Abstract
Many patients with major depressive disorder (MDD) are reported to have higher levels of multiple inflammatory cytokines including interleukin 6 (IL-6). Recent studies both pre-clinical and clinical have advocated for the functional role of IL-6 in development of MDD and suggested a great potential for targeting this cytokine to open new avenues in pharmacotherapy of depression. The purpose of the present narrative review was to provide an integrated account of how IL-6 may contribute to development of depression. All peer-reviewed journal articles published before July 2020 for each area discussed were searched by WOS, PubMed, MEDLINE, Scopus, Google Scholar, for original research, review articles, and book chapters. Publications between 1980 and July 2020 were included. Alterations in IL-6 levels, both within the periphery and the brain, most probably contribute to depression symptomatology in numerous ways. As IL-6 acts on multiple differing target tissues throughout the body, dysregulation of this particular cytokine can precipitate a multitude of events relevant to depression and blocking its effects can prevent further escalation of inflammatory responses, and potentially pave the way for opening new avenues in diagnosis, treatment, and prevention of this debilitating disorder.
Similar content being viewed by others
Background
Major depressive disorder (MDD) is a leading cause of disability throughout the world with a global prevalence of 2.6–5.9% [1]. The total estimated number of people living with depression worldwide increased by 49.86% from 1990 to 2017 [2]. According to worldwide projections, MDD will be the single major cause of burden of all health conditions by 2030 [3]. MDD is characterized by periods of low mood, altered cognition, considerable functional burden including impaired occupational functioning and psychosocial disability [4]. Despite available pharmacotherapeutic options, 30–60% of patients with MDD are not responsive to available treatments [5] and the rate of remission of the disease is often < 50% [6], while recurrence rates are more than 85% within 10 years of a depressive episode, and average about ≥ 50% within 6 months of assumed clinical remission [4]. Indeed, there exists no compelling evidence that current treatments are capable of disease modification in MDD patients. Thus, therapeutic deficiency in treatment outcomes reflects the demand for revitalizing psychiatric therapeutics with novel pharmacotherapeutic options that engage non-monoaminergic molecular targets.
A large body of evidence suggests that inflammation has central role in pathogenesis of MDD [7,8,9,10,11,12,13]. However, the exact mechanisms underlying inflammation-induced depression are not completely elucidated [3]. Historically, the “monoamine-depletion hypothesis” has been the main proposed pathophysiology [14]; nevertheless, this hypothesis alone cannot fully account for pathogenesis of MDD [15, 16]. In recent years, “inflammatory hypothesis” has been proposed [17]. However, it is noteworthy that it was probably in the early 1990s that for the first time, possible relationships between the peripheral immune system and major depression was studied [18]. Maes et al. (1992) established immune cell profile of patients with depression and advocated for the existence of a systemic immune activation during major depressive disorder [19]. Moreover, correlations between IL-6 activity, acute phase proteins, and hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis were suggested in severe depression [20].
Most proximally, inflammation is regulated by expression of immune response genes including interleukin (IL)-1B, tumor necrosis factor (TNF), and IL-6 which promote secretion of pro-inflammatory cytokines leading to systemic inflammation. Distally, inflammation is regulated in the brain where socio-environmental cues including possible threat are detected. This neuro-inflammatory link can activate the conserved transcriptional response to adversity (CTRA) before happening of a possible threat or bacterial infection. However, the negative aspect of central regulation of systemic inflammation is that it can give social and foreseen dangers (including those that have not yet occurred or may never actually happen) the ability to activate the CTRA in the absence of actual physical danger. Under normal conditions, CTRA-related inflammatory activity is downregulated by the HPA axis via the production of cortisol. Nevertheless, when prolonged actual or perceived social threat or physical danger is present, glucocorticoid resistance can develop which leads to excessive inflammation that heightens a person’s risk for development of several disorders including MDD, especially if activation of these pathways is prolonged [21]. As mentioned above, the current understanding of MDD encloses not only alterations in neurotransmitters, but also changes in immune and endocrine functioning as well as neural circuits [22]. This broadened framework has just started to inform a wide array of novel, personalized therapeutics that are showcasing great promise in a new holistic approach to MDD [23].
Cytokines are implicated in pathogenesis of MDD [24,25,26,27,28,29,30]. Risk factors of developing MDD include familial, developmental, psychological, and medical risk factors as well as molecular factors associated with genetics, epigenetics, gene expression, and also those related to the endocrine and the immune system [31, 32]. All these risk factors have been shown to be related with changes in cytokine production or signaling. In other words, cytokines are involved in almost every predisposing or precipitating risk factor associated with MDD [24]. Indeed, there is accumulating evidence in favor of involvement of pro-inflammatory cytokines in pathophysiology of depression [24, 29, 33,34,35,36]. Various studies reported higher levels of multiple inflammatory markers including IL-6 in patients with MDD [37,38,39,40,41]. Of all pro-inflammatory cytokines, changes in IL-6 serum levels have been reported as one of the most reproducible abnormalities in MDD [38].
The aim of the present narrative review is to elucidate the fundamentals, implications, challenges of cytokine research specifically IL-6 in major depressive disorder. This comprises of the following:
-) A Brief overview of cytokines
-) Cytokine categories according to immunological function.
-) IL-6 as a pleiotropic cytokine.
-) Brief overview of chemokines and their role in Depression.
-) Challenges of cytokine research in psychiatry.
-) IL-6 alterations in depression.
-) Effects of IL-6 on neurotransmitters’ synthesis, signaling, metabolism, and function.
-) Effects of IL-6 levels on brain morphology in depression.
-) Blockade of IL-6 and its receptor in the periphery as a potential therapeutic option in MDD.
-) Possible role of IL-6 together with gut microbiota in pathogenesis of depression.
-) Elevated levels of IL-6 in patients with COVID-19 infection.
Methods
The present article is a narrative review. All peer-reviewed journal articles published before July 2020 for each area discussed were searched by WOS, PubMed, MEDLINE, Scopus, Google Scholar, for original research, review articles, and book chapters. We selected articles on the basis of being comprehensive, innovative, and informative for an in-depth understanding and a critical debate on the topic. Publications between 1973 and 2020 were included.
A brief overview of cytokines
Cytokines are a broad category of released proteins that act as signaling molecules to regulate inflammation and cellular activities [24, 42]. They are produced by different immune cells (e.g., macrophages, lymphocytes, mast cells), parenchymal cells, endothelial and epithelial cells, fibroblasts, adipocytes, and stromal cells within the periphery [24, 43]. Additionally, they are produced by microglia, astrocytes, and neurons in the brain [44]. Cytokines from the periphery (peripherally produced cytokines) can exert influences on inflammatory processes in the brain [45, 46]. Indeed, they can enter blood-brain barrier (BBB) and affect the brain via humoral (accessing the brain through leaky secretions of the BBB such as choroid plexus), neural (through stimulation of primary afferent nerve fibers in the vagus nerve), and cellular (through stimulation of microglia by pre-inflammatory cytokines to produce monocyte chemottractant protein-1 and recruit monocytes to the brain) pathways [47]. Most cytokines function in their immediate microenvironment. Few of them are involved in paracrine signaling which indeed is fundamental to the control of an inflammatory response within a given tissue or organ and the activation of a coordinated immune response that involves multiple cell types [48]. Apart from navigating the immune system to defend the body from pathogens, cytokines have a modifying effect on neurotransmission [49].
It’s also noteworthy that the same cytokines can be produced by multiple cell types. For example, white blood cells, endothelium, fat cells, and other cells can produce TNF-α [50]. Additionally, one single cell can release different cytokines. For instance, T Helper type 2 (TH2) cells can produce IL-3, IL-4, IL-5, IL-6, and IL-13 [24]. Cytokines can have pleiotropic, redundant, synergistic, and antagonistic effects [51]. The phenomenon that a single cytokine can act on several different cell types is called pleiotropy [51]. For instance, IL-10 can activate TH2 cells and B cells, yet inhibit macrophages and T helper type 1 (TH1) cells. Thus, being immunostimulatory as well as being immunosuppressive [52]. Cytokines are redundant in their activity, i.e., similar functions can be exerted by different cytokines. For instance, interferon (IFN)-γ, IL-2, and TNF-α enhance cellular immunity and production of cytotoxic cell contacts [53]. Cytokines can also act synergistically, i.e., they can have combined effects when acting together. For instance, IL-3 and IL-4 amplify each other’s effects to induce growth, differentiation, and activation of mast cells in a synergistic manner [24]. Another phenomenon in cytokines signaling is antagonism. An example of cytokine antagonism is that cytokines of the IL-1 superfamily can antagonize IL-18 effects [54].
Cytokine categories according to immunological function
Four categories of cytokines are usually referred to in psychoimmunological literature. (1) TH1 cytokines (IL-2, IL-12, IFN-γ) which induce cytotoxic cell contacts. (2) TH2 cytokines (IL-4, (IL-5, IL-13) which lead to production of antibodies. (3) Pro-inflammatory cytokines (IL-1, IL-6, IL-8, IL-17, IL-21, IL-22, IFN-α, TNF-α) which further the progress of inflammation. (4) Anti-inflammatory cytokines (IL-10, transforming growth factor-beta (TGF)-β which are influenced by regulatory T cells and impede inflammatory process from escalating [24]. However, these categories are not distinct and it must be considered that cytokines can exert various effects on different cells and therefore, they may have pro- and also anti-inflammatory properties. For instance, IFN-α which has been listed as a pro-inflammatory cytokine can also have anti-inflammatory properties [55].
IL-6 as a pleiotropic cytokine
IL-6 was first identified as a differentiation factor for B cells which stimulates production of antibodies by activated B cells. Apart from regulation of acute inflammation, IL-6 is known to induce differentiation of B cells, and activation and population expansion of T cells [56]. Within the peripheral and central nervous system (CNS), IL-6 can act as a neuronal growth factor inducing neurite development and nerve regeneration [57]. IL-6 receptor (IL-6R) consists of the IL-6-binding chain which has two forms of transmembrane IL-6R and soluble IL-6R (sIL-6R) [58] and a gp130 signal-transducing chain [59]. Following binding to its receptor (IL-6R), IL-6 initiates to exert its multiple functions.
It is quite interesting that IL-6 exerts both pro- and anti-inflammatory properties [60, 61]. Indeed, its signaling is complex and can lead to both inflammatory and anti-inflammatory cascades depending upon the presence of either IL-6 receptor (IL-6R) or the membrane bound gp130 signal transducer and these are expressed at very different frequencies within specific cell type in the body [5]. Trans-signaling of IL-6, in which the soluble form of the IL-6 receptor (sIL-6R) is shed from the membrane bound receptors, is known to be pro-inflammatory [62]. The sIL-6R binds to IL-6 and is transported to any cell type on which gp130 is expressed [63]. While most soluble receptors (e.g., soluble receptor for TNFα) result in antagonistic action by competing for the ligand, the sIL-6R is agonistic and increases the types of cells through which IL-6 can signal. Additionally, IL-6 engages in classical signaling which is anti-inflammatory [63] and occurs through binding of IL-6 to the membrane bound cell surface receptor. Classical signaling of IL-6 solely occurs on some subsets of T cells, neutrophils and monocytes megakaryocytes, and hepatocytes [64]. In both classical and trans-signaling, the IL-6/IL-6R/gp130 complex uses two pathways to activate intracellular signaling namely the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway and the mitogen-activated protein kinase (MAPK) pathway [5].
Indeed, IL-6 has been mostly regarded as having pro-inflammatory properties; however, it has many anti-inflammatory functions which are necessary for resolution of inflammation [65]. For instance, IL-6 inhibits activity of the transcription factor named nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and expression of the chemokine receptor on dendritic cells which is needed for recruiting these cells to lymphoid tissues; thus, involving in resolution of inflammation [66]. Research findings showed that IL-6 has a crucial role in regulation of T helper17 (Th17)/regulatory T (Treg) cells [67]. In the presence of TGF-β, IL-6 is a vital signal for differentiation of naive T cells into Th17 cells which in turn are implicated in induction of autoimmune diseases [68, 69], and result in local tissue injury in chronic inflammatory disorders [70]. On the contrary, IL-6 can strongly inhibit the TGF-β-induced differentiation of Treg cells which in turn results in inhibition of autoimmunity and protects against tissue damage [71]. Functional dichotomy of IL-6 indicates that it may be responsible for maintaining the balance between pro- and anti-inflammatory responses, while having tissue-specific properties at the periphery and in the CNS [72].
Brief overview of chemokines and their role in depression
Chemokines are small chemotactic cytokines that are identified to have significant roles in migration of immune cells, induction of direct chemotaxis, and propagation of inflammatory responses [73]. They are classified into four sub-families based on their structural criteria (i.e., the number and spacing of their two N-terminals, disulfide bonding participating cysteine residues). These four subfamilies include CXC, CC, C, and CX3C [74]. Furthermore, they can be categorized according to their biological activity, namely, the maintenance of homeostasis and the induction of inflammation. There are also chemokines which have dual functionality [75].
These small chemotactic cytokines are known to be secreted in response to inflammatory cytokines; thereafter, selectively recruiting lymphocytes, monocytes, and neutrophil-inducing chemotaxis by activating G-protein-coupled receptors (GPCRs) [76]. A growing body of evidence suggests that chemotactic cytokines are implicated in neurobiological processes relevant to psychiatric disorders such as synaptic transmission and plasticity, neuroglia communication, and neurogenesis [77]. Indeed, disruption of any of the mentioned functions which may take place by activation of the inflammatory response system has consistently been found to be relevant in pathogenesis of depression [73].
There are indeed both pre-clinical and clinical evidence in support of linking alterations in the chemokine network to depressive behavior [73]. In an animal model of depression, namely prenatal stressed rats, levels of CCL2, and CXCL12 chemokines were found to be upregulated in the hippocampus and prefrontal cortex, which was indeed suggestive of excessive microglial activation [78]. Additionally, Trojan et al. (2017) investigated the modulatory properties of chronic administration of anti-depressants on the chemokines. According to their results, chronic administration of anti-depressants has been shown to normalize the prenatal stress-induced behavioral disturbances together with the observed alterations in CXCL12 and their receptor. Indeed, they concluded that alterations of CXCL12 and their receptor and at less extend changes in CX3CL1–CX3CR1 expression will probably be normalized following chronic treatment with anti-depressants [79].
Moreover, several clinical studies found correlations between elevated levels of circulating inflammatory chemokines and depressive symptoms in patients with major depressive disorder [73, 80,81,82,83]. According to the results of a comprehensive meta-analysis, peripheral concentrations of a number of chemokines including CCL2, CCL3, CCL4, CCL11, CXCL4, CXCL7, and CXCL8 can potentially discriminate between individuals with depression and those without [84]. Additionally, Ślusarczyk et al. (2016) provided a comprehensive account of the role of chemokines in processes underlying depressive disorder [85].
Challenges of cytokine research in psychiatry
There are some difficulties faced by researchers in conducting cytokine research in psychiatry. The major problem seems to be heterogeneity of the obtained results. In other words, research outcomes are conflicting and challenging to interpret [24]. Moreover, research in this area is largely based on measurement of cytokine levels in the periphery and it is not completely clear how serum or plasma levels of cytokines reflect the situation in the brain [47]. Compellingly, results of studies that examined both peripheral and CFS levels of IL-6 found no correlations between the mentioned measures; thus, suggesting that peripheral levels of IL-6 may not directly reflect central IL-6 levels [86, 87].
It is also noteworthy that some environmental, social, biological, and medical factors may influence peripheral cytokine changes. For instance, one of the characteristics of obesity is chronic inflammation with the increased circulating levels of cytokines [88, 89]. Indeed, adipose tissue is reported to build up and activate lymphocytes and macrophages that secrete inflammatory factors [89, 90]. Interestingly, obese people show behavioral symptoms such as MDD and cognitive dysfunction at an increased rate in comparison with the general population [91, 92]. Therefore, one may argue that alterations in cytokine levels are somehow unspecific [24]. Additionally, there is a considerable overlap in cytokine values between patients in the acute phase of depression, patients in remission, and patients who are recovered [24]. Although the use of cytokines as potential biomarkers of depression has been discussed frequently in various studies, cytokine changes have been reported in other psychiatric disorders as well. For instance, increased levels of pro-inflammatory cytokines have been reported in generalized anxiety disorder [93,94,95], obsessive-compulsive disorder [96], posttraumatic stress disorder [97, 98], and sleep disorder [99].
Moreover, cytokine levels change during pharmacotherapy of depression. Indeed, it has been suggested that treatment with anti-depressants can potentially lead to alteration in peripheral levels of cytokines. According to the results of a meta-analysis, anti-depressants, overall, cause decrease in peripheral levels of IL-6, IL-10, and TNF-α [100]. However, anti-psychotics which are used in psychotic depression, especially those with the highest risk of weight gain (e.g., clozapine and olanzapine), cause significant increase in the blood levels of pro-inflammatory cytokine [101]. Additionally, mood stabilizers such as lithium and carbamazepine have also been linked with an increase in the peripheral levels of cytokines [102]. In sum, as cytokine signaling often exhibits pleiotropic, redundant, synergistic, and antagonistic effects, it seems to be advisable to consider all cytokines that work together or against each other and therefore, take into account the whole range of cytokines instead of a single one.
On the role of interleukin-6 in depression
IL-6 alterations in depression
A growing body of evidence suggests that IL-6 has a crucial role in pathogenesis of depression [3] and is the most consistently increased cytokine in blood samples of MDD patients [38, 103]. The first promising evidence for the role of IL-6 in occurrence of depression is most probably provided by a longitudinal study in which children with higher circulating levels of IL-6 at age 9 were found to be at a 10% greater risk of developing MDD by age 18, compared to the general population or children with low IL-6 levels. Indeed, the researchers concluded that inflammation and high IL-6 levels possibly predate the occurrence of depression [104]. Another evidence for potential role of IL-6 in depression is that peripheral levels of IL-6 were found to be positively correlated with symptom severity in anti-depressant non-responders [105].
Stress-based preclinical models of depression showed that IL-6 levels are increased following the onset of depression-associated behaviors. Rodents who were exposed to chronic mild stress exhibited anhedonia and elevated circulating levels of pro-inflammatory cytokines including IL-6 [106, 107]. Moreover, in another study on male Wistar rats, serum levels of pro-inflammatory cytokines including IL-6 were reported to be higher in acute and restraint stress compared to non-stressed rats [108]. It is also noteworthy that some studies reported no significant alteration in peripheral levels of IL-6 in chronic mild stress models of depression [109]. Nevertheless, they reported elevated CNS levels of other inflammatory markers which probably reflects a time-dependent shift from peripheral to central cytokine activation or potential transport of the peripheral cytokines into CNS [109]. Another promising evidence was provided by studies on IL-6 knockout mice. Indeed, they were reported to be resistant to the development of depression-like phenotype following long-term light deprivation in the constant darkness, proposing a functional role for IL-6 in stress susceptibility [110]. Moreover, Ślusarczyk et al. (2015) found evidence for the role of prenatal stress as a priming factor that could exhibit effects on microglial cells and consequently lead to depressive-like disturbances in adult rat offspring. According to their results, the release of pro-inflammatory cytokines including IL-6 is enhanced in microglia obtained from prenatally stressed animals compared to control animals [78].
In fact, not every individual exposed to prolonged or acute stress develops a psychiatric disorder [111]. According to previous research, vulnerability to repeated social defeat stress is predicted by differences in IL-6 levels in the innate peripheral immune system [112]. Following induction of social defeat stress, two thirds of mice were reported to show depression-like behavior measured by social avoidance, anhedonia, circadian system disruptions, and metabolic changes [113] together with elevated activation of pro-inflammatory cytokines such as IL-6 [112]. Indeed, higher degrees of elevation in peripheral IL-6 levels of susceptible mice were reported in comparison with resilient mice. Moreover, it was found that this increase occurs within 20 min of the first social defeat. Interestingly, mice that later became susceptible had higher number of leukocytes and those leukocytes produced more levels of IL-6 following stimulation via LPS ex vivo [112]. Additionally, studies with non-social stress-based models found evidence for the functional role of IL-6 in the development of stress susceptibility. In these models, animals were exposed to a controllable or uncontrollable stress (e.g., shock), and their ability to actively escape a subsequent stressor was measured. According to the results, 20% of animals who were exposed to uncontrollable stress were found susceptible and developed learned helplessness and the rest were found to be resilient. Interestingly, susceptible animals showed elevated levels of peripheral IL-6 together with anhedonia in contrast to resilient animals [114].
Clinical studies have also revealed that patients with MDD have increased levels of plasma and serum concentrations of pro-inflammatory cytokines including IL-6 in comparison with healthy controls [24, 100, 115, 116]. It should be noted that three meta-analyses verified increased peripheral IL-6 levels in MDD patients compared to healthy volunteers [38, 116, 117]. Nevertheless, there are also studies reporting no significant differences in IL-6 levels in MDD patients compared to healthy volunteers [118]. However, one may argue that different subtypes of depression and certain depressive symptoms should be taken into account. For instance, Rudolf et al. (2014) compared IL-6 levels among patients with atypical and typical depression and healthy controls. According to their results, IL-6 levels were significantly increased in patients with atypical depression and not in typical MDD patients compared to healthy controls [119]. Additionally, Rush et al. (2016) studied peripheral levels of IL-6 and TGF-β in 55 melancholic depressed patients. They were found to have significantly higher baseline IL-6 levels compared to healthy controls. Moreover, these elevated levels of IL-6 did not normalize following electroconvulsive therapy (ECT) [120]. A recent systematic review conformed Rush et al.’s results. In the mentioned review, authors found that peripheral IL-6 levels are increased in patients with melancholic depression in comparison with controls [121]. Moreover, Maes et al. (1997) examined serum levels of IL-6 and IL-1 receptor antagonist in patients with chronic, treatment resistant depression both before and after subchronic treatment with anti-depressants. According to their results, subchronic treatment with anti-depressants had no significant impact on serum levels of IL-6; nevertheless, it decreased serum soluble IL-6R levels significantly [122].
Effects of IL-6 on neurotransmitters’ synthesis, signaling, metabolism, and function
The effects of cytokines on neurotransmitters have been studied extensively [49, 123]. Cytokines and their signaling pathways (e.g., p38 mitogen activated protein kinase) are reported to exhibit significant impacts on metabolism of multiple neurotransmitters such as serotonin, dopamine, and glutamate; thus, influencing their synthesis, release, and reuptake [49]. Indeed, cytokines can decrease synthesis of serotonin via activating the enzyme indoleamine 2,3 dioxygenase (IDO) which breaks the precursor of serotonin (i.e., tryptophan) to kynurenine (KYN) instead of metabolizing tryptophan to serotonin; thus, leading to serotonin depletion [3]. The process of serotonin depletion has been long associated with major depression [124, 125]. Moreover, cytokines can modulate serotonin signaling via elevating the expression and function of monoamine transporters. These transporters are known to re-uptake serotonin [126, 127].
IL-6 is known to influence neurotransmission by modulating the behavioral output of the brain; however, the exact mechanism is unknown. A previous study showed that IL-6 directly controls the levels of serotonin transporter (SERT) and therefore influences serotonin reuptake. Indeed, the researchers concluded that IL6-induced modulation of serotonergic neurotransmission through the signal transducer and activator of transcription 3 (STAT3) signaling pathway contributes to the role of IL6 in depression [128]. The activity of SERT forms serotonergic transmission which is implicated in depressive behavioral changes and pathophysiology of the disease [129]. By intensifying dopaminergic and serotonergic turnover in hippocampus and frontal cortex, IL-6 influences neurotransmission of catecholamines [130]. It seems that noradrenaline is not affected by IL-6; however, noradrenaline itself can induce expression of IL-6 in glial cells [131]. IL-6 together with other pro-inflammatory cytokines can activate kinurenine pathway which is involved in glutamatergic neurotransmission [132].
Effects of IL-6 levels on brain morphology in depression
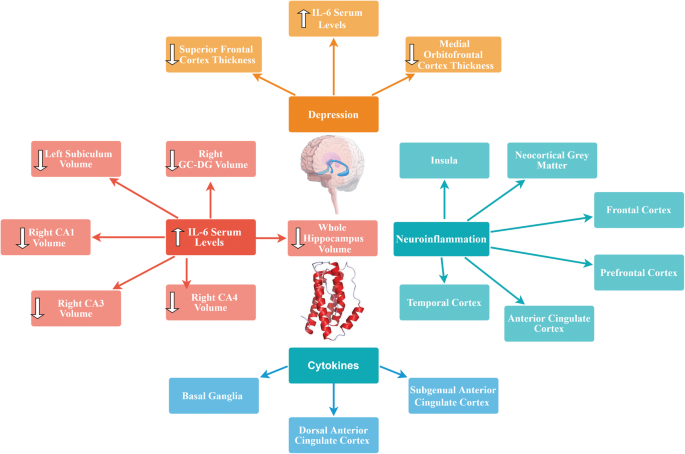
Previous studies showed that elevated levels of pro-inflammatory cytokines such as IL-6 may affect neurogenesis [133] and neural plasticity [134]. Imaging studies have shown that specific brain regions such as basal ganglia (which is involved in motor activity and motivation), the dorsal anterior cingulate cortex (ACC) (which has a central role in generation of anxiety), and the subgenual ACC (which is known to be involved in the development of depression) are influenced by cytokines [135, 136]. Additionally, high IL-6 expression levels demonstrated neuropathologic manifestations including neurodegeneration [137, 138].
There are many studies in which positron emission tomography (PET) has been applied to test translocator protein (TSPO) binding, a marker of neuroinflammation, in order to study neuroinflammatory hypothesis of depression [139,140,141,142,143]. According to their results, neuroinflammation was present in various regions of the brain (e.g., neocortical grey matter, frontal cortex, prefrontal cortex, anterior cingulate cortex, insula, temporal cortex) as well as the hippocampus [139,140,141,142,143].
In a recent study, Kakeda et al. (2018) evaluated possible relationship between serum levels of IL-1β, IL-6, IFN-γ, and TNFα and brain morphology in terms of brain cortical thinning and hippocampal subfield volumes during the first depressive episode in drug-naïve patients with MDD using a whole-brain SBM analysis. They found a significant inverse correlation between prefrontal cortex (PFC) thickness and serum IL-6 level in MDD patients. Indeed, high serum levels of IL-6 were correlated with reduced left subiculum and right CA1, CA3, CA4, GC-DG, subiculum, and whole hippocampus volumes in MDD patients. Additionally, thickness of the superior frontal and medial orbitofrontal cortices in patients with depression was significantly decreased compared to healthy controls. Since PFC contains high concentrations of IL-6 receptors, IL-6 mediated neurotoxicity might happen under conditions in which high serum IL-6 levels are present (i.e., early stages of MDD). Consequently, the authors advocated that the neuroinflammatory status in the early stage of MDD is associated with changes in the brain gray matter and IL-6 probably plays a key role in the morphological changes observed in the PFC during early stages of the disease. It is also noteworthy that serum IL-6 was the only cytokine among the tested cytokines that showed significant differences between the patients and controls in their study. Indeed, serum IL-6 levels were found to be significantly higher in MDD patients than in healthy controls [144]. In another study, Frodl et al. (2012) investigated possible effects of changes in the glucocorticoid and inflammatory systems on hippocampal volumes in patients with MDD. According to their results, MDD patients showed increased IL-6 levels and smaller hippocampal volumes compared to healthy controls. Positive effects of messenger RNA (mRNA) expression of glucocorticoid-inducible genes and further inverse effects of IL-6 concentration, on hippocampal volumes were also reported. Thus, they concluded that increased expression of IL-6 can probably predict decreased hippocampal volume [145].
As already mentioned, there is considerable amount of evidence regarding the central role of the highly plastic, stress-sensitive hippocampal region in pathogenesis of depression [146]. Indeed, grey-matter structures, including the hippocampus are vulnerable to atrophy in depression [147, 148]. Hippocampal volume reductions are most probably the result of remodeling of key cellular elements, involving retraction of dendrites, loss of glial cells, and decreased neurogenesis in the dentate gyros [149]. Factors underlying this cellular remodeling are known to be stress-induced increased levels of glucocorticoids, which are implicated in decreased neurogenesis [150]. Moreover, increased activity of the HPA axis resulting in decreased levels of glucocorticoids combined with resistance to glucocorticoid-induced negative feedback control is commonly observed in depression [151]. This dysregulation of glucocorticoid secretion along with the increased activity of excitatory neurotransmitters can potentially lead to cellular remodeling (which can be reversible) and hippocampal neurons cell death in patients with depression [152]. Since hippocampus has been identified to have a role in negative feedback inhibition of glucocorticoids, remodeling or neuronal damage may lead to less efficient inhibitory control of the corticotrophin-releasing hormone, resulting in elevated amounts of circulating glucocorticoids and further damage of the hippocampal neurons [153]. Taken together, it seems that further studies are required to elucidate the physiological mechanisms in which IL-6 might exert changes in the brain grey matter. A brief overview of the effects of cytokines including IL-6 on brain morphology is shown in Fig. 1.
Blockade of IL-6 and its receptor in the periphery as a potential therapeutic option in MDD
Growing body of evidence suggests that abnormalities in the immune system are most probably relevant to pathogenesis and potential novel treatment of psychiatric disorders. Previous studies showed that alterations in the peripheral IL-6 levels might contribute to depressive-like behavior in animal studies [3, 112, 114, 154]. Moreover, IL-6 knockout mice showed resistance to development of depressive-like behavior [155] which gives further evidence for possible role of IL-6 pathogenesis of depression. High peripheral levels of IL-6 are even more apparent in patients with treatment-resistant depression. Additionally, correlations have been found between decrease in IL-6 levels and alleviation of depressive symptoms in patients who were responsive to the pharmacotherapy [156]. Moreover, results of a study on 222 stroke patients indicated significant associations between IL-6 periphery levels and development of MDD within 2 weeks and at 1 year following stroke. Furthermore, significant correlations were found between statin use and IL-6 on the presence of a depressive disorder at the 1st year. Indeed, preventive effects of treatment with statins (which are known to possess anti-inflammatory properties and potently reduce the cytokine-mediated IL-6 release [157]) against post-stroke depression was confirmed [158]. Thus, suppression of IL-6 activity could possibly lead to clinical recovery and may be considered as a novel pharmacotherapeutic option. Utilizing IL-6 receptor antibodies (for instance, Tocilizumab) or IL-6 antibodies (for instance, Sirukumab or Siltuximab) for reduction of IL-6 activity seems to be a novel strategy.
Blockade of IL-6 receptor by the humanized anti-IL-6 antibody, Tocilizumab has been used in treatment of rheumatoid arthritis (RA) [159,160,161,162,163] and systemic juvenile idiopathic arthritis [164,165,166]. Extensive clinical studies have established both short-term and long-term efficacy and safety of Tocilizumab [±conventional disease-modifying anti-rheumatic drugs (DMARDs)] in adults with moderate to severe RA. Additionally, Tocilizumab was shown to be effective as a monotherapy in patients with systemic juvenile idiopathic arthritis and also in patients whose disease has been refractory to other therapies [164]. Moreover, the safety profile of tocilizumab was reported to be consistent over time and also consistent with safety profile of other immunomodulatory agents [162]. It is also important to note that oral tocilizumab has been shown to inhibit experimental autoimmune encephalitis by elevating Th2 anti-inflammatory cytokines and decreasing pro-inflammatory Th1 cytokines [167]. With regard to crucial role of IL-6 in regulating metabolic homeostasis, side effects such as significant weight gain followed by hypertrygliceridemia and hypercholesterolemia may be observed in patients treated with tocilizumab [168]. Blockade of IL-6 trans-signaling, while classical IL-6R signaling stays intact seems to be crucial for the goal of maintaining gut mucosal integrity and epithelial regeneration [65]. Indeed, few randomized clinical trials were conducted on anti-depressant properties of tocilizumab. According to the results of a recent meta-analysis of anti-depressant activity of anti-cytokine therapies, treatment with tocilizumab showed statistically significant improvements in depressive symptoms [169].
Another promising human monoclonal antibody against Il-6, namely Sirukumab has been reported to be a safe and well-tolerated agent, capable of modulating the immune response in healthy populations as well as in patients with inflammatory disorders (e.g., rheumatoid arthritis). It targets the IL-6 signaling pathway by inhibition of both the pro- and anti-inflammatory effects of IL-6 [170]. Effects of Sirukumab on cytokine networks provide a well-founded rationale for its potential use in pharmacotherapy of psychiatric disorders promising possible advantages across varying domains of the biobehavioral research criteria [171]. In a phase 2, double-blind, placebo-controlled trial, the efficacy of Sirukumab and Siltuximab on depressive symptoms was studied in patients with rheumatoid arthritis or multicentric Castleman’s disease respectively. Compared with placebo, both IL-6 neutralizing antibodies were found to make significantly greater improvements on depressive symptoms in the patients [172]. Results of a recent mega-analysis of 18 randomized, placebo-controlled clinical trials of efficacy of immunomodulatory drugs on depressive symptoms in patients with inflammatory disorders demonstrated promising results (N = 10,743 participants). According to their findings, anti-IL-6 antibodies (sirukumab and siltuximab) had large and statistically significant effect sizes on core depressive symptoms before correction for physical health outcomes. Additionally, their effects remained significant in non-responders for the primary disease states evaluated [173]. Although further research is needed in this area, potential application of anti-IL-6 antibodies could possibly open new avenues in pharmacotherapy of MDD.
Possible role of IL-6 together with gut microbiota in pathogenesis of depression
The human intestine harbors nearly 100 trillion bacteria [174] consisting assemblages of microorganisms associated with various niches in and on the body with long-term implications to health [175]. Evidence is emerging regarding correlations of microbial activities with progressive structural and functional processes in the brain of both animal models and humans [175]. There is a large body of evidence for the role of gut microbiota composition in pathogenesis of depression [176,177,178,179,180]. Moreover, there is growing body of literature for the influence of the gut microbiome on cytokine signaling [181, 182].
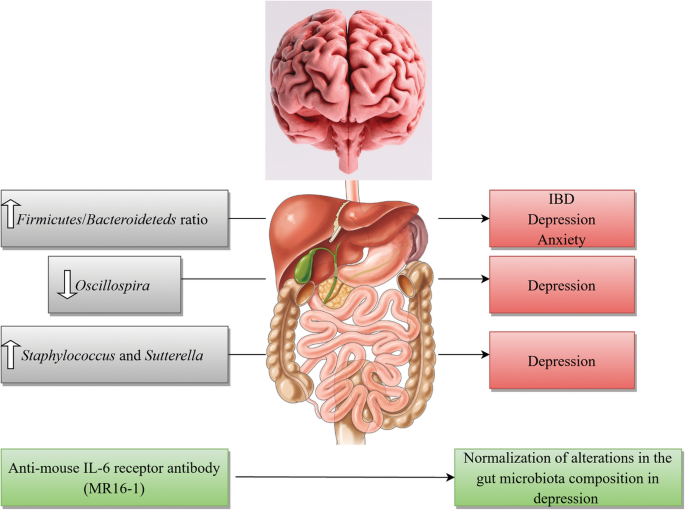
The dominant gut microbial phyla are known to be Firmicutes and Bacteroideteds [183, 184]. The Firmicutes/Bacteroideteds ratio is of great relevance in signaling human gut microbiota status [185]. For instance, increased levels of Firmicutes/Bacteroideteds ratio have been reported in patients suffering from irritable bowel syndrome (IBD) and seem to have some correlations with development of depression and anxiety [186, 187]. Additionally, Firmicutes/Bacteroideteds ratio is associated with overall alterations in bacterial profiles at different life stages [185]. In a novel study, researchers reported decreased Firmicutes/Bacteroideteds ratio in mice following social defeat stress; thus, proposing possible role of Firmicutes/Bacteroideteds in depressive-like behavior. Furthermore, administration of anti-mouse IL-6 receptor antibody (MR16-1) attenuated the decreased ratio of Firmicutes/Bacteroideteds in susceptible mice. Thus, the researchers concluded that anti-mouse IL-6 receptor antibody may have anti-depressant-like effects by normalizing the Firmicutes/Bacteroideteds ratio via modulation of the immune system [3].
Decreased number of Oscillospira was detected in patients with depression [180] which suggests for possible role of Oscillospira in pathogenesis of depression. Two animal studies yielded same results. A recent study investigated therapeutic effects of finasteride on depressive-like behavior in rats together with 1 month of treatment withdrawal. Withdrawal from finasteride was associated with increased depressive-like behavioral responses. Therapeutic use of finasteride was linked with elevations in the phylum Bacteroidetes and in the family Prevotellaceae, and withdrawal was found to be correlated with decreases in the family Ruminococcaceae and the genera Oscillospira and Lachnospira [188]. In another study, socially stressed mice developing depression-like symptoms showed increases at the genus level of fecal Oscillospira. Interestingly, IV administration of anti-mouse IL-6 receptor antibody (MR16-1) normalized depression-like behavior and resulted in significant decrease in Oscillospira levels towards pre-stressor levels [3]. Moreover, increased number of Sutterella was reported in fecal samples [189] and intestinal biopsy samples of children with Autism spectrum disorder [190]. Additionally, elevated number of Staphylococcus and Sutterella were found in mice following social defeat stress. It is likely that Staphylococcus and Sutterella play a role in the depressive-like behavior via infection-induced inflammation. Interestingly, administration of anti-mouse IL-6 receptor antibody resulted in attenuation of elevated number of Staphylococcus and Sutterella following social defeat stress in mice [3].
These findings advocate that peripheral IL-6 may have a significant role in pathogenesis of MDD and blockade of IL-6 receptor in the periphery may exhibit rapid-onset effects by attenuating the altered composition of gut microbiota. Taking into account the role of gut-microbiota in immunomodulation, it is highly probable that gut-microbiota-brain- axis plays a role in anti-depressant actions of treatment with anti-IL-6 receptor [3]. A brief overview of the role of IL-6 together with gut microbiota in pathogenesis of depression is shown in Fig. 2.
Elevated levels of IL-6 in patients with COVID-19 infection
The world-wide effect of the coronavirus disease 2019 (COVID-19) pandemic is enormous and is not solely limited to the increased mortality and morbidity rates, but also extends into the mental health of the global population [191]. Considerable amount of clinical data is emerging regarding the manifestation of depression in patients during [192,193,194] and post-COVID 19 infection [192,193,194,195]. It is estimated that about 48% of confirmed COVID-19 cases displayed overt psychological symptoms such as depression and often expressed feelings of regret, loneliness, helplessness, and irritation [196].
There is growing body of literature regarding dual role of IL-6 in COVID-19 infection and depression [192]. Normal plasma levels of IL-6 in adults range between 1 and 10 pg/ml; whereas in a systemic inflammation this amount increases to several ng/ml [197] and even higher concentrations were reported in COVID-19 patients [198]. Indeed, cytokine release syndrome (CRS) is common in COVID-19 patients and increased levels of serum IL-6 have been identified to be significantly associated with acute respiratory distress syndrome (ARDS), respiratory failure, and poor disease outcome in numerous studies [192, 199,200,201]. Studies suggest that new onset depression is most probably caused by inflammation initiated during the active phase of the infection leading to a cytokine surge [202]. Indeed, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infects primarily human monocytes and dendritic cells causing dendritic cell dysfunction, leading to T cell apoptosis and exhaustion; thus, contributing to the immunopathology [203]. Alpert et al. (2020) described two cases of COVID-19 patients with elevated amounts of IL-6 (25 pg/mL and 26.7 pg/mL respectively) who were diagnosed with major depressive disorder (according to the Diagnostic and Statistical Manual of Mental Disorder, 5th Edition (DSM-5)) during COVID 19 infection. Both patients’ depressive symptoms subsided about 6 weeks after initiation of anti-depressant pharmacotherapy and normalization of the inflammatory cytokines [192]. The authors concluded that lower cytokine activity ameliorates depressive symptoms as normalization of IL-6 plasma levels decreased depression with or without anti-depressants [192]. Moreover, Benedett et al. (2020) studied effects of treatment with cytokine-blocking agents on the psychopathological status of the patients with COVID-19 infection. Their results were in favor of the protective effects of treatment with cytokine-blocking agents in early phases of COVID-19 against the later onset of depression [204].
It is indeed crucial to maintain a multidisciplinary approach in management of the psychological effects of this debilitating pandemic. Treatment strategies addressing the immunopathology of SARS-CoV-2 infection will be promising during the acute phase of the disease [192, 205]. Currently, there are few studies considering psychological and neuropsychiatric implications of COVID-19; however, it is very likely to expect an increased incidence of mental pathologies both during and post-COVID-19 infection.
Conclusion
Preclinical and clinical studies present strong evidence that inflammation is altered in a subset of patients with MDD and there is mounting body of literature for the role of pro-inflammatory cytokines namely IL-6 in pathophysiology of depression. Nevertheless, there still exists gap in our understanding of the mechanisms by which IL-6 signaling and its molecular components could possibly contribute to depression manifestation. A number of humanized monoclonal antibodies are undergoing clinical trials for potential pharmacotherapy of mood disorders. Biologics including IL-6 receptor antibodies or IL-6 antibodies are currently approved to treat inflammatory disorders such as RA and are undergoing clinical trials as a novel target for MDD treatment. However, these novel therapeutic targets may also raise the possibility of potential side effects. By investigating the interface of peripheral cytokines, namely IL-6 and brain cellular processes contributing to depression, one might be able to develop novel therapeutic options for treatment of mood disorders by sequestering and preventing this peripherally derived inflammatory marker from acting upon mood circuits in the CNS. In sum, therapeutic deficiency in treatment outcomes reflects the growing demand for revitalizing psychiatric therapeutics with novel options that could potentially open new avenues in treatment of this debilitating disorder and enhancement of patients’ quality of life.
Availability of data and materials
Not applicable.
Abbreviations
- ACC:
-
Anterior cingulate cortex
- ARDS:
-
Acute respiratory distress syndrome
- BBB:
-
Blood-brain barrier
- CNS:
-
Central nervous system
- COVID-19:
-
Coronavirus disease 2019
- CRS:
-
Cytokine release syndrome
- CTRA:
-
Conserved transcriptional response to adversity
- DMARDs:
-
Disease-modifying anti-rheumatic drugs
- DSM-5:
-
Diagnostic and Statistical Manual of Mental Disorder, 5th Edition
- ECT:
-
Electroconvulsive therapy
- Gp130:
-
Glycoprotein 130
- GPCRs:
-
G-protein-coupled receptors
- HPA:
-
Hypothalamic-pituitary-adrenal
- IDO:
-
Indoleamine 2,3 dioxygenase
- IFN:
-
Interferon
- IL:
-
Interleukin
- IL-6R:
-
IL-6 receptor
- JAK/STAT:
-
Janus kinase/signal transducer and activator of transcription
- KYN:
-
Kynurenine
- MDD:
-
Major depressive disorder
- MAPK:
-
Mitogen-activated protein kinase
- mRNA:
-
Messenger RNA
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PFC:
-
Prefrontal cortex
- RA:
-
Rheumatoid arthritis
- SARS-CoV-2:
-
severe acute respiratory syndrome coronavirus 2
- SERT:
-
Serotonin transporter
- STAT3:
-
Signal transducer and activator of transcription 3
- sIL-6R:
-
Soluble IL-6R
- TGF:
-
Transforming growth factor
- TH2:
-
T helper type 2
- TH1:
-
T helper type 1
- Th:
-
T helper cell
- TNF:
-
Tumor necrosis factor
- TSPO:
-
Translocator protein
- Treg:
-
Regulatory T cells
References
Depression and Other Common Mental Disorders: Global Health Estimates. 2017. Geneva: World Health Organization, Licence: CC BY-NC-SA 3.0 IGO.
Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J Psychiatr Res. 2020;126:134–40.
Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, Hashimoto K. Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis. Transl Psychiatry. 2017;7:e1138.
Sim K, Lau WK, Sim J, Sum MY, Baldessarini RJ. Prevention of Relapse and Recurrence in Adults with Major Depressive Disorder: Systematic Review and Meta-Analyses of Controlled Trials. Int J Neuropsychopharmacol. 2016;19:pyv076.
Hodes GE, Ménard C, Russo SJ. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiol Stress. 2016;4:15–22.
Fonseka TM, McIntyre RS, Soczynska JK, Kennedy SH. Novel investigational drugs targeting IL-6 signaling for the treatment of depression. Expert Opin Investig Drugs. 2015;24:459–75.
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34.
Majd M, Hashemian F, Hosseini SM, Vahdat Shariatpanahi M. Sharifi A. A randomized, double-blind, placebo-controlled trial of celecoxib augmentation of sertraline in treatment of drug-naive depressed women: a pilot study. Iran J Pharm Res. 2015;14:891–9.
Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. 2016;17:497–511.
Zhao G, Liu X. Neuroimmune advance in depressive disorder. Adv Exp Med Biol. 2019;1180:85–98.
Lee CH, Giuliani F. The role of inflammation in depression and fatigue. Front Immunol. 2019;10:1696.
Liu CH, Zhang GZ, Li B, Li M, Woelfer M, Walter M, Wang L. Role of inflammation in depression relapse. J Neuroinflammation. 2019;16:90.
Cassano T, Calcagnini S, Carbone A, Bukke VN, Orkisz S, Villani R, Romano A, Avolio C, Gaetani S. Pharmacological treatment of depression in Alzheimer’s disease: a challenging task. Front Pharmacol. 2019;10:1067.
Hirschfield RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry. 2000;61:4–6.
Massart R, Mongeau R, Lanfumey L. Beyond the monoaminergic hypothesis: neuroplasticity and epigenetic changes in a transgenic mouse model of depression. PhilosTrans R Soc Lond Ser B Biol Sci. 2012;367:2485–94.
Boku S, Nakagawa S, Toda H, Hishimoto A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72:3–12.
Kopschina Feltes P, Doorduin J, Klein HC, Juárez-Orozco LE, Dierckx RA. Moriguchi- Jeckel CM, de Vries EF. Anti-inflammatory treatment for major depressive disorder: implications for patients with an elevated immune profile and non-responders to standard antidepressant therapy. J Psychopharmacol. 2017;31:1149–65.
Maes M, Van der Planken M, Stevens WJ, Peeters D, DeClerck LS, Bridts CH, Schotte C, Cosyns P. Leukocytosis, monocytosis and neutrophilia: hallmarks of severe depression. J Psychiatr Res. 1992;26:125–134.
Maes M, Lambrechts J, Bosmans E, Jacobs J, Suy E, Vandervorst C, de Jonckheere C, Minner B, Raus J. Evidence for a systemic immune activation during depression: results of leukocyte enumeration by flow cytometry in conjunction with monoclonal antibody staining. Psychol Med. 1992;22:45–53.
Maes M, Scharpé S, Meltzer HY, Bosmans E, Suy E, Calabrese J, Cosyns P. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic- pituitary-adrenal axis in severe depression. Psychiatry Res. 1993;49:11–27.
Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815.
Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–83.
Henter ID, de Sousa RT, Gold PW, Brunon AR, Zarate CA, Machado-Vieira R. Mood therapeutics: novel pharmacological approaches for treating depression. Expert Rev Clin Pharmacol. 2017;10:153-166.
Himmerich H, Patsalos O, Lichtblau N, Ibrahim MAA, Dalton B. Cytokine research in depression: principles, challenges, and open questions. Front Psychiatry. 2019;7(10):30.
Zou W, Feng R, Yang Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS One. 2018;13:e0197267.
Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatr. 2009;65:732–41.
Horowitz MA, Zunszain PA. Neuroimmune and neuroendocrine abnormalities in depression: two sides of the same coin. Ann N Y Acad Sci. 2015;1351:68–79.
Raison CL. Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13:467–75.
Young JJ, Bruno D. Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J Affect Disord. 2014;169:15–20.
Kim YK, Na KS, Shin KH, Jung HY, Choi SH, Kim JB. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1044–53.
Himmerich H, Kohls E, Hegerl U, Rummel-Kluge C. Prädiktive Faktoren der Depression und ihrer Therapie. Der Nervenarzt. 2014;85:1249–54.
Muñoz RF, Cuijpers P, Smit F, Barrera AZ, Leykin Y. Prevention of major depression. Ann Rev Clin Psychol. 2010;6:181–212.
Brebner K, Hayley S, Zacharko R, Merali Z, Anisman H. Synergistic effects of interleukin-1β, interleukin-6, and tumor necrosis factor-α: Central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacology. 2000;22:566–80.
Farooq RK, Asghar K, Kanwal S, Zulqernain A. Role of inflammatory cytokines in depression: focus on interleukin-1β. Biomed Rep. 2017;6:15–20.
Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229.
Black C, Miller BJ. Meta-analysis of cytokines and chemokines in suicidality: distinguishing suicidal versus nonsuicidal patients. Biol Psychiatry. 2015;78:28–37.
Goldsmith DR, Rapaport MH. Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–709.
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK. Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57.
Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur Neuropsychopharmacol. 2015;25:1532–43.
Fan N, Luo Y, Ou Y, He H. Altered serum levels of TNF-α, IL-6, and IL-18 in depressive disorder patient. Hum Psychopharmacol. 2017;32(4) https://doi.org/10.1002/hup.2588.
Nishuty NL, Khandoker MH, Karmoker JR, Ferdous S, Shahriar M, Qusar S, Islam S. Kadir MF, Islam R. Evaluation of serum interleukin-6 and C-reactive protein levels in drug-naïve major depressive disorder patient. cureus. 2019;11:e3868.
Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration. and neuropathic pain. Mediators Inflamm. 2013;2013:480739.
Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;7(5):491.
Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Front Neuroendocrinol. 2012;33:116–25.
Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–56.
Hopkins SJ. Central nervous system recognition of peripheral inflammation: a neural. hormonal collaboration. Acta Biomed. 2007;78:231–47.
Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–38.
Caldwell AB, Cheng Z, Vargas JD, Birnbaum HA, Hoffmann A. Network dynamics determine the autocrine and paracrine signaling functions of TNF. Genes Dev. 2014;28:2120–33.
Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306.
Perskidskii IV, Barshtein IA. Biological manifestations of the tumor necrosis factor effect and its role in the pathogenesis of various diseases. Arkh Patol. 1992;54:5–10.
Zhang JM, An J. Cytokines, inflammation. and pain. Int Anesthesiol Clin. 2007;45:27–37.
Kicielinska J, Pajtasz-Piasecka E. The role of IL-10 in the modulation of the immune response in normal conditions and the tumor environment. Postepy Higieny Med Doświadczal. 2014;68:879–92.
Munk ME, Emoto M. Functions of T-cell subsets and cytokines in mycobacterial infections. Eur Respir J Suppl. 1995;20:668s–−675s.
Krumm B, Xiang Y, Deng J. Structural biology of the IL-1 superfamily: key cytokines in the regulation of immune and inflammatory responses. Protein Sci. 2014;23:526–38.
Tilg H, Peschel C. Interferon-alpha and its effects on the cytokine cascade: a pro- and anti-inflammatory cytokine. Leukem Lymphoma. 1996;23:55–60.
Klimpel GR. Soluble factor(s) from LPS-activated macrophages induce cytotoxic T cell differentiation from alloantigen-primed spleen cells. J Immunol. 1980;125:1243–9.
Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, Haegeman G, Gerlo S. Interleukin-6. a mental cytokine. Brain Res Rev. 2011;67:157–83.
Yamasaki K, Taga T, Hirata Y, Yawata H, Kawanishi Y, Seed B, Taniguchi T, Hirano T, Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988;241:825–8.
Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149–57.
Fuster JJ, Walsh K. The good, the bad, and the ugly of interleukin-6 signaling. EMBO J. 2014;33:1425–7.
Schett G. Physiological effects of modulating the interleukin-6 axis. Rheumatology. 2018;57:ii43–ii50.
Mullberg J, Schooltink H, Stoyan T, Gunther M, Graeve L, Buse G, Mackiewicz A. Heinrich PC, Rose-John S. The soluble interleukin-6 receptor is generated by shedding. Eur J Immunol. 1993;23:473–80.
Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20.
Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–88.
Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–57.
Hegde S, Pahne J, Smola-Hess S. Novel immunosuppressive properties of interleukin- 6 in dendritic cells: inhibition of NF-kB binding activity and CCR7 expression. FASEB J. 2004;18:1439–41.
Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–5.
Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4.
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8.
Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–62.
Dominitzki S, Fantini MC, Neufert C, Nikolaev A, Galle PR, Scheller J, Monteleone G, Rose-John S, Neurath MF, Becker C. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naïve CD4+CD25 T cells. J Immunol. 2007;179:2041–5.
Milovan BM, Ivan J, Gordana R, Jelena P, Slavica MJ, Nebojsa A, Lukic ML. Interleukin-6 in schizophrenia—is there a therapeutic relevance? Front Psychiatry. 2017;8:221.
Milenkovic VM, Stanton EH, Nothdurfter C, Rupprecht R, Wetzel CH. The role of chemokines in the pathophysiology of major depressive disorder. Int J Mol Sci. 2019;20:2283.
Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–16.
Le Thuc O, Blondeau N, Nahon JL, Rovère C. The complex contribution of chemokines to neuroinflammation: switching from beneficial to detrimental effects. Ann N Y Acad Sci. 2015;1351:127–40.
Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–26.
Stuart MJ, Baune BT. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neurosci Biobehav Rev. 2014;42:93–115.
Ślusarczyk J, Trojan E, Głombik K, Budziszewska B, Kubera M, Lasoń W, Popiołek- Barczyk K, Mika J, Wędzony K, Basta-Kaim A. Prenatal stress is a vulnerability factor for altered morphology and biological activity of microglia cells. Front Cell Neurosci. 2015;9:82.
Trojan E, Ślusarczyk J, Chamera K, Kotarska K, Głombik K, Kubera M, Basta-Kaim A. The modulatory properties of chronic antidepressant drugs treatment on the brain chemokine – chemokine receptor network: a molecular study in an animal model of depression. Front Pharmacol. 2017;8:779.
Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH, Nierenberg AA, Fava M. Wong KK. A detailed examination of cytokine abnormalities in major depressive disorder. Eur Neuropsychopharmacol. 2008;18:230–3.
Piletz JE, Halaris A, Iqbal O, Hoppensteadt D, Fareed J, Zhu H, Sinacore J, Devane CL. Pro- inflammatory biomakers in depression: treatment with venlafaxine. World J Biol Psychiatry. 2009;10:313–23.
De la Peña FR, Cruz-Fuentes C, Palacios L, Girón-Pérez MI, Medina-Rivero E. Ponce- Regalado MD, Alvarez-Herrera S, Pérez-Sánchez G, Becerril-Villanueva E, Maldonado-García JL, Jiménez-Martínez MC, Pavón L. Serum levels of chemokines in adolescents with major depression treated with fluoxetine. World J Psychiatry. 2020;10:175–86.
Romero-Sanchiz P, Nogueira-Arjona R, Araos P, Serrano A, Barrios V, Argente J, Garcia- Marchena N, Lopez-Tellez A, Rodriguez-Moreno S, Mayoral F, et al. Variation in chemokines plasma concentrations in primary care depressed patients associated with Internet-based cognitive-behavioral therapy. Sci Rep. 2020;10:1078.
Leighton SP, Nerurkar L, Krishnadas R, Johnman C, Graham GJ, Cavanagh J. Chemokines in depression in health and in inflammatory illness: a systematic review and meta- analysis. Mol Psychiatry. 2018;23:48–58.
Ślusarczyk J, Trojan E, Chwastek J, Głombik K, Basta-Kaim AA. Potential Contribution of Chemokine Network Dysfunction to the Depressive Disorders. Curr Neuropharmacol. 2016;14:705–20.
Sasayama D, Hattori K, Wakabayashi C, Teraishi T, Hori H, Ota M, Yoshida S, Arima K, Higuchi T, Amano N, et al. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J Psychiatr Res. 2013;47:401–6.
Boufidou F, Lambrinoudaki I, Argeitis J, Zervas IM, Pliatsika P, Leonardou AA. Petropoulos G, Hasiakos D, Papadias K, Nikolaou C. CSF and plasma cytokines at delivery and postpartum mood disturbances. J Affect Disord. 2009;115:287–92.
Schmidt FM, Weschenfelder J, Sander C, Minkwitz J, Thormann J, Chittka T, Mergl R, Kirkby KC, Faßhauer M, Stumvoll M, et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE. 2015;10:e0121971.
Bastard JP, Jardel C, Delattre J, Hainque B, Bruckert E, Oberlin F. Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation. 1999;99:2221–2.
Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003112:1785–8.
Sweat V, Starr V, Bruehl H, Arentoft A, Tirsi A, Javier E. Convit A. C-reactive protein is linked to lower cognitive performance in overweight and obese women. Inflammation. 2008;31:198–207.
Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ, Maisano C, Jones L. Murrah NV, Vaccarino V. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol Psychiatry. 2008;64:896–900.
Hou R, Garner M, Holmes C, Osmond C, Teeling J, Lau L, Baldwin DS. Peripheral inflammatory cytokines and immune balance in Generalised Anxiety Disorder: Case- controlled study. Brain Behav Immun. 2017;62:212–8.
Costello H, Gould RL, Abrol E, Howard R. Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalised anxiety disorder. BMJ Open. 2019;9:e027925.
Vieira MMM, Ferreira TB, Pacheco PAF, Barros PO, Almeida CRM, Araújo-Lima CF, Silva-Filho RG, Hygino J, Andrade RM, Linhares UC, et al. Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J Neuroimmunol. 2010;229:212–8.
Gray SM, Bloch MH. Systematic review of proinflammatory cytokines in obsessive- compulsive disorder. Curr Psychiatr Rep. 2012;14:220–8.
Hori H, Kim Y. Inflammation and post-traumatic stress disorder. PCN Psychiatry and Clinical Neurosciences. 2019;73:143–53.
Renna ME, O’Toole MS, Spaeth PE, Lekander M, Mennin DS. The association between anxiety, traumatic stress, and obsessive-compulsive disorders and chronic inflammation: a systematic review and meta-analysis. Depress Anxiety. 2018;35:1081–94.
Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–72.
Köhler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, de Andrade NQ, Morris G, Fernandes BS, Brunoni AR, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol. 2018;55:4195–206.
Himmerich H, Minkwitz J, Kirkby K. Weight gain and metabolic changes during treatment with antipsychotics and antidepressants. Endocr Metabol Immune Disorder Drug Targets. 2015;15:252–60.
Himmerich H, Koethe D, Schuld A, Yassouridis A, Pollmacher T. Plasma levels of leptin and endogenous immune modulators during treatment with carbamazepine or lithium. Psychopharmacology. 2005;179:447–51.
Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta- analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–15.
Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71:1121–8.
Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–9.
Mutlu O, Gumuslu E, Ulak G, Celikyurt IK, Kokturk S, Kir HM, Akar F, Erden F. Effects of fluoxetine, tianeptine and olanzapine on unpredictable chronic mild stress-induced depression-like behavior in mice. Life Sci. 2012;91:1252–62.
Pan Y, Zhang WY, Xia X, Kong LD. Effects of icariin on hypothalamic-pituitary- adrenal axis action and cytokine levels in stressed Sprague-Dawley rats. Biol Pharm Bull. 2006;29:2399–403.
Himmerich H, Fischer J, Bauer K, Kirkby KC, Sack U, Krugel U. Stress-induced cytokine changes in rats. Eur Cytokine Network. 2013;24:97–103.
Farooq RK, Isingrini E, Tanti A, Le Guisquet AM, Arlicot N, Minier F, Leman S, Chalon S, Belzung C. Camus V. Is unpredictable chronic mild stress (UCMS) a reliable model to study depression-induced neuroinflammation? Behav Brain Res. 2012;231:130–7.
Monje FJ, Cabatic M, Divisch I, Kim EJ, Herkner KR, Binder BR, Pollak DD. Constant darkness induces IL-6-dependent depression-like behavior through the NF-kappaB signaling pathway. J Neurosci. 2011;31:9075–83.
Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–84.
Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014;111:16136–41.
Krishnan V. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404.
Yang C, Shirayama Y, Zhang JC, Ren Q, Hashimoto K. Peripheral interleukin-6 promotes resilience versus susceptibility to inescapable electric stress. Acta Neuropsychiatr. 2015;27:312–6.
Wang M, Wei J, Yang X, Ni P, Wang Y, Zhao L, Deng W, Guo W, Wang Q, Li T, et al. The level of IL-6 was associated with sleep disturbances in patients with major depressive disorder. Neuropsychiatric Disease and Treatment. 2019;15:1695–700.
Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86.
Liu Y, Ho RC, Mak A. Interleukin (il)-6, tumour necrosis factor alpha (tnf-alpha) and soluble interleukin-2 receptors (sil-2r) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J Affect Disord. 2012;139:230–9.
Chocano-Bedoya PO, Mirzaei F, O’Reilly EJ, Lucas M, Okereke OI, Hu FB, Rimm EB, Ascherio A. C-reactive protein, interleukin-6, soluble tumor necrosis factor alpha receptor 2 and incident clinical depression. J Affect Disord. 2014;163:25–32.
Rudolf S, Greggersen W, Kahl KG, Huppe M, Schweiger U. Elevated il-6 levels in patients with atypical depression but not in patients with typical depression. Psychiatry Res. 2014;217:34–8.
Rush G, O’Donovan A, Nagle L, Conway C, McCrohan A, O’Farrelly C, Lucey JV, Malone KM. Alteration of immune markers in a group of melancholic depressed patients and their response to electroconvulsive therapy. J Affect Disord. 2016;205:60–8.
Yang C, Tiemessen KM, Bosker FJ, Wardenaar KJ, Lie J, Schoevers RA. Interleukin, tumor necrosis factor-alpha and c-reactive protein profiles in melancholic and non-melancholic depression: A systematic review. J Psychosom Res. 2018;111:58–68.
Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL- 6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–8.
Lichtblau N, Schmidt FM, Schumann R, Kirkby KC, Himmerich H. Cytokines as biomarkers in depressive disorder: current standing and prospects. Int Rev Psychiatry. 2013;25:592–603.
Cowen PJ, Browning M. What has serotonin to do with depression? World Psychiatry. 2015;14:158–60.
Albert PR, Benkelfat C, Descarries L. The neurobiology of depression—revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philos Trans R Soc Lond B Biol Sci. 2012;367:2378–81.
Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA. Blakely RD. p38 MAPK Activation Elevates Serotonin Transport Activity via a Trafficking-independent, Protein Phosphatase 2A-dependent Proces. The Journal of Biological Chemistry. 2005;280:15649–58.
Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–31.
Kong E, Sucic S, Monje FJ, Reisinger SN, Savalli G, Diao W, Khan D, Ronovsky M, Cabatic M, Koban F, et al. STAT3 controls IL6-dependent regulation of serotonin transporter function and depression-like behavior. Sci Rep. 2015;5:9009.
Baudry A, Pietri M, Launay JM, Kellermann O, Schneider B. Multifaceted regulations of the serotonin transporter: impact on antidepressant response. Front Neurosci. 2019;13:91.
Zalcman S, Green-Johnson JM, Murray L, Nance DM, Dyck D, Anisman H, Greenberg AH. Cytokine-specific central monoamine alterations induced by interleukin-1, -2 and – 6. Brain Res. 1994;643:40–9.
Day JS, O’Neill E, Cawley C, Aretz NK, Kilroy D, Gibney SM, Harkin A, Conor TJ. Noradrenaline acting on astrocyticβ2-adrenoceptors induces neurite outgrowth in primary cortical neurons. Neuropharmacology. 2014;77:234–48.
Müller N, Myint AM, Krause D, Weidinger E, Schwarz MJ. Anti-inflammatory treatment in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:146–53.
Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–5.
Khairova RA, Machado-Vieira R, Du J. Manji HK. A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2009;12:561–78.
Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Progress Brain Res. 1991;85:119–46.
Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn Sci. 2004;8:294–300.
Rothaug M, Becker-Pauly C, Rose-John S. The role of interleukin-6 signaling in nervous tissue. Biochim Biophys Acta. 2016;1863:1218–27.
Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993;90:10061–5.
Setiawan E, Attwells S, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Xu C, Sharma S, Kish S, Houle S, et al. Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. The Lancet Psychiatry. 2018;5:339–47.
Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72:268–75.
Holmes SE, Hinz R, Conen S, Gregory CJ, Matthews JC, Anton-Rodriguez JM, Gerhard A, Talbot PS. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol Psychiatry. 2018;83:61–9.
Richards EM, Zanotti-Fregonara P, Fujita M, Newman L, Farmer C, Ballard ED, Machado- Vieira R, Yuan P, Niciu MJ, Lyoo CH, et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 2018;8:57.
Li H, Sagar AP, Kéri S. Translocator protein (18kDa TSPO) binding, a marker of microglia, is reduced in major depression during cognitive-behavioral therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2018;83:1–7.
Kakeda S, Watanabe K, Katsuki A, Sugimoto K, Igata N, Ueda I, Igata R, Abe O, Yoshimura R, Korogi Y. Relationship between interleukin (IL)-6 and brain morphology in drug-naïve, first-episode major depressive disorder using surface-based morphometry. Sci Rep. 2018;8:10054.
Frodl T, Carballedo A, Hughes MM, Saleh K, Fagan A, Skokauskas N, McLoughlin DM, Meaney J, O'Keane V, Connor TJ. Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: high IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Transl Psychiatry. 2012;2:e88.
Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–26.
MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387-1392.
Frodl T, Meisenzahl EM, Zetzsche T. Born C, Groll C, Jäger M, Leinsinger G, Bottlender R, Hahn K, Möller HJ. Hippocampal changes in patients with a first episode of major Depression. Am J Psychiatry. 2002;159:1112–8.
Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–7.
Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–7.
Young EA, Haskett RF, Grunhaus L, Pande A, Weinberg VM, Watson SJ, Akil H. Increased evening activation of the hypothalamic-pituitary-adrenal axis in depressed patients. Arch Gen Psychiatry. 1994;51:701–7.
Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000 Oct 15;48(8):755–65.
McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–89.
Yang C, Hashimoto K. Peripheral IL-6 signaling: a promising therapeutic target for depression? Expert Opin Investig Drugs. 2015;24:989–90.
Chourbaji S, Urani A, Inta I, Sanchis-Segura C, Brandwein C, Zink M, Schwaninger M, Gass P. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol Dis. 2006;23:587–94.
Ting EYC, Yang AC, Tsai AJ. Role of interleukin-6 in depressive disorder. Int J Mol Sci. 2020;21:2194.
Loppnow H, Zhang L, Buerke M, Lautenschläger M, Chen L, Frister A, Schlitt A, Luther T, Song N, Hofmann B, et al. Statins potently reduce the cytokine-mediated IL-6 release in SMC/MNC cocultures. J Cell Mol Med. 2011;15:994–1004.
Kang HJ, Bae KY, Kim SW, Kim JT, Park MS, Cho KH, Kim JM. Effects of interleukin-6, interleukin-18, and statin use, evaluated at acute stroke, on post-stroke depression during 1- year follow-up. Psychoneuroendocrinology. 2016;72:156–60.
Burmester GR, Rigby WF, van Vollenhoven RF, Kay J, Rubbert-Roth A, Kelman A, Dimonaco S, Mitchell N. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Di. 2016;75:1081-1091.
Yazici Y, Curtis JR, Ince A, Baraf H, Malamet RL, Teng LL, Kavanaugh A. Efficacy of tocilizumab in patients with moderate to severe active rheumatoid arthritis and a previous inadequate response to disease-modifying antirheumatic drugs: the ROSE study. Ann Rheum Dis. 2012;71:198–205.
Kaneko A. Tocilizumab in rheumatoid arthritis: efficacy, safety and its place in therapy. Ther Adv Chronic Dis. 2013;4:15–21.
Scott LJ. Tocilizumab: a review in rheumatoid arthritis. Drugs. 2017;77:1865–79.
Haraoui B, Casado G, Czirják L, Taylor A, Dong L, Button P, Luder Y, Caporali R. Tocilizumab patterns of use, effectiveness, and safety in patients with rheumatoid arthritis: final results from a set of multi-national non-interventional studies. Rheumatol Ther. 2019;6:231–43.
Turnier JL, Brunner HI. Tocilizumab for treating juvenile idiopathic arthritis. Expert Opin Biol Ther. 2016;16:559–66.
Machado SH, Xavier RM. Safety of tocilizumab in the treatment of juvenile idiopathic arthritis. Expert Opin Drug Saf. 2017;16:493–500.
Shepherd J, Cooper K, Harris P, Picot J, Rose M. The clinical effectiveness and cost- effectiveness of abatacept, adalimumab, etanercept and tocilizumab for treating juvenile idiopathic arthritis: a systematic review and economic evaluation. Health Technol Assess. 2016;20:1–222.
Brod SA, Bauer VL. Ingested (oral) tocilizumab inhibits EAE. Cytokine. 2014;68:86–93.
Nishimoto N, Kanakura Y, Aozasa K, Johkoh T, Nakamura M, Nakano S, Nakano N, Ikeda Y, Sasaki T, Nishioka K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627–32.
Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2018;23:335–43.
Taylor PC, Schiff MH, Wang Q, Jiang Y, Zhuang Y, Kurrasch R, Daga S, Rao R, Tak PP, Hsu B. Efficacy and safety of monotherapy with sirukumab compared with adalimumab monotherapy in biologic-naïve patients with active rheumatoid arthritis (SIRROUND-H): a randomised, double-blind, parallel-group, multinational, 52-week, phase 3 study. Annals of the Rheumatic Diseases. 2018;77:658–66.
Zhou AJ, Lee Y, Salvadore G, Hsu B, Fonseka TM, Kennedy SH, McIntyre RS. Sirukumab: a potential treatment for mood disorders? Adv Ther. 2017;34:78–90.
Sun Y, Wang D, Salvadore G, Hsu B, Curran M, Casper C, Vermeulen J, Kent JM, Singh J, Drevets WC, et al. The effects of interleukin-6 neutralizing antibodies on symptoms of depressed mood and anhedonia in patients with rheumatoid arthritis and multicentric castleman’s disease. Brain Behav. Immun. 2017;66:156–64.
Wittenberg GM, Stylianou A, Zhang Y, Sun Y, Gupta A, Jagannatha PS, Wang D, Hsu B, Curran ME, Khan S, et al. Effects of immunomodulatory drugs on depressive symptoms: A mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry. 2020;25:1275–85.
Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol. 2013;6:295–308.
Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167:915–32.
Cheung SG, Goldenthal AR, Uhlemann AC, Mann JJ, Miller JM, Sublette ME. Systematic review of gut microbiota and major depression. Front Psychiatry. 2019;10:34.
Kelly JR, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbiota axis: challenges for translation in psychiatry. Ann Epidemiol. 2016;26:366–72.
Fung T, Olson C, Hsiao E. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–55.
Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–94.
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S. Du X. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry. 2016;21:786–96.
Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, Ter Horst R, Jansen T, Jacobs L, Bonder MJ, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167:1125–36 e8.
O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O'Sullivan GC, Kiely B, Collins JK, Shanahan F, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–51.
Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MV. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet. and diseases. Microorganisms. 2019;7:14.
Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8:51.
Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, Corthier G, Furet JP. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123.
Jeffery IB, O’Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006.
Distrutti E, Monaldi L, Ricci P, Fiorucci S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J Gastroenterol. 2016;22:2219–41.
Diviccaro S, Giatti S, Borgo F, Barcella M, Borghi E, Trejo JL, Garcia-Segura LM, Melcangi RC. Treatment of male rats with finasteride, an inhibitor of 5alpha-reductase enzyme, induces long-lasting effects on depressive-like behavior, hippocampal neurogenesis, neuroinflammation and gut microbiota composition. Psychoneuroendocrinology. 2019;99:206–15.
Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism. 2013;4:42.
Williams BL, Hornig M, Parekh T, Lipkin WI. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio. 2012;3:pii: e00261–11.
Guo Q, Zheng Y, Shi J, Wang J, Li G, Li C. Immediate psychological distress in quarantined patients with COVID-19 and its association with peripheral inflammation: a mixed-method study. Brain Behav Immun. 2020;88:17–27.
Alpert O, Begun L, Garren P, Solhkhah R. Cytokine storm induced new onset depression in patients with COVID-19. A new look into the association between depression and cytokines -two case reports. Brain Behav Immun Health. 2020;9:100173.
Taquet M, Luciana S, Geddes JR, Harrison PJ. Bidirectional associations between COVID- 19 and psychiatric disorder: retrospective cohort studies of 62354 COVID-19 cases in the USA. Lancet Psychiatry. 2020. https://doi.org/10.1016/S2215-0366(20)30462-4.
Mukaetova-Ladinska, EB, Kronenberg G. Psychological and neuropsychiatric implications of COVID-19. Eur Arch Psychiatry Clin Neurosci. 2020. https://doi.org/10.1007/s00406- 020-01210-2
Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F, Rovere-Querini P, et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600.
Ahmed MZ, Ahmed O, Aibao Z, Hanbin S, Siyu L, Ahmad A. Epidemic of COVID-19 in China and associated psychological problems. Asian J Psychiatr. 2020;51:10209.
Steardo L, Steardo L, Verkhratsky A. Psychiatric face of COVID-19. Transl Psychiatry. 2020;10:261.
Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta- analysis. Rev Med Virol. 2020;30:1–9.
Mojtabavi H, Saghazadeh A, Rezaei N. Interleukin-6 and severe COVID-19: a systematic review and meta-analysis. Eur Cytokine Netw. 2020;31:44–9.
Sun H, Guo P, Zhang L, Wang F. Serum Interleukin-6 Concentrations and the Severity of COVID-19 Pneumonia: A Retrospective Study at a Single Center in Bengbu City, Anhui Province, China. in January and February 2020. Med Sci Monit. 2020;26:e926941.
Gubernatorova EO, Gorshkova EA, Polinova AI, Drutskaya MS. IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020;53:13–24.
Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020:102452.
Moore JB, June CH. Cytokine release syndrome in severe COVID- 19. Science. 2020;368:473–4.
Benedetti F, Mazza M, Cavalli G, Ciceri F, Dagna L, Rovere-Querini P. Can cytokine blocking prevent depression in COVID-19 survivors? J Neuroimmune Pharmacol. 2020:1–3.
Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Sig Transduct Target Ther. 2020;5:84.
Acknowledgements
Not applicable
Funding
Financial support was solely provided by the authors.
Author information
Authors and Affiliations
Contributions
ER contributed to the conception of the study, searched, screened, did the data extraction of the studies, drafted, and reviewed the manuscript. NJ contributed to the conception of the study, reviewed, and edited the manuscript. FH conceived the idea of the study, critically reviewed, and edited the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Roohi, E., Jaafari, N. & Hashemian, F. On inflammatory hypothesis of depression: what is the role of IL-6 in the middle of the chaos?. J Neuroinflammation 18, 45 (2021). https://doi.org/10.1186/s12974-021-02100-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12974-021-02100-7