Abstract
Background
The expression of PD-L1 in the immune microenvironment can guide the application of immunosuppressants. In order to monitor the immune status of the body, repeated biopsies have to be taken. Our research aims to find new and convenient means to evaluate this indicator.
Methods
Eighty-three cases of newly diagnosed operable breast cancer without receiving preoperative treatment, were recruited from Beijing Shijitan Hospital between November 2018 and November 2019. The expression of PD-1/PD-L1 on circulating T lymphocytes was detected by flow cytometry and the expression of PD-L1 on immune cells in tumor microenvironment was detected by immunohistochemistry.
Results
The median percentage of positive PD-1 and PD-L1 expression on circulating T lymphocytes was 15.2% and 0.7%, respectively. The peripheral PD-1 had no relationship with clinicopathological characteristics, but the peripheral PD-L1 expression had a correlation with lymph node metastasis (p = 0.005) and Her-2 expression (p = 0.034) (p < 0.05). The positive rate of PD-L1 expression was 32.9% in tumor microenvironment. PD-L1 expression in tumor microenvironment had a significant correlation with PD-1/PD-L1 expression on circulating T lymphocytes, the correlation coefficients being 0.24 (p < 0.05) and 0.26 (p < 0.05), respectively. To predict the PD-L1 expression in tumor microenvironment, the area under the receiver operating characteristic curve was 0.65 and 0.66 for peripheral PD-1 and PD-L1, respectively. High level of peripheral PD-1/PD-L1 expression was associated with the odds ratios of 5.42 and 4.76 for positive PD-L1 expression in tumor microenvironment.
Conclusion
Peripheral PD-1/PD-L1 expression had a significant consistency with PD-L1 expression in tumor microenvironment and could act as an alternative choice of tissue detection, for the patients intolerable of biopsy.
Similar content being viewed by others
Background
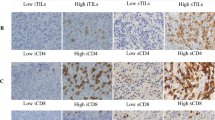
With the severe challenge from breast cancer (BC) worldwide, novel therapeutic options are emerging in recent years [1,2,3]. The immune checkpoint inhibitors (ICIs) were approved to improve the prognosis of non-small cell lung cancer, melanoma, and many solid tumors [4, 5]. Triple-negative breast cancer (TNBC) is a particular subtype of BC, characterized by negative expression of hormone receptors and Her-2. TNBC had a high number of tumor-infiltrated lymphocytes (TILs) in tumor environment (TME) and was approved for the immunotherapy of PD-1/PD-L1 inhibitors [6]. The PD-L1 positive rate was reported to range from 38 to 78% in TNBC, and the variation was related with the ethnicity of patients, previous treatment and metastasis [7,8,9,10]. TME expression of PD-1/PD-L1 was reported to be correlated to the clinicopathological characteristics and the clinical responses to ICIs [7, 11, 12].
Immune checkpoint molecules on peripheral lymphocytes changed dramatically with treatment and reflected the clinical efficacy of anti-cancer treatment [13]. However, tissue biopsy is time-consuming, and traumatic. More than half patients were fearful and anxious to biopsy, and 5.2% of them had complications. Liquid biopsy testing in peripheral blood can avoid these limitations [14, 15], and provided an alternative choice for patients intolerable to biopsy. This study evaluated the feasibility of alternative PD-1/PD-L1 on circulating T lymphocytes to TME PD-L1 expression in BC patients.
Methods
Aim
The purpose of this study was to explore the correlation and consistency of PD-1/PD-L1 expression on circulating T lymphocytes and PD-L1 expression in TME.
Ethical approval
This study was approved by the Ethics Committee of Beijing Shijitan Hospital, Capital Medical University. Patients provided the written informed consent.
Patients
Eighty-three cases of operable BC patients were recruited at Beijing Shijitan Hospital between November 2018 and November 2019. Patients did not have any invasions to skin or chest wall. Patients did not have any diagnosis of inflammatory BC, autoimmune diseases, heart, brain, kidney, or other vital organs insufficiency. Patients should have the Eastern Cooperative Oncology Group (ECOG) > 2 and did not receive any preoperative treatments. Peripheral blood samples were collected preoperatively and tumor tissues were obtained through biopsy or surgery.
Tumor size was categorized by the diameter, ≤ 2 cm (T1), ≤ 5 cm (T2) and > 5 cm (T3). Clinical tumor node metastasis (cTNM) stage was classified as I (T1N0M0), II (T0~1N1M0, T2N0~1M0, T3N0M0) and III (T0~2N2M0, T3N1~2M0, T4N0~3M0, T0~4N3M0) stage. Histological grade was defined by the scores estimated according to the glandular duct formation, nuclear pleomorphism and mitotic ability. The score range of grade I II and III was 3 to 5, 6 to 7 and 8 to 9, respectively.
Immunohistochemistry (IHC)
The positive threshold of ER and PR in IHC detection was set as 1% tumor cell staining. Positive expression of HER-2 was defined as + + + in IHC tests or positive in situ hybridization (ISH) test; negative expression was defined as -, + in IHC test or negative ISH test. Ki-67 index was detected by IHC on 4 μm-thick formalin fixed paraffin-embedded sections. Hot-spot area was determined under low-power field and the index ≥ 14% was defined as high expression of Ki-67. Molecular subtypes were defined as Luminal A (HER-2 negative, ER positive, Ki-67 low expression), Luminal B (HER-2 negative, ER positive, Ki-67 high expression or HER-2 positive, ER positive, Ki-67 arbitrary), TNBC (HER-2 negative, ER negative, Ki-67 arbitrary), and HER-2 overexpression (HER-2 positive, ER negative, Ki-67 arbitrary).
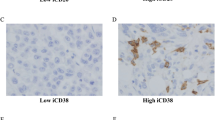
Monoclonal antibodies to PD-L1 (rabbit anti-human, #SP142) were purchased from Roche Shanghai Co. Ltd. Second antibodies were purchased from Beijing Zhongshanjinqiao biotechnology Co Ltd. The EnVision two-step method was used to detect the expression of PD-L1 on the immune cells in TME. Two pathologists interpreted the IHC staining of immune cells on the whole section. Tumor-infiltrating immune cells with brown staining accounting for more than 1% tumor area was determined as positive expression of PD-L1 in TME [16, 17].
Flow cytometry
Six milliliter venous blood was collected into EDTA-K2 anticoagulation tube (Becton, Dickinson and Company) and three-color flow cytometric analysis was performed to determine cell phenotypes. The expression of PD-L1 and PD-1 on the surface of circulating T lymphocytes was detected by Cytomics FC500 flow cytometer (Beckman-Coulter). The monoclonal antibodies of CD3-FITC (A07746, Beckman-Coulter), PD-1-PE/Cy5.5 (B30634, Beckman-Coulter) and PD-L1-PE/Cy7 (A78884, Beckman-Coulter) were added into the flow tube. PD-1+ and PD-L1+ T lymphocytes was defined as percentage of total circulating T lymphocytes (CD3+ cells). 5000 CD3+ cells were gated to calculate the percentage of PD-1/PD-L1 positive T cells by a CXP analysis software (Beckman-Coulter). PD-1/PD-L1 positive T lymphocytes were gated on PD-1/PD-L1 positive cells in CD3+ cell gate.
Statistical analysis
All data were analyzed by SPSS software (version 23.0). The Mann–Whitney U test was used to analyze the relationship between age, lymph node metastasis, ER, PR, HER-2, Ki-67 index and the positive levels of PD-1/PD-L1 on circulating T lymphocytes. Spearman correlation test was used to analyze the relationship between tumor size, cTNM stage, histological grade and the positive levels of PD-1/PD-L1 on circulating T lymphocytes. Kruskal–Wallis test was used to analyze the relationship between the molecular subtype and the positive levels of PD-1/PD-L1 on circulating T lymphocytes. χ2 test was used to analyze the relationship between age, lymph node metastasis, ER, PR, HER-2, Ki-67 index, molecular subtype and the TME PD-L1 expression; Mann–Whitney U test was used to analyze the relationship between the tumor size, cTNM stage, histological grade and the TME PD-L1 expression. Spearman correlation test was used to analyze the relationship between the positive levels of PD-1/PD-L1 on circulating T lymphocytes and the positive levels of TME PD-L1.
The receiver operative character (ROC) curve was illustrated between the peripheral PD-1/PD-L1 and TME PD-L1 expression, and the validity was estimated by the area under the curve (AUC). The cut-off value of peripheral PD-1/PD-L1 versus TME PD-L1 expression was calculated and the percentage of PD-1/PD-L1 on circulating T cells was transformed to categorical variable by the cut-off value. The odds ratios of expression of peripheral PD-1/PD-L1 in the categorical variables to TME PD-L1 expression were estimated in Logistical Regression Model with age adjustment. All analyses were two-tailed and significant level was 0.05.
Results
The positive levels of PD-1/PD-L1 in peripheral blood samples and TME
In peripheral blood, CD3+ circulating T lymphocytes had positive expression of PD-1 (Fig. 1A) and PD-L1 (Fig. 1B). The median percentage of PD-1/PD-L1 positive T lymphocytes was 15.2% and 0.7%, respectively. TME PD-L1 expression in immune cells had a heterogeneity (Fig. 2A and B) and the median percentage was 32.9%.
Correlation with clinicopathological characteristics
The positive levels of PD-1/PD-L1 on circulating T lymphocytes did not have any correlation with age, tumor size, cTNM stage, histological grade, ER and PR status, Ki-67 index and molecular subtype (Table 1). The median percentage of PD-L1 positive circulating T lymphocytes was 1.4% in patients with lymph node metastasis, significantly higher than that in patients without lymph node metastasis (0.6%, Table 1); The median percentage of PD-L1 positive circulating T lymphocytes in HER-2 positive patients was 0.5%, significantly lower than that in HER-2 negative patients (0.9%, Table 1). The median percentage of PD-1 positive circulating T lymphocytes had no relationship with lymph node metastasis and HER-2 expression (Table 1).
The percentage of PD-L1 positive immune cells in TME had no relationship with age, tumor size, lymph node metastasis, cTNM stage, histological grade, ER/PR and HER-2, Ki-67 index and molecular subtype (Table 2).
The consistency between peripheral and TME PD-1/PD-L1 expression
The correlation coefficients between percentage of PD-1/PD-L1 positive circulating T lymphocytes and percentage of PD-L1 positive immune cells in TME were 0.24 (p = 0.046) and 0.26 (p = 0.034), respectively.
The AUC between the percentage of PD-1/PD-L1 positive circulating T lymphocytes and TME PD-L1 expression was 0.65 (95%CI 0.53, 0.76) and 0.66 (95%CI 0.54, 0.77) (Fig. 3), with the cut-off values of 14.6% and 1.1%, respectively.
Higher percentage of PD-1/PD-L1 positive circulating T lymphocytes in peripheral blood was associated with a 5.42-fold (p = 0.007) and 4.76-fold high probability to be TME PD-L1 positive (p = 0.005, Table 3).
Subgroup analysis in molecular subtypes
In Luminal A subtype, the AUC value was 0.86 (95%CI = 0.62–0.98, p < 0.001) and 0.73 (95%CI = 0.47–0.91, p = 0.12) between PD-1/PD-L1 positive circulating T lymphocytes and TME PD-L1 positive expression (Fig. 4A). In Luminal B subtype, the AUC value was 0.60 (95%CI = 0.42–0.77, p = 0.317) and 0.65 (95%CI = 0.47–0.81, p = 0.209) between PD-1/PD-L1 positive circulating T lymphocytes and TME PD-L1 positive expression (Fig. 4B). In TNBC subtype, the AUC value was 0.71 (95%CI = 0.38–0.93, p = 0.313) and 0.75 (95%CI = 0.41–0.95, p = 0.175) between PD-1/PD-L1 positive circulating T lymphocytes and TME PD-L1 positive expression (Fig. 4C). In HER-2 overexpression subtype, the AUC value was 1.00 (95%CI = 0.54–1.000, p < 0.001) and 0.50 (95%CI = 0.12–0.88, p > 0.999) between PD-1/PD-L1 positive circulating T lymphocytes and TME PD-L1 positive expression (Fig. 4D).
Discussion
In this study, we observed a significant consistency of peripheral PD-1/PD-L1 expression with TME PD-L1 expression. BC patients with higher level of peripheral PD-1/PD-L1 were more likely to be PD-L1 positive in TME.
The Impassion 130 study revealed that PD-L1 inhibitor Atezolizumab combined with chemotherapy significantly prolonged the median PFS by 2.5 months in PD-L1 positive patients compared with chemotherapy alone [7, 8]. In our study, PD-1/PD-L1 expression on circulating T lymphocytes had a significant consistency with the PD-L1 expression in TME. Immune checkpoint proteins, including CTLA-4, CD28 and PD-1, are co-stimulatory or co-suppressive proteins expressed on the surface of antigen-presenting cells and T lymphocytes, and maintain the immune balance by up- or down-regulating T lymphocyte functions [18, 19]. Tumor cells express PD-L1 and bind to PD-1 on the immune cells to escape recognition and elimination from the immune system. The link between PD-1 and PD-L1 down-regulated the cell counts and function of T lymphocytes, and induced occurrence, progression and drug resistance of malignant cells [20, 21].
Higher level of TILs indicated a better prognosis of BC [22,23,24]. PD-1/PD-L1 expression in TME was the indicator for PD-1 inhibitor therapy [25, 26], and more than 1% expression rate of PD-L1 was eligible for atezolizumab treatment for BC [22, 26]. But the expression of immune checkpoint proteins in peripheral blood and TME was dynamically changing during treatment [27,28,29,30]. It is necessary to repeat tissue biopsies for the optimistic treatments. For the inconvenient tissue biopsy, liquid biopsy of peripheral blood became the alternative, and circulating T lymphocytes were reported to reflect the condition of TILs in TME [27, 31]. PD-1/PD-L1 expression on circulating T lymphocytes had a significant consistency with the TME PD-L1 expression, especially in Luminal A and HER-2 overexpression subtypes.
PD-L1 expression on circulating T lymphocytes was related to lymph node metastasis and HER-2 expression, which were not consistent with other studies [32,33,34]. This might be caused by the application of different antibodies and scoring standards. Since Atezolizumab was the first approved checkpoint inhibitor of PD-1/PD-L1 for BC immunotherapy, we chose the recommended antibody SP142 and the results are more reliable [26, 35]. Previous treatments and clinical stage affected PD-1/PD-L1 expression and immunotherapy efficacy [36,37,38,39,40]. The recruited patients were in early or mid-stage and never received any treatment preoperatively. Heterogeneity of research subjects between studies contributed to the variation in results. The positive rate of TME PD-L1 was 36%, in consistent with the Impassion130 trial in Japan [9].
The limited sample size was the first limitation in this study. The low percentage of PD-L1 positive circulating T lymphocytes was the second limitation. Lack of soluble PD-1/PD-L1 detection in peripheral blood was another limitation.
Conclusions
Peripheral PD-1/PD-L1 expression on circulating T lymphocytes had a certain correlation and consistency with TME PD-L1 expression, and was potential to be an alternative to the TME detection, especially for the patients intolerable to tissue biopsy. However, this result still needs to be verified with a larger sample size and we will definitely continue to conduct subsequent studies.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due to privacy and ethical but are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- BC:
-
Breast cancer
- cTNM:
-
Clinical tumor node metastasis
- ECOG:
-
Eastern Cooperative Oncology Group
- ER:
-
Estrogen Estrogen
- HER-2:
-
Human Epidermal Growth Factor Receptor Type 2
- ICI:
-
Immune checkpoint inhibitor
- IHC:
-
Immunohistochemistry
- ISH:
-
In situ hybridization
- PD-1:
-
Programmed Death-1
- PD-L1:
-
Programmed Death Ligand-1
- PR:
-
Progesterone Estrogen
- ROC:
-
Receiver operative character
- TIL:
-
Tumor-infiltrated lymphocyte
- TME:
-
Tumor environment
- TNBC:
-
Triple-negative breast cancer
References
Global Burden of Disease Cancer C, Fitzmaurice C, Akinyemiju TF, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4(11):1553–68.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Li N, Deng Y, Zhou L, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J Hematol Oncol. 2019;12(1):140.
Hartkopf AD, Taran FA, Wallwiener M, et al. PD-1 and PD-L1 Immune Checkpoint Blockade to Treat Breast Cancer. Breast Care (Basel). 2016;11(6):385–90.
Rauch DA, Conlon KC, Janakiram M, et al. Rapid progression of adult T-cell leukemia/lymphoma as tumor-infiltrating Tregs after PD-1 blockade. Blood. 2019;134(17):1406–14.
Emens LA. Breast Cancer Immunotherapy: Facts and Hopes. Clin Cancer Res. 2018;24(3):511–20.
Emens LA, Cruz C, Eder JP, et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019;5(1):74–82.
Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59.
Iwata H, Inoue K, Kaneko K, et al. Subgroup analysis of Japanese patients in a Phase 3 study of atezolizumab in advanced triple-negative breast cancer (IMpassion130). Jpn J Clin Oncol. 2019;49(12):1083–91.
Ho AY, Barker CA, Arnold BB, et al. A phase 2 clinical trialassessing theefficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer. 2020;126(4):850–60.
Huang W, Ran R, Shao B, Li H. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: a meta-analysis. Breast Cancer Res Treat. 2019;178(1):17–33.
Wyss J, Dislich B, Koelzer VH, et al. Stromal PD-1/PD-L1 Expression Predicts Outcome in Colon Cancer Patients. Clin Colorectal Cancer. 2019;18(1):e20–38.
Vilain RE, Menzies AM, Wilmott JS, et al. Dynamic Changes in PD-L1 Expression and Immune Infiltrates Early During Treatment Predict Response to PD-1 Blockade in Melanoma. Clin Cancer Res. 2017;23(17):5024–33.
Overman MJ, Modak J, Kopetz S, et al. Use of research biopsies in clinical trials: are risks and benefits adequately discussed? J Clin Oncol. 2013;31(1):17–22.
Agulnik M, Oza AM, Pond GR, Siu LL. Impact and perceptions of mandatory tumor biopsies for correlative studies in clinical trials of novel anticancer agents. J Clin Oncol. 2006;24(30):4801–7.
Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–74.
Vennapusa B, Baker B, Kowanetz M, et al. Development of a PD-L1 Complementary Diagnostic Immunohistochemistry Assay (SP142) for Atezolizumab. Appl Immunohistochem Mol Morphol. 2019;27(2):92–100.
Lim S, Phillips JB, Madeira da Silva L, et al. Interplay between Immune Checkpoint Proteins and Cellular Metabolism. Cancer Res. 2017;77(6):1245–9.
Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131(1):58–67.
Schutz F, Stefanovic S, Mayer L, von Au A, Domschke C, Sohn C. PD-1/PD-L1 Pathway in Breast Cancer. Oncol Res Treat. 2017;40(5):294–7.
Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–53.
Kurozumi S, Fujii T, Matsumoto H, et al. Significance of evaluating tumor-infiltrating lymphocytes (TILs) and programmed cell death-ligand 1 (PD-L1) expression in breast cancer. Med Mol Morphol. 2017;50(4):185–94.
Du H, Yi Z, Wang L, Li Z, Niu B, Ren G. The co-expression characteristics of LAG3 and PD-1 on the T cells of patients with breast cancer reveal a new therapeutic strategy. Int Immunopharmacol. 2020;78:106113.
Syed Khaja AS, Toor SM, El Salhat H, et al. Preferential accumulation of regulatory T cells with highly immunosuppressive characteristics in breast tumor microenvironment. Oncotarget. 2017;8(20):33159–71.
Incorvaia L, Fanale D, Badalamenti G, et al. Programmed Death Ligand 1 (PD-L1) as a Predictive Biomarker for Pembrolizumab Therapy in Patients with Advanced Non-Small-Cell Lung Cancer (NSCLC). Adv Ther. 2019;36(10):2600–17.
Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018;379(22):2108–21.
Pico de Coana Y, Wolodarski M, van der Haar Avila I, et al. PD-1 checkpoint blockade in advanced melanoma patients: NK cells, monocytic subsets and host PD-L1 expression as predictive biomarker candidates. Oncoimmunology. 2020;9(1):1786888.
Kamada T, Togashi Y, Tay C, et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A. 2019;116(20):9999–10008.
Zhang L, Wang J, Wei F, et al. Profiling the dynamic expression of checkpoint molecules on cytokine-induced killer cells from non-small-cell lung cancer patients. Oncotarget. 2016;7(28):43604–15.
Han JJ, Kim DW, Koh J, et al. Change in PD-L1 Expression After Acquiring Resistance to Gefitinib in EGFR-Mutant Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2016;17(4):263–70 (e262).
Callahan MK, Wolchok JD. Recruit or Reboot? How Does Anti-PD-1 Therapy Change Tumor-Infiltrating Lymphocytes? Cancer Cell. 2019;36(3):215–7.
Reisenbichler ES, Han G, Bellizzi A, et al. Prospective multi-institutional evaluation of pathologist assessment of PD-L1 assays for patient selection in triple negative breast cancer. Mod Pathol. 2020;33(9):1746–52.
Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146(1):15–24.
Sun WY, Lee YK, Koo JS. Expression of PD-L1 in triple-negative breast cancer based on different immunohistochemical antibodies. J Transl Med. 2016;14(1):173.
Kwa MJ, Adams S. Checkpoint inhibitors in triple-negative breast cancer (TNBC): Where to go from here. Cancer. 2018;124(10):2086–103.
Samanta D, Park Y, Ni X, et al. Chemotherapy induces enrichment of CD47(+)/CD73(+)/PDL1(+) immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci U S A. 2018;115(6):E1239–48.
Shigemori T, Toiyama Y, Okugawa Y, et al. Soluble PD-L1 Expression in Circulation as a Predictive Marker for Recurrence and Prognosis in Gastric Cancer: Direct Comparison of the Clinical Burden Between Tissue and Serum PD-L1 Expression. Ann Surg Oncol. 2019;26(3):876–83.
Takamori S, Toyokawa G, Takada K, Shoji F, Okamoto T, Maehara Y. Combination Therapy of Radiotherapy and Anti-PD-1/PD-L1 Treatment in Non-Small-cell Lung Cancer: A Mini-review. Clin Lung Cancer. 2018;19(1):12–6.
Rojko L, Reiniger L, Teglasi V, et al. Chemotherapy treatment is associated with altered PD-L1 expression in lung cancer patients. J Cancer Res Clin Oncol. 2018;144(7):1219–26.
Szekely B, Bossuyt V, Li X, et al. Immunological differences between primary and metastatic breast cancer. Ann Oncol. 2018;29(11):2232–9.
Acknowledgements
Not applicable.
Funding
This research was funded by Beijing Municipal Committee of Science and Technology grant number Z181100001718090 and Z19110006619041.
Author information
Authors and Affiliations
Contributions
KY and JW conducted the detection of PD-1/PD-L1 in peripheral blood, and were major contributors in writing the manuscript. YZ and SL were responsible for data collection and analysis. QZ and FS performed the detection of PD-L1 in tumor microenvironment. YL and QS designed, supervised and optimized the entire study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Beijing Shijitan Hospital (protocol code sjtky11-1x-2018(56), date 8th November, 2018). All enrolled patients have been explained in detail about the study and signed informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yuan, K., Wu, J., Zhao, Y. et al. Consistent expression of PD-L1 in tumor microenvironment with peripheral PD-1/PD-L1 in circulating T lymphocytes of operable breast cancer: a diagnostic test. Diagn Pathol 17, 68 (2022). https://doi.org/10.1186/s13000-022-01249-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-022-01249-w