Abstract
Background
In resource-limited settings, multi-experienced HIV infected patients are often prescribed raltegravir for salvage therapy. Patients failing raltegravir-containing regimens require other drugs including other integrase inhibitors. In this context, real-life data about the resistance and cross-resistance pathways between integrase inhibitors is limited. The aim of this study was to investigate integrase resistance pathways in a cohort of Mexican multi-experienced patients failing of a raltegravir-containing salvage regimen.
Methods
Twenty-five plasma samples from subjects failing antiretroviral regimens which included raltegravir were obtained from various healthcare centres from 2009 to 2017 in Mexico. Antiretroviral history and demographics were collected. Samples were processed for integrase resistance genotyping testing by sequencing. The viral sequences were analysed with the Stanford HIV drug resistance database algorithm. Data was analysed with SPSS Statistics software.
Results
We found a mean viral load of 4.17 log10 c/mL (SD 1.11) at the time of virologic failure. Forty-eight percent of the samples were raltegravir resistant. The Y143R/H/C substitutions were the most prevalent, followed by the N155H, and both Q148H/K and G140S/A in the same proportion. The Q148 + G140 combination was found in (12%) of the samples. Cross-resistance to elvitegravir was found in 83.3% and in 18.2% for both dolutegravir and bictegravir. Thirteen samples (52%) were susceptible to the four integrase strand-transfer inhibitors.
Conclusions
Our findings suggest a high occurrence of resistance and cross-resistance to other integrase inhibitors among multi-experienced subjects failing raltegravir. We found a modestly lower proportion of cross-resistance to dolutegravir than data from clinical trials. Likely this drug could be used for salvage therapy. Explanations for the absence of mutations in half of the samples, other than reduced adherence, should be further investigated. Close surveillance is needed.
Similar content being viewed by others
Background
Antiretroviral therapy (ART) has increased the survival and reduced the morbidity of patients with HIV infection [1,2,3,4]. However, a major concern is the emergence of resistance-associated mutations (RAMs) that reduce the response to treatment [5,6,7]. Viral strains from patients that are not successfully treated frequently select RAMs. This makes the selection of a new ART regimen more difficult and increases the risk of virologic failure. Resistance genotyping studies are used to determine whether or not the viral strains are susceptible to a given drug [7], however, these tests are frequently not available in resource-limited settings.
The integrase strand-transfer inhibitors (INSTIs) are the most recent class of antiretroviral drugs approved for HIV infection [8]. These drugs work through the active inhibition of the integrase enzyme, which promotes the integration of the viral genome into the host DNA.
In the context of middle and low income-countries where integrase inhibitors are not widely available, these drugs were mostly used for salvage therapy in ART-experienced patients. In Mexico and most countries in Latin America, experience with integrase resistance and cross-resistance is limited.
Most patients treated with integrase inhibitors are using raltegravir (RAL), elvitegravir (EVG) or dolutegravir (DTG). Resistance and cross-resistance among these have been described in several studies (most of them in first-line therapy studies) that found RAMs related to the viral fitness and the potential decrease of response to salvage therapy [8,9,10,11,12,13]. This brings new challenges to the selection of second line and salvage therapies.
RAL has been the most commonly prescribed in our setting and has been used as salvage therapy for highly ART-experienced patients. RAL failing cases are limited and integrase resistance testing is not widely available [14]. Besides the data from clinical trials, there is limited experience in real-life settings about INSTIs resistance [15]. Moreover, there is limited data regarding the resistance pathways in patients failing INSTIs in the context of multi-experienced patients, where some shared resistance pathways between RAL and EVG, and cross-resistance pathways to DTG and bictegravir (BIC) are not well described.
In resource-limited settings (such as in Mexico and most countries in Latin America), where the overall use of integrase inhibitors has been increasing in recent years, data for predicting the viral response to INSTIs, when switching to another treatment is limited. This study aimed to investigate the resistance-associated mutations in the HIV-1 integrase gene and to evaluate the resistance and cross-resistance pathways to INSTIs in a cohort of ART multi-experienced Mexican patients failing to RAL-containing regimens.
Methods
Study design
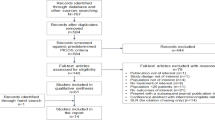
The study was conducted at the Molecular Virology Unit of the Infectious Diseases Department of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ) in Mexico City, a national reference unit for HIV positive patients. A cross-sectional analysis of 25 plasma samples from HIV-1 infected adult subjects collected from various healthcare centres throughout the country from 2009 to 2017 for viral load testing (available from our samples bank) was conducted. Samples were selected from subjects failing a RAL-containing salvage regimen (defined as two consecutive HIV-1 viral loads above the limit of detection after suppressive ART or not achieving undetectable RNA viral load after 6 months of highly active antiretroviral therapy) at the time that the sample was collected. In all cases, the patients were naive to INSTIs before using RAL and their RAL-containing regimen were prescribed as third line ART. Antiretroviral treatment history and resistance history data available from subjects were also collected.
Laboratory assays
HIV-RNA viral load (VL) was measured in all samples with the AmpliPrep/COBAS TaqMan HIV-1 Test, v2.0 (Roche Molecular Systems, Branchburg, NJ, USA); and CD4 + total cell count was measured by flow cytometry (BD Multitest™ CD3/CD8/CD45/CD4 in BD FACSCanto™ II, BD Biosciences, San Jose, CA, USA). Plasma samples stored at − 70 ℃ were used for resistance testing. The RNA viral extraction step was performed using a modified protocol of the ViroSeq HIV-1 Genotyping System (Celera Diagnostics, Alameda, CA, USA) using 1000 mL of plasma volume plus centrifugation at 17,000 rpm for 2 h, 4 ℃ temperature for viral precipitation. RNA elution was performed according to the viral load value; VL > 20,000 copies/mL, 30 mL of elution buffer; VL 1000–19,999 copies/mL, 20 mL elution buffer and < 1000 to 100 copies/mL, 15 mL of elution buffer. Amplification of the HIV-1 integrase gene region was performed either using the ViroSeq HIV-1 Integrase Genotyping System (Celera Diagnostics, Alameda, CA, USA) or our in-house standardized nested PCR strategy: A one-step reverse transcription and first round of PCR was conducted using the OneStep RT-PCR kit (QIAGEN, Hilden, Germany) with in-house designed primers, forward AOR-IN-F (5′-CAGTGCTGGAATCAGGAAAGTA-3′) and reverse AOR-IN-R (5′-CTTGGATGAGGGCTTTCATAGT-3′). PCR products from the first round were used as template for the second one that was performed using the AmpliTaq Gold DNA Polymerase with Buffer II (ThermoFisher, Foster City, CA, USA) and the primers INPS1 (5′-TAGTAGCCAGCTGTGATAAATGTC-3′) and INPR8 (5′-TTCCATGTTCTAATCCTCATCCTG-3′) from the ANRS AC11 Resistance Study Group protocol, available at: http://www.hivfrenchresistance.org/ANRS-procedures.pdf (Accessed November 11, 2015). The resulting PCR products were visualized and measured with the Agilent Bioanalyzer 2100 system and the DNA 7500 kit (Agilent Technologies, Waldbronn, Germany), products were purified with the Microcon Centrifugal Filters (Merck, Darmstadt, Germany) and prepared with the BigDye Terminator v3.1 (Applied Biosystems, Austin, TX, USA) for Sanger automated capillary sequencing using the 16-capillary 3130xl Genetic Analyzer (Applied Biosystems, Austin, TX, USA).
Data analysis
Viral sequences were analysed with the SeqScape v2.5 software and the FASTA files were analysed using the Stanford HIV Drug Resistance Database v8.8 algorithm (http://hivdb.stanford.edu/), defining resistance interpretation of penalty scores as susceptible (score 0–9), potential low-level resistance (score 10–14), low-level resistance (score 15–29), intermediate resistance (score 30–59) and high-level resistance (score ≥ 60). The REGA HIV-1 Subtyping Tool version 3.0 (http://dbpartners.stanford.edu:8080/RegaSubtyping/stanford-hiv/typingtool/) was used for the HIV subtype analysis.
Descriptive statistics, proportions and dispersion measures (with standard deviation) were described for population features, antiretroviral treatment history variables and laboratory results. Data were analysed using the IBM© SPSS© Statistics v23.0.0.0 software. Correlation and Chi square analysis were performed. A P < 0.05 was considered significant.
Results
Study population characteristics
Twenty-five plasma samples from 20 (80%) male subjects and 5 (20%) female subjects fulfilling eligible criteria (from 2009 to 2017) were successfully genotyped and included for the analysis. The mean duration of treatment prior to current failure was 22.33 months (SD 20.38, n = 15) while the mean time under ART was 10.65 years (SD 5.7, n = 12). Subjects had been exposed to a mean of 5.31 (SD 3.13, n = 16) ART combinations.
The HIV-1 subtyping analysis of the sequences indicated subtype B for all 25 samples. At RAL failure, the mean viral load was 4.17 log10 copies/mL (SD 1.11, n = 22) and the mean of CD4 + T-cell count was 328.5 cells/mm3 (SD 217.07, n = 12) (Table 1).
The most common ART combinations included NRTIs (Table 1) either alone or with a protease inhibitor (PI). Overall, tenofovir (TDF) was the most frequent drug across the subjects (58.82%, n = 17).
Integrase resistance patterns
The number of samples with genotype-predicted resistance (susceptibility score ≥ 15) to RAL was 12/25 (48%), while 11/25 (44%) were resistant to EVG and 7/25 (28%) to both DTG and BIC (Fig. 1).
Proportion of samples with genotype-predicted resistance to integrase strand-transfer inhibitors. Absolute numbers are shown above each bar. RAL raltegravir, EVG elvitegravir, DTG dolutegravir, BIC bictegravir, S Susceptible (score 0–9), PLLR Potential low-level resistance (score 10–14), LLR Low-level resistance (score 15–29), IR Intermediate resistance (score 30–59), HLR High-level resistance (score ≥ 60); N = 25
Thirteen out of the 25 samples (52%) were found to be fully susceptible to the four INSTIs (susceptibility score < 15). Of the RAL-resistant samples (n = 12), cross-resistance to EVG was found in 10/12 (83.3%) while 2/12 samples (16.7%) had resistance to DTG and BIC.
Integrase inhibitors RAMs
Concerning integrase RAMs, changes at the Y143 position were the most frequent (Fig. 2). Overall, the non-polymorphic integrase mutations occurred at positions; Y143 (28%), V151 (20%), N155 (20%), Q148 (12%), G140 (12%), G163 (12%), L74 (8%), E92 (8%), E138 (8%), T97 (4%), E157 (4%) and S230 (4%).
The Q148 + G140 combination of substitutions, which has been related to DTG resistance [10, 15] was observed in 3/25 samples (12%) (subjects 5, 8 and 14) as shown in Table 2.
Also, we found the L101I/T124A combination in 7/25 samples (28%) (subjects 3, 5, 11, 17, 19, 23 and 25). Four of these samples (subjects 3, 11, 23 and 25) were fully susceptible to INSTIs. Two out of four samples with intermediate DTG resistance had harboured such combination (subjects 5 and 19). The DTG resistance associated mutation R263K was not found. However, one sample (subject 13) had the presence of the E157Q substitution (Table 2).
We did not find any association or significant correlation of the presence of drug resistance and any of the mutations with the baseline characteristics of our study population. Data about adherence was not entirely available, and therefore it was not analysed.
Susceptibility of the RAL-containing regimen
Only 17 out of the 25 subjects had fully documented treatment history available. From these 17, only 11 (64.7%) had a regimen with ≥ 2 susceptible drugs (including RAL). Also, 11 out of those 17 (64.7%) had mutations in the reverse transcriptase and protease regions (RT-PR) that were affecting the current antiretroviral regimen, 2/17 samples (17.6%) had mutations that were not affecting the susceptibility of the regimen and 2/17 samples (17.6%) had no mutations.
Among the samples that were fully susceptible to all INSTIs, 6/9 (66.7%) were also susceptible to their respective RT-PR backbone regimen. None of these subjects had enfuvirtide (T20) in their regimen. RT-PR drug resistance mutations of the samples and their backbone regimen are summarized in Table 3.
Discussion
The antiretroviral therapy for HIV infection should not be left out from antimicrobial resistance stewardship strategies. In this regard, the World Health Organization developed a global strategy for the prevention and assessment of HIV drug resistance to support an approach for monitoring and surveillance in public health [16, 17]. We want to highlight the importance of performing resistance surveillance especially for INSTIs, drugs that will be used with worldwide. So far, multi-experienced patients failing integrase inhibitors are difficult to find in our settings. Nevertheless, they still can be found in the HIV services, and drug resistance is one of the major challenges for clinicians who treat them. Our subjects were heavily ART experienced, and so their ART salvage options were somewhat limited by the resistance data.
Despite the concentration of the HIV epidemic in Mexico among men who have sex with men (MSM) and other key populations [18], we found that 5 of our 25 samples were from female subjects, highlighting that this demographic group should not be underestimated in our setting.
The genetic analysis exhibited HIV subtype B strains for all samples. This is consistent with epidemiological data in Latin America that describes subtype B as the most prevalent subtype in the region [19]. We would like to further investigate integrase resistance patterns across different subtypes.
Forty-eight percent of our samples had resistance to RAL while the other (52%) were still susceptible. This absence of detectable resistance after treatment failure in our samples might have been the result of the presence of drug-resistant minority viral populations, a prolonged interval between the time of antiretroviral drug discontinuation and genotypic testing, poor adherence and/or drug–drug interactions (leading to sub-therapeutic drug levels), although, none of these causes could be fully documented in our study. Supporting the likely absence of selective pressure and the lower occurrence of resistance, is the comparison that our data with the BENCHMRK 1 and 2 trials that included similar cases to ours [20]. We found a lower frequency (48%) of patients who had integrase mutations at important residues (Y143, Q148 or N155) compared with the 65% reported in these studies [20].
As we suspected, cross-resistance to EVG was higher than cross-resistance to the other INSTIs in our study. The presence of the N155H substitution (one of the second most frequent in our study) reduces both RAL and EVG susceptibility but does not, alone, compromise DTG nor BIC by itself (thus, having more resistance to RAL leads to more EVG cross-resistance).
We found a similar proportion of DTG resistance (28%) to the 35% reported in the study of Cavalcanti et al. conducted in Brazil with patients failing RAL [21]. Both mean CD4+ cell count and viral load from our subjects at the time of resistance testing were very similar to the ones reported in that study (CD4+ cell count of 221 cells/mm3 and 3.99 log10 copies/mL of viral load). Those cases were exposed to RAL for a mean of 115 weeks with prior use of 7 regimens while our patients had 89.32 weeks (22.33 months) of RAL exposure and a mean of 5.3 regimens. On the other hand, we do not find a significant difference between the mean viral load of the subjects with DTG resistance compared to the subjects without DTG resistance (n = 22), nor with samples harbouring a G140S/A + Q148H/R/K combination (n = 3) as described in that study. This is important since the presence of the G140S mutation in combination with the Q148H mutation has been described to improve the viral fitness and increase the viral load in RAL-resistant subjects [22].
According to our findings, the frequency of the substitution Q148+ ≥1 secondary mutation(s) (G140A/C/S, L74I or E138A/K/T) was 12%. These combinations were found to reduce the response to DTG after RAL expose in the VIKING-3 study [15] (Table 4), while here, the proportion of patients without INSTIs mutations was higher. Drug resistance testing is recommended when DTG (and potentially BIC) is considered for salvage therapy.
Sequencing has demonstrated non-polymorphic mutations and combinations associated with INSTIs exposure, which do not, alone, cause resistance. The L101I/T124A which may vary between HIV subtypes as reported by Garrido et al. was more frequently observed in our cohort compared to theirs (for subtype B) [23]. Also, we found the L101I/T124A combination at a higher frequency in our cohort (28%) than that reported by Saladini et al. (10.8%) for RAL experienced patients [24]. This combination has been more frequently described in RAL experienced patients and has been suggested to play a role in the modulation of response to DTG in vitro by favouring the selection of other mutations [25].
The E157Q substitution (found in subject 13) is a natural polymorphism described as a compensatory mutation for R263K-mediated DTG resistance [26] and after RAL exposure [27]. More information is needed to further recommend the use of DTG in naive patients harbouring the E157Q substitution as suggested by Charpentier and Descamps [28].
This study was limited by incomplete information about; treatment history, adherence, the HIV-1 viral load and CD4+ T-cell count. Before-mentioned data, could not be located due to loss of retention in care, the transfer of care centre or the passing of the subjects. The follow-up of the subjects was also limited and clinical outcomes could not be fully described. Moreover, RAMs in the RT-PR region illustrates the high exposure to previous ART regimens. On the other hand, the absence of mutations in 66.7% of the subjects (for RT-PR and integrase regions) may be explained by poor adherence and lower drug exposure. However, we cannot exclude a significant role for minority variants, which should be further investigated.
Conclusions
This is the first study to explore the integrase inhibitors resistance profiles in ART multi-experienced subjects in our country. RAL resistance was documented in 48% of the samples. Predicted cross-resistance to EVG was very high as described in clinical trials, while cross-resistance to DTG was limited (83.33% and 18.18% respectively). The frequencies of INSTIs RAMs at RAL failure were lower than those reported in the BENCHMRK 1 and 2 studies [20]. On the other hand, the occurrence of DTG cross-resistance was also lower than that reported in the VIKING-3 study [15]. These lower proportions could be related to poor adherence or to the genetic barrier of the overall ART combinations.
We found a high frequency of the polymorphic combination L101I/T124A than that reported for subtype B strains. This combination has been suggested to be somehow related to DTG resistance and is more prevalent in RAL experienced patients in comparison to RAL-naive patients [25].
The RAL non-resistant samples were still susceptible to all INSTIs. However, it is difficult to support the continuation of RAL in those cases without strong evidence from controlled clinical trials. The role of minor viral strains and other regions outside the integrase gene should also be investigated especially in patients without documented RAMs.
In summary, we found an important occurrence of RAL drug resistance and cross-resistance in our cohort. The relationship between specific clinical features and the development of drug resistance in multi-experienced subjects should be further investigated. Given that multi-experienced patients failing integrase inhibitors are difficult to find, our cohort represents an important number of real-life cases. Furthermore, the number of people with INSTIs-based therapy will be growing in Latin-America following recent changes in international and local guidelines. The decision of using DTG (or potentially BIC) for salvage therapy in the presence of RAL failure or suspected resistance to INSTIs should be made following an integrase resistance test. Despite the similar profiles of DTG and BIC resistance, more information in real-life settings will be needed to guide future salvage regimens. Moreover, we urge reinforcing adherence as well as close surveillance in multi-experienced patients to prevent the emerging of resistance and likely transmitted resistance to integrase inhibitors.
Availability of data and materials
Integrase sequences have been submitted to GenBank and are available under accession numbers MF154570 to MF154594.
References
Lee FJ, Amin J, Carr A. Efficacy of initial antiretroviral therapy for HIV-1 infection in adults: a systematic review and meta-analysis of 114 studies with up to 144 weeks’ follow-up. PLoS ONE. 2014;9:e97482. https://doi.org/10.1371/journal.pone.0097482.
Chêne G, Sterne JAC, May M, Costagliola D, Ledergerber B, Phillips AN, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003;362:679–86.
Yoshimura K. Current status of HIV/AIDS in the ART era. J Infect Chemother. 2017;23:12–6. https://doi.org/10.1016/j.jiac.2016.10.002.
INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015. https://doi.org/10.1056/NEJMoa1506816.
Tang MW, Shafer RW. HIV-1 antiretroviral resistance: scientific principles and clinical applications. Drugs. 2012;72:e1–25. https://doi.org/10.2165/11633630-000000000-00000.
Wirden M, Simon A, Schneider L, Tubiana R, Malet I, Ait-Mohand H, et al. Raltegravir has no residual antiviral activity in vivo against HIV-1 with resistance-associated mutations to this drug. J Antimicrob Chemother. 2009;64:1087–90. https://doi.org/10.1093/jac/dkp310.
Clutter DS, Jordan MR, Bertagnolio S, Shafer RW. HIV-1 drug resistance and resistance testing. Infect Genet Evol. 2016;46:292–307. https://doi.org/10.1016/j.meegid.2016.08.031.
Mesplède T, Quashie PK, Zanichelli V, Wainberg MA. Integrase strand transfer inhibitors in the management of HIV-positive individuals. Ann Med. 2014;46:123–9. https://doi.org/10.3109/07853890.2014.883169.
Mesplède T, Wainberg MA. Resistance against integrase strand transfer inhibitors and relevance to HIV persistence. Viruses. 2015;7:3703–18. https://doi.org/10.3390/v7072790.
Malet I, Thierry E, Wirden M, Lebourgeois S, Subra F, Katlama C, et al. Combination of two pathways involved in raltegravir resistance confers dolutegravir resistance. J Antimicrob Chemother. 2015;70:2870–80. https://doi.org/10.1093/jac/dkv197.
Kobayashi M, Nakahara K, Seki T, Miki S, Kawauchi S, Suyama A, et al. Selection of diverse and clinically relevant integrase inhibitor-resistant human immunodeficiency virus type 1 mutants. Antiviral Res. 2008;80:213–22. https://doi.org/10.1016/j.antiviral.2008.06.012.
Charpentier C, Karmochkine M, Laureillard D, Tisserand P, Bélec L, Weiss L, et al. Drug resistance profiles for the HIV integrase gene in patients failing raltegravir salvage therapy. HIV Med. 2008;9:765–70. https://doi.org/10.1111/j.1468-1293.2008.00628.x.
Andreatta KN, Miller MD, White KL. Drug Susceptibility and viral fitness of HIV-1 with integrase strand transfer inhibitor resistance substitution Q148R or N155H in combination with nucleoside/nucleotide reverse transcriptase inhibitor resistance substitutions. Antimicrob Agents Chemother. 2016;60:757–65. https://doi.org/10.1128/AAC.02096-15.
Cesar C, Shepherd BE, Jenkins CA, Ghidinelli M, Castro JL, Veloso VG, et al. Use of third line antiretroviral therapy in Latin America. PLoS ONE. 2014;9:e106887. https://doi.org/10.1371/journal.pone.0106887.
Castagna A, Maggiolo F, Penco G, Wright D, Mills A, Grossberg R, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis. 2014;210:354–62. https://doi.org/10.1093/infdis/jiu051.
Bennett DE, Bertagnolio S, Sutherland D, Gilks CF. The World Health Organization’s global strategy for prevention and assessment of HIV drug resistance. Antivir Ther (Lond). 2008;13(Suppl 2):1–13.
Phillips AN, Cambiano V, Miners A, Revill P, Pillay D, Lundgren JD, et al. Effectiveness and cost-effectiveness of potential responses to future high levels of transmitted HIV drug resistance in antiretroviral drug-naive populations beginning treatment: modelling study and economic analysis. Lancet HIV. 2014;1:e85–93. https://doi.org/10.1016/S2352-3018(14)70021-9.
Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380:367–77. https://doi.org/10.1016/S0140-6736(12)60821-6.
Avila-Rios S, Sued O, Rhee S-Y, Shafer RW, Reyes-Teran G, Ravasi G. Surveillance of HIV transmitted drug resistance in Latin America and the Caribbean: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0158560. https://doi.org/10.1371/journal.pone.0158560.
Steigbigel RT, Cooper DA, Teppler H, Eron JJ, Gatell JM, Kumar PN, et al. Long-term efficacy and safety of Raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 phase III trials. Clin Infect Dis. 2010;50:605–12. https://doi.org/10.1086/650002.
de Souza Cavalcanti J, de Ferreira JL, de Guimarães PM, Vidal JE, de Brigido LF. High frequency of dolutegravir resistance in patients failing a raltegravir-containing salvage regimen. J Antimicrob Chemother. 2015. https://doi.org/10.1093/jac/dku439.
Delelis O, Malet I, Na L, Tchertanov L, Calvez V, Marcelin A-G, et al. The G140S mutation in HIV integrases from raltegravir-resistant patients rescues catalytic defect due to the resistance Q148H mutation. Nucleic Acids Res. 2009;37:1193–201. https://doi.org/10.1093/nar/gkn1050.
Garrido C, Soriano V, Geretti AM, Zahonero N, Garcia S, Booth C, et al. Resistance associated mutations to dolutegravir (S/GSK1349572) in HIV-infected patients—impact of HIV subtypes and prior raltegravir experience. Antiviral Res. 2011;90:164–7. https://doi.org/10.1016/j.antiviral.2011.03.178.
Saladini F, Meini G, Bianco C, Monno L, Punzi G, Pecorari M, et al. Prevalence of HIV-1 integrase mutations related to resistance to dolutegravir in raltegravir naïve and pretreated patients. Clin Microbiol Infect. 2012;18:E428–30. https://doi.org/10.1111/j.1469-0691.2012.03917.x.
Malet I, Wirden M, Fourati S, Armenia D, Masquelier B, Fabeni L, et al. Prevalence of resistance mutations related to integrase inhibitor S/GSK1349572 in HIV-1 subtype B raltegravir-naive and -treated patients. J Antimicrob Chemother. 2011;66:1481–3. https://doi.org/10.1093/jac/dkr152.
Anstett K, Cutillas V, Fusco R, Mesplède T, Wainberg MA. Polymorphic substitution E157Q in HIV-1 integrase increases R263K-mediated dolutegravir resistance and decreases DNA binding activity. J Antimicrob Chemother. 2016;71:2083–8. https://doi.org/10.1093/jac/dkw109.
Ghosn J, Mazet A-A, Avettand-Fenoel V, Peytavin G, Wirden M, Delfraissy J-F, et al. Rapid selection and archiving of mutation E157Q in HIV-1 DNA during short-term low-level replication on a raltegravir-containing regimen. J Antimicrob Chemother. 2009;64:433–4. https://doi.org/10.1093/jac/dkp182.
Charpentier C, Descamps D. Resistance to HIV integrase inhibitors: about R263K and E157Q mutations. Viruses. 2018. https://doi.org/10.3390/v10010041.
Acknowledgements
The authors would like to thank the Molecular Virology Unit team and the Infectious Diseases Department for their valuable help. Also to Dr. Juan José Calva Mercado, director of the Comité Nacional para el uso Racional de los Antirretrovirales (CORESAR) and to Dr. Eddie Antonio León Juárez from the Centro Nacional para la Prevención y el Control del VIH/SIDA (CENSIDA) of Mexico.
Funding
The study was funded entirely by the Molecular Virology Unit (Infectious Diseases Department) of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán in Mexico City.
Author information
Authors and Affiliations
Contributions
LES and AO designed the study protocol. AOR and RAR designed the wet lab approach for PCR amplification and Sanger sequencing of the integrase region and the design of the integrase in-house genotyping test. AO processed and sequenced the samples, created the database and conducted the analysis of the data. LAA and MH contributed with the review and quality assessment of the sequences. AO wrote the manuscript. LES and RAR critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the research and ethics boards of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. All experiments were performed in compliance with relevant laws, institutional guidelines and by the ethical standards of the Declaration of Helsinki. Being a retrospective study, based on non-identifiable archived data, informed consent was not requested.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Orta-Resendiz, A., Rodriguez-Diaz, R.A., Angulo-Medina, L.A. et al. HIV-1 acquired drug resistance to integrase inhibitors in a cohort of antiretroviral therapy multi-experienced Mexican patients failing to raltegravir: a cross-sectional study. AIDS Res Ther 17, 6 (2020). https://doi.org/10.1186/s12981-020-0262-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12981-020-0262-y