Abstract
Objective
This systematic review aimed to map the evidence evaluated the relationship between vitamin D and redox and inflammatory status during gestation.
Methods
Three databases (PubMed/MEDLINE, Scopus, and Web of Science (WoS)) and reference list of included documents were searched for related observational studies published until 2nd October 2023. To determine the quality of the selected observational studies, the Newcastle-Ottawa Scale (NOS) was used.
Results
After a primary search of three databases, 19492records were appeared. When duplicates and irrelevant documents were removed, 14 articles were found to have eligible criteria. The design of the identified studies was cross-sectional, case-control and cohort. Evidence showed an adverse association between 25(OH)D and the biomarkers of inflammation, such as high-sensitivity C-reactive protein (hs-CRP), Interleukin-1beta (IL-1β), Interleukin-6 (IL-6), and tumor necrosis factor- alfa (TNF-α) during pregnancy. On the contrary, some studies represented that 25(OH)D positively correlated with hs-CRP in the cord blood. One study suggested a direct association between serum concentrations of 25(OH)D and Interleukin-8 (IL-8), macrophage inflammatory protein (MIP), and TNF-α levels in mothers with gestational diabetes mellitus (GDM). A case-control study showed that lower serum concentration of 25(OH)D positively correlated with total antioxidant capacity (TAC) levels in participants.
Conclusions
Evidence confirmed the supposition of the direct relationship between vitamin D levels and biomarkers with anti-inflammatory and anti-oxidative properties. However, the Existence of inconsistent evidence confirms the need for further studies in mothers with GDM and hypertensive disorders.
PROSPERO registration code
CRD42020202600.
Similar content being viewed by others
Introduction
Vitamin D as a fat-soluble vitamin has many substantial functions in the body, such as the cells proliferation and differentiation, immunity regulation, and inflammatory and oxidative stress modulation in addition to its classical role regarding bone and dental health [1,2,3,4]. Vitamin D can also transfer through placenta and affect pregnancy outcomes via implementing changes in inflammatory and redox status [5,6,7,8].
Balanced levels of cytokines regulate pregnancy and parturition, while excessive amounts of cytokines with pro-inflammatory properties (e.g., IL-1β, IL-6, and TNF-α), were associated with GDM, hypertensive disorders of pregnancy (HDP), preterm birth, and fetal loss [9, 10]. Therefore, dysregulation of the cytokines can adversely influence pregnancy and increase pregnancy complications [11].
Similarly, the generation of reactive oxygen species (ROS) during pregnancy contributes to some physiological processes, such as embryo implantation. However, excessive ROS production and oxidative stress can lead to the disruption of developmental processes and impaired function of the placenta and consequently, the occurrence of pregnancy complications, like GDM, intra uterine growth restriction (IUGR), and pregnancy loss [12, 13]. Therefore, providing a balanced production of oxidants and antioxidants (redox status) throughout pregnancy is critical [13]. Documents suggested that sufficient levels of vitamin D is related to minimal oxidative stress and proper function of mitochondria and the endocrine system, that result in lower risk of adverse pregnancy outcomes [14]. Pregnant women have a high possibility for vitamin D deficiency due to the enhanced physiological requirements [5]. A few observational studies have been conducted to reveal the relationship between the levels of vitamin D and biomarkers of inflammation and oxidative stress during gestation. However, the mentioned relationship has not been assessed through systematic reviews during pregnancy. Therefore, the present systematic review aimed to collect and summarize the evidence that assessed the association between the status of vitamin D and inflammation and oxidative stress in pregnant women.
Materials & methods
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) standard was applied for writing this systematic review. The study protocol registration code is CRD42020202600 (available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020202600).
Search strategy
Three databases, including PubMed/MEDLINE, Scopus, and WoS, and reference list of included documents were used for a comprehensive search of literatures published from onset to 2nd July October 2023with no language restriction.
A combination of the following terms was searched: pregnancy, gestation, child bearing, gravidity, intrauterine pregnancy, labor presentation, pregnancy maintenance, pregnancy trimesters, and vitamin D. The supplemental information contains the details of the search strategy.
Eligibility criteria
Inclusion criteriaPopulation: Pregnant women and their infant cord blood; Exposure: Blood levels of 25(OH)D; Outcomes: Biomarkers of inflammation, such as high-sensitivity C-reactive protein (hs-CRP), TNF-α, transforming growth factor-beta (TGF-β), interferon-gama (IFN-γ ), IL-1β, IL-4, IL-6, IL-7, IL-8, IL-10, IL-13, and/or oxidative stress markers, such as malondialdehyde (MDA), TAC, glutathione (GSH), and superoxide dismutase (SOD); study design: observational studies (cross-sectional, case-control and cohort studies), and baseline data of randomized control trials (RCTs).
Exclusion criteria
-
1)
Clinical trials, animal studies, and in vitro studies.
-
2)
Studies without reports on the levels of 25(OH)D and at least one of the biomarkers of inflammation or oxidative stress.
-
3)
Studies with determination of the interested outcomes just in infants and not in their mothers.
Study selection
Titles and abstracts of the records that retrieved were read by a reviewer (SM1) in order to find relevant documents. Then, two reviewers checked the full text of the included articles for their eligibility (SM1 & RA). When two reviewers came to disagreement, they resolved it by discussion and thereafter, by counselling a third expert reviewer (ReA).
Data extraction
Two independent reviewers (SM1 & SM2) extracted the required information, such as first author, journal, date of publication, chronological and gestational age of mothers, anthropometric indices (BMI, etc.), study design, sample size, and desired outcomes (concentrations of 25(OH)D and biomarkers inflammation and oxidative stress), and reported effect sizes with their intervals.
Risk of bias assessment
The selected articles were reviewed by two independent investigators (SM1 and RA) for risk of bias or quality assessment by using the Newcastle-Ottawa Scale (NOS) that judges the quality through three main domains including selection, comparability, and exposure/outcome, and uses “star system” for scoring. The number of stars in each domain determines the final quality. The final quality ranked “Very Good” (≥ 5 points in cross-sectional studies or 7–8 points in cohort and cross-sectional studies), “Good” (4 in Cross-sectional or 5–6 in cohort and cross-sectional studies), “Satisfactory” (3 in cross-sectional studies or 4 in cohort and cross-sectional studies), or “Unsatisfactory”(0–2 points in cross-sectional studies or 0–3 points in cohort/cross-sectional studies) [15]. A third expert reviewer (ReA) was called for final decision in case of any disagreement between the reviewers.
Results
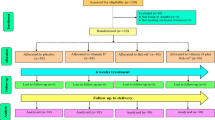
Figure 1 indicates that 19,492 records were appeared through the search (Scopus: 10,836, PubMed/MEDLINE: 1915, and WoS: 6741). After removing 8257 duplicates, titles and abstracts of the remained records were screened. Subsequently, the full-text of 244 relevant articles were assessed for eligibility. Finally, 14 eligible articles were selected and assessed in terms of quality.
Studies characteristics
The review included 5 cross-sectional (including 2 baseline data of trials), 4 case-control, and 5 cohort studies conducted in Mexico (k = 1), Indonesia (k = 1), Iran (k = 2), Iraq (k = 1), India (k = 2), USA (k = 2), Finland (k = 1), China (k = 2), Australia (k = 1), and Canada (k = 1).
Two case-control studies were conducted among GDM compared with healthy pregnant women [16, 17]. Mousa et al. (2017) studied the baseline serum samples of a large RCT conducted among overweight or obese pregnant women who were susceptible to GDM [18]. Three studies were conducted among preeclampsia or hypertensive disorders in pregnancy (HDP) cases [19,20,21]. In a case-control study by Budhwar et al. (2020), the biomarkers in cord blood samples of pre-term and term babies were measured [22]. Rosendahl examined cord blood of 939 healthy term infants [20]. One study applied in pregnant women with preterm delivery and their infants cord blood [23]. Perichart-Perera et al. examined the biomarkers of women with SGA fetus [24]. More details about the selected studies are presented in Table 1.
Serum 25(OH)D and maternal outcomes
In the study of Yaqiong et al., a significant lower serum concentration of 25(OH)D among GDM cases in comparison to the control group was observed [25]. Moreover, serum concentrations of 25(OH)D had a negative correlation with hs-CRP levels (r= -0.24, p < 0.001), and the risk of developing GDM increased by higher levels of hs-CRP (OR 1.40, 95% CI 1.09–1.80, p = 0.008) and TNF-α (OR 1.22, 95% CI 1.07–1.41, p = 0.004) [25]. In another study by Haidari et al., healthy participants had a negative association between serum concentration of 25(OH)D and hs-CRP (r= -0.44, p = 0.003) [16]. A negative relationship between the levels of 25(OH)D and hs-CRP among healthy pregnant women has been reported by Jin et al., as well [26]. The results of evaluating the association between serum vitamin D metabolites concentration and the levels of cytokine/chemokine in pregnant women with hypertensive disorders showed a positive correlation between 25(OH)D and TNF-α (r = 0.34, p = 0.010), macrophage inflammatory protein-1α (MIP-1α) (r = 0.35, p = 0.008) and MIP-1β (r = 0.27, p = 0.034) and also, a positive correlation between 1,25(OH)2D and IL-9 (r = 0.26, p = 0.047), IL-17 (r = 0.26, p = 0.045), INF-γ (r = 0.27, p = 0.039), and MIP-1β (r = 0.33, p = 0.010) [27]. Bobbitt et al., represented that 25(OH)D had a negative association with IL-1β (p = 0.002) [28]. A large cohort study among preeclamptic women showed that vitamin D deficiency and IL-6 concentration had an independent positive correlation with the risk of preeclampsia [21]. It means that the relationship between vitamin D deficiency and preeclampsia was not as a result of activating the inflammation [21]. The concentrations of 25(OH)D negatively associated with IL-6 among overweight or obese women who were at high risk for GDM (r= -0.20, p = 0.048) [29]. A case-control study by Pourghassem Gargari et al., among women with preeclampsia and their healthy counterparts showed lower serum 25(OH)D concentration in preeclampsia participants which was positively correlated with TAC levels (beta = 0.43, p = 0.010) [19].
Serum 25(OH)D and fetal outcomes
In a cohort study on 939 healthy infants with the aim of assessing the relationship between the levels of vitamin D in cord bloods and the status of inflammatory markers, a direct correlation between 25(OH)D and hs-CRP serum levels (B coefficient 1.00, 95% CI 1.00-1.01, p = 0.018) was observed [20]. Budhwar et al., found that the insufficient concentration of cord serum 25(OH)D in pre-term infants were related to the impaired balance of inflammation in their mother’s placenta [30]. A case-control study on GDM mothers (n = 36) and controls (n = 37), found that maternal serum concentration of 25(OH)D was associated with IL-8 (r = 0.52, p = 0.020) and TNF-α (r = 0.48, p = 0.030) in a significant positive manner among GDM cases [31].
Risk of bias assessment
Based on NOS, the included studies were ranked as very good (k = 7), good (k = 4), and satisfactory (k = 3) in terms of quality (Table 1).
Discussion
Overall, an adverse association between the levels of 25(OH)D and hs-CRP in GDM cases [16, 25], healthy pregnant women [32] and infants’ cord blood [20] was reported by some studies in this systematic review. In addition, vitamin D deficiency concurrent with altered levels of cytokines/chemokines has been shown in pregnant women with hypertensive disorders [33]. On the contrary, one of the included studies proposed a positive association between the levels of 25(OH)D and IL-8 and TNF-α among pregnant women with GDM [31]. Similarly, another other studies suggested an adverse relationship between 25(OH)D concentrations and IL-6 [18] and IL-1β [28].
Evidence suggests a controversial association between vitamin D status and pro-inflammatory markers which can be due to the design of the included studies which made it impossible to extract the causal relationships. Another reason can be attributed to the differences in the time and methods of measuring vitamin D status and the type of inflammatory biomarkers.
In general, some cytokines/chemokines along with other molecules including growth factors and hormones are produced by immune and placental cells to maintain pregnancy [34]. In this regard, cytokines profile is of great importance [34]. It is suggested that vitamin D has a role as immune modulator and cytokine production at the maternal-fetal interface [35]. Vitamin D can switch the immune system from pro inflammatory to anti-inflammatory responses [36, 37]. Although 25(OH) D and inflammation relationship could be U-shaped [38].
Some studies reported that pregnant women experience a rise in the levels of calcitriol during the first trimester which stayed in the same level until the end of pregnancy [39, 40]. However, the results of some longitudinal studies proved that the increment of calcitriol continues until the end of pregnancy [41, 42]. The increasing trend of calcitriol during first trimester might confirm its role in regulating immune function and inflammatory system. The results of a study have shown that calcitriol inhibited cultured trophoblast cells, which are treated with TNF-α to imitate a pro-inflammatory condition, from producing pro-inflammatory cytokines such as IL-6, INF-γ, and TNF-α [34, 43].
A mechanism that have been suggested for the effect of vitamin D on the inflammation is its role in the down-regulation of pro-inflammatory markers including IL-6 and TNF-α [44, 45]. It has been also proposed that vitamin D can affect the production of progesterone-induced blocking factor (PIBF) which poses immunomodulatory role [46, 47]. Another mechanism for the effect of vitamin D on reducing inflammatory status is its role in inhibiting cyclooxygenase-2 (COX-2) expression, increasing 15-prostaglandin dehydrogenase (15-PGDH) and subsequently stop prostaglandin (PG) pathway or reducing the production of PG. Vitamin D can also reduce the expression of EP and FP PG receptor and thus disrupt the PG signaling [48,49,50].
In this systematic review, one of the included studies showed that the levels of TAC and 25(OH)D had a positive association with each other in preeclamptic women [19]. Another systematic review declared that vitamin D supplementation increased TAC levels [51]. TAC levels represents all the antioxidants in the body and their cumulative effect [52].
Vitamin D anti-oxidant capacity and, therefore, its contribution to prevent protein and lipid peroxidation and DNA damage has been recognized [14]. Active form of vitamin D affected the expression of some genes through its nuclear receptors [14]. Therefore, vitamin D deficiency can be a reason for the complications that are related to the oxidative stress [14]. The result of a study suggested that vitamin D deficiency in pregnant women can disturb vasoconstrictor (thromboxane B-2) and vasodilator (6-keto prostaglandin F-1 alpha) eicosanoids balance and subsequently resulted in the endothelial dysfunction and increased risk of adverse pregnancy outcome [53].
Vitamin D is known to have a role in reducing oxidative stress status through deactivating nuclear transcription factor kB (NF-kB) as a result of elevating the expression of IkB and decreasing the phosphorylation of IkB-α [54,55,56]. Therefore, vitamin D can disrupt pathways that are dependent on NF-κB [57, 58] and reduce free radicals production.
The effect of vitamin D on oxidative stress status can also be explained by its role in stimulating the production of Nrf2 and subsequently anti-oxidant enzymes [59]. Moreover, Vitamin D reduces oxidative stress through lessening free radicals production and disrupting pathways that are dependent on NF-κB [57, 58]. It also regulates the generation of cellular glutathione and superoxide dismutase [60, 61], suppresses the expression of nicotinamide adenine dinucleotide phosphate (NADP) enzyme [62], and prevents the advanced glycation end products to be accumulated [63], which may in turn suppress oxidative stress.
Strengths and limitations of the study
According to the authors’ knowledge, this is the first systematic review that assessed the observational evidence regarding the association between vitamin D status and inflammatory and oxidative biomarkers in pregnant women. This systematic review met all the principles of PRISMA, such as conducting a comprehensive search strategy and assessing the quality using standard tools. The present systematic review has also some limitations like variability in study design, the characteristics of the participants, the methods of measuring vitamin D, inflammatory and oxidative stress status, time of launching the studies, and outcomes measurement. these conditions made it impossible to do meta-analysis and reach to conclusive results. Therefore,, assessing the association between vitamin D status and the levels of inflammation and/or oxidative stress biomarkers needs further works regarding the nature of different diseases.
Conclusions
According to the systematic review of the observational literature, evidence supports the hypothesis that there is a negative association between 25(OH)D levels and hs-CRP among healthy pregnant women, those with GDM, and in cord blood serum. Calcidiol levels have inverse association with serum IL-8 and TNF-α in GDM subjects. Moreover, 25(OH)D concentration of pregnant women has a positive association with TAC levels. Further observational studies are required to have a better understanding of the modulatory role of vitamin D on inflammatory and oxidative status of pregnant women and their offspring.
Data Availability
Not applicable.
Abbreviations
- NOS:
-
Newcastle-Ottawa Scale
- hs-CRPL:
-
high-sensitivity C-reactive protein
- IL-1β:
-
Interleukin-1beta
- IL-6:
-
Interleukin-6
- TNF-α:
-
tumor necrosis factor- alfa
- IL-8:
-
Interleukin-8
- MIP:
-
macrophage inflammatory protein
- GDM:
-
gestational diabetes mellitus
- TAC:
-
total antioxidant capacity
- HDP:
-
hypertensive disorders of pregnancy
- ROS:
-
reactive oxygen species
- IUGR:
-
intra uterine growth restriction
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- TGF-β:
-
transforming growth factor-beta
- IFN-γ:
-
interferon-gama
- MDA:
-
malondialdehyde
- GSH:
-
glutathione
- SOD:
-
superoxide dismutase
- MIP-1α:
-
macrophage inflammatory protein-1α
References
Schroder-Heurich B, Springer CJP, von Versen-Hoynck F. Vitamin D effects on the Immune System from Periconception through pregnancy. Nutrients. 2020;12(5).
Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: a systematic review of Immune Cell studies. PLoS ONE. 2015;10(11):e0141770.
Colotta F, Jansson B, Bonelli F. Modulation of inflammatory and immune responses by vitamin D. J Autoimmun. 2017;85:78–97.
Ao T, Kikuta J, Ishii M. The effects of vitamin D on Immune System and Inflammatory Diseases. Biomolecules. 2021;11(11).
Suárez-Varela MM, Uçar N, Peraita-Costa I, Huertas MF, Soriano JM. Vitamin D-Related risk factors for maternal morbidity during pregnancy: Syst Rev. 2022;14(15).
Motamed S, Nikooyeh B. Efficacy of two different doses of oral vitamin D supplementation on inflammatory biomarkers and maternal and neonatal outcomes. Am J Reproductive Immunol (New York NY: 1989). 2019;15(4):e12867.
Motamed S, Nikooyeh B, Kashanian M, Chamani M, Hollis BW, Neyestani TR. Evaluation of the efficacy of two doses of vitamin D supplementation on glycemic, lipidemic and oxidative stress biomarkers during pregnancy: a randomized clinical trial. BMC Pregnancy Childbirth. 2020;20(1):619.
Gilani S, Janssen P, Maternal Vitamin D. Levels during pregnancy and their effects on maternal–fetal outcomes: a systematic review. J Obstet Gynecol Can. 2020;42(9):1129–37.
Munro SK, Balakrishnan B. Cytokines and pregnancy: potential regulation by histone deacetylases. 2021;88(5):321–37.
Vesce F, Battisti C, Crudo M. The inflammatory cytokine imbalance for miscarriage, pregnancy loss and COVID-19 Pneumonia. Front Immunol. 2022;13:861245.
Welch BM, McNell EE, Edin ML, Ferguson KK. Inflammation and oxidative stress as mediators of the impacts of environmental exposures on human pregnancy: evidence from oxylipins. Pharmacol Ther. 2022;239:108181.
Hussain T, Murtaza G, Metwally E, Kalhoro DH, Kalhoro MS, Rahu BA et al. The role of oxidative stress and antioxidant balance in pregnancy. 2021;2021:9962860.
Zejnullahu VA, Zejnullahu VA, Kosumi E. The role of oxidative stress in patients with recurrent pregnancy loss: a review. Reprod Health. 2021;18(1):207.
Wimalawansa SJ, Vitamin D, Deficiency. Effects on oxidative stress, epigenetics, Gene Regulation, and aging. Biology (Basel). 2019;8(2).
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Haidari F, Jalali MT, Shahbazian N, Haghighizadeh MH, Azadegan E. Comparison of Serum Levels of Vitamin D and inflammatory markers between women with gestational Diabetes Mellitus and healthy pregnant control. J Family Reproductive Health. 2016;10(1):1–8.
McManus R, Summers K, de Vrijer B, Cohen N, Thompson A, Giroux I. Maternal, umbilical arterial and umbilical venous 25-hydroxyvitamin D and adipocytokine concentrations in pregnancies with and without gestational Diabetes. Clin Endocrinol (Oxf). 2014;80(5):635–41.
Mousa A, Abell SK, Shorakae S, Harrison CL, Naderpoor N, Hiam D, et al. Relationship between vitamin D and gestational Diabetes in overweight or obese pregnant women may be mediated by adiponectin. Molecular Nutrition and Food Research; 2017.
Pourghassem Gargari B, Pourteymour Fard Tabrizi F, Sadien B, Asghari Jafarabadi M, Farzadi L. Vitamin D status is related to oxidative stress but not high-sensitive C-Reactive protein in women with Pre-eclampsia. Gynecol Obstet Invest. 2016;81(4):308–14.
Rosendahl J, Holmlund-Suila E, Helve O, Viljakainen H, Hauta-Alus H, Valkama S, et al. 25-hydroxyvitamin D correlates with inflammatory markers in cord blood of healthy newborns. Pediatr Res. 2017;81(5):731–5.
Xu L, Lee MJ, Jeyabalan A, Roberts JM. The relationship of hypovitaminosis D and IL-6 in preeclampsia. Am J Obstet Gynecol. 2014;210(2).
Budhwar S, Verma P, Verma R, Gupta S, Rai S, Rajender S et al. Altered cord serum 25-hydroxyvitamin D signaling and placental inflammation is associated with pre-term birth. 2020;83(2):e13201.
Lisnawati Y, Marianna Y, Rohsiswatmo R. Increased levels of umbilical cord blood interleukin-6 (IL-6) and serum C-Reactive protein (CRP) in premature infants of vitamin D deficient mothers. Indonesian J Obstet Gynecol. 2021;9(1):21–5.
Perichart-Perera O, Avila-Sosa V, Solis-Paredes JM, Vitamin D, Deficiency. Excessive Gestational Weight Gain, and oxidative stress Predict Small for Gestational Age newborns using an Artificial neural Netw Model. 2022;11(3).
Yaqiong L, Guohua W, Fuyan Y, Wei L, Dan S, Yi Z. Study on the levels of 25(OH)D, inflammation markers and glucose and fat metabolism indexes in pregnant women of Han nationality in Jiangsu province with gestational Diabetes Mellitus. Medicine. 2020;99(35):e21654.
Jin D, Zhu DM, Hu HL, Yao MN, Yin WJ, Tao RX, et al. Vitamin D status affects the relationship between lipid profile and high-sensitivity C-reactive protein. Nutr Metabolism. 2020;17:57.
Adela R, Borkar RM, Mishra N, Bhandi MM, Vishwakarma G, Varma BA et al. Lower Serum Vitamin D Metabolite Levels in relation to circulating Cytokines/Chemokines and Metabolic Hormones in pregnant women with Hypertensive disorders. Front Immunol. 2017;8.
Bobbitt KR, Peters RM, Li J, Rao SD, Woodcroft KJ, Cassidy-Bushrow AE. Early pregnancy vitamin D and patterns of antenatal inflammation in African-American women. J Reprod Immunol. 2015;107:52–8.
Mousa A, Abell SK, Shorakae S, Harrison CL, Naderpoor N, Hiam D et al. Relationship between vitamin D and gestational Diabetes in overweight or obese pregnant women may be mediated by adiponectin. Mol Nutr Food Res. 2017;61(11).
Akoh CC, Pressman EK, Cooper E, Queenan RA, Pillittere J, O’Brien KO. Low Vitamin D is Associated with Infections and Proinflammatory cytokines during pregnancy. Reproductive Sci. 2018;25(3):414–23.
McManus R, Summers K, de Vrijer B, Cohen N, Thompson A, Giroux I. Maternal, umbilical arterial and umbilical venous 25-hydroxyvitamin D and adipocytokine concentrations in pregnancies with and without gestational Diabetes. Clin Endocrinol. 2014;80(5):635–41.
Nassr OA, Mohammed MM, Showman HA. Relationship between inflammatory biomarkers, vitamin D levels, and depressive symptoms in late pregnancy and during the postpartum period: a prospective, observational study. Middle East Current Psychiatry. 2022;29(1).
Adela R, Borkar RM, Mishra N, Bhandi MM, Vishwakarma G, Varma BA et al. Lower serum vitamin D metabolite levels in relation to circulating cytokines/chemokines and metabolic hormones in pregnant women with hypertensive disorders. Frontiers in Immunology. 2017.
Barrera D, Diaz L, Noyola-Martinez N, Halhali A. Vitamin D and inflammatory cytokines in healthy and preeclamptic pregnancies. Nutrients. 2015;7(8):6465–90.
Ji JL, Muyayalo KP, Zhang YH, Hu XH, Liao AH. Immunological function of vitamin D during human pregnancy. American journal of reproductive immunology (New York, NY: 1989). 2017;78(2).
Cannell JJ, Grant WB, Holick MF. Vitamin D and inflammation. Dermatoendocrinol. 2014;6(1):e983401.
Danescu LG, Levy S, Levy J. Vitamin D and Diabetes Mellitus. Endocrine. 2009;35(1):11–7.
Mellenthin L, Wallaschofski H, Grotevendt A, Völzke H, Nauck M, Hannemann A. Association between serum vitamin D concentrations and inflammatory markers in the general adult population. Metabolism. 2014;63(8):1056–62.
Kovacs CS. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. Am J Clin Nutr. 2008;88(2):520S–8S.
Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18(6):832–72.
Ardawi MS, Nasrat HA, HS BAA. Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: a longitudinal study. Eur J Endocrinol. 1997;137(4):402–9.
Halhali A, Villa AR, Madrazo E, Soria MC, Mercado E, Diaz L, et al. Longitudinal changes in maternal serum 1,25-dihydroxyvitamin D and insulin like growth factor I levels in pregnant women who developed preeclampsia: comparison with normotensive pregnant women. J Steroid Biochem Mol Biol. 2004;89–90(1–5):553–6.
Díaz L, Noyola-Martínez N, Barrera D, Hernández G, Avila E, Halhali A, et al. Calcitriol inhibits TNF-α-induced inflammatory cytokines in human trophoblasts. J Reprod Immunol. 2009;81(1):17–24.
Barbalho SM, Bechara MD, de Alvares Goulart R, Quesada K, Gasparini RG, de Cássio A et al. Reflections About Inflammatory Bowel Disease and Vitamins A and D. Journal of medicinal food. 2016;19(12):1105-10.
Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–96.
Wang P, Qiu W, Dudgeon C, Liu H, Huang C, Zambetti GP, et al. PUMA is directly activated by NF-kappaB and contributes to TNF-alpha-induced apoptosis. Cell Death Differ. 2009;16(9):1192–202.
Zheng L, Zhang YL, Dai YC, Chen X, Chen DL, Dai YT, et al. Jianpi Qingchang decoction alleviates ulcerative Colitis by inhibiting nuclear factor-κB activation. World J Gastroenterol. 2017;23(7):1180–8.
Hummel DM, Fetahu IS, Gröschel C, Manhardt T, Kállay E. Role of proinflammatory cytokines on expression of vitamin D metabolism and target genes in colon Cancer cells. J Steroid Biochem Mol Biol. 2014;144 Pt A:91–5.
Krishnan AV, Moreno J, Nonn L, Malloy P, Swami S, Peng L, et al. Novel pathways that contribute to the anti-proliferative and chemopreventive activities of calcitriol in Prostate cancer. J Steroid Biochem Mol Biol. 2007;103(3–5):694–702.
Brozek W, Manhardt T, Kállay E, Peterlik M, Cross HS. Relative expression of Vitamin D Hydroxylases, CYP27B1 and CYP24A1, and of Cyclooxygenase-2 and heterogeneity of human Colorectal Cancer in relation to age, gender, Tumor Location, and malignancy: results from factor and cluster analysis. Cancers (Basel). 2012;4(3):763–76.
Motamed S, Nikooyeh B, Anari R, Motamed S, Mokhtari Z, Neyestani T. The effect of vitamin D supplementation on oxidative stress and inflammatory biomarkers in pregnant women: a systematic review and meta-analysis of clinical trials. BMC Pregnancy Childbirth. 2022;22(1):816.
Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. 2000;29(11):1106–14.
Nandi AA, Wadhwani NS, Randhir KN, Madiwale SD, Deshpande JS, Wagh GN, et al. Maternal vitamin D deficiency influences long-chain polyunsaturated fatty acids and pregnancy outcome in association with alterations in one-carbon metabolism. Volume 86. New York, NY: Nutrition research; 2021. pp. 37–49.
Codoñer-Franch P, Tavárez-Alonso S, Simó-Jordá R, Laporta-Martín P, Carratalá-Calvo A, Alonso-Iglesias E. Vitamin D status is linked to biomarkers of oxidative stress, inflammation, and endothelial activation in obese children. J Pediatr. 2012;161(5):848–54.
Akbari M, Ostadmohammadi V, Lankarani KB, Tabrizi R, Kolahdooz F, Heydari ST et al. The Effects of Vitamin D Supplementation on Biomarkers of Inflammation and Oxidative Stress Among Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2018;50(4):271-9.
Sepidarkish M, Farsi F, Akbari-Fakhrabadi M, Namazi N, Almasi-Hashiani A, Maleki Hagiagha A, et al. The effect of vitamin D supplementation on oxidative stress parameters: a systematic review and meta-analysis of clinical trials. Pharmacol Res. 2019;139:141–52.
Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39(4):199–218.
Xin L, Che B, Zhai B, Luo Q, Zhang C, Wang J, et al. 1,25-Dihydroxy vitamin D(3) attenuates the oxidative stress-mediated inflammation Induced by PM(2.5)via the p38/NF-κB/NLRP3 pathway. Inflammation. 2019;42(2):702–13.
Nakai K, Fujii H, Kono K, Goto S, Kitazawa R, Kitazawa S, et al. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates Nephropathy in diabetic rats. Am J Hypertens. 2014;27(4):586–95.
Sun H, Wang C, Hao M, Sun R, Wang Y, Liu T, et al. CYP24A1 is a potential biomarker for the progression and prognosis of human Colorectal cancer. Hum Pathol. 2016;50:101–8.
Xiao YT, Yan WH, Cao Y, Yan JK, Cai W. Neutralization of IL-6 and TNF-α ameliorates intestinal permeability in DSS-induced Colitis. Cytokine. 2016;83:189–92.
Kim DH, Meza CA, Clarke H, Kim JS, Hickner RC. Vitamin D and endothelial function. Nutrients. 2020;12(2).
Šebeková K, Stürmer M, Fazeli G, Bahner U, Stäb F, Heidland A. Is vitamin D deficiency related to accumulation of advanced glycation end products, markers of inflammation, and oxidative stress in diabetic subjects? Biomed Res Int. 2015;2015:958097.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SM1 and SM2 contributed in searching the databases, screening the documents and data collection. SM1 and RAassessed the quality of the selected documents. SM1 prepared the original draft of the paper and ReA reviewed and revised the manuscript. The final version of the paper was read and approved by all the authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The proposal of this study was approved by the Ethics Committee of Asadabad School of Medical sciences. The ethics code is IR.ASAUMS.REC.1400.019.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Supporting information
S1. Search terms.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Motamed, S., Anari, R., Motamed, S. et al. Vitamin D and biomarkers of inflammation and oxidative stress among pregnant women: a systematic review of observational studies. BMC Immunol 24, 41 (2023). https://doi.org/10.1186/s12865-023-00577-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12865-023-00577-w