Abstract
Background
To investigate the ameliorative effects of glucosamine (GS), chondroitin sulphate (CS) and glucosamine plus chondroitin sulphate (GC) on rheumatoid arthritis (RA) in rats, and to explore the mechanism of GS, CS and GC in improving RA based on the gut microbiota.
Methods
RA rat models were effectively developed 14 days after CFA injection, and then garaged with GS, CS and GC. Body weight and paw volume of rats were monitored at multiple time points at the beginning of CFA injection. Until D36, serum and ankle tissue specimens were used to measure levels of circulating inflammatory factors (TNF-α, IL-1β, MMP-3, NO and PGE2) and local inflammatory indicators (TLR-4 and NF-κB). On D18, D25, and D36, intergroup gut microbiota was compared using 16S rRNA gene sequencing and bioinformatics analysis. We also performed the correlation analysis of gut bacteria, joint swelling and inflammatory indicators.
Results
GC, rather than GS and CS, could reduce right paw volumes, levels of TLR-4 and NF-κB in synovial tissues. In addition, enriched genera in RA model rats screened out by LEfSe analysis could be inhibited by GC intervention, including potential LPS-producing bacteria (Enterobacter, Bacteroides, Erysipelotrichaceae_unclassified and Erysipelotrichaceae_uncultured) and some other opportunistic pathogens (Esherichia_Shigella, Nosocomiicoccus, NK4A214_group, Odoribacter, Corynebacterium and Candidatus_Saccharimonas.etc.) that positively correlated with pro-inflammatory cytokines, right paw volume, and pathology scores. Furthermore, the gut microbiota dysbiosis was observed to recover before alleviating joint swelling after interventions.
Conclusions
GC could inhibit potential LPS-producing bacteria and the activation of TLR-4/NF-κB pathway in RA rats, thus alleviating RA-induced joint injury.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by synovial inflammation and is most clinically significant in terms of damage to the joints, manifested by progressive damage to the articular cartilage, causing swelling, pain, and stiffness, and eventually leading to impaired joint mobility [1, 2]. With 0.5%-1% of the world's population are suffering from RA, which is a considerable burden on individuals and society [3]. The specific pathological mechanisms involved in RA remain unclear but delayed joint dysfunction has become the mainstay of treatment for RA [4]. Most of the clinical drugs currently applied in the treatment of RA come with more or fewer side effects. As a result, attention has been focused on finding functional nutritional foods that can complement or even replace the effects of drugs.
Glucosamine (GS) and chondroitin sulfate (CS) are synthesized from natural substances and are widely marketed as functional nutritional foods for joint protection. GS is a naturally occurring monosaccharide that is a key component of glycosaminoglycans in the extracellular matrix of articular cartilage and other connective tissues. CS, on the other hand, is a complex polysaccharide consisting of repeating disaccharide chains (glucuronide and n-acetylgalactosamine), which are also structural components of articular cartilage, providing it with resistance to compression. In recent years, glucosamine plus chondroitin sulfate (GC) have been increasingly used in the improvement of osteoarthritis [5,6,7].. However, no studies had shown whether GS, CS and GC can improve RA. Intriguingly, GS and CS modulate the abundance of Prevotella, Bacteroidaceae and Clostridiaceae, which have been shown to be enriched in RA [8,9,10,11]. This suggests that the gut microbiota may play a role in the effect of GS, CS and GC on RA.
Adjuvant arthritis (AA) was used to confirm the efficacy of GS, CS and GC on RA, respectively. In addition, 16S rRNA gene sequencing and bioinformatics analysis were used to confirm the existence of a pathway by which GS, CS and GC could also regulate the gut microbial composition and thereby ameliorating the joint injury in RA. In this study, the gut microbiota was used to interrelate functional nutritional foods and diseases such as RA, which is also expected to provide a more novel perspective for future related studies.
Methods
Animals
Adult male SD rats weighing approximately 270 g were maintained in a standard laboratory environment. Two standard cages were loaded with a total of seven rats in groups of five, all of which were provided with the same potable water and standard rat chow for ad libitum feeding. Each SD rat was allowed a fourteen-day window to acclimatize to the laboratory environment before the experiment was conductedfig.
Reagents
Complete Freund’s adjuvant (CFA) containing 10 mg/ml heat-killed Mycobacterium tuberculosis H37 RA (non-viable) was purchased from Chondrex Inc. (USA) for induction of AA model. GS and CS were purchased from Jiaxing Hengjie Biopharmaceutical Co., LTD. The corresponding ELISA kits were obtained from Hushang Biotechnology Co. (Shanghai, China). Antibodies against nuclear factor kappa B (NF-κB), toll-like receptor 4 (TLR-4), and HRP-conjugated secondary anti-rat and anti-rabbit antibodies were purchased from Proteintech Group, Inc., USA.
Induction of AA model
AA, previously been used as a classic model for RA, can easily be induced in susceptible strains of rats by injection of 0.1 ml suspension of heat-killed Mycobacterium tuberculosis incomplete Freund’s adjuvant at a concentration of 10 mg/ml, intradermally at the base of the tail [12].
Study design
All rats were assigned into 5 groups of 7 rats each as follows (Fig. 1): Normal test (NT) group, non-arthritic healthy control rats receiving a daily oral dose of the saline. Model test (MT) group, AA rats receiving a daily oral dose of the saline. GS group, AA rats treated daily oral dose of GS (300 mg/kg) [13]. CS group, AA rats treated daily oral dose of CS (300 mg/kg) [14]. GC group, AA rats treated daily oral dose of GS (300 mg/kg) plus CS (300 mg/kg). In this study, the above intervention was performed on AA model successful day (D0) and remained in place for day 36 of the intervention until the end.
Assessment of arthritis
Basal right hind paw volume of the rats using a digital plethysmometer (IITC Inc. Life Science, Woodland Hills, CA, USA). The progression of arthritis was evaluated by measuring the right hind paw volume on D0, D4, D11, D18, D25, and D36 (the day of an intervention or the model successful day is recorded as “D0”).
Serum cytokines measurement
At D36, all blood specimens were collected through the posterior orbital plexus vein and placed in sterile EP tubes. Serum was obtained by centrifugation at 3500 rpm for 10 min after being placed in a refrigerator overnight at 4 °C and stored in units of 200 µl at − 80 °C for further analysis. Tumor necrosis factor (TNF)-α, Interleukin (IL)-1β, IL-10, prostaglandin E2 (PGE2), matrix metalloproteinase (MMP)-3 and nitric oxide (NO) were determined through enzyme immunosorbent assay (ELISA) or electrochemical methods according to the relevant procedures.
Histopathological parameters
At D36, right ankle joints were removed and immediately fixed with 4% paraformaldehyde for histological examination. Then, the right ankle joints were decalcified with 10% calcium nitrate, dehydrated with a gradient, series of ethanol, washed twice with xylene and embedded in paraffin. Finally, 4 μm slices were sectioned and stained with hematoxylin-eosin (H&E) for histopathological analysis and examined using light microscopy. The severity of arthritis in the joint was scored from 0 to 3 according to inflammatory cell infiltration, Pannus formation, synovial hyperplasia, destruction of articular cartilage and bone, and the total score of each mouse was calculated [15].
Immunohistochemistry
4 μm paraffin sections were baked at 60 °C for 30 min, then deparaffinized and rehydrated in xylene and descending gradient alcohol solution, followed by PBS rinsing. Sections were placed in citric acid solution (0.1 mol/L and pH 6.0) repair solution for antigen repair. Endogenous peroxidase activity was blocked by 3% H2O2 and antigen closure was performed using 5% BSA as the closure solution. After incubation in blocking buffer, these sections were treated with primary antibody against TLR-4 and NF-κB at a dilution of 1:300, respectively. Sections with sufficient peroxidase-labeled polymer secondary antibodies were re-incubated at room temperature. Finally, DAB was used as chromogen and these sections were counterstained with hematoxylin.
16S rRNA gene sequencing and bioinformatics analysis
Microbial community genomic DNA was extracted from all fecal samples using the E.Z.N.A.® soil DNA Kit (Omega Bio-Tek, Norcross, GA, U.S.) according to the manufacturer’s instructions. The DNA extract was checked on 1% agarose gel, and DNA concentration and purity were determined with NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, Wilmington, USA). The region V3-V4 of the bacterial 16S rRNA gene was amplified with primer pairs 338F: ACTCCTACGGGAGGCAGCAG’ and 806R: GGACTACHVGGGTWTCTAAT by an ABI GeneAmp® 9700 PCR thermocycler (ABI, CA, USA). Purified amplicons were pooled in equimolar and paired-end sequenced on an Illumina MiSeq PE300 platform (Illumina, San Diego, USA) according to the standard protocols by HonSunBio Technology Co. Ltd. (Shanghai, China).
Paired-end reads were quality-filtered by fastp version 0.20.0 and merged by FLASH version 1.2.11 [16, 17]. Operational taxonomic units (OTUs) with 97% similarity cutoff were clustered using UPARSE version 7.1, and chimeric sequences were identified and removed [18]. The taxonomy of each NAÏVE representative sequence was analyzed by RDP Classifier version 2.2 against the 16S rRNA gene database (e.g. Silva v138) using a confidence threshold of 0.7 [19]. The overall structure of the microbial community was analyzed by weighted UniFrac Principal Coordinate analysis (PCoA). The linear discriminant analysis effect size (LEfSe, Galaxy version 1.0) was performed to identify the significant differences between groups [20, 21].
Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM). The significance of differences between the groups was determined using one-way ANOVA or T-test using GraphPad Prism version 8.0.2 (263). P values < 0.05 were considered significant.
Results
Changes in weight of rats in each group
Rats in CFA-injected group were prone to lose weight when compared to rats in the NT group, although no significant difference was observed (Additional file 1: Fig. 1). During D0 to D36, no significant differences in body weight were observed among five groups.
Joint swelling was relieved by interventions
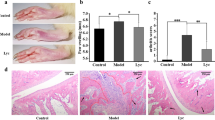
Joint swelling is one of the most important features of the AA model. At D-7, we found no significant difference in paw volume between the NT group and the MT group (Fig. 2a, P= 0.423). Although there was no significant difference in hindfoot and left paw volumes in the MT group compared to the NT group until D0, a significant difference appeared in the right paw volume (Fig. 2b, c, P = 0.002), indicating that the AA model was effectively established. The tumefaction degrees of paws swelling were diminished in the groups treated with GS, CS and GC on the end of our study (Additional file 2: Fig. 2). From D0 to D18, we found no significant difference in the right paw volume of rats between the MT group and three intervention groups, but the right paw volume of rats in the CFA-injected group was significantly high than that of rats in the NT group (Fig. 2c). At D25, there was significant difference in right paw volume between rats in the GC group compared with the MT group (Fig. 2c), indicating that GC intervention significantly improved joint swelling. Right paw volumes in the GS and CS groups were not significantly different with the MT and NT group, while fall in between the NT group and the MT group. This finding implied that GS and CS also alleviate joint swelling to some extent, although no statistically significant difference was found.
Changes of joint swelling in rats. a Hind paw volume of rats in each group at D-7 and D0. b Left paw volume of rats in each group at D0. c Swelling degree of right hind paw volume of rats in each group at several time points. d Histologic images of the talus bone and cartilage surface of ankle joints stained with H&E (40X & 100X magnification) at D36. e Pathological scores were calculated. (MT_Pre, the group of pre-intervention modeling. *p < 0.05 and **p < 0.01 compared with NT group. #p < 0.05 compared with MT group)
Joint pathology scores decreased by interventions
The HE-stained pathological sections of the right hind ankle joint of each group of rats are shown in Fig. 2d. Ankle joints of rats in the MT group exhibited pathological features such as inflammatory cell infiltration, severe synovial hyperplasia and the destruction of articular cartilage. Pathological scores in the GS and CS groups were not significantly different with the MT and NT group, while fall in between the NT group and the MT group. This finding indicated that GS and CS groups could tend to improve the histological damage of RA model rats. Rats in the GC group had considerably lower pathology scores than the MT group (Fig. 2e, P< 0.01), which indicated that GC group showed a significant improvement of pathological scores.
Changes in circulating inflammatory cytokines after interventions
Serum TNF-α, IL-1β, MMP-3, NO and PGE2 are pro-inflammatory factors, while IL-10 is an anti-inflammatory factor. At D36, a tendency for the level of serum TNF-α of rats in the MT group to be higher than in the serum of rats in the NT group (Fig. 3a, P= 0.0973), while no significant differences in other inflammatory indexes were observed. In addition, the expression level of serum IL-10 tended to be higher in the MT group than in the NT group (Fig. 3a, P= 0.0728), and no statistically significant difference was observed among three intervention groups and MT group.
Changes of inflammatory indicators. a Circulating inflammatory cytokines were determined through ELISA or electrochemical methods at D36 according to the relevant procedures. (*p < 0.05 and **p < 0.01 compared with NT group; #p < 0.05 compared with MT group) b Local inflammatory indicators (the expression of TLR-4 and NF-κB) in synovial tissues was detected by immunohistochemical methods at D36. The red arrows represent dye enrichment points
Expression levels of TLR-4 and NF-κB in synovial tissues decreased after interventions
Immunohistochemical methods were used to detect expression levels of TLR-4 and NF-κB in the synovial tissues in rats. (Fig. 3b) TLR-4 and NF-κB were highly expressed in the synovia tissues of rats in the MT group, and oral administration of GS or CS could tend to reduce the expression levels of TLR-4 and NF-κB. Notably, oral GC could significantly inhibit the expression of TLR-4 and NF-κB in the synovial tissues in RA rats.
Changes in the composition of the gut microbiota after interventions
16S rRNA gene sequencing was used to detect fecal microbiota at D18, D25, and D36 in rats in each group. Results of PCoA showed significant differences among five groups at all three time points (Fig. 4a , R2= 0.224, P = 0.001; R2 = 0.232, P = 0.001; R2 = 0.186, P = 0.001). In addition, a significant difference in the microbiota structure occurred between the MT and NT group at D18 and D25 (Fig. 4b, P < 0.05), indicating the gut microbiota dysbiosis occurred in CFA-injected rats. At D36, no significant difference in microbiota structure was observed between the MT and NT groups (Fig. 4b, P = 0.189), implying that the structure of the gut microbiota in the MT group gradually approached that of the NT group after a relatively long time. In addition, it showed that no significant differences were observed in the microbiota structure in the GS and MT group from three time points. A significant difference was found only in the CS group compared to the MT group at D25 (Fig. 4b, P = 0.002), and no significant differences were discovered at D18 and D36. Notably, there was a significant difference in the gut microbiota structure between the GC and MT group at D18, D25 and D36 (Fig. 4b, P < 0.05), suggesting that oral supplementation with GC has a durable ability to alleviate gut microbiota disorders accompanied by the pathological process of RA.
Differences in the gut micrbiota composition of rats in each group. a PCoA plots show the horizontal coordinate (PCoA1) and the vertical coordinate2 (PCoA2) representing the suspected influences on the bias of microbial composition between groups, respectively. Each point represents a sample. Different colors represent different groups. b The difference in the gut microbiota between MT group and NT, GS, CS and GC group respectively at three time points. P < 0.05 was considered to be statistically significantly different
Changes in the key gut bacteria after interventions
To further reveal changes in the key gut bacteria in rat group, LEfSe analysis was used to identify bacterial taxa that differed in abundance between groups, considering only those with an LDA score of > 2. As only GC showed a statistically significant improvement in symptoms compared to the MT group, we focused on the key differential genera that existed between the MT group and the NT and GC groups, respectively, at D18, D25 and D36 (Fig. 5).
Plots of LEfSe analysis of differential key genera between NT and MT groups and between MT and GC groups at three time points. Red boxes, key genera enriched in the MT group and reversed after GC intervention. Blue boxes, key genera enriched in both NT and GC groups. Red arrows, key genera positively correlated with LPS. Blue arrows, key genera negatively correlated with LPS
On the one hand, key genus number in NT group was 4, 17 and 5, while in MT group was 10, 19 and 12 at D18, D25 and D36, respectively. Among them, Escherichia_Shigella and Bilophila continued to show high abundance in the MT group at D18, D25 and D36.
On the other hand, at D18, the abundance of Enterobacter, Peptococcaceae_uncultured and Clostridia_vadinBB60_group_norank increased and the abundance of Lactobacillus and Clostridia_ uncultured decreased in the MT group compared to the NT group. Meanwhile, GC intervention reversed the abundance of the above genera. Moreover, Negativibacillus, Rothia and Deffuviitaleace_UCG_011 showed significantly high abundance, while Clostridia_uncultured showed low abundance in the MT group at D25. GC intervention for 25 consecutive days reversed the changes in the abundance of these genera. Similarly, GC intervention for 36 days reduced the abundance of five increased genera including RF39_norank, NK4A214_group, Alloprevotella, Candidatus_Saccharimonas and Eggerthellaceae_unclassified in the MT group, while increasing the abundance of Parabacteroides inhibited by CFA injection. Notably, at D18 and D25, GC intervention significantly increased the abundance of Lactobacillus which was considered to be negatively associated with lipopolysaccharide (LPS), and decreased the abundance of LPS-producing genera (e.g., Enterobacter, Erysipelotrichaceae_g_uncultured and Erysipelotrichaceae_g_unclassified) [22, 23].
Correlation analysis of gut microbiota, joint swelling and inflammatory indicators
We analyzed the correlation between joint swelling indicators and gut bacteria in rats (Fig. 6). We found that right paw volume was negatively correlated with the abundance of Firmicutes_unclassified (r = − 0.378, P < 0.05), but positively correlated with the abundance of Jeotgalicoccus (r = 0.335, P < 0.05), Nosocomiicoccus (r = 0.452, P < 0.01) and Candidatus_Saccharimonas (r = 0.470, P < 0.01). In addition, pathology scores were negatively correlated with the abundance of Lactobacillus (r = − 0.350, P < 0.05) and Parabacteroides (r = − 0.391, P < 0.05), but positively correlated with the abundance of NK4A214_group (r = 0.380, P < 0.05), Corynebacterium (r = 0.372, P < 0.05), RF39_norank (r = 0.360, P < 0.05), Candidatus_Saccharimona (r = 0.390, P < 0.05) and Coriobacteriaceae_UCG_002 (r = 0.365, P < 0.05). In addition, we also discovered that serum TNF-α was positively correlated with the abundance of Esherichia_Shigella (r = 0.479, P < 0.01), Eggerthellaceae_unclassified (r = 0.417, P < 0.05) and Clostridia_vadinBB60_group_norank (r = 0.406, P < 0.05). Serum IL-1β was positively correlated with the abundance of Erysipelatoclostridiaceae_unclassified (r = 0.343, P < 0.05) and Clostridia_vadinBB60_group_norank (r = 0.352, P < 0.05). Serum PGE2 was positively correlated with the abundance of Odoribacter (r = 0.461, P < 0.01) and Erysipelatoclostridiaceae_unclassified (r = 0.492, P < 0.01). Serum MMP-3 was negatively correlated with the abundance of Clostridia_uncultured (r = − 0.441, P < 0.01), but positively correlated with the abundance of Erysipelatoclostridiaceae_unclassified (r = 0.339, P < 0.05). Serum NO was negatively correlated with the abundance of Lachnospiraceae_FCS020_group (r = − 0.425, P < 0.05).
Heatmap of correlations between the gut microbiota, clinical indicators a and serological indicators b in different groups at the end of study. The correlation coefficients were calculated using Spearman correlation test. Boxes are labeled with the key genera selected in the LEfSe analysis. *p < 0.05, **p < 0.01. Red color, positive correlation. Blue color, negative correlation. Red boxes, key genera enriched in the MT group and reversed after GC intervention. Blue boxes, key genera enriched in both NT and GC groups
Discussions
RA was an autoimmune disease that affects the joints primarily, causing cartilage and bone degeneration as well as functional disability [1, 2]. TLRs and many endogenous TLR ligands were present in inflamed joints. Several microbial TLR ligands had been identified to display a synovial fibroblast response in RA, with TLR-4 being more responsive to TLR ligation in RA than other TLRs. [12] TLRs can be activated by LPS to increase the expression of M1-type macrophages in the synovial membrane [24, 25]. M1-type macrophages could release several destructive factors such as IL-1β, IL-6, and TNF-α for subsequent cartilage [25]. In addition, TNF-α, IL-1β, IL-6, and MMPs were released by activated immune cells (macrophages, mast cells, and neutrophils, etc.), which encouraged the synthesis of PGE2 and matrix degrading enzymes [26, 27].
Joint swelling is an important sign of synovial inflammation in RA [2]. In our study, the RA rat models were effectively developed 14 days after the CFA injection, and then fed with GS, CS and GC. After 25 days of continuous intervention, we found that paw volume of rats in the GC group was significantly lower than the MT group, while the paw volumes of rats in the GS and CS groups fall in between the NT and MT groups and were indifferent with the NT group. This finding suggested that combined treatment with glucosamine and chondroitin sulfate may have the better effect on alleviating joint swelling in RA. In addition, TNF-α, IL-1β, IL-10, PGE2, MMP-3 and NO were determined on day 50 of the CFA injection (i.e., D36). We discovered that levels of six serum inflammatory factors in the MT group were not different from the NT group, and also could not be influenced by GS, CS, or GC interventions. However, data from previous investigation indicated that levels of these serum inflammatory factors were increased on day 28 of the CFA injection [28, 29]. Inflammation in CFA-induced RA model rats would recede gradually [30]. The inconsistence of levels of inflammatory factors after CFA injection between our findings and others’ may be caused by the different sampling time point. In synovial tissue, however, pathology scores and expression levels of TLR-4 and NF-κB in the MT group were discovered to be higher than in the NT group and were reduced after GC intervention, suggesting that oral supplementation with GC could alleviate local inflammation levels in the joints in RA rats. We also found no significant differences in pathology scores and expression levels of TLR-4 and NF-κB in either the GS or CS groups compared with the MT group. Notably, pathological scores and expression levels of TLR-4 and NF-κB in both the GS and CS groups were not different from those in the NT group. Our findings indicated that GS and CS interventions also could alleviate the local inflammation in RA rat joints to some extent.
Furthermore, interactions between the intestinal microbiota and the host immune system were important in regulating the inflammatory state, and the disordered gut microbiota has been identified to trigger the inflammation [31, 32]. An epidemiological data have shown that clinical or subclinical symptoms of gut microbiota dysbiosis are prevalent in some RA patient [33]. Studies have shown that both GS and CS could influence the intestinal microbial composition, including RA-associated bacteria such as Prevotella, Bacteroidaceae, and Clostridiaceae [8,9,10,11]. We discovered that the intestinal microbiota composition in rats in the MT group differed from that in the NT group at D18 and D25 using PCoA and Anoism analysis, indicating that the AA model rats had intestinal microbiota dysbiosis. Compared with the MT group, no difference was seen in the intestinal microbiota composition in rats in either the GS or CS groups, but the GC group showed significant differences, which suggested that GC could improve bacteria dysbiosis. Intriguingly, we also found that GS, CS, and GC improved gut microbiota dysbiosis at D18, while joint swelling did not improve until D25, implying that gut microbiota dysbiosis generated by CFA injection recovered faster than joint swelling after GS, CS, and GC interventions.
LPS derived from bacteria had been demonstrated to cause arthritis and chronic inflammatory disorders via the TLR-4/NF-κB signaling pathway [12, 34]. We observed that LPS-producing bacteria such as Enterobacter [35] was more abundant in the MT group than in the NT group and was dramatically reduced after GC intervention at D18. Similarly, Lactobacillus [22, 23] and Parabacteroides [36], which were negatively related to LPS generation, were significantly decreased in the MT group compared to the NT group, and were significantly increased after GC intervention. Besides, we also discovered that GC group had lower abundance of some other LPS-producing bacteria, including Erysipelotrichaceae_unclassified [37], Bacteroides [38] and Erysipelotrichaceae_uncultured [37] compared with the MT group. These findings suggested that GC intervention could reduce the population of LPS-producing bacteria, lowering LPS production and thereby alleviating inflammation-induced joint damage in RA. Furthermore, the combined results of Figs. 5 and 6 show that GC intervention reduced the abundance of genera (Candidatus Saccharimonas, and Eubacterium_ruminantium_group) positively associated with joint swelling in the MT group, reduced the abundance of genera (NK4A214_group, Candidatus_Saccharimonas, and RF39_norank) positively associated with pathological parameters in the MT group, reduced the abundance of genera (Nosocomiicoccus) negatively associated with the anti-inflammatory factor IL-10 in the MT group, reduced the abundance of genera (Eggerthellaceeae_unclassfied, and Clostidia_vadinBB60_group_norank) positively associated with the pro-inflammatory factors IL-1β and TNF-α in the MT group, and reduced the abundance of genera (Eubacterium_oxidoreducens_group) positively associated with NO in the MT group. This finding suggested that the abundances of RA clinical injury-related genera and inflammation-related genera, were both reduced by GC intervention.
Collectively, GC intervention may reduce RA-induced joint inflammation and alleviate joint swelling and damage by inhibiting LPS-producing bacteria, lowering LPS synthesis, and thereby suppressing the activation of the TLR-4/NF-κB pathway in joint tissues. However, further high-quality evidence-based medical proof is required to conclusively prove the efficacy and duration of GS, CS, and GC in improving RA. More comprehensive studies are necessary to confirm whether the change in the gut microbiota is the cause or the consequence in the improvement of joint symptoms.
Conclusions
This study found that both GS and CS could reduce the symptoms of RA-related joint inflammation and swelling to some extent, with the effect of GC being more apparent, providing a theoretical foundation for expanding the usage of GS and CS. Furthermore, we discovered that the bacteria enriched in the RA model were mostly strongly correlative with pro-inflammatory cytokines, right paw volume, and pathological score using correlation analysis. After GS, CS, and GC intervention, these bacteria enriched in the RA model recovered, with GC having the most apparent beneficial impact. Notably, the gut microbiota dysbiosis could be recovered before the improvement of joint symptoms after the intervention. Our findings also indicated that GC might inhibit LPS-producing bacteria and the activation of the TLR-4/NF-κB pathway, thus alleviating RA-induced joint inflammation and ameliorating the joint swelling and injury.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. The relevant sequenced data have been deposited into a public database. Study No. SRP419979.
Abbreviations
- GS:
-
Glucosamine
- CS:
-
Chondroitin sulphate
- GC:
-
Glucosamine plus chondroitin sulphate
- RA:
-
Rheumatoid arthritis
- AA:
-
Adjuvant arthritis
- CFA:
-
Complete Freund’s adjuvant
- NF-κB:
-
Nuclear factor kappa B
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- IL:
-
Interleukin
- PGE2 :
-
Prostaglandin E2
- MMP:
-
Matrix metalloproteinase
- NO:
-
Nitric oxide
- ELISA:
-
Enzyme immunosorbent assay
- H&E:
-
Hematoxylin-eosin
- OTUs:
-
Operational taxonomic units
- PCoA:
-
Principal coordinate analysis
- LEfSe:
-
The linear discriminant analysis effect size
- SEM:
-
Standard error of the mean
- LPS:
-
Lipopolysaccharide
References
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–38.
McInnesSchett IBG. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19.
Safiri S, Kolahi AA, Hoy D, Smith E, Bettampadi D, Mansournia MA, Almasi-Hashiani A, Ashrafi-Asgarabad A, Moradi-Lakeh M, Qorbani M, et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78:1463–71.
Wang K, Zhao L, Liu X, Hao Z, Zhou Y, Yang C, Li H. Differential co-expression analysis of rheumatoid arthritis with microarray data. Mol Med Rep. 2014;10:2421–6.
Aghazadeh-Habashi A, Kohan MH, Asghar W, Jamali F. Glucosamine dose/concentration-effect correlation in the rat with adjuvant arthritis. J Pharm Sci. 2014;103:760–7.
Fransen M, Agaliotis M, Nairn L, Votrubec M, Bridgett L, Su S, Jan S, March L, Edmonds J, Norton R, et al. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann Rheum Dis. 2015;74:851–8.
Hochberg MC, Martel-Pelletier J, Monfort J, Moller I, Castillo JR, Arden N, Berenbaum F, Blanco FJ, Conaghan PG, Domenech G, et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis. 2016;75:37–44.
Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, Wu X, Li J, Tang L, Li Y, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21:895–905.
Lee JY, Mannaa M, Kim Y, Kim J, Kim GT, Seo YS. Comparative analysis of fecal microbiota composition between rheumatoid arthritis and osteoarthritis patients. Genes (Basel). 2019;10:748.
Sun Y, Chen Q, Lin P, Xu R, He D, Ji W, Bian Y, Shen Y, Li Q, Liu C, et al. Characteristics of gut microbiota in patients with rheumatoid arthritis in Shanghai, China. Front Cell Infect Microbiol. 2019;9:369.
Shmagel A, Demmer R, Knights D, Butler M, Langsetmo L, Lane NE, Ensrud K. The effects of glucosamine and chondroitin sulfate on gut microbial composition: a systematic review of evidence from animal and human studies. Nutrients. 2019;11:294.
Gowayed MA, El Achy S, Kamel MA, El-Tahan RA. Polymyxin B prevents the development of adjuvant arthritis via modulation of TLR/Cox-2 signaling pathway. Life Sci. 2020;259:118250.
Hua J, Suguro S, Hirano S, Sakamoto K, Nagaoka I. Preventive actions of a high dose of glucosamine on adjuvant arthritis in rats. Inflamm Res. 2005;54:127–32.
Bauerova K, Ponist S, Kuncirova V, Mihalova D, Paulovicova E, Volpi N. Chondroitin sulfate effect on induced arthritis in rats. Osteoarthritis Cartilage. 2011;19:1373–9.
Li X, Tang X, Wang Y, Chai C, Zhao Z, Zhang H, Peng Y, Wu L. CS-semi5 inhibits NF-kappaB activation to block synovial inflammation, cartilage loss and bone erosion associated with collagen-induced arthritis. Front Pharmacol. 2021;12:655101.
Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–90.
Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–63.
Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–8.
Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7.
Zhang C, Li S, Yang L, Huang P, Li W, Wang S, Zhao G, Zhang M, Pang X, Yan Z, et al. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun. 2013;4:2163.
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60.
Minj J, Chandra P, Paul C, Sharma RK. Bio-functional properties of probiotic Lactobacillus: current applications and research perspectives. Crit Rev Food Sci Nutr. 2021;61:2207–24.
Chen L, Li H, Li J, Chen Y, Yang Y. Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates microbiota dysbiosis in an experimental model of sepsis. Int J Mol Med. 2019;43:1139–48.
Velloso LA, Folli F, Saad MJ. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr Rev. 2015;36:245–71.
Zhou F, Mei J, Han X, Li H, Yang S, Wang M, Chu L, Qiao H, Tang T. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF-kappaB/MAPK signaling and protecting chondrocytes. Acta Pharm Sin B. 2019;9:973–85.
Seibl R, Birchler T, Loeliger S, Hossle JP, Gay RE, Saurenmann T, Michel BA, Seger RA, Gay S, Lauener RP. Expression and regulation of toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003;162:1221–7.
Liew FY, McInnes IB. The role of innate mediators in inflammatory response. Mol Immunol. 2002;38:887–90.
Uttra AM, Shahzad M, Shabbir A, Jahan S. Ephedra gerardiana aqueous ethanolic extract and fractions attenuate freund complete adjuvant induced arthritis in Sprague Dawley rats by downregulating PGE2, COX2, IL-1beta, IL-6, TNF-alpha, NF-kB and upregulating IL-4 and IL-10. J Ethnopharmacol. 2018;224:482–96.
Linghang Q, Yiyi X, Guosheng C, Kang X, Jiyuan T, Xiong L, Guangzhong W, Shuiqing L, Yanju L. Effects of atractylodes oil on inflammatory response and serum metabolites in adjuvant arthritis rats. Biomed Pharmacother. 2020;127:110130.
Choudhary N, Bhatt LK, Prabhavalkar KS. Experimental animal models for rheumatoid arthritis. Immunopharmacol Immunotoxicol. 2018;40:193–200.
Yoo JY, Groer M, Dutra SVO, Sarkar A, McSkimming DI. Gut microbiota and immune system interactions. Microorganisms. 2020;8:1587.
Scheithauer TPM, Rampanelli E, Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, Herrema H. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front Immunol. 2020;11:571731.
Lerner A, Matthias T. Rheumatoid arthritis-celiac disease relationship: joints get that gut feeling. Autoimmun Rev. 2015;14:1038–47.
Echizen K, Hirose O, Maeda Y, Oshima M. Inflammation in gastric cancer: interplay of the COX-2/prostaglandin E2 and toll-like receptor/MyD88 pathways. Cancer Sci. 2016;107:391–7.
Zhang B, Yue R, Chen Y, Huang X, Yang M, Shui J, Peng Y. The herbal medicine Scutellaria-Coptis alleviates intestinal mucosal barrier damage in diabetic rats by inhibiting inflammation and modulating the gut microbiota. Evid Based Complement Altern Med. 2020;2020:4568629.
Zhang L, Gu X, Wang J, Liao S, Duan Y, Li H, Song Z, He X, Fan Z. Effects of dietary isomaltooligosaccharide levels on the gut microbiota, immune function of sows, and the diarrhea rate of their offspring. Front Microbiol. 2020;11:588986.
Chen L, Li H, Chen Y, Yang Y. Probiotic Lactobacillus rhamnosus GG reduces mortality of septic mice by modulating gut microbiota composition and metabolic profiles. Nutrition. 2020;78:110863.
Wang W, Zhai S, Xia Y, Wang H, Ruan D, Zhou T, Zhu Y, Zhang H, Zhang M, Ye H, et al. Ochratoxin A induces liver inflammation: involvement of intestinal microbiota. Microbiome. 2019;7:151.
Acknowledgements
Not applicable.
Funding
This work was supported by Research Project of Wuxi Health Commission (2020ZHYB05); project of Wuxi Municipal Bureau on Science and Technology (N20192025); the National Natural Science Foundation of China (1522044, 31671909, 31772034, 32101033, 81870544 and 81870594); the Natural Science Foundation of Jiangsu Province (BK20181132 and BK20210060); Scientific Research Project of Jiangsu Commission of Health (M2021055); Jiangsu Scientific Research Project of Elderly Health (LK2021035); Jiangsu Scientific Research Project of Women’s and Children’s Health (F201741); Scientific Research Project of Wuxi Commission of Health (ZZ003 and Q201762); Wuxi scientific and technological development project (N20192024, N20191001 and N2020X007);Translational medicine Research Program of Wuxi Translational Medicine Centre (2020ZHYB08).
Author information
Authors and Affiliations
Contributions
HC and FZ conceived the study. XW and DL performed most of the experiments. DL, JY, XZ and QZ were involved in the rat studies, including serum cytokines and histological analyses. XW, DL, HC, FZ and QW performed 16S rRNA gene sequencing and bioinformatics analysis. YX and YX participated in data discussion and interpretation. XW and DL drafted the manuscript. All authors read, commented on and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All operations performed in this experiment in studies involving animals were conducted with the approval of the Jiangnan University Ethics Committee. (JN. No20191215S0350210[348]).
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Figure 1 Change curve of body weight from D-14 to D36
Additional file 2
. Figure 2 Photos of joint swelling in each group of rats
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, X., Liu, D., Li, D. et al. Combined treatment with glucosamine and chondroitin sulfate improves rheumatoid arthritis in rats by regulating the gut microbiota. Nutr Metab (Lond) 20, 22 (2023). https://doi.org/10.1186/s12986-023-00735-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-023-00735-2