Abstract
Background
This study aimed to investigate the association between clinicopathologic factors, mesothelin, and cancer antigen (CA) 125 in endometrial carcinoma.
Methods
Between 1989 and 2017, patients with endometrial carcinoma who underwent total hysterectomy and bilateral salpingo-oophorectomy at our hospital were identified. The association between either or both immunochemical expression of mesothelin and CA125 and clinicopathological features were retrospectively examined.
Results
Among 485 patients, 171 were positive for mesothelin, 368 were positive for CA125, and 167 were positive for mesothelin and CA125. The expression of mesothelin and CA125 was positively correlated (p < 0.01). More patients with mesothelin expression showed myometrial invasion of more than 50% (p = 0.028) and positive lymphovascular invasion (p = 0.027). Similarly, more patients with co-expression of mesothelin and CA125 had myometrial invasion of more than 50% (p = 0.016) and positive lymphovascular invasion (p = 0.02). Patients with mesothelin expression and co-expression of mesothelin and CA125 demonstrated worse progression-free survival (PFS) and overall survival (OS). In the multivariate analysis, mesothelin expression and co-expression were poor prognostic factors for PFS (mesothelin expression: hazard ratio [HR] = 2.14, p < 0.01; co-expression: HR = 2.19, p < 0.01) and OS (mesothelin expression: HR = 2.18, p < 0.01; co-expression: HR = 2.22, p < 0.01).
Conclusions
Mesothelin expression and co-expression might be associated with tumor aggressiveness and poor prognosis in patients with endometrial carcinoma. Persons with mesothelin-expressing endometrial cancers present a particularly high medical unmet need.
Similar content being viewed by others
Background
The incidence of endometrial carcinoma (EC) has gradually increased, and ECs is one of the major types of gynecologic carcinomas [1]. The standard treatments for EC are surgery and adjuvant therapy including chemotherapy and radiotherapy according to the recurrent risk factor [2]. Due to the advances in treatment, the prognosis of these patients has dramatically improved, but those with advanced disease stage of aggressive histological subtypes such as serous carcinoma showed worse prognosis [3].
Mesothelin, a 40-kDa protein, is normally expressed in mesothelial cells of the pleural cavity, peritoneal cavity, and peritoneum [4, 5]. Furthermore, mesothelin has a strong affinity to cancer antigen 125 (CA125) with N-linked glycans [6]. Several studies reported that co-expression of mesothelin and CA125 correlated with aggressive features of tumors and poor prognosis of several carcinomas such as ovarian carcinomas [7,8,9,10,11,12,13,14,15,16]. However, only a few studies have investigated the association between mesothelin and CA125 expression in ECs [17,18,19,20]. Therefore, we considered the need to study the relationship between these expressions and clinicopathological features.
Herein, this study aimed to evaluate the correlation between the clinicopathological factors and either the expression of mesothelin or CA125 or co-expression of these biomarkers in endometrial carcinoma.
Methods
Patients
Patients with endometrial carcinoma who underwent total hysterectomy and bilateral salpingo-oophorectomy in our hospital between 1989 and 2017 were identified. All patients were re-evaluated pathologically according to the 2020 World Health Organization criteria [21]. Those with grades 1, 2, and 3 endometrioid carcinoma, serous carcinoma, clear cell carcinoma, carcinosarcoma, and mixed carcinoma were included in this study. Patients with other histological types, complicated with other carcinomas, and lack of either clinical information or surgical specimens were excluded. All patients qualified in the period were included. The clinicopathological factors were obtained from the medical records.
Immunohistochemical analysis
Four-hundred and eighty-five formalin-fixed paraffin-embedded tissues were used for tissue microarray (TMA). All slides were stained immunohistochemically as previously reported [20]; anti-mesothelin (clone 5B2 diluted 1:50 Leica: NCL-L-MESO) and anti-CA125 (clone M11 diluted 1:50 DAKO:M3520) antibodies were used under the same conditions.
Immunohistochemical evaluation
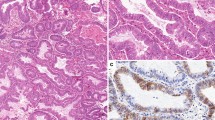
The evaluation methods used were those described in prior literature and our previous studies [10, 20]. The expression of mesothelin and CA125 was determined by evaluating the tumor proportion score and staining intensity score. The tumor proportion score, defined as the percentage of mesothelin- or CA125-positive cells in carcinoma tissues, was as follows:0, no tumor cells stained in the entire carcinoma tissue; 1+, ≥1 to < 10% of cells stained in the entire carcinoma tissue; 2+, ≥10 to < 50% of cells stained in the entire carcinoma tissue; and 3+, ≥50% of cells stained in the entire carcinoma tissue. The staining intensity score was defined as follows: 0, no tumor cells stained in the entire carcinoma tissue; 1+, incomplete membrane staining and/or faint or barely perceptible cytoplasmic staining detected; and 2+, entire circumference of the cell membrane stained and/or moderate to strong cytoplasmic staining. A tumor proportion score of 3+ and/or a staining intensity score of 2+ was considered positive for mesothelin (Fig. 1a) or CA125 expression (Fig. 1b). On the contrary, other cases were considered negative for mesothelin (Fig. 1c) or CA125 expression (Fig. 1d). Additionally, presence of both mesothelin-positive and CA125 cells was considered as co-expression. Two observers evaluated immunoreactivity without prior information of clinical data. In the interpretation of immunohistochemistry, any discrepancies between 2 observers were resolved through discussion over a multiviewer microscope.
The expression variation of mesothelin and cancer antigen (CA) 125. a Mesothelin-positive cells in grade 1 endometrioid carcinoma. The percentage of mesothelin-positive cells is ≥50% (tumor proportion score: 3+), and the entire circumference of the cancer cell membrane is stained (intensity score: 2+). b CA125-positive cells of grade1, endometrioid carcinoma. The percentage of CA125-positive cells is ≥50% (proportion score: 3+), and the cytoplasm of cancer cell is highly stained (intensity score: 2+). c Mesothelin-positive cells in grade 2 endometrioid carcinoma. The percentage of mesothelin-positive cells is ≥50% (tumor proportion score: 3+), and the entire circumference of the cancer cell membrane is stained (intensity score: 2+). d CA125-positive cells of grade2, endometrioid carcinoma. The percentage of CA125-positive cells is ≥50% (proportion score: 3+), and the cytoplasm of cancer cell is highly stained (intensity score: 2+). e Mesothelin-positive cells in grade 3 endometrioid carcinoma. The percentage of mesothelin-positive cells is ≥50% (tumor proportion score: 3+), and the entire circumference of the cancer cell membrane is stained (intensity score: 2+). f CA125-positive cells of grade3, endometrioid carcinoma. The percentage of CA125-positive cells is ≥50% (proportion score: 3+), and the cytoplasm of cancer cell is highly stained (intensity score: 2+). g Mesothelin-negative cells in grade 1 endometrioid carcinoma. No staining is noted in the cancer cell’s membrane. h CA125-negative cells in grade 1 endometrioid carcinoma. No staining is noted in the cancer cell’s membrane. Magnification for all slides: × 100. *HE, hematoxylin-eosin
Statistical analysis
Histological subtypes were classified into four types: grade 1, endometrioid carcinoma; grade 2, endometrioid carcinoma; grade 3, endometrioid carcinoma, and other carcinomas including serous carcinoma, clear cell carcinoma, carcinosarcoma, and mixed carcinoma. The χ2 test and Fisher’s exact test were used to confirm the correlation between the clinicopathological data and mesothelin and CA125 expression. Progression-free survival (PFS) and overall survival (OS) curves were drawn using the Kaplan-Meier method. The differences between the survival curves were analyzed using the Cox proportional hazard test. In the statistical analysis, all p-values of < 0.05 were considered significant. The software JMP® 14.0 (SAS Institution Inc., Cary, NC, USA) was used to perform all statistical analyses.
Results
The details of the tumor proportion and intensity scores are shown in Table 1. Two-hundred and sixteen patients (45%) showed absence of mesothelin expression. A total of 68 (14%) patients had a tumor proportion score of 3+, while 166 had a staining intensity score of 2+ on mesothelin staining Among the 485 patients, 171 (35%) were positive for mesothelin expression. Similarly, 56 patients (11%) showed absence of CA125 expression. Two hundred seventy (56%) patients had a tumor proportion score of 3+, while 364 (75%) had a staining intensity score of 2+ on CA125 staining. Moreover, 368 (75%) patients showed were positive for CA 125 expression, while 167 (34%) demonstrated co-expression of mesothelin and CA 125. The relationship between the histological subtypes and expression is shown in Table 2. Only a few patients had grade 1 endometrioid carcinoma and more had other types of carcinomas in the groups with positive mesothelin expression than in those with negative mesothelin expression (p = 0.027). More patients had grade 1 endometrioid carcinoma in groups with positive CA125 expression than in those with negative CA125 expression (p < 0.01). In the groups with positive co-expression, only a fewer patients had grade 1 endometrioid carcinoma and more had other types of carcinomas than in those with negative co-expression (p = 0.048). Among 171 patients with positive mesothelin expression, 168 (98%) showed co-expression of mesothelin and CA125 (p < 0.01).
First, we investigated the association between mesothelin expression and clinical parameters. More patients in groups with positive mesothelin expression had myometrial invasion of more than 50% (p = 0.028) and positive lymphovascular invasion (p = 0.027) than those with negative mesothelin expression (Table 3). No significant correlations were observed with the other factors. Patients with positive mesothelin expression showed worse PFS and OS than those with negative mesothelin expression (Fig. 2a: PFS, p < 0.01; Fig. 2b: OS, p < 0.01). Multivariate analysis showed that positive mesothelin expression was a worse prognostic factor for PFS (Table 4: hazard ratio [HR] = 2.14, 95% confidence interval [CI] = 1.45–3.16, p < 0.01) and OS (Table 5; HR = 2.18, 95% CI = 1.31–3.71, p < 0.01).
Progression-free survival (PFS) and overall survival (OS) of patients according to their differences in expression of mesothelin and CA125, and in the co-expression of both mesothelin and CA125. PFS (a) and OS (b) according to mesothelin expression, PFS (c) and OS (d) according to CA125 expression, and PFS (e) and OS (f) co-expression of both were shown
Second, we examined the relationship between CA125 expression and the clinical features. No significant associations were noted between CA125 expression and the clinicopathological factors (Table 3). In addition, PFS and OS were not different between the two groups according to CA125 expression (Fig. 2c: PFS, p = 0.97; Fig. 2d: OS, p = 0.64).
Thirdly, Analysis to compare patients without both mesothelin and CA 125 expression with other patients was performed. There were no statistical significances of several clinicopathological features, PFS (p = 0.83), and OS (p = 0.76) between two groups.
Finally, the relationship between mesothelin and CA125 co-expression and clinicopathological factors was examined. Patients in the group with positive co-expression had myometrial invasion of more than 50% (p = 0.016) and positive lymphovascular invasion (p = 0.02) compared with those with negative co-expression (Table 3). Patients with positive co-expression had worse PFS (Fig. 2d: PFS, p < 0.01) and OS (Fig. 2e: OS, p < 0.01) than those with negative co-expression. Multivariate analyses for PFS and OS demonstrated that positive co-expression was a worse prognostic factor for PFS (Table 4: HR = 2.19, 95% CI = 1.49–3.23; p < 0.01) for OS (Table 5: HR = 2.22, 95% CI = 1.33–3.77, p < 0.01).
Discussion
In our study, mesothelin expression and co-expression of mesothelin and CA125 were more often observed in patients with other histological types – including serous carcinoma, clear cell carcinoma, and carcinosarcoma –mixed carcinoma, and associated deeper myometrial invasion and lymphovascular invasion. Furthermore, multivariate analysis demonstrated that mesothelin expression and co-expression of mesothelin and CA125 were independent prognostic factors.
Mesothelin expression and co-expression of mesothelin and CA125 were associated with tumor aggressiveness in several types of carcinomas [7,8,9,10,11,12,13,14,15]. In endometrial carcinoma, serous carcinoma, clear cell carcinoma, carcinosarcoma, and mixed carcinoma are more aggressive histological subtypes than grade 1 and 2 endometrioid carcinoma [22,23,24,25]. In addition, the depth of myometrial invasion and lymphovascular invasion were the factors associated with tumor aggressiveness and worse prognosis [26,27,28]. The relationships between mesothelin expression and co-expression and clinicopathological features in our study were related to these facts. Evaluating for mesothelin expression and co-expression of mesothelin and CA125 might be useful for the detection of aggressive subtypes of endometrial carcinomas.
Our previous reports demonstrated co-expression was associated with tumor malignancy such as lymphovascular invasion and lymph node metastasis in serous carcinoma [20]. In our current study, co-expression was associated with tumor aggressiveness. Mesothelin and co-expression might be the common important factors to enhance malignant potential regardless of histological subtypes.
Mesothelin independently activate epithelial-to-mesenchymal transition and tumor progression in some malignant tumors [29]. Overexpression of mesothelin alone could constitutively activate the nuclear factor-kappa B, mitogen-activated protein kinase, and phosphoinositide 3-kinase intracellular pathways, promoting cell proliferation and resistance to apoptosis [30]. Besides, mesothelin-accelerated tumor progression is accelerated by CA125 [6, 11]. In our study, almost all patients with positive mesothelin were positive for CA125 expression, which indicated that mesothelin might play an important role in the development of ECs. Also, the association that almost all patients with positive mesothelin were positive for CA125 expression was observed in pancreatic carcinoma [10]. There was the possibility that only CA125 positive cancer cell could stabilize mesothelin expression and CA125-mesothelin complex. However, because it was just a hypothesis, we considered further study to examine the fact.
Only mesothelin expression was associated with worse prognosis in several carcinomas [7,8,9, 12,13,14,15]. Moreover, co-expression was correlated with worse prognosis in pancreatic, ovarian, and endometrial serous carcinomas [10, 11, 20]. On the other hand, mesothelin expression was better prognosis factor in gastric carcinoma, ovarian carcinoma, and mesothelioma [31,32,33]. Clinical significances of mesothelin might be different among several carcinomas. In our study, only mesothelin expression and co-expression of mesothelin and CA125 were worse prognostic factors in several histological subtypes of endometrial carcinoma. Therefore, mesothelin may be a potential candidate for a new target therapy in endometrial carcinomas.
Mesothelin have been studied as a biomarker for targeted therapy including antibody-based drugs, cancer vaccine, and chimeric antigen receptor T cell therapies in several types of solid tumors [34, 35]. In phase I study for mesothelin targeted therapies, 2.4 mg/kg of mesothelin-targeting antibody-based drug administered three times a week showed safety and partial responses in 3 of 10 (30%) patients with platinum-resistant ovarian cancer [36]. Furthermore, the in vivo proliferation of human uterine -cancer cells with mesothelin expression was inhibited by treatment with a mesothelin-targeting antibody-based drug [37]. In our study, mesothelin were used to determine patients with worse prognosis. Therefore, using mesothelin as a biomarker by immunohistochemical analysis, mesothelin-targeted therapies might be helpful in the treatment of EC.
This study has some limitations. It was performed in a single institution and was retrospective in nature. Our study could not include all patients during observational periods. Among a total of 675 patients during observational periods, 130 patients without our surgical specimen and 60 patients without clinical information were excluded. Finally, 485 patients were included in our study. Our study included selection bias to some extent but minimized it because the reason of exclusion was not our intention. Also, there were several confounding factors associated with PFS and OS. However, in our study, multivariate analysis was performed. In results, mesothelin and co-expression was identified as the independent prognostic factors. Therefore, we considered our study could minimized the effect of confounding factors. However, the strength point of our study included relatively large samples. Our study showed that mesothelin and co-expression of mesothelin and CA125 might be important factors in EC.
Conclusions
Co-expression mesothelin and CA125 might be a better biomarker for predicting the prognosis of EC. Only mesothelin expression might be the better biomarker to predict the prognosis. Further large-scale studies are needed to confirm these findings.
Availability of data and materials
All data analyzed in this study are available from the corresponding author upon reasonable request.
Abbreviations
- CA:
-
Cancer antigen
- EC:
-
Endometrial carcinoma
- OS:
-
Overall survival
- PFS:
-
Progression free survival
- TMA:
-
Tissue microarray
- HR:
-
Hazard ratio
References
Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW, Son CH, et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. 2019;69(4):258–79. https://doi.org/10.3322/caac.21561.
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387(10023):1094–108. https://doi.org/10.1016/S0140-6736(15)00130-0.
Sorosky JI. Endometrial cancer. Obstet Gynecol. 2012;120(2, Part 1):383–97. https://doi.org/10.1097/AOG.0b013e3182605bf1.
Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992;50(3):373–81. https://doi.org/10.1002/ijc.2910500308.
Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A. 1996;93(1):136–40. https://doi.org/10.1073/pnas.93.1.136.
Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault M, Ho M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50.
Li YR, Xian RR, Ziober A, Conejo-Garcia J, Perales-Puchalt A, June CH, et al. Mesothelin expression is associated with poor outcomes in breast cancer. Breast Cancer Res Treat. 2014;147(3):675–84. https://doi.org/10.1007/s10549-014-3077-5.
Shiraishi T, Shinto E, Mochizuki S, Tsuda H, Kajiwara Y, Okamoto K, et al. Mesothelin expression has prognostic value in stage ΙΙ/ΙΙΙ colorectal cancer. Virchows Arch. 2019;474(3):297–307. https://doi.org/10.1007/s00428-018-02514-4.
Nahm CB, Turchini J, Jamieson N, Moon E, Sioson L, Itchins M, et al. Biomarker panel predicts survival after resection in pancreatic ductal adenocarcinoma: a multi-institutional cohort study. Eur J Surg Oncol. 2019;45(2):218–24. https://doi.org/10.1016/j.ejso.2018.10.050.
Einama T, Kamachi H, Nishihara H, Homma S, Kanno H, Takahashi K, et al. Co-expression of mesothelin and CA125 correlates with unfavorable patient outcome in pancreatic ductal adenocarcinoma. Pancreas. 2011;40(8):1276–82. https://doi.org/10.1097/MPA.0b013e318221bed8.
Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279(10):9190–8. https://doi.org/10.1074/jbc.M312372200.
Einama T, Homma S, Kamachi H, Kawamata F, Takahashi K, Takahashi N, et al. Luminal membrane expression of mesothelin is a prominent poor prognostic factor for gastric cancer. Br J Cancer. 2012;107(1):137–42. https://doi.org/10.1038/bjc.2012.235.
Einama T, Kamachi H, Nishihara H, Homma S, Kanno H, Ishikawa M, et al. Importance of luminal membrane mesothelin expression in intraductal papillary mucinous neoplasms. Oncol Lett. 2015;9(4):1583–9. https://doi.org/10.3892/ol.2015.2969.
Yildiz Y, Kabadayi G, Yigit S, Kucukzeybek Y, Alacacioglu A, Varol U, et al. High expression of mesothelin in advanced serous ovarian cancer is associated with poor prognosis. J BUON. 2019;24(4):1549–54.
Inoue S, Tsunoda T, Riku M, Ito H, Inoko A, Murakami H, et al. Diffuse mesothelin expression leads to worse prognosis through enhanced cellular proliferation in colorectal cancer. Oncol Lett. 2020;19(3):1741–50. https://doi.org/10.3892/ol.2020.11290.
Felder M, Kapur A, Gonzalez-Bosquet J, Horibata S, Heintz J, Albrecht R, et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014;13(1):129. https://doi.org/10.1186/1476-4598-13-129.
Dainty LA, Risinger JI, Morrison C, Chandramouli GV, Bidus MA, Zahn C, et al. Overexpression of folate binding protein and mesothelin are associated with uterine serous carcinoma. Gynecol Oncol. 2007;105(3):563–70. https://doi.org/10.1016/j.ygyno.2006.10.063.
Ginath S, Menczer J, Fintsi Y, Ben-Shem E, Glezerman M, Avinoach I. Tissue and serum CA125 expression in endometrial cancer. Int J Gynecol Cancer. 2002;12(4):372–5. https://doi.org/10.1046/j.1525-1438.2002.01007.x.
Metindir J, Dilek GB, Pak I. Staining characterization by immunohistochemistry of tumor cancer antigen in patients with endometrial cancer. Eur J Gynaecol Oncol. 2008;29(5):489–92.
Kakimoto S, Miyamoto M, Einama T, Matsuura H, Iwahashi H, Ishibashi H, et al. Co-expression of mesothelin and CA125 is associated with the poor prognosis of endometrial serous carcinoma and mixed carcinomas including serous carcinoma. Pathol Oncol Res. 2020;26(4):2299–306. https://doi.org/10.1007/s12253-020-00823-1.
WHO. Classification of tumors editorial board: female Genital Organs. 5th ed. Lyon: International Agency for Research on Cancer; 2020. p. 245–66.
Chen W, Husain A, Nelson GS, Nelson GS, Rambau PF, Liu S, et al. Immunohistochemical profiling of endometrial serous carcinoma. Int J Gynecol Pathol. 2017;36(2):128–39. https://doi.org/10.1097/PGP.0000000000000291.
Li W, Li L, Wu M, Lang J, Bi Y. The prognosis of stage ia mixed endometrial carcinoma. Am J Clin Pathol. 2019;152(5):616–24. https://doi.org/10.1093/ajcp/aqz083.
DeLair DF, Burke KA, Selenica P, Lim RS, Scott SN, Middha S, et al. The genetic landscape of endometrial clear cell carcinomas. J Pathol. 2017;243(2):230–41. https://doi.org/10.1002/path.4947.
Cantrell LA, Blank SV, Duska LR. Uterine carcinosarcoma: a review of the literature. Gynecol Oncol. 2015;137(3):581–8. https://doi.org/10.1016/j.ygyno.2015.03.041.
Singh N, Hirschowitz L, Zaino R, Alvarado-Cabrero I, Duggan MA, Ali-Fehmi R, et al. Pathologic prognostic factors in endometrial carcinoma (other than tumor type and grade). Int J Gynecol Pathol. 2019;38:S93–S113. https://doi.org/10.1097/PGP.0000000000000524.
Ørtoft G, Lausten-Thomsen L, Høgdall C, Hansen ES, Dueholm M. Lymph-vascular space invasion (LVSI) as a strong and independent predictor for non-locoregional recurrences in endometrial cancer: a Danish gynecological Cancer group study. J Gynecol Oncol. 2019;30(5):e84. https://doi.org/10.3802/jgo.2019.30.e84.
van der Putten LJ, van de Vijver K, Bartosch C, Davidson B, Gatius S, Matias-Guiu X, et al. Reproducibility of measurement of myometrial invasion in endometrial carcinoma. Virchows Arch. 2017;470(1):63–8. https://doi.org/10.1007/s00428-016-2035-5.
He X, Wang L, Riedel H, Wang K, Yang Y, Dinu CZ, et al. Mesothelin promotes epithelial-to-mesenchymal transition and tumorigenicity of human lung cancer and mesothelioma cells. Mol Cancer. 2017;16(1):63. https://doi.org/10.1186/s12943-017-0633-8.
Bharadwaj U, Marin-Muller C, Li M, Chen C, Yao Q. Mesothelin confers pancreatic cancer cell resistance to TNF-α-induced apoptosis through Akt/PI3K/NF-κB activation and IL-6/Mcl-1 overexpression. Mol Cancer. 2011;10(1):106. https://doi.org/10.1186/1476-4598-10-106.
Baba K, Ishigami S, Arigami T, Uenosono Y, Okumura H, Matsumoto M, et al. Mesothelin expression correlates with prolonged patient survival in gastric cancer. J Surg Oncol. 2012;105(2):195–9. https://doi.org/10.1002/jso.22024.
Yen MJ, Hsu CY, Mao TL, Wu TC, Roden R, Wang TL, et al. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin Cancer Res. 2006;12(3):827–31. https://doi.org/10.1158/1078-0432.CCR-05-1397.
Vizcaya D, Farahmand B, Walter AO, Kneip C, Jöhrens K, Tukiainen M, et al. Prognosis of patients with malignant mesothelioma by expression of programmed cell death 1 ligand 1 and mesothelin in a contemporary cohort in Finland. Cancer Treat Res Commun. 2020;25:100260. https://doi.org/10.1016/j.ctarc.2020.100260.
Hassan R, Thomas A, Alewine C, Le DT, Jaffee EM, Pastan I. Mesothelin immunotherapy for cancer: ready for prime time? J Clin Oncol. 2016;34(34):4171–9. https://doi.org/10.1200/JCO.2016.68.3672.
Lv J, Li P. Mesothelin as a biomarker for targeted therapy. Biomark Res. 2019;7(1):18. https://doi.org/10.1186/s40364-019-0169-8.
Weekes CD, Lamberts LE, Borad MJ, Voortman J, McWilliams RR, Diamond JR, et al. Phase I study of DMOT4039A, an antibody-drug conjugate targeting mesothelin, in patients with unresectable pancreatic or platinum-resistant ovarian cancer. Mol Cancer Ther. 2016;15(3):439–47. https://doi.org/10.1158/1535-7163.MCT-15-0693.
Lazzerini L, Jöhrens K, Sehouli J, Cichon G. Favorable therapeutic response after anti-mesothelin antibody-drug conjugate treatment requires high expression of mesothelin in tumor cells. Arch Gynecol Obstet. 2020;302(5):1255–62. https://doi.org/10.1007/s00404-020-05734-9.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This study was conducted without any financial support.
Author information
Authors and Affiliations
Contributions
SK, MM, and TE contributed to protocol/project development. YT, HM, HI, HI, TS, TH, JS, TI, RS, and AS contributed to data collection and management. SK and, MM performed data analysis. SK, MM, and MT wrote and edited the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional review board of the National Defense Medical College, Tokorozawa, Japan (approval no.3084). Records/information of all women in the study were completely anonymized and de-identified prior to analysis. The present study was exempt from collecting informed consent from all participants.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kakimoto, S., Miyamoto, M., Einama, T. et al. Significance of mesothelin and CA125 expression in endometrial carcinoma: a retrospective analysis. Diagn Pathol 16, 28 (2021). https://doi.org/10.1186/s13000-021-01093-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-021-01093-4