Abstract
Background
This study aimed to determine the clinical burden, spatial dynamics, and associated risk factors of Plasmodium infection among the natives of Chakwal, Punjab Pakistan from 2019 to 2023 to guide targeted screening and treatment interventions.
Method
A community-based cross-sectional study was conducted using primary and secondary data sources. Participants were recruited using a multi-stage cluster sampling by taking informed consent for the primary data sources. Whereas a secondary dataset of Plasmodium infection was obtained from the database of surveyed hospitals after ethical consideration. All the participants living in the study area for at least 3 months were included, while patients with chronic illness were excluded. Sociodemographic and environmental data were collected using a structured questionnaire. Plasmodium infection was diagnosed using microscopy, rapid diagnostic tests (RDTs), and nested polymerase chain reaction (PCR) as the reference standard. A Zero-inflated negative binomial logistic regression model and interpolation of malaria distribution to environmental variables were employed to determine the association and spatiotemporal patterns of malaria in Chakwal.
Results
Among the 2457 participants, the prevalence of Plasmodium vivax infection (99%) was significantly higher than P. falciparum (0.8%) and mixed infection (0.2%). Females had a higher infection rate than males and the infection rate was higher in adults (21–40 years) and children (0–20 years). Age category (61–100), P. vivax species, temperature, rainfall, monsoon season, and mixed infection factors were significantly associated (p < 0.05) with increased Plasmodium infection risk. Geospatial mapping indicated potential malaria hotspots in Sarkal Kasar, Chak Baili, Mulkwal, Chohan, and other close areas of the Potohar region.
Conclusion
The clinical burden of P. vivax was the highest during monsoon season in Chakwal. Age, gender, climatic factors, and mixed infection were significant risk factors for malaria transmission in this region. Targeted screening and treatment strategies should prioritize identified hotspots and high-risk groups to effectively control malaria in Potohar region of Pakistan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Backgrounds
Malaria remains a significant public health challenge, predominantly in South Asia, where effective screening and treatment strategies are critical for limiting the disease burden [1, 2]. Pakistan is one of the countries in this region experiencing high malaria endemicity [3]. Plasmodium vivax and P. falciparum are the common malaria-causing species in Pakistan [4]. While P. falciparum is associated with chronic and fatal clinical outcomes, P. vivax has a broader geographic range due to its ability to survive at higher elevations and lower temperatures [5]. In Pakistan, a recent shift in species dominance has been seen, with P vivax accounting for 77% of the laboratory-confirmed malaria cases, whereas P. falciparum accounts for 23% [4, 6]. This shifting epidemiology emphasizes a demand for targeted malaria control strategies and an inclusive understanding of the malaria burden and associated risk factors.
Previous epidemiological studies in Pakistan have primarily focused on regions such as Khyber Pakhtunkhwa, Baluchistan, the Federally Administrated Tribal Areas (FATA), and Sindh [4, 6,7,8,9,10,11]. However, an all-inclusive assessment of the malaria burden in the Potohar region of Punjab has been hindered due to a lack of exploration in many areas within the province. In addition, malaria is re-emerging in the highly endemic and under-resourced areas of Punjab, Pakistan [12]. This resurgence can be attributed to various factors, including environmental conditions favoring disease transmission, socioeconomic challenges, limited access to healthcare facilities, and the emergence of antimalarial drug resistance. Environmental factors (temperature, precipitation, vegetation cover, and relative humidity) and climatic conditions (tropical and sub-tropical) in Pakistan with major irrigation systems support the transmission and perseverance of vector-borne infectious diseases [9]. Malaria severity increases in Pakistan continuously throughout the year after the monsoon rainfalls [13].
The district of Chakwal, an economic transition zone in the Potohar region, may be at risk of malaria transmission due to factors such as the transportation of goods and the influx of people from other endemic regions. The district is located in the Potohar Plateau, adjacent to Attock, with low-lying, wet, and often swampy areas in the Northern part of Punjab. Despite its potential vulnerability, no epidemiological study has been reported previously to elucidate the status of Plasmodium infection in this region.
Therefore, the current study aims to determine the clinical burden, spatial dynamic, and associated risk factors of Plasmodium infection among the local population of Chakwal, Punjab, Pakistan using a comprehensive approach involving microscopy, rapid diagnostic tests (RDTs), and nested polymerase chain reaction (PCR) as the reference standard. Understanding the microepidemiology of malaria in this region is crucial for developing targeted control interventions and allocating resources effectively.
2 Methods
2.1 Study design and data collection
A community-based cross-sectional study investigating the clinical burden of malaria and associated risk factors among the local population belonging to various villages across the Potohar region of district Chakwal, Pakistan was conducted using the primary and secondary datasets. Participants were recruited using a multi-stage cluster sampling by taking informed consent for the primary data sources.
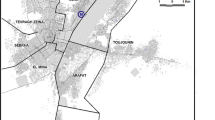
Primary data, including demographic characteristics (age, gender, village) and blood sample (n = 350) by venipuncture method in the presence of a medical nurse, were collected from January to August 2023 at different healthcare centers and diagnostic laboratories in district Chakwal. The data were collected using a structured questionnaire that was validated through expert review and pilot testing to ensure clarity and content validity. All the participants living in the study area for at least 3 months were included, while patients with chronic illness were excluded. Whereas a secondary dataset of Plasmodium infection was obtained from the sentinel sites established by the national malaria combat program in the Potohar region from 2019 to 2023 and recruited 1363 malaria cases with their demographic factors such as the village, gender, and age (Fig. 1). These sentinel sites are district headquarter hospitals and diagnostic private laboratories that routinely maintain malarial patient data. Whereas climatic data of the study area were extracted from the website (https://worldclim.org/) of worldclim at 30-s resolution.
2.2 Ethical consideration and consent to participants
The current study protocol was reviewed and approved by the institutional ethical review committee and all procedures adhered to ethical standards for human subjects. The collection of malarial patients’ data was ethically approved and assigned a protocol (BEC-FBS-QAU-22) by the Bio-Ethical Committee (BEC) of Quaid-i-Azam University and the Medical Superintendent of Health Care Centers.
An informed consent was obtained from all participants before collecting data and samples. Furthermore, study aims and procedures were explained to participants in their local language. Participants were guaranteed the confidentiality of their demographic information and consent was obtained in a private setting at the sentinel sites to ensure privacy.
2.3 Plasmodium infection diagnosis
Three diagnostic methods were used to get an accurate diagnosis of Plasmodium infections, including submicroscopic infection that could be missed by conventional microscopy and rapid diagnostic tests (RDTs).
Microscopy For initial diagnoses, microscopy was performed using thick and thin both kinds of smears (Qureshi et al. [9]). Acetone exposure was given for 1 min in case of a thin smear, and 10 min for a thick smear. After methanol fixation for 1 min, smears were stained with Giemsa and examined under oil immersion (100X magnification) using a trinocular digital microscope (Optika® B-500, Italy).
Rapid Diagnostic Test (RDTs) RDTs were performed on all suspected blood samples using a commercial kit Bioline Malaria Ag Pf (Abbott Diagnostic Inc. Korea) following the manufacturer’s instructions.
Molecular diagnosis Molecular confirmation and detection of submicroscopic infections, Plasmodium DNA was extracted from blood samples using GeneJet Genomic DNA Purification Kit (Thermo Scientific™, EU Lithuania) according to manufacturer’s guidelines. DNA extraction was confirmed by gel electrophoresis. The 18S rRNA gene was amplified using the nested polymerase chain reaction (PCR) method as reported by Qureshi et al. [9]. In the first round, a 50 µl reaction mixture containing the following reagents was used for genus-specific Plasmodium amplification: 32.5 µl PCR water, 4 µl MgCl2 (25 mM), 5 µl phosphate buffer (10X), 1 µl dNTPs (10 mM), 2 µl of each forward primer PLU5 (10 mM, 5′- CCT GTT GTT GCC TTA AAC TTC -3′) and reverse PLU6 (10 mM 5′- TTA AAA TTG TTG CAG TTA AAACG -3′), 0.5 µl Taq polymerase (5 U/µl) and 3 µl DNA template. In the second round, 4 µl of the first-round product was used as a template along with the same amount of 1st round’s master mix and species-specific primers under nested PCR conditions (details in Supplementary Tables 1 and 2). The final products were analyzed on 1.5% agarose gel and the confirmation Plasmodium genus and species were confirmed by visualizing the gel bands (Supplementary Fig. 1) under a gel dock system (BioRad®, USA).
2.4 Data analysis
The count of malaria cases was run with the primary data comprising age, gender, Plasmodium spp., and season of infection to determine the malarial incidence to spatial–temporal patterns in R 4.3.2. Missing data were handled using mean imputation techniques to perform the statistical power. Initial data exploration showed that secondary data is zero-inflated and over-dispersed, which revealed observed variance (327.256) in data was significantly greater than the mean (7.91667). Therefore, we run the following three models in R software for better prediction of the model.
-
1.
M1 = Zero-inflated negative binomial regression with random effect of season.
Summary (m1 <- zeroinfl (Incidences ~ age + sex + specie+ 1 | season, data = Disease, dist = "negbin")
-
2.
M2 = Zero-inflated negative binomial regression without any random effect.
Summary (m2 <- zeroinfl (Incidences ~ age + sex + specie, data = Disease, dist = "negbin")
-
3.
M3 = general linear model with simple binomial regression.
Summary (m3 <- glm.nb (Incidences ~ age + sex + specie + season, data = Disease)
We also applied the Voung test (Supplementary file II) to compare the models, which suggested that the zero-inflated negative binomial model is a more significant (p = 0.000621) choice than the simple general linear Poisson regression model. The annual malarial incidence rate across the Chakwal district was plotted to average annual temperature and rainfall data retrieved from the Worldclim website (https://worldclim.org/) at 30 s using a spatial analyst tool in ArcGIS 10.2. A smoothed map (neighboring type smooth, smoothing factor, 0.5 angles zero) of the spatial pattern of malarial prevalence in District Chakwal was constructed using the inverse distance weighting (IDW) spatial interpolation tool in ArcGIS 10.2. This is a deterministic estimation method where values at unsampled points are determined by a linear combination of values at known sampled points. It employs Tobler’s Law (everything is related to everything else, but near things are more related than a distant thing) by estimating unknown measurements as weighted averages over the known measurements at nearby points, giving the greatest weight to the nearest. More specifically, IDW assumes that each measured point has a local influence that weakens with distance.
3 Results
3.1 Diagnosis and clinical burden of Plasmodium infection
A total of 350 individuals in the primary dataset were tested through the rapid diagnostic test (RDT) and microscopic technique out of which 36% (n = 126) were detected positive and 64% (n = 224) negatives by RDT, while 65.71% (n = 230) were positive by microscopy and 34.28% (n = 120) negative. All suspected cases (n = 350) tested by microscopy and RDT were also confirmed by PCR amplification under nested conditions, where results revealed 84% (n = 294) positive cases. A significant difference (F(df) = 3.42(3) = p < 0.05) has been observed between the malaria-positive cases diagnosed by RDT, microscope, and PCR amplification. The molecular results confirmed that 88.43% of malaria-positive cases were because of P. vivax infection followed by 11.55% mixed-infection and 1.03% only due to P. falciparum among the local population of district Chakwal (Table 1).
3.2 Species-wise distribution of malaria burden (2019–2023)
Plasmodium vivax was the most prevalent and the highest malaria-causing species in all village areas except Siral and Baagh villages where the mixed infection was high or equal to P. vivax. Malarial infection due to P. falciparum was only observed in Sarkal Kasar and approximately disappeared in Chakwal. The abundance of P. vivax was 99.0% as compared to P. falciparum and mixed infection. P. vivax was not only distributed in specific villages but it was observed across the whole district of Chakwal (Fig. 2a). Five-year (2019–2023) secondary data indicated 1363 suspected malaria patients visited the different public and private clinics for diagnosis, among which 640 (46.95%) cases were positive for Plasmodium infection and 723 (53.03%) suspected cases were negative for malaria.
The highest number of suspected cases 422(30.96%) was observed in 2019, among which 153 (11.22%) were positive malaria cases. After 2019 Govt and Public malaria control practices reduced malarial cases, and a consecutive reduction in suspected and positive cases was observed in 2020, 248 (18.19%) > 2021, 242 (17.75%) > 2022, 240 (17.60%) > 2023, 204(15%) as shown in Fig. 2b.
The gradual decrease in malaria suspected cases from 2019 to 2023 might be linked to several positive factors such as better basic health facilities and improved education status in district Chakwal. In addition, malaria infection was reduced significantly (p < 0.05) from 2020 onwards.
3.3 Malaria occurrence by characteristics using a zero-inflated negative binomial model
Independent factors such as gender, age, and Plasmodium species were used in zero-inflated binomials to determine the expected occurrence of malaria cases in the Chakwal district. Considering the gender factor, the male has an expected log (incidence) of − 0.0234 lower than that of the base-level (female) holding other variables constant with a p-value of 0.941 which is insignificant (Fig. 3; Table 2).
The second major patient characteristic used as an indicator for malaria prevalence is the age of the individual, adults (21–40 years) and younger (1–20) were found more susceptible to malaria infection, and the risk declined gradually with older age classes (Fig. 3). For example, the age group 21–40 has an expected log (incidence) of 0.4017 higher as compared to the base level (age = 1–20) holding other variables constant. Likewise, age groups 41–60 and 61–100 have an expected log (incidence) of -0.4689 and -1.5117 lower as compared to the base level (age = 1–20), holding other variables constant.
The age groups 21–40, and 41–60 were insignificant predictors i.e., the p-value for the 21–40 age group was 0.3483, and that of the 41–60 age group was 0.2012 insignificant, except the p-value for 61–100 age group, which is highly significant (p < 0.002). Therefore, the incidence rate in different age groups was like 21–40 > have a high rate of infection followed by 1–20 > 41–60 > 61–100 age groups. It indicated that the incidence rate was high in the adult age group (21–40 years) in both genders followed by other age groups and the distribution of malaria parasites was least common in older age groups (Table 2; Fig. 3).
The P. vivax has an expected log (incidence) of 5.2238 higher mixed infection holding other variables constant. The p-value is 0.00021 which is highly significant predicting that P. vivax was a major and highly abundant malaria-causing species than P. falciparum and mixed infection (Table 2).
3.4 Impacts of environmental factors on malaria occurrence
Results indicated that the occurrence of malaria was unevenly distributed to temperature in Chakwal (Supplementary Fig. 2). In 2019, the average temperature ranged from 27 to 35 °C, however, the maximum malaria cases were observed in the area where the temperature class was 29 °C. In 2023, the malaria incidence counts decreased, just in most optimal temperature areas, the malaria count decreased up to 17–29 count. The maximum count of malaria in 2019, was observed in Dudhial (48) and followed by Jethal village (22), where the temperature ranged from 25 to 30 °C. In 2020 and 2021 same trend was in Dudhial and Jethal village areas, but the number of patients decreased as compared to 2019 (Supplementary Fig. 2).
Similarly, maximum and minimum annual precipitation also influenced the malaria count across the study area. In 2019, suitable precipitation was found at 55–70 mm on which growth for the malarial parasite was found at a maximum than other areas. The precipitation increased year-wise, however, the malaria incidence increased only with optimal precipitation (50–80 mm). In 2023, precipitation increased; however, the vector caused less malaria in the population of Chakwal (Supplementary Fig. 3).
3.5 Month-wise malaria distribution
Among the total of 1363 suspected cases, the peak value of infection was reported in September (127 positive cases) followed by August and October (Fig. 4). The coldest months of the year such as December, January, February, and March carried the least parasitic incidence with a similar exposition of malarial cases. The high proportion of negative cases in suspected cases indicated that a fall in temperature reduces malaria incidence. However, the rate of infection was found to drastically increase as the warmest months of the year started such as May (n = 56), June (n = 64), July (n = 96), and August (n = 124). Males were consecutively found prominent to the malaria infection during these months (Fig. 4).
3.6 Interpolation of malaria distribution
Malaria interpolation using spatial tool inverse distance weighting showed that in district Chakwal, the disease burden was high in the northeast border of district Chakwal which is adjacent to other Punjab areas, however, decreased in the southern and central sides of Chakwal. The weighted average of disease is decreased when the distance from the high-threat area is increased. The red color in the maps illustrates the area (Dudhial) followed by Jethal with a large number of malaria cases and it is the hub of the risk as shown in Fig. 5. The disease radiates from this village to the surrounding neighboring villages. Other neighboring villages such as Fim Kasar and Hasola are hotspots too and these villages also radiate incidences to the precinct villages.
4 Discussion
More than 3.4 million suspected malaria cases have been reported in 2022 and 2.6 million in 2021 from Pakistan (WHO, 2022). Such intense transmission may occur due to high population displacement, devastating flooding (> 33 million people affected in 81 districts), health infrastructure damages, poor implementation of integrated disease surveillance and response (IDSR), politicized irregular distribution of long-lasting insecticidal nets (LLINs) in flooded region, no vector surveillance support, restrained rapid diagnosed tests (RDTs) and inadequate supply of antimalarial drugs [14].
The present study highlights the clinical burden of Plasmodium infection and associated factors among the local population of an untapped district Chakwal, in the Potohar region of Punjab, Pakistan. The prevalence of P. vivax over P. falciparum has significant clinical consequences and underscores the need for tailored control strategies. Malaria by P. vivax often though considered less fatal than P. falciparum, however, it can still cause severe illnesses such as distress respiratory, severe anemic conditions, and multiorgan failures among young children and pregnant women [15]. The current study revealed that 99% of the malaria burden was due to P. vivax followed by mixed infection, while P. falciparum species infection was just noticed in the patients belonging to the village Sarkal Kasar. Whereas, Khattak et al. [16] reported the distribution of P. vivax, P. falciparum, and mixed infection incidence rates at 76%, 18%, and 6% respectively in overall Pakistan. They observed 73% as P. vivax, 22% as P. falciparum, and 5% as mixed infection in Punjab in 2013. Likewise, Qureshi et al. [9] reported that 66.7% of the malaria burden was due to P. vivax, followed by P. falciparum (23.7%) and mixed infection (9.6%) in the Punjab Province of Pakistan. This skewness towards P. vivax may be due to long-lasting survival and compatibility of P. vivax with vector and patient body environment. Furthermore, the ability of P. vivax to form dormant liver stages (hypnozoites) contributes to its resilience and potential for relapsing infections, posing challenges for effective treatment and elimination efforts [15].
Primary data screening revealed that out of 350 suspected cases, 230 were positive by microscopy, and 120 were negative. But when cross-checked by PCR, 294 cases were positive, all with P. vivax, and the rest 56 negatives. This discrepancy can be explained by the reason that as compared to thick and thin films, PCR is a more sensitive and specific technique especially when the parasite rate is low or asymptomatic and in case of mixed infection [14].
The study of five-year data showed a varied trend of malaria, the highest number of malaria suspected cases were reported in 2019 followed by 2021 and 2020. These changes may be due to control measures that were conducted by the provincial government and WHO support under the dengue vector control program, which was started after the major outbreak of dengue infection in 2019 in Punjab. In the present study, the malarial infection was found more prevalent in females as compared to males, these consequences are dissimilar to the findings of various studies [7, 13, 17]. The multitude prevalence among females in this study may be justified by the detail that females have more exposure to malaria vector than males due to their household activities, as they work in shades, home maintenance, home gardening, or fields at peak mosquito biting times or migrate to areas of high endemicity for work [10]. Malaria mostly affected young and adult ones such as the 0–20 and 21–40 age groups with 21.24 and 56.82% cases [6, 18]. Since they were more exposed to the environment and susceptible to the disease, however, the rate of infection declined in the older age group 41–60 and 61–100 (13.45 and 8.52%) respectively. This malaria burden decline in elders may be due to adaptive immunity against malaria over time [19]. Moreover, a conceivable reason for infection in young age groups can be outdoor activities under unhygienic environments, weak immune systems of children, living under dense vegetation, and damp areas making them more susceptible to infection [20].
The month-wise distribution of malaria in Chakwal district showed the high profile of Plasmodium infection in the rainfall season (July–September) using secondary data. Rainfall season and high temperatures give rise to maximum vegetation that supplies suitable habitats for the vector of malaria [21]. The most suitable temperature and precipitation were seen in villages Dudhial, Baagh, Jabba, Siral, Jand, Chohan, and Karyala. Other main explanations can be pollution and decreased availability of standing water and damp habitats which supply a breeding place for mosquito survival and breeding. Similarly, spatiotemporal distribution in Hainan China revealed that malaria incidences are highly distributed in the central south of the province such as Boating, Baisha, and Qionzhong due to differences in temperature and altitude in these districts [15]. Overall, this update provides early intimations on anticipated malaria breakouts in adjacent areas to Chakwal for The Punjab Government of Pakistan and international health agencies can perform vector and malaria control measures in advance.
5 Conclusion
The study highlights the clinical burden and associated risk factors with malaria transmission in the Potohar region. Results showed that age groups (0–20 and 21–40 years) and females were the most susceptible to Plasmodium infection in the study region. Seasonal distribution reveals a peak in malaria cases during the monsoon season (July–September), driven by promising environmental conditions such as high humidity, temperature, and vegetation cover. Spatial analysis revealed hotspots of malaria transmission in specific villages, including Dudhial and Jethal, where optimal temperature and rainfall levels supported the mosquito’s growth. The zero-inflated negative binomial regression model identified significant risk factors of malaria such as age-groups, Plasmodium spp., temperature, rainfall, monsoon season, and mixed infections. Overall current study findings underscore the need for tailored malaria control programs in Pakistan, with a particular focus on active case detection and vector control measures.
Data availability
The corresponding author will provide the raw data on a specific and reasonable demand.
References
Kumar A, et al. Malaria in South Asia: prevalence and control. Acta Trop. 2012. https://doi.org/10.1016/j.actatropica.2012.01.004.
Verma R, et al. Safety and efficacy of primaquine in patients with Plasmodium vivax malaria from South Asia: a systematic review and individual patient data meta-analysis. BMJ Glob Heal. 2023. https://doi.org/10.1136/bmjgh-2023-012675.
Karim AM, et al. Prevalence of clinical malaria and household characteristics of patients in tribal districts of Pakistan. PLoS Negl Trop Dis. 2021. https://doi.org/10.1371/journal.pntd.0009371.
Khattak AA, et al. Prevalence and distribution of human Plasmodium infection in Pakistan. Malar J. 2013. https://doi.org/10.1186/1475-2875-12-297.
Kevin Baird J. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin Microbiol Rev. 2013. https://doi.org/10.1128/CMR.00074-12.
Jahan F, Khan NH, Wahid S, Ullah Z, Kausar A, Ali N. Malaria epidemiology and comparative reliability of diagnostic tools in Bannu; an endemic malaria focus in south of Khyber Pakhtunkhwa, Pakistan. Pathog Glob Health. 2019. https://doi.org/10.1080/20477724.2019.1595904.
Tareen AM, et al. Malaria burden in human population of Quetta, Pakistan. Eur J Microbiol Immunol. 2012. https://doi.org/10.1556/EuJMI.2.2012.3.5.
Hussain I, Qureshi NA, Afzal M, Shaheen N, Ali A, Ashraf A. Prevalence and distribution of human Plasmodium infection in federally administrative tribal areas of Pakistan. Acta Parasitol. 2016. https://doi.org/10.1515/ap-2016-0071.
Qureshi NA, Fatima H, Afzal M, Khattak AA, Nawaz MA. Occurrence and seasonal variation of human Plasmodium infection in Punjab Province, Pakistan. BMC Infect Dis. 2019. https://doi.org/10.1186/s12879-019-4590-2.
S. Akram, M. A. Khan, and H. B. U. Shah, “Frequency, seasonal variation and treatment outcome of malaria in Panjgur, Pakistan,” J. Postgrad. Med. Inst., 2020.
Ullah I, et al. Pcr-rflp based genetic diversity of plasmodium vivax genotypes in district mardan, Pakistan. Brazilian J Biol. 2022. https://doi.org/10.1590/1519-6984.241110.
Nieto NC, Khan K, Uhllah G, Teglas MB. The emergence and maintenance of vector-borne diseases in the khyber pakhtunkhwa province, and the federally administered tribal areas of Pakistan. Front Physiol. 2012. https://doi.org/10.3389/fphys.2012.00250.
Khan AQ, et al. Surveillance of genetic markers associated with Plasmodium falciparum resistance to artemisinin-based combination therapy in Pakistan, 2018–2019. Malar J. 2020. https://doi.org/10.1186/s12936-020-03276-8.
Khattak AA, Awan UA, Nadeem MF, Yaqoob A, Afzal MS. Chloroquine-resistant Plasmodium falciparum in Pakistan. Lancet Infect Dis. 2021. https://doi.org/10.1016/S1473-3099(21)00700-3.
Bantuchai S, Imad H, Nguitragool W. Plasmodium vivax gametocytes and transmission. Parasitol Int. 2022. https://doi.org/10.1016/j.parint.2021.102497.
Khattak AA, et al. Prevalence and patterns of antifolate and chloroquine drug resistance markers in Plasmodium vivax across Pakistan. Malar J. 2013. https://doi.org/10.1186/1475-2875-12-310.
Shaikh MS, et al. Plasmodium in the bone marrow: case series from a hospital in Pakistan, 2007–2015. Malar J. 2021. https://doi.org/10.1186/s12936-021-03792-1.
Ferraresi M, Panzieri DL, Leoni S, Cappellini MD, Kattamis A, Motta I. Therapeutic perspective for children and young adults living with thalassemia and sickle cell disease. Eur J Pediatr. 2023. https://doi.org/10.1007/s00431-023-04900-w.
Penman BS, Gandon S. Adaptive immunity selects against malaria infection blocking mutations. PLoS Comput Biol. 2020. https://doi.org/10.1371/journal.pcbi.1008181.
Morales F, Montserrat-de la Paz S, Leon MJ, Rivero-Pino F. Effects of malnutrition on the immune system and infection and the role of nutritional strategies regarding improvements in children’s health status: a literature review. Nutrients. 2024. https://doi.org/10.3390/nu16010001.
Gouda KC, Pernaje N, Benke M. Climate parameter and malaria association in north-east India. J Parasit Dis. 2023. https://doi.org/10.1007/s12639-023-01585-8.
Acknowledgements
We acknowledge all participants involved in this study. We would also like to thank the paramedical staff of the surveyed hospital in District Chakwal, Pakistan.
Funding
This study had no funding source.
Author information
Authors and Affiliations
Contributions
M.A. and B.L. carried out surveys, performed laboratory work and wrote the main manuscript text. M.A. revised and formatted the whole manuscript according to the reviewers’ comments. While N.A.Q and MA supervised and conceptualized this research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Afzal, M., Latif, B. & Qureshi, N.A. Clinical burden of Plasmodium infection and associated risk factors among the local population of Chakwal, Punjab, Pakistan (2019–2023). Discov Public Health 21, 65 (2024). https://doi.org/10.1186/s12982-024-00190-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12982-024-00190-1