Abstract
Objective
To evaluate the impact of the residual setup errors from differently shaped region of interest (ROI) and investigate if surface-guided setup can be used in radiotherapy with concurrent tumor treating fields (TTFields) for glioblastoma.
Methods
Fifteen patients undergone glioblastoma radiotherapy with concurrent TTFields were involved. Firstly, four shapes of region of interest (ROI) (strip-shaped, T-shaped, \(\perp\)-shaped and cross-shaped) with medium size relative to the whole face were defined dedicate for patients wearing TTFields transducer arrays. Then, ROI-shape-dependent residual setup errors in six degrees were evaluated using an anthropomorphic head and neck phantom taking CBCT data as reference. Finally, the four types of residual setup errors were converted into corresponding dosimetry deviations (including the target coverage and the organ at risk sparing) of the fifteen radiotherapy plans using a feasible and robust geometric-transform-based method.
Results
The algebraic sum of the average residual setup errors in six degrees (mm in translational directions and ° in rotational directions) of the four types were 6.9, 1.1, 4.1 and 3.5 respectively. In terms of the ROI-shape-dependent dosimetry deviations, the D98% of PTV dropped off by (3.4 ± 2.0)% (p < 0.05), (0.3 ± 0.5)% (p < 0.05), (0.9 ± 0.9)% (p < 0.05) and (1.1 ± 0.8)% (p < 0.05). The D98% of CTV dropped off by (0.5 ± 0.6)% (p < 0.05) for the strip-shaped ROI while remained unchanged for others.
Conclusion
Surface-guided setup is feasible in radiotherapy with concurrent TTFields and a medium-sized T-shaped ROI is appropriate for the surface-based guidance.
Similar content being viewed by others
Introduction
Maintenance therapy with tumor treating fields (TTFields) has confirmed advantage to improve both overall survival (OS) and progression-free survival (PFS) without significant deteriorate of health-related quality of life (HRQoL) for glioblastoma (GBM) [1,2,3]. Technically, four transducer arrays (each consists of 9 button-type ceramic electrodes arranged in 3 × 3 adhered on a gel patch) are attached to skull of patient to establish the intra-cranial treating field [4]. At present, “radiotherapy + concurrent/adjuvant Temozolomide + TTFields” has been preferred in the NCCN guidelines for the treatment of newly diagnosed GBM as a Class I recommendation (Level 1 evidence) [5].
However, there have been increasing interests on the timing of intervention of TTFields in recent years. It has been found that there are therapeutic synergies when TTFields is used with concurrent radiotherapy (TTFields intervenes on the first day of radiotherapy). In 2017, it was reported that the outcome of radiotherapy was enhanced by TTFields at the cellular level [6]. Clinically, safety and feasibility have been proven for radiotherapy and concurrent TTFields [7, 8]. Furthermore, the phase II clinical trial initially examined the therapeutic synergies and observed the improved local control [9]. The ongoing phase III randomized clinical trial, EF32, aims to definitively confirm whether synergistic effect exists [10]. Moreover, taking off the disposable and expensive transducer arrays during each fraction of radiotherapy will be too costly to practice in clinical scenes, which will have an impact on the compliance and the final survival outcome [11]. Thus, facing the coexistence of imaging and radiation and TTFields electrodes may be inevitable from the point of view of feasibility and economy in these clinical trial as well as the future practice. The clinical efforts and initial results also arouse the research interests in technical aspect about the compatibility between the two treatment modalities and several efforts have been made in order to deal with the challenges such as the dosimetry issue [12,13,14,15].
Patient positioning is one of the decisive factor for the precise dose delivery. High-density metal leads to kV-CT artifacts due to the beam-hardening and the photon starvation [16]. In radiotherapy with concurrent TTFields, artifacts from the transducer arrays lead to the vague of the bony structure of the patient and affect the registering and evaluation of the kV-CBCT-guided patient positioning [17]. MV-CBCT may be a potential alternative to deal with this challenge [18]. However, little dada is published recently about the application of MV-CBCT-guided radiotherapy with concurrent TTFields. Moreover, the lower popularizing rate of the on-board MV-CBCT also limits its usage in this field. Thus, new modality and workflow need be developed and verified for accurate and robust patient positioning in the image-guided radiotherapy with concurrent TTFields.
Surface-guided radiotherapy (SGRT) is a unique technology characterized by real-time capability and non-radiation used to verify and monitor the position of patients before or during the dose delivery [19]. Technically, patient’s surface is reconstructed using images captured by optic cameras in different directions and, then, is co-registered with the reference surface in the user defined region of interest (ROI) to obtain the positioning error. Since cranium can be viewed by and large as rigid without significant deformation of the internal atomical structure, the facial surface is generally corelative well to the inner tumor and organs compared with other anatomical sites (e.g. chest and abdomen). Therefore, for the head & neck and brain radiotherapy, due to the convenience and non-radiation characteristics of the surface-guidance, it can be regarded as a supplement to CBCT-guidance, reducing the use frequency of CBCT-guidance. Recently, studies on SGRT for the head and neck and brain tumor were reported with improved accuracy of patient positioning and dose delivery as well as the treatment time reduction [20,21,22]. What is more, in particular cases where patients wear transducer arrays and, thus, CBCT-guidance is negatively impacted, surface-guidance may be a promising alternative and play a more important role than in the conventional head & neck or brain radiotherapy situations. Thus, there may be just right promising advantage to apply surface-based guidance to radiotherapy with concurrent TTFields. However, situation of patients undergoing radiotherapy combined with TTFields differs from sole head and neck or brain radiotherapy in two sides: (1) high incidence of the scalp toxicity may lead to the involuntary movement of patients’ heads and limit the open-faced size for the fixation consideration [8]; (2) transducer arrays coverage leaves part of the facial vertex data unavailable [4]. However, it is not clear whether the two limitations challenge the potential application of SGRT, especially the surface-guided setup, in the context that patients have to wear the transducer arrays during the radiotherapy.
The aim of this study is to preliminarily evaluate the feasibility of the surface-guided setup in the radiotherapy with concurrent TTFields. Firstly, four shapes of ROIs with medium size relative to the whole face were defined dedicate for patients wearing TTFields transducer arrays. Then, ROI-shape-dependent residual setup errors in six degrees were evaluated using an anthropomorphic head & neck phantom taking CBCT data as reference. Finally, the four types of residual setup errors were converted into corresponding dosimetry deviations (including the target coverage and the organ at risk sparing) of the fifteen radiotherapy plans using a feasible and robust geometric-transform-based method. Through this method, the performance of different ROIs in the surface-guided setup in the radiotherapy with concurrent TTFields as well as the feasibility were evaluated.
Material and method
Patient cohort
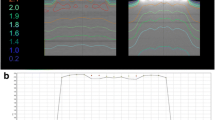
Fifteen patients undergone radiotherapy with concurrent TTFields were involved in this study. The immobilization of patients were based on the combination of the thermoplastic head supporter and the thermoplastic mask (Klarity Medical System, Guangzhou, China) with customed sponge medium adhered for the transducer arrays accommodating [7]. The 2 mm slice thickness planning CT-scan (Siemens Healthineers, Erlangen, Germany) were performed in the absence of the transducer arrays in order to avoid beam hardening artifacts. The gross target volume (GTV) and the clinical target volume (CTV) were contoured by experienced clinicians and the planned target volume (PTV) were uniformly expanded by 3 mm from the CTV. The positions of the tumor centers of the fifteen patients covered the frontal, occipital, parietal and temporal lobes and PTV volumes ranged from 160.7 cc to 604.2 cc. All radiotherapy plans were convention-fractionated (60 Gy in 30 fractions) coplanar intensity-modulated radiotherapy (IMRT) plans with 7–9 fields and were generated and approved by experienced dosimetrists in the Eclipse15.6 Treatment Planning System (TPS) (Varian Medical Systems, Palo Alto, CA, USA). The placements of the four transducer arrays (Novocure GmbH, Lucerne, Switzerland) were optimized based on the shape and size of the patient's skull and PTV, as well as the geometric relationship between them and located generally in the frontal lobe, posterior occipital, and left and right temporal lobes, respectively. Figure 1 shows two examples of TTFields plans generated in the NovoTALTM software (Novocure GmbH, Lucerne, Switzerland) in which patients were wearing grouped transducer arrays on their heads. The main part of forehead of each patient was covered by the front array consisting of button-type electrodes and the gel patch. The lower patch edge of the front array was cut off after the attachment of arrays were done (dashed line represented the shear line) in order to improve the patient comfort and enlarge the open-face area during radiotherapy. In final, the radiation dose were delivered to patients wearing the transducer arrays on the TrueBeam Linac (Varian Medical Systems, Palo Alto, CA, USA). This study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Nanjing Medical University, and the ethics number is 2023-071.
Equipment and predefined ROIs
Optical Surface Monitor System (OSMS, VisionRT, London, UK) was used as the surface-based guiding equipment [23]. Four shapes of ROIs with medium size relative to the whole face were defined dedicate for patients wearing TTFields transducer arrays as candidates. The first type was approximately strip-shaped located under the inferior orbits and above the upper lip with the left and right boundaries to auricles. The second type added the upper jaw bone to the first one (T-shaped ROI); and whilst the third type added the interocular area and a few part of lower forehead to the first one (\(\perp\)-shaped ROI). The last type was the union of the T-shaped and \(\perp\)-shaped ones, which showed cross-shaped. All shapes of ROIs could be contoured manually on the workstation of OSMS with high convenience.
ROI-shape-dependent residual setup error (Roi-Se)
CIRS-038 phantom (SunNuclear, Florida, USA) was used for Roi-Se evaluation of SGRT. CIRS-038 is a head & neck phantom with verisimilar male facial surface in optic and anthropomorphic internal anatomical structure in CT, which is appropriate for both surface registration and 3D registration. Before the test, the phantom was scanned in CT simulator with 1 mm slice thickness and the CT image was imported into Eclipse 15.6 Treatment Planning System (TPS) to generate surface point cloud. Then, CT image and surface point clouds were imported into the ARIA and OSMS as references of the surface registration and 3D registration. The four predefined medium-size ROIs were contoured in the surface in the work station of OSMS (Fig. 2). The visible part of the strip-shaped ROI shown in Fig. 2a was a rectangle with the approximate size (width × height) of 10 × 4 cm2, while each of the bilateral invisible parts in the side face was also a rectangle with the approximate size of 3 × 4 cm2. The added rectangle of either the T-shaped ROI (the lower part in Fig. 2b) or the \(\perp\)-shaped ROI (the upper part in Fig. 2c) compared with the strip-shaped ROI was approximately 4 × 3 cm2. And so on to the size of the cross-shaped ROI in Fig. 2d. 10 groups of preset setup errors (each contained 3 translational and 3 rotational degrees) were defined for the test, as is shown in Table 1.
In the Roi-Se test, CBCT-guided setup of CIRS-038 was firstly performed using CT as reference. Then, the 10 groups of preset setup errors was introduced one by one by moving the 6D couch. In final, different ROI-based surface-guided guidance were performed for each introduced setup error and the corresponding residual setup errors were recorded.
ROI-shape-dependent dosimetry deviation (Roi-De)
A common method to introduce the translational setup errors into a radiotherapy plan (with a single isocenter) is to move the isocenter of each field, which was adopted in this study. As for the rotational setup errors, we used a geometric-transform-based method for realization. Yue et al. proposed that targeted adjustment to the three geometric parameters (the gantry angle, the collimator angle and the table angle) of each field of a IMRT plan can compensate the rotational setup errors in geometry before the dose delivery [24]. The specific formula of the geometric transform was also given in their study. In this study, we use the inverse operation to transform the resulted rotational components of the Roi-Se to the corresponding three geometric parameters of each IMRT field, through which the measured rotational Roi-Se were introduced into the fifteen plans of glioblastoma in TPS with a simple and robust method. In fact, this method can be described as to find the proper orientation parameters (the three angles) to modify the IMRT beam (but the MLC sequence remains unchanged) to match the orientation of the patient in the view of the modified beam with the orientation of the patients with rotational setup error in the view of the original beam. Figure 3 shows the 3D views (from Eclipse 15.6) of the geometric-transform-based method to introduce the rotational setup error of − 10° in three directions into an IMRT plan for glioblastoma for an intuitive presentation. The fields arrangement with modified geometric parameters remained coplanar and unchanged under the transformed machine coordinate system (Fig. 3b), while the patient orientation changed a certain amount relative to that of the reference plan (Fig. 3a). Finally, the modified plans were recalculated with the preset Mu values in order to obtain the dosimetry deviations as Roi-De.
3D views (from Eclipse 15.6) of the geometric-transform-based method to introduce the rotational setup error of − 10° in three directions into an IMRT plan for glioblastoma (a: the reference plan; b: the modified plan). (Setup error of − 10° here is to make the difference of the patient orientations between (a) and (b) obvious and does not mean that it happened in clinical scenes.)
The dosimetric metrics of the modified plans were extracted including the D98%, D2% and Dmean of CTV and PTV and organ at risk (OAR) sparing (the bilateral optical nerves, brainstem and chiasm). All work were completed using an in-house ESAPI-based C# program [25, 26].
Result
Roi-Se
Figure 4 shows four types of ROI-shape-dependent residual setup errors in six degrees (mm in translational directions and ° in rotational directions) as well as the dimensionless algebraic sums of the averages (for intuitive assessment). The standard deviation lower than 0.2 mm in translational direction and 0.2° in rotational direction showed high stability and implied that the systematic component was larger than the random component. The strip-shaped Roi-Se was as high as 3.6 mm in longitudinal direction and 2.2° in pitch direction, while absolute values in other directions were lower than 1 (mm or °) (Fig. 4a). Other metrics with absolute value larger than 1 (mm or °) included the \(\perp\)-shaped ROI in longitudinal and pitch directions and the cross-shaped ROI in vertical direction (Fig. 4c and d). As for the T-shaped Roi-Se, the algebraic values in all directions were in the range of [− 1, 1] (lower than 0.3° in rotational directions in especial), representing higher consistency with CBCT (Fig. 4b). It was also found that the dimensionless algebraic sum of the strip-shaped Roi-Se was the largest on the whole with value of 6.9 (Fig. 4e). Oppositely, the T-shaped Roi-Se had the smallest value of 1.1. In addition, the sum values of \(\perp\)-shaped ROI and cross-shaped ROI were 4.1 and 3.5 respectively.
Roi-De
The strip-shaped Roi-Se lead to the (3.4 ± 2.00)% (p < 0.05) decrease of the PTV D98% and (0.5 ± 0.6)% (p < 0.05) decrease of the D98% of CTV, given 3 mm margin from CTV to PTV. However, the reduction of PTV D98% resulting from other three types of Roi-Se were (0.3 ± 0.5)% (p < 0.05), (0.9 ± 0.9)% (p < 0.05) and (1.1 ± 0.8)% (p < 0.05), and did not cause significant deterioration of CTV D98% (p > 0.05). Figure 5 gives four types of ROI-shape-dependent dosimetry deviation compared with the reference plans. The deviations of D2% and Dmean of the PTV and CTV were not statistically significant (p > 0.05). As for the OAR sparing, all types of Roi-Se changed none of metrics significantly. Figure 6 shows an example of the four types of ROI-shape-dependent dose-volume histogram of PTV (a and b) and CTV (c and d). In this case, D98% of CTV using strip-shaped-ROI guidance dropped to a value lower than 60 Gy while the CTV coverage remained sufficient in other types of ROI guidance (seen in Fig. 6d).
Discussion
Feasibility of SGRT for the head & neck and brain tumors have been reported in several studies. Wei et al. studied 60 head & neck SGRT cases and found that surface-guidance improved setup accuracy and efficiency [20]. Lee et al. examined 269 surface-guided stereo-tactic radiosurgery (SRS) cases and concluded that surface-guidance has an extra advantage of real-time cranial motion monitoring beside of accuracy and efficiency [21]. Victoria used the 3D anthropomorphic gel phantoms to perform end-to-end verification on multiple metastases surfaced-guided SRS and demonstrates good accuracy of setup and dose delivery [22]. These studies also hint at the feasibility of non-radiation and real-time SGRT for glioblastoma. Moreover, since setup uncertainty resulting from artifacts of the TTFields arrays in kV-CBCT guidance may increase, the alternative use of surface guidance is even more important compared with other treatment modality. Nevertheless, to our known, this is the first investigation about surface-guided setup in radiotherapy with concurrent TTFields for glioblastoma.
The imaging devices, reconstruction algorithm and registration algorithm synthetically determines the guiding performance in SGRT. However, accuracy does not rely solely on the system feature but also the user decision, in which the ROI selection plays an important role. Similar with registrations of other image modalities, surface registration is a numerical optimization process, of which the accuracy depends on the included vertex points contained in user-defined ROI [27]. The lower standard deviations of the results from the Roi-Se test demonstrate that the surface-guided system is relatively stable and the effective input range is enough to cover most clinical situations in case of the medium-size ROIs. Meanwhile, a systematic bias compared with CBCT does exist with dependence on the selected ROI style. Therefore, attentions should be paid to the of ROI-dependent features in order to find the optimal ones in SGRT with concurrent TTFields.
ROI strategies for other sites have been discussed in earlier studies [28,29,30]. For the facial surface, ROI were usually defined as the combination of the mid-face area above lip and the front of the forehead [20,21,22]. This type of ROI were considered balanced between the immobility of open-faced thermoplastic mask and the sufficiency of the optical vertex points data. However, for patients undergoing radiotherapy combined with TTFields, the forehead data are unavailable for surface reconstruction due to the coverage of the transducer arrays (as is shown in Fig. 1). Moreover, since the scalp adverse reactions exists commonly in patients undergoing TTFields, the open-faced area of the thermoplastic mask cannot be too large for the consideration of immobility. Thus, a medium-size ROI rather than a larger-size ROI containing whole face may be more suitable. In this study, four medium-size ROIs with or without a little part of forehead were defined and verified. Moreover, for the facial surface, it has been reported that appropriate ROI contains sufficient vertex points, obvious 3D feature and excludes regions reflecting sole local motion [31, 32]. Bry et al. reported that facial motion lead to false positional corrections in SGRT and the adverse impact is greater in smaller-size ROI [33]. The conclusion is partly followed in this study: the predefined four types of ROI were designed to avoid eyebrows, the lower cheeks and the lower jaw that moves sensitively with human’s expressions like fear. However, the nose was retained for the considerations of the signal stability and 3D feature robustness.
Since each dimension of setup error affects the dose to a different degree, the information given by sole ROI-shape-dependent residual setup error is limited. Through a software developed previously, we further introduced the 6D ROI-shape-dependent residual setup error into fifteen IMRT plans for glioblastoma and converted it into corresponding dosimetry deviation with a simple but reliable geometric-transformation-based method. On one hand, this method realized the “rotation” of the reference CT image relative to the field arrangement in effect inside TPS, and directly obtained the simulated dose distribution after recalculation, avoiding the calculation uncertainty caused by additional image processing. On the other hand, since the simulation process did not require external software and radiotherapy file import/export, it improved the efficiency and feasibility of the setup error simulation [34,35,36].
Strip-shaped ROI resulted in largest setup errors among the defined ROI especially in longitudinal and pitch directions and thus diminished not only the PTV coverage but also the CTV coverage, which made itself unsuitable for the surface guidance. T-shaped Roi-Se corresponds well to the data from CBCT and proves to have impact on the PTV coverage rather than the CTV coverage. Compared with the T-shaped ROI, the cross-shaped ROI contains vertex points on the forehead, but does not improve the accuracy of the surface registration. This may be because the vertex points from a flat forehead does not contain enough information about 3D feature except in vertical direction and results in extra setup errors. In other words, forehead information may not be as important for head and neck SGRT as often thought. Just from the point of view of the setup error, the performance of the \(\perp\)-shaped ROI and cross-shaped is not as good as the T-shaped ROI. Nevertheless, it as well does not affect the CTV coverage. The reason may be that the dosimetry deviation of the CTV coverage ought to exist is compensated by the 3 mm margin from CTV to PTV. Although, it is difficult to analytically explain the specific dosimetric effects of the various dimensions of Roi-Se on the glioblastoma radiotherapy plans, the evidence-based result may give insights to aid decision-making. However, due to the other non-ideal factors during radiotherapy, caution is required when using the \(\perp\)-shaped ROI and the cross-shaped ROI to perform the surface-guided setup in the glioblastoma radiotherapy with concurrent TTFields. A IMRT plan with a larger setup error may be more sensitive to other non-ideal factors in the dose delivery process. For a given ROI, the smaller slice thickness of the CT scan compared with those for real patients in this study caused a larger number of surface points. However, Dong et al. reported that the accuracy of the surface-guided setup was sufficient regardless of the slice thickness of the CT scan based on a phantom study [37]. Thus, the results can be generalized to the real patient despite of the different slice thickness of the CT scan.
This study has several limitations. Phantom-based investigation demonstrated that the accuracy of the surface-guided setup depends on the selection of ROI. However, the facial surface used may be too unitary to generalize to the population. In the future, ROI study of 3D-printed personalized surface may lead more profound understanding in this field and serve as a dedicated part of the overall QA program for glioblastoma SGRT with concurrent TTFields. The other main limitation is the lack of research about the performance of the selected ROI in the surface-guided monitoring of the intra-fraction motion in the radiotherapy with concurrent TTFields for glioblastoma. A combination of the head & neck phantom and a motion platform may provide a useful tool to study this issue. On the other hand, actual-case-based study need to be performed to verify the effectiveness of the movement reduction consideration in this study. In addition, further research is needed on the technical feasibility and performance (stability and comfort) of facial thermoplastics dedicated to T-shaped openings.
Conclusion
In this study, the feasibility of the surface-guided setup in radiotherapy with concurrent TTFields for glioblastoma with focus on the ROI selection was investigated from views of both setup accuracy and inferred dose delivery accuracy. It demonstrates that a relatively medium-size T-shaped ROI without forehead area is appropriate for the surface-based guidance. However, actual-case-based study need be performed to further verify the feasibility in clinical scenes.
References
Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–43.
Stupp R, Taillibert S, Kanner AA, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–16.
Taphoorn MJB, Dirven L, Kanner AA, et al. Influence of treatment with tumor-treating fields on health-related quality of life of patients with newly diagnosed glioblastoma: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):495–504.
Lok E, Swanson KD, Wong ET. Tumor treating fields therapy device for glioblastoma: physics and clinical practice considerations. Expert Rev Med Devices. 2015;12(6):717–26.
Nabors LB, Portnow J, Ahluwalia M, et al. Central nervous system cancers, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2020;18(11):1537–70.
Giladi M, Munster M, Schneiderman RS, et al. Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat Oncol. 2017;12(1):1–13.
Bokstein F, Blumenthal D, Limon D, et al. Concurrent tumor treating fields (TTFields) and radiation therapy for newly diagnosed glioblastoma: a prospective safety and feasibility study. Front Oncol. 2020;10:411.
Song A, Bar-Ad V, Martinez N, et al. Initial experience with scalp sparing radiation with concurrent temozolomide and tumor treatment fields (SPARE) for patients with newly diagnosed glioblastoma. J Neurooncol. 2020;147:653–61.
Ali AS, Lombardo J, Niazi MZ, et al. Concurrent chemoradiation and Tumor Treating Fields (TTFields, 200 kHz) for patients with newly diagnosed glioblastoma: patterns of progression in a single institution pilot study. J Neurooncol. 2022;160(2):345–50.
Shi W, Roberge D, Kleinberg L, et al. Phase 3 TRIDENT study (EF-32): Tumor treating fields (TTFields; 200 kHz) concomitant with chemoradiation, and maintenance TTFields therapy/temozolomide in newly diagnosed glioblastoma. J Clin Oncol. 2023;41(16):TPS2083.
Toms SA, Kim CY, Nicholas G, et al. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: a subgroup analysis of the EF-14 phase III trial. J Neurooncol. 2019;141:467–73.
Straube C, Oechsner M, Kampfer S, et al. Dosimetric impact of tumor treating field (TTField) transducer arrays onto treatment plans for glioblastomas—a planning study. Radiat Oncol. 2018;13(1):1–10.
Guberina N, Pöttgen C, Kebir S, et al. Combined radiotherapy and concurrent tumor treating fields (TTFields) for glioblastoma: dosimetric consequences on non-coplanar IMRT as initial results from a phase I trial. Radiat Oncol. 2020;15(1):1–11.
Nour Y, Pöttgen C, Kebir S, et al. Dosimetric impact of the positioning variation of tumor treating field electrodes in the PriCoTTF-phase I/II trial. J Appl Clin Med Phys. 2021;22(1):242–50.
Biswas S, Kapitanova I, Divekar S, et al. Targeting accuracy considerations for simultaneous tumor treating fields antimitotic therapy during robotic hypofractionated radiation therapy. Technol Cancer Res Treat. 2021;20(1):1–9.
Barrett JF, Keat N. Artifacts in CT: recognition and avoidance. Radiographics. 2004;24(6):1679–91.
Pan M, Xiao Y, Zhu L, et al. Evaluation of interfraction setup uncertainty of patients with glioblastoma wearing TTFields (Tumor Treating Fields) during radiation therapy. Pract Radiat Oncol. 2023;13(6):522–30.
Lin T, Ma CMC. Positioning errors of metal localization devices with motion artifacts on kV and MV cone beam CT. BJR Open. 2019;1:20190013.
Al-Hallaq HA, Cerviño L, Gutierrez AN, et al. AAPM task group report 302: surface-guided radiotherapy. Med Phys. 2022;49(4):e82–112.
Wei W, Ioannides PJ, Sehgal V, et al. Quantifying the impact of optical surface guidance in the treatment of cancers of the head and neck. J Appl Clin Med Phys. 2020;21(6):73–82.
Lee SK, Huang S, Zhang L, et al. Accuracy of surface-guided patient setup for conventional radiotherapy of brain and nasopharynx cancer. J Appl Clin Med Phys. 2021;22(5):48–57.
Bry V, Saenz D, Pappas E, et al. End to end comparison of surface-guided imaging versus stereoscopic X-rays for the SRS treatment of multiple metastases with a single isocenter using 3D anthropomorphic gel phantoms. J Appl Clin Med Phys. 2022;23(5): e13576.
Pietro M, et al. Accuracy evaluation of the optical surface monitoring system on EDGE linear accelerator in a phantom study. Med Dosim. 2016;41(2):173–9.
Yue NJ, Knisely JPS, Song H, et al. A method to implement full six-degree target shift corrections for rigid body in image-guided radiotherapy. Med Phys. 2006;33(1):21–31.
Schreibmann E, Crocker I, Dhabaan A, et al. Automated plan quality assurance integrated with eclipse using varian’s ESAPI interface. Med Phys. 2016;43(6):3712–3712.
Lucido JJ, Shiraishi S, Seetamsetty S, et al. Automated testing platform for radiotherapy treatment planning scripts. J Appl Clin Med Phys. 2023;24(1): e13845.
Li P, Wang R, Wang Y, et al. Evaluation of the ICP algorithm in 3D point cloud registration. IEEE Access. 2020;8:68030–48.
Laaksomaa M, Moser T, Kritz J, et al. Comparison of three differently shaped ROIs in free breathing breast radiotherapy setup using surface guidance with AlignRT®. Rep Pract Oncol Radiother. 2021;26(4):545–52.
Sauer TO, Ott OJ, Lahmer G, et al. Region of interest optimization for surface guided radiation therapy of breast cancer. J Appl Clin Med Phys. 2021;22(10):152–60.
Song Y, Zhai X, Liang Y, et al. Evidence-based region of interest (ROI) definition for surface-guided radiotherapy (SGRT) of abdominal cancers using deep-inspiration breath-hold (DIBH). J Appl Clin Med Phys. 2022;23(11): e13748.
Kang HJ, Grelewicz Z, Wiersma RD. Development of an automated region of interest selection method for 3D surface monitoring of head motion. Med Phys. 2012;39(6 Part 1):3270–82.
Wiersma RD, Tomarken SL, Grelewicz Z, et al. Spatial and temporal performance of 3D optical surface imaging for real-time head position tracking. Med Phys. 2013;40(11): 111712.
Bry V, Licon AL, McCulloch J, et al. Quantifying false positional corrections due to facial motion using SGRT with open-face masks. J Appl Clin Med Phys. 2021;22(4):172–83.
Sagawa T, Ohira S, Ueda Y, et al. Dosimetric effect of rotational setup errors in stereotactic radiosurgery with HyperArc for single and multiple brain metastases. J Appl Clin Med Phys. 2019;20(10):84–91.
Tsujii K, Ueda Y, Isono M, et al. Dosimetric impact of rotational setup errors in volumetric modulated arc therapy for postoperative cervical cancer. J Radiat Res. 2021;62(4):688–98.
Isobe A, Usui K, Hara N, et al. The effects of rotational setup errors in total body irradiation using helical tomotherapy. J Appl Clin Med Phys. 2021;22(7):93–102.
Dong T, Zhou Z, Liang Y, et al. PO-1718: the effect of CT slice thickness and HUs on DICOM reference surface accuracy. Radiother Oncol. 2023;182:S1433–4.
Acknowledgements
The authors wish to thank the colleagues and peers who kindly agreed to share their data and provide help.
Funding
National Key Research & Development Program of China (2022YFC2404605); Spark Basic Research Program of Jiangsu Cancer Hospital (ZJ202309).
Author information
Authors and Affiliations
Contributions
Jiajun Zheng and Geng Xu were involved in the conception and design of the study. Wenjie Guo, Yuanyuan Wang, Jianfeng Wu, Dan Zong and Boyang Ding were involved in the analysis and interpretation of the data, the drafting and critical revision of the manuscript. Jiajun Zheng, Li Sun and Xia He were involved in the final approval of the version submitted for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All patients provided verbal informed consent prior to inclusion in the study for the research use and publishing of their clinical data. This study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Nanjing Medical University, and the ethics number is 2023-071.
Competing interest
No conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zheng, J., Xu, G., Guo, W. et al. Preliminary study of feasibility of surface-guided radiotherapy with concurrent tumor treating fields for glioblastoma: region of interest. Radiat Oncol 19, 129 (2024). https://doi.org/10.1186/s13014-024-02525-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-024-02525-3