Abstract
Background
The aim of this study was to compare pain-scores in three targeted treatment-strategies in JIA-patients and to identify characteristics predicting persistent pain.
Methods
In the BeSt-for-Kids-study 92 DMARD-naïve JIA-patients were randomized in 3 treatment-strategies: 1) initial sequential DMARD-monotherapy 2) initial methotrexate (MTX)/prednisolone-bridging or 3) initial MTX/etanercept. Potential differences in VAS pain scores (0-100 mm) over time between treatment-strategies were compared using linear mixed models with visits clustered within patients. A multivariable model was used to assess the ability of baseline characteristics to predict the chance of high pain-scores during follow-up.
Results
Pain-scores over time reduced from mean 55.3 (SD 21.7) to 19.5 (SD 25.3) mm after 24 months. On average, pain-scores decreased significantly with β -1.37 mm (95% CI -1.726; -1.022) per month. No significant difference was found between treatment-strategies (interaction term treatment arm*time (months) β (95% CI) arm 1: 0.13 (-0.36; 0.62) and arm 2: 0.37 (-0.12; 0.86) compared to arm 3). Correction for sex and symptom duration yielded similar results. Several baseline characteristics were predictive for pain over time. Higher VAS pain [β 0.44 (95% CI 0.25; 0.65)] and higher active joint count [0.77 (0.19; 1.34)] were predictive of higher pain over time, whereas, low VAS physician [ -0.34 (-0.55; -0.06)], CHQ Physical [ -0.42 (-0.72; -0.11)] and Psychosocial summary Score [ -0.42 (-0.77; -0.06)] were predictive of lower pain.
Conclusions
Treatment-to-target seems effective in pain-reduction in non-systemic JIA-patients irrespective of initial treatment-strategy. Several baseline-predictors for pain over time were found, which could help to identify patients with a high risk for development of chronic pain.
Trial registration
Dutch Trial Registry number 1574.
Similar content being viewed by others
Background
Juvenile Idiopathic Arthritis (JIA) is the most common auto-immune disease in children, with an estimated prevalence of 33 per 100.000 children [1, 2]. Pain is a common and distressing symptom of JIA [3, 4]. It can cause sleep disturbances, disrupts school attendance and leads to a decline in quality of life that can persist into adulthood [5,6,7]. Disease activity is a well-known contributor to pain severity, but not the only contributing factor [3, 8]. In recent years, introduction of (biologic) DMARDs earlier in the disease course has improved the outcome for JIA patients [9,10,11]. However, this has not directly translated into better pain outcomes as some children experience pain during inactive disease despite adequate treatment and effective disease control [12,13,14]. A possible explanation for persistent pain with low or no visible disease activity is a decreased pain threshold. Children with JIA were found to have a lower pain threshold than their healthy peers, even when they had no detectable joint inflammation after treatment [15], with longer disease duration as a possible predictor [16, 17]. It has been hypothesized that peripheral and central sensitization, causing prolonged hypersensitivity of pain circuits in the nerve system, contribute to a lower pain threshold [18]. This hypothesis suggests that pain, and the causes of pain should be treated as soon and adequately as possible to prevent sensitization.
In JIA, treatment to target (T2T) with clinical remission as a treatment goal has been widely recommended [19, 20]. Treating JIA to target makes drug-free inactive disease a feasible outcome for an increasing number of children with non-systemic JIA [11, 21]. However, improved patient reported outcomes, such as less fatigue and pain, have not yet been confirmed for treat to target therapy [22].
The aim of this subanalysis is to compare pain scores over two years in three treatment strategies in non-systemic JIA patients who were treated to target aimed at inactive disease [21]. Furthermore, we aim to determine the effect of inactive disease and time to inactive disease on pain in the Best for Kids cohort and to explore and identify baseline characteristics predicting unfavorable pain trajectories.
Methods
Patients
The BeSt for Kids study (NTR 1574) is a Dutch multicenter randomized single-blinded trial. It was designed to investigate the effectiveness of three different treat to target strategies for non-systemic JIA patients. Newly diagnosed patients between 2 and 16 years old with JIA (oligoarticular JIA, RF negative polyarticular JIA and juvenile psoriatic arthritis) were included. Exclusion criteria were a disease duration of more than 18 months, uveitis at enrolment and prior DMARD therapy.
Patients were randomized between three strategy arms. Patients in arm 1 were initially treated with methotrexate(MTX) or sulfasalazine(SSZ) monotherapy. Patients in arm 2 initially received MTX and 6 weeks prednisolone bridging. Patients in arm 3 were initially treated with etanercept (ETN) and MTX. Patients were treated to target, with inactive disease (as defined by Wallace [23]) as treatment goal. If inflammation was not enough suppressed and inactive disease was not achieved, treatment was intensified according to the treatment protocol as previously described [21]. Patients were followed every three months until two years. After at least 6 months of inactive disease, treatment was tapered and stopped [21].
The BeSt for Kids study was approved by the Institutional Review Board at Leiden University Medical Center and written informed consent was obtained from all participants before enrolment.
Outcome measures
The primary outcome measure was pain intensity. This was assessed using a 100 mm visual analogue scale (VAS), where 0 mm is ‘no pain’ and 100 mm is ‘unbearable pain’. Patients were asked to rate their pain over the last 7 days. Under the age of 12, pain was estimated by the parents. A VAS pain of ≤ 35mm is considered ‘mild pain’, whereas ‘moderate pain’ is defined as 36–60 mm and ≥ 61mm as ‘severe pain’ [24].
Inactive disease was defined by the adjusted Wallace criteria [23], where Physician’s Global assessment was measured on a 100 mm VAS and < 10mm indicated no active disease. Baseline number of active joints, Physician Global assessment, VAS patient/parent general wellbeing (0-100mm, where 0 is worst and 100 is best), health related quality of life (HRQoL), symptom duration (time from the first recollection of symptoms) and the use of non-steroid anti-inflammatory drug (NSAID) were tested as possible baseline predictors. HRQoL was measured using the Child Health Questionnaire Parent Form 50 (CHQ-PF50), that includes the physical summary score (PhS) and the psychosocial summary score (PsS) (range 0–100, where 0 is worst and mean (SD) of general US population is 50 (10)) [25, 26]. The number of active joints at each visit was assessed by a physiotherapist or physician who was blinded to the treatment allocation.
Statistical methods
Descriptive statistics were used with mean and standard deviations (SDs) for continuous variables and absolute frequency percentage for categorical variables. Potential differences in VAS pain scores over time between treatment arms were compared using linear mixed models with random intercept and random slope for visits clustered within patients. The third arm was treated as reference arm since we hypothesized that arm 3 would be superior compared with arm 1 or arm 2, based on earlier research [27]. Possible confounders that were taken into account in this model were sex and age, as some studies suggest these factors might influence pain [28]. This model was performed for the complete 24 months and for the first 3 months separately, as arm 3 proved superior to the other arms concerning disease activity in the first 3 months [29]. A similar multivariable mixed model was used to determine the association between inactive disease and pain intensity, and between time to inactive disease and pain intensity (with all visits except baseline clustered within patients). This model was adjusted for the possible confounders age, sex, treatment group, baseline VAS pain, Physical summary score, Psychosocial summary score, baseline JADAS10 and diagnosis, based on clinical reasoning and previous research [28, 30, 31].
Additionally, a third mixed model was used in an exploratory analysis to assess the ability of several baseline characteristics to predict the chance of high pain levels during follow-up. For this model multiple imputation was used to deal with missing values of variables with missing data from patients who were still in follow-up, as well as data from the 2 patients that were lost to follow-up and performed based on predictive mean matching (with 5 observations to draw from, resulting in 30 imputation sets). Imputation variables were treatment group, age at inclusion, sex, NSAID use, VAS pain, PGA, VAS patient/parent general wellbeing, VAS disease activity, symptom duration at diagnosis, Juvenile Arthritis Disease Activity Score 10 (JADAS10), number of active joints, number of limited joints, Child Health Assessment Questionnaire (CHAQ) score, CHQ- PhS, CHQ-PsS, JIA category, Erythrocyte Sedimentation Rate (ESR), baseline ESR and all baseline outcome measures. We first evaluated the possible predictive variables in a univariable model and subsequently added them to a multivariable model and evaluated its predictive value for reaching a lower VAS pain during follow-up.
For all statistical analyses p-value < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS version 25 (SPSS, Chicago, IL., USA) and Stata SE version 16 (StataCorp LP).
Results
Patient characteristics
Ninety-four patients were randomized between 2009 and 2014 into the three treatment arms: 32 patients were assigned initial monotherapy (arm 1), 32 patients received initial methotrexate (MTX) with 6 weeks prednisolone bridging therapy (arm 2) and 30 patients initial MTX and etanercept (ETN) (arm 3). Baseline demographics, including proportions of missing data, are summarized in Table 1. Two patients received a revised diagnosis during follow-up and were therefore excluded (one patient in arm 1 and one in arm 3). Two patients who were lost-to-follow-up (one at visit 2, one at visit 5) were included in the analyses.
VAS pain
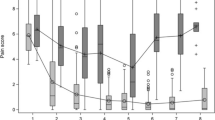
Mean (SD) pain at 24 months was 17.3 (25.5) mm in arm 1, 25.6 (28.5) mm in arm 2 and 15.8 (21.0) mm in arm 3 (overall 19.5(25.3)). When comparing pain scores over time per treatment arm, pain decreased significantly in every arm with β -1.37 mm (95% CI -1.73;-1.02) per month. No significant difference was found in pain over 24 months between treatment arms (interaction term treatment arm*time (months) in arm 1 with β (95% CI) 0.13 (-0.36; 0.62) and in arm 2 with β 0.37 (-0.12; 0.86) compared to arm 3). Correction for age and sex yielded similar results. Pain trajectories over time are depicted in Fig. 1.
When performing the model for the first three months separately, pain decreased at a similar rate in each treatment group with β(95% CI) -7.82 mm (-11.12; -4.53) per month. Arm 1 compared to arm 3 with β(95% CI) 1.45(-3.14; -6.03) and arm 2 with β(95% CI) 2.33(-2.19; 6.85).
Over 24 months, more than 70 percent of patients reached inactive disease [21]. The effect of inactive disease on VAS pain was β -16.87 mm (95% CI -19.65; -14.10) for inactive versus active disease. After adjustment for possible confounders, this effect was β -11.36 mm (95% CI -13.80; -8.93). The effect of time to inactive disease was β 1.33 mm (95% CI 0.60; 2.07) and β 0.52 mm (95% CI 0.11; 0.93) after adjustment for possible confounders. At 24 months, 5 children (8 percent) experienced moderate pain and 2 children (3 percent) experienced severe pain during inactive disease, these seven patients had a mean baseline VAS pain that was 10 mm higher than the overall group. Figure 2 shows VAS pain scores in active and inactive disease at 12 and 24 months.
VAS pain active and inactive disease. VAS pain in children with active disease and inactive disease at 12 and 24 months with median and interquartile range. Inactive disease was determined based on adjusted Wallace criteria. At 12 months inactive disease n = 48, at 24 months n = 65. AD = active disease, ID = inactive disease
Baseline predictors
Several baseline characteristics were selected beforehand and tested for predictive value for pain over time, first in a univariable and subsequently in a multivariable prediction model. In the multivariable model, higher VAS pain and number of active joints at baseline were significantly predictive of higher pain over time, whereas higher VAS physician, CHQ PhS and PsS were predictive of lower pain. Baseline symptom duration, NSAID use and VAS patient/parent were not predictive of pain during follow-up (Table 2). VAS pain showed a β of 0.46 (95%CI 0.25–0.65), indicating that every additional 10 mm VAS pain at baseline is associated with a 4.6 mm higher VAS pain during follow-up.
Discussion
The BeSt for Kids is one of the first treat-to-target studies in newly diagnosed, DMARD naïve, non-systemic JIA patients investigating pain as outcome measure. In the current subanalysis, three frequently used initial treatment strategies were compared for effectiveness in treating pain [21, 32]. In the BeSt for Kids study, the treatment target was inactive disease. Pain was not a treatment target, and therefore treatment adjustments were, at least partly, independent of whether patients had residual pain. Nevertheless, our results show that targeted treatment can significantly reduce pain in children with JIA. After 24 months the mean(SD) VAS pain of JIA patients decreased from 55.3(SD 21.7) to 19.5(SD 25.3) mm, at a similar rate irrespective of initial treatment. Pain scores after two years are lower than in earlier studies assessing pain during non-targeted treatment with DMARDs [13, 33]. This suggests that treat-to-target is beneficial in achieving lower pain scores, although direct comparisons are lacking.
Although few studies have addressed pain over time as an outcome, we know from cross sectional data and reports from trials and prospective observational studies that pain scores can remain high after treatment. These high pain levels are reported after treatment according to clinical practice before the availability of biologic treatment [34, 35], but also in patients in whom disease activity has declined after successful treatment with abatacept and other biologicals [3, 13, 33]. Even in the biologic era, pain can remain present in the long term, and high pain perception affects quality of life [12, 14]. The decrease in pain seen in patients participating in our treat to target strategy is therefore promising.
Even though we saw a decrease of active disease and pain in the BeSt for kids study, still some of our patients who did achieve inactive disease continued to have pain. It is this group of patients that deserves additional attention [36, 37]. Central sensitization could be a factor involved in persistence of pain in chronic conditions like JIA [38]. Our analyses showed that longer time to inactive disease has a small, but significant association with more pain over time. Further research should focus on how to identify patients who are at risk for chronic pain even after the inflammation is abrogated. When we are able to recognize children with chronic pain in an early stage, they might benefit from education about the cause of their pain, relevant pain mechanisms, and the role of psychosocial and physical factors in precipitating and maintaining chronic pain [39, 40].
In addition to this, we could consider adding an extra patient reported outcome, to investigate whether the clinical improvement we find corresponds to what our patients regard as a satisfactory improvement in pain levels [37, 41, 42]. In adult RA it is recognized that clinicians may have another perspective on how a favorable outcome is defined than their patients, and research is increasingly directed towards more patient reported measures and a value based perception of health care [43, 44]. Recently, in adult rheumatology, the dual target strategy was proposed to enhance patient satisfaction, with one target representing control of inflammation (biological remission) and the other control of disease impact (symptom remission) [45].
We performed an exploratory analysis to identify baseline factors that predict pain over time. In univariable analyses we identified VAS pain, PGA, VAS patient/parent, number of active joints, PhS and PsS as significant predictors of pain over time. In a multivariable model including all previously tested variables, identified VAS pain, PGA, number of active joints, PhS and PsS remained predictive. This is in accordance with previous literature [31, 46,47,48].
Counterintuitively and in contrast to previous studies, in our multivariable model, a lower PGA at baseline was associated with more pain during the two years follow up [8, 46]. This reversal of the effect compared to the results of our univariable analyses is related to the combination of predictors in our multivariable model. Due to the predictive nature of our model, this relationship with pain should not be interpreted causally.
Contrary to previous studies we did not find symptom duration as a significant baseline predictor for more pain over time. This could be due to lack of power, as these studies included more patients and the effect that was found was very small [8, 46].
In our previous publication [21], we reported intraarticular corticosteroid injections that were administered outside the study protocol. This happened rarely, but more frequently in arm 2 than in other treatment arms. Despite this difference in frequency, we do not see a difference in pain course over time, which could be attributed to the intraarticular injections.
There are some limitations to our study. The sample size of our study was limited, therefore more subtle differences might have been found in a larger study cohort. Second, pain is multifactorial in nature and affected by many psychosocial patient factors [39]. This study only looked at pain intensity and did not take a possible response shift during the study period into consideration [49]. Third, VAS pain scores were completed by patients or their parents. Parents were encouraged to obtain self-report by the child, but in young children this was not always possible. Patient-proxy reports are known to not always correspond, especially in children with a high disease burden [50]. Fourth, pain can be influenced by sensitization [18, 38]. This might have occurred prior to start of treatment. In our analyses, we have only looked at time to start of treatment, but other factors may have influenced sensitization and pain. And lastly, the prediction model was based upon a randomized cohort with strict inclusion criteria. To participate in the BeSt for kids study, symptom duration had to be less than 18 months. This and other inclusion criteria might limit the generalizability of the results.
Conclusions
We conclude that treat to target therapy is effective in reducing pain over time for DMARD-naïve non-systemic JIA patients, irrespective of initial treatment. On the other hand, some children still experience pain despite achieving clinically inactive disease. This emphasizes the necessity of addressing patient related outcomes in addition to targeted treatment to reduce disease activity. Several baseline predictors for pain over time were found, which could help to identify and support non-systemic JIA-patients with a high risk of pain in addition to a treat-to-target strategy treatment.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369(9563):767–78. https://doi.org/10.1016/S0140-6736(07)60363-8. [publishedOnlineFirst:2007/03/06].
Thierry S, Fautrel B, Lemelle I, et al. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine. 2014;81(2):112–7. https://doi.org/10.1016/j.jbspin.2013.09.003. [publishedOnlineFirst:2013/11/12].
Schanberg LE, Anthony KK, Gil KM, et al. Daily pain and symptoms in children with polyarticular arthritis. Arthritis Rheum. 2003;48(5):1390–7. https://doi.org/10.1002/art.10986.
Palman J, Shoop-Worrall S, Hyrich K, et al. Update on the epidemiology, risk factors and disease outcomes of Juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol. 2018;32(2):206–22. https://doi.org/10.1016/j.berh.2018.10.004. [publishedOnlineFirst:2018/12/12].
Weiss JE, Luca NJ, Boneparth A, et al. Assessment and management of pain in juvenile idiopathic arthritis. Paediatr Drugs. 2014;16(6):473–81. https://doi.org/10.1007/s40272-014-0094-0. [publishedOnlineFirst:2014/10/22].
Giancane G, Alongi A, Rosina S, et al. Open issues in the assessment and management of pain in juvenile idiopathic arthritis. Clin Exp Rheumatol 2017;35 Suppl 107(5):123–26. [published Online First: 2017/10/03].
Tollisen A, Selvaag AM, Aulie HA, et al. Physical functioning, pain, and health-related quality of life in adults with juvenile idiopathic arthritis: a longitudinal 30-year followup study. Arthritis Care Res (Hoboken). 2018;70(5):741–9. https://doi.org/10.1002/acr.23327. [publishedOnlineFirst:2017/07/22].
Malleson PN, Oen K, Cabral DA, et al. Predictors of pain in children with established juvenile rheumatoid arthritis.Arthritis Rheum 2004;51(2):222-7. doi: https://doi.org/10.1002/art.20238. [published Online First: 2004/04/13].
Prince FH, Geerdink LM, Borsboom GJ, et al. Major improvements in health-related quality of life during the use of etanercept in patients with previously refractory juvenile idiopathic arthritis. Ann Rheum Dis2010;69(1):138-42. doi: https://doi.org/10.1136/ard.2009.111260. [published Online First: 2009/07/08].
Minden K, Niewerth M, Zink A, et al. Long-term outcome of patients with JIA treated with etanercept, results of the biologic register JuMBO. Rheumatology (Oxford). 2012;51(8):1407-15. https://doi.org/10.1093/rheumatology/kes019. [published Online First: 2012/03/27].
Klein-Wieringa IR, Brinkman DMC, Ten Cate R, et al. Update on the treatment of nonsystemic juvenile idiopathic arthritis including treatment-to-target: is (drug-free) inactive disease already possible? Curr Opin Rheumatol. 2020;32(5):403-13. https://doi.org/10.1097/bor.0000000000000727. [published Online First: 2020/07/14].
Lomholt JJ, Thastum M, Herlin T. Pain experience in children with juvenile idiopathic arthritis treated with anti-TNF agents compared to non-biologic standard treatment. Pediatr Rheumatol Online J. 2013;11(1):21. https://doi.org/10.1186/1546-0096-11-21. [published Online First: 2013/05/07].
Bromberg MH, Connelly M, Anthony KK, et al. Self-reported pain and disease symptoms persist in juvenile idiopathic arthritis despite treatment advances: an electronic diarystudy. Arthritis Rheumatol. 2014;66(2):462-9. https://doi.org/10.1002/art.38223. [published Online First: 2014/02/08].
Anink J, Prince FH, Dijkstra M, et al. Long-term quality of life and functional outcome of patients with juvenile idiopathic arthritis in the biologic era: a longitudinal follow-up study in the Dutch Arthritis and Biologicals in Children Register. Rheumatology (Oxford). 2015;54(11):1964-9. doi: https://doi.org/10.1093/rheumatology/kev195. [published Online First: 2015/06/17].
Hogeweg JA, Kuis W, Huygen AC, et al. The pain threshold in juvenile chronic arthritis. Br J Rheumatol. 1995;34(1):61-7. https://doi.org/10.1093/rheumatology/34.1.61. [published Online First: 1995/01/01].
Arnstad ED, Iversen JM, Uglem M, et al. Pain sensitivity in young adults with juvenile idiopathic arthritis: a quantitative sensory testing study. Arthritis Res Ther. 2020;22(1):262. doi: https://doi.org/10.1186/s13075-020-02345-2. [published Online First: 2020/11/07].
Leegaard A, Lomholt JJ, Thastum M, et al. Decreased Pain Threshold in Juvenile Idiopathic Arthritis: A Cross-sectional Study. J Rheumatol. 2013;40(7):1212–7. https://doi.org/10.3899/jrheum.120793.
La Hausse de Lalouviere L, Ioannou Y, Fitzgerald M. Neural mechanisms underlying the pain of juvenile idiopathic arthritis. Nat Rev Rheumatol. 2014;10(4):205–11. doi: https://doi.org/10.1038/nrrheum.2014.4. [published Online First: 2014/02/05].
Ravelli A, Consolaro A, Horneff G, et al. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2018;77(6):819-28. https://doi.org/10.1136/annrheumdis-2018-213030. [published Online First: 2018/04/13].
Hinze C, Gohar F, Foell D. Management of juvenile idiopathic arthritis: hitting the target. Nat Rev Rheumatol. 2015;11(5):290-300. https://doi.org/10.1038/nrrheum.2014.212. [published Online First: 2015/01/07].
Hissink Muller P, Brinkman DMC, Schonenberg-Meinema D, et al. Treat to target (drug-free) inactive disease in DMARD-naive juvenile idiopathic arthritis: 24-month clinical outcomes of a three-armed randomised trial. Ann Rheum Dis 2019;78(1):51-59. https://doi.org/10.1136/annrheumdis-2018-213902. [published Online First: 2018/10/13].
Schoemaker CG, Swart JF, Wulffraat NM. Treating juvenile idiopathic arthritis to target: what is the optimal target definition to reach all goals? Pediatr Rheumatol Online J. 2020;18(1):34. https://doi.org/10.1186/s12969-020-00428-7. [published Online First: 2020/04/18].
Wallace CA, Ruperto N, Giannini E, et al. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31(11):2290–4. [published Online First: 2004/11/02].
Hirschfeld G, Zernikow B. Cut points for mild, moderate, and severe pain on the VAS for children and adolescents: what can be learned from 10 million ANOVAs? Pain. 2013;154(12):2626-32. https://doi.org/10.1016/j.pain.2013.05.048. [published Online First: 2013/06/08].
Wulffraat N, van der Net JJ, Ruperto N, et al. The Dutch version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ). Clin Exp Rheumatol. 2001;19(4 Suppl 23):S111–5. [published Online First: 2001/08/21].
Landgraf JM, Abetz L, Ware JEJ. The CHQ: A User's Manual. Boston, MA: The Health Institute, New England Medical Center 1996.
Klotsche J, Minden K, Thon A, et al. Improvement in health-related quality of life for children with juvenile idiopathic arthritis after start of treatment with etanercept. Arthritis Care Res (Hoboken). 2014;66(2):253-62. https://doi.org/10.1002/acr.22112. [published Online First: 2013/08/29].
Anthony KK, Schanberg LE. Pediatric pain syndromes and management of pain in children and adolescents with rheumatic disease. Pediatr Clin North Am. 2005;52(2):611–39, vii. https://doi.org/10.1016/j.pcl.2005.01.003. [published Online First: 2005/04/12].
Hissink Muller PC, Brinkman DM, Schonenberg D, et al. A comparison of three treatment strategies in recent onset non-systemic Juvenile Idiopathic Arthritis: initial 3-months results of the BeSt for Kids-study. Pediatr Rheumatol Online J. 2017;15(1):11. https://doi.org/10.1186/s12969-017-0138-4. [published Online First: 2017/02/09].
Glerup M, Herlin T, Twilt M. Clinical Outcome and Long-term Remission in JIA. Curr Rheumatol Rep. 2017;19(12):75. https://doi.org/10.1007/s11926-017-0702-4. [published Online First: 2017/11/05].
Arnstad ED, Rypdal V, Peltoniemi S, et al. Early Self-Reported Pain in Juvenile Idiopathic Arthritis as Related to Long-Term Outcomes: results from the Nordic Juvenile Idiopathic Arthritis Cohort Study. Arthritis Care Res (Hoboken). 2019;71(7):961-69. https://doi.org/10.1002/acr.23715. [published Online First: 2018/07/29].
Ringold S, Weiss PF, Colbert RA, et al. Childhood Arthritis and Rheumatology Research Alliance Consensus Treatment Plans for New-Onset Polyarticular Juvenile Idiopathic Arthritis. Arthritis Care Res. 2014;66(7):1063–72. https://doi.org/10.1002/acr.22259.
Ruperto N, Lovell DJ, Li T, et al. Abatacept improves health-related quality of life, pain, sleep quality, and daily participation in subjects with juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2010;62(11):1542-51. doi: https://doi.org/10.1002/acr.20283. [published Online First: 2010/07/03].
Schanberg LE, Lefebvre JC, Keefe FJ, et al. Pain coping and the pain experience in children with juvenile chronic arthritis. Pain 1997;73(2):181-9. doi: https://doi.org/10.1016/s0304-3959(97)00110-3. [published Online First: 1998/02/07].
Bowyer SL, Roettcher PA, Higgins GC, et al. Health status of patients with juvenile rheumatoid arthritis at 1 and 5 years after diagnosis. J Rheumatol. 2003;30(2):394–400. [published Online First: 2003/02/04].
Schoemaker CG, Armbrust W, Swart JF, et al. Dutch juvenile idiopathic arthritis patients, carers and clinicians create a research agenda together following the James Lind Alliance method: a study protocol. Pediatr Rheumatol Online J. 2018;16(1):57. doi: https://doi.org/10.1186/s12969-018-0276-3.
Schoemaker CG, de Wit MPT. Treat-to-Target From the Patient Perspective Is Bowling for a Perfect Strike. Arthritis Rheumatol. 2021;73(1):9-11. https://doi.org/10.1002/art.41461. [published Online First: 2020/08/03].
Pas R, Ickmans K, Van Oosterwijck S, et al. Hyperexcitability of the Central Nervous System in Children with Chronic Pain: A Systematic Review. Pain Med. 2018;19(12):2504-14. https://doi.org/10.1093/pm/pnx320. [published Online First: 2018/01/06].
Brandelli YN, Chambers CT, Mackinnon SP, et al. A systematic review of the psychosocial factors associated with pain in children with juvenile idiopathic arthritis. Pediatr Rheumatol. 2023;21(1):57. https://doi.org/10.1186/s12969-023-00828-5.
Robins H, Perron V, Heathcote LC, et al. Pain Neuroscience Education: State of the Art and Application in Pediatrics. Children (Basel). 2016;3(4) https://doi.org/10.3390/children3040043. [published Online First: 2016/12/24].
Lunt LE, Shoop-Worrall S, Smith N, et al. Validation of novel patient-centred juvenile idiopathic arthritis-specific patient-reported outcome and experience measures (PROMs/PREMs).Pediatr Rheumatol Online J. 2020;18(1):91. https://doi.org/10.1186/s12969-020-00481-2. [published Online First: 2020/11/21].
Beckers E, Webers C, Boonen A, et al. Validation and implementation of a patient-reported experience measure for patients with rheumatoid arthritis and spondyloarthritis in the Netherlands. Clin Rheumatol. 2020;39(10):2889-97. doi: https://doi.org/10.1007/s10067-020-05076-6. [published Online First: 2020/04/23].
Consolaro A, Ruperto N, Bazso A, et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009;61(5):658–66. https://doi.org/10.1002/art.24516.
Ten Klooster PM, Vonkeman HE, Oude Voshaar MA, et al. Predictors of satisfactory improvements in pain for patients with early rheumatoid arthritis in a treat-to-target study.Rheumatology (Oxford). 2015;54(6):1080-6. doi: https://doi.org/10.1093/rheumatology/keu449. [published Online First: 2014/11/30].
Ferreira RJO, Ndosi M, de Wit M, et al. Dual target strategy: a proposal to mitigate the risk of overtreatment and enhance patient satisfaction in rheumatoid arthritis. Ann Rheum Dis. 2019;78(10):e109. https://doi.org/10.1136/annrheumdis-2018-214199. [published Online First: 2018/08/22].
Rashid A, Cordingley L, Carrasco R, et al. Patterns of pain over time among children with juvenile idiopathic arthritis. Arch Dis Child. 2018;103(5):437–43. https://doi.org/10.1136/archdischild-2017-313337. [published Online First:2017/11/28].
Shiff NJ, Tupper S, Oen K, et al. Trajectories of pain severity in juvenile idiopathic arthritis: results from the Research in Arthritis in Canadian Children Emphasizing Outcomes cohort. Pain. 2018;159(1):57-66. https://doi.org/10.1097/j.pain.0000000000001064. [published Online First: 2017/09/25].
Tarkiainen M, Tynjälä P, Vähäsalo P, et al. Health-related quality of life during early aggressive treatment in patients with polyarticular juvenile idiopathic arthritis: results from randomized controlled trial. Pediatr Rheumatol Online J. 2019;17(1):80. https://doi.org/10.1186/s12969-019-0370-1. [published Online First: 2019/12/18].
Barclay-Goddard R, Epstein JD, Mayo NE. Response shift: a brief overview and proposed research priorities. Qual Life Res. 2009;18(3):335-46. https://doi.org/10.1007/s11136-009-9450-x. [published Online First: 2009/02/26].
Lal SD, McDonagh J, Baildam E, et al. Agreement between proxy and adolescent assessment of disability, pain, and well-being in juvenile idiopathic arthritis. J Pediatr. 2011;158(2):307-12. https://doi.org/10.1016/j.jpeds.2010.08.003. [published Online First: 2010/09/28].
Acknowledgements
We would like to thank Bastiaan van Dijk and Joy van der Pol for their support in formatting the graphs.
Funding
The BeSt for Kids study is an investigator-initiated study which received financial support from Pfizer, who had no role in study design, data collection, data analysis, data interpretation, writing of an abstract, or decision to submit a manuscript for submission.
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content. All authors approved the final version to be published. Ms Spekking and dr Hissink Muller had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: KS, PB, SB, DB, CA, RC, LS, MR, PHM. Acquisition of data: PHM, DB, CA, DS, YK, MR, LS, MB. Critically revising the manuscript; KS, JA, PB, SB, MB, DS, YK, MR, LS, CA, RC, DB, PHM.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval of the Medical Ethical Committee of the Leiden University Medical Center and local Ethical Committees was obtained prior to start at each study site. Written Informed consent was obtained from patients above 12 years of age and parents of all participating patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Spekking, K., Anink, J., de Boer, P. et al. Significant pain decrease in children with non-systemic Juvenile Idiopathic Arthritis treated to target: results over 24 months of follow up. Pediatr Rheumatol 21, 90 (2023). https://doi.org/10.1186/s12969-023-00874-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12969-023-00874-z