (153 of 477 patients) were women. The mean defect size was 1.7 ± 0.4 cm2. Few studies reported data with regard to the location of the lesion: medial 73% (99 of 135), lateral 27% (36 of 135), central 0% (0 of 135). Patient demographics at baseline is shown in greater detail in Table 1 (BMC—bone marrow concentrate; PBC—peripheral blood concentrate; MAST—matrix-associated stem cell transplantation; AMIC—autologous matrix-induced chondrogenesis; PRP—platelet-rich plasma; mACI—membrane-assisted autologous chondrocyte implantation; PRGF—platelet-rich grow factors) (Table 2).
Outcomes of interest
At last follow-up, the VAS had improved of -4.4/10 (P = 0.002) and the AOFAS of 31.1/100 (P = 0.0001). No improvement was found in Tegner score (P = 0.4).
Complications
Few articles reported data on complications. At last follow-up the rates of reoperation and failure were 0.06% and 0.03%, respectively. No graft delamination or hypertrophy were observed. Table 3 shows the frequency of complication (data are based on the total number of patients that were included in the articles reporting quantitative data on such complication).
Quality of the recommendations
The overall quality of evidence of collective outcomes according to the GRADE approach was very low (Fig. 4).
Discussion
According to the main findings of the present study, cell therapies augmentation for surgical procedures may enhance cartilage regeneration in chondral defects of the talus. PROMs were significant improved from baseline to the last follow-up, indicating that these procedures may be effective in restoring ankle function, reducing the symptoms and improving the physical activity of the patients. The risk of reoperations and failures were 5.2% and 3.3%, respectively, and no delamination or hypertrophy were evidenced at last follow-up, indicating that these procedures may be safely performed.
The type of surgical procedures and cell therapies used for augmentation were heterogeneous. Guney et al. [53] enhanced MFx with platelet rich plasma (PRP) 6 to 24 h after the arthroscopic procedure. They found an improvement of the AOFAS and VAS scores at a mean of 42 months follow-up [53]. However, they reported greater pain control in the control group (mosaicplasty) [53]. PRP is obtained by centrifugation of platelets extracted by peripheral venous blood [58, 59]. PRP was introduced in the early 50’s: since then, it has been employed in regenerative medicine, and extended to musculoskeletal disorders [58, 60, 61]. PRP has a high concentration of growth factors, such as TGF-β, VEGF, EGF, IGF-1, b-FGF, [62, 63]. These growth factors and mediators enhance chemotaxis, angiogenesis, cell proliferation, and matrix formation of MSCs, accelerating tissue heling and improve regeneration [63,64,65,66,67,68]. Moreover, previous studies found that PRP reduces catabolism and increases the anabolic activity of hyaline cartilage [69]. Given its regenerative potential, PRP has been advocated in the conservative management of several musculoskeletal ailments [69,70,71,72]. Nguyen et al. [55] augmented osteochondral autograft transplantation (OAT) with bone marrow aspirate concentrate or platelet-rich grow factors in a cohort of 38 athletes. 87% (33 of 38) of athletes returned to sport at their previous level within a mean of 8.2 months, 11% (4 of 38) returned at a lower level, and 2% (1 of 38) did not return to sport [55]. Although they did not report data separately according to their augmentation procedure, overall good outcome and patient satisfaction were observed [55].
Most of the cell therapy modalities included in the present study promoted MSCs migration and proliferation [51, 52, 54,55,56,57]. In addition to their cellular differentiation potential, the paracrine activity of MSCs interact with the microenvironment, enhancing tissue regeneration and modulating inflammation, promoting T and B cell proliferation, and NK cell activity [25, 26, 73]. The power of regenerative medicine relies in the signalling and mutual interaction patterns between stem cells and environment [74,75,76]. MSCs can act as pericytes, releasing factors with reparative, anti-inflammatory, and immunomodulatory effect [25, 77]. These characteristics suggest that, beyond their interation with the environment and differentiation potential, MSCs also modulate inflammation, which is pivotal in pain control. MSCs release trophic, anabolic, and chemotactic cytokines which attract further MSCs to the defect, enhancing neocartilage integration and collagen type II expression 78. This function of MSCs as chemofactors and supervisors is essential for tissue repair and regeneration. Richter et al. [57] compared 129 patients who underwent matrix-associated stem cell transplantation (MAST) versus 129 patients who underwent AMIC augmented with peripheral blood concentrate. At two-year follow-up, they evidenced no difference between the two procedures in the rate of revision and failures [57]. Isolated AMIC for chondral defects of the talus reported promising outcomes [79, 80], with results superior to isolated MFx [81].
This study certainly has limitations. The limited number of included studies and the retrospective design of most of studies represent an important weakness of the present investigation. However, we point out that such limitations are intrinsic of the published scientific literature, which lacks high level of evidence investigations. Inter-rater agreement during study selection has not been conducted. The evidence on surgical strategies for chondral defects of the talus augmented with cell therapies is limited. Multiple surgical metodolodiges were considered for analysis, with marked variability in indications, procedures, and protocols. This surely leads to greater risk of bias, and hence the results from the present study should be considered cautiously. Given the heterogeneous nature of the treatments, along with the limited quantitative data available for inclusion, no further subgroup analyses were conducted. The nature of the membrane used to coat the cells was also heterogeneous: some author used a hyaluronic acid based membrane [51, 52], other a collagen I/III porcine derived scaffold [54, 56, 57]. Primary surgery and revision settings were not considered as separate, as some authors did not specify it or mixed the interventions. Only Guney et al. [53] considered solely primary interventions. The authors of the included studies did not adequately specify whether the lesions were acute or chronic, or considered them separately in the analyses. Chondral damage of the talus is typically caused by an acute injury, such as a sudden pivot or twist, a fall, or direct blow to the ankle [82]. Less common causes have been described, such as prolonged immobilization, osteochondritis, alteration in the forces exerted on the articular cartilage, nutritional inadequacies [83, 84]. The surgical access (arthroscopy, mini-arthrotomy, arthrotomy) has not been analysed separately. A recent systematic review compared 421 arthroscopic versus 349 mini-arthrotomy approach for mACI in the knee, with no difference between the two groups in Tegner, Lysholm, and International Knee Documentation Committee (IKDC) Score, and in the rate of failures and revisions [85]. PRP preparation and processing protocols have not been yet established. The initial whole blood volume, centrifugation rate, and duration of centrifugation is heterogeneous, and no consensus has been reached [86,87,88,89,90,91]. Evidence in support of the use of an activator for PRP (calcium chloride) is limited, and its implementation unclear [92, 93]. The best method to enhance surgical procedures addressing chondral defect is still unknown. Standardization of surgical procedures, methods of cell harvesting and delivery, and timing of outcome measures assessment must be better standardised. To optimize the performance of chondral procedures augmented with cell-based therapies, stricter eligibility criteria for such techniques must be also clarified. The current literature should be enriched with randomized controlled clinical trials with larger population size and longer follow-up times.
Conclusions
The current evidence suggests that cell therapies may be effective and safe to enhance surgical procedures for chondral defects of the talus. These results should be considered within the limitations of the present study. The current literature should be enriched with randomized controlled clinical trials with larger population size and longer follow-up.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available throughout the manuscript.
Abbreviations
- MFx:
-
Microfractures
- ACI:
-
Autologous chondrocyte implantation
- OAT:
-
Osteochondral allograft and autograft transplantation
- BMAC:
-
Stem cells (MSC) or bone marrow aspirate concentrate
- IKDC:
-
International Knee Documentation Committee
- MD:
-
Mean difference
- BMC:
-
Bone marrow concentrate
- PBC:
-
Peripheral blood concentrate
- PRP:
-
Platelet-rich plasma
- mACI:
-
Membrane-assisted autologous chondrocyte implantation
- PRGF:
-
Platelet-rich grow factor
- MAST:
-
Matrix-associated stem cell transplantation
- AMIC:
-
Autologous matrix-induced chondrogenesis
References
Orr JD, Dawson LK, Garcia EJ, et al. Incidence of osteochondral lesions of the talus in the United States military. Foot Ankle Int. 2011;32:948–54.
Williamson ERC, Shimozono Y, Toale J, et al. Incidence of chondral and osteochondral lesions in ankle fracture patients identified with ankle arthroscopy following rotational ankle fracture: a systematic review. J Foot Ankle Surg. 2021;11:11578.
Mei-Dan O, Carmont MR, Laver L, et al. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. 2012;40:534–41.
Migliorini F, Maffulli N, Baroncini A, et al. Allograft versus autograft osteochondral transplant for chondral defects of the talus: systematic review and meta-analysis. Am J Sports Med. 2021;36:35465211037349.
Litwic A, Edwards MH, Dennison EM, et al. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–99.
Migliorini F, Eschweiler J, Maffulli N, et al. Management of patellar chondral defects with autologous matrix induced chondrogenesis (AMIC) compared to microfractures: a four years follow-up clinical trial. Life (Basel). 2021;11:1145.
D’Ambrosi R, di Silvestri C, Manzi L, et al. Post-traumatic ankle osteoarthritis: quality of life, frequency and associated factors. Muscle Ligament Tendon J. 2019;9:363–71.
Anders S, Volz M, Frick H, et al. A randomized, controlled trial comparing autologous matrix-induced chondrogenesis (AMIC(R)) to microfracture: analysis of 1- and 2-year follow-up data of 2 centers. Open Orthop J. 2013;7:133–43.
Aigner J, Tegeler J, Hutzler P, et al. Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester. J Biomed Mater Res. 1998;42:172–81.
Devitt BM, Bell SW, Webster KE, et al. Surgical treatments of cartilage defects of the knee: systematic review of randomised controlled trials. Knee. 2017;24:508–17.
Karuppal R. Current concepts in the articular cartilage repair and regeneration. J Orthop. 2017;14:A1–3.
Migliorini F, Maffulli N, Baroncini A, et al. Matrix-induced autologous chondrocyte implantation versus autologous matrix-induced chondrogenesis for chondral defects of the talus: a systematic review. Br Med Bull. 2021;138:144–54.
Orr JD, Dunn JC, Heida KA Jr, et al. Results and functional outcomes of structural fresh osteochondral allograft transfer for treatment of osteochondral lesions of the talus in a highly active population. Foot Ankle Spec. 2017;10:125–32.
Park KH, Hwang Y, Han SH, et al. Primary versus secondary osteochondral autograft transplantation for the treatment of large osteochondral lesions of the talus. Am J Sports Med. 2018;46:1389–96.
Carey JL, Remmers AE, Flanigan DC. Use of MACI (autologous cultured chondrocytes on porcine collagen membrane) in the United States: preliminary experience. Orthop J Sports Med. 2020;8:2325967120941816.
Correa Bellido P, Wadhwani J, Gil ME. Matrix-induced autologous chondrocyte implantation grafting in osteochondral lesions of the talus: evaluation of cartilage repair using T2 mapping. J Orthop. 2019;16:500–3.
Migliorini F, Maffulli N, Schenker H, et al. Surgical management of focal chondral defects of the talus: a bayesian network meta-analysis. Am J Sports Med. 2021;3635:465211029642.
Migliorini F, Berton A, Salvatore G, et al. Autologous chondrocyte implantation and mesenchymal stem cells for the treatments of chondral defects of the knee-a systematic review. Curr Stem Cell Res Ther. 2020;11:5589.
Andriolo L, Reale D, Di Martino A, et al. Long-term results of arthroscopic matrix-assisted autologous chondrocyte transplantation: a prospective follow-up at 15 years. Am J Sports Med. 2020;48:2994–3001.
Calvi M, Curti M, Ossola C, et al. Knee articular cartilage injury treatment with matrix-induced autologous chondrocyte implantation (MACI): correlation at 24 and 120 months between clinical and radiological findings using MR arthrography. Skeletal Radiol. 2021;22:114.
Dai X, Fang J, Wang S, et al. Short- to midterm clinical and radiological outcomes after matrix-associated autologous chondrocyte implantation for chondral defects in Knees. Orthop J Sports Med. 2021;9:2325967120982139.
Ayyaswamy B, Salim M, Sidaginamale R, et al. Early to medium term outcomes of osteochondral lesions of the talus treated by autologous matrix induced chondrogenesis (AMIC). Foot Ankle Surg. 2021;27:207–12.
Puddu L, Altamore F, Santandrea A, et al. Surgical treatment of talar osteo-chondral lesions with micro-fractures, mesenchymal cells grafting on membrane, or allograft: mid-term clinical and magnetic resonance assessment. J Orthop. 2020;21:416–20.
Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42:648–57.
de Windt TS, Vonk LA, Slaper-Cortenbach ICM, et al. Allogeneic MSCs and recycled autologous chondrons mixed in a one-stage cartilage cell transplantion: a first-in-man trial in 35 patients. Stem Cells. 2017;35:1984–93.
de Windt TS, Vonk LA, Slaper-Cortenbach IC, et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells. 2017;35:256–64.
Buda R, Baldassarri M, Perazzo L, et al. A useful combination for the treatment of patellofemoral chondral lesions: realignment procedure plus mesenchymal stem cell-retrospective analysis and clinical results at 48 months of follow-up. Eur J Orthop Surg Traumatol. 2019;29:461–70.
Azam A, Forster M, Robertson A. Clinical and radiological outcome for Trufit Plug in the treatment of chondral and osteochondral lesions at a minimum of 2 years. J Orthop. 2018;15:47–51.
Migliorini F, Tingart M, Maffulli N. Progress with stem cell therapies for tendon tissue regeneration. Expert Opin Biol Ther. 2020;22:77802.
Longo UG, Rizzello G, Berton A, et al. Potential of adipose derived stem cells in orthopaedic surgery. Curr Stem Cell Res Ther. 2013;8:418–21.
Goto K, Aoyama T, Toguchida J, et al. Ten-year results of mesenchymal stromal cell transplantation augmented with vascularised bone grafts for advanced osteonecrosis of the femoral head. J Orthop. 2021;26:67–71.
Eltorai AE, Susai CJ, Daniels AH. Mesenchymal stromal cells in spinal fusion: current and future applications. J Orthop. 2017;14:1–3.
Hollander AP. Cell therapies and regenerative medicine-the dawn of a new age or more hype than hope? Clin Transl Med. 2012;1:12.
Enea D, Cecconi S, Calcagno S, et al. Single-stage cartilage repair in the knee with microfracture covered with a resorbable polymer-based matrix and autologous bone marrow concentrate. Knee. 2013;20:562–9.
Murata D, Tokunaga S, Tamura T, et al. A preliminary study of osteochondral regeneration using a scaffold-free three-dimensional construct of porcine adipose tissue-derived mesenchymal stem cells. J Orthop Surg Res. 2015;10:35.
Qian JJ, Xu Q, Xu WM, et al. Expression of VEGF-A signaling pathway in cartilage of ACLT-induced osteoarthritis mouse model. J Orthop Surg Res. 2021;16:379.
Liu H, Rui Y, Liu J, et al. Hyaluronic acid hydrogel encapsulated BMP-14-modified ADSCs accelerate cartilage defect repair in rabbits. J Orthop Surg Res. 2021;16:657.
Yang J, Li Y, Liu Y, et al. Role of the SDF-1/CXCR4 signaling pathway in cartilage and subchondral bone in temporomandibular joint osteoarthritis induced by overloaded functional orthopedics in rats. J Orthop Surg Res. 2020;15:330.
Tang JZ, Nie MJ, Zhao JZ, et al. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. J Orthop Surg Res. 2020;15:403.
Elksnins-Finogejevs A, Vidal L, Peredistijs A. Intra-articular platelet-rich plasma vs corticosteroids in the treatment of moderate knee osteoarthritis: a single-center prospective randomized controlled study with a 1-year follow up. J Orthop Surg Res. 2020;15:257.
Karakaplan M, Elmali N, Mirel E, et al. Effect of microfracture and autologous-conditioned plasma application in the focal full-thickness chondral defect of the knee: an experimental study on rabbits. J Orthop Surg Res. 2015;10:110.
Chen P, Huang L, Ma Y, et al. Intra-articular platelet-rich plasma injection for knee osteoarthritis: a summary of meta-analyses. J Orthop Surg Res. 2019;14:385.
Yausep OE, Madhi I, Trigkilidas D. Platelet rich plasma for treatment of osteochondral lesions of the talus: A systematic review of clinical trials. J Orthop. 2020;18:218–25.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142.
Howick JCI, Glasziou P, Greenhalgh T, Carl H, Liberati A, Moschetti I, Phillips B, Thornton H, Goddard O, Hodgkinson M. The 2011 Oxford CEBM Levels of Evidence. Oxford Centre for Evidence-Based Medicine. 2011. Available at https://wwwcebmnet/indexaspx?o=5653.
Briggs KK, Lysholm J, Tegner Y, et al. The reliability, validity, and responsiveness of the Lysholm score and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37:890–7.
Kitaoka HB, Alexander IJ, Adelaar RS, et al. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15:349–53.
Brozek JL, Akl EA, Alonso-Coello P, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64:669–77.
Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490.
Buda R, Vannini F, Castagnini F, et al. Regenerative treatment in osteochondral lesions of the talus: autologous chondrocyte implantation versus one-step bone marrow derived cells transplantation. Int Orthop. 2015;39:893–900.
Desando G, Bartolotti I, Vannini F, et al. Repair potential of matrix-induced bone marrow aspirate concentrate and matrix-induced autologous chondrocyte implantation for talar osteochondral repair: patterns of some catabolic, inflammatory, and pain mediators. Cartilage. 2017;8:50–60.
Guney A, Yurdakul E, Karaman I, et al. Medium-term outcomes of mosaicplasty versus arthroscopic microfracture with or without platelet-rich plasma in the treatment of osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2016;24:1293–8.
Murphy EP, Fenelon C, Egan C, et al. Matrix-associated stem cell transplantation is successful in treating talar osteochondral lesions. Knee Surg Sports Traumatol Arthrosc. 2019;27:2737–43.
Nguyen A, Ramasamy A, Walsh M, et al. Autologous osteochondral transplantation for large osteochondral lesions of the talus is a viable option in an athletic population. Am J Sports Med. 2019;47:3429–35.
Richter M, Zech S, Andreas MS. Matrix-associated stem cell transplantation (MAST) in chondral defects of the ankle is safe and effective - 2-year-followup in 130 patients. Foot Ankle Surg. 2017;23:236–42.
Richter M, Zech S, Meissner S, et al. Comparison matrix-associated stem cell transplantation (MAST) with autologous matrix induced chondrogenesis plus peripheral blood concentrate (AMIC+PBC) in chondral lesions at the ankle-a clinical matched-patient analysis. Foot Ankle Surg. 2020;26:669–75.
Wu PI, Diaz R, Borg-Stein J. Platelet-Rich plasma. Phys Med Rehabil Clin N Am. 2016;27:825–53.
Kemmochi M, Sasaki S, Takahashi M, et al. The use of platelet-rich fibrin with platelet-rich plasma support meniscal repair surgery. J Orthop. 2018;15:711–20.
Tayapongsak P, O’Brien DA, Monteiro CB, et al. Autologous fibrin adhesive in mandibular reconstruction with particulate cancellous bone and marrow. J Oral Maxillofac Surg. 1994;52:161–5 (discussion 166).
Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–8.
Goncalves NJN, Frantz N, de Oliveira RM. Platelet-rich plasma (PRP) therapy: an approach in reproductive medicine based on successful animal models. Anim Reprod. 2020;16:93–8.
Wasterlain AS, Braun HJ, Harris AH, et al. The systemic effects of platelet-rich plasma injection. Am J Sports Med. 2013;41:186–93.
Boswell SG, Cole BJ, Sundman EA, et al. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28:429–39.
Weber AE, Bolia IK, Trasolini NA. Biological strategies for osteoarthritis: from early diagnosis to treatment. Int Orthop. 2021;45:335–44.
Centeno CJ, Pastoriza SM. Past, current and future interventional orthobiologics techniques and how they relate to regenerative rehabilitation: a clinical commentary. Int J Sports Phys Ther. 2020;15:301–25.
Mazzocca AD, McCarthy MB, Chowaniec DM, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012;40:1742–9.
Harris SE, Bonewald LF, Harris MA, et al. Effects of transforming growth factor beta on bone nodule formation and expression of bone morphogenetic protein 2, osteocalcin, osteopontin, alkaline phosphatase, and type I collagen mRNA in long-term cultures of fetal rat calvarial osteoblasts. J Bone Miner Res. 1994;9:855–63.
Braun HJ, Wasterlain AS, Dragoo JL. The use of PRP in ligament and meniscal healing. Sports Med Arthrosc Rev. 2013;21:206–12.
Nourissat G, Mainard D, Kelberine F, et al. Current concept for the use of PRP in arthroscopic surgery. Orthop Traumatol Surg Res. 2013;99:S407-410.
Bennell KL, Hunter DJ, Paterson KL. Platelet-rich plasma for the management of Hip and Knee osteoarthritis. Curr Rheumatol Rep. 2017;19:24.
Scotti C, Hirschmann MT, Antinolfi P, et al. Meniscus repair and regeneration: review on current methods and research potential. Eur Cell Mater. 2013;26:150–70.
Alessandri-Bonetti M, Egro FM, Persichetti P, et al. The role of fat grafting in alleviating neuropathic pain: a critical review of the literature. Plast Reconstr Surg Glob Open. 2019;7:e2216.
Maidhof R, Rafiuddin A, Chowdhury F, et al. Timing of mesenchymal stem cell delivery impacts the fate and therapeutic potential in intervertebral disc repair. J Orthop Res. 2017;35:32–40.
Strassburg S, Hodson NW, Hill PI, et al. Bi-directional exchange of membrane components occurs during co-culture of mesenchymal stem cells and nucleus pulposus cells. PLoS ONE. 2012;7:e33739.
Shim EK, Lee JS, Kim DE, et al. Autogenous mesenchymal stem cells from the vertebral body enhance intervertebral disc regeneration via paracrine interaction: an in vitro pilot study. Cell Transplant. 2016;25:1819–32.
Gobbi A, Karnatzikos G, Scotti C, et al. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness Knee cartilage lesions: results at 2-year follow-up. Cartilage. 2011;2:286–99.
Makris EA, Gomoll AH, Malizos KN, et al. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21–34.
Gotze C, Nieder C, Felder H, et al. AMIC for focal osteochondral defect of the talar shoulder. Life (Basel). 2020;10:1147.
Gotze C, Nieder C, Felder H, et al. AMIC for traumatic focal osteochondral defect of the talar shoulder: a 5 years follow-up prospective cohort study. BMC Musculoskelet Disord. 2021;22:638.
Migliorini F, Eschweiler J, Maffulli N, et al. Autologous matrix induced chondrogenesis (AMIC) compared to microfractures for chondral defects of the talar shoulder: a five-year follow-up prospective cohort study. Life (Basel). 2021;11:5140.
Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95:1045–54.
Tan XW, Joukhadar N, Leduc S, et al. Outcome of retroarticular drilling for osteochondritis dissecans of the talus in a pediatric population. Foot Ankle Surg. 2021;22:9147.
Allahabadi S, Allahabadi S, Allala R, et al. Osteochondral lesions of the distal tibial plafond: a systematic review of lesion locations and treatment outcomes. Orthop J Sports Med. 2021;9:2325967121997120.
Migliorini F, Eschweiler J, Spiezia F, et al. Arthroscopy versus mini-arthrotomy approach for matrix-induced autologous chondrocyte implantation in the knee: a systematic review. J Orthop Traumatol. 2021;22:23.
Migliorini F, Driessen A, Quack V, et al. Comparison between intra-articular infiltrations of placebo, steroids, hyaluronic and PRP for Knee osteoarthritis: a Bayesian network meta-analysis. Arch Orthop Trauma Surg. 2021;141:1473–90.
Migliorini F, Kader N, Eschweiler J, et al. Platelet-rich plasma versus steroids injections for greater trochanter pain syndrome: a systematic review and meta-analysis. Br Med Bull. 2021;139:86–99.
Kirchner F, Pinar A, Milani I, et al. Vertebral intraosseous plasma rich in growth factor (PRGF-Endoret) infiltrations as a novel strategy for the treatment of degenerative lesions of endplate in lumbar pathology: description of technique and case presentation. J Orthop Surg Res. 2020;15:72.
Rodas G, Soler R, Balius R, et al. Autologous bone marrow expanded mesenchymal stem cells in patellar tendinopathy: protocol for a phase I/II, single-centre, randomized with active control PRP, double-blinded clinical trial. J Orthop Surg Res. 2019;14:441.
Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of Knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16.
Kim SJ, Yeo SM, Noh SJ, et al. Effect of platelet-rich plasma on the degenerative rotator cuff tendinopathy according to the compositions. J Orthop Surg Res. 2019;14:408.
Toyoda T, Isobe K, Tsujino T, et al. Direct activation of platelets by addition of CaCl2 leads coagulation of platelet-rich plasma. Int J Implant Dent. 2018;4:23.
Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–35.
Acknowledgements
None
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
FM contributed to conception and design, drafting, interpretation of the data, final approval; NM contributed to supervision, revision, final approval; PT contributed to supervision, final approval; JE contributed to drafting, final approval; CG contributed to supervision, final approval; FH contributed to supervision, final approval. All authors have agreed to the final version to be published and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study complies with ethical standards.
Consent for publication
Not applicable.
Competing interests
Professor Maffulli is the Editor in Chief of the Journal of Orthopaedic Surgery and Research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Migliorini, F., Eschweiler, J., Goetze, C. et al. Cell therapies for chondral defects of the talus: a systematic review. J Orthop Surg Res 17, 308 (2022). https://doi.org/10.1186/s13018-022-03203-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-022-03203-4