Abstract
Current evidence suggests that non-traditional serum lipid ratios are more effective than traditional serum lipid parameters in predicting vascular diseases, and both of them are associated with dietary patterns. Therefore, this study aimed to investigate the relationship between the dietary inflammatory index (DII) and atherogenic indices using traditional serum lipid parameters (triglyceride (TG), total cholesterol (TC), LDL cholesterol (LDL–c), high-density lipoprotein cholesterol (HDL–c)) and non-traditional serum lipid ratios (atherogenic index of plasma (AIP), Castelli's index-I (CRI_I), Castelli's index-II (CRI_II), the lipoprotein combination index (LCI), and the atherogenic coefficient (AC)). Basic information from the Ravansar Non-Communicable Diseases cohort study was utilized in the present cross-sectional observational study. The study included 8870 adults aged 35–65 years. A validated food frequency questionnaire (FFQ) was used to measure DII. We compared the distributions of outcomes by DII score groups using multivariable linear regression. The difference between DII score groups was evaluated by the Bonferroni test. The mean ± SD DII was − 2.5 ± 1.43, and the prevalence of dyslipidemia was 44%. After adjusting for age, sex, smoking status, alcohol consumption status, physical activity, systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting blood sugar (FBS), body mass index (BMI) and socioeconomic status (SES), participants in the highest quartile of DII had a greater risk for CRI_I (β = 0.11, CI 0.05, 0.18), CRI_II (β = 0.06, CI 0.01, 0.11), LCI (β = 0.11, CI 288.12, 8373.11), AC (β = 0.11, CI 0.05, 0.17) and AIP (β = 0.06, CI 0.02, 0.10). Moreover, according to the adjusted logistic regression model, the risk of dyslipidemia significantly increased by 24% (OR: 1.24, 95% CI 1.08–1.41), 7% (OR: 1.07, 95% CI 0.94, 1.21) and 3% (OR: 1.03, 95% CI 0.91, 1.16) in Q4, Q3 and Q2 of the DII, respectively. Finally, diet-related inflammation, as estimated by the DII, is associated with a higher risk of CRI-I, CRI-II, LCI, AC, and AIP and increased odds of dyslipidemia.
Similar content being viewed by others
Introduction
Dyslipidemia, characterized by an imbalance in lipid profiles, is a recognized factor in the development of atherosclerosis and cardiovascular diseases (CVDs)1. The World Health Organization (WHO) estimates that CVDs are a major cause of mortality, with 7.9 million deaths annually2. Furthermore, the global prevalence of lipid profile abnormalities is increasing worldwide3. Elevated levels of low-density lipoprotein cholesterol (LDL-C) and a decrease in high-density lipoprotein (HDL-C) cholesterol result in the accumulation of plaque throughout the arteries and increase atherosclerotic CVD risk4. Alongside these prime parameters, lipoprotein ratios such as total-C/HDL-C and LDL-C/HDL-C and the logarithm of the triglyceride (TG)/HDL-C ratio have greater predictive capacity for CVD incidence and the severity of atherosclerosis5,6. Atherosclerosis is attributed to several inflammatory cells and factors throughout the initiation, progression and formation of atherosclerotic plaques4,7.
Previous evidence indicates that the intake of various dietary agents can lead to the modulation and attenuation of systemic inflammation8,9,10,11. Indeed, certain dietary patterns, such as Mediterranean and prudent diets or high loads of dietary intake of fruits, vegetables, whole grains, polyunsaturated fatty acids, fiber, and nonnutritive compounds (e.g., polyphenols), were inversely associated with biomarkers of inflammation and endothelial activation, such as interleukin-18 (IL-18), fibrinogen, C-reactive protein (CRP), interleukin-6 (IL-6), and cell adhesion molecules, which can lead to CVD and atherosclerosis12,13,14. In contrast, Western dietary patterns with high loadings of saturated fatty acids, red meat, processed meat, dessert, beer, sugar-sweetened beverages and low-fiber foods were positively related to inflammatory biomarkers12. The Dietary Inflammatory Index (DII) was designed as an applicable diet-related, literature-based tool in human populations15. The DII evaluates the overall inflammatory potential of dietary components, such as macronutrients, micronutrients, phytochemicals, and other dietary components, based on pro- and anti-inflammatory aspects16.
In recent years, there have been several Umbrella reviews of systematic reviews and meta-analyses of observational studies of links between noncommunicable diseases and all causes of mortality with DII. Higher DII scores were related to harmful effects on health outcomes16,17,18,19. However, few studies have investigated the association between DII and lipoprotein ratios in developing Middle Eastern countries20,21. Frequent studies have shown that lipoprotein ratios are a greater predictor of cardiovascular risk than lipid profiles are in developing countries22,23,24. Moreover, lipoprotein ratios better reflect metabolic and clinical interactions with lipid fractions. To reduce the economic and clinical burden of dyslipidemia, dietary modifications may be a crucial strategy. The DII can be used to evaluate overall inflammation related to nutritional patterns. This study aimed to examine whether the DII score is related to dyslipidemia and atherogenic risk.
Methods and materials
Data source and study population
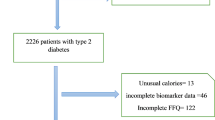
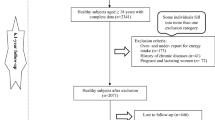
We carried out an analysis using data from 10,047 adults aged 35–65 years who participated in the Ravansar Noncommunicable Diseases Cohort Study (RaNCD) as a part of the Prospective Epidemiological Research Studies in Iran (PERSIAN). The present cross-sectional study included 4030 men and 4840 women and was started in November 2014 until February 2017. The study design, objectives, and procedures of the RaNCD study have been published elsewhere before25,26,27. In this observational study, data were retrieved from adults who completed the dietary interview, and the exclusion criteria were as follows: individuals with energy intake above 4200 and less than 800 kcal per day (n = 814), cancer (n = 83), pregnancy (n = 138), and incomplete information (n = 142). Overall, 8870 participants were included in the study (Fig. 1).
Dietary data and dietary inflammatory index calculations
In total, 118 food items were assessed by the validated food frequency questionnaire based on portion size and daily, weekly, monthly, or annual food consumption through face-to-face interviews. The questionnaire was previously validated in the Iranian population28. We assessed the inflammatory potential of the whole diet by DII scores according to the recent method proposed by Shivappa et al.15. The DII is a score-based algorithm that scores 45 dietary constituents in the range of –1 (maximally anti-inflammatory) to + 1 (maximally proinflammatory). The DII evaluates the inflammatory status of the whole diet by predicting the levels of specific inflammatory and anti-inflammatory markers, including C-reactive protein (CRP), interleukin-1 (IL-1), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), and tumor necrosis factor α (TNF-α). Similarly, in this study, the DII was calculated using a total of 31 available food parameters from the FFQ of the 45 possible food variables composing the DII: retinol, beta-carotene, pyridoxine, cobalamin, ascorbic acid, calciferol, tocopherol, folic acid, niacin, thiamine, riboflavin, iron, zinc, selenium, magnesium, omega 3, omega 6, total fat, saturated fats (SFAs), cholesterol, monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs), fiber, protein, total fat, carbohydrate and energy, caffeine, onion, garlic, and tea.
Lipid profile measurements
The traditional and nontraditional lipid profile parameters used for atherogenic indices included triglyceride (TG), total cholesterol (TC), LDL cholesterol (LDL–c), high-density lipoprotein cholesterol (HDL–c), the atherogenic index of plasma (AIP), Castelli's index-I (CRI_I), Castelli's index-II (CRI_II), the lipoprotein combination index (LCI), and the atherogenic coefficient (AC).
Lipid profile measurements have been previously described in detail27. Lipoprotein ratio measurements were calculated as AIP: log (TG/HDL–C)29, AC: ((TC- HDL–C)/HDL–C)30, CRI-I: (TC/HDL–C)31, CRI-II: (LDL–C/HDL–C) and LCI: (TC*TG*LDL-C)/HDL-C31. Dyslipidemia was defined as LDL-C ≥ 160 mg/dL and/or TC ≥ 240 mg/dL and/or HDL-C < 40 mg/dL and/or TG > 200 mg/dL32.
Anthropometric and physical activity data
Weight, height, body mass index (BMI), waist-to-hip ratio (WHR), visceral fatty acid (VFA), and percentage of body fat (PBF) were collected and analyzed in this study. The anthropometric data were measured using an automated bioelectric impedance machine (In Body 770, BIOSPACE KOREA). Physical activity levels were evaluated according to the PERSIAN cohort self-reported questionnaire, and participants' responses were measured in terms of the metabolic equivalent of task per hour per day (MET/h per day), following a methodology from a separate study25. Physical activity was categorized into three levels: low (24–36.5 MET/hour per day), moderate (36.6–44.4 MET/hour per day) and high (≥ 44.5 MET/hour per day)25,33.
Other variables
In the present study, the sociodemographic information included sex, age, region (rural or urban), socioeconomic status (poor, middle, or rich) and alcohol consumption (yes or no). Patients were divided into four groups according to their self-reported smoking status: current smokers, former smokers, passive smokers, and nonsmokers. Blood pressure and fasting blood glucose levels were measured.
Statistical analysis
All the statistical analyses were performed using the software STATA version 14.2 (Stata Corp., College Station, TX, USA). ANOVA and chi-square tests were used to determine the significance of differences between continuous variables and categorical variables across quartiles of DII scores. To compare the differences in the continuous variables among four DII score groups, Bonferroni (post-hoc test) was used after one-way analysis of variance, while categorical variables were compared using the χ2 test. The associations between DII scores and dyslipidemia were determined via logistic regression models, while the relationships between DII scores and atherogenic risk scores were determined via linear regression models. P values < 0.05 and 95% confidence intervals (CIs) were considered to indicate statistical significance.
Ethics approval and consent to participate
The study was approved by the ethics committee of Kermanshah University of Medical Sciences (IR.KUMS.REC.1401.560). All methods were carried out in accordance with relevant guidelines and regulations. All the participants provided oral and written informed consent.
Results
The demographic and socioeconomic characteristics of our participants are shown in Table 1. The mean age of the participants at baseline was 47.49 ± 8.23 years. Fifty-four percent of the participants were female. The prevalence of dyslipidemia was 44%. The means ± SDs of LDL-c, HDL-c, TC and TG were 111.63 ± 31.33 (mg/dl), 46.60 ± 11.37 (mg/dl), 185.59 ± 37.73 (mg/dl) and 136.82 ± 81.64 (mg/dl), respectively. The mean ± SD DII was − 2.5 ± 1.43. The participants with dyslipidemia had greater pro-inflammatory scores (-2.42 ± 1.46) than those with a normal lipid profile (-2.57 ± 1.41) (P < 0.001). Participants with dyslipidemia reported having a greater BMI, WHR, VFA, SBP and DBP (P < 0.001). Moreover, those in the dyslipidemia category were more likely to have higher CRI_I (4.89 ± 1.06 vs. 3.58 ± 0.73) and CRI_II (2.92 ± 0.84 vs. 2.17 ± 0.62) scores than were those in the non-dyslipidemia category (P < 0.001), as were those in the LCI (108,769.8 ± 84,434.68 vs. 44,135.68 ± 28,228.21), AIP (1.41 ± 0.56 vs. 0.62 ± 0.46) and AC (3.88 ± 1.06 vs. 0.62 ± 0.46).
As shown in Table 2, in the highest quartile of the DPI, the intake of saturated fat, dairy products, calcium, and selenium was lower than that in the lowest quartile (P < 0.001). Conversely, individuals in the higher DII quartile had significantly greater intakes of energy, protein, MUFAs, PUFAs, whole grains, fruits, vegetables, red and white meat, legumes, eggs, fibers, nuts, vitamin E, vitamin A, vitamin D, vitamin K, vitamin C, vitamin B6, vitamin B12, magnesium, zinc, and iron (P < 0.001). Additionally, there were no statistically significant differences in carbohydrate or fat consumption across the DII quartiles. Moreover, the difference between DII score groups was evaluated by the Bonferroni test.
The lipid profile and atherogenic indices of the participants across DII quartiles are shown in Table 3 and the difference between DII score groups was evaluated by Bonferroni test.
A higher DII was related to higher TG, CRI_I, CRI_II, LCI, AC and AIP (P < 0.001) in patients in the highest DII quartiles than in those in the lowest DII quartile. Participants in the upper quartile had lower levels of high-density lipoprotein cholesterol (HDL-C) and total cholesterol (TC) than those in the lower quartile.
There was a direct association between DII and the risk of prevalent dyslipidemia according to the crude odds ratios (ORs) (Table 4).
After adjustment for age and sex, a higher DII was associated with a greater likelihood of dyslipidemia. The corresponding odds ratios were 1.32 (95% CI 1.16, 1.50), 1.11 (95% CI 0.098, 1.25) and 1.04 (95% CI (0.92, 1.17)) for the fourth, third and second quintiles of the DII, respectively, compared with the first. According to the fully adjusted logistic regression models and after adjustment for participant age, sex, smoking status, alcohol consumption status, physical activity, SBP, DBP, FBS, BMI and SES, the risk of dyslipidemia significantly increased by 24% (OR: 1.24, 95% CI 1.08–1.41), 7% (OR: 1.07, 95% CI 0.94, 1.21) and 3% (OR: 1.03, 95% CI 0.91, 1.16) in quartile 4, quartile 3 and quartile 2 of the DII, respectively, compared with the first quartile.
We also analyzed the linear relationship between DII and atherogenic indices (Table 5). The linear regression model showed that the DII was positively associated with atherogenic indices (CRI_I, CRI_II, LCI, AC and AIP). Indeed, in the highest DII quartile, atherogenic indices were significantly greater than those in the lowest quartile according to both unadjusted and adjusted models.
A DII in the fourth quartile compared with the first quartile was positively associated with a higher risk of CRI_I (β = 0.11, CI 0.05, 0.18), CRI_II (β = 0.06, CI 0.01, 0.11), LCI (β = 0.11, CI 288.12, 8373.11), AC (β = 0.11, CI 0.05, 0.17) and AIP (β = 0.06, CI 0.02, 0.10) according to the fully adjusted linear regression model.
Discussion
The results revealed that there was direct bonding between the DII score and dyslipidemia risk (based on crude ORs), and dyslipidemia risk (after adjustment for potentially confounding factors) significantly increased by 3%, 7% and 24% in Q2, Q3 and Q4 of the DII, respectively, compared with the first quartile. In addition, the DII was positively associated with atherogenic indices. A DII in the fourth quartile was positively associated with a higher risk of CRI_I, CRI_II, LCI, AC and AIP than was a DII in the first quartile.
Investigations of adults with metabolic syndrome34,35 or atherosclerosis21, children36,37, elderly individuals38,39 and overweight or obese women40,41 have also shown that following a diet with a high DII may significantly increase dyslipidemia, which is in line with our findings. A survey of the elderly population in Taiwan reported that the inflammatory dietary pattern in the male group was linked to a 20% increase in dyslipidemia risk, while in the female group, it showed a borderline rising trend in the index studied (OR: 1.12, 95% CI 0.99–1.26, p = 0.052)42.
Furthermore, a survey of Mexican adults demonstrated that an inflammatory diet could considerably increase hypertriglyceridemia risk (HRQ4 vs Q1 = 2.28; 95% CI 1.13, 4.57; P-trend = 0.01)43.
A cohort study of elderly individuals (55 years and older) over 5 years also demonstrated that there was a direct relationship between a diet with a higher DII and increasing plasma triglyceride levels, especially in people with abdominal obesity (per score increment: 1.62%, 95% CI 0.58–2.76%; pFDR = 0.01)44.
The bulk of evidence in the literature indicates that habitual intake of an inflammatory diet is directly related to unfavorable lipid profiles as well as an increase in the level of inflammatory indicators, which could lead to elevated metabolic syndrome risk45.
A systematic review of the relationship between DII score and lipid profiles revealed that there was a direct association between the dietary inflammatory index score and plasma triglyceride levels in healthy subjects46. Although the outcomes of the abovementioned investigations confirmed the correlation between DII score and dyslipidemia incidence, nontraditional lipid indices have not been fully investigated. Therefore, additional studies of various age groups with different genetic backgrounds are necessary to investigate this correlation.
On the other hand, an investigation of individuals with metabolic syndrome revealed that increasing the DII had no significant effect on blood HDL-C levels47. Moreover, studies on individuals with type 2 diabetes have shown that adherence to a diet with a higher inflammatory index score has no significant relationship with HDL or triglyceride levels48,49. Moreover, a study of women with diabetes did not reveal a significant connection between following a proinflammatory diet and improving LDL-C/HDL-C50.
The present study emphasizes the correlation between DII score and atherogenic risk. Research on children has revealed that dietary inflammation, as evaluated by the C-DII, is directly related to atherogenic risk51,52. Additionally, studies on Dutch elderly individuals highlighted that atherogenic indices were more desirable in people fed anti-inflammatory diets39. Research on healthy adults45,53, children and adolescents54,55 has also emphasized the direct relationship between DII scores and atherogenic risk. In contrast, a study conducted by Behbahani et al. showed no significant relationship between the dietary DII score and atherogenic risk21.
However, further studies are needed to assess the interdependency of DII scores with dyslipidemia and atherogenic risk in individuals with different levels of inflammatory indices as well as different disorders.
In the present study, individuals with the highest DII score (fourth quartile) had significantly greater intake of energy and less intake of selenium, dairy products, and calcium. (Compared to the lowest level, P value ≤ 0.001). Studies have revealed that increased energy intake, especially at the end of the day (simultaneously with a decrease in insulin sensitivity that has a daily rhythm), may lead to a decrease in lipoprotein lipase activity and ultimately could increase plasma LDL cholesterol levels. However, additional studies are needed to investigate the link between energy consumption patterns and the studied complications56.
It should be noted that Selenium intake is necessary for physiological processes (in combination with certain proteins called selenoproteins). Hence, consuming an appropriate amount could play an effective role in improving plasma triglyceride and cholesterol levels57,58.
Selenium increases 5-deoxy prostaglandin J2A production, which is shown in the legend. It is known for its role as a peroxisome proliferator-activated receptor (PPAR-γ). Research has shown that its activity can reduce cholesterol synthesis by decreasing the sterol regulatory element-binding protein-2 (SREBP-2) concentration59,60.
In addition, a low selenium level reduces the expression of HMG-COA reductase (an enzyme necessary for cholesterol synthesis), which subsequently leads to a rise in the concentration of lipids that breakdown in the bloodstream. Additionally, studies have shown a positive correlation between selenium levels in erythrocytes and cardiovascular risk parameters61,62,63.
In addition, it is assumed that there is a direct correlation between dietary calcium intake and lipid profile improvement64. The beneficial effects of calcium on ameliorating serum lipids and lipoprotein concentrations can be justified through various mechanisms, including reducing the absorption of fatty acids and increasing their excretion through feces (by forming insoluble calcium-fatty soaps in the gut, the ability to bond with bile acids, and boosting the conversion of cholesterol into bile acids, which leads to increased cholesterol excretion)65,66. Moreover, intracellular calcium promotion in hepatocytes could stimulate microsomal triacylglycerol transfer protein, which is involved in VLDL synthesis and excretion from hepatocytes64. If dietary calcium intake is limited, calcitrophic hormones increase calcium intake from adipocyte cells, which ultimately leads to a decrease in calcium in adipocytes and a subsequent increase in lipolysis67.
According to recent studies, dairy products are rich sources of calcium and other nutrients, including proteins and fatty acids, and there seems to be an inverse relationship between dairy product consumption and the investigated indicators (dyslipidemia and atherogenic risk)68. Dairy products contain whey proteins (including whey and casein), which are regarded as crucial factors for improving lipid profiles and reducing CVD risk (through reducing postprandial triglycerides)69. Although these products are rich in saturated fatty acids, monounsaturated fatty acids (such as oleic acid) constitute approximately 25% of the fat content in these products and may improve lipid profiles through insulin resistance and limitation pathways70. Moreover, the high mineral content in dairy products (calcium, potassium and magnesium) is related to their effectiveness in improving lipid profiles and reducing atherogenic risk71,72.
Strengths and limitations
This study has several noteworthy strengths. First, the sample size was large, providing greater statistical power to detect associations. Second, confounding factors were adjusted for in the analysis, which enhances the validity of the findings. Moreover, this study is the first to investigate the relationship between nontraditional lipid profiles and diet, providing novel insights into the potential impact of dietary habits on lipid metabolism. Despite its strengths, there are some limitations. First, the causal relationships cannot be inferred because of the cross-sectional design of the current study, which means more prospective studies are needed to establish the results conclusively. Second, dietary intake was assessed by food-frequency questionnaire (FFQ), hence we could not deny recall bias and misclassification of study participants. Additionally, owing to the questionnaire setting, only 31 out of 45 parameters were extracted from FFQ. However, a previous study found that there would be no drop-off in the predictive ability of the DII if only 28 parameters were used in predictive ability73. As a result, conducting further well-designed longitudinal studies while tackling these limitations is highly recommended.
In summary, the study findings showed that adherence to a diet with a high inflammatory score increases the atherogenic indices.
Data availability
The data sets generated during this study are available from the corresponding author upon reasonable request via email.
References
Pappan, N. & Rehman, A. Dyslipidemia (StatPearls Publishing, 2022).
World Health Organization. World Health Statistics 2018: Monitoring Health for the SDGs, Sustainable Development Goals (World Health Organization, 2018).
Pirillo, A., Casula, M., Olmastroni, E., Norata, G. D. & Catapano, A. L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 18(10), 689–700 (2021).
Zhu, Y. et al. Research progress on the relationship between atherosclerosis and inflammation. Biomolecules 8(3), 80 (2018).
Millán, J. et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. https://doi.org/10.2147/VHRM.S6269 (2009).
Wu, J., Zhou, Q., Wei, Z., Wei, J. & Cui, M. Atherogenic index of plasma and coronary artery disease in the adult population: A meta-analysis. Front. Cardiovasc. Med. 8, 817441 (2021).
Hertiš Petek, T., Petek, T., Močnik, M. & Marčun, V. N. Systemic inflammation, oxidative stress and cardiovascular health in children and adolescents: A systematic review. Antioxidants 11(5), 894 (2022).
Aleksandrova, K., Koelman, L. & Rodrigues, C. E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 42, 101869 (2021).
Pirouzeh, R. et al. Effect of DASH diet on oxidative stress parameters: A systematic review and meta-analysis of randomized clinical trials. Diabetes Metab. Syndrome Clin. Res. Rev. 14(6), 2131–2138 (2020).
Deng, F. E., Shivappa, N., Tang, Y., Mann, J. R. & Hebert, J. R. Association between diet-related inflammation, all-cause, all-cancer, and cardiovascular disease mortality, with special focus on prediabetics: Findings from NHANES III. Eur. J. Nutr. 56, 1085–1093 (2017).
Hlebowicz, J. et al. Food patterns, inflammation markers and incidence of cardiovascular disease: The Malmö Diet and Cancer study. J. Intern. Med. 270(4), 365–376 (2011).
Barbaresko, J., Koch, M., Schulze, M. B. & Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 71(8), 511–527 (2013).
Bonaccio, M. et al. Mediterranean diet, dietary polyphenols and low grade inflammation: Results from the MOLI-SANI study. Br. J. Clin. Pharmacol. 83(1), 107–113 (2017).
Nettleton, J. A. et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 83(6), 1369–1379 (2006).
Shivappa, N., Steck, S. E., Hurley, T. G., Hussey, J. R. & Hébert, J. R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 17(8), 1689–1696 (2014).
Cavicchia, P. P. et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 139(12), 2365–2372 (2009).
Farazi, M., Jayedi, A. & Shab-Bidar, S. Dietary inflammatory index and the risk of non-communicable chronic disease and mortality: An umbrella review of meta-analyses of observational studies. Crit. Rev. Food Sci. Nutr. 63(1), 57–66 (2023).
Liu, F.-H. et al. Dietary inflammatory index and health outcomes: An umbrella review of systematic review and meta-analyses of observational studies. Front. Nutr. 8, 647122 (2021).
Marx, W. et al. The dietary inflammatory index and human health: An umbrella review of meta-analyses of observational studies. Adv. Nutr. 12(5), 1681–1690 (2021).
Ayeneh Pour, A. et al. Association of Dietary Inflammatory Index with cardiovascular disease in Kurdish adults: Results of a prospective study on Ravansar non-communicable diseases. BMC Cardiovasc. Disorders 20, 1–8 (2020).
Behbahani, H. B. et al. The Dietary Inflammatory Index is positively associated with cardiometabolic risk parameters in atherosclerosis patients. Nutr. Res. 107, 26–36 (2022).
Fernández-Macías, J. C., Ochoa-Martínez, A. C., Varela-Silva, J. A. & Pérez-Maldonado, I. N. Atherogenic index of plasma: Novel predictive biomarker for cardiovascular illnesses. Arch. Med. Res. 50(5), 285–294 (2019).
Zhu, L. et al. Lipoprotein ratios are better than conventional lipid parameters in predicting coronary heart disease in Chinese Han people. Kardiologia Polska (Polish Heart J.) 73(10), 931–938 (2015).
Collaboration, A. P. C. S. A comparison of lipid variables as predictors of cardiovascular disease in the Asia Pacific region. Ann. Epidemiol. 15(5), 405–413 (2005).
Poustchi, H. et al. Prospective epidemiological research studies in Iran (the PERSIAN Cohort Study): Rationale, objectives, and design. Am. J. Epidemiol. 187(4), 647–655 (2018).
Eghtesad, S. et al. The PERSIAN cohort: Providing the evidence needed for healthcare reform. Arch. Iran. Med. 20(11), 691–695 (2017).
Pasdar, Y. et al. Cohort profile: Ravansar Non-Communicable Disease cohort study: The first cohort study in a Kurdish population. Int. J. Epidemiol. 48(3), 682–683 (2019).
Mirmiran, P., Esfahani, F. H., Mehrabi, Y., Hedayati, M. & Azizi, F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 13(5), 654–662 (2010).
Dobiasˇova, M. Atherogenic Index of Plasma [log (triglycerides/HDL-cholesterol)]: Theoretical and Practical Implications 1113–1115 (Oxford University Press, 2004).
Abid, H., Abid, Z. & Abid, S. Atherogenic indices in clinical practice and biomedical research: A short review. Baghdad J. Biochem. Appl. Biol. Sci. 2(02), 60–70 (2021).
Castelli, W. P., Abbott, R. D. & McNamara, P. M. Summary estimates of cholesterol used to predict coronary heart disease. Circulation 67(4), 730–734 (1983).
Rezaei, M., Fakhri, N., Pasdar, Y., Moradinazar, M. & Najafi, F. Modeling the risk factors for dyslipidemia and blood lipid indices: Ravansar cohort study. Lipids Health Dis. 19(1), 1–8 (2020).
Jetté, M., Sidney, K. & Blümchen, G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin. Cardiol. 13(8), 555–565 (1990).
Kim, H.-Y., Lee, J. & Kim, J. Association between dietary inflammatory index and metabolic syndrome in the general Korean population. Nutrients 10(5), 648 (2018).
Neufcourt, L. et al. Prospective association between the dietary inflammatory index and metabolic syndrome: Findings from the SU. VI. MAX study. Nutr. Metab. Cardiovasc. Dis. 25(11), 988–996 (2015).
Rauber, F., Campagnolo, P. D., Hoffman, D. J. & Vitolo, M. R. Consumption of ultra-processed food products and its effects on children’s lipid profiles: A longitudinal study. Nutr. Metab. Cardiovasc. Dis. 25(1), 116–122 (2015).
Wang, Y., Armijos, R. X. & Weigel, M.-M. Dietary inflammatory index and cardiometabolic risk in Ecuadorian school-age children. J. Am. Nutr. Assoc. 42(6), 618–627 (2023).
Zhao, Q. et al. The Relationship between the dietary inflammatory index (DII) and metabolic syndrome (MetS) in middle-aged and elderly individuals in the United States. Nutrients 15(8), 1857 (2023).
Szypowska, A., Regulska-Ilow, B., Zatońska, K. & Szuba, A. Comparison of intake of food groups based on dietary inflammatory index (DII) and cardiovascular risk factors in the middle-age population of lower Silesia: Results of the PURE Poland study. Antioxidants 12(2), 285 (2023).
ElhamKia, M. et al. The interaction between dietary total antioxidant capacity and MC4R gene and HOMA-IR in metabolically healthy and unhealthy overweight and obese women. Nutr. Metab. Insights 15, 11786388221105984 (2022).
Yarizadeh, H. et al. The interaction between the dietary inflammatory index and MC4R gene variants on cardiovascular risk factors. Clin. Nutr. 40(2), 488–495 (2021).
Kurniawan, A. L., Hsu, C.-Y., Rau, H.-H., Lin, L.-Y. & Chao, J. C. Inflammatory dietary pattern predicts dyslipidemia and anemia in middle-aged and older Taiwanese adults with declined kidney function: A cross-sectional population study from 2008 to 2010. Nutrients 11(9), 2052 (2019).
Shu, L., Zhao, Y., Shen, Y., Zhang, J. & Li, L. The dietary inflammatory index and metabolic health of population-based Chinese elderly. Asia Pacific J. Clin. Nutr. 31(2), 305–311 (2022).
Chuang, S.-C. et al. Dietary inflammatory patterns are associated with serum TGs and insulin in adults: A community-based study in Taiwan. J. Nutr. 153(6), 1783–1792 (2023).
Phillips, C. M., Shivappa, N., Hébert, J. R. & Perry, I. J. Dietary inflammatory index and biomarkers of lipoprotein metabolism, inflammation and glucose homeostasis in adults. Nutrients 10(8), 1033 (2018).
Vajdi, M., Farhangi, M.A., Mahmoudi-Nezhad, M. Dietary inflammatory index significantly affects lipids profile among adults: An updated systematic review and meta-analysis. Int. J. Vitam. Nutr. Res. (2020).
Sokol, A. et al. Association between the dietary inflammatory index, waist-to-hip ratio and metabolic syndrome. Nutr. Res. 36(11), 1298–1303 (2016).
Karimi, E. et al. A personalised diet study: The interaction between ApoA2− 265T> C polymorphism and dietary inflammatory index on oxidative and inflammatory markers and lipid profile in patients with type 2 diabetes mellitus: A cross-sectional study. Int. J. Clin. Pract. 75(7), e14178 (2021).
Mohamadinarab, M., Yekaninejad, M. S., Siassi, F. & Koohdani, F. Association between dietary inflammatory index and lipid profiles with consideration of Apo B Ins/Del SNP in type 2 diabetic patients. Meta Gene 26, 100811 (2020).
Mohamadi Narab, M., Siassi, F., Koohdani, F. The association between dietary inflammatory pattern and body weight, lipid profile in Iranian diabetic adults. (2020).
Suhett, LG. Children’s Dietary Inflammatory Index: Association with sociodemographic and behavioral factors, cardiometabolic risk, and inflammatory markers (PASE study). (2021).
Suhett, L. G. et al. Dietary inflammatory index scores are associated with atherogenic risk in Brazilian schoolchildren. Public Health Nutr. 24(18), 6191–6200 (2021).
Mazidi, M. et al. Dietary inflammatory index and cardiometabolic risk in US adults. Atherosclerosis 276, 23–27 (2018).
Correa-Rodríguez, M. et al. Dietary inflammatory index and cardiovascular risk factors in Spanish children and adolescents. Res. Nurs. Health 41(5), 448–458 (2018).
Lydakis, C. et al. Correlation of blood pressure, obesity, and adherence to the Mediterranean diet with indices of arterial stiffness in children. Eur. J. Pediatr. 171, 1373–1382 (2012).
Song, X. et al. Trajectories of energy intake distribution and risk of dyslipidemia: Findings from the China health and nutrition survey (1991–2018). Nutrients 13(10), 3488 (2021).
Al-Mubarak, A. A. et al. High selenium levels associate with reduced risk of mortality and new-onset heart failure: Data from PREVEND. Eur. J. Heart Fail. 24(2), 299–307 (2022).
Hasani, M. et al. Effect of selenium supplementation on lipid profile: A systematic review and meta-analysis. Hormone Metab. Res. 50(10), 715–727 (2018).
Khalil, H. S., Mansour, A. T., Goda, A. M. A. & Omar, E. A. Effect of selenium yeast supplementation on growth performance, feed utilization, lipid profile, liver and intestine histological changes, and economic benefit in meagre, Argyrosomus regius, fingerlings. Aquaculture 501, 135–143 (2019).
Assarzadeh, S., Vahdat, S., Seirafian, S., Pourfarzam, M. & Badri, S. Effect of selenium supplementation on lipid profile, Anemia, and inflammation indices in Hemodialysis Patients. J. Res. Pharmacy Pract. 11(3), 103 (2022).
Rad, E. Y. et al. Effect of selenium supplementation on lipid profile levels: An updated systematic review and meta-analysis of randomized controlled clinical trials. Obesity Med. 15, 100113 (2019).
Huang, Y. et al. Association of circulating selenium concentration with dyslipidemia: Results from the NHANES. J. Trace Elem. Med. Biol. 58, 126438 (2020).
Liu, A. et al. High serum concentration of selenium, but not calcium, cobalt, copper, iron, and magnesium, increased the risk of both hyperglycemia and dyslipidemia in adults: A health examination center based cross-sectional study. J. Trace Elem. Med. Biol. 59, 126470 (2020).
Mulet-Cabero, A.-I. & Wilde, P. J. Role of calcium on lipid digestion and serum lipids: A review. Crit. Rev. Food Sci. Nutr. 63(6), 813–826 (2023).
Kashkooli, S., Choghakhori, R., Hasanvand, A. & Abbasnezhad, A. Effect of calcium and vitamin D co-supplementation on lipid profile of overweight/obese subjects: A systematic review and meta-analysis of the randomized clinical trials. Obesity Med. 15, 100124 (2019).
Heshmati, J. et al. Impact of dietary calcium supplement on circulating lipoprotein concentrations and atherogenic indices in overweight and obese individuals: A systematic review. J. Diet. Suppl. 16(3), 357–367 (2019).
Derakhshandeh-Rishehri, S.-M., Ghobadi, S., Akhlaghi, M. & Faghih, S. The effect of calcium supplement intake on lipid profile: A systematic review and meta-analysis of randomized controlled clinical trials. Crit. Rev. Food Sci. Nutr. 62(8), 2093–2102 (2022).
Lin, L.-Y. et al. Dietary patterns in relation to components of dyslipidemia and fasting plasma glucose in adults with dyslipidemia and elevated fasting plasma glucose in Taiwan. Nutrients 11(4), 845 (2019).
Shi, N. et al. Associations of dairy intake with circulating biomarkers of inflammation, insulin response, and dyslipidemia among postmenopausal women. J. Acad. Nutr. Dietetics 121(10), 1984–2002 (2021).
Ardisson Korat, A. V. et al. Circulating very-long-chain SFA concentrations are inversely associated with incident type 2 diabetes in US men and women. J. Nutr. 150(2), 340–349 (2020).
Formisano, E. et al. Effects of a Mediterranean diet, dairy, and meat products on different phenotypes of dyslipidemia: A preliminary retrospective analysis. Nutrients 13(4), 1161 (2021).
Schmidt, K. A. et al. Impact of low-fat and full-fat dairy foods on fasting lipid profile and blood pressure: Exploratory endpoints of a randomized controlled trial. Am. J. Clin. Nutr. 114(3), 882–892 (2021).
Shivappa, N. et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 17(8), 1825–1833 (2014).
Acknowledgements
The authors thank the PERSIAN cohort study collaborators and Kermanshah University of Medical Sciences. The Iranian Ministry of Health and Medical Education has also contributed to the funding used in the PERSIAN Cohort through Grant no. 700/534.
Funding
This research was supported by the Kermanshah University of Medical Sciences (Proposal number: 4020054).
Author information
Authors and Affiliations
Contributions
NHE and SS designed the study. MB, FN, MMN, and YP implemented the survey and, helped to manage the data collection. MD conducted the data analyses, and NHE interpreted the results. NHE, SH, and RFB wrote the first draft of the manuscript. NHE, SS, MD, FN, and YP revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heidarzadeh-Esfahani, N., Hajahmadi, S., Pasdar, Y. et al. Diet-related inflammation is positively associated with atherogenic indices. Sci Rep 14, 13190 (2024). https://doi.org/10.1038/s41598-024-63153-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63153-1
- Springer Nature Limited