Abstract
Primary pulmonary hyalinizing clear cell carcinoma (HCCC) is a very rare lung tumor that accounts for less than 0.09% of all primary lung tumors and has no specific epidemiology. The correct diagnosis requires imaging, laboratory, pathological, immunohistochemical, and molecular examination. The most typical feature of pulmonary HCCC is the clear cell component with clear stroma. In addition, the fusion gene EWSR1::ATF1 due to t(12;22)(q13;q12) is essential for the pathological diagnosis of pulmonary HCCC. The main treatment for pulmonary HCCC is surgery. This review focus on the pathological features, immunohistochemical examination, mutation analysis and treatment of pulmonary HCCC.
Similar content being viewed by others
Introduction
Hyalinizing clear cell carcinoma (HCCC), also referred to as clear cell carcinoma (CCC), is a rare salivary gland tumor usually caused by the salivary glands of the head and neck [1]. Salivary gland-type tumor (SGT) presenting outside the head and neck is a rare phenomenon, with pulmonary SGT being less than 1% of all lung tumors [2, 3]. Primary pulmonary hyalinizing clear cell carcinoma, a type of pulmonary SGT, accounts for less than 0.09% of all primary lung tumors and was first reported in 2015 [4, 5]. Pulmonary HCCC is a low-grade malignant tumor [6] with histological, immunophenotypic and molecular features similar to those of salivary gland CCC of the head and neck [7]. The epidemiological and clinical features of pulmonary HCCC are neither specific nor representative [5]. Therefore, the correct diagnosis of pulmonary HCCC requires a combination of histopathological examination, immunohistochemistry (IHC) and molecular mutation analysis. Pathologically, there are several distinctive features such as a clear cell components, hyalinizing stroma, and low-grade cytological atypia [5]. In terms of molecular fusion, Ewing’s sarcoma break region 1 (EWSR1) rearrangement was detected in 87–91% of HCCCs [8]. The most common genetic variant is t(12;22)(q13;q12), leading to the gene fusion EWSR1 activation of transcription factor 1 (ATF1) [9, 10]. Other gene fusions, such as EWSR1::CREM and ATF1::SPLTC2, have been reported in pulmonary HCCC [6]. To date, surgery is recognized as the main treatment of pulmonary HCCC [7], while the effectiveness of chemotherapy and radiotherapy is unclear [11].
Methods
We used a comprehensive computer search of literature in the National Institutes of Health PubMed database, using combinations of the terms pulmonary hyalinizing clear cell carcinoma, clear cell carcinoma, clear cell and salivary gland tumor to search for studies published from 2015 to December 2023. After a careful review of each published case, tumors were selected for inclusion if they were diagnosed as pulmonary HCCC. Then, extract data related to demographics, clinical presentation, radiological presentation, gene fusion, treatment and outcomes.
Clinical characteristics
HCCC usually originates from the salivary glands of the head and neck and is a common low-grade malignant tumor of the salivary gland [1, 4]. Uncommon locations include trachea, bronchi, and nasopharynx [12]. Pulmonary HCCC accounts for less than 0.09% of primary lung tumors [5]. Our review of the previous literature showed that the average age of pulmonary HCCC patients is 54 years old, and tumors seem to be more common in females, accounting for 61.8% of cases. As is well known, lung cancer is closely related to smoking; however, 76.5% of pulmonary HCCC patients have no history of smoking. The detailed information of pulmonary HCCC cases is shown in Table 1. Pulmonary HCCC originates in the submucosal bronchial glands and presents as a central lesion of an intrabronchial mass, so patients usually present with signs and symptoms of bronchial obstruction, including shortness of breath, cough, chest pain, hemoptysis, pneumonia or fever [11]. Sometimes, patients are asymptomatic and can only be detected by physical examination [13].
The Ki-67 index of tumor cells in the pulmonary HCCC is low, with a positive index of 1% ~ 15%, indicating a slow growth rate [5, 6]. The mitotic rate is usually low, and necrosis is rare [25], indicating that this tumor is of low grade. In the head and neck, HCCC commonly shows inert clinical behavior, local recurrence and locoregional nodal metastasis of 49% and 15%, respectively, have been reported [26]. Although pulmonary HCCC is similar to salivary gland HCCC and has a lower likelihood of metastasis, local recurrence may still occur [1, 4]. In some cases of pulmonary HCCC, tumor cells infiltrating the lung parenchyma [14], cartilage [4, 15, 16], and nerves [5, 16, 17] have been confirmed, which may indicate an aggressive clinical course that can also lead to metastasis and recurrence [14]. For example, Wang et al. [14] diagnosed a patient with pulmonary HCCC who underwent right upper lobe lobectomy. 16 years later, four recurrent, 1.5 cm diameter hard white pulmonary HCCC tumors were found in the right lower lobe, and enlarged group 3/4 lymph node metastases were observed.
Taken together, pulmonary HCCC is a rare tumor that occurs unrelated to smoking and originates from submucosal bronchial glands. In addition, due to the lack of relevant reports and the long duration of metastasis and recurrence, little is known about pulmonary HCCC. Therefore, we need more case studies to illuminate the clinical characteristics of pulmonary HCCC.
Imaging examination
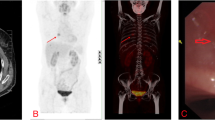
Chest imaging is the preferred method to detect pulmonary HCCC, especially computed tomography (CT) scan is advantageous as it can clearly display the lesion site, size, morphology, margin, density, presence of pleura, pericardial invasion, and enlargement of mediastinal lymph nodes in the hilum. However, the imaging features of pulmonary SGT were not distinct. CT shows a well-defined single mass or nodule in the bronchus, causing bronchial wall thickening and/or intramural polyp-like growth [11]. On positron emission tomography—computed tomography (PET-CT), this tumor shows FDG avidity like squamous cell carcinoma [27]. All pulmonary HCCC masses are central, involving the trachea, main bronchus, lobar bronchus and segmental bronchial mucosa [6]. Endobronchial lesions are often observed as imaging characteristic of obstructive pneumonia and/or pulmonary atelectasis [11].
Pathology and immunohistochemistry examinations
The histological, immunophenotypic and molecular characteristics of pulmonary HCCC are similar to those of salivary gland CCC in the head and neck [7]. Primary salivary gland tumors of the tracheobronchial tree originate from the submucosal glands of the bronchi [18]. Pulmonary HCCC is often located near the bronchus or extends into the bronchial lumen [5], obstructing the bronchus and a large amount of mucus is observed in the sample [4]. Grossly, pulmonary HCCC tumors has a tan-white [4, 11], gray-white [11] or brownish [7] cut with a fibrous and firm consistency, well-defined margins [4, 7, 11, 15, 16], and no epithelium [6].
Pathological examination reveals infiltrative pulmonary HCCC neoplasms composed of irregular nests, cords and trabeculae of polygonal tumor cells [15]. The tumor cells are rather monotonous, small to medium sized, and contain oval nuclei with irregular nuclear contours and ample clear cytoplasm [11]. Sometimes, very rare intranuclear cytoplasmic inclusions found in some pulmonary HCCCs [15]. There is no or only very concentrated necrosis in the tumor cells, and mitotic activity is low [11].Focal mucin pools may be evident [11]. The edge of the tumors is often surrounded by a lymphocytic infiltrate [4, 28]. It is worth noting that, despite the name "clear", most cases of pulmonary HCCC are eosinophilic, and the proportion of cells with clear or eosinophilic cytoplasm varies from case to case, with a clear gradual transition, so the diagnosis of pulmonary HCCC was previously based on the growth pattern (cords, nests, trabeculae, etc.) and the interstitial glassy appearance [5, 6]. Moreover, most pulmonary HCCC tumor cells have clear boundaries, however, under a microscope, it can be observed that pulmonary HCCC tumor cells invade adjacent alveolar parenchyma and lymph nodes around the bronchi or damages bronchial cartilage. In addition to the above situation, tumor cells were seen spreading along the bronchial wall below the respiratory epithelium in the case demonstrated by Feng et al. [17]. Given the rarity of pulmonary HCCC, if these morphological details do not provide a definitive diagnosis, other techniques such as molecular studies or immunohistochemical markers can be applied [11].
The immunohistochemical features of pulmonary HCCC can be summarized by reviewing previous literature. In most cases, p63, p40, CK7, CK5 /6 were positive, while thyroid transcription factor-1 (TTF-1), smooth muscle actin (SMA) and S-100 protein were negative. Immunohistochemical results for patients with pulmonary HCCC are specified in Table 2. P63 was positive, indicating a proliferative potential of the lesion [29]. Since pulmonary HCCC tumor cells were S-100-negative and epithelial membrane antigen-positive, it was suggested that pulmonary HCCC was predominantly epithelial rather than myoepithelial in nature [4]. The absence of focal clear cell features of S-100 and SMA expression in the background of transparent fibrosis indicates that pulmonary HCCC may originate from bronchial submucosal glands [4]. SOX10 was consistently negative in HCCC and mucinous epidermoid carcinoma. Furthermore, SOX10 is a marker of bronchial glandular differentiation, suggesting that pulmonary HCCC are not of acinar cell origin [5]. According to reports, a patient with pulmonary HCCC developed tumor metastases 16 years after right upper lung lobectomy. Interestingly, although the morphological features of this metastatic case were similar to primary pulmonary HCCC, immunohistochemistry was altered and p63 expression was absent in this case [14].
Molecular characteristics
Fusion genes are important molecular genetic features of many tumors. Translocations is considered to be the first step in tumor development [30], leading to gene fusions that often produce new, tumor-specific chimeric transcription factors [31], which in turn might disrupt gene expression [32]. Many translocations are associated with specific tumor types that have unique clinical features and gene expression profiles [30]. Antonescu et al. [9] identified the EWSR1 rearrangement in pulmonary HCCC by fluorescence in situ hybridization (FISH), the fusion transcript EWSR1::ATF1 by reverse transcription-polymerase chain reaction (RT-PCR) [19]. The fusion of EWSR1::ATF1 in most pulmonary HCCCs is due to t(12; 22)(q13; q12) [9, 10]. Translocation of EWSR1 is a determinant molecular feature and is present in approximately 80% of salivary CCCs [15]. The EWSR1::ATF1 fusion is the most common form of fusion in the salivary CCCs and is usually detected in 80% to 90% of cases [6]. EWSR and ATF1 genes are rearranged not only in primary pulmonary HCCC but also in pulmonary HCCC metastases [14]. We reviewed previous literature and found that EWSR1::ATF1 gene fusion was present in 19/34 cases and EWSR1::CREM fusion in 3/34 cases, with one case showing EWSR1::ATF1 gene fusion and ATF1::SPLTC2 gene fusion. The detailed gene fusions of the cases are shown in Table 1. Recently, Grosjean et al. [20] reported a case of pulmonary HCCC with a final diagnosis of EWSR::CREM fusion after analysis by RNASeq. Diagnosis can be confirmed by demonstrating rearrangements involving EWSR1::ATF1 or, more rarely, EWSR1::CREM. Although, EWSR1 exhibits the ability to fuse with various partner genes such as POU5F1, PBX1 and ZNF444, fusion with ATF1 at a specific breakpoint (EWSR1 exon 11:ATF1 exon 3) was detected only in salivary gland HCCC [9]. In the research by Di et al. [21], fusion of EWSR1::ATF1 (exon 2: exon 12) was found in a patient, which was the first fusion found in primary lung HCCC except for exon 11 and exon 3, but the significance of fusion is still unclear.
EWSR1 rearrangements can distinguish pulmonary HCCC from other pulmonary salivary gland tumors. To date, other gene fusions in pulmonary HCCC include EWSR1::CREM and ATF1::SPLTC2. EWSR1::ATF1 plays a certain role in the diagnosis of pulmonary HCCC. However, EWSR1 rearrangements (with different binding partners) are also detected in other tumors, such as hematolymphoid neoplasms, Ewing sarcoma/ primitive neuroectodermal tumors, desmoplastic small round cell tumor, clear cell sarcoma, myxoid chondrosarcoma, myxoid liposarcoma, and melanocytic neoplasms [4]. Although EWSR1::ATF1 is not specific for the diagnosis of pulmonary HCCC, such as in clear cell sarcomas, angiomatoid fibrous histiocytoma, angiosarcoma, malignant gastrointestinal neuroectodermal tumor, and the soft tissue myoepithelial tumor, EWSR1::ATF1 can distinguish pulmonary HCCC from other tumors without this gene fusion [4]. In addition, our review of previous literature shows that EWSR1::CREM is observed in clear cell sarcomas, clear cell sarcomatoid tumors of the gastrointestinal tract, myxoid fibrous histiocytomas, unclassified spindle cell tumors, and unclassified small round cell tumors [6]. Therefore, the combination of morphological and immunophenotypic features and tumor location deems EWSR1 rearrangement as a promising indicator for the diagnosis of pulmonary HCCC [8].
Differential diagnosis
The primary salivary gland type of CCC needs to be distinguished morphologically from solid sheet-like carcinomas such as squamous cell carcinoma of lung, mucinous epidermoid carcinoma, myoepithelial carcinoma, solid subtype adenocarcinoma and other metastatic clear cell carcinomas [6].
Squamous cell carcinoma of lung: pulmonary HCCC can be squamous epithelial metaplasia and needs to be differentiated from squamous cell carcinoma. Squamous cell carcinoma is usually more heterogeneous, with more nuclear schizophrenia and more aggressive growth, and may have FGFR1, DDR2 and PIK3CA mutations. The absence of the EWSR1 fusion gene helps to differentiate it from salivary gland CCC [6].
Mucinous epidermoid carcinoma: pulmonary HCCC may present with mucus secretion and squamous or epidermis-like differentiation and needs to be differentiated from mucinous epidermoid carcinoma. Mucinous epidermoid carcinoma usually presents with distinct mucinous, epidermis-like and intermediate cells, forming solid nests and often cystic changes. Most importantly, mucinous epidermoid carcinoma exist a MAML2 gene fusion, which helps to differentiate it from pulmonary HCCC [6].
Clear cell myoepithelial carcinoma: the cytoplasm of tumor cells is transparent, mildly atypic, nest-like growth, which is similar to pulmonary HCCC in morphology. EWSR1 gene transmutation also exists in myoepithelial carcinoma, but the fusion form is different from pulmonary HCCC. EWSR1::PBX1, EWSR1::ZNF444 and FUS::KLF17 are common [33]. In addition, myoepithelial carcinoma cells are often spindle-shaped, plasma cell-like and epithelioid, and are positive for markers of myoepithelial differentiation (S-100 protein, GFAP, SMA, Calponin, etc.) to varying degrees, whereas in pulmonary HCCC, these markers are negative [6].
Solid subtype adenocarcinoma solid subtype of lung adenocarcinoma cells may appear as clear cells, and it often express TTF-1 and Napsin A, which are markers of lung adenocarcinoma [6] and not of pulmonary HCCC.
Pulmonary metastatic CCC: pulmonary metastatic renal CCCs and ovarian CCCs, all of which may present with clear cells, but positive for PAX8 in metastatic kidney cancer and ovarian cancer. The differential diagnosis can be made by combining the clinical history, histopathological features and immunophenotype [6].
In addition, since pulmonary HCCC and salivary gland CCC share the same features, consisting mainly of vacuolated cells with large amounts of clear cytoplasm, which might be confused with metastatic salivary CCC. Therefore, thorough clinical examination is essential for excluding metastatic salivary CCC [5, 34]. Finally, PET-CT would clarify the location of the primary site, thus excluding CCC originating from the salivary gland at the primary site.
Treatment
Due to the low prevalence of pulmonary HCCC, there is no consensus on the best treatment strategy. Nevertheless, it is currently believed that complete surgical resection is the best treatment option [7, 35]. On the aspect of HCCC of the head and neck, Albergotti et al. [36] suggest that for patients with positive margins, re-excision should be attempted to obtain negative margins, and if this is not a feasible option, adjuvant radiotherapy would be appropriate to consider. Gubbiotti et al. [19] had applied radiotherapy and chemotherapy to a patient with pulmonary HCCC, but the efficacy was not very clear and the patient died 6 years after the first consultation. The efficacy of chemotherapy and radiotherapy is unknown because of the small number of cases in which they have been applied [11]. Radiotherapy alone or in combination with radio-sensitization chemotherapy may be useful for recurrent disease, but the evidence is insufficient [37]. Therefore, Radiotherapy and chemotherapy are current directions worth exploring.
Treatment options for pulmonary HCCC include not only surgery, but also laser therapy and cryotherapy. Icard et al. [1] found a 66-year-old woman in respiratory failure due to a pulmonary HCCC mass that blocked 60% of the tracheal lumen. Hence, neodymium-doped: yttrium aluminum garnet (Nd: YAG) laser treatment was performed, and continuous cryotherapy was performed within one to two minute freeze thaw cycles. Cryotherapy to the areas of cauterization offers a localized hemostasis to the damaged mucosa and potentially localized treatment to the tumor. In addition, this local tumor treatment reduces the recurrence rate of the tumor and allows for sloughing of the remaining mucosa, which may help decrease restenosis associated with endothermic damage caused from laser therapy.
Hitherto, the main treatment for pulmonary HCCC is surgery, with cryotherapy and laser treatment also being options, while the effectiveness of radiotherapy and chemotherapy is unknown. Radiotherapy and chemotherapy may be a direction to try when patients are not well treated with surgery. In addition to this, as gene fusions almost always occur in pulmonary HCCC, then targeted therapy is a future direction that could be explored.
Prognosis
We reviewed previous literature and found that 34 patients were followed up for 2–192 months with only 2 recurrences. Details of the pulmonary HCCC cases can be found in Table 1. Patients with HCCC usually have a relatively good prognosis. A good to excellent prognosis has been reported for head and neck HCCC [7]. The local recurrence rate is 11% to 20%, metastatic disease is rare [9, 28, 36, 38,39,40,41,42]. Of the 111 patients with head and neck HCCC collected by Albergotti et al. [36], 21 had local recurrence. The time to recurrence ranged from 5 to 180 months, with a mean time of 42.1 months. Necrosis, positive cut margins and lymph node status are risk factors associated with recurrence of head and neck HCCC. In particular, lymph node invasion, positive surgical margin status, and tumor cell necrosis are associated with an increased risk of recurrence, with the highest risk of recurrence in the first 2 years and years 5 to 7 [7]. HCCCs with high-grade features (necrosis, nuclear pleomorphism, high mitotic index, atypical mitogram) should be considered with more caution when considering prognosis [7]. At present, the prognosis of head and neck HCCC is clear, and as for pulmonary HCCC, which has similarities to head and neck, the prognosis of pulmonary HCCC still needs to be further investigated through the accumulated cases due to the limited number of cases of pulmonary HCCC. As of now, the prognosis for pulmonary HCCC is good.
However, it should be remembered that although the prognosis for pulmonary HCCC is good, metastases and recurrences can still be expected. A case of late recurrence of bronchial HCCC was reported by Wang et al. [14]. These findings suggest that bronchial HCCC risks late postoperative metastasis, even if it is an occult cancer, and therefore careful long-term follow-up should be advisable [5].
Conclusion
Pulmonary HCCC is a low-grade malignant tumor. The majority of pulmonary HCCC occurs in middle-aged and elderly women over 40 years old. Clinical symptoms of pulmonary HCCC are non-specific, and some patients with pulmonary HCCC are even found accidentally during routine physical examinations. Pulmonary HCCC is most typically characterised by clear to eosinophilic tumour cells arranged in nests, ropes and trabeculae. IHC showed that all cases expressed epithelial markers, while myoepithelial markers were not expressed in all cases. EWSR1::ATF1 is the most common gene fusion induced by t(12; 22)(q13; q12) mutations. Patients with pulmonary HCCC have a high survival rate, a good prognosis and recurrence is rare. Surgery is currently the main treatment for pulmonary HCCC, with other treatments including cryotherapy and laser therapy. The role of adjuvant chemotherapy and radiotherapy is unknown. Targeted therapies and immunotherapy should be explored in the future.
Availability of data and materials
All data generated or analyses during this study are included in this published article and its supplementary information files.
References
Icard B, Grider DJ, Aziz S, et al. Primary tracheal hyalinizing clear cell carcinoma. Lung Cancer. 2018;125:100–2.
Molina JR, Aubry MC, Lewis JE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer. 2007;110:2253–9.
Moran CA. Primary salivary gland-type tumors of the lung. Semin Diagn Pathol. 1995;12:106–22.
García JJ, Jin L, Jackson SB, et al. Primary pulmonary hyalinizing clear cell carcinoma of bronchial submucosal gland origin. Hum Pathol. 2015;46:471–5.
Takamatsu M, Sato Y, Muto M, et al. Hyalinizing clear cell carcinoma of the bronchial glands: presentation of three cases and pathological comparisons with salivary gland counterparts and bronchial mucoepidermoid carcinomas. Mod Pathol. 2018;31:923–33.
Xue QQ, Huang Y, Zuo SY, et al. [Clinicopathological features and molecular genetic changes of lung salivary gland-type clear cell carcinoma]. Zhonghua Bing Li Xue Za Zhi. 2021;50:728–33.
Jeffus SK, Gardner JM, Steliga MA, et al. Hyalinizing clear cell carcinoma of the lung: case report and review of the literature. Am J Clin Pathol. 2017;148:73–80.
Roden AC. Recent updates in salivary gland tumors of the lung. Semin Diagn Pathol. 2021;38:98–108.
Antonescu CR, Katabi N, Zhang L, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50:559–70.
Aljerian K. EWSR1 rearrangement is not specific for hyalinizing clear cell carcinoma of salivary glands. Ann Diagn Pathol. 2022;59:151946.
Falk N, Weissferdt A, Kalhor N, et al. Primary pulmonary salivary gland-type tumors: a review and update. Adv Anat Pathol. 2016;23:13–23.
Thakur S, Nambirajan A, Larsen BT, et al. Primary pulmonary hyalinizing clear cell carcinoma: case series with Review of Literature. Int J Surg Pathol. 2023;31:1187–94.
Zhang Y, Han W, Zhou J, et al. Primary lung hyalinizing clear cell carcinoma: a diagnostic challenge in biopsy. Diagn Pathol. 2022;17:35.
Weinreb I. Hyalinizing clear cell carcinoma of salivary gland: a review and update. Head Neck Pathol. 2013;7(Suppl 1):20–9.
Hernandez-Prera JC, Kwan R, Tripodi J, et al. Reappraising hyalinizing clear cell carcinoma: a population-based study with molecular confirmation. Head Neck. 2017;39:503–11.
Wang H, Li WY, Kuo YJ, et al. Primary pulmonary hyalinising clear cell carcinoma with mucin production and delayed metastases after 16 years. Pathology. 2016;48:518–21.
Feng L, Han Y, Wang Y, et al. Primary pulmonary hyalinizing clear cell carcinoma with pseudopapillary structures and abundant cysts filled with mucus. Pathol Res Pract. 2023;241:154237.
Doxtader EE, Shah AA, Zhang Y, et al. Primary salivary gland-type tumors of the tracheobronchial tree diagnosed by transbronchial fine needle aspiration: clinical and cytomorphologic features with histopathologic correlation. Diagn Cytopathol. 2019;47:1168–76.
Gubbiotti MA, Montone K, Zhang P, et al. A contemporary update on hyalinizing clear cell carcinoma: compilation of all in-house cases at our institution and a literature review spanning 2015–2020. Hum Pathol. 2021;111:45–51.
Grosjean V, Fournel P, Picot T, et al. Hyalinizing clear cell carcinoma of the lung with EWSR1::CREM fusion. Histopathology. 2023;83:333–5.
Di J, Xiong Y, Li D, et al. Primary pulmonary hyalinising clear cell carcinoma: two cases and literature review. Malays J Pathol. 2022;44:509–16.
Chapman E, Skalova A, Ptakova N, et al. Molecular profiling of hyalinizing clear cell carcinomas revealed a subset of tumors harboring a Novel EWSR1-CREM Fusion: report of 3 cases. Am J Surg Pathol. 2018;42:1182–9.
Li Z, Li W, Xue L. Primary pulmonary hyalinizing clear cell carcinoma with vocal-cord squamous cell carcinoma: a case report with systematic review. Diagn Pathol. 2023;18:90.
Chen L, Zhou N, Hu S, et al. Hyalinising clear cell carcinoma of the lung: a case report and review of literature. Med (Baltim). 2023;102:e34101.
Shah AA, Mehrad M, Kelting SM, et al. An uncommon primary lung tumour: hyalinizing clear cell carcinoma, salivary gland-type. Histopathology. 2015;67:274–6.
Shahi M, Dolan M, Murugan P. Hyalinizing clear cell carcinoma of the bronchus. Head Neck Pathol. 2017;11:575–9.
Park CM, Goo JM, Lee HJ, et al. Tumors in the tracheobronchial tree: CT and FDG PET features. Radiographics. 2009;29:55–71.
Solar AA, Schmidt BL, Jordan RC. Hyalinizing clear cell carcinoma: case series and comprehensive review of the literature. Cancer. 2009;115:75–83.
Baghirath PV, Kumar JV, Vinay BH. Hyalinizing clear cell carcinoma: a rare entity. J Oral Maxillofac Pathol. 2011;15:335–9.
Mitelman F, Johansson B, Mertens F. The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer. 2007;7:233–45.
Ladanyi M. The emerging molecular genetics of sarcoma translocations. Diagn Mol Pathol. 1995;4:162–73.
Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer. 2003;3:685–94.
Leduc C, Zhang L, Öz B, et al. Thoracic myoepithelial tumors: a pathologic and molecular study of 8 cases with review of the literature. Am J Surg Pathol. 2016;40:212–23.
Li HY, Yu SL, Sun PL, et al. [Hyalinising clear cell carcinoma of the thymus with EWSR1 translocation: report of a case]. Zhonghua Bing Li Xue Za Zhi. 2021;50:1291–3.
Kang DY, Yoon YS, Kim HK, et al. Primary salivary gland-type lung cancer: surgical outcomes. Lung Cancer. 2011;72:250–4.
Albergotti WG, Bilodeau EA, Byrd JK, et al. Hyalinizing clear cell carcinoma of the head and neck: Case series and update. Head Neck. 2016;38:426–33.
Daniele L, Nikolarakos D, Keenan J, et al. Clear cell carcinoma, not otherwise specified/hyalinising clear cell carcinoma of the salivary gland: the current nomenclature, clinical/pathological characteristics and management. Crit Rev Oncol Hematol. 2016;102:55–64.
Milchgrub S, Gnepp DR, Vuitch F, et al. Hyalinizing clear cell carcinoma of salivary gland. Am J Surg Pathol. 1994;18:74–82.
Jin R, Craddock KJ, Irish JC, et al. Recurrent hyalinizing clear cell carcinoma of the base of tongue with high-grade transformation and EWSR1 gene rearrangement by FISH. Head Neck Pathol. 2012;6:389–94.
Wang B, Brandwein M, Gordon R, et al. Primary salivary clear cell tumors–a diagnostic approach: a clinicopathologic and immunohistochemical study of 20 patients with clear cell carcinoma, clear cell myoepithelial carcinoma, and epithelial-myoepithelial carcinoma. Arch Pathol Lab Med. 2002;126:676–85.
O’Regan E, Shandilya M, Gnepp DR, et al. Hyalinizing clear cell carcinoma of salivary gland: an aggressive variant. Oral Oncol. 2004;40:348–52.
Yang S, Zhang J, Chen X, et al. Clear cell carcinoma, not otherwise specified, of salivary glands: a clinicopathologic study of 4 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:712–20.
Acknowledgements
Not applicable.
Funding
This study was supported by Hui Lan Public Foundation (HL-HS2020-128).
Author information
Authors and Affiliations
Contributions
Xinyuan Wang: Writing—Original Draft, Writing—Review & Editing, Investigation. Shumin Hu: Visualization, Formal analysis. Hongyang Lu: Conceptualization, Supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, X., Hu, S. & Lu, H. Pulmonary salivary gland tumor–hyalinizing clear cell carcinoma: a literature review. Diagn Pathol 19, 37 (2024). https://doi.org/10.1186/s13000-024-01460-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-024-01460-x