Abstract
Background
To systematically review the literature and provide a comprehensive understanding of the preemptive effects of oral pregabalin on perioperative pain management in lower limb orthopedic surgery.
Method
We searched three electronic databases for randomized controlled trials comparing the results of preoperative pregabalin and placebo in patients undergoing lower limb orthopedic surgery. Data analyses were conducted using RevMan 5.4.
Results
Twenty-one randomized controlled trials met our inclusion criteria. The cumulative opioid consumption within 24 and 48 h postoperatively in the pregabalin group was significantly less than that in the placebo group. The pooled static pain intensity at all time points within the first day was significantly lower in the pregabalin group than in the placebo group. Lower dynamic pain intensity at 48 h was detected in the pregabalin group than in the placebo group. Meanwhile, pregabalin led to a lower incidence of nausea but appeared to be associated with a higher incidence of dizziness and sedation. Subgroup analyses showed that no difference was detected between subgroups stratified by dosing regimen or pregabalin dose in the results of opioid consumption, pain intensity and incidence of complications.
Conclusion
This meta-analysis supports the use of pregabalin preoperatively in patients undergoing lower limb orthopedic surgery. However, it was wary of the resulting increase in dizziness and sedation. There is no evidence to support the continued use of pregabalin postoperatively or using more than 150 mg of pregabalin per day.
Trial registration: This study was registered on 09 November 2021 with INPLASY (registration number: INPLASY2021110031).
Similar content being viewed by others
Background
Acute postoperative pain is a common problem faced by patients undergoing surgical treatment and arises after activation of nociceptors, inflammation, and nerve injury [1, 2]. Unrelieved postoperative pain may lead to prolonged hospital stay and recovery time [3, 4]. Moreover, approximately 10–50% of patients may develop chronic pain after surgery, which could further affect their quality of life [2]. Considering the increased opioid consumption and its adverse effects as well as the complications caused by poor postoperative pain control, multimodal analgesia has been recommended in pain management [5, 6].
Pregabalin can reduce the release of excitatory neurotransmitters and the excitability of synapses by inhibiting calcium influx through high-voltage gated channels [7, 8]. It has been proven that pregabalin can exert its analgesic effect by reducing the hyperexcitability of dorsal horn neurons caused by tissue damage rather than reducing pain transmission from the injury site [9, 10]. Considering its analgesic properties, preoperative administration of pregabalin has been widely used in perioperative pain management, and its analgesic effectiveness in procedures (such as spinal surgery) has been confirmed by existing meta-analyses [3, 11].
Pregabalin has been used in lower limb orthopedic surgery for more than 10 years, and several previous randomized trials have explored its effect on perioperative pain management [12,13,14,15]. However, there is no official consensus as to whether pregabalin is effective in perioperative pain management for patients undergoing lower limb orthopedic surgery. A meta-analysis is warranted.
In this analysis, we aim to conduct a meta-analysis to compare the cumulative opioid consumption, pain intensity and incidence of complications after surgery between the pregabalin group and the placebo group to provide recommendations for surgeons and anesthesiologists.
Materials and methods
Inclusion and exclusion criteria
This meta-analysis was performed following the PRISMA guidelines (www.prisma-statement.org) and was registered on 10 November 2021 with INPLASY (registration number: INPLASY2021110031) (Details were displayed in Additional file 1: Appendix 1). The studies were selected based on the PICO criteria. Studies comparing the outcomes of cumulative opioid consumption, pain intensity or complication incidence following preoperative administration of oral pregabalin and placebo in patients undergoing lower limb orthopedic surgery were included in the current analysis.
Search strategy
The PubMed, EMBASE, and Cochrane Library databases were systematically searched (search trials are displayed in Additional file 2: Appendix 2). No restrictions on the publication status or language were applied.
Studies were assessed by two of the authors independently, and data were extracted with a standard data extraction form. The risk of bias was assessed for the included studies based on the Cochrane risk-of-bias criteria. Any disagreement was resolved through discussion.
Statistical analysis
The primary outcomes in this analysis were defined as the cumulative opioid consumption, static pain intensity and dynamic pain intensity. For opioid consumption, the reported data were converted to the oral morphine equivalent dose. For pain intensity, the data of VAS (0, no pain; 10, worst imaginable pain) were pooled. The secondary outcomes included the incidence of complications, such as nausea, vomiting, dizziness and sedation. Mean differences (MD) with a 95% CI were calculated using the inverse variance method for continuous variables, and risk ratios (RR) with a 95% CI were calculated using the Mantel–Haenszel analysis method for dichotomous variables. Heterogeneity was assessed using the Chi2 and I2 tests, and an I2 of > 50% was identified as substantial heterogeneity. Sensitivity analysis was performed for variables presenting with substantial heterogeneity by sequentially excluding individual studies. Subgroup analysis was conducted by stratifying studies according to dosing regimen (receiving pregabalin preoperatively only vs receiving pregabalin both pre- and postoperatively) and pregabalin dose (> 150 mg/day vs ≤ 150 mg/day). We used the Chi2 test to test for subgroup interactions. Analysis was undertaken using RevMan 5.4 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) with a significance threshold of P < 0.05.
Results
Study retrieved and characteristics
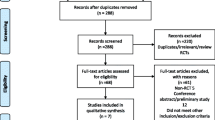
Twenty-one randomized controlled trials from 11 countries with a total sample size of 1520 (453 for the pregabalin group, 1067 for the placebo group) were included in the current analysis (Fig. 1) [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Characteristics including the author’s name, year, origin, anesthesia, and dosing regimen were extracted and are displayed in Table 1. The studies by Nimmaanrat et al. and Yik et al. were not included in the quantitative analysis because of the inconsistent results they reported [26, 31]. The studies by Martinez et al. and Niruthisard et al. were treated as two comparisons due to the combined use of other drugs [14, 27].
Study quality assessment
In the current analysis, we defined the trials as high quality when selection bias was graded as low risk, with the others as low or unclear. When selection bias was assessed as high risk, trials were graded as low quality. Trials were defined as moderate quality if they did not meet the above criteria. Sixteen trials were graded as high quality [12,13,14, 16,17,18, 20,21,22,23, 25, 27, 28, 30,31,32], and five trials were graded as moderate quality [15, 19, 24, 26, 29]. The details of the quality assessment are displayed in Fig. 2.
Cumulative opioid consumption at 24 h and 48 h postoperatively
The reported opioid consumption was uniformly converted to the oral morphine equivalent dose. Patients who received pregabalin preoperatively had significantly lower opioid consumption than patients receiving placebo at 24 h after surgery (MD − 34.47, 95% CI [− 52.32, − 16.63], P = 0.0002), and substantial heterogeneity was observed (I2 = 84%). For the results at 48 h after surgery, a similarly lower morphine equivalent dose was detected in patients receiving pregabalin than in those receiving placebo (MD − 46.69, 95% CI [− 75.11, − 18.28], P = 0.001). High heterogeneity was also seen (I2 = 68%) (Fig. 3). Sensitivity analyses were performed for the results, and no individual studies detected a significant influence on the pooled results of morphine consumption within 24 and 48 h (details are displayed in Additional file 3: Appendix 3).
Postoperative pain intensity
Significantly lower static pain intensity favoring patients who received pregabalin preoperatively was noted at 2 h (MD − 0.26, 95% CI [− 0.50, − 0.02], P = 0.04), 6 h (MD − 0.30, 95% CI [− 0.31, − 0.29], P < 0.00001), 12 h (MD − 0.53, 95% CI [− 0.78, − 0.29], P < 0.0001), and 24 h (MD − 0.24, 95% CI [− 0.38, − 0.10], P = 0.0008). However, no difference in static pain intensity was observed at 48 h between groups (MD − 0.15, 95% CI [− 0.49, 0.18], P = 0.38), with no substantial heterogeneity seen for static pain intensity (Fig. 4).
Patients who received preoperative pregabalin had significantly lower dynamic pain intensity than patients receiving placebo at 48 h (MD − 0.47, 95% CI [− 0.88, − 0.07], P = 0.02). However, no difference in dynamic pain intensity at 24 h was seen between groups (MD − 0.41, 95% CI [− 0.81, 0.00], P = 0.05), with no heterogeneity seen for dynamic pain intensity (Fig. 5).
Incidence of complications
Preoperative use of pregabalin lowered the incidence of nausea after surgery (RR 0.76, 95% CI [0.58, 0.99], P = 0.04). An increased postoperative incidence of dizziness and sedation was observed in patients treated with pregabalin (RR 1.67, 95% CI [1.21, 2.30], P = 0.002) for the incidence of dizziness; RR 1.69, 95% CI [1.08, 2.63], P = 0.03 for the incidence of sedation). No difference in the incidence of vomiting and drowsiness was seen in patients receiving pregabalin (RR 1.06, 95% CI [0.65, 1.70], P = 0.82 in the incidence of vomiting; RR 1.04, 95% CI [0.64, 1.69], P = 0.87 in the incidence of drowsiness). No substantial heterogeneity was seen for the outcomes of complications (Fig. 6).
Two trials included in this study reported the outcomes of chronic neuropathic pain assessed by the Leeds Assessment of Neuropathic Symptoms and Signs pain scale (LANSS) [13, 17]. Quantitative analysis of chronic pain was not performed because of the paucity of data.
Subgroup analyses
To compare the effectiveness of pregabalin in patients taking various dosing regimens, subgroup analyses were performed. A minimum of two comparisons per subgroup were available in the current analysis. There was no statistically significant difference in the results of cumulative opioid consumption within 24 h (test for subgroup differences, P = 0.30) in the subgroups of patients receiving pregabalin only before surgery or in patients receiving pregabalin both before and after surgery. Similarly, no significant difference between subgroups was shown in the results of static pain intensity at 24 h and incidence of complications. Details are displayed in Table 2.
To further explore the effect of pregabalin dose in patients undergoing lower limb orthopedic surgery, post hoc subgroup analyses were conducted in studies stratified by pregabalin dose (> 150 mg/day vs ≤ 150 mg/day). A minimum of two arms per subgroup was available in the current analysis. There was no statistically significant difference in opioid consumption within 24 h (test for subgroup differences, P = 0.62) between subgroups. Comparable results between subgroups were also detected in static pain intensity at 24 h (test for subgroup differences, P = 0.30), dynamic pain intensity at 24 h (test for subgroup differences, P = 0.72) and incidence of complications. Details are displayed in Table 3.
Discussion
The main findings of the current analysis were that the use of pregabalin preoperatively in patients undergoing lower limb orthopedic surgery appeared to be associated with lower morphine consumption at 24 h and 48 h. Similarly, decreased static pain intensity within 24 h and dynamic pain intensity at 48 h were detected in patients taking pregabalin. Compared with placebo, the use of pregabalin was associated with a reduction in the incidence of nausea but an increase in dizziness. No evidence was found with subgroup analyses to support the continued use of pregabalin postoperatively or using more than 150 mg of pregabalin per day.
To our knowledge, this was the first meta-analysis focused on the efficacy of pregabalin in perioperative pain management in patients undergoing lower limb orthopedic surgery. The primary outcomes in the current analysis were pain intensity and cumulative opioid consumption. A small reduction in the pain intensity at rest, as described in existing research [33], was detected in patients receiving pregabalin at all time points within the first day after surgery. Similar outcomes of pain intensity on movement at 48 h were also found. In addition, lower heterogeneity was noted among the studies. All the aforementioned outcomes confirm the analgesic effectiveness of pregabalin in patients undergoing lower limb orthopedic surgery, which has been known to induce hyperalgesia [34]. The test for subgroup differences was not significant, suggesting that altered pregabalin dose or dosing regimen does not affect the analgesic effectiveness of pregabalin.
Significantly lower morphine consumption within 24 h and 48 h was observed in patients receiving pregabalin, indicating that preoperative pregabalin could effectively reduce opioid consumption postoperatively. A similar result was also detected in a study by Lee et al. [24], who reported that pregabalin led to a reduction in fentanyl consumption during the first or the second days after surgery. Considering the reduction in pain intensity, the opioid-sparing effect of pregabalin can be regarded as a manifestation of analgesic effectiveness. Substantial heterogeneity was observed in the results, and sensitivity analyses were performed by subsequently excluding individual studies. However, no individual studies detected a significant influence on these pooled results, suggesting that the results were stable. Subgroup analyses were conducted on morphine consumption within 24 h by stratifying studies according to the pregabalin dose and dosing regimen. The heterogeneity persisted in each subgroup, suggesting that variations in pregabalin dose and dosing regimen could not explain the heterogeneity in opioid consumption. Although we converted the reported data to the oral morphine equivalent dose uniformly, the variations in the types of opioids and their routes of administration may be the possible source of heterogeneity in opioid consumption. Other possible explanations may be the various surgical sites, incision lengths, surgical experiences, perioperative analgesic regimens, and patient factors. However, it was impossible to perform subgroup analyses with regard to the aforementioned items because of the paucity of data. Additionally, the test for subgroup differences (stratified by pregabalin dose and dosing regimen) was not significant, suggesting that an increased pregabalin dose or long-term dosing regimen seemed to be ineffective in further reducing the consumption of opioids.
Recently, perioperative pain management has sought to reduce the incidence of opioid-related adverse effects [33]. In the current analysis, a lower incidence of nausea was observed in patients receiving pregabalin, indicating that pregabalin can reduce the incidence of opioid-related adverse effects through its opioid-sparing effect. The incidence of dizziness and sedation, however, was significantly higher in the pregabalin group, which is considered to be an adverse effect associated with pregabalin [35]. Hence, we should use pregabalin with caution. Moreover, the test for subgroup differences regarding the results of dizziness stratified by pregabalin dose was not significant, indicating that pregabalin had no dose effect on the incidence of dizziness. These results are contrary to the outcomes of previous research, which reported that the adverse effects of pregabalin are dose-dependent [36]. The difference in results may be due to the variations in the types of surgery. Moreover, considering the indirect comparisons that existed in our current analysis, we cannot exclude a possible dose effect of pregabalin on adverse effects. Hence, considering that there is no detectable difference in the results of pregabalin’s analgesic effect and opioid-sparing effect between subgroups stratified by pregabalin dose, we recommend that the use of pregabalin does not exceed 150 mg per day.
Only two trials that reported the incidence of chronic neuropathic pain were identified in this analysis. Formal meta-analysis was not performed because of the paucity of data. Buvanendran et al. [17] reported a lower incidence of chronic neuropathic pain in patients receiving pregabalin. However, no significant reduction in the incidence of chronic neuropathic pain was noted in the study by Yadeau et al. [13]. At present, no clear evidence has been proposed with regard to the beneficial effects of pregabalin on the prevention of chronic neuropathic pain. More research that focuses on chronic neuropathic pain is needed to solve this problem.
Several limitations were detected in the current analysis. First, substantial heterogeneity among studies was observed regarding the results of morphine consumption, and further studies are needed to enhance the strength of the evidence or find the source of heterogeneity. Second, comparisons of the outcomes stratified by pregabalin dose or dosing regimen were indirect in the current analysis, and studies that compared different doses or dosing regimens directly would have to further evaluate the optimal dosage and dosing regimen of pregabalin. Third, the data presented in some studies were not suitable for pooling for meta-analyses. Finally, although the results were statistically significant, whether it is clinically significant requires further investigation, randomized controlled trials with a larger sample size are needed in future work.
In summary, this meta-analysis supports the use of pregabalin preoperatively in patients undergoing lower limb orthopedic surgery with regard to opioid-sparing and analgesic effects. However, it is wary of the resulting increase in the incidence of dizziness and sedation. At present, there is no evidence to recommend the continued use of pregabalin postoperatively or the use of more than 150 mg of pregabalin per day.
Availability of data and materials
As a meta-analysis, there are no patient data sets.
References
Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–40.
Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25.
Yu L, Ran B, Li M, Shi Z. Gabapentin and pregabalin in the management of postoperative pain after lumbar spinal surgery: a systematic review and meta-analysis. Spine (Phila Pa 1976). 2013;38:1947–52.
Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82:679–84.
Pitchon DN, Dayan AC, Schwenk ES, Baratta JL, Viscusi ER. Updates on multimodal analgesia for orthopedic surgery. Anesthesiol Clin. 2018;36:361–73.
Dunkman WJ, Manning MW. Enhanced recovery after surgery and multimodal strategies for analgesia. Surg Clin N Am. 2018;98:1171–84.
Shneker BF, McAuley JW. Pregabalin: a new neuromodulator with broad therapeutic indications. Ann Pharmacother. 2005;39:2029–37.
Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, et al. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A. 2006;103:17537–42.
Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission. Trends Pharmacol Sci. 2007;28:75–82.
Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol. 2007;20:456–72.
Jiang HL, Huang S, Song J, Wang X, Cao ZS. Preoperative use of pregabalin for acute pain in spine surgery: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96:e6129.
Buvanendran A, Kroin JS, Della VC, Moric M, Tuman KJ. Cerebrospinal fluid neurotransmitter changes during the perioperative period in patients undergoing total knee replacement: a randomized trial. Anesth Analg. 2012;114:434–41.
YaDeau JT, Lin Y, Mayman DJ, Goytizolo EA, Alexiades MM, Padgett DE, et al. Pregabalin and pain after total knee arthroplasty: a double-blind, randomized, placebo-controlled, multidose trial. Br J Anaesth. 2015;115:285–93.
Martinez V, Cymerman A, Ben AS, Fiaud JF, Rapon C, Poindessous F, et al. The analgesic efficiency of combined pregabalin and ketamine for total hip arthroplasty: a randomised, double-blind, controlled study. Anaesthesia. 2014;69:46–52.
Singla NK, Chelly JE, Lionberger DR, Gimbel J, Sanin L, Sporn J, et al. Pregabalin for the treatment of postoperative pain: results from three controlled trials using different surgical models. J Pain Res. 2015;8:9–20.
Akhavanakbari G, Entezariasl M, Isazadehfar K, Mirzarahimi T. The effects of oral pregabalin on post-operative pain of lower limb orthopedic surgery: a double-blind, placebo-controlled trial. Perspect Clin Res. 2013;4:165–8.
Buvanendran A, Kroin JS, Della VC, Kari M, Moric M, Tuman KJ. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg. 2010;110:199–207.
Clarke H, Pagé GM, McCartney CJ, Huang A, Stratford P, Andrion J, et al. Pregabalin reduces postoperative opioid consumption and pain for 1 week after hospital discharge, but does not affect function at 6 weeks or 3 months after total hip arthroplasty. Br J Anaesth. 2015;115:903–11.
Damirchi AN, Kamali A, Azami M, Monfared ME. Comparison of the effect of apotel and pregabalin on postoperative pain among patients undergoing lower limb orthopedic surgeries. J Family Med Prim Care. 2019;8:2405–8.
Jain P, Jolly A, Bholla V, Adatia S, Sood J. Evaluation of efficacy of oral pregabalin in reducing postoperative pain in patients undergoing total knee arthroplasty. Indian J Orthop. 2012;46:646–52.
Kavak AF, Baran AI, Altinsoy S, Özkan D, Ergil J. The effects of pregabalin and adductor canal block on postoperative pain in arthroscopic anterior cruciate ligament reconstruction. Turk J Med Sci. 2020;50:195–204.
Kheirabadi D, Safavi MR, Taghvaei M, Habibzadeh MR, Honarmand A. Comparing the prophylactic effects of oral gabapentin, pregabalin, and celecoxib on postoperative pain management in orthopedic surgery of the lower extremity: a double-blind randomized controlled trial. J Res Med Sci. 2020;25:9.
Khetarpal R, Kataria AP, Bajaj S, Kaur H, Singh S. Gabapentin vs pregabalin as a premedication in lower limb orthopaedics surgery under combined spinal epidural technique. Anesth Essays Res. 2016;10:262–7.
Lee JK, Chung KS, Choi CH. The effect of a single dose of preemptive pregabalin administered with COX-2 inhibitor: a trial in total knee arthroplasty. J Arthroplasty. 2015;30:38–42.
Mathiesen O, Jacobsen LS, Holm HE, Randall S, Adamiec-Malmstroem L, Graungaard BK, et al. Pregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplasty. Br J Anaesth. 2008;101:535–41.
Nimmaanrat S, Tangtrakulwanish B, Klabklay P, Boonriong T. Perioperative administration of pregabalin in patients undergoing arthroscopic anterior cruciate ligament reconstruction: does it help to relieve postoperative pain? J Med Assoc Thail. 2012;95:1297–301.
Niruthisard S. Preoperative pregabalin and/or celecoxib for pain management after total knee arthroplasty under intrathecal morphine: a randomized controlled trial. Asian Biomed. 2013;7:579–85.
Omara AF, Ahmed SA, Abusabaa MM. The effect of the use of pre-emptive oral pregabalin on the postoperative spinal analgesia in patients presented for orthopedic surgeries: randomized controlled trial. J Pain Res. 2019;12:2807–14.
Rahat DA, Moosavi A, Nasir-Al-Din TS, Ordoni AJ, Sistanizad M. The effect of a single dose oral pregabalin on hemodynamic changes and duration of analgesia after spinal anesthesia in orthopedic surgeries of tibial fractures. Iran J Pharm Res. 2018;17:2–7.
Sebastian B, Talikoti AT, Nelamangala K, Krishnamurthy D. Effect of oral pregabalin as preemptive analgesic in patients undergoing lower limb orthopedic surgeries under spinal anaesthesia. J Clin Diagn Res. 2016;10:C1-4.
Yik JH, Tham W, Tay KH, Shen L, Krishna L. Perioperative pregabalin does not reduce opioid requirements in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2019;27:2104–10.
Yadeau JT, Paroli L, Kahn RL, Jules-Elysee KM, Lasala VR, Liu SS, et al. Addition of pregabalin to multimodal analgesic therapy following ankle surgery: a randomized double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2012;37:302–7.
Eipe N, Penning J, Yazdi F, Mallick R, Turner L, Ahmadzai N, et al. Perioperative use of pregabalin for acute pain-a systematic review and meta-analysis. Pain. 2015;156:1284–300.
Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: a meta-analysis. Br J Anaesth. 2011;106:454–62.
Van Ameringen M, Mancini C, Pipe B, Bennett M. Antiepileptic drugs in the treatment of anxiety disorders: role in therapy. Drugs. 2004;64:2199–220.
White PF, Tufanogullari B, Taylor J, Klein K. The effect of pregabalin on preoperative anxiety and sedation levels: a dose-ranging study. Anesth Analg. 2009;108:1140–5.
Acknowledgements
The authors want to thank the family for their support.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 31870961).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. ZC had the idea for the article. A literature search and data analysis were performed by ZC, JC and RL. The first draft of the manuscript was written by ZC, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. The details of the registration information.
Additional file 2
. The details of the search trial.
Additional file 3
. The results of the sensitivity analyses,
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Z., Chen, J., Luo, R. et al. The preemptive effects of oral pregabalin on perioperative pain management in lower limb orthopedic surgery: a systematic review and meta-analysis. J Orthop Surg Res 17, 237 (2022). https://doi.org/10.1186/s13018-022-03101-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-022-03101-9