Abstract
Background
Attention deficit hyperactivity disorder (ADHD) is a prevalent and multifactorial neuropsychiatric disorder in the human population worldwide. Complex etiology and clinical heterogeneity have challenged the research, diagnostics and treatment of the disease. Hyperactive and impulsive behaviour has also been observed in dogs, and they could offer a physiologically relevant model for human ADHD. As a part of our ongoing study to understand the molecular etiology of canine anxiety traits, this study was aimed to pilot an approach to identify metabolic biomarkers in canine ADHD-like behaviours for research, diagnostics and treatment purposes.
Methods
We collected fresh plasma samples from 22 German Shepherds with varying ADHD-like behaviours. All dogs were on the same controlled diet for 2 weeks prior to sampling. A liquid chromatography combined with mass spectrometry (LC–MS)-based non-targeted metabolite profiling was performed to identify plasma metabolites correlating with the ADHD-like behaviour of the dogs.
Results
649 molecular features correlated with ADHD-like behavioural scores (praw < 0.05), and three of them [sn-1 LysoPC(18:3), PC(18:3/18:2) and sn-1 LysoPE(18:2)] had significant correlations also after FDR correction (pFDR < 0.05). Phospholipids were found to negatively correlate with ADHD-like behavioural scores, whereas tryptophan metabolites 3-indolepropionic acid (IPA) and kynurenic acid (KYNA) had negative and positive correlations with ADHD-like behavioural scores, respectively.
Conclusions
Our study identified associations between canine ADHD-like behaviours and metabolites that are involved in lipid and tryptophan metabolisms. The identified metabolites share similarity with earlier findings in human and rodent ADHD models. However, a larger replication study is warranted to validate the discoveries prior to further studies to understand the biological role of the identified metabolites in canine ADHD-like behaviours.
Similar content being viewed by others
Background
Attention deficit hyperactivity disorder (ADHD) is a multifactorial neuropsychiatric disorder with a high prevalence (5–10 %) among children worldwide. It is also increasingly reported in adults [1–3]. In general, ADHD is defined by age-inappropriate levels of inattention, impulsivity and hyperactivity [4]. The symptoms can be disabling, interfering with normal everyday life [5]. Moreover, ADHD tends to comorbid with other neuropsychiatric disorders such as anxiety disorders [4]. Although we know that ADHD is highly heritable with mean heritability estimates of 0.7, the exact molecular mechanisms underlying ADHD pathology are still not properly understood due to the genetic complexity and interactions between genetic and environmental factors [3, 4, 6]. However, studies suggest that disruptions in normal functions of dopaminergic, serotonergic and noradrenergic systems may play a key role in ADHD pathogenesis [1, 3, 4].
One potential approach to unravel the biological pathways of ADHD is to utilise animal models, like dogs, which spontaneously show ADHD-like behaviours, such as hyperactivity, impulsivity and inattention. Many young dogs often show hyperactive and impulsive behaviour, but some hunting and working breeds, like the German Shepherd and the Belgian Shepherd, may continue to show these behavioural extremes later in life as well [7]. Due to the physiological similarities, dogs may serve as excellent natural large animal models of human ADHD. Moreover, genetic and pharmacological studies have already suggested that the underlying molecular mechanisms of ADHD behaviours may be shared in dogs and humans [8]. Dogs with high impulsivity scores have been observed to have reduced tolerance for a delay in reward and also lower levels of urinary serotonin and serotonin/dopamine ratio levels, thus, demonstrating convergent validity for canine model of impulsivity [9]. A dopamine transporter polymorphism was also found to associate with high activity levels in Belgian Malinois [10].
One of the challenges in ADHD research lies in the genetic complexity of the disorder. It is expected that multiple small effect genes contribute to ADHD susceptibility [3, 4]. There is a need either for large study cohorts or new natural models with a simpler genetic architecture such as dogs combined with complementary omics approaches. High-throughput technologies, such as metabolomics, may have the potential to facilitate ADHD research. Non-targeted metabolite profiling offers a hypothesis-free approach to detect altered metabolites and pathways in neuropsychiatric disorders. Few successful examples exist and suggest genetic and environmental contributions to diseases [11–13].
In this study, we investigated the association between plasma metabolites and ADHD-like behavioural scores in diet-controlled dogs with varying ADHD-like behaviours in order to identify ADHD-related pathways and biomarker candidates using a LC-qTOF-MS –based approach. Our results reveal associations between ADHD-like behaviours and plasma phospholipids and tryptophan metabolites in dogs.
Methods
Animals and study design
Data on dog ADHD-like behaviour was collected using our validated owner-completed behavioural survey [14]. The questionnaire was advertised to Finnish dog owners and breed clubs of all breeds via Facebook. Both dogs with hyperactive, impulsive and inattentive behaviours, as well as dogs with no sign of hyperactivity, impulsivity or inattention, were invited to participate in the survey.
The owner-completed behavioural survey included both general questions concerning the details of their dogs’ background, daily routines, and everyday behaviour and 13 more specific questions concerning the activity, impulsivity and inattention behaviour of the dog (Additional file 1: Table S1). In the questionnaire, we utilised the previously validated questions on impulsivity and activity levels in dogs [15, 16]. During data collection, the questionnaire was modified three times, resulting in four slightly different versions of the questionnaire (the first survey was a paper version, whereas the three others were online questionnaires). The main questions regarding our target trait, ADHD-like behaviours, were not changed between the versions. The main difference came from adding further background questions to versions three and four (maternal care, place of birth, type of food, extra nutrients, time spent alone/day, daily exercise) to better document the early life experiences and conditions of the dogs. To sort out the dogs with extreme ADHD-like behaviours, factor analysis was used to explore the factorial structure of the questionnaire, and to reveal possible interconnection between the questions concerning activity, impulsivity and inattention. The factor analysis (PROC FACTOR) was performed with SAS (version 9.3) and was conducted using the principal factor method with VARIMAX rotation. Based on the criterion of eigenvalues >1, all 13 questions were grouped into two factors, identified as ‘inattention’ and ‘impulsivity-activity’ (‘impulsivity-activity’ referred to as ‘impulsivity’ from now on). These two factors were very similar to factors found in the earlier studies [15, 16]. The ‘inattention’—factor consisted of questions #1,2,3,4,7,10 and 12, and the ‘impulsivity’—factor of questions #5,6,8,9 and 13. Question #11 did not load with either factor and was excluded. Average scores for both inattention and impulsivity factors were calculated for each dog by summing up the points in each individual question and dividing the result by the number of questions in the factor (7 for ‘inattention’ and 5 for ‘impulsivity’). The total ADHD score consisting of the mean of the answers to all the 13 questions concerning the hyperactive, impulsive and inattentive behaviour of the dog was defined for each dog to reflect the total ADHD-like status. All questions had four choices of increasing frequency (never = 1, sometimes = 2, often = 3, very often = 4). Higher scores represented higher ADHD-like behaviours.
Based on the ADHD-like behavioural scores, 22 privately-owned German Shepherds with scores varying from high to low, reflecting the ADHD-like behaviour of the dog, were recruited for the metabolomics study (Additional file 2: Table S2). Furthermore, the behaviour of the selected dogs was confirmed by owner interviews. The age of the dogs varied from 16 to 91 months, where the mean age was 62 months (median 64.6 months). The data consisted of six males (mean age 58.3 months, ranging from 16 to 86 months) and sixteen females (mean age 63.4 months, ranging from 21 to 91 months). To control the possible effects of diet on the metabolite profiles, all recruited dogs were fed with the same commercial dry food (Royal Canin Maxi Sensible) for 2 weeks prior to sampling with 1 week as a run-in period, during which, the dogs adapted to the diet change. The owners were instructed not to use any other foods or dietary supplements during the two-week period and were asked to report any changes. To investigate the metabolite profiles of the dogs, blood samples were collected from each dog by the same trained person followed by immediate isolation of plasma by a portable centrifuge. Plasma samples were kept on ice during shipping and stored in −20 degrees (max 2 months) prior to metabolomics analysis. Most of the samples were taken at the dog’s home and two samples were taken in our laboratory. Samples were collected during the day and in the evening. Most of the dogs (19 out of 22) fasted 12 h before sampling. Samples were collected with the owners’ consent under valid ethical licenses (ESAVI/6054/04.10.03/2012 and Royal Canine ethical board 30052016).
Non-targeted LC–MS metabolite profiling analysis
The sample preparation, instrument parameters and pre-processing of raw data were performed in the LC–MS Metabolomics Center at Biocenter Kuopio (University of Eastern Finland), and they are previously presented in detail [17]. Briefly, methanol (300 µl) was used to precipitate the proteins and extract the metabolites from plasma (100 µl). The non-targeted metabolite profiling was carried out using the UHPLC-qTOF-MS system (Agilent Technologies, Waldbronn, Karlsruhe, Germany), which consisted of a 1290 LC system, a Jetstream electrospray ionization (ESI) source and a 6540 UHD accurate-mass quadrupole-time-of-flight (qTOF) mass spectrometry. All samples were analysed using two different chromatographic techniques; i.e., reversed phase (RP) and hydrophilic interaction chromatography (HILIC). In addition, data were acquired in both ionization polarities; i.e., ESI positive (ESI+) and ESI negative (ESI−).
Non-targeted metabolomics data analysis
Data collection and statistical analysis
The LC–MS data was collected using the vendor’s software MassHunter Qualitative Analysis B.05.00 (Agilent Technologies), where the ions were extracted to compounds utilising the “Find by molecular feature” algorithm. The data were output as compound exchange format (.cef) files into the Mass Profiler Professional software (MPP 2.2, Agilent Technologies) for compound alignment and data pre-processing. In order to reduce noise and remove insignificant metabolite features, only the features found in at least 50 % of the samples were included in the analysis. This resulted in a dataset comprising 7058 features in four separate analytical runs [1462 in HILIC ESI(+), 1483 in HILIC ESI(−), 2624 in RP ESI(+), and 1489 in RP ESI(−)].
The four datasets were exported into Microsoft Excel (2013), and filtered according to peak area >20,000 to exclude small and insignificant features from further analysis. To investigate the associations between the peak areas of the metabolites and each of the ADHD-like behavioural scores (total, inattention and impulsivity scores), the Spearman correlation analysis was used. The results were adjusted for multiple comparisons by the Benjamini-Hochberg false discovery rate (FDR) correction [18] used in each of the four analytical approaches. Only metabolites with praw <0.05 were included in the identification analysis, and metabolites with pFDR <0.05 were considered statistically significant. Finally, the remaining features in the lists were inspected in the LC–MS chromatograms and spectra using the MassHunter software to locate chromatographic peaks with poor retention time accuracy and peak symmetry, which were removed from downstream analysis. Peak lists were also investigated to ensure that the molecular ion of a compound was included into automatic MS/MS fragmentation, and if not, targeted MS/MS analysis was performed.
To investigate whether sex, age or fasting status had any effects on the associations between the metabolites and ADHD-like behavioural scores, a partial correlation analysis including sex, age and fasting status as covariates was performed. All the statistical analyses were performed using the R project for Statistical Computing version 3.0.1.
Identification of the molecular features in the LC–MS data
The identification of metabolites was based on the accurate mass and isotope information; i.e., ratios, abundances and spacing, as well as product ion spectra (MS/MS) acquired either in the automatic MS/MS analysis, during the initial data acquisition, or via re-injection of the samples in targeted MS/MS mode. The spectra were compared with the METLIN Metabolite Database [19], the Human Metabolome Database (HMDB) [20], and LipidMaps [21] or fragmentation patterns reported in earlier publications. The identification of lipids was based on their characteristic fragmentation patterns reported in earlier publications [22–25]. Briefly, the key elements for identification were the protonated head group (m/z 184.07 for PCs and LysoPCs and m/z 196.03 for LysoPEs), as well as the deprotonated fatty acid fragments visible in the negative ionization mode. For LysoPCs, the product ion at m/z 104 was used to distinguish sn-1 and sn-2 isomers. The MS/MS fragmentation data for all of the identified metabolites is presented in Table 1.
Results
In this study, we used non-targeted LC–MS-based metabolite profiling to create the plasma metabolite profiles of 22 German Shepherds with varying ADHD-like behavioural scores describing the hyperactive, impulsive and inattentive behaviour of the dog. The variation in the dietary profiles was controlled by feeding the same food to all dogs for a 2-week period prior to sampling.
From the LC–MS measurements, we were able to extract 7058 molecular features to downstream statistical analysis. Of these, 649 features correlated with ADHD-like behavioural scores (praw <0.05). These molecular features were subjected to manual inspection to identify metabolites, resulting in a set of 22 identified and five unidentified metabolites (Table 1).
Tryptophan metabolites are associated with ADHD-like behaviour
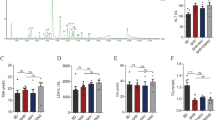
Three molecules related to tryptophan metabolism correlated with the ADHD-like behavioural scores (Table 2; Fig. 1). Compounds with m/z 190.086 and retention time (rt) 5.77 min and m/z 190.050 and rt 3.23 min in RP ESI(+) analysis showed identical fragmentation with 3-indolepropionic acid (IPA) (CAS No. 830-96-6) and kynurenic acid (KYNA) (CAS No. 492-27-3), respectively, according to the METLIN database (Table 1). Compound with m/z 176.071 and rt 5.01 in RP ESI(+) analysis was identified as indoleacetic acid (IAA) (CAS No. 87-51-4), based on its MS/MS fragmentation pattern. Higher ADHD-like behavioural scores were associated with lower plasma levels of IPA (total: rs = −0.565, praw = 0.006, pFDR = 0.381; inattention: rs = −0.618, praw = 0.002, pFDR = 0.241; impulsivity: rs = −0.452, praw = 0.035, pFDR = 0.560) and IAA (inattention: rs = −0.474, praw = 0.030, pFDR = 0.433) (Table 2; Fig. 1). In contrast, high ADHD-like behavioural scores were associated with high plasma levels of KYNA (total: rs = 0.511, praw = 0.015, pFDR = 0.426; inattention: rs = 0.505, praw = 0.017, pFDR = 0.392; impulsivity: rs = 0.498, praw = 0.018, pFDR = 0.447).
More intense ADHD-like behaviour is associated with decreased plasma phospholipids, but increased fatty acids
The majority of the identified metabolites in the plasma of dogs with ADHD-like behaviours were phospholipids, including five phosphatidylcholines (PC) (PC(18:3/18:2), PC(20:5/18:3), PC(20:4/14:0), PC(18:2/16:1) and PC(15:0/18:2)), five lysophosphatidylcholines (LysoPC) (sn-1 LysoPC(18:3), sn-1 LysoPC(14:0), sn-1 LysoPC(15:0), sn-1 LysoPC(17:0) and sn-1 LysoPC(20:3)) and four lysophosphatidylethanolamines (LysoPE) (sn-1 LysoPE(18:2), sn-1 LysoPE(20:5), sn-1 LysoPE(18:1) and sn-1 LysoPE(18:0)) (Table 1). All of these detected phospholipids negatively correlated with the ADHD-like behavioural scores, of which sn-1 LysoPC(18:3), sn-1 LysoPE(18:2) and PC(18:3/18:2) had the strongest associations (Table 2; Fig. 1). The relationships between sn-1 LysoPC(18:3) and all three ADHD-like behavioural scores (total: rs = −0.769, praw = 2.93E − 05, pFDR = 0.016; inattention: rs = −0.740, praw = 8.26E-05, pFDR = 0.030; impulsivity: rs = −0.765, praw = 3.32E-05, pFDR = 0.036), sn-1 LysoPE(18:2) and inattention score (rs = −0.697, praw = 3.1E-04, pFDR = 0.044), and PC(18:3/18:2) and total and inattention scores (total: rs = −0.804, praw = 1.91E-05, pFDR = 0.016; inattention: rs = −0.798, praw = 2.53E-05, pFDR = 0.019) also remained significant after FDR correction. Significant correlations were not due to outliers (Additional file 3: Figure S1).
In addition, two fatty acids were identified as arachidonic acid (C20:4; m/z 303.235) and C18:1 (m/z 283.26428) (Table 1). In contrast to phospholipids, higher ADHD-like behavioural scores were associated with higher plasma levels of C20:4 (total: rs = 0.508, praw = 0.016, pFDR = 0.163; inattention: rs = 0.579, praw = 0.005, pFDR = 0.102) and C18:1 (total: rs = 0.509, praw = 0.016, pFDR = 0.426; inattention: rs = 0.532, praw = 0.011, pFDR = 0.342; impulsivity: rs = 0.447, praw = 0.025, pFDR = 0.492) (Table 2; Fig. 1).
ADHD-like behavioural scores also correlate with other plasma metabolites
The metabolite with m/z 585.270 and rt 11.35 in the RP ESI(+) analysis correlated positively with all three ADHD-like behavioural scores (total: rs = 0.513, praw = 0.015, pFDR = 0.426; inattention: rs = 0.580, praw = 0.005, pFDR = 0.271; impulsivity: rs = 0.452, praw = 0.035, pFDR = 0.560) and was identified as bilirubin (CAS No. 635-65-4) (Tables 1, 2; Fig. 1). The metabolite with m/z 137.071 and rt 2.22 in the HILIC(+) analysis was identified as 1-methylnicotinamide (CAS No. 3106-60-3). It had a positive association with all three ADHD-like behavioural scores (total: rs = 0.647, praw = 0.001, pFDR = 0.618; inattention: rs = 0.668, praw = 6.86E-04, pFDR = 0.455; impulsivity: rs = 0.571, praw = 0.005, pFDR = 0.461).
The majority of the identified plasma metabolites correlate with all three ADHD-like behavioural scores
Twenty out of the 27 reported metabolites correlated with all three ADHD-like behavioural scores (total, inattention and impulsivity) (Table 2; Fig. 1). Twenty-two metabolites correlated with the total ADHD score and 26 with the inattention score. The metabolites sn-1 LysoPC(14:0), sn-1 LysoPC(18:1) and unknown PC with m/z 796.516 correlated only with the inattention score. The impulsivity score correlated with 21 metabolites, and phenylalanine was found to specifically correlate only with the impulsivity score.
Age, sex and fasting have minor effects on the association between metabolites and ADHD-like behavioural scores
Since there were differences in the fasting status of the dogs (Additional file 2: Table S2), we wanted to determine whether this had any effects on the observed correlations between the plasma metabolites and ADHD-like behavioural scores. Also, the possible effects of age and sex were analysed. Correlation coefficients and p-values adjusted for age, sex and fasting are represented in Table 3 together with the original p-values. Most of the associations between the metabolites and ADHD-like behavioural scores remained after controlling for age, sex and fasting status (age, sex and fasting adjusted p value < 0.05). However, age, sex and fasting had significant effects on associations between IPA and the impulsivity score (original p = 0.035, adjusted p = 0.058), sn-1 LysoPC(14:0) and the inattention score (original p = 0.049, adjusted p = 0.102), sn-1 LysoPC(17:0) and the inattention score (original p = 0.041, adjusted p = 0.055), unknown PC with m/z 796.516 and the inattention score (original p = 0.029, adjusted p = 0.087), IAA and the inattention score (original p = 0.03, adjusted p = 0.062), phenylalanine and the impulsivity score (original p = 0.049, adjusted p = 0.063), and unknown metabolite with m/z 221.150 and the total ADHD score (original p = 0.039, adjusted p = 0.069).
Discussion
ADHD is a prevalent and severe neuropsychiatric disorder, but yet poorly characterised for underlying genes and molecular networks. Genetic complexity and clinical heterogeneity have challenged the research, warranting new approaches to identify novel biomarkers and pathways. An alternative approach would be a study of a physiologically relevant large animal model with natural ADHD-like behaviours such as the dog [15]. Here, we applied a methodologically well-controlled pilot non-targeted metabolite profiling of canine ADHD-like behaviours in order to investigate the correlation between the plasma metabolite profiles and ADHD-like behavioural scores of German Shepherds with varying ADHD-like behaviours. We report 27 metabolites that correlated with at least one of the three ADHD-like behavioural scores (total, inattention, impulsivity). The identified ADHD-like behaviour-related candidate metabolites indicate alterations in tryptophan and phospholipids metabolisms. The same pathways have been suggested in human ADHD [26–30], and the possible important overlap in the human and canine pathways warrants a larger replication study in dogs prior to further conclusions of the metabolic similarity of the ADHD models.
We found three interesting metabolites in the tryptophan pathway, IPA, IAA and KYNA. Lower plasma IPA and IAA levels were associated with higher ADHD-like behavioural scores. IPA is a microbial deamination product of dietary tryptophan with antioxidant capacity [31, 32], produced solely by enteric bacteria (including Clostridium sporogenes) in the intestines [33–35] (Fig. 2). IAA is a plant hormone of the auxin class synthesised via multiple pathways in the plants [36], but it can also be produced in mammals from tryptophan in tissues, and in the intestines by enteric bacteria (including Clostridium bartlettii) [37, 38]. Due to the intestinal bacterial production of both IPA and IAA by bacteria belonging to the same genus (Clostridium), negative correlations between the ADHD-like behavioural scores and these metabolites suggest differences in intestinal microbiota of dogs with different ADHD-like behaviours, since there were no differences in the diets (and intake of tryptophan) of the dogs. Gut microbiota may have a bidirectional effect on the nervous and the immune systems [39–41]. Since IPA is capable of crossing the blood–brain barrier (BBB) and is known to have neuroprotective actions in the central nervous system (CNS) [35], decreased IPA may predispose dogs to neurological and behavioural abnormalities due to oxidative stress, for example. Alternatively, stress caused by hyperactive and impulsive behaviour may lead to altered gut microbiota and result in changed plasma IPA. Changes in the gut microbiota have been suggested in neuro behavioural disorders such as mood disorders and autism [40].
Simplified illustration of the possible metabolic pathways of dietary tryptophan in the intestines. Dietary tryptophan can be degraded in the intestines by enteric bacteria to produce IAA and IPA or KYNA via kynurenine. From the intestines, IPA, IAA and KYNA are transferred to circulation. IPA is known to cross BBB, and thus, can migrate to CNS and act there, but the ability of IAA and KYNA to cross BBB is uncertain. In addition to the degradation of dietary tryptophan in the intestines, KYNA and IAA can also be synthetised from tryptophan in various other tissues in the body, but IPA is solely produced in the intestines by enteric bacteria. BBB blood–brain barrier, CNS central nervous system, IAA indoleacetic acid, IDO indoleamine 2,3-dioxygenase, IPA 3-indolepropionic acid, KYNA kynurenic acid
The third metabolite in the tryptophan pathway, KYNA, was positively correlated with the ADHD-like behavioural scores. KYNA is a neuroactive metabolite produced in the kynurenine pathway of tryptophan metabolism [42, 43]. It acts as an endogenous antagonist of the cholinergic nicotinic α7-receptor (α7nAChR) and the glutamatergic N-methyl-d-aspartate receptor (NMDAR) in the CNS and as an agonist of a particular G-protein coupled receptor, GPR35, in immune cells and in the gastrointestinal (GI) tract [44]. Interestingly, altered levels of KYNA have been proposed in schizophrenia [45, 46], depression [47] and bipolar disorder [48]. A recent study demonstrated a reduction in serum KYNA levels in adult ADHD patients, when compared with controls [26]. Interestingly, our data showed a positive relationship between the plasma KYNA levels and ADHD-like behavioural scores in adult dogs, indicating that dogs with more intense ADHD-like behaviour had higher KYNA levels. KYNA is mainly produced via tryptophan catabolism in various tissues, but enteric bacteria in the intestines are also capable of converting dietary tryptophan into KYNA [49, 50] (Fig. 2). Higher plasma KYNA levels may be due to increased bacterial production of KYNA in the intestines or increased catabolism of tryptophan via the kynurenine pathway in the tissues resulting, for example, from inflammation or oxidative stress [51]. Thus, there may be less tryptophan available for transport into the brain for serotonin synthesis. Serotonin, in turn, is an important neurotransmitter, regulating impulsivity and social behaviour [52]. Decreased serotonin levels have been reported in ADHD patients [52]. Thus, the positive correlation between the plasma KYNA levels and ADHD-like behavioural scores may indirectly reflect decreased serotonin levels with characteristic behavioural symptoms in dogs with higher ADHD-like behavioural scores.
We observed strong and negative correlations between ADHD-like behavioural scores and fifteen phospholipids, including five PCs, five LysoPCs, four LysoPEs and one unknown PC. The blood lipid composition is affected by nutrition and fasting [53], and we, therefore, changed the diet of all study dogs and controlled fasting in the statistical analysis. Thus, the observed difference suggests alterations in the endogenous phospholipid metabolism or in the absorption of dietary lipids between the study groups. Phospholipids are important signalling molecules and major components of cell membranes regulating membrane fluidity, charge and receptor function [54, 55]. The adequate amount of brain phospholipids and their right fatty acid composition is especially important to ensure optimal membrane functionality in the brain [55]. Regarding this, the lower plasma phospholipids may have negative effects on the physiology and behaviour of dogs with more intense ADHD-like behaviour.
Phospholipids are composed of hydrophobic and hydrophilic parts, which are joined together by glycerol [56]. The hydrophilic part consists of a phosphate group combined with a characteristic head group (choline in PCs and ethanolamine in PEs), whereas the hydrophobic part consists of different kinds of combinations of fatty acids, PCs containing two fatty acids, whereas LysoPCs and LysoPEs contain only one fatty acid [56]. Thus, the composition of phospholipids depends on the availability of free fatty acids, which are incorporated into phospholipids. Interestingly, both human [27, 28, 30, 57] and rodent [58–61] studies have suggested that the omega-3 polyunsaturated fatty acid (PUFA) status may contribute to the etiology of ADHD. Both children and adult ADHD patients have been demonstrated to have decreased proportions of long chain PUFAs (LC-PUFAs) and especially omega-3 PUFAs, like eicosapentaenoic acid (EPA; 20:5 n-3) and docosahexaenoic acid (DHA; 22:6 n-3), in plasma lipids when compared with control subjects [28, 29, 62–65].
It has been suggested that the relationship between decreased omega-3 PUFAs and ADHD would lie in dopamine neurotransmission, as rodent studies have proposed that chronic omega-3 PUFA deficiency is associated with significantly decreased concentrations of endogenous dopamine and reduced dopamine-2 (D2) receptor binding in the frontal cortex [59, 61], accompanied by attentional and behavioural problems when compared to rodents fed with diet containing adequate levels of PUFAs [66]. In this study, ADHD-like behavioural scores negatively correlated with plasma phospholipids containing combinations of omega-3 (18:3 n-3 (ALA), 20:5 n-3 (EPA)) and omega-6 (18:2 n-6 (LA)) fatty acids. Our results are not directly comparable to those findings made in ADHD patients, but indicate that phospholipid abnormalities could contribute to canine ADHD-like behaviours too. In contrast to phospholipids, ADHD-behavioural scores were positively associated with plasma levels of free fatty acids C20:4 and C18:1. This may refer to an increased breakdown of phospholipids or problems in the phospholipid synthesis, rather than problems in the intestinal absorption of dietary fatty acids.
Our study also suggests the involvement of oxidative stress in canine ADHD-like behaviours. The ADHD-like behavioural scores showed a positive relationship with fatty acid C20:4, an oxidative stress marker, but a negative relationship with the antioxidant IPA. Impaired balance between the oxidant and antioxidant systems and increased levels of oxidative stress have already been demonstrated in ADHD, although precise mechanisms remain to be characterised [67–70].
We have previously demonstrated the promise of the metabolomics approach in canine fear research [17], and now, we have applied this approach to study canine ADHD-like behaviours with better optimised methodology and preparation of the study cohort. Instead of whole blood, we used fresh plasma samples from dogs that had undergone a controlled diet change before the sampling. However, we have several limitations in our study. First, our sample size is small for conclusive results. Second, we must acknowledge that the behavioural survey is based on the owner reports of the behaviours and activity levels of the dogs, and no behavioural testing was performed to verify the owner reports. Thirdly, fasting control was incomplete, as some dogs failed it. Finally, the time of sampling varied from daytime to evening and should be less variable in the future to better control possible variations due to the circadian rhythm.
Conclusions
Our metabolomics study suggests associations between canine ADHD-like behaviours and tryptophan and phospholipid metabolisms. A replication study with a larger sample size is needed to validate our findings and to confirm the overlap in the affected pathways between human ADHD and canine ADHD-like behaviours.
Abbreviations
- ADHD:
-
attention deficit hyperactivity disorder
- BBB:
-
blood-brain barrier
- CNS:
-
central nervous system
- ESI:
-
electrospray ionization
- HILIC:
-
hydrophilic interaction
- IAA:
-
indoleacetic acid
- IPA:
-
3-indolepropionic acid
- KYNA:
-
kynurenic acid
- LC:
-
liquid chromatography
- LysoPC:
-
lysophosphatidylcholine
- LysoPE:
-
lysophosphatidylethanolamine
- MS:
-
mass spectrometry
- PC:
-
phosphatidylcholine
- RP:
-
reversed phase
References
Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatr. 2005;57(11):1215–20.
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatr. 2007;164(6):942–8.
Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin North Am. 2010;33(1):159–80.
Tarver J, Daley D, Sayal K. Attention-deficit hyperactivity disorder (ADHD): an updated review of the essential facts. Child Care Health Dev. 2014;40(6):762–74.
Hoegl T, Heinrich H, Barth W, Losel F, Moll GH, Kratz O. Time course analysis of motor excitability in a response inhibition task according to the level of hyperactivity and impulsivity in children with ADHD. PLoS ONE. 2012;7(9):e46066.
Li Z, Chang SH, Zhang LY, Gao L, Wang J. Molecular genetic studies of ADHD and its candidate genes: a review. Psychiatr Res. 2014;219(1):10–24.
Lindsay S. Handbook of applied dog behavior and training. Iowa: Iowa State University Press; 2001.
Hejjas K, Vas J, Topal J, Szantai E, Ronai Z, Szekely A, et al. Association of polymorphisms in the dopamine D4 receptor gene and the activity-impulsivity endophenotype in dogs. Anim Genet. 2007;38(6):629–33.
Wright HF, Mills DS, Pollux PM. Behavioural and physiological correlates of impulsivity in the domestic dog (Canis familiaris). Physiol Behav. 2012;105(3):676–82.
Lit L, Belanger JM, Boehm D, Lybarger N, Oberbauer AM. Differences in behavior and activity associated with a poly(a) expansion in the dopamine transporter in Belgian Malinois. PLoS ONE. 2013;8(12):e82948.
Theodoridis GA, Gika HG, Want EJ, Wilson ID. Liquid chromatography-mass spectrometry based global metabolite profiling: a review. Anal Chim Acta. 2012;711:7–16.
Zhou B, Xiao JF, Tuli L, Ressom HW. LC-MS-based metabolomics. Mol BioSyst. 2012;8(2):470–81.
Kaddurah-Daouk R, Krishnan KR. Metabolomics: a global biochemical approach to the study of central nervous system diseases. Neuropsychopharmacology. 2009;34(1):173–86.
Tiira K, Lohi H. Reliability and validity of a questionnaire survey in canine anxiety research. Appl Anim Behav Sci. 2014;155:82–92.
Vas J, Topál J, Péch É, Miklósi Á. Measuring attention deficit and activity in dogs: a new application and validation of a human ADHD questionnaire. Appl Anim Behav Sci. 2007;103:105–17.
Lit L, Schweitzer JB, Iosif AM, Oberbauer AM. Owner reports of attention, activity, and impulsivity in dogs: a replication study. Behav Brain Funct. 2010;6(1):1.
Puurunen J, Tiira K, Lehtonen M, Hanhineva K, Lohi H. Non-targeted metabolite profiling reveals changes in oxidative stress, tryptophan and lipid metabolisms in fearful dogs. Behav Brain Funct. 2016;12(1):1.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57(1):289–300.
The METLIN metabolite database. https://metlin.scripps.edu/index.php. Accessed 8 Aug 2015.
The Human Metabolome Database. http://www.hmdb.ca/. Accessed 8 Aug 2015.
LIPID Metabolites And Pathways Strategy (LIPID MAPS). http://www.lipidmaps.org/. Accessed 8 Aug 2015.
Murphy RC, Axelsen PH. Mass spectrometric analysis of long-chain lipids. Mass Spectrom Rev. 2011;30(4):579–99.
Xu F, Zou L, Lin Q, Ong CN. Use of liquid chromatography/tandem mass spectrometry and online databases for identification of phosphocholines and lysophosphatidylcholines in human red blood cells. Rapid Commun Mass Spectrom. 2009;23(19):3243–54.
Xia YQ, Jemal M. Phospholipids in liquid chromatography/mass spectrometry bioanalysis: comparison of three tandem mass spectrometric techniques for monitoring plasma phospholipids, the effect of mobile phase composition on phospholipids elution and the association of phospholipids with matrix effects. Rapid Commun Mass Spectrom. 2009;23(14):2125–38.
Hsu FF, Turk J. Characterization of ceramides by low energy collisional-activated dissociation tandem mass spectrometry with negative-ion electrospray ionization. J Am Soc Mass Spectrom. 2002;13(5):558–70.
Aarsland TI, Landaas ET, Hegvik TA, Ulvik A, Halmoy A, Ueland PM, et al. Serum concentrations of kynurenines in adult patients with attention-deficit hyperactivity disorder (ADHD): a case-control study. Behav Brain Funct. 2015;11(1):1.
Antalis CJ, Stevens LJ, Campbell M, Pazdro R, Ericson K, Burgess JR. Omega-3 fatty acid status in attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids. 2006;75(4–5):299–308.
Stevens LJ, Zentall SS, Deck JL, Abate ML, Watkins BA, Lipp SR, et al. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am J Clin Nutr. 1995;62(4):761–8.
Widenhorn-Muller K, Schwanda S, Scholz E, Spitzer M, Bode H. Effect of supplementation with long-chain omega-3 polyunsaturated fatty acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): a randomized placebo-controlled intervention trial. Prostaglandins Leukot Essent Fatty Acids. 2014;91(1–2):49–60.
Spahis S, Vanasse M, Belanger SA, Ghadirian P, Grenier E, Levy E. Lipid profile, fatty acid composition and pro- and anti-oxidant status in pediatric patients with attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids. 2008;79(1–2):47–53.
Karbownik M, Reiter RJ, Garcia JJ, Cabrera J, Burkhardt S, Osuna C, et al. Indole-3-propionic acid, a melatonin-related molecule, protects hepatic microsomal membranes from iron-induced oxidative damage: relevance to cancer reduction. J Cell Biochem. 2001;81(3):507–13.
Hwang IK, Yoo KY, Li H, Park OK, Lee CH, Choi JH, et al. Indole-3-propionic acid attenuates neuronal damage and oxidative stress in the ischemic hippocampus. J Neurosci Res. 2009;87(9):2126–37.
Attwood G, Li D, Pacheco D, Tavendale M. Production of indolic compounds by rumen bacteria isolated from grazing ruminants. J Appl Microbiol. 2006;100(6):1261–71.
Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106(10):3698–703.
Zhang LS, Davies SS. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 2016;8(1):1.
Zhao Y. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol. 2010;61:49–64.
Weissbach H, King W, Sjoerdsma A, Udenfriend S. Formation of indole-3-acetic acid and tryptamine in animals: a method for estimation of indole-3-acetic acid in tissues. J Biol Chem. 1959;234(1):81–6.
Russell WR, Duncan SH, Scobbie L, Duncan G, Cantlay L, Calder AG, et al. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res. 2013;57(3):523–35.
de Theije CG, Bavelaar BM, Lopes da Silva S, Korte SM, Olivier B, Garssen J, et al. Food allergy and food-based therapies in neurodevelopmental disorders. Pediatr Allergy Immunol. 2014;25(3):218–26.
Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37(5):984–95.
Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–42.
Myint AM, Kim YK. Network beyond IDO in psychiatric disorders: revisiting neurodegeneration hypothesis. Prog Neuropsychopharmacol Biol Psychiatr. 2014;48:304–13.
Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, et al. Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37(4):939–49.
Moroni F, Cozzi A, Sili M, Mannaioni G. Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. J Neural Transm. 2012;119(2):133–9.
Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in Schizophrenia. Schizophr Bull. 2010;36(2):211–8.
Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 2011;37(6):1147–56.
Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord. 2007;98(1–2):143–51.
Savitz J, Dantzer R, Wurfel BE, Victor TA, Ford BN, Bodurka J, et al. Neuroprotective kynurenine metabolite indices are abnormally reduced and positively associated with hippocampal and amygdalar volume in bipolar disorder. Psychoneuroendocrinology. 2015;52:200–11.
Kuc D, Zgrajka W, Parada-Turska J, Urbanik-Sypniewska T, Turski WA. Micromolar concentration of kynurenic acid in rat small intestine. Amino Acids. 2008;35(2):503–5.
Wu W, Nicolazzo JA, Wen L, Chung R, Stankovic R, Bao SS, et al. Expression of tryptophan 2,3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer’s disease brain. PLoS ONE. 2013;8(4):e59749.
Patrick RP, Ames BN. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015;29(6):2207–22.
Banerjee E, Nandagopal K. Does serotonin deficit mediate susceptibility to ADHD? Neurochem Int. 2015;82:52–68.
Dougherty RM, Galli C, Ferro-Luzzi A, Iacono JM. Lipid and phospholipid fatty acid composition of plasma, red blood cells, and platelets and how they are affected by dietary lipids: a study of normal subjects from Italy, Finland, and the USA. Am J Clin Nutr. 1987;45(2):443–55.
Muller CP, Reichel M, Muhle C, Rhein C, Gulbins E, Kornhuber J. Brain membrane lipids in major depression and anxiety disorders. Biochim Biophys Acta. 2015;1851(8):1052–65.
Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH. Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids. 2000;35(12):1305–12.
Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th ed. London: Garland Science; 2002.
Richardson AJ, Puri BK. The potential role of fatty acids in attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids. 2000;63(1–2):79–87.
Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. 2006;75(4–5):259–69.
Delion S, Chalon S, Herault J, Guilloteau D, Besnard JC, Durand G. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotoninergic neurotransmission in rats. J Nutr. 1994;124(12):2466–76.
Zimmer L, Delpal S, Guilloteau D, Aioun J, Durand G, Chalon S. Chronic n-3 polyunsaturated fatty acid deficiency alters dopamine vesicle density in the rat frontal cortex. Neurosci Lett. 2000;284(1–2):25–8.
Zimmer L, Hembert S, Durand G, Breton P, Guilloteau D, Besnard JC, et al. Chronic n-3 polyunsaturated fatty acid diet-deficiency acts on dopamine metabolism in the rat frontal cortex: a microdialysis study. Neurosci Lett. 1998;240(3):177–81.
Chen JR, Hsu SF, Hsu CD, Hwang LH, Yang SC. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. J Nutr Biochem. 2004;15(8):467–72.
Bekaroglu M, Aslan Y, Gedik Y, Deger O, Mocan H, Erduran E, et al. Relationships between serum free fatty acids and zinc, and attention deficit hyperactivity disorder: a research note. J Child Psychol Psychiatr. 1996;37(2):225–7.
Young GS, Maharaj NJ, Conquer JA. Blood phospholipid fatty acid analysis of adults with and without attention deficit/hyperactivity disorder. Lipids. 2004;39(2):117–23.
Hawkey E, Nigg JT. Omega-3 fatty acid and ADHD: blood level analysis and meta-analytic extension of supplementation trials. Clin Psychol Rev. 2014;34(6):496–505.
Frances H, Monier C, Bourre JM. Effects of dietary alpha-linolenic acid deficiency on neuromuscular and cognitive functions in mice. Life Sci. 1995;57(21):1935–47.
Ceylan MF, Sener S, Bayraktar AC, Kavutcu M. Changes in oxidative stress and cellular immunity serum markers in attention-deficit/hyperactivity disorder. Psychiatr Clin Neurosci. 2012;66(3):220–6.
Ceylan M, Sener S, Bayraktar AC, Kavutcu M. Oxidative imbalance in child and adolescent patients with attention-deficit/hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatr. 2010;34(8):1491–4.
Selek S, Bulut M, Ocak AR, Kalenderoglu A, Savas HA. Evaluation of total oxidative status in adult attention deficit hyperactivity disorder and its diagnostic implications. J Psychiatr Res. 2012;46(4):451–5.
Kul M, Unal F, Kandemir H, Sarkarati B, Kilinc K, Kandemir SB. Evaluation of Oxidative Metabolism in Child and Adolescent Patients with Attention Deficit Hyperactivity Disorder. Psychiatr Investig. 2015;12(3):361–6.
Authors’ contributions
HL, SS and KT designed the experiment, JP, ML and KH performed the experiment, JP analysed the data. HL, ML, CA and KH contributed to reagents/materials/analysis tools. JP and HL wrote the manuscript with others’ contributions. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Petra Jaakonsaari for technical assistance in sample collection and Miia Reponen for maintaining the LC–MS-qTOF instrument. Royal Canin is thanked for donating food for the participating dogs and The German Shepherd breed club and owners are thanked for participation.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Anonymous raw metabolite data files from each dog are available from the corresponding author upon request.
Ethics approval and consent to participate
Samples were collected with the owners’ consent under valid ethical licenses (ESAVI/6054/04.10.03/2012 and Royal Canine ethical board 30052016).
Funding
This study was funded partly by the ERCStG, ERANET-NEURON Mental Disorders, the Finnish Kennel Club, the Academy of Finland, the Orion-Farmos Research Foundation, the Sigrid Juselius Foundation, the Jane and Aatos Erkko Foundation, Biocenter Finland and the Dog Health Research Fund, University of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Additional files
12993_2016_112_MOESM3_ESM.docx
Additional file 3: Figure S1. Sample distributions for each metabolite having significant correlation coefficients (pFDR < 0.05).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Puurunen, J., Sulkama, S., Tiira, K. et al. A non-targeted metabolite profiling pilot study suggests that tryptophan and lipid metabolisms are linked with ADHD-like behaviours in dogs. Behav Brain Funct 12, 27 (2016). https://doi.org/10.1186/s12993-016-0112-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12993-016-0112-1