Abstract
Background
Primary pulmonary hyalinizing clear cell carcinoma (HCCC) is a low-grade salivary gland-type carcinoma. Until now, 23 cases of pulmonary HCCC have been reported.
Case presentation
Here, we present a patient with primary pulmonary HCCC along with vocal-cord squamous cell carcinoma (SCC) revealed by biopsy examination. The patient underwent radiotherapy for vocal-cord SCC, followed by right upper lobectomy and lymph node dissection 10 months later. Histology revealed polygonal cells with eosinophilic or clear cytoplasm in the myxoid matrix together with hyaline degeneration. The tumor involved the whole layer of the segmental bronchus and regionally involved the alveolar tissue along with one intrapulmonary lymph node. Targeted RNA sequencing revealed Ewing Sarcoma Breakpoint Region 1 (EWSR1)- activating transcription factor 1 (ATF1) fusion. We analyzed the data on pulmonary malignant tumors between 2000 and 2019 in the Surveillance, Epidemiology, and End Results (SEER) database and reviewed all cases of pulmonary HCCC with EWSR1 fusion by searching PubMed. The results showed that head and neck (HN) adenoid cystic carcinoma (ACC) (47.89%) and HNSCC (22.54%) were the most common carcinomas occurring with pulmonary salivary gland-type malignant tumors. Screening of 24 cases of pulmonary HCCC with EWSR1 fusion revealed that five cases demonstrated lymph node metastases and only two had documented tumor recurrences. HCCC is rare and easily misdiagnosed as SCC, but the treatment regimen differs between pulmonary HCCC and SCC.
Conclusions
Hence, pulmonary tumors with clear cells must be diagnosed with caution. Next-generation sequencing (NGS) may be useful for diagnosis, especially in cases with a history of squamous cell carcinoma (SCC).
Similar content being viewed by others
Background
Multiple primary malignant neoplasms refer to the appearance of two or multiple malignant neoplasms in the same individual, excluding metastasis from initial primary cancers, and include synchronous and metachronous cancers [1]. Synchronous malignancy of the upper aerodigestive tract is commonly associated with lung cancer [2]. Since lung is a common site of metastasis [3,4,5], it is difficult to differentiate between a multiple primary tumor and metastasis. A history of primary cancer may play a helpful or interfering role in the diagnosis. Pulmonary salivary gland-type carcinoma is a rare type of pulmonary carcinoma, accounting for less than 1.0% of all pulmonary carcinomas [6]. The differentiation of primary pulmonary salivary gland-type carcinoma from common pulmonary carcinomas such as squamous cell carcinoma (SCC), adenocarcinoma, and intrapulmonary metastases is essential in clinic practice, especially in cases with a history of malignancy [7].
Pulmonary hyalinizing clear cell carcinoma (HCCC) is a salivary gland-type malignancy with good prognosis. Pulmonary HCCC, as a new entity, has been listed in the World Health Organization grading system of thoracic tumors (2021 WHO) [7]. According to the 2021 WHO classification criteria, Ewing Sarcoma Breakpoint Region 1 (EWSR1) gene fusion has been identified as a criterion for HCCC. Thus far, 23 cases of pulmonary HCCC with EWSR1 fusion from 13 articles have been reported. This report describes the first case of pulmonary HCCC with vocal-cord SCC.
Case presentation
An 81-year-old man presented to the Cancer Hospital of the Chinese Academy of Medical Sciences with hoarseness, without a history of smoking, occupational or environmental exposures, or a family history of cancer. Bronchoscopic findings showed a neoplasm arising from the opening of the right upper lobe posterior segmental bronchi (Fig. 1A); and laryngoscopy revealed a polyp on the posterior-portion of the right vocal fold with leukoplakia (Fig. 1B). Computed tomography (CT) revealed a well-demarcated, irregular mass, 2.9 cm in size, in the posterior segmental bronchial root of posterior apex of the upper right lung. The tumor was adjacent to the artery and vein of the right upper lobe (Fig. 1C). Biopsy specimens were pathologically diagnosed as right vocal-cord squamous cell carcinoma with intraepithelial neoplasia and pulmonary HCCC. He underwent radiotherapy 33 times for vocal-cord SCC without any additional therapy for the pulmonary tumor. CT reexamination demonstrated no obvious change 10 months later. He underwent right upper lobectomy and lymph node dissection 10 months later. Histopathologic examination of the resected specimen revealed that the lesion was HCCC with involvement of one lymph node.
Preoperative representative radiologic and bronchoscopic images from the current case. (A) Bronchoscopic findings show a neoplasm arising from the opening of the posterior segmental bronchi of the right upper lobe (arrow). (B) Laryngoscope findings showing a polyp on the posterior portion of the right vocal fold with leukoplakia (arrow) (C) Computed tomography scan showing a well-demarcated irregular mass in the posterior segmental bronchial root of the posterior apex of the upper right lung (arrow)
Histopathologic evaluation and immunohistochemistry (IHC)
A fully automated system was used to carry out immunohistochemistry (IHC) on formalin-fixed, paraffin-embedded (FFPE) sections cut at 3 μm. The following antibodies were analyzed on Leica Biosystems: Calponin (EP63, ZSGB-BIO), SMA (1A4, MXB), p53 (MX008, MXB), and p40 (ZR8, MXB). Ki-67 (GM027, genetech), p63 (4A4, Ventana), and Desmin (MX046, MXB) were analyzed on a Discovery Ultra VENTANA systems automated Stainer (Roche), while CK5/6 (MX040, MXB) was analyzed with Dako Autostained Stain System.
Targeted gene sequencing
Next, 56-gene targeted next-generation sequencing (NGS) was conducted according to the previously described procedure [8]. A QIAamp DNA FFPE Tissue Kit (Qiagen, Düsseldorf, Germany) was used to extract DNA from FFPE tissue. Quantification of DNA was performed using a Qubit 3.0 Fluorometer (Thermo Fisher Scientific, Carlsbad, CA), and quality assessment was performed using a 1% agarose gel electrophoresis. Fragmented genomic DNA was processed to create libraries with barcodes that were used to hybridize with DNA panels. Data from sequenced libraries was analyzed to determine genetic alternations. The closest genes in both directions were defined as the predicted fusion partners when breakpoints were detected in the intergenic regions.
Patient population
Dataset 22 from the Surveillance, Epidemiology, and End Results (SEER) database was used to analyze the incidence rate of lung malignant neoplasms between 2000 and 2019. International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) was used to identify tumors based on the histological findings. Neoplasms were included according to the following criteria: (1) neoplasms in the head and neck (HN) (C00-C14, C32) and lung (C33, C34) by the ICD-O-3 primary sites code; (2) neoplasms with malignant behavior; (3) primary tumors diagnosed by international criteria; (4) synchronous tumors and metachronous tumors defined as multiple primary tumors diagnosed within 1 year and more than 1 year, respectively.
Statistical analysis
In this study, multiple primary tumors in the lung and HN were analyzed based on sex, age, sex, primary site, histologic type ICD-O-3, and tumor behavior. The data were compiled by SEER*Stat version 8.4.0.1. and R software (version 4.1.02) was used to generate the boxplot and network.
Pathologic and IHC findings
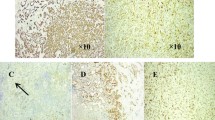
Biopsy samples obtained by bronchoscopy revealed a neoplasm with infiltrative growth in the bronchial submucosa (Fig. 2A). It was composed of polygonal cells, which were arranged as flaky, adenoid clusters in the myxoid matrix with hyaline degeneration. The polygonal cells had an eosinophilic or clear cytoplasm with light atypical round-to-oval nuclei (Fig. 2B). On IHC, tumor cells demonstrated strongly positive staining for CK7 and CK5/6; mildly positive staining for p40, and negative staining for SMA, Desmin, and Calponin. Ki67 proliferative index was approximately 8% (Fig. 2C-F). According to the morphology of this tumor, NGS was performed. NGS showed EWSR1-ATF1 fusion in 14.4% of the tumor cells (Fig. 2G). In addition, a frameshift mutation (p. T13fs) in exon 2 of the FANCI gene was observed. Based on the above-mentioned features, the diagnosis of primary pulmonary HCCC was confirmed.
Transbronchial biopsy revealed hyalinizing clear cell carcinoma (HCCC). Tumor cells exhibit infiltrative growth in the myxoid matrix with hyaline degeneration in the bronchial submucosa. (A). Tumor cells demonstrate eosinophilic to clear cytoplasm and light atypical round-to-oval nuclei (B). Tumor cells express CK7 (C), CK5/6 (D), and p40 (E). Ki-67 index is around 8% (F). Targeted next-generation sequencing results show EWSR1-ATF1 fusion in 14.4% of the tumor cells (G)
Meanwhile, biopsy samples under the laryngoscope yielded a well-differentiated squamous cell carcinoma with high grade dysplasia and epidermoid metaplasia in the right processus vocalis (Fig. 3).
A right upper lobectomy with lymphadenectomy was performed. Grossly, a yellowish, firm, solid mass adjacent to the segmental bronchus was identified (Fig. 4A). Hematoxylin and eosin (H&E)-stained sections showed a morphology which was similar to that of bronchial biopsy sections (Fig. 4B-E). Polygonal cells with eosinophilic to clear cytoplasm were arranged as flaky nests or adenoid clusters in the myxoid matrix with hyaline degeneration. Mucous secretion was observed in a gland-like space with no bona fide glandular differentiation. Abundant extracellular mucus with absent mucinous cells was confirmed by alcian blue and periodic acid schiff staining (Fig. 4F). Nuclei were light atypical with a small blue nucleolus and rare nuclear divisions. The neoplasm mainly involved the whole layer of the segmental bronchus and regionally involved the alveolar tissue and one intrapulmonary lymph node. There was evidence of perineural invasion but no pleural involvement or necrosis. The resection margin was negative. In the study period, this patient was free of any local recurrence and required no additional therapy after surgery.
Pathological analysis of the radical resection specimen. On gross examination, the tumor appears yellowish, firm, and solid (A). Low power magnification shows prominent endobronchial growth (B). Tumor cells are arranged as flaky nests and adenoid clusters in the matrix with hyaline degeneration (C). Nuclei are lightly atypical with a small blue nucleolus (D). Mucous secretion was observed in the gland-like space (E) and confirmed by alcian blue and periodic acid schiff staining (F)
Multiple primary tumors in the SEER dataset
A total of 1,776,599 primary lung cancers from 1,752,949 patients were selected and analyzed from the SEER dataset. Among them, 21,451 (1.224%) patients had a primary head and neck malignancy tumors before a primary lung malignancy, including 6,049 and 16,689 cases of synchronous and metachronous primary malignant tumors, respectively. Unlike the incidence of total pulmonary malignant tumors, squamous cell carcinoma (SCC) had the highest incidence rate among all pulmonary malignant tumors occurring with HN malignant tumors (Fig. 5). The most frequent patterns were pulmonary SCC with HNSCC and pulmonary adenocarcinoma with HNSCC. Additionally, HN adenoid cystic carcinoma (ACC) (n = 10, 37.04%) and HNSCC (n = 9, 33.33%) were the most common carcinomas occurred with pulmonary salivary gland-type malignant tumors, including ACC, mucoepidermoid carcinoma (MEC), and epithelial myoepithelial carcinoma (EMC).
SEER data analysis 2000–2019. Total number of patients with primary pulmonary malignant tumors and patients with metachronous and synchronous primary head and neck malignant tumors (A). Sankey diagram shows the relationship of primary pulmonary malignant tumors with metachronous and synchronous head and neck malignant tumors, and the widths of the bands are directly proportional to the number of cases (B). HN, head and neck; SCC, squamous cell carcinoma; AdCC, adenocarcinoma; LCC, large cell carcinoma; NET, neuroendocrine tumor; AdSCC, adenosquamous carcinoma; SaC, sarcromatoid carcinoma; ACC, adenoid cystic carcinoma; CCC, clear cell carcinoma; MEC, mucoepidermoid carcinoma; EMC, epithelial myoepithelial carcinoma; LEC lymphoepithelial carcinoma
Discussion and conclusions
HCCC is a rare salivary gland-type carcinoma with a good prognosis that comprises 1.2% of all primary carcinomas of the salivary gland. According to the 2021 WHO classification criteria, EWSR1 gene fusion has been identified as one of the criteria for HCCC. Thus far, 23 cases of pulmonary HCCC with EWSR1 gene fusion from 13 articles have been reported.
The 23 cases of pulmonary HCCC cases with EWSR1 gene fusion reported in PubMed are summarized in Table 1, along with the present case. Among them, 13 (54.17%) patients were women and 11 (45.83%) were men, with age ranging from 32 to 81 (average, 45.7) years at the time of diagnosis. History of neoplasms was not mentioned in all 23 cases in the literature. Almost all tumors were in the trachea, bronchi, and segmental bronchi (n = 22, 91.67%). Twenty-three patients were treated with resection, only one patient received adjuvant chemoradiation therapy, and one patient received additional adjuvant radiation therapy. Five of the 20 patients who underwent resection and lymph node dissection had lymph node involvement or metastases. Of the 22 patients with available follow-up data, there were only two patients with tumor recurrence. HCCC comprises of polygonal cells arranged as flaky, nested, adenoid clusters in the myxoid matrix with hyaline degeneration. Mucous secretion was observed in gland-like spaces with no bona fide glandular differentiation [8]. Takamatsu et al. found that mucin production and gland-like spaces were slightly higher in frequency in pulmonary HCCC compared with that in salivary HCCC [9].
As far as we know, this is the first reported case of pulmonary HCCC with synchronous vocal-cord SCC. Recent studies have found a significant association between HN carcinoma and pulmonary carcinoma [2]. Data from SEER pulmonary cancer analysis showed that pulmonary SCC was the most common carcinoma occurring with HN malignant tumors. HCCC is rare and easily misdiagnosed as SCC [10]. However, pulmonary HCCC is a low-grade malignancy. The treatment regimen differs between pulmonary HCCC and SCC. Most patients with pulmonary HCCC recover after radical surgery, while a proportion of patients with pulmonary SCC require radical surgery with additional chemoradiotherapy or targeted therapy [11]. Hence, pulmonary tumors with clear cells have to be diagnosed with caution, especially cases with a history of SCC, and NGS may be useful for diagnosis.
In conclusion, the first reported case of pulmonary HCCC with vocal-cord SCC has been described in this report. Our patient had pulmonary HCCC with synchronous vocal-cord SCC, thereby causing significant diagnostic confusion. Multiple tumors with a similar morphology require careful pathological diagnosis.
Data Availability
The dataset 22 of SEER analyzed during the current study was derived from the SEER database. [https://seer.cancer.gov/statistics/]
Abbreviations
- SCC:
-
Squamous cell carcinoma
- HCCC:
-
Hyalinizing clear cell carcinoma
- ATF1:
-
Activating transcription factor 1
- SEER:
-
Surveillance, Epidemiology, and End Results
- HN:
-
Head and neck
- ACC:
-
Adenoid cystic carcinoma
- NGS:
-
Next-generation sequencing
- CT:
-
Computed tomography
- FFPE:
-
Formalin-fixed paraffin-embedded
- IHC:
-
Immunohistochemistry
References
Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, et al. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2(2):e000172.
Tanjak P, Suktitipat B, Vorasan N, Juengwiwattanakitti P, Thiengtrong B, Songjang C, et al. Risks and cancer associations of metachronous and synchronous multiple primary cancers: a 25-year retrospective study. BMC Cancer. 2021;21(1):1045.
Zhang H, Yu Y, Zhou L, Ma J, Tang K, Xu P, et al. Circulating Tumor Microparticles promote lung metastasis by reprogramming inflammatory and mechanical niches via a macrophage-dependent pathway. Cancer Immunol Res. 2018;6(9):1046–56.
Yücel OT, Yilmaz T, Unal OF, Turan E. Distant metastases in laryngeal squamous cell carcinoma. J Exp Clin Cancer Res. 1999;18(3):285–8.
Ali S, Bryant R, Palmer FL, DiLorenzo M, Shah JP, Patel SG, et al. Distant metastases in patients with carcinoma of the major salivary glands. Ann Surg Oncol. 2015;22(12):4014–9.
Kim BG, Lee K, Um SW, Han J, Cho JH, Kim J, et al. Clinical outcomes and the role of bronchoscopic intervention in patients with primary pulmonary salivary gland-type tumors. Lung Cancer. 2020;146:58–65.
WHO Classification of Tumours Editorial Board. Thoracic tumours. 5th ed. Lyon, France: International Agency for Research on Cancer; 2021.
Jeffus SK, Gardner JM, Steliga MA, Shah AA, Stelow EB, Arnaoutakis K. Hyalinizing Clear Cell Carcinoma of the lung: Case Report and Review of the literature. Am J Clin Pathol. 2017;148(1):73–80.
Takamatsu M, Sato Y, Muto M, Nagano H, Ninomiya H, Sakakibara R, et al. Hyalinizing clear cell carcinoma of the bronchial glands: presentation of three cases and pathological comparisons with salivary gland counterparts and bronchial mucoepidermoid carcinomas. Mod Pathol. 2018;31(6):923–33.
Gubbiotti MA, Montone K, Zhang P, Livolsi V, Baloch Z. A contemporary update on hyalinizing clear cell carcinoma: compilation of all in-house cases at our institution and a literature review spanning 2015–2020. Hum Pathol. 2021;111:45–51.
Liu J, Li C, Seery S, Yu J, Meng X. Identifying optimal first-line interventions for advanced non-small cell lung carcinoma according to PD-L1 expression: a systematic review and network meta-analysis. Oncoimmunology. 2020;9(1):1746112.
Doxtader EE, Shah AA, Zhang Y, Wang H, Dyhdalo KS, Farver C. Primary salivary gland-type tumors of the tracheobronchial tree diagnosed by transbronchial fine needle aspiration: clinical and cytomorphologic features with histopathologic correlation. Diagn Cytopathol. 2019;47(11):1168–76.
Shah AA, Mehrad M, Kelting SM, Lewis JS, Stelow EB. An uncommon primary lung tumour: hyalinizing clear cell carcinoma, salivary gland-type. Histopathology. 2015;67(2):274–6.
Garcia JJ, Jin L, Jackson SB, Larsen BT, Lewis JE, Sukov WR, et al. Primary pulmonary hyalinizing clear cell carcinoma of bronchial submucosal gland origin. Hum Pathol. 2015;46(3):471–5.
Wang H, Li WY, Kuo YJ, Yeh YC, Hsieh MS. Primary pulmonary hyalinising clear cell carcinoma with mucin production and delayed metastases after 16 years. Pathology. 2016;48(5):518–21.
Shahi M, Dolan M, Murugan P. Hyalinizing Clear Cell Carcinoma of the Bronchus. Head Neck Pathol. 2017;11(4):575–9.
Icard B, Grider DJ, Aziz S, Rubio E. Primary tracheal hyalinizing clear cell carcinoma. Lung Cancer. 2018;125:100–2.
Komatsu M, Sakai Y, Nishikubo M, Tane S, Nishio W, Kajimoto K, et al. EWSR1-CREM fusion in pulmonary mesenchymal neoplasm showing distinctive clear cell morphology. Pathol Int. 2020;70(12):1020–6.
Zhang Y, Han W, Zhou J, Yong X. Primary lung hyalinizing clear cell carcinoma: a diagnostic challenge in biopsy. Diagn Pathol. 2022;17(1):35.
Xue QQ, Huang Y, Zuo SY, Zheng Q, Ji G, Zhou XY, et al. Clinicopathological features and molecular genetic changes of lung salivary gland-type clear cell carcinoma. Zhonghua Bing Li Xue Za Zhi. 2021;50(7):728–33.
Chapman E, Skalova A, Ptakova N, Martinek P, Goytain A, Tucker T, et al. Molecular profiling of Hyalinizing Clear Cell Carcinomas revealed a subset of tumors harboring a Novel EWSR1-CREM Fusion: report of 3 cases. Am J Surg Pathol. 2018;42(9):1182–9.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the CAMS Innovation Fund for Medical Sciences (grant numbers 2021-I2M-1-067 and 2016-I2M-3-005).
Author information
Authors and Affiliations
Contributions
Zhuo Li: Data collection, Writing – original draft. Weihua Li: Data analysis. Liyan Xue: Writing – review & editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Z., Li, W. & Xue, L. Primary pulmonary hyalinizing clear cell carcinoma with vocal-cord squamous cell carcinoma: a case report with systematic review. Diagn Pathol 18, 90 (2023). https://doi.org/10.1186/s13000-023-01376-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-023-01376-y