Abstract
Background
Endurance flight impose substantial oxidative costs on the avian oxygen delivery system. In particular, the accumulation of irreversible damage in red blood cells can reduce the capacity of blood to transport oxygen and limit aerobic performance. Many songbirds consume large amounts of anthocyanin-rich fruit, which is hypothesized to reduce oxidative costs, enhance post-flight regeneration, and enable greater aerobic capacity. While their antioxidant benefits appear most straightforward, the effects of anthocyanins on blood composition remain so far unknown. We fed thirty hand-raised European starlings (Sturnus vulgaris) two semisynthetic diets (with or without anthocyanin supplement) and manipulated the extent of flight activity in a wind tunnel (daily flying or non-flying for over two weeks) to test for their interactive effects on functionally important haematological variables.
Results
Supplemented birds had on average 15% more and 4% smaller red blood cells compared to non-supplemented individuals and these diet effects were independent of flight manipulation. Haemoglobin content was 7% higher in non-supplemented flying birds compared to non-flying birds, while similar haemoglobin content was observed among supplemented birds that were flown or not. Neither diet nor flight activity influenced haematocrit.
Conclusion
The concerted adjustments suggest that supplementation generally improved antioxidant protection in blood, which could prevent the excess removal of cells from the bloodstream and may have several implications on the oxygen delivery system, including improved gas exchange and blood flow. The flexible haematological response to dietary anthocyanins may also suggest that free-ranging species preferentially consume anthocyanin-rich fruits for their natural blood doping, oxygen delivery-enhancement effects.
Similar content being viewed by others
Background
Fruits provide frugivores with energy, vitamins and minerals [1, 2] as well as phytochemicals that may yield specific physiological benefits for animals [3]. For example, anthocyanins are pigments responsible for the dark-red colours of fruit and serve as strong free radical scavengers and metal chelators [4,5,6], that could benefit both the plants and their consumers. Specifically, anthocyanins may either directly mitigate oxidative stress by quenching free radicals and disrupting free radical formation [4], or indirectly affect oxidative balance by activating signalling pathways involved in endogenous antioxidant responses [7, 8]. In addition, anthocyanins are known to improve energy metabolism [9,10,11,12], increase vasodilation [13,14,15,16,17], regulate iron metabolism [18, 19], attenuate the endocrine stress response [20], and stimulate reproductive hormones and behaviour during the breeding season [21]. The wide range of health-stimulating actions identifies anthocyanins as a valuable dietary component that could be particularly rewarding during energy-demanding events, when the risk of oxidative stress is considered high [22,23,24,25].
The benefits of consuming dietary anthocyanins could be relevant for birds during the migratory season, which is known to impose distinct oxidative and metabolic challenges on migrants [26,27,28,29,30,31]. For example, during endurance flight birds may operate at five to seven times higher metabolic rate than when at rest [32,33,34,35] and this extreme metabolic effort is known to increase generation of reactive oxygen species that could damage tissues and in turn tradeoff aerobic metabolism and performance during migration [28, 36,37,38,39]. To minimize the oxidative stress risk, birds, like other vertebrates including humans, can rely on endogenous antioxidants [28, 37, 40] that quench and balance the excess production of reactive oxygen species under most exercise conditions [37, 41]. In addition, birds may also consume dietary antioxidants, which have been proposed to relieve oxidative stress-related costs [42,43,44]. Specifically, dietary antioxidants, including anthocyanins, may be consumed prophylactically to increase antioxidant capacity or therapeutically to prevent the accumulation of damage in tissues and speed up the recovery following damage [22, 25, 28, 37, 42, 45, 46]. Presumably, the reduction of oxidative costs and enhanced regeneration of metabolically active tissues provided by dietary anthocyanins could enable greater aerobic capacity and thus promote phenotypic adjustments that support more efficient oxygen supply during exercise. However, the effects of dietary anthocyanins on the oxygen delivery system have not yet been investigated.
The oxidative hazard during intense physical activity can be acute to metabolically active tissues such as skeletal muscles, heart [41, 47] and blood [48]. Specifically, red blood cells are vulnerable to oxidative damage because of their continuous exposure to high oxygen fluxes, the high content of heme–iron (a potential catalyst of free radicals formation), and membranes that are rich in polyunsaturated fatty acids [49,50,51,52]. Moreover, mature erythrocytes are unable to repair or synthesize damaged proteins de novo [53], hence the oxidative stress might eventually impair the oxygen delivery system by permanent alterations in red blood cell properties and/or by accelerated removal of unfunctional erythrocytes from the bloodstream. The alterations in erythrocytes may include loss of cell volume and surface [54,55,56]. When cell shrinkage is non-uniform (i.e., involves changes in the shape) and entails a decreased surface-area-to-volume ratio (e.g. due to a greater degree of loss of surface than volume), then it can reduce cell deformability [56,57,58]. In consequence, this can substantially impede the passage of red blood cells through the microcirculation [59, 60]. In addition, oxidative damage is known to cause haemolysis [54, 61,62,63], which, if not compensated by increased erythropoiesis, may lead to anaemia that have organism-wide consequences including fatigue and impaired physical performance. In birds, the exercise-induced anaemia could occur during migration as indicated by low haematocrit and haemoglobin content of bar-tailed godwits (Limosa lapponica taymyrensis) sampled upon their arrival at a migratory stopover [64, 65]. To prevent the potential oxidative costs in blood, birds may enhance their antioxidant defenses through increased or selective consumption of dietary anthocyanins and other antioxidants, which are known to increase the resistance of erythrocytes to free radical oxidation [61, 66,67,68,69,70].
Despite evidence that dietary anthocyanins are beneficial for antioxidant capacity in avian blood [71], to our best knowledge, their effects on haematological variables have not been investigated in the context of intense and regular physical activity like that associated with continuous flight of migratory birds. Our research study focuses on whether migratory birds can benefit from the consumption of an anthocyanin-rich diet during the energy-demanding event. Specifically, we tested the hypothesis that long-term anthocyanin supplementation and regular flying interactively affect functionally important blood properties in a short-distance migratory bird, the European starling (Sturnus vulgaris). We hand-raised thirty hatchling birds and employed a two-factorial experiment that involved manipulation of dietary anthocyanin availability (with or without supplementation) and extent of flying (daily flying or non-flying) to test for their effects on a suite of common haematological variables. Flights were performed during natural spring migration season of starlings living in Germany, in an advanced research wind tunnel that enables precise control of steady flight conditions [32, 72] and has been successfully used in numerous flight-related studies on birds [20, 32, 73]. We predicted that starlings flown each day for several weeks would have reduced red blood cell number, lower haematocrit and haemoglobin content, and smaller cell size compared to more sedentary starlings. We also expected that these flight-induced changes in blood parameters would be mitigated in anthocyanin-supplemented starlings and that the more sedentary, non-flying groups would have similar haematological profiles because of their reduced physical activity and associated lower risk of oxidative stress in blood. Captivity and a sedentary lifestyle in birds can also cause oxidative stress, although it is apparently lower when compared to a more active lifestyle [74]. It is important to note, however, that oxidative stress is not necessarily increased with increased physical activity but rather mitochondrial functioning and increased protonmotive force [75]. Therefore, our prediction that sedentary birds would be better protected from oxidative stress and consequently would have similar haematological profiles as anthocyanin-supplemented birds should be viewed with caution.
Results
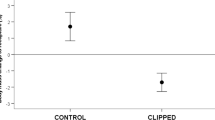
Out of the four primary haematological variables, only the number of red blood cells (RBCcount) was increased and the size (RBCarea) decreased due to diet treatment (Table 1, Fig. 1C, D, Additional file 1: Fig. S1). Haematocrit (Hct) did not respond to any of the experimental treatments (Table 1, Fig. 1A, Additional file 1: Fig. S1), whereas haemoglobin (Hb) content was the only variable for which we detected a statistically significant diet × flight interaction (Additional file 1: Table S1, Fig. 1B, Additional file 1: Fig. S1). Specifically, starlings supplemented with anthocyanin had similar Hb content when flown compared to unflown (t1,25 = 1.20, p = 0.242), whereas starlings not fed the dietary supplement had 7% higher Hb content when flown compared to unflown (t1,25 = − 2.00, p = 0.057). Otherwise, we found clear effects of anthocyanin supplement but not flight on all secondary and shaped-related haematological variables except mean corpuscular haemoglobin content (MCHC) and red blood cell major axis length (RBCmajor, Table 1, Additional file 1: Fig. S1).
The effect of anthocyanin supplementation and wind-tunnel flight activity on the primary haematological variables (upper row) and selected secondary haematological variables (lower row). White and black colours represent non-flying and flying birds respectively. Data points are least-squares means (LSM) with 95% confidence intervals (CI) derived from the linear models. Hct Haematocrit; Hb Haemoglobin content; RBCcount Red blood cell number; RBCarea Red blood cell surface area; MCH Mean cell haemoglobin; MCHC Mean cell haemoglobin concentration; MCV Mean cell volume; SA:V ratio Surface-area-to-volume ratio
Anthocyanin supplemented starlings compared to non-supplemented ones had on average 15% higher RBCcount (Table 2, Fig. 1D, Additional file 1: Fig. S1) and smaller erythrocyte size indicated by about 4% lower RBCarea (Table 2, Fig. 1C, Additional file 1: Fig. S1) and 13% lower mean cell volume (MCV, Table 2, Fig. 1D, Additional file 1: Fig. S1). The reduction of cell size was also associated with about 13% lower mean corpuscular haemoglobin (MCH, Table 2, Fig. 1E, Additional file 1: Fig. S1). Birds fed with anthocyanin-rich diet had increased surface-area-to-volume ratio (SA:V ratio, Table 2, Fig. 1H, Additional file 1: Fig. S1) and total surface area of erythrocytes (TSAE) by about 11% and decreased Hb-content-to-TSAE ratio (Hb:TSAE ratio) ratio by 9%, compared to birds fed with control diet (Table 2, Additional file 1: Fig. S1). In terms of shape, smaller erythrocytes of supplemented birds were more elongated, characterized by a higher aspect ratio (by about 4%) and lower cell circularity (by about 3%), which have resulted from about 3% shorter length of red blood cell minor axis length (RBCminor) compared to erythrocytes of non-supplemented birds (Table 2, Additional file 1: Fig. S1). Hct, Hb content, RBCcount and TSAE were additionally influenced by the date of sampling (Table 1, Additional file 1: Fig. S1), with all four variables consistently decreasing over the course of the spring season.
Discussion
Our study provides novel evidence for the effects of anthocyanin supplementation on functionally important haematological variables in songbirds. Long-term supplementation with anthocyanins in starlings resulted in substantially smaller and more numerous red blood cells compared to control birds not fed the anthocyanin supplement. Contrary to our predictions and except for the interaction effect on haemoglobin content, we detected no clear effect of wind tunnel flight activity on the haematological variables. We acknowledge that the unclear flight-induced effects may be due to the potentially mild nature of the exercise, which in turn may not be sufficient to induce oxidative stress and haematological changes in starlings. However, using similar flight training regimens, previous studies have successfully simulated the exercise nature of migratory flights and its effects on bird physiology [20, 32, 76] including the effects on oxidative status [71, 73]. Worth noting, the flying group in our study was exposed to an additional 600–700 km of continuous flapping flight compared to non-flying birds, which likely increases the risk of oxidative damage compared to sedentary birds, although it may not have been sufficient to induce acute effects on haematological variables, such as typically associated with strenuous activities. The observed changes in size and number could have several implications related to the exchange of respiratory gases, blood flow behaviour and efficiency of haemoglobin use.
Consuming dietary anthocyanins produces smaller and more abundant red blood cells with implications for oxygen delivery
Erythrocytes are continuously exposed to various stressors and oxidative challenges in circulation [48, 52] and thus may particularly require effective antioxidant safeguards [54, 56, 59, 60]. Anthocyanin supplementation has been demonstrated to increase the resistance of red blood cells to oxidative stress [70] and thereby may prevent excessive destruction and removal of erythrocytes from the bloodstream. Alternatively, it is also possible that dietary anthocyanins induce changes in blood independently of their effects on oxidative stress mitigation. For example, anthocyanin supplementation can increase testosterone levels in starlings [21], which in turn can enhance erythropoietic processes in birds (Herrick et al.[77] but see Kern et al. [78]). Considering these various possibilities, we found accordingly that birds fed dietary anthocyanins had about 15% more red blood cells (Fig. 1D) compared to non-supplemented birds. The higher number of erythrocytes in anthocyanin supplemented birds was accompanied by size effects; erythrocytes were with about 4% lower surface area (Fig. 1C) and about 13% lower volume (Fig. 1G) substantially smaller than that of control birds. The adjustments in the two size-related traits resulted in about 11% higher surface-area-to-volume ratio (Fig. 1H). However, it is important to note that the increase in the surface-area-to-volume ratio was due to non-uniform shrinkage, which favorably impacted both cell size and shape (Table 2). Possibly, if non-uniform shrinkage had caused a larger loss of surface area than volume, it could have had a negative effect on oxygen delivery processes through a decrease in the surface-area-to-volume ratio (see next paragraphs). Interestingly, in mammals, senescent erythrocytes appear to decrease cell surface area by a similar degree as cell volume and this may consequently lead to constant surface-area-to-volume ratio and more dense cells [56, 79, 80]. Assuming that similar mechanisms are involved in birds, the considerable increase in surface-area-to-volume ratio found in our study may suggest that the cell size changes not only result from a higher proportion of older cells since they were better protected from oxidative damage, but could be also accompanied by additional structural changes in the cytoskeleton and shape of erythrocytes. The bullet-like shape of erythrocytes (i.e., shorter minor axis, higher aspect ratio, lower circularity, Table 2) found in anthocyanin-supplemented birds might also suggest that the cells are less resistant to flow and may have larger contact area with surrounding tissues [81]. Taken together, the increase in red blood cell number with the paralleled decrease in cell size and adjustments in shape may support the view that circulating erythrocytes of anthocyanin supplemented birds are better protected against oxidative damage. Yet, the physiological significance of changes in cell number, size, and shape of avian erythrocytes requires further investigation.
One of the most important implications of adjustments in the size of red blood cells is the effect on the exchange of respiratory gases. In general, smaller erythrocytes are known to have a higher rate of oxygen uptake and release, which is attributed to a larger surface-area-to-volume ratio of smaller cells [82,83,84]. However, due to relatively high energy demands for the maintenance of ion gradients, smaller cells may promote higher basal metabolism at the whole animal level [85, 86]. The negative relationship between basal metabolic rate and erythrocyte size has been demonstrated in various vertebrates at the inter- [87,88,89] and also the intra-specific level [90,91,92,93,94]. High surface-area-to-volume ratio found in anthocyanin-supplemented birds thus could facilitate considerably faster gas exchange, although this might come at higher energy costs required for maintenance of metabolism. However, it is important to note that increased energy metabolism of the cell does not necessarily increase the production of reactive oxygen species, and can actually have the opposite effect depending on the balance between mitochondrial efficiency and uncoupling processes [75]. Further studies should address the question of how red blood cell size and surface-area-to-volume ratio affect mitochondrial functioning and oxidative stress.
In addition to the effects on the exchange of respiratory gases, the decreased cell size and the increased surface-area-to-volume ratio could also promote more efficient blood flow. Specifically, there is a positive relationship between surface-area-to-volume and the degree to which erythrocytes can deform when passing through narrow capillaries [95,96,97]. Furthermore, the suspensions of smaller red blood cells were found to have lower resistance to flow than that of larger cells [98]. Accordingly, the here observed changes in red blood cell size and surface-area-to-volume ratio may not only suggest the improved erythrocyte deformability but also serve as the mechanism to reduce blood flow resistance [96, 99,100,101]. Importantly, blood flow can be considerably reduced by high haematocrit, a well-known factor that increases blood viscosity [96, 102,103,104]. In this study, despite the substantial increase in red blood cell number, haematocrit remained unchanged (Fig. 1A), which might be explained by the concomitant decrease in erythrocyte size (Fig. 1A, G) or an expansion of plasma volume [104, 105]. The maintenance of constant haematocrit can thus represent the additional mechanism of blood viscosity optimization and could help to minimize the cost of blood circulation at a given flow rate [106, 107], promoting more efficient oxygen transport during exercise [108].
The adjustments in cell size and number could also enable more efficient haemoglobin use, especially when birds encounter increased blood flow [109, 110]. Full haemoglobin saturability in the lungs depends on haemoglobin-oxygen affinity [111], the rate of gas exchange [82] and the available exchange surface of erythrocytes relative to the haemoglobin content in the blood (haemoglobin-to-total-surface-area-of-erythrocytes ratio) [109, 110, 112]. A rapid capillary transit associated with increased blood flow may reduce the time required for full haemoglobin saturation, hence, a larger total surface of erythrocytes area per blood volume unit may help to overcome eventual limitations of increased blood flow and enable more efficient haemoglobin use. We found about 11% larger total surface area of erythrocytes among anthocyanin supplemented birds (Table 2, Supplementary Additional file 1: Fig. S1), which combined with relatively small changes in haemoglobin content, also resulted in a 9% lower haemoglobin-to-total surface area of erythrocytes ratio of supplemented birds compared to non-supplemented ones (Table 2, Supplementary Additional file 1: Fig. S1). This may suggest that the full haemoglobin saturation in non-supplemented starlings could be limited by the rapid capillary transit during flight. Kostelecka-Myrcha [109] found that birds, independently of physiological and environmental conditions, have a constant haemoglobin-to-total surface area of erythrocytes ratio. This constancy has been suggested optimal for haemoglobin saturation with oxygen, which in birds takes place under constant conditions due to cross-current gas exchange in their tubular lungs [109, 113]. Our study challenges this view and demonstrates that the haemoglobin-to-total-surface-area of erythrocytes ratio in birds can be modulated by appropriate adjustments in size and number of red blood cells in response to diet. The increase in total surface area of erythrocytes found among anthocyanin supplemented birds could represent one of the mechanisms to overcome limitations in haemoglobin saturability potentially associated with elevated blood flow, but may also compensate for an eventual reduction in the oxygen-carrying capacity of the blood, ensuring the more efficient haemoglobin use during flight.
No effect of physical activity (flight) on haematological variables
Haematological variables in birds, like in other vertebrates, are portrayed as phenotypically flexible traits that can respond to the increased levels of physical activity. This response may differ depending on the duration of exercise (i.e. short exercise bouts vs periods of repeated exercise) [114] and it was demonstrated that red blood cell number, haemoglobin content and haematocrit decrease after weeks of increased flight activity in zebra finches [114]. Similarly, reduced haematocrit and haemoglobin content was found in bar-tailed godwits (Limosa lapponica taymyrensis) at the beginning of migratory stopover [64, 65]. One explanation could be that migrants suffer from haemolytic anaemia incurred by oxidative stress during endurance flights. Alternatively, birds may increase plasma volume—and thus decrease haematocrit—to reduce blood viscosity and costs associated with circulation [115, 116]. Because in our study the changes in blood haemoglobin occurred without a change in haematocrit (Fig. 1A), birds could have altered the amount of haemoglobin within a single erythrocyte, indicated by the analogous change in mean corpuscular haemoglobin concentration. Although we did not find such an effect (Fig. 1F), there is a relatively large uncertainty around the effect size estimates (Additional file 1: Fig. S1), that may suggest we did not have enough statistical power to detect the effect. Collectively, the interaction effect on haemoglobin content and lack of clear effects of flight activity in other haematological variables may suggest that starlings in our study did not experience flight-induced anaemia or had quickly recovered from flights each day during the wind tunnel flight training period.
Phenotypic flexibility in erythrocyte size and number and its implications for migratory birds
Simultaneous adjustments in red blood cell number, haematocrit and haemoglobin content often occur in response to changes in environmental and physiological conditions [114, 117,118,119]. Our study shows that such parallel responses are not consistent and might depend on additional changes in other blood-related traits. Importantly, if we would have studied only the routinely measured variables in avian studies—haematocrit (for review see [120]) and haemoglobin content (for review see [121, 122])—we would have missed the response in red blood cell size and number to the dietary anthocyanin manipulation. Considering this potential bias of many studies that have only single variables, comparable phenotypic flexibility in erythrocyte size in concert with cell number changes like that reported here may in fact occur more often in response to other environmental or endogenous conditions. Clearly, more comprehensive investigations on haematological variables beyond the easily obtained measurements are required.
Independent of flight activity and diet, several erythrocyte traits (i.e., red blood cell number, haemoglobin content and haematocrit, total surface area of erythrocytes; Table 1) decreased from February to March. These temporal trends are consistent with seasonal changes of haematological variables documented in other northern hemisphere birds, with higher values of blood-related traits during winter and lower values during spring and summer [123,124,125]. The observed effect could be associated with genetically preprogrammed seasonal adjustments in body mass and metabolism [126], which are known to affect energy requirements and, consequently, modulate oxygen demands of the tissues [125].
While our study provides evidence that dietary anthocyanins can have a positive impact on blood parameters, measuring the uptake of anthocyanins into the blood and assessing the total antioxidant capacity of the blood or plasma could have provided more comprehensive insights into the effectiveness of the supplemented diet in preventing oxidative damage to the blood cells. Due to limitations in our experimental design, we were unable to measure these parameters in the current study. However, as previously reported in our companion study [71], we found that anthocyanin supplementation can have mixed effects on the oxidative status of birds. Specifically, we found that there were no consistent changes in oxidative status between birds on diets with and without anthocyanin supplement. Starlings on the anthocyanin-rich diet decreased in non-enzymatic antioxidant capacity during the 15-day flight training period and this decrease occurred regardless of whether the birds were trained or untrained. In contrast, birds on the control diet maintained their non-enzymatic antioxidant capacity over the course of this period [71]. These results suggest that anthocyanin-supplemented birds may have utilized non-enzymatic antioxidants more efficiently than birds fed with the control diet. In future studies, it would be beneficial to include measurements of the uptake of anthocyanins into the blood. This would allow for a better understanding of the observed effects. Additionally, assessing the total antioxidant capacity of the blood or plasma would provide more conclusive evidence that the effects seen are due to greater antioxidant protection. Finally, it is important to note that the elderberry extract we used as the supplement contains other phytochemicals in addition to anthocyanins, which can display a range of biochemical effects [127], and thus contribute to the observed changes in haematological parameters. Although we cannot retrospectively measure the total antioxidant capacity and phytochemical profile of the control and anthocyanin-rich diets, we suggest that future studies could include these measurements in order to fully understand the extent of possible diet-treatment effects.
The flexible response of blood composition to dietary anthocyanins is novel and suggests that free-ranging species may preferentially consume anthocyanin-rich fruits for their natural blood doping, oxygen delivery-enhancement effects. Because our study focused exclusively on female birds, it is important to note that further research is needed to assess potential gender-specific differences in response to dietary anthocyanins. Nevertheless, our findings are likely generalizable to males, similar to what was previously observed in the effects of dietary flavonoids (including anthocyanins) on the immune responsiveness of black caps [42]. Although the underlying mechanism, as well as the ecological relevance for this phenotypic flexibility, remains to be directly investigated, the observed adjustments in the size and number of red blood cells may impact several variables associated with the efficiency of supply and removal of respiratory gasses to and from the tissues. A combination of potential effects on the exchange of respiratory gasses, blood flow behaviour and efficiency of haemoglobin use, may set up the physiological machinery for an energy-efficient metabolism. Such slow but enduring effects established through dietary effects could still be modulated by short-term effects, for example in response to immediate responses like shown for other song birds (e.g., [114]). In other words, an additional decrease in red blood cell size, as well as the increased haematocrit and haemoglobin content at the moment of peak aerobic metabolism, may further enhance oxygen supply or may provide assets against the eventual limitations in the oxygen delivery system during flight [114]. Considering the dietary preferences of many songbirds during the migratory season [26, 27], our study also supports the view that migrants could select anthocyanin-rich fruits for their natural oxygen delivery-enhancements effects.
Methods
Hand-raising of hatchlings from a local colony of migratory European starlings
Thirty female five-to-eight-day old European starlings (Sturnus vulgaris) were collected from nest boxes in late-April to early-May 2015 from a native colony in Upper Bavaria, South Germany (47° 58′ N, 11° 13′ 142 E) and brought into an animal care facility at the Max Planck Institute for Ornithology (MPIO), Seewiesen, Germany. We chose to focus on female starlings as they may face unique physiological challenges during migration in relation to their reproductive success. Migratory birds prepare to breed shortly after spring migration and depositing antioxidants into eggs has been shown to lead to higher hatching and fledging rates for offspring [128, 129]. Female birds may thus face substantial oxidative tradeoff during migration, as they must balance preventing oxidative damage with their future reproductive success [44]. Hatchlings were hand-raised and fed a high protein diet consisting of bee larvae, crickets, wax worms, green bottle fly larvae (pinkies and buffaloes), beef heart with vitamin mixture and calcium carbonate powder. Once hatchlings were able to feed independently, we additionally offered them live mealworms, fresh fruits and vegetables. The diet has been successfully used to hand-raise starlings at the MPIO in previous experiments [32, 73, 130]. From about the age of 35–100 days, the starlings were moved to outdoor aviaries, kept under a natural light cycle, and maintained on an MPIO diet that consisted of insect powder, lettuce, fresh apples and oranges, dried fruit pellets, and mealworms.
Experimental diets and supplementation with dietary anthocyanins from elderberry fruits
Starting from August 2016, birds were fed with a semi-synthetic (agar-based) diet that simulated a relatively high lipid natural fruit diet (41% carbohydrates: 23% protein: 20% fat) [1, 131]. In October 2016, starlings were randomly assigned to either control-diet (n = 16) or anthocyanin-diet (n = 14) group. The two semi-synthetic diets were identical except 1.64 mg of standardized elderberry extract (6.5% Powder; Artemis International, Inc., Fort Wayne, IN, USA) per gram of food was included in the anthocyanin diet (see [21] for the detailed composition of these diets). Elderberries are one of the most anthocyanin-rich fruits found in nature [132] and are commonly consumed by starlings and other passerine birds during autumn [133]. The anthocyanin concentration was chosen to reflect a natural level of consumption that birds would experience during periods of lower fruit intake [42]. This was determined to be equivalent to a bird consuming around 17 berries per day, based on a daily food intake of 35 g. A standard vitamin-mineral mix was also included in both diets to ensure the nutritional adequacy of other micronutrients and to provide the necessary Vitamin C for efficient use of the dietary anthocyanin supplement [21].
Housing conditions prior to the start of flight-training
After ca. 100 days old, birds were provided ad libitum fresh semi-synthetic diet and drinking and bathing water each day. From August 2016 to at least February 9, 2017, birds were housed in separate outdoor aviaries (two aviaries per each experimental diet) in groups of 15–20 individuals (the number includes other starlings fed with the same diet, that were used in study by [71]. Outdoor aviaries were 3.0 × 4.0 × 2.0 m with a 3 m long half-wall subdividing the aviary into sections: one larger (1.9 × 4.0 m) and one smaller (1.0 × 4.0 m). During the colder winter months (November to February), food and water were placed on a heater mat to avoid its freezing. The outdoor aviaries were maintained on a 13D:11N light cycle, until December 22, 2016, when the light schedule was temporarily changed (until February 14, 2017) to 10D:14N to simulate the natural light cycle of starlings wintering in Rome. After January 21, 2017, the natural light transmitted through the opaque ceiling and front window of each outdoor aviary was longer than the provided aviary lights so thereafter the birds were exposed to natural photoperiod in the outdoor aviaries (for details see [21]).
Flight training of cohorts in a wind-tunnel and blood sampling
The following experimental procedures and measurements took place during natural spring migration season of starlings. Prior to February 9, 2017, starlings in each of the two diet groups were randomly assigned to cohorts of four–six individuals each of which included at least two–three birds to be flown in the wind tunnel or not flown. Starting on February 9, 2017, and continuing every three–nine days, birds from a randomly selected cohort were transferred to the wind tunnel facility and housed in indoor aviaries (1.5 × 2.5 × 2.5 m) that surrounded the wind tunnel. These indoor aviaries were maintained on a 13D:11N light cycle and at relatively constant temperature (18–22 °C). Non-flying birds in a given cohort were placed in cloth-covered cages (25 × 50 × 30 cm) with perch and without access to food and water while flying birds were flight trained each day for 15 consecutive days in the wind tunnel. Each day during flight training, flown birds from a given cohort were allowed to fly out of their aviary, into an enclosed area adjacent to the wind tunnel, and then into the working section (L × W: 2 m × 1.2 m; octagon-shaped). Wind speed was a constant 12 m s−1 and ambient temperature was 18–22 °C for all experimental flights. Numerous other recent studies have used a similar wind speed and temperature, and a 15-day flight-training regime to successfully fly small groups of starlings for relatively long durations [20, 32, 71, 73, 76, 134, 135]. After five days of habituation to the wind tunnel, all flown birds were exposed to the same 15-day flight-training regime as follows: days 1–3 = 20 min; day 4 = 30 min; day 5 = 60 min; day 6 = 30 min; day 7 = 60 min; day 8 = 90 min; day 9 resting = no flight; day 10 = 120 min; day 11 = 180 min; day 12 resting = no flight; day 13 = 60 min; day 14 = 30 min. On day 15, starlings flew for up to 6 h until they refused to stay aloft and attempted to perch on the ground or netting at least three times within a five-min period.
After the 15 day flight-training period, females in each cohort were transferred to outdoor aviaries that contained seven–eight male starlings (sex ratio of 1.5 males: 1 females) as part of a companion study focusing on the effects of dietary antioxidants on breeding behavior [21]. For the next five consecutive days, females could interact with males while having constant access to their original diet. In the afternoon (~ 15:00) of day 20, birds were transferred back to the wind tunnel facility for overnight measurements of basal metabolic rate (BMR) at 25 °C temperature (data not covered in this paper). Food was removed at ~ 15:00 on Day 20 so that BMR was measured in post-absorptive birds. At ~ 8:00 on day 21, we sampled ~ 400 µL of blood from the brachial vein of each bird using heparinized capillary tubes after puncture with a 17G needle. Blood samples were immediately transferred to a 0.5 mL heparinized Eppendorf tube for temporary storage. Collecting blood samples 5 days after two weeks of daily flying and with males present mimics the arrival to the breeding grounds following the completion of spring migration.
Measurements of haematological variables
Measurements of haemoglobin (Hb) content, haematocrit (Hct) and red blood cell count (RBCcount), as well as preparations of blood smears for measurements of red blood cells surface area (RBCarea), were done within 30–60 min of blood sampling each bird, hereafter referred as primary haematological variables. Haemoglobin content (g/L) was measured with HemoCue AB (Angelholm, Sweden) and for the reliability of this method, we additionally cross-calibrated the device with reference to Drabkin’s solution method. To assess Hct, we filled micro-capillaries with ~ 9 µL of blood and then centrifuged them for 6 min at 11,500 rpm (Eickemeyer, Tuttlingen, Germany) and then measured with callipers the fraction of red blood cells to the nearest hundredth of a millimetre. RBC count (per µL) was counted using a standard microscope and Bürker’s chambers. If possible, Hb, Hct and RBCcount were measured in duplicate subsamples to increase reliability and the mean was used as estimates of each variable. To measure RBCarea (µm2) we fixed and stained blood smears on glass slides (staining with Gill II Hematoxylin, Sigma, Aldrich, Germany; Eosin Y, Analab, Warszawa, Poland). Microscope photographs were taken four–five months after preparation of glass slides, under 100-fold magnification of a light microscope (Nikon Eclipse 801, Tokyo, Japan) connected to a camera (Nikon DS-Ri2, Tokyo, Japan) and NIS Elements software (Nikon, Tokyo, Japan). Finally, we used Fiji software (imagej.nig.gov/ij; fiji.sc/Fiji) to measure area from 50 erythrocytes and the mean value was used as an estimate of RBCarea. The software was set to automatically measure the cell area after image thresholding. Cells with a distorted shape and those deformed due to touching other cells were excluded from the measurements. In addition to cell surface area, the software also measured length (RBCmajor) and width of the cells (RBCminor) and calculated the aspect ratio (RBCar; the ratio of major to minor axis) and cell circularity (RBCcirc; 4π × area × perimeter–2; a value of 1 indicates a perfect circle cell shape and as the value approaches 0, cells are increasingly elongated in shape), which we refer to shape-related variables. Based on the four primary haematological variables, we calculated a set of secondary haematological variables that could help us in the interpretation of eventual alterations in erythrocyte properties: Mean Corpuscular Haemoglobin Content (MCHC, pg/cell) calculated from Hb and RBCcount, Mean Corpuscular Haemoglobin (MCH, g/dL) calculated from Hb and Hct and Mean Corpuscular Volume (MCV, fL) calculated from Hct and RBCcount. The surface-area-to-volume ratio of erythrocytes (SA:V ratio, µm2/µm3) was calculated by dividing the RBCarea by MCV, while the total surface area of erythrocytes (TSAE, µm2/µm3) by multiplying SA:V by RBCcount. The haemoglobin-to-total-surface-area-of-erythrocytes ratio was calculated by dividing Hb content by TSAE (Hb:TSAE ratio, pg/mm3).
Statistical analysis
Statistical analyses were performed in R (version 4.0.3, R Core Team 2020). We used linear models to test the effects of diet (control-diet vs anthocyanin-diet), migratory flight activity (non-flying vs flying), and their interactive effect on all haematological variables measured or calculated in this study. To control for potential temporal changes, all models included the date of sampling as a fixed covariate. Initially, all the models contained first and second-order interactions between the covariate and main effects; however, all these interactions were not significant and were removed from the final models. We checked the assumptions of normality, homogeneity and lack of remaining patterns on residuals. To infer the fixed effects, we used Type III F-test from the R package “car” (function “Anova”; [136]). We used the R package “emmeans” [137] to estimate (a) the Least Squares Means with 95% confidence intervals for each experimental group (conditional and marginal means), and (b) the standardized effect sizes (Cohen’s d [138]; function “eff_size”) following Nakagawa and Cuthill [139]. In the case of statistically significant interaction between main effects, we further analyzed simple effects (Tukey-adjusted) of flight activity (non-flying vs flying) separately within the two diet groups.
Availability of data and materials
We agree to archive the data associated with this manuscript should the manuscript be accepted. We expect to archive the data at Dryad Digital Repository.
References
Johnson RA, Willson MF, Thompson JN, Bertin RI. Nutritional values of wild fruits and consumption by migrant frugivorous birds. Ecology. 1985;66:819–27.
Jordano P. Fruits and frugivory. In: Seeds: the ecology of regeneration in plant communities. United Kingdom: Cabi Wallingford; 2014. p. 18–61.
Villalba JJ, Costes-Thiré M, Ginane C. Phytochemicals in animal health: diet selection and trade-offs between costs and benefits. Proc Nutr Soc. 2017;76:113–21.
González-Paramás AM, Ayuda-Durán B, Martínez S, González-Manzano S, Santos-Buelga C. The mechanisms behind the biological activity of flavonoids. Curr Med Chem. 2018;26:6976–90.
Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–33.
Landi M, Tattini M, Gould KS. Multiple functional roles of anthocyanins in plant-environment interactions. Environ Exp Bot. 2015;119:4–17.
Cimino F, Speciale A, Anwar S, Canali R, Ricciardi E, Virgili F, Trombetta D, Saija A. Anthocyanins protect human endothelial cells from mild hyperoxia damage through modulation of Nrf2 pathway. Genes Nutr. 2013;8:391–9.
Yan F, Chen Y, Azat R, Zheng X. Mulberry anthocyanin extract ameliorates oxidative damage in HepG2 cells and prolongs the lifespan of Caenorhabditis elegans through MAPK and Nrf2 pathways. Oxid Med Cell Longev. 2017;2017.
Gomes JVP, Rigolon TCB, da Silveira Souza MS, Alvarez-Leite JI, Della Lucia CM, Martino HS, de Rosa OB. Antiobesity effects of anthocyanins on mitochondrial biogenesis, inflammation, and oxidative stress: a systematic review. Nutrition. 2019;66:192–202.
Jia Y, Wu C, Kim YS, Yang SO, Kim Y, Kim JS, Jeong MY, Lee JH, Kim B, Lee S, et al. A dietary anthocyanin cyanidin-3-O-glucoside binds to PPARs to regulate glucose metabolism and insulin sensitivity in mice. Commun Biol. 2020;3:2–11.
Matsukawa T, Motojima H, Sato Y, Takahashi S, Villareal MO, Isoda H. Upregulation of skeletal muscle PGC-1α through the elevation of cyclic AMP levels by Cyanidin-3-glucoside enhances exercise performance. Sci Rep. 2017;7:1–12.
You Y, Han X, Guo J, Guo Y, Yin M, Liu G, Huang W, Zhan J. Cyanidin-3-glucoside attenuates high-fat and high-fructose diet-induced obesity by promoting the thermogenic capacity of brown adipose tissue. J Funct Foods. 2018;41:62–71.
Edwards M, Czank C, Woodward GM, Cassidy A, Kay CD. Phenolic metabolites of anthocyanins modulate mechanisms of endothelial function. J Agric Food Chem. 2015;63:2423–31.
Matsumoto H, Takenami E, Iwasaki-Kurashige K, Osada T, Katsumura T, Hamaoka T. Effects of blackcurrant anthocyanin intake on peripheral muscle circulation during typing work in humans. Eur J Appl Physiol. 2005;94:36–45.
Sorrenti V, Mazza F, Campisi A, Di Di Giacomo C, Acquaviva R, Vanella L, Galvano F. Heme oxygenase induction by cyanidin-3-O-β-glucoside in cultured human endothelial cells. Mol Nutr Food Res. 2007;51:580–6.
Speciale A, Cimino F, Saija A, Canali R, Virgili F. Bioavailability and molecular activities of anthocyanins as modulators of endothelial function. Genes Nutr. 2014;9(4):1–9.
Xu JW, Ikeda K, Yamori Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension. 2004;44:217–22.
Mu M, Wu A, An P, Du X, Wu Q, Shen X, Wang F. Black soyabean seed coat extract regulates iron metabolism by inhibiting the expression of hepcidin. Br J Nutr. 2014;111:1181–9.
Villaño D, Vilaplana C, Medina S, Algaba-Chueca F, Cejuela-Anta R, Martínez-Sanz JM, Ferreres F, Gil-Izquierdo A. Relationship between the ingestion of a polyphenol-rich drink, hepcidin hormone, and long-term training. Molecules. 2016;21(10):1333.
Casagrande S, DeMoranville KJ, Trost L, Pierce BJ, Bryla A, Dzialo M, Sadowska ET, Bauchinger U, McWilliams SR. Dietary antioxidants attenuate the endocrine stress response during long-duration flight of a migratory bird. Proc Natl Acad Sci B. 2020;18:1–8.
Carbeck KM, DeMoranville KJ, D’Amelio PB, Goymann W, Trost L, Pierce B, Bryła A, Dzialo M, Bauchinger U, McWilliams SR. Environmental cues and dietary antioxidants affect breeding behavior and testosterone of male European starlings (Sturnus vulgaris). Horm Behav. 2018;103:36–44.
Bowtell J, Kelly V. Fruit-derived polyphenol supplementation for athlete recovery and performance. Sport Med. 2019;49:3–23.
Braakhuis AJ, Somerville VX, Hurst RD. The effect of New Zealand blackcurrant on sport performance and related biomarkers: a systematic review and meta-analysis. J Int Soc Sports Nutr. 2020;17:1–10.
Cook MD, Willems MET. Dietary anthocyanins: a review of the exercise performance effects and related physiological responses. Int J Sport Nutr Exerc Metab. 2019;29:322–30.
Kashi DS, Shabir A, Da Boit M, Bailey SJ, Higgins MF. The efficacy of administering fruit-derived polyphenols to improve health biomarkers, exercise performance and related physiological responses. Nutrients. 2019;11:2389.
Alan RR, McWilliams SR, McGraw KJ. The importance of antioxidants for avian fruit selection during autumn migration. Wilson J Ornithol. 2013;125:513–25.
Bolser JA, Alan RR, Smith AD, Li L, Seeram NP, McWilliams SR. Birds select fruits with more anthocyanins and phenolic compounds during autumn migration. Wilson J Ornithol. 2013;125:97–108.
Cooper-Mullin C, McWilliams SR. The role of the antioxidant system during intense endurance exercise: lessons from migrating birds. J Exp Biol. 2016;219:3684–95.
Costantini D. Oxidative stress and hormesis in evolutionary ecology and physiology: a marriage between mechanistic and evolutionary approaches. 2014.
Jenni-Eiermann S, Jenni L, Smith S, Costantini D. Oxidative stress in endurance flight: an unconsidered factor in bird migration. PLoS ONE. 2014;9:1–6.
McWilliams SR, Guglielmo C, Pierce B, Klaassen M. Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. J Avian Biol. 2004;35:377–93.
Engel S, Biebach H, Visser GH. Metabolic costs of avian flight in relation to flight velocity: a study in rose coloured starlings (Sturnus roseus, Linnaeus). J Comp Physiol B Biochem Syst Environ Physiol. 2006;176:415–27.
Klaassen M, Kvist A, Lindström Å. Flight costs and fuel composition of a bird migrating in a wind tunnel. Condor. 2000;102:444–51.
Lindström Å, Klaassen M, Kvist A. Variation in energy intake and basal metabolic rate of a bird migrating in a wind tunnel. Funct Ecol. 1999;13:352–9.
Schmidt-Wellenburg CA, Biebach H, Daan S, Visser GH. Energy expenditure and wing beat frequency in relation to body mass in free flying Barn Swallows (Hirundo rustica). J Comp Physiol B Biochem Syst Environ Physiol. 2007;177:327–37.
Beaulieu M, Reichert S, Le Maho Y, Ancel A, Criscuolo F. Oxidative status and telomere length in a long-lived bird facing a costly reproductive event. Func Ecol. 2011;25:577–85.
Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford: Oxford University Press; 2015.
Morosinotto C, Rainio M, Ruuskanen S, Korpimäki E. Antioxidant enzyme activities vary with predation risk and environmental conditions in free-living passerine birds. Physiol Biochem Zool. 2018;91:837–48.
Pingitore A, Lima GPP, Mastorci F, Quinones A, Iervasi G, Vassalle C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition. 2015;31:916–22.
Skrip MM, Mcwilliams SR. Oxidative balance in birds: An atoms-to-organisms-to-ecology primer for ornithologists. J F Ornithol. 2016;87:1–20.
Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–76.
Catoni C, Schaefer HM, Peters A. Fruit for health: the effect of flavonoids on humoral immune response and food selection in a frugivorous bird. Funct Ecol. 2008;22:649–54.
Larcombe SD. Roles of dietary antioxidants and oxidative stress in mediating fitness related traits in birds. In: Division of Environmental and Evolutionary Biology, Institute of Biomedical and Life Sciences. 2008; p. 205.
Skrip MM, Seeram NP, Yuan T, Ma H, McWilliams SR. Dietary antioxidants and flight exercise in female birds affect allocation of nutrients to eggs: How carry-over effects work. J Exp Biol. 2016;219:2716–25.
Beaulieu M, Schaefer HM. Rethinking the role of dietary antioxidants through the lens of self-medication. Anim Behav. 2013;86:17–24.
Schaefer HM, McGraw K, Catoni C. Birds use fruit colour as honest signal of dietary antioxidant rewards. Funct Ecol. 2008;22:303–10.
Powers SK, Ji LL, Kavazis AN, Jackson MJ. Reactive oxygen species: impact on skeletal muscle. Compr Physiol. 2011;1:941–69.
Petibois C, Déléris G. Evidence that erythrocytes are highly susceptible to exercise oxidative stress: FT-IR spectrometric studies at the molecular level. Cell Biol Int. 2005;29:709–16.
Clemens MR, Waller HD. Lipid peroxidation in erythrocytes. Chem Phys Lipids. 1987;45:251–68.
Edwards CJ, Fuller J. Oxidative stress in erythrocytes. Comp Haematol Int. 1996;6:24–31.
Petibois C, Déléris G. Erythrocyte adaptation to oxidative stress in endurance training. Arch Med Res. 2005;36:524–31.
Smith JA. Exercise, training and red blood cell turnover. Sport Med. 1995;19:9–31.
Williams AF. DNA synthesis in purified populations of avian erythroid cells. J Cell Sci. 1972;10:27–46.
Lutz HU, Bogdanova A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front Physiol. 2013;4:1–15.
Wagner G, Chiu D, Qju J, Heath R, Lubin B. Spectrin oxidation correlates with membrane vesiculation in stored RBCs. Blood. 1987;69:1777–81.
Waugh RE, Narla M, Jackson CW, Mueller TJ, Suzuki T, Dale GL. Rheologic properties of senescent erythrocytes: loss of surface area and volume with red blood cell age. Blood. 1992;79:1351–8.
Roussel C, Monnier S, Dussiot M, Farcy E, Hermine O, Le Van Kim C, Colin Y, Piel M, Amireault P, Buffet PA. Fluorescence exclusion: a simple method to assess projected surface, volume and morphology of red blood cells stored in blood bank. Front Med. 2018;5:164.
Waugh RE, Sarelius IH. Effects of lost surface area on red blood cells and red blood cell survival in mice. Am J Physiol Cell Physiol. 1996;271:C1847–52.
Hale JP, Winlove CP, Petrov PG. Effect of hydroperoxides on red blood cell membrane mechanical properties. Biophys J. 2011;101:1921–9.
Mohanty JG, Nagababu E, Rifkind JM. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol. 2014;5 FEB:1–6.
Sentürk ÜK, Gündüz F, Kuru O, Aktekin MR, Kipmen D, Yalçin Ö, Bor-Küçükatay M, Yeşilkaya A, Başkurt OK. Exercise-induced oxidative stress affects erythrocytes in sedentary rats but not exercise-trained rats. J Appl Physiol. 2001;91:1999–2004.
Şentürk ÜK, Gündüz F, Kuru O, Koçer G, Özkaya YG, Yeşilkaya A, Bor-Küçükatay M, Üyüklü M, Yalçin Ö, Başkurt OK. Exercise-induced oxidative stress leads hemolysis in sedentary but not trained humans. J Appl Physiol. 2005;99:1434–41.
Sivilotti MLA. Oxidant stress and haemolysis of the human erythrocyte. Toxicol Rev. 2004;23:169–88.
Landys-Ciannelli MM, Jukema J, Piersma T. Blood parameter changes during stopover in a long-distance migratory shorebird, the bar-tailed godwit Limosa lapponica taymyrensis. J Avian Biol. 2002;33:451–5.
Piersma T, Everaarts JM, Jukema J. Build-up of red blood cells in refuelling bar-tailed Godwits in relation to individual migratory quality. Condor. 1996;98:363–70.
Dai F, Miao Q, Zhou B, Yang L, Liu ZL. Protective effects of flavonols and their glycosides against free radical-induced oxidative hemolysis of red blood cells. Life Sci. 2006;78:2488–93.
Delesderrier E, Curioni C, Omena J, Macedo CR, Cople-Rodrigues C, Citelli M. Antioxidant nutrients and hemolysis in sickle cell disease. Clin Chim Acta. 2020;510:381–90.
Manthou E, Georgakouli K, Deli CK, Sotiropoulos A, Fatouros IG, Kouretas D, Haroutounian S, Matthaiou C, Koutedakis Y, Jamurtas AZ. Effect of pomegranate juice consumption on biochemical parameters and complete blood count. Exp Ther Med. 2017;14:1756–62.
Senturk UK, Yalcin O, Gunduz F, Kuru O, Meiselman HJ, Baskurt OK. Effect of antioxidant vitamin treatment on the time course of hematological and hemorheological alterations after an exhausting exercise episode in human subjects. J Appl Physiol. 2005;98:1272–9.
Youdim KA, Shukitt-Hale B, MacKinnon S, Kalt W, Joseph JA. Polyphenolics enhance red blood cell resistance to oxidative stress: in vitro and in vivo. Biochim Biophys Acta Gen Subj. 2002;1523:117–22.
Frawley AE, Demoranville KJ, Carbeck KM, Trost L, Bryła A, Dzialo M, Sadowska ET, Bauchinger U, Pierce BJ, Mcwilliams SR. Season, anthocyanin supplementation, and flight training have mixed effects on the antioxidant system of migratory European Starlings. Auk. 2021;138:1–16.
Hedenström A, Lindström Å. Wind tunnel as a tool in bird migration research. J Avian Biol. 2017;48:37–48.
McWilliams S, Pierce B, Wittenzellner A, Langlois L, Engel S, Speakman JR, Fatica O, DeMoranville K, Goymann W, Trost L, et al. The energy savings-oxidative cost trade-off for migratory birds during endurance flight. Elife. 2020;9:1–18.
Larcombe SD, Tregaskes CA, Coffey J, Stevenson AE, Alexander LG, Arnold KE. Oxidative stress, activity behaviour and body mass in captive parrots. Conserv Physiol. 2015;3(1):cov045.
Koch RE, Buchanan KL, Casagrande S, Crino O, Dowling DK, Hill GE, Hood WR, McKenzie M, Mariette MM, Noble DW, Pavlova A. Integrating mitochondrial aerobic metabolism into ecology and evolution. Trends Ecol Evol. 2021;36:321–32.
Nebel S, Bauchinger U, Buehler DM, Langlois LA, Boyles M, Gerson AR, Price ER, McWilliams SR, Guglielmo CG. Constitutive immune function in European starlings, Sturnus vulgaris, is decreased immediately after an endurance flight in a wind tunnel. J Exp Biol. 2012;215:272–8.
Herrick EH, Lockhart CH, Martin EP, Nusser WL. Influence of sex hormones on blood cell counts and hemoglobin levels in fowls. Trans Kansas Acad Sci. 1954;57:483.
Kern MD, De Graw WA, King JR. Effects of gonadal hormones on the blood composition of white-crowned sparrows. Gen Comp Endocrinol. 1972;18:43–53.
Bartosz G. Erythrocyte aging: physical and chemical membrane changes. Gerontology. 1991;37:33–67.
Linderkamp O, Meiselman H. Geometric, osmotic, and membrane mechanical properties of density-separated human red cells. Blood. 1982;59:1121–7.
Janiga M, Haas M, Kufelová M. Age, sex and seasonal variation in the shape and size of erythrocytes of the alpine accentor, Prunella collaris (Passeriformes: Prunellidae). Eur Zool J. 2017;84:583–90.
Holland RA, Forster RE. The effect of size of red cells on the kinetics of their oxygen uptake. J Gen Physiol. 1966;49:727–42.
Jones DA. The importance of surface area/volume ratio to the rate of oxygen uptake by red cells. J Gen Physiol. 1979;74:643.
Yamaguchi K, Jürgens KD, Bartels H, Piiper J. Oxygen transfer properties and dimensions of red blood cells in high-altitude camelids, dromedary camel and goat. J Comp Physiol B Biochem Syst Environ Physiol. 1987;157:1–9.
Kozlowski J, Konarzewski M, Gawelczyk AT. Cell size as a link between noncoding DNA and metabolic rate scaling. Proc Natl Acad Sci U S A. 2003;100:14080–5.
Szarski H. Cell size and the concept of wasteful and frugal evolutionary strategies. J Theor Biol. 1983;105:201–9.
Gregory TR. A bird’s-eye view of the C-value enigma: genome size, cell size, and metabolic rate in the class aves. Evolution (N Y). 2002;56:121–30.
Opazo JC, Soto-Gamboa M, Fernández MJ. Cell size and basal metabolic rate in hummingbirds. Rev Chil Hist Nat. 2005;78:261–5.
Starostová Z, Kubická L, Konarzewski M, Kozlowski J, Kratochvíl L. Cell size but not genome size affects scaling of metabolic rate in eyelid geckos. Am Nat. 2009;174(3):E100–5.
Huang Q, Zhang Y, Liu S, Wang W, Luo Y. Intraspecific scaling of the resting and maximum metabolic rates of the crucian carp (Carassius auratus). PLoS ONE. 2013;8:2–9.
Luo Y, He D, Li G, Xie H, Zhang Y, Huang Q. Intraspecific metabolic scaling exponent depends on red blood cell size in fishes. J Exp Biol. 2015;218:1496–503.
Maciak S, Janko K, Kotusz J, Choleva L, Boroń A, Juchno D, Kujawa R, Kozłowski J, Konarzewski M. Standard metabolic rate (SMR) is inversely related to erythrocyte and genome size in allopolyploid fish of the Cobitis taenia hybrid complex. Funct Ecol. 2011;25:1072–8.
Starostová Z, Konarzewski M, Kozłowski J, Kratochvíl L. Ontogeny of metabolic rate and red blood cell size in eyelid geckos: species follow different paths. PLoS ONE. 2013;8:21–3.
Zhang Y, Huang Q, Liu S, He D, Wei G, Luo Y. Intraspecific mass scaling of metabolic rates in grass carp (Ctenopharyngodon idellus). J Comp Physiol B Biochem Syst Environ Physiol. 2014;184:347–54.
Chien S. Red cell deformability and its relevance to blood flow. Annu Rev Physiol. 1987;49:177–92.
Chien S, Usami S, Dellenback RJ, Bryant CA. Comparative hemorheology–hematological implications of species differences in blood viscosity. Biorheology. 1971;8:35–57.
Stuart J, Nash GB. Red cell deformability and haematological disorders. Blood Rev. 1990;4:141–7.
Chen D, Kaul DK. Rheologic and hemodynamic characteristics of red cells of mouse, rat and human. Biorheology. 1994;31:103–13.
Gaehtgens P, Schmidt F, Will G. Comparative rheology of nucleated and non-nucleated red blood cells-I. Microrheology of avian erythrocytes during capillary flow. Pflügers Arch Eur J Physiol. 1981;390:278–82.
Nikinmaa M. Vertebrate red blood cells. Berlin, Heidelberg: Springer Berlin Heidelberg; 1990.
Usami S, Magazinovic V, Chien S, Gregersen MI. Viscosity of turkey blood: rheology of nucleated erythrocytes. Microvasc Res. 1970;2:489–99.
Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost. 2003;29:435–50.
Chien S. Determinants of blood viscosity and red cell deformability. Scand J Clin Lab Invest. 1981;41:7–12.
El-Sayed MS, Ali N, Ali ZES. Haemorheology in exercise and training. Sport Med. 2005;35:649–70.
Mairbäurl H. Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front Physiol. 2013;4 NOV:1–13.
Birchard GF. Optimal hematocrit: theory, regulation and implications. Am Zool. 1997;37:65–72.
Schuler B, Arras M, Keller S, Rettich A, Lundby C, Vogel J, Gassmann M. Optimal hematocrit for maximal exercise performance in acute and chronic erythropoietin-treated mice. Proc Natl Acad Sci U S A. 2010;107:419–23.
Connes P, Yalcin O, Baskurt O, Brun JF, Hardeman M. In health and in a normoxic environment, V̇O2 max is not limited primarily by cardiac output and locomotor muscle blood flow. J Appl Physiol. 2006;100:2099.
Kostelecka-Myrcha A. The ratio of amount of haemoglobin to total surface area of erythrocytes in birds in relation to body mass, age of nestlings, and season of the year. Physiol Zool. 1997;70:278–82.
Kostelecka-Myrcha A. The ratio of amount of haemoglobin to total surface area of erythrocytes in mammals. Acta Theriol (Warsz). 2002;47:209–20.
Storz JF. Hemoglobin-oxygen affinity in high-altitude vertebrates: Is there evidence for an adaptive trend? J Exp Biol. 2016;219:3190–203.
Maciak S, Kostelecka-Myrcha A. Regularities of variation of the red blood indices characterizing the respiratory function of blood in selected fish. Zool Pol. 2012;56:35–48.
Kostelecka-Myrcha A, Chołostiakow-Gromek J. Body mass dependence of the haemoglobin content to surface area ratio of avian erythrocytes. Acta Ornithol. 2001;36:123–8.
Bury A, Niedojadlo J, Sadowska ET, Bauchinger U, Cichon M. Contrasting response of haematological variables between long-term training and short exercise bouts in zebra finches (Taeniopygia guttata). J Exp Biol. 2019a;222(4):jeb193227.
Convertino VA. Blood volume: its adaptation to endurance training. Med Sci Sport Exerc. 1991;23:1338–48.
Hellsten Y, Nyberg M. Cardiovascular adaptations to exercise training. Compr Physiol. 2016;6:1–32.
Balog JM, Huff GR, Rath NC, Huff WE. Effect of dietary aspirin on ascites in broilers raised in a hypobaric chamber. Poult Sci. 2000;79:1101–5.
Bury S, Bury A, Sadowska ET, Cichoń M, Bauchinger U. More than just the numbers—contrasting response of snake erythrocytes to thermal acclimation. Sci Nat. 2019b;106:1–6.
Rosenmann M, Ruiz G. Seasonal changes of blood values in the andean mouse Abrothrix andinus. Comp Biochem Physiol Part A Physiol. 1993;105:119–22.
Fair J, Whitaker S, Pearson B. Sources of variation in haematocrit in birds. Ibis (Lond 1859). 2007;149:535–52.
Minias P. The use of haemoglobin concentrations to assess physiological condition in birds: a review. Conserv Physiol. 2015;3:1–15.
Minias P. Ecology and evolution of blood oxygen-carrying capacity in birds. Am Nat. 2020;195:788–801.
Carey C, Morton ML. Aspects of circulatory physiology of montane and lowland birds. Comp Biochem Physiol Part A Physiol. 1976;54:61–74.
Clemens DT. Interspecific variation and effects of altitude on blood properties of Rosy Finches ( Leucosticte arctoa ) and House Finches ( Carpodacus mexicanus ). Physiol Zool. 1990;63:288–307.
Swanson DL. Seasonal variation of vascular oxygen transport in the dark-eyed Junco. Condor. 1990;92:62–6.
Versteegh MA, Helm B, Gwinner E, Tieleman BI. Annual cycles of metabolic rate are genetically determined but can be shifted by phenotypic flexibility. J Exp Biol. 2012;215:3459–66.
Liu D, He XQ, Wu DT, Li HB, Feng YB, Zou L, Gan RY. Elderberry (Sambucus nigra L.): Bioactive compounds, health functions, and applications. J Agric Food Chem. 2022;70:4202–20.
McGraw KJ, Adkins-Regan E, Parker RS. Maternally derived carotenoid pigments affect offspring survival, sex ratio, and sexual attractiveness in a colorful songbird. Naturwissenschaften. 2005;92:375–80.
Norte AC, Ramos JA, Sampaio HL, Sousa JP, Sheldon BC. Physiological condition and breeding performance of the great tit. The Condor. 2010;112:79–86.
Schmidt-Wellenburg CA, Engel S, Visser GH. Energy expenditure during flight in relation to body mass: effects of natural increases in mass and artificial load in rose coloured starlings. J Comp Physiol B Biochem Syst Environ Physiol. 2008;178:767–77.
Smith SB, McPherson KH, Backer JM, Pierce BJ, Podlesak DW, McWilliams SR. Fruit quality and consumption by songbirds during autumn migration. Wilson J Ornithol. 2007;119:419–28.
de Pascual-Teresa S, Moreno DA, García-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci. 2010;11:1679–703.
Snow BK, Snow D. Birds and berries: a study of an ecological interaction. Calton [Scotland]: T & A D Poyser; 1988.
Carter WA, DeMoranville KJ, Pierce BJ, McWilliams SR. The effects of dietary linoleic acid and hydrophilic antioxidants on basal, peak, and sustained metabolism in flight-trained European starlings. Ecol Evol. 2020;10:1552–66.
Price ER, Bauchinger U, Zajac DM, Cerasale DJ, McFarlan JT, Gerson AR, McWilliams SR, Guglielmo CG. Migration- and exercise-induced changes to flight muscle size in migratory birds and association with IGF1 and myostatin mRNA expression. J Exp Biol. 2011;214:2823–31.
Fox J, Weisberg S. Package ‘car’. Companion to applied regression. 2nd ed. Thousand Oaks: CA Sage; 2011.
Lenth R (2021) Emmeans: estimated marginal means, aka least-squares means. R Packag version 1.5.4.34. https://cran.r-project.org/web/packages/emmeans/index.html.
Cohen J. Statistical power analysis for the behavioural science. 2nd ed. New Jersey: Lawrence Erlbaum; 1988.
Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev. 2007;82:591–605.
Acknowledgements
We are especially grateful to the Max Planck Institute for Ornithology animal care staff for handling much of the animal husbandry responsibilities, to Susan Forrest and Aleksandra Małochleb for helping raise the starlings, and to Wiola Oleś, Alicja Witwicka and Joanna Sulich for priceless help in wind tunnel flight trainings and feeding of birds. We also thank two anonymous reviewers for their thoughtful feedback on the manuscript. The open-access publication of this article was funded by the programme “Excellence Initiative—Research University” at the Faculty of Biology of the Jagiellonian University in Kraków, Poland.
Funding
The experiment was supported by a Grant from the National Science Foundation to S.R.M. and B.J.P. (IOS-1354187); by OPUS Grant from National Science Foundation (NCN), Poland, to U.B. (UMO-2015/19/B/NZ8/01394) and DS Grant from the Institute of Environmental Sciences, Jagiellonian University to U.B. (N18/DBS/000003).
Author information
Authors and Affiliations
Contributions
Study conception and design: SRM, UB, BP, ETS, LT. Acquisition of haematological data: MD, AB, KMC, OF, KJD, ETS, UB. Analysis and interpretation of data: MD, AB, ETS, UB, SRM. Drafting of the manuscript: MD, UB, SRM. Critical revision: (all authors). All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the experimental protocols were approved by the University of Rhode Island IACUC (Protocol #AN08-02-014) and the Government of Upper Bavaria, Germany (AZ 55.2-1-54-2532-216-2014).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Standardized effect sizes in response to anthocyanin-rich diet among non-flying (A) and flying starlings (B); in response to wind-tunnel flight activity among control-(C) and anthocyanin-diet (D) fed starlings. Effect size higher or lower than 0 indicates respectively higher or lower values among anthocyanin supplemented (panel A and B) or flying birds (panel C and D). Circles and squares depict directly measured and calculated variables respectively. Colors: light grey, dark grey and black indicate small (d ≥ 0.2), medium (d ≥ 0.5) and large effect (d ≥ 0.8) sizes following Cohen [138]. Standardized effect size (Cohen’s d) are reported with their 95% confidence interval. The detailed effect size estimates are described in the Additional file 1: Table S1. Hct Haematocrit; Hb content Haemoglobin content; RBCcount Red blood cell number; RBCarea Red blood cell surface area; MCH Mean cell haemoglobin; MCHC Mean cell haemoglobin concentration; MCV Mean cell volume; SA:V ratio Surface-area-to-volume ratio; TSAE Total surface area of erythrocytes; Hb:TSAE ratio Haemoglobin-to-total-surface-area-of-erythrocytes ratio. RBCmajor Red blood cell major length; RBCminor Red blood cell minor axis length; RBCar Red blood cell aspect ratio; RBCcirc Red blood cell circularity. Table S1. Effect size estimates (% change, absolute change and standardized Cohen’s d) for the comparisons of experimental groups (conditional effects) for all haematological variables considered in this study. Hct Haematocrit; Hb content Haemoglobin content; RBCcount Red blood cell number; RBCarea Red blood cell surface area; MCH Mean cell haemoglobin; MCHC Mean cell haemoglobin concentration; MCV Mean cell volume; SA:V ratio Surface-area-to-volume ratio; TSAE Total surface area of erythrocytes; Hb:TSAE ratio Haemoglobin-to-total-surface-area-of-erythrocytes ratio. RBCmajor Red blood cell major length; RBCminor Red blood cell minor axis length; RBCar Red blood cell aspect ratio; RBCcirc Red blood cell circularity.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dzialo, M., Bryła, A., DeMoranville, K.J. et al. Concerted phenotypic flexibility of avian erythrocyte size and number in response to dietary anthocyanin supplementation. Front Zool 20, 9 (2023). https://doi.org/10.1186/s12983-023-00487-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12983-023-00487-y