Abstract
Background
The biological pathways through which vitamin D is involved in the regulation of systemic inflammation remain largely unknown.
Objective
The objective of this study was to evaluate the role of vitamin D status on the relationship between lipid profile and high-sensitivity C-reactive protein (hs-CRP) in pregnant women.
Design
Serum 25-hydroxyvitamin D (25(OH)D), hs-CRP, and indicators of lipid profiles (total cholesterol, TC; triglyceride, TG; high-density lipoprotein cholesterol, HDL-C; low-density lipoprotein cholesterol, LDL-C), were measured in 2479 pregnant women during the second trimester. Potential confounding including maternal sociodemographic characteristics, perinatal health status, diet, and lifestyle was prospectively collected. Multiple regression models and cubic models were used to evaluate the associations.
Results
There was a significant non-linear relationship between lipid profile (TC, TG, HDL-C, LDL-C) and hs-CRP (P < 0.05). Increased serum 25(OH)D was significantly associated with decreasing TC, TG, HDL-C, LDL-C, and hs-CRP levels. Compared with medium levels of lipids group, pregnant women with higher levels of TC or TG have higher levels of hs-CRP, and pregnant women with lower levels of TC, HDL-C or LDL-C also have higher levels of hs-CRP in the vitamin D deficient group, and there was a significant correlation between low levels of TG and decreased hs-CRP (adjusted β for TG: -0.063, 95%CI: − 0.120,-0.007) in the non-vitamin D deficient group. Mediators that had appreciable shares of the associations between 25(OH)D and hs-CRP was TG (10.2% of the association; β = − 0.011; total indirect effect: 95% CI: − 0.019, − 0.002). The cubic model suggested that a steep increase in the adjusted regression coefficient of lipid with hs-CRP up to 50 nmol/L of 25(OH)D, and the highest adjusted regression coefficients were observed in pregnant women with 25(OH)D above 50 nmol/L.
Conclusion
Our findings suggest that high levels of vitamin D during pregnancy may improve lipid profile levels and inhibit elevated hs-CRP induced by high lipid metabolism.
Similar content being viewed by others
Introduction
Increasing evidence suggests that dyslipidemia is often associated with elevated levels of inflammatory markers (e.g., tumor necrosis factor-β, interleukin 6 and hs-CRP) and is closely relationship with metabolic disease including obesity, type 2 diabetes, and cardiovascular disease [1,2,3], this metabolically triggered inflammation is called metabolic inflammation [4]. Although physiological changes of lipids during pregnancy may have specific causes for fetus development [5], studies revealed that some factors such as obesity [6], physical activity [7], diet and lifestyle [8] negatively influence lipid profile increase, and it is possible to further enhance the systemic inflammatory response, causing the occurrence of metabolic inflammation. Metabolic inflammation may play an important role in the mechanism of gestational diabetes [9].
Vitamin D may have immunomodulatory effects, serum 25(OH)D concentrations were inversely related to systemic inflammatory markers such as hs-CRP [10, 11]. However, biological pathways through which vitamin D participates in the regulation of systemic inflammation remain largely unknown. Previous studies have shown that vitamin D deficiency exacerbates dyslipidemia. Therefore, vitamin D deficiency may further enhance the systemic inflammatory response through aggravating dyslipidemia. To date, some studies on the associations among vitamin D, lipid profile and systemic inflammatory response are mostly from the general population. It is not clear whether the conclusion of the general population was applicable to pregnant women. In addition, the role of pregnancy-related vitamin D on lipid profile and systemic inflammatory markers remains inconsistent during pregnancy from several small sample studies [12,13,14]. Therefore, more high-quality research is needed to clarify.
In this study, based on a larger sample, we aimed to assess the role of vitamin D during pregnancy on the relationship between lipid profile and hs-CRP, stratifications by vitamin D status was performed to further examine the associations.
Methods
Study design and participants
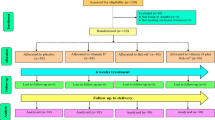
This study was conducted based on a prospective birth cohort. A total of 6712 pregnant women aged 18–45, with gestational ages from 14 to 27 weeks, were recruited in Hefei (latitude 32), China, between March 2015 and September 2018. At the time of enrollment, participants completed a self-designed structured questionnaire including sociodemographic characteristics, perinatal health status, diet, and lifestyle. Midwives or study nurses collected the pregnant anthropometric details and blood samples. The exclusion criteria included: multiple pregnancies, severe pregnancy complications (e.g., heart failure, severe anemia, gestational hypertension, etc.), abnormal function in liver, kidney or thyroid, artificially assisted reproduction, incomplete follow-up data during pregnancy, a toll of 5456 effective research subjects was obtained. We randomly selected half of the samples (2728 samples) were assayed for serum 25(OH)D, lipid profile and hs-CRP, and 249 samples in which there at least one index lower than the lower limit of detection was excluded. Finally, there were 2479 pregnant women in our study (Supplemental Figure 1). Written informed consent was obtained from all pregnant women, and the research protocol was approved by the Ethics Committee of Anhui Medical University (batch number: 2015002).
Blood samples
Blood samples collection (5 mL) was performed between 7:00 am and 9:00 am by a trained technician (nurse) after 8–12 h of overnight fasting. Serum samples were immediately centrifuged (3000 rpm for 5 min) and stored at − 80 °C until analysis.
Serum 25(OH)D and hs-CRP were measured by using the Electrochemical Luminescence Detection Kit for Roche E601 (Sandhofer, Mannheim, Germany) and the enzyme-linked immunosorbent assay (ELISA) kits (Cusabio Biotech, Wuhan, China) according to manufacturer’s instructions. Interclass and intraclass coefficients of variation were less than 10%.
The concentrations of serum TC, TG, HDL-C, and LDL-C were evaluated using an automatic analyzer (Beckman Coulter, Brea, CA, USA) and a commercial kit (Leadman, Beijing, China). TG level was tested by the glycerol-3-phosphate oxidase peroxidase-anti-peroxidase (PAP) method. TC was measured using the cholesterol oxidase PAP method. HDL-C and LDL-C were measured by direct measurement. The coefficient of variation (CV) for inter- and intra-assay was 4 and 3% for TC, 6 and 5% for TG, 6 and 4% for HDL-C, 4 and 3% for LDL-C.
Serum 25(OH)D concentrations were classified as vitamin D deficiency (< 50 nmol/L) and non-vitamin D deficiency (≥50 nmol/L) by the Institute of Medicine (IOM) [15]. In the absence of pregnancy-specific reference ranges, in this study, the lipid profile (TC, TG, HDL-C, and LDL-C) grouped by the equal quartile method (P25, P50, P75) were combined into the following three groups from the perspective of exploration: low level (<P25), medium-level (P25-P75) and high level (≥P75), the specific number of participants in each category was shown in Table 2.
Potential confounders
The following potential confounding was obtained from interviews or medical records. Maternal sociodemographic characteristics included age(years), an education level (≥9 and < 9 years of completed schooling), income (less than 4000 and greater than 4000 RMB Yuan/month). Perinatal health status included pre-pregnancy body mass index (Pre-pregnancy BMI, based on self-reported weight divided by the measured squared height at prenatal visit), systolic and diastolic blood pressure (mmHg), parity (nulliparous or multiparous). Diet and lifestyle included the frequency of physical exercise for no less than 10 min per week (< 3 and ≥ 3 days/week), the time to sedentary time every day (< 4 and ≥ 4 h/day), paternal alcohol (yes or no ) and smoking (less or more than 6 cigarettes/day) in the last 3 months. Frequency of food intake (e.g., mike, soy product, and dessert intake), fish oil, and multivitamin supplement in the past week. The season was designated as summer (June, July, August), autumn (September, October, November), winter (December, January, February) or spring (March, April, May).
Statistical analysis
The characteristics and laboratory statistics of 2479 pregnant women were described as the percentage or means (standard deviations, SD). In this study, non-normally distributed variables of hs-CRP were logarithmically transformed for further regression analysis.
Non-linear relations of lipid profile and hs-CRP have fitted the data in non-linear regression models using a cubic polynomial fitting. We divided the lipid metabolism index into three groups (low level: <P25; medium-level: P25-P75; high level:≥P75) based on the observed non-linear relationship. Multiple linear regression models were used to estimate associations between lipid profile status and hs-CRP with adjusting for potential confounding factors.
Crude and adjusted regression coefficients were generated for the associations of 25(OH)D with lipid profile and hs-CRP by using multiple linear regression analyses. Stratifying by vitamin D status (25(OH)D < 50 nmol/L and 25(OH)D ≥ 50 nmol/L), we further assessed the associations between lipid profile and hs-CRP by using multiple linear models. Using cubic fitting to test the association of serum 25(OH)D levels and adjusted regression coefficients for lipid profile and inflammatory marker hs-CRP.
Supplemental Figure 2 shows the direct and indirect effects from 25(OH)D to hs-CRP through intervening variables (TC, TG, HDL-C, and LDL-C) by using a path analysis model (Analysis of Moment Structures27). The bootstrap method (sample = 5000) was used to estimate the mediating effect, and the indirect effect was obtained by bias-corrected CI estimation. Statistical analyses were performed using SPSS 21.0 software (Statistical Package for the Social Sciences version 21.0, IBM Corp: Armonk, NY, USA).
Results
Sociodemographic and laboratory characteristics of the participants
The study included 2479 pregnant women, the mean age of the study population was 29.3 ± 4.2 years, the percentage of more frequency of maternal education (≥9 years) was 60.7% (n = 1505). The mean pre-pregnancy BMI was 21.2 ± 2.8 kg/m2, and 42.3% (n = 1049) of participants were multipara. The mean of serum 25(OH)D and hs-CRP concentrations were 40.08 nmol/L (SD:16.69, range:10.00–114.00) and 3.03 mg/L (SD:2.07, range:0.20–10.00), the percentage of the participants who had vitamin D deficiency was 75.6% (n = 1875). The mean of serum TC, TG, HDL-C, and LDL-C concentrations were 5.99 mmol/L (SD:1.01, range:2.90–9.71), 2.61 mmol/L (SD:1.02, range:0.80–9.87), 2.02 mmol/L (SD:0.35, range:0.76–3.67), and 2.85 mmol/L (SD:0.70, range:0.70–7.42), respectively (Table 1).
Association of lipid profile and hs-CRP levels in the second trimester
Polynomial fitting revealed a significant non-linear correlation between lipids level (TC: R2 = 0.520, P = 0.036; TG: R2 = 0.931, P <0.001; HDL-C: R2 = 0.457, P = 0.001; LDL-C: R2 = 0.750, P = 0.006) and hs-CRP, respectively (Fig. 1). The U-shaped nature of the association of TC and LDL-C with hs-CRP was confirmed with cubic-fitting model. After adjustment for confounding, compared with the medium level of lipid profile group, the hs-CRP levels in the low (adjusted β: 0.040, 95%CI: 0.009,0.071) or high (adjusted β: 0.043, 95%CI: 0.012,0.074) level group of TC, high leaves of TG (adjusted β: 0.040, 95%CI: 0.010,0.070), or low LDL-C (adjustedβ: 0.050, 95%CI: 0.020,0.080) levels were significantly elevated, while hs-CRP was significantly reduced only at low TG (adjustedβ: -0.077, 95%CI:-0.108,-0.047) levels. However, there was no significant association between HDL-C and hs- CRP (Table 2).
Association of 25(OH)D status with lipid profile and hs-CRP in the second trimester
Table 3 shows the relationship between 25(OH)D status with lipid profile and hs-CRP in the second trimester of pregnancy. Adjusted multiple linear regression analyses revealed a significant negative correlation between serum 25(OH)D with TC, TG, HDL-C, LDL-C, and hs-CRP. Compared with vitamin D deficiency group, the levels of TC, TG, HDL-C, LDL-C and hs-CRP in the non-vitamin D deficiency group significant decrease by 8.690 mg/dL (95%CI: 5.046,12.333), 13.254 mg/dL (95%CI: 4.963,21.544), 1.910 mg/dL (95%CI: 0.644,3.176), 4.933 mg/dL (95%CI: 2.409,7.458), and 0.034 mg/L (95%CI: 0.004,0.064), respectively.
Relationship between lipid profile and hs-CRP in different levels of vitamin D
Stratification by 25(OH)D level was performed to further explore the association of lipid profile and hs-CRP (Table 4). Compared with medium levels of lipids group, hs-CRP levels are higher in pregnant women with high TC or TG levels, and pregnant women with lower levels of TC, HDL-C or LDL-C also have higher hs-CRP levels in the vitamin D deficient group. However, in the non-vitamin D deficient group, a higher lipid profile was not associated with increased hs-CRP levels. Additionally, there was a significant association of lower levels of TG with decreased hs-CRP.
HDL-C was not included in path analysis because no significant association between 25(OH)D and HDL-C was found. For the comparative fit index, normal fit index, and the root mean square error of approximation of our path model, values were 0.781, 0.870, 0.001, respectively, reflecting an acceptable fit. Supplemental Figure 2 shows that a 1 nmol/L increase in 25(OH)D was associated with a 20%, a 10%, and a 23% decrease in TC, TG and LDL-C concentrations (P < 0.05). If the TG concentration increased by 1%, we would expect hs-CRP to increase by 0.02 mg/L (P < 0.05). Mediators that had appreciable shares of the associations between 25(OH)D and hs-CRP was TG (10.2% of the association; β = − 0.011; total indirect effect: 95% CI: − 0.019, − 0.002).
The role of 25(OH)D in the relationship between lipid profile and hs-CRP in the second trimester
We further assessed the role of 25(OH)D status in the relationship between lipid profile and hs-CRP in the second trimester by cubic model, the solid black line shows the best fit analysis (TC: R2 = 0.68, P = 0.03; TG: R2 = 0.14, P = 0.81; HDL- C: R2 = 0.63, P = 0.03; LDL-C: R2 = 0.41, P = 0.21) (Fig. 2). After adjusting for confounds, this model suggested a steep increase in the adjusted regression coefficient of lipid with hs-CRP up to 50 nmol/L of 25(OH)D, and the highest adjusted regression coefficients were observed in pregnant women with 25(OH)D above 50 nmol/L.
The role of vitamin D in the relationship between lipid profile and inflammation in the second trimester. a.25(OH)D, 25-hydroxyvitamin D; hs-CRP, high-sensitivity C-reactive protein; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; Note: The ordinates denote the adjusted regression coefficient of the multiple linear regression of lipid profile and hs-CRP, and the abscissa denote the 25(OH)D level in the second trimester; the solid black line denote the correlation between vitamin D levels with lipid profile and hs-CRP in the second trimester, and the shaded area indicates a 95% confidence interval. The closer the distance of the solid line from the X axis, the stronger the correlation strength between the two. Adjusted for sociodemographic characteristics (maternal age, education, income, season, parental diabetes and parental rheumatism), perinatal health status(pre-pregnancy BMI, systolic blood pressure and diastolic blood pressure), lifestyle(sitting or lying time, physical activities, paternal alcohol and smoking consumption, fish oil supplement, multivitamin supplement, milk intake, soy product intake and dessert intake)
Discussion
In the present study, we first evaluated the relationship between lipid profile and hs-CRP in a large sample. Results showed that lipid metabolic indicators were significantly associated with elevated inflammation during pregnancy, and pregnant women with high levels of TC and TG had higher hs-CRP. Notably, there was a significant non-linear relationship between lipid profile (TC, TG, HDL-C, LDL-C) and hs-CRP, lower levels of TC or LDL-C were also significantly associated with elevated hs-CRP. Secondly, we simultaneously evaluated the relationships among maternal 25(OH)D, lipid profile, and hs-CRP. Pregnant women with non-vitamin D deficiency have lower TC, TG, HDL-C, LDL-C, and hs-CRP levels compared with those deficient. Most importantly, based on stratified analysis, the significant association between high levels of TC, TG and elevated hs-CRP were only observed in pregnant women with vitamin D deficiency, but not the non-vitamin D deficiency. This study implicates the adequate vitamin D during pregnancy may inhibit high hs-CRP levels induced by hyperlipidemia.
To the best of our knowledge, some studies support the process of metabolic inflammatory, abnormal lipid profile levels may activate the systemic inflammatory response marker such as hs-CRP [16,17,18,19], which is similar to our findings in pregnant women. In addition, this is a study with the largest sample examining the role of vitamin D status in the association of lipid profile - hs-CRP during pregnancy, it was interesting that the intensity of the association steep ascended with the increasing levels of 25(OH)D up to 50 nmol/L and the strongest association was observed in 25(OH)D level above 50 nmol/L. This result further highlights that adequate vitamin D seems to enhance the effect on inhibition of lipid metabolism-inflammation. Given the adverse outcomes due to metabolic inflammation [20,21,22], taking vitamin D supplements during pregnancy for women with vitamin D deficiency may have clinical benefits in improving lipid and hs-CRP.
Our results are in agreement with previous results [23,24,25,26], which reported a significant correlation between abnormal lipid profile and elevated hs-CRP. However, there is no consensus on the relationship between lipids (TC, TG, HDL-C, or LDL-C) and inflammation. A cross-sectional study of 168 healthy school students found a positive correlation between TC/HDL-C ratio and hs-CRP levels [27]. Results in the general population of China indicate hs-CRP correlated positively with TG level, and negatively with the levels of HDL-C [28]. Another study showed that higher CRP levels correlated with lower levels of TC, LDL-C, and HDL-C in patients with rheumatoid arthritis [29]. Different findings might be explained by different factors such as ethnicity of the study population, geography, observation of gestational weeks and pre-pregnancy BMI. Notably, a non-linear relationship between lipid profile and hs-CRP may partly explain the inconsistency of the correlation. Our result suggested that lipid profile may have a dual biological effect, lipid metabolism indicators TC and LDL-C are beneficial to pregnant women within a certain range, exceeding this threshold may cause the level of inflammation marker hs-CRP to increase [30, 31]. Therefore, the dual biological effect of lipid metabolism indicators on inflammation during pregnancy is needed to further discussion.
Although observational studies showed that serum 25(OH)D level was negatively correlated with TC, and LDL-C, anther study have shown a positive correlation between vitamin D and lipids such as TC and TG in early pregnancy [12, 14]. It is worth noting that our results provided data that pregnant women with non-vitamin D deficiency had decreased lipid profile (TC, TG, HDL-C, LDL-C) compared with vitamin D deficient group. Inconsistent results may be related to vitamin D status. It suggests that adequate vitamin D may play an important role in lipid metabolism. And this report also concluded that the percentage of the pregnant women who had vitamin D deficiency has reached 75.6%, if vitamin D status has an effect on the lipid profile, this would be most easily observed that those with vitamin D deficiency combined with high lipid profile levels [32]. Unfortunately, prior studies assessing the effectiveness of vitamin D supplementation in reducing lipid profile found limited information and inconsistent results [33,34,35], most of the participants came from the same cohort, the sample size was small, different doses and the duration of the studies was different. Therefore, there is a need for the future of large-sample, multi-center, and high-quality interventional studies testing the effects of vitamin D supplementation on the lipid profile.
Additionally, a significant correlation between adequate vitamin D during pregnancy and low level of hs-CRP compared with those deficient group was found, and the association was previously observed in the general adult population [36], asymptomatic individuals [37], and neonates [10]. This result further supports the anti-inflammatory effect of vitamin D [38], and it seems to be important to avoid vitamin D deficiency during pregnancy. In this study, we further evaluated the potential biological pathway, and it supports the pathway of vitamin D deficiency may further enhance the systemic inflammatory response through aggravating dyslipidemia because of the significant positive association between lipid profile and hs-CRP was only observed among pregnant women with vitamin D deficiency. Although the mechanisms involved in this relationship are not understood, there is evidence that vitamin D limits the expression of microRNA in adipocytes in vitro and in adipose tissue in vivo through its impact on NF-κB signaling pathway [39]. These finding raised the possibility of vitamin D supplementation in pregnant women with vitamin D deficiency may be beneficial to improve metabolic inflammation.
This study elucidates the role of vitamin D status in the relationship between lipid profile and hs-CRP during pregnancy by using stratified analysis and nonlinear fitting models. The role of vitamin D in metabolic inflammation during pregnancy it was novel. Furthermore, an important strength is that this study relies on a prospective birth cohort study, the number of participants is relatively large, and confounding factors relevant to vitamin D status and lipid profile were strictly controlled to avoid confounding in statistical analyses. However, some limitations should also be mentioned in this study. First, there are no validated cutoff points to define abnormal lipid metabolism in pregnancy, a percentile cutoff is used to define elevated lipids, which might lead to misclassification, and the clinical significance of lipids is difficult to interpret. Second, due to the observational nature of our study, we cannot draw any causal relationship between vitamin D, lipid profile and inflammation. Third, we only measured hs-CRP and few lipid profile concentrations, which might not adequately represent the inflammatory or lipid metabolism of the participants. Finally, the generalizability of the results may be reduced by the fact that this study was conducted in one city of Hefei, and caution is necessary for generalizing the findings to other regions.
In conclusion, this study shows that there is a significant non-linear relationship between the lipid profile and the inflammation marker hs-CRP. Vitamin D may be a critical factor relevant to the association between lipid profiles and inflammation. Adequate vitamin D status during pregnancy may improve TC, TG, LDL-C levels and inhibit elevated hs-CRP induced by hyperlipidemia. In future research, the randomized controlled trial should be conducted to confirm the role of vitamin D supplementation on inhibition of inflammation through improving dyslipidemia.
Availability of data and materials
All data generated are included in this published article.
Abbreviations
- 25(OH)D:
-
25-Hydroxyvitamin D
- hs-CRP:
-
High-sensitivity C-reactive protein
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- HDL-C:
-
High-density lipoprotein-cholesterol
- LDL-C:
-
Low-density lipoprotein-cholesterol
References
Ouchi N, Parker JL, Lugus JJ, Walsh K: Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011 F;11:85–97.
Katsiki N, Mantzoros C, Mikhailidis DP. Adiponectin, lipids and atherosclerosis. Curr Opin Lipidol. 2017;28:347–54.
Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7.
An-Na C, Man-Li Y, Jeng-Hsiu H, Pesus C, Shin-Kuo S, Heung-Tat N. Alterations of serum lipid levels and their biological relevances during and after pregnancy. Life Sci. 1995;56:2367–75.
Farias DR, Franco Sena AB, Vilela A, Lepsch J, Mendes RH, Kac G. Lipid changes throughout pregnancy according to pre-pregnancy BMI: results from a prospective cohort. BJOG Int J Obstet Gynaecol. 2016;123:570–8.
Swift DL, Houmard JA, Slentz CA, Kraus WE. Effects of aerobic training with and without weight loss on insulin sensitivity and lipids. PLoS One. 2018;13:e196637.
Johansson I, Nilsson LM, Esberg A, Jansson JH, Winkvist A. Dairy intake revisited - associations between dairy intake and lifestyle related cardio-metabolic risk factors in a high milk consuming population. Nutr J. 2018;17:110.
Lekva T, Norwitz ER, Aukrust P, Ueland T. Impact of systemic inflammation on the progression of gestational diabetes mellitus. Curr Diab Rep. 2016;16:26.
Tao RX, Zhou QF, Xu ZW, Hao JH, Huang K, Mou Z, Jiang XM, Tao FB, Zhu P. Inverse correlation between vitamin D and C-reactive protein in newborns. Nutrients. 2015;7:9218–28.
Haidari F, Jalali MT, Shahbazian N, Haghighizadeh MH, Azadegan E. Comparison of serum levels of vitamin D and inflammatory markers between women with gestational diabetes mellitus and healthy pregnant control. J Family Reprod Health. 2016;10:1–8.
Tao M, Shao H, Gu J, Zhen Z. Vitamin D status of pregnant women in Shanghai, China. J Matern Fetal Neonatal Med. 2012;25:237–9.
Lepsch J, Eshriqui I, Farias DR, Vaz JS, Cunha Figueiredo AC, Adegboye ARA, Brito A, Mokhtar R, Allen LH, Holick MF, Kac G. Association between early pregnancy vitamin D status and changes in serum lipid profiles throughout pregnancy. Metabolism. 2017;70:85–97.
Al-Ajlan A, Krishnaswamy S, Alokail MS, Aljohani NJ, Al-Serehi A, Sheshah E, Alshingetti NM, Fouda M, Turkistani IZ, Al-Daghri NM. Vitamin D deficiency and dyslipidemia in early pregnancy. BMC Pregnancy Childbirth. 2015;15:314.
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8.
Wang T, Fu X, Chen Q, Patra JK, Wang D, Wang Z, Gai Z. Arachidonic acid metabolism and kidney inflammation. Int J Mol Sci. 2019;20:3683.
Ali L, Schnitzler JG, Kroon J. Metabolism: the road to inflammation and atherosclerosis. Curr Opin Lipidol. 2018;29:474–80.
Ferrante AJ. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–14.
Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–16.
Singer K, Lumeng CN. The initiation of metabolic inflammation in childhood obesity. J Clin Invest. 2017;127:65–73.
Janket SJ, Javaheri H, Ackerson LK, Ayilavarapu S, Meurman JH. Oral infections, metabolic inflammation, genetics, and Cardiometabolic diseases. J Dent Res. 2015;94:119S–27S.
Connaughton RM, McMorrow AM, McGillicuddy FC, Lithander FE, Roche HM. Impact of anti-inflammatory nutrients on obesity-associated metabolic-inflammation from childhood through to adulthood. Proc Nutr Soc. 2016;75:115–24.
Ebrahimi M, Heidari-Bakavoli AR, Shoeibi S, Mirhafez SR, Moohebati M, Esmaily H, Ghazavi H, Saberi KM, Parizadeh SM, Mohammadi M, Mohaddes AH, Ferns GA, Ghayour-Mobarhan M. Association of Serum hs-CRP levels with the presence of obesity, diabetes mellitus, and other cardiovascular risk factors. J Clin Lab Anal. 2016;30:672–6.
Bi X, Loo YT, Ponnalagu S, Henry CJ. Obesity is an independent determinant of elevated C-reactive protein in healthy women but not men. Biomarkers. 2019;24:64–9.
Gutaj P, Krzyżanowska P, Brązert J, Wender-Ożegowska E. Determinants of C-reactive protein concentrations in pregnant women with type 1 diabetes. Pol Arch Med Wewn. 2016;126:230–6.
Gan L, He Y, Liu L, Ou Q, Lin J. Association of serum lipids with autoantibodies and inflammatory markers in rheumatoid arthritis patients. Clin Chim Acta. 2018;486:282–90.
Agirbasli MM, Tanrikulu AM, Acar Sevim BM, Azizy M, Bekiroglu NP. Total cholesterol-to-high-density lipoprotein cholesterol ratio predicts high-sensitivity C-reactive protein levels in Turkish children. J Clin Lipidol. 2015;9:195–200.
Xu W, Li R, Zhang S, Gong L, Wang Z, Ren W, Xia C, Li Q. The relationship between high-sensitivity C-reactive protein and ApoB, ApoB/ApoA1 ratio in general population of China. Endocrine. 2012;42:132–8.
Toms TE, Panoulas VF, Douglas KMJ, Nightingale P, Smith JP, Griffiths H, Sattar N, Symmons DPM, Kitas GD. Are lipid ratios less susceptible to change with systemic inflammation than individual lipid components in patients with rheumatoid arthritis? Angiology. 2011;62:167–75.
Hong W, Zimmer V, Basharat Z, Zippi M, Stock S, Geng W, Bao X, Dong J, Pan J, Zhou M. Association of total cholesterol with severe acute pancreatitis: a U-shaped relationship. Clin Nutr. 2020;39:250–7.
Madsen CM, Varbo A, Tybjærg-Hansen A, Frikke-Schmidt R, Nordestgaard BG. U-shaped relationship of HDL and risk of infectious disease: two prospective population-based cohort studies. Eur Heart J. 2018;39:1181–90.
Al Mheid I, Quyyumi AA. Vitamin D and cardiovascular disease: controversy unresolved. J Am Coll Cardiol. 2017;70:89.
Jafari T, Fallah AA, Barani A. Effects of vitamin D on serum lipid profile in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Clin Nutr. 2016;35:1259–68.
Yazdchi R, Gargari BP, Asghari-Jafarabadi M, Sahhaf F. Effects of vitamin D supplementation on metabolic indices and hs-CRP levels in gestational diabetes mellitus patients: a randomized, double-blinded, placebo-controlled clinical trial. Nutr Res Pract. 2016;10:328–35.
Ponda MP, Liang Y, Kim J, Hutt R, Dowd K, Gilleaudeau P, Sullivan-Whalen MM, Rodrick T, Kim DJ, Barash I, Lowes MA, Breslow JL. A randomized clinical trial in vitamin D-deficient adults comparing replenishment with oral vitamin D3 with narrow-band UV type B light: effects on cholesterol and the transcriptional profiles of skin and blood. Am J Clin Nutr. 2017;105:1230–8.
Mellenthin L, Wallaschofski H, Grotevendt A, Völzke H, Nauck M, Hannemann A. Association between serum vitamin D concentrations and inflammatory markers in the general adult population. Metabolism. 2014;63:1056–62.
Amer MM, Qayyum RMM. Relation between serum 25-Hydroxyvitamin D and C-reactive protein in asymptomatic adults (from the continuous National Health and nutrition examination survey 2001 to 2006). Am J Cardiol. 2012;109:226–30.
Sassi F, Tamone C, D'Amelio P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients. 2018;10:1656.
Karkeni E, Bonnet L, Marcotorchino J, Tourniaire F, Astier J, Ye J, Landrier JF. Vitamin D limits inflammation-linked microRNA expression in adipocytes in vitro and in vivo: a new mechanism for the regulation of inflammation by vitamin D. Epigenetics. 2018;13:156–62.
Acknowledgements
We acknowledge the support of participants and the staff of in the Health Management Center of the First Affiliated Hospital of Anhui Medical University, Maternal and Child Health Hospital of Anhui Province,and the Hefei First People’s Hospital.
Ethical approval and consent to participate
Written informed consent was obtained from all pregnant women, and the research protocol was approved by the Ethics Committee of Anhui Medical University (batch number: 2015002).
Funding
This study received funding by grants from the National Natural Science Foundation of China (81872631, 81472991), Key Projects of Excellent Young Talents Fund in universities of Anhui Province (gxyqZD2018025), Public welfare technology application linkage fund of Anhui provincial science and technology department (1704F0804026), Hefei Municipal Health Planning Commission Applied Medicine Research Project (No. hwk2018zd002), National College Student Innovation Training Program (201710366029).
Author information
Authors and Affiliations
Contributions
PZ and RX T designed the study; DJ and DM Z carried out the initial analyses, drafted the initial manuscript, and revised the manuscript; DJ, HL H, MN Y, and WJ Y contributed to the data interpretation and reviewed the manuscript. PZ: critically reviewed the manuscript for accuracy and intellectual content; All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Competing interests
DJ, DM Z, HL H, MN Y, WJ Y, RX T and PZ, no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Supplementary Figure 1.
Participants flow chart.
Additional file 2: Supplemental Figure 2.
Estimated path analysis model showing the associations between serum 25(OH)D concentrations and hs-CRP.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jin, D., Zhu, DM., Hu, HL. et al. Vitamin D status affects the relationship between lipid profile and high-sensitivity C-reactive protein. Nutr Metab (Lond) 17, 57 (2020). https://doi.org/10.1186/s12986-020-00455-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-020-00455-x