Abstract
Background
Since the emergence of the COVID-19 infection in China, it has caused considerable morbidity, mortality, and economic burden. It causes the vast majority of clinical manifestations, ranging from mild or even no symptoms to severe respiratory failure. There are many risk factors for severe COVID-19, such as old age, male gender, and associated comorbidities. A major role for genetic factors may exist. The SARS-CoV-2 virus enters the cell primarily through ACE2 receptors. rs2285666 is one of many polymorphisms found in the ACE2 receptor gene. To enable endosome-independent entry into target cells, the transmembrane protease serine-type 2 (TMPRSS2) is necessary to cleave the virus’ spike (S) glycoprotein. TMPRSS2 is characterized by an androgen receptor element. The rs12329760 polymorphism in TMPRSS2 may explain different genetic susceptibilities to COVID-19.
Method
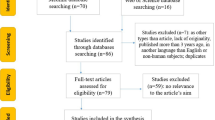
This cross-sectional study was held in Mansoura University Hospitals during the period from June 2020 to April 2022 on patients who had mild and severe COVID-19. Demographic, clinical, and laboratory data were collected, and the TaqMan real-time polymerase chain was used for allelic discrimination in the genotyping of rs2285666 and rs12329760.
Results
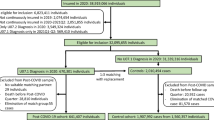
This study included 317 Egyptian patients, aged from 0.2 to 87 years. Males were 146, while females were 171. They were divided into mild and severe groups (91 and 226 patients, respectively) based on their clinical symptoms. There was a significant association between COVID-19 severity and male gender, hypertension, diabetes mellitus, and high CRP. The genotype and allele frequency distributions of the ACE2 rs2285666 polymorphism showed no significant association with the severity of COVID-19 in both. In contrast, in TMPRSS2 rs12329760 minor T allele and CT, TT genotypes were significantly associated with a reduced likelihood of developing severe COVID-19.
Conclusion
Our study indicates that the ACE2 rs2285666 polymorphism is not related to the severity of COVID-19, whether genotypes or alleles. In TMPRSS2 rs12329760, the dominant model and T allele showed significantly lower frequencies in severe cases, with a protective effect against severity. The discrepancies with previous results may be due to variations in other ACE2 receptor-related genes, inflammatory mediators, and coagulation indicators. Haplotype blocks and differences in racial makeup must be taken into consideration. Future research should be done to clarify how ethnicity affects these polymorphisms and how other comorbidities combine to have an additive effect.
Similar content being viewed by others
Introduction
One of the deadliest pandemics in the past hundred years was the Coronavirus Disease 2019 (COVID-19) pandemic [1]. By January 2023, there were more than 670 million infections and more than 6.5 million fatalities worldwide [2]. The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), responsible for COVID-19, has spread quickly and steadily, affecting human health and the stability of the global economy [3]. This has triggered a crisis that has spread across the globe. The clinical spectrum of SARS-CoV-2 infection is wide, ranging from asymptomatic or mildly symptomatic to severe symptoms that require admission to intensive care [4].
The known risk factors for increased COVID-19 morbidity and mortality include older age, male gender, and associated comorbidities like diabetes, obesity, and cardiovascular disease. Another important risk factor for COVID-19 is the influence of a person’s genetics [4]. The discovery of host genetic pathways and DNA polymorphisms that regulate the risk of infection and disease severity will significantly aid in the development of new COVID-19 preventive and/or therapeutic strategies [5].
SARS-CoV-2 utilizes the angiotensin-converting enzyme 2 (ACE2) receptor for entry into the cells and the host transmembrane serine protease (TMPRSS2) for S protein priming [6, 7]. Therefore, the study of polymorphisms in ACE2 and TMPRSS2 in various populations could open the way for precision medicine and individualized COVID-19 treatment plans [8].
ACE2 is a type I transmembrane enzyme with homology to ACE, which plays a key role in the Renin-Angiotensin system and is a target for the treatment of hypertension [9]. ACE2 receptors are the main host cell receptors responsible for viral entry into the cell [6]. This occurs by binding of viral spike glycoprotein to ACE2 receptors of the host cells [10].
It was hypothesized that higher susceptibility to COVID-19 infection is related to the expression of the target ACE2 receptor in the epithelium exposed to the virus [11]. Age affects the expression of the ACE2 receptor gene in the nasal epithelium. which is the first site of SARS-CoV2 contact [12]. The lower expression of the ACE2 receptor in children may explain the reduced risk [13]. However, ACE2 expression in the oral cavity mucosa may enable the virus to cause infection more easily [14]. Smoking and chronic obstructive pulmonary disease have been shown to increase the expression of ACE2 receptors in the lower respiratory tract and thus the risk of COVID-19 infection [15, 16].
The expression level of ACE2 receptor gene is largely influenced by genetic variations. rs2285666 is one of the numerous polymorphisms found in the ACE2 receptor gene [17]. It is located in the third intron’s fourth base and the intron next to the exon, and it can change messenger RNA alternate splicing and affect the expression of the ACE2 receptor gene [18]. It had population-based frequency differences [19].
The transmembrane protease serine-type 2 (TMPRSS2) plays a significant role in coronavirus infections. It is necessary for priming the glycoprotein of the virus spike by its cleavage for easier entry into target cells in an endosome-independent way [6]. There are androgen receptor elements upstream of the transcription site of TMPRSS2 [20]. Type I alveolar epithelial cells and ciliated cells were found to have the highest levels of TMPRSS2 expression [21]. Additionally, it is co-expressed with ACE2 [22], which is the SARS-CoV-2 cellular receptor [15].
SARS-CoV-2 infection has been shown to be inhibited in vivo by TMPRSS2 knockout [23]. This was associated with a diminished pro-inflammatory viral response [24]. Studies conducted in vitro have demonstrated that TMPRSS2 inhibitors protect against SARS-CoV-2 infections of primary airway cells. Mice infected with SARS-CoV and given the serine protease inhibitor survived [25]. Based on these results, it was proposed that a genetic change in TMPRSS2 may have an impact on the severity of the infection. The rs12329760 polymorphism in TMPRSS2 may play an important role [8].
Considering the role of ACE2 and TMPRSS2 in COVID-19 pathogenesis and the variation in disease severity, rs2285666 and rs12329760 polymorphisms have attracted attention. Since there were discrepancies between previous results, which may be attributed to host factors, including ethnicity, we aimed to study the polymorphisms of rs2285666 and rs12329760 in COVID-19-positive Egyptian patients and their relationship to the severity of the disease. This may pave the way for precision medicine and personalized treatment strategies for COVID-19.
Patients and methods
Study population
This cross-sectional study included 317 Egyptian patients with SARS-CoV-2 infection confirmed by RT-PCR testing in at least one biological sample. Mild cases included 91 patients, while severe cases included 226 patients. Severity of COVID-19 cases was considered with a diagnosis of viral pneumonia or myocardial infarction within 14 days after the SARS-CoV-2 positive test, hospitalization for 7 days or longer, or intensive care unit admission with clinical and laboratory findings suggesting a decrease in oxygen saturation, respiratory distress, and signs of pneumonia according to World Health Organization (WHO) severity guidelines [26]. Mild cases exhibited signs and symptoms like loss of taste and odor, dry cough, exhaustion, fever, diarrhea, chills, nasal congestion, sore throat, conjunctivitis, headache, musculoskeletal pain, skin rashes, and dizziness with a history of COVID-19 contact and confirmed by positive PCR. All severe cases who were admitted to the isolation unit at Mansoura University Hospitals during the study period were included, while mild cases were obtained from medical residents, laboratory personnel, nurses, and employees who had close contact with COVID-19 cases. This study was conducted at the Department of Medical Biochemistry and Molecular Biology, Faculty of Medicine, Mansoura. Informed consent was obtained from all study participants or their relatives. The Institutional Research Board approved the protocol (RP.20.05.70). A complete, comprehensive medical history was obtained, and a complete clinical examination was performed, including age, disease duration, comorbidities, signs of infection, and complications.
Laboratory analyses
The blood samples were collected during the period from June 2020 to April 2022. Samples were processed cautiously, and laboratory parameters were assessed, including CBC, C-reactive protein (CRP), ferritin, D-dimer, AST (aspartate aminotransferase), ALT (alanine aminotransferase), and creatinine.
Genetic analysis
Two milliliters of each subject’s blood were drawn via venipuncture and put into an ethylene diamine tetraacetic acid (EDTA) tube. Until DNA extraction, blood was kept at -20°. Genomic DNA was extracted from blood samples using the QIAamp DNA Extraction Micro Kit (Qiagen, Germany) according to the manufacturer’s instructions. The NanoDrop 2000c Spectrophotometer from Thermo Scientific (USA) was used to assess DNA concentration and purity. The DNA purity of the examined samples is accepted if it ranges from 1.6 to 1.9. This study focused on genotyping of two SNPs, ACE2 C/T (rs2285666) and TMPRSS2 C/T (rs12329760). This was performed using pre-designed TaqMan SNP genotyping assays (Thermo Fisher Scientific, C___2551626_1_ and C__25622353_20, respectively). Each assay contains two distinct forward and reverse primers that flank each SNP, and two TaqMan probes each of which was labeled with a fluorescent dye (either VIC or FAM) that differed only at the SNP site (Table 1). One probe was complementary to the wild-type allele and the other to the variant allele (Figs. 1 and 2). This was done by allelic discrimination using TaqMan real-time polymerase chain instrument (Azure Cielo 6, Azure, USA).
Real-time polymerase chain reaction of the three genotypes of ACE2 (rs2285666): (a) homozygous CC wild-type genotype, (b) homozygous TT variant genotype, (c) heterozygous CT genotype. The multicomponent plot analysis displays the result of each sample based on the dye released. For example, when the VIC green dye is released, this indicates a homozygous wild-type genotype. The release of FAM blue dye alone refers to homozygous variant genotype. In the case of the release of both dyes, this refers to heterozygosity for both alleles
Statistical analysis
Statistical analysis of the data was done using Statistical Package for Social Science (SPSS) version 21 and SNP Stats software. To compare qualitative data, the Chi-square test was employed. The Mann-Whitney U test was used as a test of significance for the comparison of the two groups. The quantitative data were expressed as the median and interquartile range (IQR). Odds ratios (OR), P values, and 95% confidence intervals (CI) were used to present the data. Results were regarded as statistically significant if the p-value was < 0.05.
Results
Out of 317 COVID-19 patients enrolled in this study; 146 were males and 171 were females. Age ranged from 0.2 to 87 years (Table 2). They were classified into mild and severe patients (91 and 226, respectively). Table 3 demonstrates that males experienced a greater increase in disease severity than females (P < 0.001). Severity also increased with history of COVID contact, hypertension, and DM and it was significantly associated with hospitalization, oxygen therapy, ICU admission, mechanical ventilation, respiratory distress, pneumonia, ARDS, shock, sepsis, multi-organ failure, acute kidney injury, and hepatitis and seizures (p < 0.05) (Table 4). Severe cases were also significantly associated with anemia, leukocytosis, neutrophilia, lymphopenia, decreased platelet count, increased CRP, D-dimer, ALT, and creatinine (p < 0.001) (Table 5). The association between the two examined SNPs and the severity of COVID-19 was displayed in Table 6. Regarding rs2285666, males and females were analyzed in a separate way because of the location of ACE2 receptor gene on X chromosome and males are hemizygous for ACE2. Neither genotypes nor allele frequencies were statistically associated with COVID-19 severity in rs2285666. Regarding rs12329760, the dominant model and T allele showed significantly lower frequency in severe cases, with a protective effect against severity.
The current study showed an interesting finding which is the gender-specific differential effect of rs12329760 alleles. A sexual dimorphic effect in the genetic association of rs12329760 with the severity of COVID-19 was noticed. In males, the rs12329760 T allele was significantly associated with more severe cases (p = 0.014, 95% CI: 1.124–2.801), while in females, the rs12329760 C allele was associated with more severe cases and the presence of T allele seems to be protective (p < 0.001, 95% CI: 0.198–0.448). The rs2285666 was not significantly associated with severity among males and females (Table 7).
Regression analysis was conducted for the prediction of COVID-19 severity. Older age, male gender, presence of comorbidities, and high CRP, were associated with the risk of severe cases, while the rs12329760 dominant model was associated with a protective effect against COVID-19 severity in univariable analysis. However, in multivariable analysis, only older age, male gender, presence of comorbidities, and a high CRP were considered as risk predictors of COVID-19 severe cases (Table 8).
Discussion
COVID-19 is the second pandemic in the twenty-first century, accounting for more than 100 million cases and more than two million fatalities [27]. On August 20th, 2022, the number of documented patients in Egypt was 515,198, with nearly 24,786 deaths [28]. Variation in the severity of COVID-19 can be partially explained by the genetic background of the host and other risk factors like age, gender, and underlying clinical conditions [2]. The analysis of about 81,000 human genomes suggests a possible association between susceptibility, severity, and clinical outcomes of COVID-19 and ACE2 and TMPRSS2 DNA polymorphisms. This may help to explain related epidemiological observations and direct the individualized treatment of COVID-19 [8].
It was reported that infection with SARS-CoV-2 is related to the male gender [29,30,31]. Our results advocate the association between the severity of COVID-19 and the male gender. Similarly, Jin et al. (2020) found that males with COVID-19 are more likely to experience poorer outcomes, regardless of age [32]. However, Alimoradi et al. (2022) reported that the incidence and severity of COVID-19 infection were not significantly related to gender [33]. Higher incidences of hypertension and diabetes mellitus were significantly correlated with COVID-19 severity, and this is in line with studies from China and Italy that proved they are the most prevalent comorbidities associated with SARS-CoV-2. These co-morbidities were known to be linked to ACE2 deficiency [33, 34], which is possibly an effect of glycosylation in diabetes mellitus [35] and maybe a causative factor for hypertension [36].
The severity of COVID-19 may be affected by a high nasopharyngeal viral load or by the host’s immune response. A high viral load and diminished virus-shedding are related to severe COVID-19. This leads to macrophage activation syndrome and cytokine storm [37, 38]. This is in accordance with our results, which showed a significant increase in inflammatory markers (CRP, ferritin, and D-dimer) in severe cases versus mild. Pro-inflammatory cytokine overproduction worsens acute respiratory distress syndrome and causes extensive tissue damage that eventually causes death by causing multiple organ failure [17]. We also found a significant decrease in lymphocyte number in severe cases. This confirms the results of Chen et al. (2020) and may be explained by the ability of SARS-CoV-2 to inhibit hematopoiesis in the bone marrow [39].
rs2285666 is a possible risk factor for type 2 diabetes, hypertension, and coronary artery disease [19]. The significant relationship between these variables and COVID-19 severity in our study supports the idea that rs2285666 may be a predisposing factor associated with the comorbidities seen in COVID-19 patients. The prevalence and risk of SARS-CoV-2 infection in the Indian, Caucasian, and Iranian populations were significantly correlated with the wild genotype of variant rs2285666 [3, 33]. A lower infection rate and case fatality were strongly correlated with the mutant allele in Indian populations [3].
Sequencing of the ACE2 receptor gene revealed that there is no strong evidence linking variations in the ACE2 coding sequence to the severity of COVID-19 [40, 41]. However, severity may be affected by the genetic variations in the noncoding regions of the ACE2 receptor gene or in other noncoding DNAs that control the expression levels of ACE genes [42]. The intronic location of the rs2285666 SNP may change mRNA splicing, gene expression, and ACE2 protein levels [36]. However, in our study, we found no significant association between genetic variants of rs2285666 and COVID-19 severity in all groups. This confirms the results of Karakaş Çelik et al. (2021) and Alimoradi et al. (2022), who found no association between rs2285666 and intron variants of the ACE2 receptor gene [17, 33]. On the contrary, Möhlendick et al. (2021) found that the GG genotype or G-allele was significantly associated with increased severity of SARS-CoV-2 [43]. Moreover, the meta-analysis done by Keikha and Karbalaei (2022) concluded that in people possessing the rs2285666 GG genotype, the risk of progression to severe infection is high, while the rs2285666 GA genotype has a protective role in patients against severe COVID-19 [44].
The susceptibility might not be an ACE2 receptor gene polymorphism. It may also be influenced by other variables, such as different ethnicities, genders, comorbidities, humidity, density of population, temperature, social isolation, or other polymorphisms. Variations in epigenetic mechanisms related to the expression of ACE2 receptors may play a role. There may also be a role for histone methylation [23]. Lambert et al. (2008) found that miR421 suppresses the gene of the ACE2 receptor [42]. The ACE2 receptor gene is post-translationally modified by phosphorylation and glycosylation [45].
The spread and pathogenesis of coronavirus depend on the activity of the TMPRSS2 enzyme [46]. Black people have an increased burden of COVID-19 [13], and this may be related to the increased nasal expression of TMPRSS2. Bioinformatics methods applied to large public domain datasets identified the rs12329760 in TMPRSS2 as a functionally significant variant in COVID-19 [19, 47]. To explain the high incidence and mortality rate of COVID-19 in the Italian population in comparison to Asian and other European countries, this variant has been put forth as one of the candidate gene variants [19].
TMPRSS2 gene expression is one of the potential mechanisms explaining the difference in COVID-19 severity in males versus females [48]. Androgen hormones are known to regulate the expression of the TMPRSS2 gene [20]. Our goal was to ascertain whether there was a connection between the severity of COVID-19 in the Egyptian population and the TMPRSS2 rs12329760 variant.
Our research revealed a significant association between rs12329760, and the severity of COVID-19 and that the T allele showed a significantly lower frequency in severe cases. This is supported by several studies [4, 49,50,51] that found that the minor T allele of this variant is linked to a decrease in the severity of symptoms of COVID-19, and this is in line with the results of the meta-analysis that showed a significant association between the TMPRSS2 rs12329760 C-allele and an increased risk of developing severe COVID-19 [46]. However, our results are in contrast to the previous studies in Iran [2, 52] and Egypt [53] that found that the T allele is a risk allele for the severe form of COVID-19. Also, earlier research done in Germany and Indonesia failed to discover any correlation between this TMPRSS2 gene variant and the severity of COVID-19 [46, 54].
The frequency of each of the studied genotypes and alleles was done in subgroups and showed that the C allele is significantly higher in females with severe infection. In contrast, the T allele was significantly associated with more severe cases in males.
This discrepancy with the results of various studies may be explained by the absence of androgen hormones dependent control of TMPRSS2 gene expression in lungs [55]. The patient’s ancestry has been postulated as a possible factor in the T allele effect on the severity of COVID-19 [2]. Additionally, variations in ethnicity, the presence of haplotype blocks with a unique combination of other risk loci, and variations of other host factors affecting immunity may have a role.
Conclusion
Our study concludes there is no evidence linking the ACE2 rs2285666 to the severity of COVID-19. While the results of the TMPRSS2 rs12329760 polymorphism showed that the minor T allele and CT and TT genotypes are protective against COVID-19 severity. Additional research is required to explain the gender-specific differential effect of rs12329760 alleles. The differences between our results and other studies may be due to the existence of haplotype blocks and racial differences. The impact of ethnicity on these polymorphisms and their relationship to COVID-19 severity should be clarified in upcoming multiethnic studies.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Azarpazhooh MR, Morovatdar N, Avan A, et al. COVID-19 pandemic, and burden of non-communicable diseases: an ecological study on data of 185 countries. J Stroke Cerebrovasc Dis. 2020;29(9):105089.
Yaghoobi A, Lord JS, Rezaiezadeh JS et al. TMPRSS2 polymorphism (rs12329760) and the severity of the COVID-19 in Iranian population. PLoS ONE 2023; 18(2), e0281750.
Srivastava A, Bandopadhyay A, Das D et al. Genetic association of ACE2 rs2285666 polymorphism with COVID-19 spatial distribution in India. Front Genet 2020; 1163.
David A, Parkinson N, Peacock TP et al. (2022). A common TMPRSS2 variant has a protective effect against severe COVID-19. Current research in translational medicine 2022; 70(2), 103333.
Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–91.
Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80.
Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26(7):1586.
Hou Y, Zhao J, Martin W, et al. New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020;18:1–8.
Shi A, Liu H, Liu L et al. Isolation, purification, and molecular mechanism of a peanut protein-derived ACE-inhibitory peptide. PLoS ONE 2014; 9(10), e111188.
Lu R, Zhao X, Li J et al. (2020). Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395, 565–574.
Wan Y, Shang J, Graham R, et al. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020;94:e00127–20.
Rodriguez GE, Shin BC, Abernathy RS, et al. Serum angiotensinconverting enzyme activity in normal children and in those with sarcoidosis. J Pediatr. 1981;99:68–72.
Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensinconverting enzyme 2 in children and adults. JAMA. 2020;323:2427–9.
Xu Y, Al-Mualm M, Terefe EM, et al. Prediction of COVID-19 manipulation by selective ACE inhibitory compounds of Potentilla reptant root: in silico study and ADMET profile. Arab J Chem. 2022;15(7):103942.
Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3.
Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID‐19. Eur Respir J. 2020;55:2000688.
Karakaş Çelik S, Çakmak Genç G, Pişkin N, Aet al, et al. Polymorphisms of ACE (I/D) and ACE2 receptor gene (Rs2106809, Rs2285666) are not related to the clinical course of COVID-19: a case study. J Med Virol. 2021;93(10):5947–52.
Kramkowski K, Mogielnicki A, Buczko W. The physiological significance of the alternative pathways of angiotensin II production. J Physiol Pharmacol. 2006;57:529–39.
Asselta R, Paraboschi EM, Mantovani A, et al. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging. 2020;12(11):10087.
Lin B, Ferguson C, White JT, et al. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999;59(17):4180–4.
Schuler BA, Habermann AC, Plosa EJ et al. Lung Biological Network. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 infection in the lung epithelium. bioRxiv. 2020; 2020-05.
Lukassen S, Chua RL, Trefzeret T, et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39(10):e10511.
Li Y, Li H, Zhou L. EZH2-mediated H3K27me3 inhibits ACE2 expression. Biochem Biophys Res Commun. 2020;526:947–52.
Iwata-Yoshikawa N, Okamura T, Shimizu Y, et al. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93(6):e01815–18.
Peacock TP, Goldhill DH, Zhou J et al. The furin cleavage site of SARS-CoV-2 spike protein is a key determinant for transmission due to enhanced replication in airway cells. BioRxiv. 2020; 2020-09.
World Health Organization. Therapeutics and COVID-19: Living Guideline—World Health Organization (WHO), 2021.
Izmailova O, Shlykova O, Kabaliei A et al. (2023). Polymorphism of tmprss2 (rs12329760) but not ace2 (rs4240157), tmprss11a (rs353163) and cd147 (rs8259) is associated with the severity of COVID-19 in the Ukrainian population. Acta Bio Medica: Atenei Parmensis 2023, 94(1).
Kandil S, Tharwat AI, Mohsen SM, et al. Developing a mortality risk prediction model using data of 3663 hospitalized COVID-19 patients: a retrospective cohort study in an Egyptian University Hospital. BMC Pulm Med. 2023;23(1):57.
Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–81.
Wu C, Chen X, Cai Y, et al. Risk factors Associated with Acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–43.
Acheampong DO, Barffour IK, Boye A, et al. Male predisposition to severe COVID-19: review of evidence and potential therapeutic prospects. Biomed Pharmacother. 2020;131:110748.
Jin JM, Bai P, He W et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health2020;152.
Alimoradi N, Sharqi M, Firouzabadi D, et al. SNPs of ACE1 (rs4343) and ACE2 (rs2285666) genes are linked to SARS-CoV-2 infection but not with the severity of disease. Virol J. 2022;19(191):48.
Verdecchia P, Cavallini C, Spanevello A, et al. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20.
Pal R, Bhansali A. COVID-19, Diabetes Mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020; 162.
Patel SK, Velkoska E, Freeman M, et al. From gene to protein—experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Front Physiol. 2014;5:227.
Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–7.
Liu Y, Liao W, Wan L, et al. Correlation between relative nasopharyngeal virus RNA load and lymphocyte count disease severity in patients with COVID-19. Viral Immunol. 2021;34(5):330–5.
Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13.
Gómez J, Albaiceta GM, García-Clemente M, et al. Angiotensin-converting enzymes (ACE, ACE2) gene variants and COVID‐19 outcome. Gene. 2020;762:145102.
Novelli A, Biancolella M, Borgiani P, et al. Analysis of ACE2 genetic variants in 131 Italian SARS-CoV‐2‐positive patients. Hum Genomics. 2020;14:29.
Lambert DW, Clarke NE, Hooper NM, et al. Calmodulin interacts with angiotensin-converting enzyme‐2 (ACE2) and inhibits shedding of its ectodomain. FEBS Lett. 2008;582:385–90.
Möhlendick B, Schönfelder K, Breuckmann K, et al. ACE2 polymorphism and susceptibility for SARS-CoV-2 infection and severity of COVID-19. Pharmacogenet Genomics. 2021;31(8):165–71.
Keikha M, Karbalaei M. Global distribution of ACE1 (rs4646994) and ACE2 (rs2285666) polymorphisms associated with COVID-19: a systematic review and meta-analysis. Microb Pathog 2022; 105781.
Saponaro F, Rutigliano G, Sestito S, et al. ACE2 in the era of SARS-CoV‐2: controversies and novel perspectives. Front Mol Biosci. 2020;7:588618.
Shirato K, Kawase M, Matsuyama S. Wild-type human coronaviruses prefer cell-surface TMPRSS2 to endosomal cathepsins for cell entry. Virology. 2018;517:9–15.
Wulandari L, Hamidah B, Pakpahan C, et al. Initial study on TMPRSS2 p. Val160Met genetic variant in COVID-19 patients. Hum Genomics. 2021;15(1):1–9.
Wadman M. Sex hormones signal why virus hits men harder. Science. 2020;368:1038–9.
Jeon CY, Yang HW. (2021). The structural changes of a local tourism network: Comparison of before and after COVID-19. Current Issues in Tourism 2021; 24(23), 3324–3338.
Ravikanth V, Sasikala M, Naveen V. (2021). A variant in TMPRSS2 is associated with decreased disease severity in COVID-19. Meta Gene 2021; 29, 100930.
Andolfo I, Russo R, Lasorsa VA, et al. Common variants at 21q22. 3 locus influence MX1 and TMPRSS2 gene expression and susceptibility to severe COVID-19. Iscience. 2021;24(4):102322.
Rokni M, Heidari Nia M, Sarhadi M, et al. Association of TMPRSS2 gene polymorphisms with COVID-19 severity and mortality: a case-control study with computational analyses. Appl Biochem Biotechnol. 2022;194(8):3507–26.
Abdelsattar S, Kasemy ZA, Ewida SF, et al. ACE2 and TMPRSS2 SNPs as determinants of susceptibility to, and severity of, a COVID-19 infection. Br J Biomed Sci. 2022;79:10238.
Schönfelder K, Breuckmann K, Elsner C, et al. Transmembrane serine protease 2 polymorphisms and susceptibility to severe acute respiratory syndrome coronavirus type 2 infection: a German case-control study. Front Genet. 2021;12:667231.
Li F, Han M, Dai P, et al. Distinct mechanisms for TMPRSS2 expression explain organ-specific inhibition of SARS-CoV-2 infection by enzalutamide. Nat Commun. 2021;12(1):866.
Acknowledgements
We acknowledge the Mansoura University Research Unit for supporting this research.
Funding
The Research is funded by Mansoura University Research Unit.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to the data collection and manuscript writing, and they all read and approved the final version that was published.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations. The study was approved by the Institutional Review Board at Mansoura University faculty of Medicine, Egypt (Approval No. RP.20.05.70). Informed consent from the participating patients or their guardians was obtained.
Consent for publication
Not Applicable.
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elnagdy, M.H., Magdy, A., Eldars, W. et al. Genetic association of ACE2 and TMPRSS2 polymorphisms with COVID-19 severity; a single centre study from Egypt. Virol J 21, 27 (2024). https://doi.org/10.1186/s12985-024-02298-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-024-02298-x