Abstract
Healthy dietary patterns are associated with lower inflammation and cardiovascular disease (CVD) risk and adherence can be measured using diet quality scores. Inflammation is traditionally measured with C-reactive protein (hsCRP), however there is interest in novel pro-inflammatory markers platelet-activating factor (PAF) and lipoprotein-associated phospholipase A2 (Lp-PLA2) that are specifically involved in endothelial dysfunction and inflammation. This cross-sectional study investigated the association between PAF, Lp-PLA2, hsCRP, and six diet scores. One hundred adults (49 ± 13 years, 31% male) with variable CVD risk were recruited. Fasting PAF, Lp-PLA2 and hsCRP and usual dietary intake were measured. Adherence to Dietary Approaches to Stop Hypertension (DASH), Dairy-adjusted DASH, Vegetarian Lifestyle Index, Healthy Eating Index for Australians (HEIFA), Mediterranean Diet Adherence Screener (MEDAS) and PREDIMED-Plus (erMedDiet) scores were calculated. Correlations and multiple regressions were performed. hsCRP, but not PAF, independently correlated with several diet scores. Lp-PLA2 independently correlated with Vegetarian Lifestyle Index only in unadjusted models. A one-point increase in adherence to the DASH Index, the Dairy-adjusted DASH Index and the Vegetarian Lifestyle Index was associated with a 30%, 30%, and 33% reduction in hsCRP levels, respectively. Smaller effects were seen with the other diet scores with a one-point increase in adherence resulting in a 19%, 22% and 16% reduction in hsCRP with HEIFA, MEDAS, erMedDiet scores, respectively. The lack of stronger associations between the novel markers of inflammation and diet scores may be due to confounding by COVID-19 infection and vaccination programs, which prevents any firm conclusion on the relationship between PAF, Lp-PLA2 and healthy dietary patterns. Future research should aim to examine the relationship with these novel markers and healthy dietary patterns in a non-pandemic setting.
Similar content being viewed by others
Introduction
Vascular inflammation plays a key role in atherosclerosis formation and progression resulting in the development of cardiovascular disease (CVD) [1]. Individual dietary components have been shown to modulate inflammation, [2, 3] however, research on dietary patterns better captures how people eat, and the synergy that exists between individual foods when combined in an overall diet [4]. Diet quality scores have been developed to measure the healthfulness of diets and can be used to assess adherence to specific heathy dietary patterns. Higher quality diets, measured by several diet quality indices, are associated with a lower risk of CVD and all-cause mortality [5].
Numerous inflammatory biomarkers have been identified to detect and measure inflammatory processes involved in atherosclerosis, one of which is high-sensitivity C-reactive protein (hsCRP). However, this is a non-specific marker of inflammation and can be elevated with both acute and chronic inflammation [6]. CRP has numerous isoforms, some more atherogenic than others, which current assays are not able to differentiate well [7]. In addition CRP has significant intraindividual variability and requires repeated measurements for accurate assessment [8].
Two novel biomarkers related to endothelial damage that have been identified in atherosclerosis research are platelet-activating factor (PAF) and lipoprotein-associated phospholipase A2 (Lp-PLA2) [9]. PAF is an ether-linked glycerophospholipid and is a potent inflammatory mediator involved in endothelial dysfunction and permeability, cell signalling and initiation of the inflammatory cascade within the intima [10]. PAF has been shown to be associated with numerous CVD events including myocardial infarction, heart failure, stroke and coronary heart disease [11,12,13,14].
Lp-PLA2 is a vascular specific marker with a low biologic fluctuation that is involved in plaque growth and thrombosis [15]. Lp-PLA2 hydrolyses the sn-2 ester bond of glycerophospholipids such as the acetyl group on PAF, in addition to oxidised phospholipids on the surface of LDL particles [16]. Elevated levels of Lp-PLA2 have been shown to be indicative of vascular inflammation associated with the formation of plaque within the arteries [17] and is involved in several CVD events including stroke, myocardial infarction, coronary artery disease and aortic stenosis [18,19,20,21].
Compliance with healthy dietary patterns such as Dietary Approach to Stop Hypertension (DASH) diet, Mediterranean diet, vegetarian diet and diets based on country specific dietary guidelines are associated with lower levels of inflammatory biomarkers and CVD risk [22,23,24,25]. However, research examining dietary patterns and inflammation measured by PAF and Lp-PLA2 is limited. A recent systematic review of 16 studies found Mediterranean diet scores, vegetarian diets and heart healthy diets to be associated with lower levels of PAF and Lp-PLA2 [26]. However, most of the included studies had numerous unadjusted confounders such as the inclusion of participants on medications and/or supplements known to lower levels of these markers such as cholesterol-lowering medications, hormone replacement therapy, niacin, orlistat, fish oils and omega-3 supplements [27,28,29,30,31,32].
Therefore, the aim of this study was to examine the relationship between hsCRP, PAF and Lp-PLA2 activity and healthy dietary patterns utilising strict exclusion criteria. Specifically, this study aimed to investigate the relationship between these novel markers of inflammation and hsCRP, and six diet quality scores including DASH Index, Dairy-adjusted DASH Index, Vegetarian Lifestyle Index, Healthy Eating Index for Australian Adults (HEIFA), original Mediterranean Dietary Adherence Screener (MEDAS), and modified MEDAS from the PREDIMED-Plus Study (erMedDiet) in Australian adults at varied levels of CVD risk.
Material and methods
Methodology for this study, excluding the assessment of dietary intake and calculation of dietary scores, has been previously published [9].
Study design and setting
This study used a cross-sectional design and a convenience sampling technique and was carried out on the Gold Coast, Queensland, Australia. Participants were recruited through non-health community settings such as sporting clubs, surf life savings clubs, shopping centres, fitness centres, council libraries, community centres, university setting and through social media and online/email methods to obtain a representative community sample of healthy adults at varying risk of cardiovascular disease. Recruitment for the study began in February 2021 and samples were collected from May 2021 to April 2022, over four two-week periods.
This study protocol was approved by the Bond University Human Research Ethics Committee (approval DR03194) and conforms to the ethical guidelines of the 1975 (revised in 1983) Declaration of Helsinki. All participants provided written informed consent.
Study population and sample size
Eligible participants included adults aged 18–70 years old who were classified as either low or high risk of CVD. In order to obtain a more robust estimate of the relationship between diet quality and inflammatory markers we aimed to get variety in CVD risk from low to high. In order to be classified as high risk of CVD, participants had to either have confirmed type 2 diabetes OR have two or more of the following risk factors for CVD: systolic blood pressure ≥ 140 mm Hg or diastolic ≥ 90 mm Hg or receiving medication for high blood pressure; total cholesterol ≥ 5.2 mmol/L; LDL cholesterol ≥ 4.1 mmol/L; HDL cholesterol ≤1 mmol/L; family history of premature CHD (≤ 60 years); or excess weight, BMI ≥ 25 kg/m2. To be classified low risk, participants had to report the absence of any chronic disease and not be on any routine medication. They also needed to be below the cut-offs listed for high-risk individuals for BMI, blood pressure, cholesterol and report no family history of premature CHD.
Exclusion criteria included any participant who reported a history of angina, myocardial infarction, peripheral vascular disease, congenital heart disease or stroke or who were current smokers. Additionally, any participant who was taking medications or supplements known to impact measurements of PAF and/or Lp-PLA2, including cholesterol lowering medications such as statins, fenofibrate, and ezetimibe; or niacin, orlistat, omega- 3, fish oil supplements and hormone replacement therapy were excluded. Participants who reported Asian or African ethnicity were also excluded as these ethnic groups have been shown in studies to have lower levels of Lp-PLA2, possibly due to genetic polymorphisms [33,34,35].
Similar studies with PAF and/or Lp-PLA2 as outcome measures have used sample sizes ranging from 10 to 106 participants [36,37,38,39]. With 100 participants there was an 80% power to detect a correlation between inflammation level and diet scores of 0.3 or greater assuming a level of significance of 5%. A correlation of 0.3 is a medium effect size for a correlation according to Cohen [40].
Data collection
Data were collected at the Bond Institute of Health and Sport through face-to-face visits and included anthropometric, biochemical, and clinical measurements.
Anthropometric data were measured with normal clothing but without shoes and in the fasted state. A wall mounted stadiometer was used to measure standing height to the nearest 0.1 cm. Weight was measured to the nearest 0.1 kg with a calibrated digital scale. Waist circumference was measured six times, three times at minimum waist and three times at the umbilicus and was averaged [41]. The formula (weight (kg)/height (m2) was used to calculate BMI.
Clinic blood pressure was measured in triplicate, two minutes apart, in the non-dominate arm, and seated with a clinical cuff using a Creative Medical PC-900 Pro Vital Signs Monitor. The first measurement was disregarded and the second and third measurements were averaged [42].
Participants completed self-administered questionnaires at the study visit which gathered information on age, sex, medical history, medication and supplement intake, menopausal status, smoking status and alcohol consumption.
The World Health Organization’s (WHO’s) Global Physical Activity Questionnaire (GPAQ) was used to measure physical activity (PA) levels of the participants [43]. The questionnaire was self-administered and included 16 questions assessing time spent physically active during work, travel, and recreation in addition to sedentary time. Participants’ metabolic equivalent (MET) minutes per weeks were then calculated based on the participant scores in accordance with the GPAQ Analysis Guide [44]. PA levels were categorised into tertiles using the MET minutes based on WHO’s PA recommendation where 0 = low, MET < 600 min/week; 1 = moderate, MET ≥ 600 to < 1500 min/week; and 2 = high, MET ≥ 1500 min/week.
Methods for blood collection and treatment and assay procedures for hsCRP, PAF and Lp-PLA2 were previously described and reported elsewhere [9].
Dietary assessment
The usual dietary intake of participants was assessed by administering the European Prospective Investigation into Cancer and Nutrition (EPIC) food frequency questionnaire (FFQ) [45] modified for the Australian food environment. The EPIC FFQ is a validated tool developed to measure habitual food and nutrient intake in adults and children during the past year. This FFQ is a semi-quantitative paper-based questionnaire that includes 130 common and less common food items in addition to an open section where participants can add additional items not assessed in the questionnaire such as breakfast cereal brand, type and quantity of milk consumed, type of fat used in cooking and baking and the amount of visible fat on meat consumed. Participants responded by reporting the consumption frequency of each food item using a 9-point scale from never or less than once a month to 6 or more times per day. The frequency of each food item was manually entered into a spreadsheet, multiplied by the portion fraction based on frequency and multiplied by the quantified serving size of the food in grams, and was further converted into serving sizes.
In order to calculate energy intake and nutrient intake such as saturated and unsaturated fats and sodium that were needed to calculate some of the diet scores, the FFQ EPIC Tool for Analysis (FETA) software was utilised. The FETA software is an open source, cross-platform tool that processes dietary data from the food frequency questionnaire used by the EPIC-Norfolk [46]. The software contains 10 data files that contain all the individual foods, nutrients, and serving sizes based on European food composition data. Adaptations were made to the original FETA files to replace the European food composition data with the Australian Food Composition Database and AUSNUT values [47, 48]. This involved manually replacing all food items and each food item’s nutrients (energy, fat, carbohydrate, protein, and potassium, phosphorus, and sodium) according to the Australian Food Composition Database.
Participants completed an estimated three-day food diary in household measures the week following their study visit. The diaries were completed on 2 consecutive weekdays and a weekend day. This diary was used to calculate water intake for some of the diet scores.
DASH Index
The adherence to the DASH dietary pattern was assessed by a DASH diet score designed by Gunther et al [49]. This score is comprised of 10 components to assess the participant’s compliance to the DASH dietary pattern (see Additional file 1: Table S1). Six of the components were weighted on a 10-point scale, which include: fruits and fruit juice; vegetables and potato; meat, poultry, fish and eggs; nuts, seeds and legumes; fats and oils; sweets.
Two additional components are grain and dairy and are assessed based on a qualitative aspect in addition to a quantitative one. Each is weighted on a 5-point scale and include: total dairy; low-fat dairy; whole grains; high-fibre grains.
Each participant was assigned the energy level that was closest to their estimated energy requirement based on age, sex, and physical activity level [50] and categorised into 1600, 2000, 2600 or 3100 cal as outlined in the National Heart, Lung and Blood Institute’s Dash Eating Plan [51]. Each dietary component was then standardised to the assigned energy level. Lower intakes were scored proportionally. Reverse scoring was applied where lower intakes are favoured by DASH, such as meats, poultry fish, eggs, fats, oils and sweets. The total DASH score was calculated by summing all the points from each respective section to generate a composite score ranging in values from 0 to 80.
Dairy-Adjusted DASH Index
As recent research has highlighted the anti-inflammatory capabilities of full fat dairy [52] and that a DASH diet with full fat dairy consumption may be just as effective as low fat dairy in reducing blood pressure and lipids in clinical trials, [53] a sub analysis was performed to assess the relationship of the DASH index (with an adjustment for the dairy component) on inflammatory markers. This adjustment was performed by removing the scoring component for 75% of dairy consumed to be low fat.
Vegetarian Lifestyle Index
Adherence to a vegetarian dietary pattern was assessed using the Vegetarian Lifestyle Index, a score developed to measure adherence to the Loma Linda University Vegetarian Food Guide Pyramid [54]. This score measures adherence to both diet and lifestyle recommendations. The score is comprised of fourteen components with eleven measuring aspects of diet and three measuring compliance to lifestyle habits (see Additional file 1: Table S2). The dietary components assessed included: whole grains; legumes and soy; vegetables; fruits; nuts and seeds; vegetable oils; dairy products; eggs; sweets; reliable sources of vitamin B-12; flesh foods. The following lifestyle habits were also assessed: daily exercise; water intake; adequate sunlight exposure.
Each dietary component was standardised to 2000 kcal (8368 kJ) per day specific to the lacto-ovo recommendations of the Vegetarian Food Guide Pyramid Guidelines for Healthful Vegetarian Diets [54]. This adjustment was based on BMR multiplied by a physical activity factor to standardised energy intake [50]. Each component was scored with 0, 0.5, or 1 point depending on adherence, with lower intakes receiving lower scores. Reverse scoring was applied where lower intakes are favoured by the vegetarian diet, such as dairy, eggs, sweets, and flesh foods. Daily exercise was assessed using the World Health Organization’s Global Physical Activity Questionnaire. All participants received full marks for sunlight exposure due to the location of this study as it has been shown that, even in dark skinned people, adequate vitamin D is produced in as little as 4 min of daily exposure to sunlight in winter, for people living in southeast Queensland [55]. The total vegetarian score was calculated by summing all of the points from each respective section to generate a composite score ranging in values from 0 to 14.
HEIFA Index
Adherence to the Australian Guide to Healthy Eating (AGHE) was assessed using the HEIFA index [56]. This score is comprised of 11 components based on different characteristics of a healthy diet (see Additional file 1: Table S3). The degree to which each participant’s diet conformed to the serving recommendations for each food group of the AGHE was measured by each component. These components included the five core food groups, which include: vegetables; fruits; grain (cereal) foods; milk and milk alternatives; meat and protein food alternatives.
Three of the food groups, vegetables, fruits and grains, were assessed based on a qualitative aspect in addition to a quantitative one. Fruits and vegetables were assessed for variety and grains were assessed on whether 50% of total grains consumed are wholegrains.
One component assessed intake of discretionary foods which were high in saturated fat and/or added sugars, added salt, or alcohol. Additional components included alcohol intake and adequate water consumption. Water was assessed as a percentage of water to total beverages where 50% water consumed relative to total beverages received a full score.
Specific nutrients were assessed as separate components and include fats such monounsaturated, polyunsaturated and saturated fats, added sugars, and sodium. Saturated fat intake was assessed as a percentage of energy intake, with an intake of less than 10% required for a full score and a 0 was given when the ratio was 15% or greater. Nine of the components (core food groups, discretionary foods, saturated fat, added sugar and sodium) were scored from 0–10. Two components (water and alcohol) were scored from 0 to 5. All components were summed to reach a composite score which ranged from 0 to 100.
Mediterranean diet adherence screener (MEDAS)
A 14-item MEDAS tool was used to assess adherence to the Mediterranean Diet [57]. Five questions assessed intake of favourable food groups such as fruit, vegetable, legumes, fish and shellfish, nuts (see Additional file 1: Table S4). Two questions assessed olive oil intake, both quantity and whether olive oil was used as the main cooking oil. One question assessed how many times per week boiled vegetables, pasta, rice, or other dishes were consumed with a sofrito sauce of tomato, garlic, onion, or leeks sauteed in olive oil. Four questions assessed intake of foods that are not favourable in the Mediterranean Diet such as red and processed meat, sugar sweetened beverages, butter, margarine or cream intake, and consumption of commercial sweets and pastries. One question assessed whether chicken and turkey meat were preferentially consumed over red meat, pork and processed meat. One question assessed wine intake. Each question had a single adherence criterion and was worth 1 point if that criteria was met, or 0 points if not met. Scores were summed with a total composite score ranging from 0 to 14.
erMedDiet Score
Adherence to the Mediterranean Diet was also assessed using the 17-item erMedDiet score [58]. This score is an adapted version of the original 14-item MEDAS that is based on a randomised control trial of the Mediterranean diet that allowed for energy restriction and body mass reduction. Similar to the original MEDAS, five questions assessed intake of favourable food groups such as fruit, vegetable, legumes, fish and shellfish, nuts (see Additional file 1: Table S5). One question assessed whether olive oil was used as the main cooking oil. One question assessed how many times per week boiled vegetables, pasta, rice, or other dishes were consumed with a sofrito sauce of tomato, garlic, onion, or leeks sauteed in olive oil. Three questions assessed grain intake with one question assessing whole grain intake and two questions assessing refined grains and white bread. Four questions assessed intake of foods that are not favourable in the Mediterranean Diet such as red and processed meat, sugar sweetened beverages, butter, margarine or cream intake, and consumption of commercial sweets and pastries. One question assessed whether chicken and turkey meat were preferentially consumed over red meat, pork and processed meat. One question assessed whether sugar was added to coffee and tea and one question assessed wine intake.
Each question was scored using the same method as the MEDAS and scores were summed with a total composite score ranging from 0 to 17. For questions based on the same components as the MEDAS some were adjusted with regards to their frequency/serve intake criteria to accommodate energy restriction.
Data analysis
All data were analysed using SPSS version 28.0.0.0 (190) (SPSS Inc., Chicago, USA). Data were assessed for normality by examining distributions via Q–Q plots. Variables that were not normally distributed were log transformed before data analysis (PAF and hsCRP). Independent t-tests were performed on normally distributed variables to test for differences between males and females, and participants with high-risk and low-risk of CVD. Pairwise Pearson’s correlations were calculated between each of the six measured diet scores as well as between the diet scores and markers of inflammation.
Multiple linear regression was performed to examine associations between markers of inflammation and diet scores. A first model was run that adjusted for age, gender and year of data collection. A second model adjusted for age, gender, year of data collection, waist circumference, physical activity and level of risk. Models which examined the Vegetarian Lifestyle Index were not adjusted for physical activity as this was accounted for in the formulation of the Vegetarian Lifestyle Index score. Checks for multicollinearity were conducted using variance inflation factor (VIF) and tolerance indices and revealed no evidence of multicollinearity. Results of multiple linear regression are reported as standardised coefficients ß and significance is reported as P values where P < 0.05 is considered statistically significant. Mean scores for each inflammatory marker concentration were calculated for quartiles of each of the diet scores for descriptive purposes only and were not used in regression analysis (see Additional File 1: Table S7).
To estimate the effect of a one-point change in diet scores that were reported as statistically significant in model 1, the β coefficients were back transformed by exponentiating the coefficient. This allowed interpretation on a multiplicative scale, e.g., a back transformed value of 0.70 means a 1 unit increase in diet score is associated with a 1–0.70 = 30% decrease in the inflammation measure.
To explore the effects that year of data collection (and potential COVID-19 illness and/or vaccine) had on results for PAF and Lp-PLA2, a separate multiple regression was performed (Table 5).
Results
Clinical characteristics
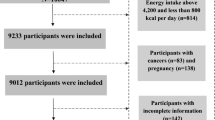
A total of 132 people were recruited; four did not meet inclusion criteria and 28 declined to participate, leaving 100 participants who attended a study data collection visit and were included in analysis (Additional file 1: Table S6). Forty-six participants (44 high-risk, 2 low-risk) attended study visits in 2021 and 54 participants (24 high-risk and 30 low-risk) attended in 2022. Demographic and clinical characteristics for the total cohort, males and females, and individuals at high- versus low- CVD risk are shown in Table 1. The mean age was 49 (range 20–69) years and 92% of the cohort were Caucasian. Mean HDL cholesterol was 1.84 ± 0.48 mmol/L and was significantly higher in females compared to males. Mean LDL cholesterol was 3.16 ± 1.11 mmol/L and triglycerides were 1.40 ± 0.82 mmol/L.
Inflammatory marker results
PAF
Median PAF level was 7.96 (3.89–16.77) ng/mL (Table 1). No significant difference was seen between males and females. Median PAF was higher for those at low-risk of CVD compared to those at high-risk (13.27 [9.59–21.63] ng/mL vs. 4.84 [3.24–14.57] ng/mL, p < 0.001).
Lp-PLA2
Mean circulating levels of Lp-PLA2 activity were 14.91 ± 4.29 nmol/min/mL and were significantly higher in males than females (16.98 ± 4.90 nmol/min/mL vs. 13.98 ± 3.65 nmol/min/mL, p < 0.001). There was no significant difference in Lp-PLA2 activity between those at low risk versus high risk of CVD.
hsCRP
Median hsCRP levels were 0.96 (0.49–2.98) mg/L (Table 1). No significant difference was seen between males and females however hsCRP levels were higher in those at high risk of CVD 1.79 (0.64–3.80) mg/L compared to those at low risk 0.56 (0.22–1.01) mg/L, p < 0.001.
Diet Scores
All diet scores significantly correlated with each other with correlations ranging from medium to strong as shown in Table 2. The mean biomarker concentration according to each quartile of diet score is shown in Additional file 1: Table S7.
As shown in Table 1, mean diet scores were DASH 43.86 ± 8.59, Vegetarian Lifestyle Index 7.64 ± 1.58, HEIFA 60.04 ± 11.61, MEDAS 6.45 ± 2.30 and erMedDiet Score 8.30 ± 2.25. There was no significant difference in scores between males and females for any of the scores except for the Vegetarian Lifestyle Index where females reported higher mean scores 7.89 ± 1.65 vs. 7.08 ± 1.29. The high-risk group reported significantly lower mean values for all diet scores compared to the lower risk group except for erMedDiet Score.
PAF and Diet Scores
No significant correlations were seen for any of the diet scores and logPAF (Tables 3 and 4). Results stratified based on year of data collection are shown in Table 5.
Lp-PLA2 and Diet Scores
There was a small negative correlation between the Vegetarian Lifestyle Index and Lp-PLA2 however no significant association remained in either adjusted model. There were no associations found for the other five diet scores. Results stratified based on year of data collection are shown in Table 5.
hsCRP and Diet Scores
There was a significant medium negative correlation between logCRP and DASH Index, Dairy-adjusted DASH Index, and Vegetarian Lifestyle Index and a small negative correlation with HEIFA and MEDAS. In model 1 adjusting for age, gender, and year of data collection, the significant medium correlations between logCRP and DASH Index, Dairy-adjusted DASH Index and Vegetarian Lifestyle Index remained and there was a significant small negative correlation with HEIFA, MEDAS and erMedDiet Score. In the second model, which adjusted for age, gender, year of data collection, waist circumference, physical activity and level of risk, only a small negative correlation remained with DASH Index and Dairy-adjusted DASH Index.
As shown in Table 4, a one-point increase in adherence to the DASH Index, the Dairy-adjusted DASH Index and the Vegetarian Lifestyle Index was associated with a 30%, 30%, and 33% reduction in hsCRP levels, respectively. Smaller effects were seen with the other diet scores with a one-point increase in adherence resulting in a 19%, 22% and 16% reduction in hsCRP with HEIFA, MEDAS, erMedDiet scores, respectively.
Discussion
This cross-sectional study examined the relationship between six diet quality scores (DASH Index, Dairy-adjusted DASH Index, Vegetarian Lifestyle Index, HEIFA, MEDAS and erMedDiet Score) and novel markers of inflammation PAF and Lp-PLA2 and hsCRP in 100 Australian adults at varying levels of risk of CVD. It is the first study to examine various healthy dietary patterns using strict exclusion criteria and analysing PAF and Lp-PLA2 activity in a broadly Caucasian population. Although the novel biomarkers lacked the expected associations with dietary quality scores, the key finding from this study was that around a 1/3 reduction in hsCRP was predicted by an incremental increase in adherence to the DASH, Dairy-adjusted DASH and Vegetarian Lifestyle Index. This is encouraging as it suggests small improvements in diet may make a large contribution to address inflammatory CVDs. These findings support previous research which found adherence to healthy diets is associated with lower levels of inflammation [60].
Adherence to the Vegetarian Lifestyle Index was shown to be associated with lower levels of Lp-PLA2, and hsCRP in unadjusted and hsCRP in adjusted models, which was unsurprising due to the numerous bioactive compounds found in plants shown to modulate inflammation [61]. However, the lack of association between Lp-PLA2 and PAF is surprising and is in contrast to findings from a recent systematic review [26]. These unexpected results could be due to confounding of the COVID-19 vaccine and Omicron outbreak in Australia during data collection. Briefly, PAF and Lp-PLA2 are thought to be affected by COVID-19 and its related vaccines as shown in previous research including some mechanistic studies [9, 62,63,64,65,66]. Levels of PAF in the current study significantly differed based on the year of blood sample collection, with higher levels seen in 2022, which coincided with the Omicron variant COVID-19 outbreak in Australia, and a boost in vaccination rates with adenovirus vector and mRNA vaccines. Higher levels of PAF were seen in the low-risk group, the majority of whom were recruited in 2022, whereas 65% of the higher risk group were recruited in 2021 when vaccination and diagnosed COVID-19 rates were low. Although vaccination status was not collected, the vaccination rate in the Gold Coast region was 90.9% for first dose and 88.5% for second dose by the end of January 2022 (unchanged by April 2022), which was the time frame for 2022 data collection [67]. Similarly, there was no difference between the high-risk and low-risk groups for Lp-PLA2, suggesting Lp-PLA2 levels could also have been elevated due to COVID-19 vaccine and/or infection. This phenomenon is described in more detail elsewhere [9, 62,63,64,65,66]. Attempts were made to control for this effect through our regression models however, adjustment for confounding by a particular variable does not always remove all confounding by that variable and there may be residual confounding [68]. Nevertheless, plant-based dietary interventions have been shown to lower levels of Lp-PLA2 in a high risk population [69], and a recent review has highlighted that numerous foods included in a vegetarian diet such as vegetables, eggs, cheese, and animal- and plant-based yoghurts plus plant nutrients such as carotenoids, vitamins C and E, have antithrombotic activity against PAF [70].
The relationship between Vegetarian Lifestyle Index and CRP was strengthened after adjusting for age, gender and year of data collection. However, this relationship disappeared when controlling for other variables such as risk of CVD and waist circumference. This could be due to CVD risk and waist circumference acting as mediators rather than confounders as poor diet may lead to higher waist circumference and increased CVD risk, ultimately leading to inflammation [71,72,73]. The results of the current study are supported by two recent systematic reviews finding significant negative associations between vegetarian diets and inflammatory markers including CRP [24, 74]. The significant results of the current study could be due to the overall increased number and variety of plant foods consumed, each of which have different bioactive and coloured pigments, many of which have been shown to lower inflammation and confer health benefits [75].
Adherence to the DASH diet was shown to be associated with lower levels of hsCRP in unadjusted and adjusted models but not with PAF or Lp-PLA2. The lack of association with PAF and Lp-PLA2 and DASH Index was unexpected [26] however this could be due to the COVID-19 confounding previously mentioned and reported elsewhere [9, 76, 77]. The results for hsCRP in the current study align with a systematic review of 7 DASH trials [23].
Interestingly, our sub analysis of DASH where we removed the requirement for 75% of dairy consumed to be low fat, showed a strengthened relationship with hsCRP. This aligns with other research, including a recent prospective cohort study whereby saturated fats from dairy were associated with lower cardiometabolic risk and CRP, [78] and a randomised crossover trial of DASH vs dairy-modified DASH, where the dairy-modified DASH was as effective as DASH in reducing blood pressure and lipids [79]. Our findings also support that the fat in dairy may be less inflammatory than previously thought [80,81,82,83,84,85]. This is an area for ongoing research, given the continued message to consume low fat dairy products in global dietary guidelines [86,87,88], which may require revision.
Surprisingly, given previous research on PAF and the Mediterranean diet, [26, 89, 90] there was no association between MEDAS and erMedDietScore and PAF and Lp-PLA2. The small association between hsCRP and HEIFA, MEDAS and erMedDiet Score was also unexpected due to previous research showing diets consistent with country-specific guidelines and Mediterranean diets were associated with significantly lower levels of this marker [25, 91]. This could be due to the AGHE requiring fewer fruit serves and not prioritising legumes and nuts, both of which are emphasised in the DASH Index and Vegetarian Lifestyle Index, and which showed stronger relationships in the current study. In addition, a previous MEDAS study found that the food groups emphasised in the DASH dietary pattern had the strongest association with CRP [92].
Strengths and limitations
Strengths of this study include the strict exclusion criteria to prevent confounding and the use of validated FFQ and diet quality scores for assessing diet.
However, there were some limitations. Dietary assessment is difficult and prone to error. FFQs overestimate some food groups like fruit and vegetables [93]. Some of the scores did not adjust for energy intake potentially confounding results as higher consumption of energy can result in higher intakes generally and therefore scores. PAF and Lp-PLA2 levels may have been elevated in some of the participants due to the COVID-19 vaccine and/or infection [9]. Further, PAF and Lp-PLA2 may be associated with more severe forms of CVD and this study’s sample only included disease-free individuals.
Conclusion
In conclusion, this study found that higher adherence to several dietary patterns was associated with lower levels of inflammation as measured by hsCRP, with the strongest relationship seen with Vegetarian Lifestyle Index, DASH Index and Dairy-adjusted DASH Index. The novel inflammatory marker findings were unexpected with no association in adjusted models for either Lp-PLA2 or PAF, possibly confounded by COVID-19 infections and/or vaccinations. Future research should aim to examine the relationship with these novel markers and healthy dietary patterns in larger, more diverse samples and in a non-pandemic setting.
Availability of data and materials
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval. Requests should be emailed to the corresponding author.
Change history
27 November 2023
A Correction to this paper has been published: https://doi.org/10.1186/s12986-023-00771-y
Abbreviations
- CVD:
-
Cardiovascular disease
- DASH:
-
Dietary approach to stop hypertension
- erMED:
-
Predimed-Plus Diet Score
- FETA:
-
FFQ EPIC tool for analysis
- HEIFA:
-
Healthy Eating Index for Australian
- hsCRP:
-
High-sensitivity C-reactive protein
- Il-6:
-
Interleukin 6
- Lp-PLA2 :
-
Lipoprotein-associated phospholipase A2
- LysoPC:
-
Lysophophatidylcholine
- MEDAS:
-
Mediterranean diet adherence screener
- oxNEFA:
-
Oxidized, non-esterified fatty acids
- PAF:
-
Platelet-activating factor
- TNF-α:
-
Tumour necrosis factor alpha
References
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease. Circulation. 2003;107(3):499–511.
Li J, Lee DH, Hu J, Tabung FK, Li Y, Bhupathiraju SN, et al. Dietary inflammatory potential and risk of cardiovascular disease among men and women in the U.S. J Am Coll Cardiol. 2020;76(19):2181–93.
Luvián-Morales J, Varela-Castillo FO, Flores-Cisneros L, Cetina-Pérez L, Castro-Eguiluz D. Functional foods modulating inflammation and metabolism in chronic diseases: a systematic review. Crit Rev Food Sci Nutr. 2022;62(16):4371–92.
Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9.
Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, et al. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr. 2014;144(6):881–9.
Luan Y-y, Yao Y-m. The Clinical Significance and Potential Role of C-Reactive Protein in Chronic Inflammatory and Neurodegenerative Diseases. Front Immunol. 2018;9.
Banait T, Wanjari A, Danade V, Banait S, Jain J. Role of high-sensitivity C-reactive protein (Hs-CRP) in non-communicable diseases: a review. Cureus. 2022;14(10): e30225.
Bower JK, Lazo M, Juraschek SP, Selvin E. Within-person variability in high-sensitivity C-reactive protein. Arch Intern Med. 2012;172(19):1519–21.
English CJ, Lohning AE, Mayr HL, Jones M, Reidlinger DP. Interrelationships among platelet-activating factor and lipoprotein-associated phospholipase A2 activity and traditional cardiovascular risk factors. BioFactors. 2022;n/a(n/a).
Handley DA, Arbeeny CM, Lee ML, Van Valen RG, Saunders RN. Effect of platelet activating factor on endothelial permeability to plasma macromolecules. Immunopharmacology. 1984;8(3):137–42.
Satoh K, Imaizumi T, Yoshida H, Hiramoto M, Takamatsu S. Increased levels of blood platelet-activating factor (PAF) and PAF-like lipids in patients with ischemic stroke. Acta Neurol Scand. 1992;85(2):122–7.
Chen H, Zheng P, Zhu H, Zhu J, Zhao L, El Mokhtari NE, et al. Platelet-activating factor levels of serum and gingival crevicular fluid in nonsmoking patients with periodontitis and/or coronary heart disease. Clin Oral Investig. 2010;14(6):629–36.
Zheng G-H, Xiong S-Q, Mei L-J, Chen H-Y, Wang T, Chu J-F. Elevated plasma platelet activating factor, platelet activating factor acetylhydrolase levels and risk of coronary heart disease or blood stasis syndrome of coronary heart disease in chinese: a case control study. Inflammation. 2012;35(4):1419–28.
Detopoulou P, Fragopoulou E, Nomikos T, Antonopoulou S, Kotroyiannis I, Vassiliadou C, et al. Baseline and 6-Week follow-up levels of PAF and activity of its metabolic enzymes in patients with heart failure and healthy volunteers–a pilot study. Angiology. 2013;64(7):522–8.
Corson MA, Jones PH, Davidson MH. Review of the evidence for the clinical utility of lipoprotein-associated phospholipase A2 as a cardiovascular risk marker. Am J Cardiol. 2008;101(12a):41f–50f.
Dennis EA. Allosteric regulation by membranes and hydrophobic subsites in phospholipase A(2) enzymes determine their substrate specificity. J Biol Chem. 2022;298(5):101873.
Cojocaru M, Cojocaru IM, Silosi I. Lipoprotein-associated phospholipase A2 as a predictive biomarker of sub-clinical inflammation in cardiovascular diseases. Maedica (Buchar). 2010;5(1):51–5.
Garza CA, Montori VM, McConnell JP, Somers VK, Kullo IJ, Lopez-Jimenez F. Association between lipoprotein-associated phospholipase A2 and cardiovascular disease: a systematic review. Mayo Clin Proc. 2007;82(2):159–65.
Chung H, Kwon HM, Kim J-Y, Yoon Y-W, Rhee J, Choi E-Y, et al. Lipoprotein-associated phospholipase A2 is related to plaque stability and is a potential biomarker for acute coronary syndrome. Yonsei Med J. 2014;55(6):1507–15.
Hu G, Liu D, Tong H, Huang W, Hu Y, Huang Y. Lipoprotein-associated phospholipase A2 activity and mass as independent risk factor of stroke: a meta-analysis. Biomed Res Int. 2019;2019:1–11.
Perrot N, Thériault S, Rigade S, Chen HY, Dina C, Martinsson A, et al. Lipoprotein-associated phospholipase A2 activity, genetics and calcific aortic valve stenosis in humans. Heart. 2020;106(18):1407–12.
Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis. 2014;24(9):929–39.
Soltani S, Chitsazi MJ, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: A systematic review and meta-analysis of randomized trials. Clin Nutr. 2018;37(2):542–50.
Menzel J, Jabakhanji A, Biemann R, Mai K, Abraham K, Weikert C. Systematic review and meta-analysis of the associations of vegan and vegetarian diets with inflammatory biomarkers. Sci Rep. 2020;10(1):21736.
Reidlinger DP, Darzi J, Hall WL, Seed PT, Chowienczyk PJ, Sanders TA. How effective are current dietary guidelines for cardiovascular disease prevention in healthy middle-aged and older men and women? A randomized controlled trial. Am J Clin Nutr. 2015;101(5):922–30.
English CJ, Mayr HL, Lohning AE, Reidlinger DP. The association between dietary patterns and the novel inflammatory markers platelet-activating factor and lipoprotein-associated phospholipase A2: a systematic review. Nutr Rev. 2022;80(6):1371–91.
Packard CJ, O’Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343(16):1148–55.
Oei HH, van der Meer IM, Hofman A, Koudstaal PJ, Stijnen T, Breteler MM, et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation. 2005;111(5):570–5.
Persson M, Hedblad B, Nelson JJ, Berglund G. Elevated Lp-PLA2 levels add prognostic information to the metabolic syndrome on incidence of cardiovascular events among middle-aged nondiabetic subjects. Arterioscler Thromb Vasc Biol. 2007;27(6):1411–6.
Yokote K, Suzuki A, Li Y, Matsuoka N, Teramoto T. Pharmacokinetics and exploratory efficacy biomarkers of bococizumab, an anti-PCSK9 monoclonal antibody, in hypercholesterolemic Japanese subjects. Int J Clin Pharmacol Ther. 2019;57(12):575–89.
Gajos G, Zalewski J, Mostowik M, Konduracka E, Nessler J, Undas A. Polyunsaturated omega-3 fatty acids reduce lipoprotein-associated phospholipase A(2) in patients with stable angina. Nutr Metab Cardiovasc Dis. 2014;24(4):434–9.
Stojanovic M, Radenkovic M. Omega-3 fatty acids are capable to decrease the lipoprotein-associated phospholipase A2 blood level. Atherosclerosis. 2018;275: e237.
Brilakis ES, Khera A, McGuire DK, See R, Banerjee S, Murphy SA, et al. Influence of race and sex on lipoprotein-associated phospholipase A2 levels: observations from the Dallas Heart Study. Atherosclerosis. 2008;199(1):110–5.
Enkhmaa B, Anuurad E, Zhang W, Pearson TA, Berglund L. Association of Lp-PLA(2) activity with allele-specific Lp(a) levels in a bi-ethnic population. Atherosclerosis. 2010;211(2):526–30.
Lee KK, Fortmann SP, Varady A, Fair JM, Go AS, Quertermous T, et al. Racial variation in lipoprotein-associated phospholipase A2 in older adults. BMC Cardiovasc Disord. 2011;11(1):38.
Detopoulou P, Nomikos T, Fragopoulou E, Panagiotakos DB, Pitsavos C, Stefanadis C, et al. Lipoprotein-associated phospholipase A2 (Lp-PLA2) activity, platelet-activating factor acetylhydrolase (PAF-AH) in leukocytes and body composition in healthy adults. Lipids Health Dis. 2009;8(1):19.
Detopoulou P, Nomikos T, Fragopoulou E, Antonopoulou S. Association of PAF and its metabolic enzymes with GGT and the Fatty Liver Index in healthy volunteers. Curr Vasc Pharmacol. 2021;19(6):663–72.
Antonopoulou S, Tsoupras A, Baltas G, Kotsifaki H, Mantzavinos Z, Demopoulos CA. Hydroxyl-platelet-activating factor exists in blood of healthy volunteers and periodontal patients. Mediators Inflamm. 2003;12(4):221–7.
Detopoulou P, Nomikos T, Fragopoulou E, Antonopoulou S, Kotroyiannis I, Vassiliadou C, et al. Platelet activating factor (PAF) and activity of its biosynthetic and catabolic enzymes in blood and leukocytes of male patients with newly diagnosed heart failure. Clin Biochem. 2009;42(1–2):44–9.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. London: Routledge; 1988.
Brown RE, Randhawa AK, Canning KL, Fung M, Jiandani D, Wharton S, et al. Waist circumference at five common measurement sites in normal weight and overweight adults: Which site is most optimal? Clin Obes. 2018;8(1):21–9.
O’Brien E, Coats A, Owens P, Petrie J, Padfield PL, Littler WA, et al. Use and interpretation of ambulatory blood pressure monitoring: recommendations of the British hypertension society. BMJ. 2000;320(7242):1128–34.
Armstrong T, Bull F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J Public Health. 2006;14(2):66–70.
World Health Organization. Global Physical Activity Questionnaire (GPAQ) analysis guide. Geneva: World Health Organization; 2010.
Riboli E. Nutrition and cancer: background and rationale of the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann Oncol. 1992;3(10):783–91.
Mulligan AA, Luben RN, Bhaniani A, Parry-Smith DJ, O’Connor L, Khawaja AP, et al. A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open. 2014;4(3):e004503.
Food Standards Australia and New Zealand. Australian Food Composition Database, monitoring nutrients in our food supply. 2013.
European Food Safety Authority. Food composition database for nutrient intake: selected vitamins and minerals in selected European countries Zenodo; 2013. Available from: https://zenodo.org/record/438313.
Günther AL, Liese AD, Bell RA, Dabelea D, Lawrence JM, Rodriguez BL, et al. Association between the dietary approaches to hypertension diet and hypertension in youth with diabetes mellitus. Hypertension. 2009;53(1):6–12.
Jakes RW, Day NE, Luben R, Welch A, Bingham S, Mitchell J, et al. Adjusting for energy intake—What measure to use in nutritional epidemiological studies? Int J Epidemiol. 2004;33(6):1382–6.
US Government. DASH Eating Plan. In: National Heart, Lung, and Blood Institute, Editor. 2021.
Bruno RS, Pokala A, Torres-Gonzalez M, Blesso CN. Cardiometabolic health benefits of dairy-milk polar lipids. Nutr Rev. 2021;79(Supplement_2):16–35.
Chiu S, Bergeron N, Williams PT, Bray GA, Sutherland B, Krauss RM. Comparison of the DASH (Dietary Approaches to Stop Hypertension) diet and a higher-fat DASH diet on blood pressure and lipids and lipoproteins: a randomized controlled trial. Am J Clin Nutr. 2016;103(2):341–7.
Le LT, Sabaté J, Singh PN, Jaceldo-Siegl K. The design, development and evaluation of the vegetarian lifestyle index on dietary patterns among vegetarians and non-vegetarians. Nutrients. 2018;10(5).
Samanek AJ, Croager EJ, Gies P, Milne E, Prince R, McMichael AJ, et al. Estimates of beneficial and harmful sun exposure times during the year for major Australian population centres. Med J Aust. 2006;184(7):338–41.
Roy R, Hebden L, Rangan A, Allman-Farinelli M. The development, application, and validation of a Healthy eating index for Australian Adults (HEIFA-2013). Nutrition. 2016;32(4):432–40.
Martínez-González MA, García-Arellano A, Toledo E, Salas-Salvadó J, Buil-Cosiales P, Corella D, et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS ONE. 2012;7(8):e43134.
Martínez-González MA, Buil-Cosiales P, Corella D, Bulló M, Fitó M, Vioque J, et al. Cohort profile: design and methods of the PREDIMED-Plus randomized trial. Int J Epidemiol. 2019;48(2):387–8.
Cohen J. A power primer. Psychol Bull. 1992;112(1):155–9.
Hart MJ, Torres SJ, McNaughton SA, Milte CM. Dietary patterns and associations with biomarkers of inflammation in adults: a systematic review of observational studies. Nutr J. 2021;20(1):24.
Samtiya M, Aluko RE, Dhewa T, Moreno-Rojas JM. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods. 2021;10(4).
Ostrowski SR, Søgaard OS, Tolstrup M, Stærke NB, Lundgren J, Østergaard L, et al. Inflammation and platelet activation after COVID-19 vaccines—Possible mechanisms behind vaccine-induced immune thrombocytopenia and thrombosis. Front Immunol. 2021;12:779453.
Antonopoulou S, Petsini F, Detopoulou M, Theoharides TC, Demopoulos CA. Is there an interplay between the SARS-CoV-2 spike protein and Platelet-Activating factor? BioFactors. 2022.
Yan B, Chu H, Yang D, Sze K-H, Lai P-M, Yuan S, et al. Characterization of the Lipidomic Profile of Human Coronavirus-Infected Cells: Implications for Lipid Metabolism Remodeling upon Coronavirus Replication. Viruses [Internet]. 2019; 11(1).
Li Y, Jiang Y, Zhang Y, Li N, Yin Q, Liu L, et al. Abnormal upregulation of cardiovascular disease biomarker PLA2G7 induced by proinflammatory macrophages in COVID-19 patients. Sci Rep. 2021;11(1):6811.
Dua P, Mishra A, Reeta KH. Lp-PLA2 as a biomarker and its possible associations with SARS-CoV-2 infection. Biomark Med. 2022;16(10):821–32.
Australian Government. COVID-19 vaccination – Geographic vaccination rates – LGA. In: Department of Health and Aged Care, Editor: Commonwealth of Australia; 2022.
Jager KJ, Zoccali C, Macleod A, Dekker FW. Confounding: what it is and how to deal with it. Kidney Int. 2008;73(3):256–60.
Najjar RS, Moore CE, Montgomery BD. Consumption of a defined, plant-based diet reduces lipoprotein(a), inflammation, and other atherogenic lipoproteins and particles within 4 weeks. Clin Cardiol. 2018;41(8):1062–8.
Harishkumar R, Hans S, Stanton JE, Grabrucker AM, Lordan R, Zabetakis I. Targeting the Platelet-Activating Factor Receptor (PAF-R): Antithrombotic and Anti-Atherosclerotic Nutrients. Nutrients. 2022;14(20).
Mulligan AA, Lentjes MAH, Luben RN, Wareham NJ, Khaw KT. Changes in waist circumference and risk of all-cause and CVD mortality: results from the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) cohort study. BMC Cardiovasc Disord. 2019;19(1):238.
Romaguera D, Angquist L, Du H, Jakobsen MU, Forouhi NG, Halkjaer J, et al. Dietary determinants of changes in waist circumference adjusted for body mass index - a proxy measure of visceral adiposity. PLoS ONE. 2010;5(7):e11588.
Battineni G, Sagaro GG, Chintalapudi N, Amenta F, Tomassoni D, Tayebati SK. Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD). Int J Mol Sci. 2021;22(9).
Craddock JC, Neale EP, Peoples GE, Probst YC. Vegetarian-based dietary patterns and their relation with inflammatory and immune biomarkers: a systematic review and meta-analysis. Adv Nutr. 2019;10(3):433–51.
Blumfield M, Mayr H, De Vlieger N, Abbott K, Starck C, Fayet-Moore F, et al. Should We 'Eat a Rainbow'? An Umbrella Review of the Health Effects of Colorful Bioactive Pigments in Fruits and Vegetables. Molecules. 2022;27(13).
Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–27.
Juárez-Chairez MF, Meza-Márquez OG, Márquez-Flores YK, Jiménez-Martínez C. Potential anti-inflammatory effects of legumes: a review. Br J Nutr. 2022;128(11):2158–69.
Yuan M, Singer MR, Pickering RT, Moore LL. Saturated fat from dairy sources is associated with lower cardiometabolic risk in the Framingham Offspring Study. Am J Clin Nutr. 2022;116(6):1682–92.
Schmidt KA, Cromer G, Burhans MS, Kuzma JN, Hagman DK, Fernando I, et al. Impact of low-fat and full-fat dairy foods on fasting lipid profile and blood pressure: exploratory endpoints of a randomized controlled trial. Am J Clin Nutr. 2021;114(3):882–92.
Bordoni A, Danesi F, Dardevet D, Dupont D, Fernandez AS, Gille D, et al. Dairy products and inflammation: a review of the clinical evidence. Crit Rev Food Sci Nutr. 2017;57(12):2497–525.
Lordan R, Tsoupras A, Mitra B, Zabetakis I. Dairy fats and cardiovascular disease: Do we really need to be concerned? Foods. 2018;7(3):29.
Lordan R, Zabetakis I. Invited review: the anti-inflammatory properties of dairy lipids. J Dairy Sci. 2017;100(6):4197–212.
Drouin-Chartier JP, Côté JA, Labonté M, Brassard D, Tessier-Grenier M, Desroches S, et al. Comprehensive review of the impact of dairy foods and dairy fat on cardiometabolic risk. Adv Nutr. 2016;7(6):1041–51.
Vors C, Joumard-Cubizolles L, Lecomte M, Combe E, Ouchchane L, Drai J, et al. Milk polar lipids reduce lipid cardiovascular risk factors in overweight postmenopausal women: towards a gut sphingomyelin-cholesterol interplay. Gut. 2020;69(3):487.
Anto L, Warykas SW, Torres-Gonzalez M, Blesso CN. Milk Polar Lipids: Underappreciated Lipids with Emerging Health Benefits. Nutrients [Internet]. 2020; 12(4).
U.S. Department of Agriculture and U.S. Department of. Health and Human Services. Dietary Guidelines for Americans, 2020–2025.
National Health and Medical Research Council. Australian dietary guidelines. Canberra: NHMRC; 2013.
UK Government. Eatwell Guide.
Nomikos T, Fragopoulou E, Antonopoulou S, Panagiotakos DB. Mediterranean diet and platelet-activating factor; a systematic review. Clin Biochem. 2018;60:1–10.
Lordan R, Tsoupras A, Zabetakis I. Phospholipids of animal and marine origin: structure, function, and anti-inflammatory properties. Molecules. 2017;22(11).
Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: the Attica study. J Am Coll Cardiol. 2004;44(1):152–8.
Lahoz C, Castillo E, Mostaza JM, de Dios O, Salinero-Fort MA, González-Alegre T, et al. Relationship of the adherence to a Mediterranean diet and its main components with CRP levels in the Spanish Population. Nutrients. 2018;10(3).
Michels KB, Welch AA, Luben R, Bingham SA, Day NE. Measurement of fruit and vegetable consumption with diet questionnaires and implications for analyses and interpretation. Am J Epidemiol. 2005;161(10):987–94.
Radtke MD, Poe M, Stookey J, Jilcott Pitts S, Moran NE, Landry MJ, et al. Recommendations for the Use of the Veggie Meter® for Spectroscopy-Based Skin Carotenoid Measurements in the Research Setting. Curr Develop Nutr. 2021;5(8):nzab104.
Acknowledgements
We acknowledge Kawther al-Tamimi and Malika Abdul who were involved in the preliminary calculation of diet scores.
Funding
C.J.E. was supported by an Australian Government Research Training Program Scholarship.
Author information
Authors and Affiliations
Contributions
CJE and DPR conceived the study and collected the data. CJE and AEL performed the laboratory analyses. MJ and CJE analysed the data. CJE, DPR, HLM and HM analysed the dietary data. CJE wrote the initial draft of the manuscript. All authors interpreted the data and critically reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol was approved by the Bond University Human Research Ethics Committee (approval DR03194) and conforms to the ethical guidelines of the 1975 (revised in 1983) Declaration of Helsinki.
Consent to participate
All participants provided written informed consent.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: the sentence and table 1 have been updated.
Supplementary Information
Additional file 1
. Dietary intake scoring details, flow of study participants, and table of unadjusted values for biomarker concentrations according to quartile of diet score.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
English, C.J., Lohning, A.E., Mayr, H.L. et al. The association between dietary quality scores with C-reactive protein and novel biomarkers of inflammation platelet-activating factor and lipoprotein-associated phospholipase A2: a cross-sectional study. Nutr Metab (Lond) 20, 38 (2023). https://doi.org/10.1186/s12986-023-00756-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-023-00756-x