Abstract
Background
Despite the increased risk of cervical cancer (CC) among women living with HIV (WLHIV), CC screening and treatment (CCST) rates remain low in Africa. The integration of CCST services into established HIV programs in Africa can improve CC prevention and control. However, the paucity of evidence on effective implementation strategies (IS) has limited the success of integration in many countries. In this study, we seek to identify effective IS to enhance the integration of CCST services into existing HIV programs in Nigeria.
Methods
Our proposed study has formative and experimental activities across the four phases of the Exploration, Preparation, Implementation, and Sustainment (EPIS) framework. Through an implementation mapping conducted with stakeholders in the exploration phase, we identified a core package of IS (Core) and an enhanced package of IS (Core+) mostly selected from the Expert Recommendations for Implementing Change. In the preparation phase, we refined and tailored the Core and Core+ IS with the implementation resource teams for local appropriateness. In the implementation phase, we will conduct a cluster-randomized hybrid type III trial to assess the comparative effectiveness of Core versus Core+. HIV comprehensive treatment sites (k = 12) will be matched by region and randomized to Core or Core+ in the ratio of 1:1 stratified by region. In the sustainment phase, we will assess the sustainment of CCST at each site. The study outcomes will be assessed using RE-AIM: reach (screening rate), adoption (uptake of IS by study sites), IS fidelity (degree to which the IS occurred according to protocol), clinical intervention fidelity (delivery of CC screening, onsite treatment, and referral according to protocol), clinical effectiveness (posttreatment screen negative), and sustainment (continued integrated CCST service delivery). Additionally, we will descriptively explore potential mechanisms, including organizational readiness, implementation climate, CCST self-efficacy, and implementation intentions.
Discussion
The assessment of IS to increase CCST rates is consistent with the global plan of eliminating CC as a public health threat by 2030. Our study will identify a set of evidence-based IS for low-income settings to integrate evidence-based CCST interventions into routine HIV care in order to improve the health and life expectancy of WLHIV.
Trial registration
Prospectively registered on November 7, 2023, at ClinicalTrials.gov no. NCT06128304. https://classic.clinicaltrials.gov/ct2/show/study/NCT06128304
Similar content being viewed by others
Background
Cervical cancer (CC) is the second most common cancer in women in Africa [1] and is a large contributor to cancer deaths in the region [2]. While age-standardized mortality trends have declined in high-income countries, mortality is rising in many low-income countries [3]. By 2030, CC will account for more than 106,000 deaths annually in Africa, an increase of 38% from the estimated 77,000 deaths in 2020 [4]. Compared with HIV-negative women, women living with HIV (WLHIV) are six times more likely to develop CC [5], and are at increased risk of mortality from CC [6,7,8,9]. About 25% of CC cases in Africa are diagnosed in WLHIV, and 21% are attributable to HIV [10]. With a 5-year prevalence of 22,500 cases [11] and 960,000 WLHIV [12], Nigeria has one of the largest burdens of CC and HIV in Africa.
CC is completely preventable. It is also curable if diagnosed and treated early. The availability of effective interventions, including human papillomavirus (HPV) vaccination and screening and treatment of precancerous lesions of CC [13], underpins the current global effort to eliminate CC as a public health problem [14]. The World Health Organization (WHO) has set measurable global targets of 90–70–90 (90% of girls fully vaccinated with HPV vaccine by 15 years of age, 70% of women screened using a high-performance test, and 90% of women identified with preinvasive cancer and invasive CC treated) to prevent and treat CC by 2030 [15].
CC screening and treatment (CCST) is an important evidence-based intervention for CC prevention and control. However, many African countries have not been able to implement and sustain organized national CC screening programs [16]. Although the availability of low-cost screening methods such as visual inspection with acetic acid (VIA) or Lugol’s iodine (VILI) for CC screening [17, 18] offers opportunities to improve early detection of CC in Africa, screening rates among women, including WLHIV, remain low [19]. In Nigeria, despite the recommendations of routine CC screening of WLHIV [20], studies have found screening coverage of < 10% among WLHIV [21,22,23,24]. CC screening in Nigeria is affected by several factors such as the unavailability of screening services, screening not being offered by healthcare providers, and poor awareness of the disease and screening services [25,26,27,28]. Efforts to improve the control of CC and other cancers in Nigeria have included the designation of one federal tertiary hospital in each of the country’s six geopolitical regions as oncology centers of excellence, where comprehensive cancer screening, diagnosis, and treatment can be accessed.
In low- and middle-income countries (LMIC) with a high burden of HIV, the integration of CCST services into the established HIV programs can improve CC prevention and control [29,30,31]. Investment in the HIV response has improved access to antiretroviral therapy (ART) in many African countries [32, 33]. For example, the US President’s Emergency Plan for AIDS Relief (PEPFAR) HIV care program in Nigeria has invested more than US $6 billion in Nigeria’s national HIV response [34]. PEPFAR has contributed to health systems strengthening through human capacity development, establishing electronic health management information systems, and providing state-of-the-art laboratories to adequately respond to the HIV epidemic and other diseases in Nigeria [35]. These existing infrastructures for HIV can be leveraged to deliver CCST services. Depending on the availability of physical and human resources, the integration of CCST services into HIV services may be within the same clinic or through referral [36].
While evidence suggests that the integration of CCST into HIV services is feasible and acceptable [36,37,38,39,40], it has not been widely implemented at scale or yielded the desired outcomes in many low-resource, high-burden countries, including Nigeria [15]. The gap in knowledge of effective implementation strategies (IS) to enhance integration of CCST into HIV services has limited its success and scale-up [30, 41]. Findings from previous reports [40, 42, 43] indicate that effective integration of CCST for WLHIV will require tailored IS that will simultaneously address multilevel barriers in the outer context of the country health systems and the inner organizational contexts of HIV clinics [44, 45].
Accordingly, guided by the Exploration, Preparation, Implementation, and Sustainment (EPIS) framework [44, 46], we will determine the barriers to and facilitators of CCST implementation and sustainment and identify and test promising implementation strategies to enhance the integration of CCST into existing HIV programs in Nigeria. EPIS is both a process and determinant framework that describes four phases of the implementation process to guide researchers and implementers in the process. We utilized the EPIS process phases to stage the study while also invoking consideration of determinants and mechanisms (i.e., barriers and facilitators). Determinants are identified in the outer system context (e.g., cultural and populations variation in regions), inner organizational context (i.e., clinic operations), innovation factors (i.e., characteristics of CCST), and bridging factors that connect outer and inner contexts [47]. Importantly, EPIS also recognizes the importance of relationships, interconnections, and linkages among and between actors in outer and inner contexts. Our implementation and clinical outcomes are informed by the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework. As part of study development, the EPIS exploration phase was completed in which we collaborated with stakeholders in Nigeria who participated in the following: (1) identifying and ranking implementation barriers and facilitators and (2) matching and ranking IS to the identified determinants [48]. As shown in Table 1, the results from the implementation mapping and the refinement are a set of a core package of IS (Core) and an enhanced package of IS (Core+).
Our specific aims are as follows:
-
Aim 1: Refine strategies to integrate CCST of cervical precancer within existing comprehensive HIV treatment programs.
-
Aim 2: Determine the comparative effectiveness of the Core vs Core+ implementation strategies on CCST.
-
Aim 3: Assess the sustainment of the integration of CCST into comprehensive HIV treatment programs.
Methods
Overview of study preparation and design
This is a cluster randomized trial using hybrid type III implementation-effectiveness study type [49] to assess the comparative effectiveness of a set of Core and enhanced (i.e., Core+) implementation strategies on the implementation and sustainment of cervical cancer screening and treatment for WHLIV. The hybrid type III design focuses primarily on testing IS effectiveness while observing clinical effectiveness outcomes [49]. HIV comprehensive treatment centers (k = 12) will be matched by region and randomized 1:1 to Core or Core+ strategies. We will assess both implementation and clinical outcomes and explore potential mechanisms that affect the study outcomes, including organizational readiness, implementation climate, CCST self-efficacy, and implementation intentions. The study is guided by two implementation science frameworks — EPIS and RE-AIM.
Exploration, preparation, implementation, and sustainment (EPIS) framework
EPIS was selected because it is useful in guiding implementation in different settings including low-income countries [44, 46, 50]. Figure 1 shows the activities within each phase.
Prior to grant submission and funding, we completed the exploration phase [48] where we used survey and modified nominal group techniques (NGT) [51] to conduct a rapid and facilitated implementation mapping [52] exercise. Collaborators identified and ranked barriers and identified, selected, and ranked implementation strategies starting with the Expert Recommendations for Implementing Change (ERIC) [53] and other identified additional strategies. The final set of strategies were selected to address the barriers to integration of CCST into HIV treatment programs to form the Core IS. Stakeholders identified two additional strategies to be included in the Core+ IS condition [48].
In the preparation phase, we refined the IS and convened implementation resource team (IRT) [54] meetings to further reflect on the refined Core and Core+ IS fit with local context. Implementation resource teams were first conceptualized and tested in the dynamic adaptation process model and the Interagency Collaborative Team model for scaling up evidence-based practices [54, 55]. The IRT for this study is a community-engaged co-creation collaborative comprising the research team and the doctors and nurses from the study sites. Twelve site research coordinators (SRCs) were also recruited and trained during this phase.
The process of refinement of our proposed implementation strategies occurred in two phases. In the first phase, the research team developed an IS table which included the identified implementation determinants (barriers/facilitators), the initially proposed IS for each determinant, a definition of each IS, the population targeted by the IS, the proposed actions for the IS, and the responsible person(s) for these proposed actions. For each IS, we discussed its feasibility and importance in addressing the implementation determinant based on the IRT’s current knowledge of the context in the implementation sites. For each IS that IRT identified as not being the most feasible or important to address the implementation determinant, we selected another IS from the same category in the ERIC grouping of implementation strategies [53], and we included some strategies not included in ERIC. The refined strategies are shown in Table 1.
In the second phase, 12 doctors and 12 nurses, 1 from each study site, participated in 2 focus-group discussions (FGDs) to further refine the IS. Each FGD was facilitated by a member of the research team with experience in qualitative data collection, using a semi-structured FGD guide, while two members of the research team took notes. Written informed consent was obtained from all the participants after reading the information about the study and the FGDs and given the opportunity to ask questions. Verbal consent was also obtained from the participants for the audio recording of the FGDs. The participants were presented with the refined implementation strategies table developed in the first phase and were asked to contribute to further refinement of the table, particularly tailoring the target population, planned actions, actor, dose, and mode of delivery. They were also asked to state if any of the IS or their activities would not be feasible at their sites. For any IS where the participants had different views about tailoring, discussions were held, and a consensus was reached. Each FGD lasted for about 2 h. Details of the further refined strategies are shown in Table 2. All of this work was in preparation for the cluster randomized trial to be described in the next section.
In the implementation phase, we will implement the Core and Core+ IS and test their comparative effectiveness on select implementation outcomes while collecting data on potential mechanisms of effects and clinical effectiveness outcomes. We will also catalog any ad hoc adaptations and contextual factors affecting implementation, services, and clinical outcomes with a focus on sustainment.
In the sustainment phase, we will assess sustainment of CCST and IS at each site and provide summaries to stakeholders regarding the planned and ad hoc adaptations that occurred during implementation.
Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM)
We selected RE-AIM as our evaluation framework for assessing individual- and setting-level outcomes important to program impact and sustainment [56, 57]. As shown in Table 3, we address relevant RE-AIM constructs: Reach, Effectiveness, Adoption, Implementation, and Maintenance (also known as Sustainment) [56, 57]. In operationalizing these five dimensions, we adapted the metrics recommended by the Integrative Systems Praxis for Implementation Research (INSPIRE) model [58]. We defined the constructs as follows: reach (screening rate); clinical effectiveness (posttreatment screen negative); adoption (uptake of IS by study sites); IS fidelity (degree to which the IS occurred according to protocol); implementation clinical intervention fidelity (delivery of CC screening, onsite treatment, and referral according to protocol); and maintenance (or sustainment) (the degree to which integration of CCST continued).
Site selection
The study will leverage the 21 comprehensive HIV treatment sites across the six geopolitical zones of Nigeria that have been designated as Nigeria Implementation Science Alliance (NISA) Model Innovation and Research Centers (NISA-MIRC) [65]. The NISA-MIRC sites are part of the ICON-3 Practice-based Research Network which is made up of the 21 NISA-MIRCs and the 6 Regional Centers of Excellence. The NISA-MIRCs have over 60,000 WLHIV in care who are eligible for enrollment into clinical trials and other implementation research studies [65]. Our proposed study will be anchored at 12 sites (see Figure 2 and Additional file 1) from the 21 NISA-MIRCs. The criteria for selection were based on the following: (1) geographical spread (two sites per region), (2) highest proportion of consented WLHIV for enrolment into clinical trials and other implementation research studies, and (3) match of the same type of site per region (secondary or tertiary).
Randomization
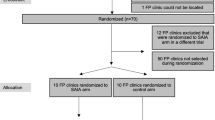
The unit of randomization will be the sites. Each site represents a cluster in the randomization representing the clinic site and the WLHIV receiving treatment at the site. We will conduct simple random allocation with the sites randomized to either the Core or Core+ IS. There are six geographic regions in Nigeria, and sites will first be matched by region, and then each site in a matched pair will be randomized to Core or Core+ IS. A random number generator will be used to assign the sites. A flow diagram for the randomization and intervention allocation is shown in Figure 3. The healthcare workers and the patients will be blinded to whether their sites are allocated to the core package of IS or an enhanced package of IS.
Methods for each study aim
-
Aim 1: To refine strategies to integrate cervical cancer screening and treatment of cervical precancers within existing comprehensive HIV treatment programs. This has been described above in the EPIS preparation phase, and the findings are included in Tables 1 and 2.
-
Aim 2: To determine the comparative effectiveness of the Core vs Core+ Implementation Strategies on CSST
Study population
To be eligible for enrolment into the trial, a woman must meet all the following criteria:
-
Be between 25 and 63 years
-
Have been diagnosed with HIV
-
Enrolled into HIV care in the sites
WLHIV will not be eligible to be enrolled into the trial if they have been previously diagnosed with preinvasive or invasive CC, received CC screening at any time within the previous 12 months, or are not willing to participate in the study procedures.
Recruitment and retention
The medical records team will provide a list of potentially eligible WLHIV based on the eligibility criteria. The eligible WLHIV will be approached by the SRCs for recruitment into the study. For those who indicate interest, the SRCs will provide more details about the study, after which signed informed consent will be obtained electronically using REDCap. The consent forms will be read to all the participants in the English language. When there is a language barrier, the multilingual SRCs will interact with the potential participants in their local dialect. At enrollment, appointments for their next visit (data collection) will be scheduled.
Data collection
Sociodemographic and baseline data will be collected from the enrolled participants who will also be followed up to assess the outcomes of interest using structured follow-up questionnaires in addition to their electronic medical records. Data on potential determinants and mechanisms will be obtained from the healthcare providers, using the following validated instruments (see Additional file 2).
Organizational Readiness for Implementing Change (ORIC)
The ORIC assesses organizational readiness through two subscales, change commitment, and change valence. The ORIC has established high inter-item consistency and inter-rater reliability for both subscales [66].
Implementation climate measure (ICM)
The six-item ICM has three subscales that assess perceptions of the degree to which a specific evidence-based practice (EBP) or innovation is expected, supported, and rewarded (i.e., recognition) in a clinic or organization [67]. The ICM has established strong psychometric properties.
Measure of innovation-specific implementation intentions (MISII)
The MISII is a three-item pragmatic measure of direct service providers’ (e.g., physicians, nurses, providers) intentions to engage with and use a specific innovation such as CCST [68]. The MISII has strong psychometric properties.
Self-Efficacy/EBP Beliefs Scale
This scale assesses the perceived value of the EBP and self-efficacy regarding the ability to implement the EBP. We will use the seven items that directly assess self-efficacy and we will tailor them to refer to CCST [69].
Outcome measures
The measures for the implementation and clinical outcomes and the potential mechanisms are summarized in Table 3.
Sample size and power calculation
We examine the sample size for the primary implementation outcome of “reach,” indicated by the CC screening rate in WLHIV. Based on preliminary data from previous studies, we assumed a conservative screen rate of 14% in the core implementation strategy group [70]. We plan to enroll 12 sites (6 sites per intervention group). Hade et al. [71] reported the range of intraclass correlation (ICC) fall in 0.02–0.07 in the group-randomized breast and CC screening studies. We performed a sensitivity analysis by assuming a range of ICC of 0.02–0.07 for site cluster effect on the outcome and a feasible sample size of 200 subjects per site. We estimated that we could achieve at least 80% power for ICC of 0.02–0.06 (84% for ICC = 0.06 and > 99.9% for ICC = 0.02) to detect a minimum improvement of 20 percentage points in screening rate (that is, 34% screening rate) in Core+ group using a two-sided type I error of 0.05, and we could still achieve 78.6% power for ICC = 0.07. The 34% screening rate is achievable and a conservative estimate for the Core+ group based on our prior related work [61]. The power analysis was conducted using the statistical software R package cluster Power [72].
Data analysis
Preliminary analyses will begin with an examination of the distribution of variables to assess and describe their characteristics (means, standard deviations, quartiles, ranges, frequencies, and percentages) for overall and for Core and Core+ groups separately and to allow assessment of randomization. Randomization will be tested by performing a series of Wilcoxon rank-sum tests for continuous variables and chi-square (or Fisher’s exact) tests for categorical variables to compare the groups on baseline demographic and clinical variables. Variables on which the groups differ initially will be explored as covariates in subsequent analysis. Intent-to-treat analysis will be conducted as the primary analysis. All estimates (point estimates and 95% confidence intervals) for the study outcomes will be adjusted by the cluster effect of clinics as described below.
The screening rate (primary outcome) will be summarized by frequency and proportion. A generalized linear mixed-effect model (GLMM) [73] with a binomial link will be used to examine the difference in screening rate (reach outcome) between Core and Core+. A random intercept will be included in the model to account for the cluster effect of the site. Multivariable random effects models will be used to examine the impact of covariates on estimated intervention effects. The GLMM will be also used to compare secondary outcome measures related to adoption, implementation strategies fidelity, and clinical intervention fidelity between two intervention groups, with a binomial link for a binary outcome and a Gaussian link for a continuous outcome. The ICM, MISII, and Self-Efficacy/EBP Beliefs Scale data collected from providers will also be analyzed similarly using the GLMM.
Covariate prescreening and variable selection procedure
In the multivariable model described above, adjustments are typically made to correct for baseline imbalances between groups and to adjust for variables known to influence the outcome independent of the intervention. Baseline characteristics will be pre-screened and assessed for an imbalance between the two intervention groups (Wilcoxon rank-sum test or Fisher’s exact test) and their association with the outcome (univariable mixed-effects model). Only those variables found to be moderately associated (p < 0.15) with the outcome or imbalanced (p < 0.10) between groups will be considered as potential covariates in the initial multivariable model. Backward elimination of insignificant variables will be used to select the main effects in the final model; all covariates that are significant at p < 0.10 will be kept in the final model.
Missing data
In the case of missing data, missing patterns will be assessed by comparing patient characteristics between patients with and without missing data. Appropriate data analytic techniques will be used for analysis, which may include deletion, imputation, and inclusion of an indicator of missing values.
-
Aim 3: To assess the sustainment of the integration of CCST into HIV programs
Study population
This will include healthcare providers (doctors and nurses) that provided CCST for WLHIV and healthcare administrators (medical directors) in the NISA-MIRCs study sites.
Recruitment
Healthcare providers/administrators will be invited via letters and emails to participate in a survey and FGD.
Data collection
Quantitative data will be collected from the healthcare providers/administrators using the Provider REport of Sustainment Scale (PRESS) [74]. The PRESS is a brief and pragmatic three-item measure of sustainment that can be used across different EBP, provider types, and settings. The PRESS captures clinic staff’s report of their clinic, team, or agency’s continued use of an EBP or innovation in practice. The PRESS has excellent psychometric characteristics. Items are measured on a 5-point Likert scale ranging from (0 [not at all] to 4 [to a very great extent]. Qualitative data regarding sustainment will be collected using FGD with doctors, nurses, and administrators in each of the sites.
Sample size
About 36–48 health care providers (3–4 per site) will be surveyed using PRESS. We will conduct 2 FGDs (Core and Core+) with 12 participants per group.
Data analysis
We will analyze data using a mixed-methods approach. From the quantitative data, we will classify sustainment status according to Wiltsey Stirman and colleague’s recommendations [75] and as used by Aarons and colleagues [76]. For the mixed-methods integration, we will use a QUAN+QUAL approach in which data are gathered and given equal weight [77]. We will triangulate qualitative and quantitative data to examine convergence, expansion, and complementarity when developing overall interpretations and conclusions [77,78,79,80,81,82,83]. We will create matrices to identify and summarize convergences and divergences in analyses of all data sources integrating results into a comprehensive picture [80]. We will first consider each type of analysis on its own terms and how they differ or converge [79, 84] by linking qualitative and quantitative databases and embedding one within the other so that each has a supportive function to play.
Study procedures
Due to the available infrastructure in the 12 study sites, CC screening in this study will be performed using VIA. In line with the standard of care in the sites, the decision to treat preinvasive cancer lesions will be based on the result of the screening test only, and not on a histologically confirmed diagnosis (screen and treat approach). Treatment of preinvasive cancer lesions will be through ablative therapy. Participants with preinvasive lesions that are not eligible for ablative treatment, or with suspected cancer lesions, will be referred to one of the regional oncology centers of excellence (Figure 4).
Schedule of measurement
We will collect baseline data on the potential mechanisms (ORIC and MISII) and CC screening rates among WLHIV in the 12 facilities (Figure 5). In aim 2, each participant will be assessed at 12 months after enrollment for the receipt of CC screening (study M33). Treatment of precancerous lesions and referral of suspected cancer or lesions ineligible for treatment will be assessed at 12 and 18 months after enrollment (study M33 and M39). Posttreatment follow-up screening will be assessed at 27 and 33 months after recruitment (study M48 & M54). Data on specific potential mechanisms will be collected from the healthcare providers after recruitment and then 6 months, 12 months, and annually.
Tracking adaptations
We will use quarterly “periodic reflections” [85] with the entire investigative team to consider any ad hoc adaptations that occurred at any of the sites. We will adapt periodic reflection focus-group guides for the discussion around what adaptations may have occurred, why they occurred, at what system or organizational level, who was involved, and the time frame. We will catalog adaptations and place them within the EPIS framework identifying the phase when they occurred and whether they arose from or impacted EPIS determinants or mechanisms in outer context, inner context, bridging factors, or the innovation itself (i.e., CCST). We will also place adaptations within the FRAME-IS model for cataloging and reporting adaptations [86, 87].
Data handling
We will manage study data using REDCap (Research Electronic Data Capture; http://project-redcap.org/). REDCap provides user-friendly secure web-based case report forms and real-time data entry. We will use password-protected and encrypted tablets, laptops, and external drives that are institutionally certified and issued for data storage. Study data will be shared with only principal investigator-approved study staff via secure file transfer platforms.
Discussion
In this study, we will conduct a cluster randomized, hybrid type III trial among comprehensive HIV treatment centers in Nigeria (k = 12) for WLHIV (N = 2400) to assess the comparative effectiveness of an enhanced package of IS (Core+) versus a core package of IS (Core) on the integration of CCST into HIV programs. Upon conclusion, our study will identify a set of evidence-based IS for low-income settings to integrate CCST interventions into routine HIV care to improve the health and life expectancy of WLHIV.
Meeting the WHO targets of ending CC requires countries to identify innovative implementation strategies that address contextual barriers to integration [14]. Several implementation strategies such as training of providers, community outreach, educational materials, changing service sites, task shifting, ongoing consultation, patient reminder systems, and audit-feedback mechanisms have been used in improving cervical cancer prevention in sub-Saharan Africa [41]. However, most of the studies reporting these IS did not evaluate their effectiveness or report implementation-specific outcomes [41].
To our knowledge, our study will be the first to test the effectiveness of tailored IS on the implementation of CCST among WLHIV in Nigeria. The use of hybrid type III trial design will allow our study to gather reliable evidence on implementation and clinical effectiveness outcomes. Our approach of conducting a multicenter study comprising 12 sites across the six geopolitical regions of Nigeria will ensure the representativeness of findings. Also, leveraging the PEPFAR-supported programs in Nigeria and our established partnerships will increase the likelihood of successful study implementation and service sustainment. The use of IRT as a part of the EPIS process can serve as a stakeholder engagement approach as it has been utilized in other studies [54]. We have engaged the IRT to refine and tailor the IS for local appropriateness. They will also facilitate the documentation of planned and ad hoc adaptations during the study.
Nonetheless, there are potential limitations to this study. The inadequate number of fully equipped laboratories, and the current high cost of HPV DNA tests, limits the implementation of WHO-recommended HPV screen, triage, and treat (HPV-STT) approach for WLHIV [88]. However, if the landscape changes, we will work to incorporate HPV testing instead of VIA/VILI. The 12 NISA-MIRCs sites selected for this study are secondary health facilities; thus, our findings may not be applicable to primary and tertiary healthcare facilities due to varying inner contexts.
Conclusion
There has been a significant increase in the uptake of ART among WLHIV in Africa; however, the coverage of CCST among WLHIV who are at increased risk of CC remains low. Efforts to successfully leverage the available infrastructure for HIV care and treatment programs in low-income countries for integrated service delivery have been limited by the paucity of evidence on effective IS. With this cluster randomized, hybrid type III trial design in Nigeria, we will test and identify effective IS to integrate CCST services into routine HIV care. Results from this study could inform better implementation of CC secondary prevention intervention to improve survival among WLHIV in resource-limited settings.
Availability of data and materials
Not applicable.
Abbreviations
- CC:
-
Cervical cancer
- CCST:
-
Cervical cancer screening and treatment
- EPIS:
-
Exploration, Preparation, Implementation, and Sustainment framework
- IRT:
-
Implementation resource team
- IS:
-
Implementation strategy
- NISA:
-
Nigeria Implementation Science Alliance
- PEPFAR:
-
US President’s Emergency Plan for AIDS Relief
- SRC:
-
Site research coordinators
- WLHIV:
-
Women living with HIV
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/CAAC.21660.
World Health Organization. Africa: Global Cancer Observatory. 2021. https://gco.iarc.fr/today/data/factsheets/populations/903-africa-fact-sheets.pdf
Yang M, Du J, Lu H, Xiang F, Mei H, Xiao H. Global trends and age-specific incidence and mortality of cervical cancer from 1990 to 2019: an international comparative study based on the Global Burden of Disease. BMJ Open. 2022;12(7):e055470. https://doi.org/10.1136/BMJOPEN-2021-055470.
International Agency for Research on Cancer. Cancer Tomorrow. 2023. https://gco.iarc.fr/tomorrow/en/dataviz/trends
Stelzle D, Tanaka LF, Lee KK, Khalil AI, Baussano I, Shah ASV, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health. 2021;9(2):e161–9. https://doi.org/10.1016/S2214-109X(20)30459-9.
Dryden-Peterson S, Bvochora-Nsingo M, Suneja G, Efstathiou JA, Grover S, Chiyapo S, et al. HIV infection and survival among women with cervical cancer. J Clin Oncol. 2016;34(31):3749–57. https://doi.org/10.1200/JCO.2016.67.9613.
Ferreira MP, Coghill AE, Chaves CB, Bergmann A, Thuler LC, Soares EA, et al. Outcomes of cervical cancer among HIV-infected and uninfected women treated at the Brazilian National Institute of Cancer (2001–2013). AIDS. 2017;31(4):523–31. https://doi.org/10.1097/QAD.0000000000001367.
Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated cancer-specific mortality among HIV-infected patients in the United States. J Clin Oncol. 2015;33(21):2376–83. https://doi.org/10.1200/JCO.2014.59.5967.
Coghill AE, Newcomb PA, Madeleine MM, Richardson BA, Mutyaba I, Okuku F, et al. Contribution of HIV infection to mortality among cancer patients in Uganda. AIDS. 2013;27(18):2933–42. https://doi.org/10.1097/01.AIDS.0000433236.55937.CB.
Ibrahim Khalil A, Mpunga T, Wei F, Baussano I, de Martel C, Bray F, et al. Age-specific burden of cervical cancer associated with HIV: a global analysis with a focus on sub-Saharan Africa. Int J Cancer. 2022;150(5):761–72. https://doi.org/10.1002/IJC.33841.
International Agency for Research on Cancer. Nigeria. Nigeria. 2021. https://gco.iarc.fr/today/data/factsheets/populations/566-nigeria-fact-sheets.pdf
Joint United Nations Programme on HIV/AIDS. AIDSinfo. 2022. http://aidsinfo.unaids.org/
World Health Organization. Human papillomavirus (HPV) and cervical cancer. 2020. Available: https://www.who.int/en/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
Simelela PN. WHO global strategy to eliminate cervical cancer as a public health problem: an opportunity to make it a disease of the past. Int J Gynecol Obstet. 2021;152(1):1–3. https://doi.org/10.1002/IJGO.13484.
World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem and its associated goals and targets for the period 2020 – 2030. Geneva: WHO; 2020.
Anaman-Torgbor J, Angmorterh SK, Dordunoo D, Ofori EK. Cervical cancer screening behaviours and challenges: a sub-Saharan Africa perspective. Pan Afr Med J. 2020;36:97. https://doi.org/10.11604/PAMJ.2020.36.97.19071.
Fokom-Domgue J, Combescure C, Fokom-Defo V, Tebeu P, Vassilakos P, Kengne A, et al. Performance of alternative strategies for primary cervical cancer screening in sub-Saharan Africa: systematic review and meta-analysis of diagnostic test accuracy studies. BMJ. 2015;351:h3084. https://doi.org/10.1136/BMJ.H3084.
Sankaranarayanan R, Anorlu R, Sangwa-Lugoma G, Denny LA. Infrastructure requirements for human papillomavirus vaccination and cervical cancer screening in sub-Saharan Africa. Vaccine. 2013;31(Suppl 5):F47–52. https://doi.org/10.1016/J.VACCINE.2012.06.066.
Yimer NB, Mohammed MA, Solomon K, Tadese M, Grutzmacher S, Meikena HK, et al. Cervical cancer screening uptake in sub-Saharan Africa: a systematic review and meta-analysis. Public Health. 2021;195:105–11. https://doi.org/10.1016/J.PUHE.2021.04.014.
Federal Ministry of Health, Nigeria. National AIDS and STIs Control Programme. National Guidelines for HIV Prevention Treatment and Care. Abuja: FMOH; 2020.
Adibe MO, Aluh DO. Awareness, knowledge and attitudes towards cervical cancer amongst HIV-positive women receiving care in a tertiary hospital in Nigeria. J Cancer Educ. 2017;33(6):1189–94. https://doi.org/10.1007/S13187-017-1229-0.
Ezechi OC, Gab-Okafor CV, Ostergren PO, Odberg PK. Willingness and acceptability of cervical cancer screening among HIV positive Nigerian women. BMC Public Health. 2013;13:46. https://doi.org/10.1186/1471-2458-13-46.
Rabiu KA, Akinbami AA, Adewunmi AA, Akinola OI, Wright KO. The need to incorporate routine cervical cancer counselling and screening in the management of HIV positive women in Nigeria. Asian Pac J Cancer Prev. 2011;12(5):1211–4.
Dim CC, Onyedum CC, Dim NR, Chukwuka JC. Cervical cancer screening among HIV-positive women in Nigeria: an assessment of use and willingness to pay in the absence of donor support. J Int Assoc Provid AIDS Care. 2013;14(3):241–4. https://doi.org/10.1177/2325957413488191.
Onyenwenyi AOC, Mchunu GG. Barriers to cervical cancer screening uptake among rural women in South West Nigeria: a qualitative study. S Afr J Obstet Gynaecol. 2018;24(1):19–23. https://doi.org/10.7196/SAJOG.1290.
Okolie EA, Barker D, Nnyanzi LA, Anjorin S, Aluga D, Nwadike BI. Factors influencing cervical cancer screening practice among female health workers in Nigeria: a systematic review. Cancer Rep. 2021;5(5):e1514. https://doi.org/10.1002/CNR2.1514.
Nwobodo H, Ba-Break M. Analysis of the determinants of low cervical cancer screening uptake among Nigerian women. J Public Health Africa. 2015;6(2):484. https://doi.org/10.4081/JPHIA.2015.484.
Modibbo FI, Dareng E, Bamisaye P, Jedy-Agba E, Adewole A, Oyeneyin L, et al. Qualitative study of barriers to cervical cancer screening among Nigerian women. BMJ Open. 2016;6(1):e008533. https://doi.org/10.1136/BMJOPEN-2015-008533.
Belhadj H, Rasanathan J, Denny L, Broutet N. Sexual and reproductive health and HIV services: integrating HIV/AIDS and cervical cancer prevention and control. Int J Gynaecol Obstet. 2013;121(Suppl 1):S29–34. https://doi.org/10.1016/J.IJGO.2013.02.002.
Huchko MJ, Maloba M, Nakalembe M, Cohen CR. The time has come to make cervical cancer prevention an essential part of comprehensive sexual and reproductive health services for HIV-positive women in low-income countries. J Int AIDS Soc. 2015;18(Suppl 1):20282. https://doi.org/10.7448/IAS.18.6.20282.
Joint United Nations Programme on HIV/AIDS. HPV, HIV and cervical cancer: leveraging synergies to save women’s lives. Geneva: UNAIDS; 2016.
Forsythe SS, McGreevey W, Whiteside A, Shah M, Cohen J, Hecht R, et al. Twenty years of antiretroviral therapy for people living with HIV: global costs, health achievements, economic benefits. Health Aff. 2019;38(7):1163–72. https://doi.org/10.1377/HLTHAFF.2018.05391.
El-Sadr WM, Holmes CB, Mugyenyi P, Thirumurthy H, Ellerbrock T, Ferris R, et al. Scale-up of HIV treatment through PEPFAR: a historic public health achievement. J Acquir Immune Defic Syndr. 2012;60(Suppl 3):S96–104. https://doi.org/10.1097/QAI.0B013E31825EB27B.
U.S. Embassy and Consulate in Nigeria. PEPFAR: 20 Years of Impact. 2023. https://ng.usembassy.gov/pepfar-20-years-of-impact/
U.S. Embassy and Consulate in Nigeria. PEPFAR. 2018. https://ng.usembassy.gov/pepfar/
Sigfrid L, Murphy G, Haldane V, Chuah FLH, Ong SE, Cervero-Liceras F, et al. Integrating cervical cancer with HIV healthcare services: a systematic review. PLoS One. 2017;12(7):e0181156. https://doi.org/10.1371/JOURNAL.PONE.0181156.
Mwanahamuntu MH, Sahasrabuddhe VV, Pfaendler KS, Mudenda V, Hicks ML, Vermund SH, et al. Implementation of ‘see-and-treat’ cervical cancer prevention services linked to HIV care in Zambia. AIDS. 2009;23(6):N1–5. https://doi.org/10.1097/QAD.0B013E3283236E11.
Odafe S, Torpey K, Khamofu H, Oladele E, Adedokun O, Chabikuli O, et al. Integrating cervical cancer screening with HIV care in a district hospital in Abuja. Nigeria Niger Med J. 2013;54(3):176–4. https://doi.org/10.4103/0300-1652.114590.
Franceschi S, Jaffe H. Cervical cancer screening of women living with HIV infection: a must in the era of antiretroviral therapy. Clin Infect Dis. 2007;45(4):510–3. https://doi.org/10.1086/520022.
Godfrey C, Prainito A, Lapidos-Salaiz I, Barnhart M, Watts DH. Reducing cervical cancer deaths in women living with HIV: PEPFAR and the Go Further partnership. Prev Med. 2021;144:106295. https://doi.org/10.1016/J.YPMED.2020.106295.
Johnson LG, Armstrong A, Joyce CM, Teitelman AM, Buttenheim AM. Implementation strategies to improve cervical cancer prevention in sub-Saharan Africa: a systematic review. Implement Sci. 2018;13:28. https://doi.org/10.1186/S13012-018-0718-9.
Shiferaw N, Salvador-Davila G, Kassahun K, Brooks MI, Weldegebreal T, Tilahun Y, et al. The single-visit approach as a cervical cancer prevention strategy among women with HIV in Ethiopia: successes and lessons learned. Glob Health Sci Pract. 2016;4(1):87–98. https://doi.org/10.9745/GHSP-D-15-00325.
Rohner E, Mulongo M, Pasipamire T, Oberlin AM, Goeieman B, Williams S, et al. Mapping the cervical cancer screening cascade among women living with HIV in Johannesburg. South Africa Int J Gynecol Obstet. 2021;152(1):53–9. https://doi.org/10.1002/IJGO.13485.
Aarons GA, Hurlburt M, Horwitz SMC. Advancing a conceptual model of evidence-based practice implementation in public service sectors. Adm Policy Ment Health Ment. 2011;38(1):4–23. https://doi.org/10.1007/s10488-010-0327-7.
Pitpitan EV, Semple SJ, Aarons GA, Palinkas LA, Chavarin CV, Mendoza DV, et al. Factors associated with program effectiveness in the implementation of a sexual risk reduction intervention for female sex workers across Mexico: results from a randomized trial. PLoS One. 2018;13(9):e0201954. https://doi.org/10.1371/JOURNAL.PONE.0201954.
Moullin JC, Dickson KS, Stadnick NA, Rabin B, Aarons GA. Systematic review of the Exploration, Preparation, Implementation, Sustainment (EPIS) framework. Implement Sci. 2019;14:1. https://doi.org/10.1186/s13012-018-0842-6.
Lengnick-Hall R, Stadnick NA, Dickson KS, Moullin JC, Aarons GA. Forms and functions of bridging factors: specifying the dynamic links between outer and inner contexts during implementation and sustainment. Implement Sci. 2021;16:34. https://doi.org/10.1186/S13012-021-01099-Y.
Itanyi IU, Viglione C, Rositch AF, Olawepo JO, Olakunde BO, Ikpeazu A, et al. Rapid implementation mapping to identify implementation determinants and strategies for cervical cancer control in Nigeria. Front public. Health. 2023:11. https://doi.org/10.3389/FPUBH.2023.1228434.
Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs. Med Care. 2012;50:217–26.
EPIS Framework. https://episframework.com/
Hatch MR, Carandang K, Moullin JC, Ehrhart MG, Aarons GA. Barriers to implementing motivational interviewing in addiction treatment: a nominal group technique process evaluation. Implement Res Pract. 2021;2:26334895211018400. https://doi.org/10.1177/26334895211018400.
Fernandez ME, ten Hoor GA, van Lieshout S, Rodriguez SA, Beidas RS, Parcel G, et al. Implementation mapping: using intervention mapping to develop implementation strategies. Front Public Health. 2019;7:158. https://doi.org/10.3389/FPUBH.2019.00158.
Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. https://doi.org/10.1186/S13012-015-0209-1.
Aarons GA, Green AE, Palinkas LA, Self-Brown S, Whitaker DJ, Lutzker JR, et al. Dynamic adaptation process to implement an evidence-based child maltreatment intervention. Implement Sci. 2012;7:32. https://doi.org/10.1186/1748-5908-7-32.
Hurlburt M, Aarons GA, Fettes D, Willging C, Gunderson L, Chaffin MJ. Interagency collaborative team model for capacity building to scale-up evidence-based practice. Child Youth Serv Rev. 2014;39:160–8. https://doi.org/10.1016/j.childyouth.2013.10.005.
Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–7. https://doi.org/10.2105/AJPH.89.9.1322.
Kwan BM, McGinnes HL, Ory MG, Estabrooks PA, Waxmonsky JA, Glasgow RE. RE-AIM in the real world: use of the RE-AIM framework for program planning and evaluation in clinical and community settings. Front Public Health. 2019;7:345. https://doi.org/10.3389/fpubh.2019.00345.
Gravitt P, Rositch A, Jurczuk M, Meza G, Carillo L, Jeronimo J, et al. Integrative Systems Praxis for Implementation Research (INSPIRE): an implementation methodology to facilitate the global elimination of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2020;29(9):1710–9. https://doi.org/10.1158/1055-9965.EPI-20-0501.
Ogunsola OO, Ajayi OA, Ojo TO, Osayi E, Wudiri K, Amoo B, et al. Cervical cancer screening and treatment for PLWHIV: experiences from an innovative program in Nigeria. Reprod Health. 2023;20(1):125. https://doi.org/10.1186/S12978-023-01658-0.
Ezeanolue EE, Obiefune MC, Yang W, Obaro SK, Ezeanolue CO, Ogedegbe GG. Comparative effectiveness of congregation- versus clinic-based approach to prevention of mother-to-child HIV transmission: study protocol for a cluster randomized controlled trial. Implement Sci. 2013;8:62. https://doi.org/10.1186/1748-5908-8-62.
Ezeanolue EE, Obiefune MC, Ezeanolue CO, Ehiri JE, Osuji A, Ogidi AG, et al. Effect of a congregation-based intervention on uptake of HIV testing and linkage to care in pregnant women in Nigeria (baby shower): a cluster randomised trial. Lancet Glob Health. 2015;3(11):e692–700. https://doi.org/10.1016/S2214-109X(15)00195-3.
Montandon M, Efuntoye T, Itanyi IU, Onoka CA, Onwuchekwa C, Gwamna J, et al. Improving uptake of prevention of mother-to-child HIV transmission services in Benue State, Nigeria through a faith-based congregational strategy. PLoS One. 2021;16(12):e0260694. https://doi.org/10.1371/JOURNAL.PONE.0260694.
Gbadamosi SO, Eze C, Olawepo JO, Iwelunmor J, Sarpong DF, Ogidi AG, et al. A patient-held smartcard with a unique identifier and an mHealth platform to improve the availability of prenatal test results in rural Nigeria: Demonstration study. J Med Internet Res. 2018;20(1):e18. https://doi.org/10.2196/jmir.8716.
Ezeanolue EE, Gbadamosi SO, Olawepo JO, Iwelunmor J, Sarpong D, Eze C, et al. An mHealth Framework to Improve Birth Outcomes in Benue State, Nigeria: a study protocol. JMIR Res Protoc. 2017;6(5):e100. https://doi.org/10.2196/resprot.7743.
Olawepo JO, Ezeanolue EE, Ekenna A, Ogunsola OO, Itanyi IU, Jedy-Agba E, et al. Building a national framework for multicentre research and clinical trials: experience from the Nigeria Implementation Science Alliance. BMJ Glob Health. 2022;7(4):e008241. https://doi.org/10.1136/BMJGH-2021-008241.
Shea CM, Jacobs SR, Esserman DA, Bruce K, Weiner BJ. Organizational readiness for implementing change: a psychometric assessment of a new measure. Implement Sci. 2014;9:7. https://doi.org/10.1186/1748-5908-9-7.
Jacobs SR, Weiner BJ, Bunger AC. Context matters: measuring implementation climate among individuals and groups. Implement Sci. 2014;9:46. https://doi.org/10.1186/1748-5908-9-46.
Moullin JC, Ehrhart MG, Aarons GA. Development and testing of the Measure of Innovation-Specific Implementation Intentions (MISII) using Rasch measurement theory. Implement Sci. 2018;13:89. https://doi.org/10.1186/S13012-018-0782-1.
Melnyk BM, Fineout-Overholt E, Mays MZ. The evidence-based practice beliefs and implementation scales: psychometric properties of two new instruments. Worldviews evidence-based Nurs. 2008;5(40):208–16. https://doi.org/10.1111/J.1741-6787.2008.00126.X.
Anderson J, Lu E, Sanghvi H, Kibwana S, Lu A. Cervical cancer screening and prevention for HIV-infected women in the developing world. In: Georgakilas AG, editor. Cancer prevention - from mechanisms to translational benefits. Rijeka, Croatia: IntechOpen; 2012. p. 231–60. https://doi.org/10.5772/31593.
Hade EM, Murray DM, Pennell ML, Rhoda D, Paskett ED, Champion VL, et al. Intraclass correlation estimates for cancer screening outcomes: estimates and applications in the design of group-randomized cancer screening studies. J Natl Cancer Inst Monogr. 2010;2010(40):97–103. https://doi.org/10.1093/JNCIMONOGRAPHS/LGQ011.
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. Available: https://www.r-project.org/
Donner A, Klar N. Design and analysis of cluster randomization trials in health research. London: Arnold; 2000.
Moullin JC, Sklar M, Ehrhart MG, Green A, Aarons GA. Provider REport of Sustainment Scale (PRESS): development and validation of a brief measure of inner context sustainment. Implement Sci. 2021;16:86. https://doi.org/10.1186/S13012-021-01152-W.
Wiltsey Stirman S, Kimberly J, Cook N, Calloway A, Castro F, Charns M. The sustainability of new programs and innovations: a review of the empirical literature and recommendations for future research. Implement Sci. 2012;7:17.
Aarons GA, Green AE, Trott E, Willging CE, Torres EM, Ehrhart MG, et al. The roles of system and organizational leadership in system-wide evidence-based intervention sustainment: a mixed-method study. Admin Pol Ment Health. 2016;43(6):991–1008. https://doi.org/10.1007/s10488-016-0751-4.
Palinkas LA, Aarons GA, Horwitz S, Chamberlain P, Hurlburt M, Landsverk J. Mixed method designs in implementation research. Admin Pol Ment Health. 2011;38(1):44–53. https://doi.org/10.1007/S10488-010-0314-Z.
Onwuegbuzie A, Teddlie C. A framework for analyzing data in mixed methods research. Handbook of Mixed Methods in Social and Behavioral Research. 2003;2:397–430.
Creswell J, Plano CV. Designing and conducting mixed methods research. Thousand Oaks, CA: SAGE Publications; 2007.
Aarons GA, Fettes DL, Sommerfeld DH, Palinkas LA. Mixed methods for implementation research: application to evidence-based practice implementation and staff turnover in community based organizations providing child welfare services. Child Maltreat. 2012;17(1):67–79. https://doi.org/10.1177/1077559511426908.
Teddlie C, Tashakkori A. Major issues and controversies in the use of mixed methods in the social and behavioral sciences. In: Teddlie C, Tashakkori A, editors. Handbook of Mixed Methods in Social and Behavioral Research. Thousand Oaks, CA: SAGE Publications Inc.; 2003. p. 3–50.
Bryman A. Integrating quantitative and qualitative research: how is it done?: Qual Res. 2006;6(1):97–113. https://doi.org/10.1177/1468794106058877.
Tashakkori A, Creswell JW. The new era of mixed methods. SAGE Publications; 2007.
Patton MQ. Qualitative research and evaluation methods. Thousand Oaks, CA: SAGE Publications; 2002.
Finley EP, Huynh AK, Farmer MM, Bean-Mayberry B, Moin T, Oishi SM, et al. Periodic reflections: a method of guided discussions for documenting implementation phenomena. BMC Med Res Methodol. 2018;18:153. https://doi.org/10.1186/S12874-018-0610-Y.
Wiltsey Stirman S, Baumann AA, Miller CJ. The FRAME: an expanded framework for reporting adaptations and modifications to evidence-based interventions. Implement Sci. 2019;14:58. https://doi.org/10.1186/S13012-019-0898-Y.
Miller CJ, Barnett ML, Baumann AA, Gutner CA, Wiltsey-Stirman S. The FRAME-IS: a framework for documenting modifications to implementation strategies in healthcare. Implement Sci. 2021;16:36. https://doi.org/10.1186/S13012-021-01105-3.
World Health Organization. WHO guideline for screening and treatment of cervical pre- cancer lesions for cervical cancer prevention, second edition. Geneva: WHO; 2021.
Acknowledgements
We thank our collaborators and partners at each of the participating sites for their engagement and co-creation in this project. We thank the Nigeria Implementation Science Alliance and ICON-3 PBRN for facilitating this work.
Funding
The study is funded by the National Cancer Institute grant U01 CA275118 (MPIs Gregory Aarons and Echezona Ezeanolue). Drs. Aarons and Ezeanolue are also supported by Fogarty International Center grant G11TW011841. Dr. Aarons is also supported by National Institute of Drug Abuse grant R01DA049891 and National Institute of Mental Health grant P50MH126231. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number U01CA275118. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
GAA and EEE are the principal investigators of the grant, and conceptualized the study. GAA, EEE, BOO, IUI, JOO, and NNL contributed to the overall study design and development and of the implementation strategies. GAA, EEE, BOO, IUI, JOO, NNL, CB, NI, TCO, CCD, and COC contributed to the refinement of the implementation strategies. LL developed the analysis plan. BOO and IUI wrote the first draft of the manuscript. All authors reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study has been approved by the National Health Research Ethics Committee of Nigeria (NHREC) (Aim 1: NHREC/01 January 2007-17 February 2023; Aims 2 & 3: NHREC/01/01/2007-20/07/2023) and University of California, San Diego, USA (Aim 1: IRB no. 804338 and Aims 2 & 3: IRB no. 808626).
Consent for publication
Not applicable.
Competing interests
GAA is a co-editor in chief at Implementation Science and is on the editorial board of Implementation Science Communications. All decisions on this paper were made by another editor. The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Description of study facilities.
Additional file 2.
Measurement Instruments.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Olakunde, B.O., Itanyi, I.U., Olawepo, J.O. et al. Comparative effectiveness of implementation strategies for Accelerating Cervical Cancer Elimination through the integration of Screen-and-treat Services (ACCESS study): protocol for a cluster randomized hybrid type III trial in Nigeria. Implementation Sci 19, 25 (2024). https://doi.org/10.1186/s13012-024-01349-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13012-024-01349-9