Abstract
Background
Maintaining static balance is relevant and common in everyday life and it depends on a correct intersegmental coordination. A change or reduction in postural capacity has been linked to increased risk of falls. People with Parkinson’s disease (pwPD) experience motor symptoms affecting the maintenance of a stable posture. The aim of the study is to understand the intersegmental changes in postural sway and to apply a trend change analysis to uncover different movement strategies between pwPD and healthy adults.
Methods
In total, 61 healthy participants, 40 young (YO), 21 old participants (OP), and 29 pwPD (13 during medication off, PDoff; 23 during medication on, PDon) were included. Participants stood quietly for 10 s as part of the Short Physical Performance Battery. Inertial measurement units (IMU) at the head, sternum, and lumbar region were used to extract postural parameters and a trend change analysis (TCA) was performed to compare between groups.
Objective
This study aims to explore the potential application of TCA for the assessment of postural stability using IMUs, and secondly, to employ this analysis within the context of neurological diseases, specifically Parkinson’s disease.
Results
Comparison of sensors locations revealed significant differences between head, sternum and pelvis for almost all parameters and cohorts. When comparing PDon and PDoff, the TCA revealed differences that were not seen by any other parameter.
Conclusions
While all parameters could differentiate between sensor locations, no group differences could be uncovered except for the TCA that allowed to distinguish between the PD on/off. The potential of the TCA to assess disease progression, response to treatment or even the prodromal PD phase should be explored in future studies.
Trial registration
The research procedure was approved by the ethical committee of the Medical Faculty of Kiel University (D438/18). The study is registered in the German Clinical Trials Register (DRKS00022998).
Similar content being viewed by others
Introduction
Maintaining an upright posture, or static balance, is a fundamental aspect of human life that underscores the intricate interconnections of the vestibular, visual, and somatosensory systems within the central nervous system [1]. Posture is more than the mere static alignment of body segments; it represents a dynamic process characterized by continuous adjustments to maintain stability while performing various tasks. Maintaining upright posture becomes increasingly critical with aging and neurological disorders due to the gradual decline in postural control, predisposing individuals to an elevated risk of falls and associated injuries. This decline is influenced by a multitude of factors, encompassing alterations in sensory input, muscle strength, joint flexibility, and neural processing [2]. As an example pwPD present profound challenges to postural control [3] which is based on the neurodegenerative character of the disease characterized by the loss of dopaminergic neurons. The difficulties with balance are linked to the loss of dopaminergic neurons affecting the basal ganglia which are essential to control upright posture.
A particularly intriguing aspect of postural control is the necessity for specific body segments to remain stable while others adapt to accommodate external demands. For instance, the head must remain stable to preserve visual focus and spatial orientation [4], while the pelvis may need to make adjustments to accommodate changes in terrain or task requirements [5]. Unconsciously, humans stabilize their visual focus or gaze and maintain awareness of their body position [6] but also stabilize their head to ensure balance [7]. For example Wallard et al. [8] found that children with cerebral palsy exhibit greater head angle variability, suggesting a compensatory strategy and Pozzo et al. [5] observed significant head stabilization during various locomotor tasks, with the head compensating for translation and rotation. People with mild traumatic brain injury revealed increased sway of the center of mass and less head stabilization compared with healthy controls [9]. In addition Israeli-Korn et al. [10] showed that intersegmental coordination patterns differ e.g. between Parkinson’s disease and cerebellar ataxia. Honegger et al. [11] investigated the coordination of the head with respect to the trunk, pelvis, and lower leg during quiet stance after vestibular loss. They argue that such simplification, as proposed by Fitzpatrick et al. [12] and Pinter et al. [13], may not fully capture the complexity of postural control in these populations. Contrary to expectations, their findings reveal synchronous movements of the head and trunk among healthy controls, suggesting that the presence of an intact vestibular system does not necessarily confer greater stability to the head in space. Instead, the pelvis emerges as a key stabilizing factor, as supported by earlier studies [13, 14] and the present investigation. These studies collectively highlight the role of aligning of body segments in postural control, particularly in individuals with motor impairments introducing another layer of complexity to our understanding of static balance. This raises the question of how the body segments sway and are controlled within the realm of quiet stance in different pathologies.
Inertial measurement units (IMUs) are small body-mounted sensors containing accelerometers, gyroscopes and magnetometers that can track 3D human movement on a very granular level e.g. to measure balance [15, 16] based on center of mass movements [17, 18]. Their reliability and validity have been extensively examined [19, 20] and provide a tool to be used in combination with a trend change analysis (TCA) [21]. TCA can detect the small number of quick corrections, an increased frequency of longer-duration corrections, and an elongation in the displacement between successive postural corrections. Adapted from techniques originally employed in stock exchange analyses, the TCA facilitates the quantification of postural corrections in both the anteroposterior (A/P) and mediolateral (M/L) directions. Moreover, it allows for the calculation of the number of adaptations, the time interval between successive posture corrections [21] providing insights about the body’s responses to postural challenges [22].
The research presented herein aims to delve into the intricate relationship between maintaining an upright posture, PD, aging, and the dynamic adjustments involving intersegmental control. The objectives of this study are twofold: Firstly, to explore the potential application of TCA for the assessment of postural stability using IMUs, and secondly, to employ this analysis within the context of neurological diseases, specifically PD. We hypothesized that the TCA could differentiate between persons with PD (pwPD) and healthy adults and also distinguish, in pwPD, between dopaminergic on (PDon) and dopaminergic off phases (PDoff).
Methods
Participants
The experimental groups consisted of 61 healthy participants, 40 young (YO), 21 old (OP) and 29 pwPD. The demographic characteristics of the study participants are presented in Table 1.
All participants were either inpatients at the neurogeriatric ward of the Neurology Center at the University Hospital Schleswig-Holstein, Campus Kiel, or spouses of the patients or members of the professional team. pwPD were diagnosed according to the Movement Disorder Society clinical diagnostic criteria for Parkinson’s disease [23, 24]. Thirteen pwPD participated as PDoff (UPDRS III score 24 ± 10), 23 as PDon (UPDRS III score 30 ± 20), and 7 as both PDon (UPDRS III score 26 ± 10) and PDoff (UPDRS III score 27 ± 10). The sample size for this study was predetermined based on prior research and the current analysis is a secondary analysis of the previously published data set [25,26,27].
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Kiel University (D438/18) and all participants provided written informed consent before participation. Participants were excluded when their fall risk was determined to be too high (> 2 falls in the previous week), corrected visual acuity was below 60%, they scored ≤ 15 points in the Montreal Cognitive Assessment (MoCA) test [24, 28], had current or past chronic substance abuse (except nicotine), and were not able to perform at least one of the walking tasks [25].
Protocol
Data from the IMU sensors were recorded using a motion capture system (Noraxon USA Inc., myoMOTION 3.16, Scottsdale, AZ, USA) [25, 26]. The participants were asked to stand in an upright position with their feet together, side-by-side and fix their gaze on a point on a white wall for 10 s as part of the Short Physical Performance Battery [25].
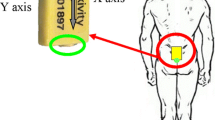
Three IMUs were attached to the body (pelvic, sternum and head) using elastic bands with a special housing for the IMU to clip into (see Fig. 1). The research procedure was approved by the ethical committee of the Medical Faculty of Kiel University (D438/18). The study is registered in the German Clinical Trials Register (DRKS00022998).
Sensor data processing
The IMU data was processed by custom written scripts using MATLAB (MathWorks, Nantick, MA) based on methodology described by Mancini et al. [29]. The parameters provided information about the sway jerkiness (JERK) (cm2/s5), the sway area (SURFACE) (cm2), path (PATH) (cm), mean velocity (MV) (cm/s), range of acceleration (RANGE) (cm/s2) and root mean square of the acceleration (RMS) (cm/s2).
In addition, the TCA was applied. Acceleration signals were filtered with a low-pass filter (7 Hz low-pass Butterworth filter). The method is based on a Moving Average Convergence Divergence (MACD) indicator calculation algorithm and evaluates the relationships of exponential moving averages (EMAs) for the recorded signal [21]. Calculations can be performed for any time-varying signal. In the case of the tests used, recorded acceleration signals were used, the S signal is the acceleration signal.
In the first step of calculations, for the signal S, the MACD line was determined as the difference between two EMAs (Eq. 2) with lengths of 12 and 26 samples according to Eq. 1.
Where EMAS,12 - faster exponential moving average for signal S,
EMAS,26 - slower exponential moving average for signal S
Where, p0 – ultimate value, p1 – penultimate value, pN – value preceding N periods, N = number of periods, α = a smoothing coefficient equal to 2/(N + 1).
In the next step, the signal line is calculated as an EMA with a length of 9 samples from the MACD line signal in accordance with Eq. 3.
The intersection of the MACD line and the Signal line determines the trend change points in the S signal. The number of intersections determines the TCI (trend changes index).
In the next step, the time intervals between successive points of trend changes in the S signal were calculated. In this way, the MACD_dT array was determined, the average value of which is the value of the TCI_dT. As a consequence, the displacement between subsequent trend change points were calculated and the results constitute the MACD_dS array. The average value of the array is the value of the TCI_dS (Fig. 2). Finally, the corresponding elements of the MACD_dS array were divided by MACD_dT to obtain the MACD_dV array. The average value of the array is the value of the TCI_dV. In this study, the displacement of the signal is the difference in the acceleration values between successive points of trend change on the acceleration signal.
Graphical explanation of the Trend Change Index (TCI), the delta time between successive TCIs (MACD_dT) as well as the delta space between successive TCIs (MACD_dS) in an acceleration signal from a sensor on the pelvis with an observation phase of about 3 s. Seven trend changes (indicated by the seven red dots) are shown. All determined MACD_dTs were used to calculate TCI_dT and all MACD_dSs to calculate TCI_dS according to the procedure described in the text
To summarize, TCI determines the number of trend changes in the assumed research period, TCI_dT defines the average time between detected trend changes, and TCI_dS determines the average value of the acceleration change between subsequent trend changes. Indices were determined for each of the three directions of measurement, and then the resultant values were determined i.e. for TCI as the sum of the number of trend changes detected in each direction of the measured accelerations (in the X, Y and Z axes), and for TCI_dT, TCI_dS, TCI_dV as the square root of the sum of squares of the values calculated in each direction.
Statistical analysis
The analyses were performed using Matlab R2022a and JASP (Version 0.16.1 JASP Team (2022)) for all statistical analyses.
The analysis aimed to investigate differences between sensor positions and cohorts within the dataset. Shapiro-Wilk tests revealed significant deviations from normality (p < 0.05) across multiple groups and sensor positions, thus prompting the utilization of non-parametric tests. Subsequently, a Kruskal-Wallis H Test were employed to evaluate variations between cohorts and sensor positions. In case of statistically significant differences (p < 0.05) post-hoc analyses, utilizing Dunn’s test with Bonferroni correction, were conducted to ascertain specific group disparities.
Results
When comparing the individual parameters for each sensor and each cohort (Table 2), no differences could be found between the cohorts but significant differences were uncovered between the sensor positions (Additional file 1).
The sensor position differed for all cohorts and all parameters except TCI and TCI_dT for PDon (Table 3).
When comparing the PDon and PDoff cohort (Table 4) only TCI & TCI_dT differed between the PDon and PDoff cohort. Significant differences were found between the three sensor locations (Table 5).
Discussion
This study investigated postural stability of healthy young, old controls and persons with PD in a static balance task using three different sensor locations. The aim of the study was to analyze the upright posture and intersegmental adjustments, to evaluate whether the parameters could uncover distinct postural sway behavior between the different cohorts. Our results confirmed that both, the postural parameters and TCA, could uncover sway differences between the segments but only the TCA could differentiate between PDon and PDoff.
The results of the current study show no group differences between the healthy adults and pwPD, confirming results from a previous study investigating static sway with increasing task difficulty [27]. This is of interest as PD is known for its altered postural reflexes with a disruption of the precisely coordinated execution of agonist and antagonist muscles (associated with bradykinesia and rigidity), which results in difficulty to maintain static postural stability [30,31,32] due to a reduced margin of stability [33].
While pwPD have shown larger values for sway acceleration, jerk and sway velocity during postural balance compared to age-matched healthy controls [29, 34] they also show an increased jerkiness during the performance of cognitive task [35], suggesting an interaction of cognitive functions, including multisensory integration, with static balance mechanisms. Our results highlight larger motions from the head compared to the sternum and the pelvis. The results convey with previous findings [14] basing their findings upon the biomechanical principal of a double-inverted pendulum. The double-inverted pendulum allows to be controlled by the ankles, the hip or both, while assuming a rigid head-on-trunk coupling. Almost all parameters were able to distinguish between sensor position indicating the complex relationship between the dynamic intersegmental adjustments and upright posture. The results suggest that for a relative simple and short balance tasks pwPD can perform control-like, which could be related to the location of the pathology within the central nervous system and its extensive compensation possibilities [36] and by using alternative pathways or even networks [37].
There is some evidence that dopaminergic medication can improve static sway [38, 39]. However, there are not many IMU-based studies available that can show these differences. One reason may be that the parameters currently assessed for this performance are not covering disease-relevant changes. Here we introduced TCA in the analysis of static sway in PDon and PDoff, and could in fact detect significant differences only with this approach (but not with the conventional parameters). We found a higher number of TCIs and smaller TCI_dT values in PDoff compared to PDon. This is coherent with previous results obtained for COP measurements showing an increase in TCIs and reduction of TCI_dT in pwPD compared to healthy individuals [40]. In our view, this perspective also aligns with a pathomechanistic standpoint. Previous research, as indicated by Bizid et al. [41], suggests that low frequencies are predominantly associated with visuo-vestibular regulation, while high frequencies are associated with proprioceptive regulation. Additionally, it is well-established that visual perception and integration are strongly dopamine-dependent [42]. Therefore, we hypothesize that the results observed through TCA most likely reflect visual deficits resulting from a dopaminergic deficit. This is particularly evident, given that lower leg proprioceptive performance does not appear to be influenced by dopaminergic treatment [43].
Limitations
It would be worthwhile to mention limitations of the current study. First, the number of pwPD measured in both medication states was relatively low, potentially limiting the generalizability of findings and the ability to capture the full spectrum of balance-related issues in PD. Another constraint lies in the brief 10-second measurement duration, which may not provide a comprehensive representation of individuals’ balance control capabilities, particularly in dynamic real-world scenarios. Additionally, the use of a side-by-side stance as a measure may cause limitations as it may not be challenging enough to detect subtle differences between cohorts or uncover changes in postural control based on intersegmental coordination. These limitations emphasize the need for cautious interpretation of results and highlight areas for future research to address these constraints and provide a more nuanced understanding of balance control in Parkinson’s disease and other relevant populations. Nevertheless, considering these limitations, it is all the more remarkable given that the TCA parameters were effective in distinguishing between PD on and PD off.
Clinical implication
This study investigated static sway in healthy individuals and pwPD using three sensor locations. Results show that postural parameters effectively distinguish between segments. However, and even more relevant, the introduction of TCA proves instrumental in detecting significant differences between PDon and off medication, showcasing its potential in assessing disease-relevant changes not captured by conventional parameters.
Data availability
No datasets were generated or analysed during the current study.
References
Winter D. Human balance and posture control during standing and walking. Gait Posture. 1995;3:193–214.
Chen X, Qu X. Age-related differences in the relationships between Lower-Limb Joint Proprioception and Postural Balance. Hum Factors. 2019;61:702–11.
Balestrino R, Schapira AHV. Parkinson disease. Euro J Neurol. 2020;27:27–42.
Guitton D, Kearney RE, Wereley N, Peterson BW. Visual, vestibular and voluntary contributions to human head stabilization. ExpBrain Res. 1986;64:59–69.
Pozzo T, Berthoz A, Lefort L. Head stabilization during various locomotor tasks in humans: I. Normal subjects. Exp Brain Res [Internet]. 1990 [cited 2023 Dec 7]; 82(1):97–106. http://link.springer.com/https://doi.org/10.1007/BF00230842.
Nikolaus T. Gait, balance and falls - reasons and consequences [Gait, balance and falls–causes and consequences]. Dtsch Med Wochenschr. 2005;130:958–68.
Hansson EE, Beckman A, Håkansson A. Effect of vision, proprioception, and the position of the vestibular organ on postural sway. Acta Otolaryngol. 2010;130:1358–63.
Wallard L, Bril B, Dietrich G, Kerlirzin Y, Bredin J. The role of head stabilization in locomotion in children with cerebral palsy. Annals Phys Rehabilitation Med. 2012;55:590–600.
Fino PC, Raffegeau TE, Parrington L, Peterka RJ, King LA. Head stabilization during standing in people with persisting symptoms after mild traumatic brain injury. J Biomech. 2020;112:110045.
Israeli-Korn SD, Barliya A, Paquette C, Franzén E, Inzelberg R, Horak FB, et al. Intersegmental coordination patterns are differently affected in Parkinson’s disease and cerebellar ataxia. J Neurophysiol. 2019;121:672–89.
Honegger F, van Spijker GJ, Allum JH. Coordination of the head with respect to the trunk and pelvis in the roll and pitch planes during quiet stance. Neuroscience. 2012;213:62–71.
Fitzpatrick RC, Taylor JL, McCloskey DI. Ankle stiffness of standing humans in response to imperceptible perturbation: reflex and task-dependent components. J Physiol. 1992;454:533–47.
Pinter IJ, van Swigchem R, van Soest AJ, Rozendaal LA. The dynamics of postural sway cannot be captured using a one-segment inverted pendulum model: a PCA on segment rotations during unperturbed stance. J Neurophysiol. 2008;100(6):3197–208.
Horlings CG, Küng UM, Honegger F, Van Engelen BG, Van Alfen N, Bloem BR, Allum JH. .Vestibular and proprioceptive influences on trunk movements during quiet standing. Neuroscience. 2009;161(3):904–14.
Bernhard FP, Sartor J, Bettecken K, Hobert MA, Arnold C, Weber YG et al. Wearables for gait and balance assessment in the neurological ward - study design and first results of a prospective cross-sectional feasibility study with 384 inpatients. 2018;18:114.
Spain RI, St. George RJ, Salarian A, Mancini M, Wagner JM, Horak FB, et al. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture. 2012;35:573–8.
Mancini M, Horak FB, Zampieri C, Carlson-Kuhta P, Nutt JG, Chiari L. Trunk accelerometry reveals postural instability in untreated Parkinson’s disease. Parkinsonism Relat Disorders. 2011;17:557–62.
Mancini M, Carlson-Kuhta P, Zampieri C, Nutt JG, Chiari L, Horak FB. Postural sway as a marker of progression in Parkinson’s disease: a pilot longitudinal study. Gait Posture. 2012;36:471–6.
Al-Amri M, Nicholas K, Button K, Sparkes V, Sheeran L, Davies J, et al. Inertial Measurement Units for Clinical Movement Analysis: reliability and concurrent validity. Sensors. 2018;18:719.
Hansen C, Beckbauer M, Romijnders R, Warmerdam E, Welzel J, Geritz J, et al. Reliability of IMU-Derived Static Balance parameters in Neurological diseases. Int J Environ Res Public Health. 2021;18:3644.
Wodarski P. Trend Change Analysis as a New Tool to complement the evaluation of human body balance in the time and frequency domains. J Hum Kinet. 2023;87:51–62.
Takakusaki K. Functional neuroanatomy for posture and Gait Control. JMD. 2017;10:1–17.
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease: MDS-PD Clinical Diagnostic Criteria. Mov Disord. 2015;30:1591–601.
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9.
Warmerdam E, Romijnders R, Geritz J, Elshehabi M, Maetzler C, Otto JC, et al. Proposed mobility assessments with Simultaneous Full-Body Inertial Measurement Units and Optical Motion capture in healthy adults and neurological patients for future validation studies: study protocol. Sens (Basel). 2021;21:5833.
Warmerdam E, Hansen C, Romijnders R, Hobert MA, Welzel J, Maetzler W. Full-body mobility data to Validate Inertial Measurement Unit Algorithms in healthy and neurological cohorts. Data. 2022;7:136.
Warmerdam E, Schumacher M, Beyer T, Nerdal PT, Schebesta L, Stürner KH, et al. Postural sway in Parkinson’s Disease and multiple sclerosis patients during tasks with different complexity. Front Neurol. 2022;13:857406.
Geritz J, Welzel J, Hansen C, Maetzler C, Hobert MA, Elshehabi M, Knacke H, Aleknonytė-Resch M, Kudelka J, Bunzeck N, Maetzler W. Cognitive parameters can predict change of walking performance in advanced Parkinson’s disease - chances and limits of early rehabilitation. Front Aging Neurosci. 2022;14:1070093.
Mancini M, Salarian A, Carlson-Kuhta P, Zampieri C, King L, Chiari L, et al. ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil. 2012;9:1–8.
Kim SD, Allen NE, Canning CG, Fung VSC. Postural instability in patients with Parkinson’s Disease: Epidemiology, Pathophysiology and Management. CNS Drugs. 2013;27:97–112.
Palakurthi B, Burugupally SP. Postural instability in Parkinson’s disease: a review. Brain Sci. 2019;9:239.
Scholz E, Diener HC, Noth J, Friedemann H, Dichgans J, Bacher M. Medium and long latency EMG responses in leg muscles: Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1987;50:66–70.
Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with Parkinson’s disease. Exp Neurol. 2005;193:504–21.
Adkin AL, Bloem BR, Allum JHJ. Trunk sway measurements during stance and gait tasks in Parkinson’s disease. Gait Posture. 2005;22:240–9.
Chen T, Fan Y, Zhuang X, Feng D, Chen Y, Chan P, et al. Postural sway in patients with early Parkinson’s disease performing cognitive tasks while standing. Neurol Res. 2018;40:491–8.
Nackaerts E, Michely J, Heremans E, Swinnen SP, Smits-Engelsman BCM, Vandenberghe W, et al. Training for Micrographia Alters Neural Connectivity in Parkinson’s Disease. Front Neurosci. 2018;12:3.
Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Internal vs external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. NeuroImage. 2003;19:764–76.
Beuter A, Hernández R, Rigal R, Modolo J, Blanchet PJ. Postural sway and effect of Levodopa in Early Parkinson’s Disease. Can j Neurol sci. 2008;35:65–8.
Menant JC, Latt MD, Menz HB, Fung VS, Lord SR. Postural sway approaches center of mass stability limits in Parkinson’s disease. Mov Disord. 2011;26:637–43.
Wodarski P, Jurkojć J, Michalska J, Kamieniarz A, Juras G, Gzik M. Balance assessment in selected stages of Parkinson’s disease using trend change analysis. J Neuroeng Rehabil. 2023;20:99.
Bizid R, Jully JL, Gonzalez G, François Y, Dupui P, Paillard T. Effects of fatigue induced by neuromuscular electrical stimulation on postural control. J Sci Med Sport. 2009;12(1):60–6.
Nieto-Escamez F, Obrero-Gaitán E, Cortés-Pérez I. Visual dysfunction in Parkinson’s Disease. Brain Sci. 2023;13:1173.
Valkovič P, Krafczyk S, Bötzel K. Postural reactions to soleus muscle vibration in Parkinson’s disease: scaling deteriorates as disease progresses. Neurosci Lett. 2006;401:92–6.
Acknowledgements
We thank all study participants for their support and engagement.
Funding
Open Access funding enabled and organized by Projekt DEAL. The publication is supported by the Rector’s habilitation grant implemented under the Excellence Initiative - Research University program. Silesian University of Technology, grant number: 07/030/SDU/10-07-01.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
RR and MAH and WM and CH and EW and MCh and PW and JJ: made the conception RR and MAH and EW and CH: data acquisitionPW and JJ and MCh and CH: analysisPW and WM and JJ and CH: interpretation of dataPW and JJ and KC: creation of new software used in the workWM and JJ and PW and CH: have drafted the work PW and JJ and RR and MAH and WM and CH and EW and MCh and KC: substantively revised the manuscrypt.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research procedure was approved by the ethical committee of the Medical Faculty of Kiel University (D438/18). The study is registered in the German Clinical Trials Register (DRKS00022998).
Consent for publication
All authors express their full consent to publication of the material.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wodarski, P., Jurkojć, J., Chmura, M. et al. Trend change analysis of postural balance in Parkinson’s disease discriminates between medication state. J NeuroEngineering Rehabil 21, 112 (2024). https://doi.org/10.1186/s12984-024-01411-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12984-024-01411-z