Abstract
The disease (COVID-19) novel coronavirus pandemic has so far infected millions resulting in the death of over a million people as of Oct 2020. More than 90% of those infected with COVID-19 show mild or no symptoms but the rest of the infected cases show severe symptoms resulting in significant mortality. Age has emerged as a major factor to predict the severity of the disease and mortality rates are significantly higher in elderly patients. Besides, patients with underlying conditions like Type 2 diabetes, cardiovascular diseases, hypertension, and cancer have an increased risk of severe disease and death due to COVID-19 infection. Obesity has emerged as a novel risk factor for hospitalization and death due to COVID-19. Several independent studies have observed that people with obesity are at a greater risk of severe disease and death due to COVID-19. Here we review the published data related to obesity and overweight to assess the possible risk and outcome in Covid-19 patients based on their body weight. Besides, we explore how the obese host provides a unique microenvironment for disease pathogenesis, resulting in increased severity of the disease and poor outcome.
Similar content being viewed by others
Introduction
COVID-19 disease is caused by a novel coronavirus (SARS-COV-2) that emerged in the Wuhan province of china [1, 2]. The first documented human infection was reported in Dec 2019 and since then, the disease has spread at an unprecedented speed and magnitude to become the greatest healthcare concern of the twenty-first century [3,4,5]. Even with the implementation of major interventions to contain the spread of the disease, COVID-19 has progressed worldwide resulting in significant morbidity and mortality [6,7,8]. As of Oct 5, 2020, the total number of infected patients stands at 35 million resulting in more than a million deaths. As a consequence, intense efforts are on to understand the epidemiology and pathobiology of this disease. The global fatality rate of CoVID-19 is ~ 3%, although great differences exist with some countries (France and the United Kingdom) recording a high death rate of ~ 10% and others (India, Israel, Russia) reporting less than 2% mortality rates [9,10,11,12,13,14]. Several epidemiological studies strongly suggest a link between age and severity of the illness [15,16,17,18,19,20]. More than 75% of the deaths have been reported in patients aged 65 years or above. Also, people with co-morbidities such as diabetes, cardiovascular diseases, hypertension, and cancer have significantly higher mortality rates [21,22,23]. Recent studies emerging from multiple countries have shown that obesity may be an independent factor to predict the risk and outcome of COVID-19 patients [24,25,26,27,28,29,30,31,32,33,34,35,36,37]. High body mass index (BMI) has particularly been found to be a strong indicator of disease severity in patients younger than 60 years of age [38,39,40,41,42].
Here we summarize the available epidemiological data with a particular focus on obesity and its impact on disease severity. We also discuss possible mechanism(s) that make obese host susceptible to severe disease as a result of SARS-CoV-2 infection.
Impact of obesity on disease severity of COVID-19 patients
There have been several reports indicating obesity to be a strong factor for becoming seriously ill with COVID-19 [43,44,45]. A retrospective study from Lille, France analyzed the relationship between body mass index BMI and requirement for invasive mechanical ventilation (IMV) in 124 consecutive patients admitted in intensive care for SARS-COV-2. Out of 124 patients, 84 (75.8%) were obese (BMI > 30 kg/m2), indicating a high incidence of obesity among patients admitted to intensive care for SARS-COV-2 [46]. When compared to ICU admissions the previous year for the severe acute pulmonary condition in the same institution, the distribution of BMI categories was strikingly different in patients admitted with COVID-19. Patients admitted with non-SAR-COV-2 conditions showed a lower prevalence of obesity (25.8%) compared to patients with SAR-COV-2. The prevalence of obesity observed in the non-SARS-COV-2 patients was similar to that observed in the general population from Nord and Pas de Calais. In contrast, sex distribution and age were not significantly different from participants in non-SARS-CoV-2 controls vs SARS-COV-2 subjects. Interestingly, obesity was also a standout factor for the requirement of Intermittent Mandatory Ventilation (IMV). Of 124 patients, 85 (68.6%) needed IMV and their BMI was higher than those who didn’t need IMV. Close to 90% of the patients with a BMI of > 35 required IMV. In a study from three hospitals in Wenzhou, China, Zheng et al. demonstrated that obesity was a major risk factor for the severity of COVID-19 in a group of patient’s metabolic associated fatty liver disease (MAFLD) [24]. The authors analyzed data from Covid-19 patients with confirmed MAFLD and showed that out of Sixty-six patients, Forty-five were overweight/obese (BMI > 25 kg/m2). Out of these 17 (37.8%) showed severe disease. Compared to only 2 (9.5%) non-obese patients that have severe disease. The authors concluded that obesity is a major risk factor for disease severity in COVID-19 patients having MAFLD. A recent review addressed the role of MAFLD in the outcome of COVID-19 patients [47]. Another study from Rhode Island, USA showed a strong association between obesity and disease severity. The authors analyzed data from 103 adult consecutive patients, admitted with COVID-19 to the hospital. The authors concluded that patients with extreme obesity (BMI of > 35 kg/m2) are at high risk of severe COVID-19. Besides, Obesity (BMI > 30 kg/m2) was strongly and independently associated with the use of invasive mechanical ventilation in patients with COVID-19 [48]. Similar results were shown by a study conducted by New York University health center on a large cohort of COVID 19 patients (N = 3615) [38]. The authors performed a prospective analysis of BMI stratified by age in COVID-19 positive symptomatic patients who showed up at the hospital. The authors showed that younger patients (Age < 60 years) with a BMI > 30 kg/m2 were more than twice likely to be admitted to hospital and develop critical illness compared to patients with a BMI < 30 kg/m2. The likelihood of admission to ICU increased to 3.6 times in patients with severe obesity (BMI ≥ 35 kg/m2) [38]. Another study from the same hospital with a larger sample size (N = 5279) showed similar results. The authors concluded that after age, obesity was the single most important factor for hospitalized patients with COVID-19 [49]. A report from the United Kingdom (a pre-print without peer-review) evaluated the fate of 16,749 hospitalized COVID-19 patients in the UK [50]. The authors concluded that Obesity was associated with a higher probability of mortality. A single center study from Italy on a cohort of 482 patients found obesity to be a strong, independent risk factor for severe diease and dealth due to COVID-19. While patients with a BMI ≥ 30 kg/m2 had a high risk for severe illness, a BMI ≥ 35 kg/m2 radically increased the risk of death [35]. Zhang et. al. reported that obesity predisposed young COVID-19 patients (14–45 Years of age) to the risk of significantly higher mortality [41]. Cai et.al. examined the association of Obesity with the severity of COVID-19 in a designated hospital in Shenzhen, China and concluded that obese patients has increased odds of progressing to severe disease due to COVID-19 [51]. Reports from other countries severely affected by the pandemic including Mexico [52], Germany [53] an Spain [54] have also found a significant association between BMI and the increasing severity of the disease and mortality due to COVID-19.
Table 1 shows the association of BMI with disease severity and mortality in COVID-19 patients from different studies. Together, these data strongly suggest obesity to be an important factor in disease severity and outcome of COVID-19 patients.
Dysregulated fatty acid metabolism, cellular hypertrophy and death, ER stress, Hypoxia and mitochondrial dysfunction, because of excess fat leads to a substantial alteration of cellular architecture of adipose tissue. This rearrangement favors a pro-inflammatory environment and perpetuates local as well as systemic inflammation
What makes the obese host so vulnerable?
People with obesity have an increased prevalence of diseases like renal insufficiency, cardiovascular diseases, Type 2 diabetes mellitus, certain types of cancers, and a significant degree of endothelial dysfunction. These conditions are major risk factors for disease severity and mortality associated with COVID-19. This makes obesity, particularly ominous in COVID-19. However, there is enough evidence to suggest that obesity is an additional risk factor associated with worse outcomes in COVID-19 patients. Caussy et al. specifically looked at whether obesity was associated with worse outcomes in COVID-19 patients with other risk factors. The authors found that obesity remained a significant factor for poor outcome of patients having other chronic issues like Hypertension, Dyslipidemia, Type 2 diabetes, Cardiomyopathy, Chronic pulmonary diseases, and malignancy. The analysis is shown in Table 2.
Therefore, it is reasonable to assume that there are additional factors that make obese host vulnerable to severe disease and worse outcomes as a result of COVID-19 infection.
Obesity-associated inflammation and its impact on SARS-CoV-2 infection
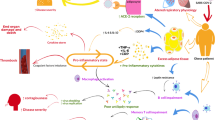
Until recently adipose tissue was merely considered to be an inert organ that stored energy in the form of lipids, which could be utilized in the state of fasting/starvation. However, adipose tissue is now being recognized as a key endocrine organ that secretes a plethora of factors (Adipokines, Chemokines, and Cytokines) that profoundly impact metabolism and immune system [61, 62]. Normal lean adipose is composed of a comprehensive set of immune cells that maintain a balance between pro-inflammatory and anti-inflammatory environment [63]. Excess calorie intake and/or reduced energy expenditure leads to a rapid expansion of adipose tissue to accommodate and store excess nutrients. However, obesity-induced expansion alters the function and architecture of adipose tissue and enlarged adipocytes become apoptotic and attract macrophages and other cells to form inflammatory adipose [64, 65]. Normal adipose tissue contains a population of three anti-inflammatory cell types associated with normal adipose function. T helper (Th2) cells, M-2 macrophages and regulatory T-cells (Treg) are important negative regulators of inflammation. Obesity is associated with significant alteration and abundance of immune cells in the adipose tissues with a marked decrease in Th2 cells, Treg cells, and M-2 macrophages. Instead, there is a significant increase in the abundance of pro-inflammatory cells like CD8+ T cells and M-1 macrophages [66,67,68,69]. Obese, inflamed adipose comprises of > 40% M-1 macrophages, which are the source of an array of pro-inflammatory cytokines leading to a local as well as systemic inflammation. Several other cell types like neutrophils, dendritic cells, and mast cells also contribute to inflammation by releasing several pro-inflammatory factors. The ultimate result is a state of chronic inflammation both at local as well at the systemic level [68, 70]. Inflammation is at the forefront of COVID-19 research and major complication of COVID-19 infection are directly associated with systemic inflammation [71,72,73,74,75,76]. Recent studies have indicated that disease severity and outcome of COVID-19 patients are directly associated with dysregulation of pro-inflammatory cytokines. Therefore, it is plausible to suggest that acute inflammation arising from COVID-19, may amplify existing chronic inflammation secondary to obesity and lead to more severe disease phenotype and poorer outcomes. A similar hypothesis was proposed in a recent paper by Paul MacDaragh Ryan and Noel M. Caplice [77]. The authors suggested that obese subjects have higher levels of various inflammatory signals and, are more likely to overreact to coronavirus infection. Zhang et al. analyzed 16 retrospective studies and found that inflammatory markers were positively correlated with the severity of COVID-19 [78]. Hamer et.al. specifically looked at the role of low inflammation in the severity of COVID-19 disease [79]. The authors found that a high rate of hospital admission in obese subjects can be partly explained by low-grade inflammation (Fig. 1).
Cellular immune function is impaired in obesity
Several lines of evidence have strongly indicated that obesity results in significant changes in both innate and adaptive immune response and individuals with obesity are in a state of chronic and low-grade inflammation [80,81,82]. The overall result is a reduced immune response to infectious agents, resulting in poorer outcomes post-infection [83,84,85,86].
Excess fat deposition disrupts lymphoid tissue architecture and integrity
Blood cells (both lymphoid and myeloid) lineages are generated from bone marrow-derived pluripotent hematopoietic stem cells. Lymphoid cells undergo further processing in the thymus to generate mature T-Lymphocytes. Mature lymphocytes reside in secondary lymphoid tissues including lymph nodes and spleen, where they take part in immune surveillance and wait for activation by pathogens. Therefore, any change in the lymphoid tissue architecture can adversely affect its functioning resulting in an alteration in the distribution of immune cell populations, impaired T cells activity, and diminished immune defense. Interestingly, Obesity and metabolic syndrome have a profound impact on the functioning of lymphoid tissue [87, 88]. Ectopic lipid deposition in tissues other than adipose is a hallmark of obesity and this is not restricted to metabolic tissues. Several studies have reported that obesity leads to increased lipid deposition in primary lymphoid organs (bone marrow and thymus). Excess lipid deposition in these tissues impacts the distribution of leukocyte population, the activity of lymphocytes resulting in a marked change in the overall immune defense [87, 89,90,91]. Lipid accumulation of lymphoid organs is known to occur in older people and adversely affect their immunity. Consequently, obesity is assumed to promote premature “aging” of the immune system [92]. Also, diet-induced obesity in mice adversely impacts the dynamics of secondary lymphoid tissues leading to alteration of effector/memory T cell ratio and an overall constraint in T cell receptor variety [90, 91, 93]. Therefore, T cells in obese mice are capable of responding to a smaller range of pathogens as compared to the normal chow-fed mice. Obesity also reduces inguinal lymph node size, hampers lymphatic fluid transport, and dendritic cell movement and reduces the number of T lymphocytes in lymph nodes [94]. Overall, obesity disturbs immune system integrity and significantly alters leukocyte growth, movement, and diversity. Indeed, a recent study showed that BMI was inversely correlated with total lymphocyte count in COVID-19 patients [95].
Insulin resistance negatively impact immune function
Multiple lines of evidence suggest that Insulin may be a key regulator of T-cell metabolism and function [96,97,98,99]. Insulin signaling exerts critical immune-stimulatory effects on T-cells, positively controlling their growth and proliferation, glucose metabolism, and production of cytokines, which results in the strengthening of host defense against infections. Obesity often leads to systemic “insulin resistance” a phenomenon that is characterized by reduced insulin signaling in peripheral tissues resulting in several metabolic abnormalities [100,101,102,103]. Insulin resistance is a complex phenomenon and multiple factors are involved but obesity induced adipose dysfunction plays a central role in the development of systemic insulin resistance [100,101,102,103,104,105,106]. Obesity leads to a significant expansion of adipose mass that radically influences adipose function, which causes disruption of insulin signaling in peripheral tissues including immune cells. Insulin-stimulated signaling pathway is impaired in lymphocytes of individuals with obesity [107] and Type 2 diabetes [108]. Francis M. Finucane and Colin Davenport in a recent paper discussed the possible relationship between insulin resistance with COPVID-19 disease severity [109, 110]. The authors suggested that markers of insulin resistance should be assessed for their prognostic efficacy. No study has specifically looked at the association between insulin resistance and the severity of CoVID-19 disease because clinical and biochemical markers of insulin resistance are not routinely measured in CoVID-19 patients. Ren et. al. used triglyceride and glucose index (TyG) as a marker of insulin resistance and showed that TyG index was significantly associated with an increased risk of severe case and mortality in CoVID-19 patients [110]. Although TyG index is a useful surrogate marker, it is not considered a gold standard for assessing insulin resistance. More studies are needed to utilize more acceptable insulin resistance models like Homeostatic Model Assessment (HOMA) or Quantitative insulin sensitivity check index (QUICKI) to investigate the contribution of insulin resistance on disease severity and mortality in CoVID-19 patients [111,112,113].
Leptin resistance in obesity impairs immune functioning
Besides insulin, leptin the hormone that is secreted from adipocytes exerts profound effects on innate and adaptive immunity. Leptin is a key regulator of metabolic homeostasis and it primarily exerts its effects via Leptin receptors (LEPR) that are highly expressed in POMC neurons in the hypothalamus, which is the epicenter of appetite and energy expenditure regulation. Leptin has also been shown to regulate several other physiological processes in the body. Interestingly, LEPRs are expressed in cells of the immune system and several studies have documents the role of leptin in regulating various aspects of immune cell development and activity [114,115,116]. Leptin has been shown to regulate both innate and adaptive immune responses via the modulation of immune cell metabolism, proliferation, and activity. Circulating leptin levels are markedly increased in obese subjects but the response of target tissues to leptin is severely compromised due to leptin resistance [117,118,119]. Therefore, leptin resistance would profoundly impact the proper development and activity of immune cells in obese subjects, weaken the host defense, and increase the chances of severe disease and poor outcome in COVID-19 patients. A recent paper provided a detailed analysis of the role of leptin in COVID-19 disease severity in obese subjects [120]. The authors describe how leptin plays a vital role in immune regulation and how chronically elevated leptin (as seen in obese subjects) impairs host immune defense. The authors conclude by suggesting studies to explore the possible role of leptin in the pathogenesis of SARS-CoV-2
Altered ACE2 expression in obese subjects may impact COVID-19 disease severity
Angiotensinogen Converting Enzyme (ACE-2) is required for the entry of COVID-19 into the cells. The receptors are expressed on cells in the nose lining, the lungs, pancreas, kidneys and gut, adipose, and in the lining of blood vessels, in the heart muscle, and cells circulating in the blood. It is assumed that increased expression of ACE-2 would boost the entry of the virus into the cells and therefore, cause severe disease with worse clinical outcomes. Emerging evidence indicates that ACE2 expression is increased in individuals who are obese and overweight. Higham et al. have demonstrated increased ACE2 expression in the bronchial epithelium of COPD patients who are overweight or obese compared to lean subjects [121]. The authors suggested that increased ACE-2 expression may be related to increased disease severity in COVID-19 patients who are overweight or obese. Interestingly, ACE-2 expression is higher in adipose compared to lung tissue, which is the primary target of COVID-19 [122]. Moreover, adipose ACE-2 expression is up-regulated in animal models of diet-induced obesity [123]. This raises the prospect of adipose tissue being an important target and a possible reservoir for COVID-19. Adipose tissue has been shown to act as a reservoir for other human pathogens [124]. More importantly, lipid droplets that are present in adipose tissue have been shown to play a key role in the production of the Hepatitis C virus [125,126,127,128]. Therefore, it is reasonable to assume that adipose tissues might act as a reservoir for COVID-19 and lipid droplets might facilitate viral production and spread. Consequently, excess adipose as seen in obesity would make it an easy target for the virus entry and spread and therefore, cause severe disease with bad clinical outcomes. More research is needed to understand the functional significance of adipose ACE-2 and its association with obesity in COVID-19 patients.
Role of coagulopathy /thrombosis in SARS-CoV-2 pathogenesis
Several studies have shown that obesity is associated with a hypercoagulable state and obese subjects have elevated levels of prothrombin factors and reduced levels of anti-thrombin molecules [129,130,131]. Since, severely ill COVID-19 patients are often associated with coagulopathy/thrombosis and obesity could potentially make it worse. A study by Gazzaruso et al. on a cohort of 49 patients hospitalized with COVID-19 infection and reported that low antithrombin (AT) levels were strongly associated with increased mortality [132]. The authors further show that BMI was the only variable that showed a significant difference between patients with low and high levels of AT. The authors documented an inverse correlation between AT levels and BMI and obese patients had significantly lower AT levels as compared to non-obese patients. The authors suggested that AT may be the connecting factor behind increased mortality in obese COVID-19 patients. More studies are needed to confirm this finding.
Does obesity survival paradox occur in COVID-19 patients?
Obese subjects are at an increased risk of developing pneumonia but ironically, obese patients with pneumonia have a lower mortality as compared to non-obese subjects. This phenomenon is known as “Obesity survival paradox” and has been the subject of several independent studies [133,134,135,136]. Obesity survival paradox in COVID-19 patients is still a matter of debate. Biscarini et al. analyzed a cohort of 331 patients admitted to hospital with COVID-19. The authors reported that obese COVID-19 patients were more likely to be admitted to ICU than non-obese subjects but obesity was not significantly associated with mortality, mortality in ICU and length of hospital stay [137]. However, majority of the studies have reported that obese subjects are an increased risk of severe disease and increased mortality due to COVID-19 [59, 132, 138,139,140,141,142,143].
Conclusion
Obesity is a huge healthcare concern because it is associated with several chronic diseases including type 2 diabetes, heart diseases, stroke, and certain types of cancers. Obesity significantly reduces the quality of life and is one of the leading causes of death, worldwide. Recent evidence has shown that obesity weakens the immune system and therefore, making the host vulnerable to infectious diseases. Indeed, Obesity has emerged as a strong risk factor for severe disease in the current pandemic disease, COVID-19. Several independent studies have demonstrated that obese subjects with COVID-19 have a higher risk of severe disease, hospitalization, and increased probability of death. During the 2009 HIN1 pandemic, patients with severe obesity were more likely to require hospitalization, ICU admission, and death due to the disease. Data over the years have indicated that obesity negatively impacts host immune defense making it vulnerable to infectious disease. Excess adiposity is associated with significant changes in the resident immune cell composition of adipose tissue, which disrupts the balance between pro-inflammatory and anti-inflammatory immune cells in favor of the former. This leads to a state of chronic low-grade inflammation. This chronic inflammation is likely amplified by acute inflammation arising out of COVID-19 resulting in a more severe disease phenotype and poorer outcomes. One the other hand excess lipid deposition alters the integrity and architecture of primary lymphoid tissues and thereby impacting the immune cell development and activation. Besides, metabolic changes associated with obesity such as insulin and leptin resistance negatively impact immune cell function. Together these changes have a substantial influence on immune cell growth and proliferation, glucose metabolism, and activation which ultimately results in impairment of host immune defense. Finally, adipose ACE-2 could also play a vital role in the spread of COVID-19 to other tissues but more work is needed to investigate this possibility. Fig. 2 illustrates the possible mechanism(s) that could explain increasing susceptibility of the obese subject to severe disease and poor clinical outcome as a result of COVID-19 infection.
Availability of data and materials
Agreed.
Abbreviations
- SARS-COV- 2:
-
Severe acute respiratory syndrome coronavirus 2
- COVID-19:
-
Coronavirus disease
- BMI:
-
Body Mass Index
- IMV:
-
Intermittent Mandatory Ventilation
- ICU:
-
Intensive Care Unit
- MAFLD:
-
Metabolic associated fatty liver disease
- H1N1:
-
Influenza A virus subtype H1N1
- ACE-2:
-
Angiotensin-converting enzyme 2
- Th cells:
-
T helper cells
- T-Reg cells:
-
Regulatory T cells
- TLR:
-
Toll-like receptors
- TCR:
-
T-cell receptor
- INSR:
-
Insulin receptor
- LEPR:
-
Leptin receptor
- POMC:
-
Pro-opiomelanocortin
References
Simon AC. COVID-19, a graphic account. Emergencias. 2020;32(3):206–9.
Sylaja PN, Srivastava MVP, Shah S, Bhatia R, Khurana D, Sharma A, et al. The SARS-CoV-2/COVID-19 pandemic and challenges in stroke care in India. Ann N Y Acad Sci. 2020;1473(1):3–10.
As We Went to Press. COVID-19 Continues to Spread. Am J Nurs. 2020;120(4):15.
Centor RM, Fisman DN. Annals on call - understanding the spread of COVID-19. Ann Intern Med. 2020;172(6):OC1.
Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020;368:m1036.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400.
Daw MA, El-Bouzedi AH. Modelling the epidemic spread of COVID-19 virus infection in northern African countries. Travel Med Infect Dis. 2020;35:101671.
Gatto M, Bertuzzo E, Mari L, Miccoli S, Carraro L, Casagrandi R, et al. Spread and dynamics of the COVID-19 epidemic in Italy: effects of emergency containment measures. Proc Natl Acad Sci U S A. 2020;117(19):10484–91.
Yang S, Cao P, Du P, Wu Z, Zhuang Z, Yang L, et al. Early estimation of the case fatality rate of COVID-19 in mainland China: a data-driven analysis. Ann Transl Med. 2020;8(4):128.
Stafford N. Covid-19: why Germany's case fatality rate seems so low. BMJ. 2020;369:m1395.
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776-7.
Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–6.
Mi YN, Huang TT, Zhang JX, Qin Q, Gong YX, Liu SY, et al. Estimating instant case fatality rate of COVID-19 in China. Int J Infect Dis. 2020;97:1–6.
Giangreco G. Case fatality rate analysis of Italian COVID-19 outbreak. J Med Virol. 2020;92(7):919–23.
Fischer F, Raiber L, Boscher C, Winter MH. COVID-19 and the elderly: who cares? Front Public Health. 2020;8:151.
Etard JF, Vanhems P, Atlani-Duault L, Ecochard R. Potential lethal outbreak of coronavirus disease (COVID-19) among the elderly in retirement homes and long-term facilities, France, March 2020. Euro Surveill. 2020;25(15):2000448.
Sinclair AJ, Abdelhafiz AH. Age, frailty and diabetes - triple jeopardy for vulnerability to COVID-19 infection. EClinical Medicine. 2020;22:100343.
Osama T, Pankhania B, Majeed A. Protecting older people from COVID-19: should the United Kingdom start at age 60? J R Soc Med. 2020;113(5):169–70.
Nickel CH, Rueegg M, Pargger H, Bingisser R. Age, comorbidity, frailty status: effects on disposition and resource allocation during the COVID-19 pandemic. Swiss Med Wkly. 2020;150:w20269.
Mahase E. Covid-19: death rate is 0.66% and increases with age, study estimates. BMJ. 2020;369:m1327.
Singh AK, Gupta R, Misra A. Comorbidities in COVID-19: outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab Syndr. 2020;14(4):283–7.
Gidlof S, Savchenko J, Brune T, Josefsson H. COVID-19 in pregnancy with comorbidities: more liberal testing strategy is needed. Acta Obstet Gynecol Scand. 2020;99(7):948–9.
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–9.
Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, et al. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244.
Sattar N, McInnes IB, McMurray JJV. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142(1):4–6.
Kassir R. Risk of COVID-19 for patients with obesity. Obes Rev. 2020;21(6):e13034.
Caussy C, Wallet F, Laville M, Disse E. Obesity is associated with severe forms of COVID-19. Obesity (Silver Spring). 2020;28(7):1175.
Ong SWX, Young BE, Leo YS, Lye DC. Association of higher body mass index (BMI) with severe coronavirus disease 2019 (COVID-19) in younger patients. Clin Infect Dis. 2020;71(16):2300–2.
Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: a systematic review and meta-analysis. J Med Virol. 2020. https://doi.org/10.1002/jmv.26237.
Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996.
Belanger MJ, Hill MA, Angelidi AM, Dalamaga M, Sowers JR, Mantzoros CS. Covid-19 and disparities in nutrition and obesity. N Engl J Med. 2020;383(11):e69.
Devecchi A, Ippolito M, Merlo FD, Pira C, Rahimi F. COVID-19 and obesity. Minerva Gastroenterol Dietol. 2020.
Mauvais-Jarvis F. Aging, male sex, obesity, and metabolic inflammation create the perfect storm for COVID-19. Diabetes. 2020;69(9):1857–63.
Muniyappa R, Wilkins KJ. Diabetes, obesity, and risk prediction of severe COVID-19. J Clin Endocrinol Metab. 2020;105(10):dgaa442.
Rottoli M, Bernante P, Belvedere A, Balsamo F, Garelli S, Giannella M, et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? Results from a single Italian Centre. Eur J Endocrinol. 2020;183(4):389–97.
Syed AA, Soran H, Adam S. Obesity and covid-19: the unseen risks. BMJ. 2020;370:m2823.
Vaughan CJ, Cronin H, Ryan PM, Caplice NM. Obesity and COVID-19: a Virchow's triad for the 21st century. Thromb Haemost. 2020;120(11):1590–3.
Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020;71(15):896–7.
Deng M, Qi Y, Deng L, Wang H, Xu Y, Li Z, et al. Obesity as a Potential Predictor of Disease Severity in Young COVID-19 Patients: A Retrospective Study. Obesity (Silver Spring). 2020;28(10):1815–25.
Iacobellis G, Malavazos AE, Ferreira T. COVID-19 rise in Younger adults with Obesity: Visceral Adiposity can predict the Risk. Obesity (Silver Spring). 2020;28(10):1795.
Zhang F, Xiong Y, Wei Y, Hu Y, Wang F, Li G, et al. Obesity predisposes to the risk of higher mortality in young COVID-19 patients. J Med Virol. 2020;92(11):2536–42.
Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, et al. Letter to the editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244.
Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity is associated with higher in-hospital mortality in a cohort of patients with COVID-19 in the Bronx. New York. Metabolism. 2020;108:154262.
Gao F, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care. 2020;43(7):e72–4.
Chiappetta S, Sharma AM, Bottino V, Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. Int J Obes. 2020;44(8):1790–2.
Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020;28(7):1195–9.
Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and nonalcoholic fatty liver disease: two intersecting pandemics. Eur J Clin Investig. 2020;50(10):e13338.
Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, et al. Association of Obesity with Disease Severity among Patients with COVID-19. Obesity (Silver Spring). 2020;28(7):1200–4.
Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. 2020;369:m1966.
AB Docherty EH, Green CA, Hardwick H, Pius R, Norman L, Holden KA, Read JM, Dondelinger CG, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, ISARICC Investigators, Dunning J, Openshaw PJM, Baillie JK, Semple MG. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. 2020;369:m1985.
Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, et al. Obesity and COVID-19 severity in a designated Hospital in Shenzhen. China. Diabetes Care. 2020;43(7):1392–8.
Bello-Chavolla OY, Bahena-Lopez JP, Antonio-Villa NE, Vargas-Vazquez A, Gonzalez-Diaz A, Marquez-Salinas A, et al. Predicting Mortality Due to SARS-CoV-2: A Mechanistic Score Relating Obesity and Diabetes to COVID-19 Outcomes in Mexico. J Clin Endocrinol Metab. 2020;105(8):dgaa346.
Petersen A, Bressem K, Albrecht J, Thiess HM, Vahldiek J, Hamm B, et al. The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317.
Urra JM, Cabrera CM, Porras L, Rodenas I. Selective CD8 cell reduction by SARS-CoV-2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin Immunol. 2020;217:108486.
Giacomelli A, Ridolfo AL, Milazzo L, Oreni L, Bernacchia D, Siano M, et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158:104931.
Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Morbid Obesity as an Independent Risk Factor for COVID-19 Mortality in Hospitalized Patients Younger than 50. Obesity (Silver Spring). 2020;28(9):1595–9.
Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx. New York. Metabolism. 2020;108:154262.
Hamer M, Kivimaki M, Gale CR, David BG. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184–7.
Pettit NN, MacKenzie EL, Ridgway J, Pursell K, Ash D, Patel B, et al. Obesity is Associated with Increased Risk for Mortality Among Hospitalized Patients with COVID-19. Obesity (Silver Spring). 2020;28(10):1806–10.
Caussy C, Pattou F, Wallet F, Simon C, Chalopin S, Telliam C, et al. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8(7):562–4.
Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–39.
Booth A, Magnuson A, Fouts J, Foster MT. Adipose tissue: an endocrine organ playing a role in metabolic regulation. Horm Mol Biol Clin Invest. 2016;26(1):25–42.
Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab. 2012;23(8):407–15.
Duffaut C, Galitzky J, Lafontan M, Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun. 2009;384(4):482–5.
Lu J, Zhao J, Meng H, Zhang X. Adipose tissue-resident immune cells in obesity and type 2 diabetes. Front Immunol. 2019;10:1173.
Serena C, Keiran N, Ceperuelo-Mallafre V, Ejarque M, Fradera R, Roche K, et al. Obesity and type 2 diabetes alters the immune properties of human adipose derived stem cells. Stem Cells. 2016;34(10):2559–73.
Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. 2014;222(3):R113–27.
Liu R, Nikolajczyk BS. Tissue immune cells fuel obesity-associated inflammation in adipose tissue and beyond. Front Immunol. 2019;10:1587.
Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cell. 2014;37(5):365–71.
Vorotnikov AV, Stafeev IS, Menshikov MY, Shestakova MV, Parfyonova YV. Latent inflammation and defect in adipocyte renewal as a mechanism of obesity-associated insulin resistance. Biochemistry (Mosc). 2019;84(11):1329–45.
Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327–31.
Garcia LF. Immune response, inflammation, and the clinical Spectrum of COVID-19. Front Immunol. 2020;11:1441.
Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46–e7.
Leisman DE, Deutschman CS, Legrand M. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020;46(6):1105–8.
Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–74.
Vepa A, Bae JP, Ahmed F, Pareek M, Khunti K. COVID-19 and ethnicity: a novel pathophysiological role for inflammation. Diabetes Metab Syndr. 2020;14(5):1043–51.
Ryan PM, Caplice NM. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity (Silver Spring). 2020;28(7):1191–4.
Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393.
Hamer M, Kivimaki M, Gale CR, Batty GD. Lifestyle Risk Factors for Cardiovascular Disease in Relation to COVID-19 Hospitalization: A Community-Based Cohort Study of 387,109 Adults in UK. medRxiv. 2020;2020.05.09.20096438.
Karagiannides I, Pothoulakis C. Obesity, innate immunity and gut inflammation. Curr Opin Gastroenterol. 2007;23(6):661–6.
Da Costa LA, Arora P, Garcia-Bailo B, Karmali M, El-Sohemy A, Badawi A. The association between obesity, cardiometabolic disease biomarkers, and innate immunity-related inflammation in Canadian adults. Diabetes Metab Syndr Obes. 2012;5:347–55.
de Heredia FP, Gomez-Martinez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71(2):332–8.
Rojas-Osornio SA, Cruz-Hernandez TR, Drago-Serrano ME, Campos-Rodriguez R. Immunity to influenza: impact of obesity. Obes Res Clin Pract. 2019;13(5):419–29.
Chng MH, Alonso MN, Barnes SE, Nguyen KD, Engleman EG. Adaptive immunity and antigen-specific activation in obesity-associated insulin resistance. Mediat Inflamm. 2015;2015:593075.
Bharath LP, Ip BC, Nikolajczyk BS. Adaptive immunity and metabolic health: harmony becomes dissonant in obesity and aging. Compr Physiol. 2017;7(4):1307–37.
Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7(1):66–75.
Adler BJ, Kaushansky K, Rubin CT. Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol. 2014;10(12):737–48.
van den Berg SM, Seijkens TT, Kusters PJ, Beckers L, den Toom M, Smeets E, et al. Diet-induced obesity in mice diminishes hematopoietic stem and progenitor cells in the bone marrow. FASEB J. 2016;30(5):1779–88.
Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity in mice reduces the maintenance of influenza-specific CD8+ memory T cells. J Nutr. 2010;140(9):1691–7.
Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, et al. Obesity accelerates thymic aging. Blood. 2009;114(18):3803–12.
Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13(8):707–12.
Castelo-Branco C, Soveral I. The immune system and aging: a review. Gynecol Endocrinol. 2014;30(1):16–22.
Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185(3):1836–45.
Weitman ES, Aschen SZ, Farias-Eisner G, Albano N, Cuzzone DA, Ghanta S, et al. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One. 2013;8(8):e70703.
Cai SH, Liao W, Chen SW, Liu LL, Liu SY, Zheng ZD. Association between obesity and clinical prognosis in patients infected with SARS-CoV-2. Infect Dis Poverty. 2020;9(1):80.
Tsai S, Clemente-Casares X, Zhou AC, Lei H, Ahn JJ, Chan YT, et al. Insulin receptor-mediated stimulation boosts T cell immunity during inflammation and infection. Cell Metab. 2018;28(6):922–34 e4.
Mirdamadi Y, Bommhardt U, Goihl A, Guttek K, Zouboulis CC, Quist S, et al. Insulin and insulin-like growth factor-1 can activate the phosphoinositide-3-kinase /Akt/FoxO1 pathway in T cells in vitro. Dermatoendocrinol. 2017;9(1):e1356518.
Helderman JH, Strom TB. Specific insulin binding site on T and B lymphocytes as a marker of cell activation. Nature. 1978;274(5666):62–3.
Fischer HJ, Sie C, Schumann E, Witte AK, Dressel R, van den Brandt J, et al. The insulin receptor plays a critical role in T cell function and adaptive immunity. J Immunol. 2017;198(5):1910–20.
Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94(2):206–18.
Rebolledo OR, Marra CA, Raschia A, Rodriguez S, Gagliardino JJ. Abdominal adipose tissue: early metabolic dysfunction associated to insulin resistance and oxidative stress induced by an unbalanced diet. Horm Metab Res. 2008;40(11):794–800.
Cherneva RV, Georgiev OB, Petrova DS, Mondeshki TL, Ruseva SR, Cakova AD, et al. Resistin - the link between adipose tissue dysfunction and insulin resistance in patients with obstructive sleep apnea. J Diabetes Metab Disord. 2013;12(1):5.
Vernochet C, Damilano F, Mourier A, Bezy O, Mori MA, Smyth G, et al. Adipose tissue mitochondrial dysfunction triggers a lipodystrophic syndrome with insulin resistance, hepatosteatosis, and cardiovascular complications. FASEB J. 2014;28(10):4408–19.
Kadowaki T, Hara K, Yamauchi T, Terauchi Y, Tobe K, Nagai R. Molecular mechanism of insulin resistance and obesity. Exp Biol Med (Maywood). 2003;228(10):1111–7.
Hulver MW, Dohm GL. The molecular mechanism linking muscle fat accumulation to insulin resistance. Proc Nutr Soc. 2004;63(2):375–80.
Bhattacharya S, Dey D, Roy SS. Molecular mechanism of insulin resistance. J Biosci. 2007;32(2):405–13.
Viardot A, Heilbronn LK, Samocha-Bonet D, Mackay F, Campbell LV, Samaras K. Obesity is associated with activated and insulin resistant immune cells. Diabetes Metab Res Rev. 2012;28(5):447–54.
Stentz FB, Kitabchi AE. Transcriptome and proteome expressions involved in insulin resistance in muscle and activated T-lymphocytes of patients with type 2 diabetes. Genomics Proteomics Bioinformatics. 2007;5(3–4):216–35.
Finucane FM, Davenport C. Coronavirus and obesity: could insulin resistance mediate the severity of Covid-19 infection? Front Public Health. 2020;8:184.
Ren H, Yang Y, Wang F, Yan Y, Shi X, Dong K, et al. Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc Diabetol. 2020;19(1):58.
Singh B, Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes. 2010;1(2):36–47.
Mahajan R. Insulin resistance: quest for surrogate markers. Int J Appl Basic Med Res. 2017;7(3):149.
Kim TJ, Kim HJ, Kim YB, Lee JY, Lee HS, Hong JH, et al. Comparison of surrogate markers as measures of uncomplicated insulin resistance in Korean adults. Korean J Fam Med. 2016;37(3):188–96.
Matarese G. Leptin and the immune system: how nutritional status influences the immune response. Eur Cytokine Netw. 2000;11(1):7–14.
Kim SY, Lim JH, Choi SW, Kim M, Kim ST, Kim MS, et al. Preferential effects of leptin on CD4 T cells in central and peripheral immune system are critically linked to the expression of leptin receptor. Biochem Biophys Res Commun. 2010;394(3):562–8.
Fernandez-Riejos P, Najib S, Santos-Alvarez J, Martin-Romero C, Perez-Perez A, Gonzalez-Yanes C, et al. Role of leptin in the activation of immune cells. Mediat Inflamm. 2010;2010:568343.
Zhou Y, Rui L. Leptin signaling and leptin resistance. Front Med. 2013;7(2):207–22.
Liu J, Yang X, Yu S, Zheng R. The Leptin resistance. Adv Exp Med Biol. 2018;1090:145–63.
Enriori PJ, Evans AE, Sinnayah P, Cowley MA. Leptin resistance and obesity. Obesity (Silver Spring). 2006;14(Suppl 5):254S–8S.
Rebello CJ, Kirwan JP, Greenway FL. Obesity, the most common comorbidity in SARS-CoV-2: is leptin the link? Int J Obes. 2020;44(9):1810–7.
Higham A, Singh D. Increased ACE2 Expression in the Bronchial Epithelium of COPD Patients who are Overweight. Obesity (Silver Spring). 2020;28(9):1586–9.
Jia XY, Lu C, Chen S, Liu Y, Bai Q, Lu JY. Two things about COVID-19 might need attention. Preprints. 2020;2020020315.
Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, et al. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Phys Regul Integr Comp Phys. 2008;295(3):R781–8.
Bourgeois C, Gorwood J, Barrail-Tran A, Lagathu C, Capeau J, Desjardins D, et al. Specific biological features of adipose tissue, and their impact on HIV persistence. Front Microbiol. 2019;10:2837.
Boulant S, Douglas MW, Moody L, Budkowska A, Targett-Adams P, McLauchlan J. Hepatitis C virus core protein induces lipid droplet redistribution in a microtubule- and dynein-dependent manner. Traffic. 2008;9(8):1268–82.
Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, et al. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9(9):1089–97.
Ogawa K, Hishiki T, Shimizu Y, Funami K, Sugiyama K, Miyanari Y, et al. Hepatitis C virus utilizes lipid droplet for production of infectious virus. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(7):217–28.
Roingeard P, Hourioux C, Blanchard E, Prensier G. Hepatitis C virus budding at lipid droplet-associated ER membrane visualized by 3D electron microscopy. Histochem Cell Biol. 2008;130(3):561–6.
Targher G, Zoppini G, Moghetti P, Day CP. Disorders of coagulation and hemostasis in abdominal obesity: emerging role of fatty liver. Semin Thromb Hemost. 2010;36(1):41–8.
De Pergola G, Pannacciulli N. Coagulation and fibrinolysis abnormalities in obesity. J Endocrinol Investig. 2002;25(10):899–904.
Abdollahi M, Cushman M, Rosendaal FR. Obesity: risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive use. Thromb Haemost. 2003;89(3):493–8.
Gazzaruso C, Paolozzi E, Valenti C, Brocchetta M, Naldani D, Grignani C, et al. Association between antithrombin and mortality in patients with COVID-19. A possible link with obesity. Nutr Metab Cardiovasc Dis. 2020;30(11):1914–9.
Braun N, Hoess C, Kutz A, Christ-Crain M, Thomann R, Henzen C, et al. Obesity paradox in patients with community-acquired pneumonia: is inflammation the missing link? Nutrition. 2017;33:304–10.
Cho WH, Oh JY, Yeo HJ, Han J, Kim J, Hong SB, et al. Obesity survival paradox in pneumonia supported with extracorporeal membrane oxygenation: analysis of the national registry. J Crit Care. 2018;48:453–7.
Corrales-Medina VF, Valayam J, Serpa JA, Rueda AM, Musher DM. The obesity paradox in community-acquired bacterial pneumonia. Int J Infect Dis. 2011;15(1):e54–7.
Nie W, Zhang Y, Jee SH, Jung KJ, Li B, Xiu Q. Obesity survival paradox in pneumonia: a meta-analysis. BMC Med. 2014;12:61.
Biscarini S, Colaneri M, Ludovisi S, Seminari E, Pieri TC, Valsecchi P, et al. The obesity paradox: analysis from the SMAtteo COvid-19 REgistry (SMACORE) cohort. Nutr Metab Cardiovasc Dis. 2020;30(11):1920–5.
Ahmed SI, Hasan SMT, Ahmed T. Obesity is a potential risk factor for covid-19 associated morbidity and mortality in urban Bangladesh. BMJ. 2020;370:m2811.
Asare S, Sandio A, Opara IN, Riddle-Jones L, Palla M, Renny N, et al. Higher obesity trends among African Americans are associated with increased mortality in infected COVID-19 patients within the City of Detroit. SN Compr Clin Med. 2020:1–3.
de Siqueira JVV, Almeida LG, Zica BO, Brum IB, Barcelo A, de Siqueira Galil AG. Impact of obesity on hospitalizations and mortality, due to COVID-19: a systematic review. Obes Res Clin Pract. 2020;14(5):398–403.
Hussain A, Mahawar K, Xia Z, Yang W, El-Hasani S. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract. 2020;14(4):295–300.
Rapp J, Lieberman-Cribbin W, Tuminello S, Taioli E. Male sex, severe obesity, older age, and chronic kidney disease are associated with COVID-19 severity and mortality in New York City. Chest. 2020;S0012-3692(20)34288-4.
Tartof SY, Qian L, Hong V, Wei R, Nadjafi RF, Fischer H, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173(10):773–81.
Acknowledgements
The authors acknowledge support of KAIMRC operations and logistics.
Funding
King Abdullah International Medical Research Center (KAIMRC) supported this study through research grant RC13/268/R awarded to SM.
Author information
Authors and Affiliations
Contributions
SM, RA, SAM, SSM, EH, TSK, AK, and AB searched and scrutinized the literature. SM wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed to publish this study.
Competing interests
The authors confirm that this article content has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mohammad, S., Aziz, R., Al Mahri, S. et al. Obesity and COVID-19: what makes obese host so vulnerable?. Immun Ageing 18, 1 (2021). https://doi.org/10.1186/s12979-020-00212-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12979-020-00212-x