Abstract
Background
In the recent years, musculoskeletal ultrasound (MSUS) has been regarded as especially promising in the assessment of juvenile idiopathic arthritis (JIA), as a reliable method to precisely document and monitor the synovial inflammation process.
Main content
MSUS is particularly suited for examination of joints in children due to several advantages over other imaging modalities. Some challenges should be considered for correct interpretation of MSUS findings in children, due to the peculiar features of the growing skeleton. MSUS in JIA is considered particularly useful for its ability to detect subclinical synovitis, to improve the classification of patients in JIA subtypes, for the definition of remission, as guidance to intraarticular corticosteroid injections and for capturing early articular damage. Current evidence and applications of MSUS in JIA are documented by several authors. Recent advances and insights into further investigations on MSUS in healthy children and in JIA patients are presented and discussed in the present review.
Conclusions
MSUS shows great promise in the assessment and management of children with JIA. Nonetheless, anatomical knowledge of sonographic changes over time, underlying immunopathophysiology, standardization and validation of MSUS in healthy children and in patients with JIA are still under investigation. Further research and educational efforts are required for expanding this imaging modality to more clinicians in their daily practice.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Juvenile idiopathic arthritis (JIA) is the most common chronic rheumatic disease of childhood and an important cause of acquired disability in children [1]. Despite the heterogeneity, all forms of JIA are characterized by prolonged synovial inflammation that can cause cartilage and bone damage, with severe impairment of physical function and impact on the quality of life. In the recent years, the availability of powerful and expensive drugs increased the need to identify patients with a high likelihood of developing erosive damage early and patients with a less aggressive disease, so as to institute the appropriate therapy at and for the most convenient time. This induced to search for sensitive methods for reliable documentation and precise monitoring of the synovial inflammation process [2–4]. Musculoskeletal ultrasound (MSUS) demonstrated to be a valid and reliable tool in the assessment of chronic inflammatory arthropathies in adults [5–7]. Therefore, it has been regarded as especially promising in the assessment of joints in children with JIA [8–11].
Advantages of MSUS in children
Though MSUS has some limitations, it is particularly suited for use in children for several advantages over other imaging techniques (Table 1). It is quick, it does not expose the child to ionizing radiation, it does not require sedation or general anesthesia, it allows for multisite assessment in the same session, comparison between symptomatic and asymptomatic sites, dynamic study, and it is easily repeatable. Moreover, it is well accepted by both children and their parents, and it is the only imaging technique that can be coupled with the conventional approach to patient assessment in the clinic (Fig. 1).
Challenges with MSUS in children
Like other imaging modalities, MSUS is an operator dependent imaging technique. Further, it is highly dependent on the properties and sensitivity of the machine used, which can range widely (Fig. 2). As in adults, MSUS in children requires continuous practice after appropriate training and has a limited value in some musculoskeletal areas, such as the axial skeleton.
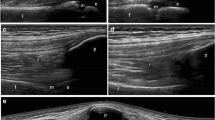
When dealing with musculoskeletal imaging in childhood, it is noteworthy to emphasize the unique feature of the growing skeleton, which include age-related variation of the thickness of the articular cartilage and incomplete ossification. Moreover, in children the epiphysis are vascularized and metaphyseal vessels anastomose with epiphyseal vessels through the growth plate. Depending on the properties and sensitivity of the ultrasound machine, vascularization in this area can be physiologically detected by MSUS in healthy children [12], whereas it would be regarded as pathological in adults. Several pitfalls can lead the ultrasonographer with little experience in pediatric joints to embarrassing misinterpretation of images (Fig. 3). Therefore, the awareness of a high ratio cartilage/bone, that changes during child growth, and the anatomical knowledge of the feeding vessels are of foremost value and cannot be overtaken.
Metaphysis (M) look like erosions; epiphysis (E) and unossified bones are anechoic, like synovial effusion (a,b). Physiological vascularization at insertion of enthesis to the cartilage can be frequently detected, resembling enthesitis (c). Feeding vessels can be intraarticular or close to ossifying nuclei, and must not be considered as signs of active synovitis (d). a. Dorsal longitudinal scan of the II metacarpophalangeal joint in a 2 years old child, grey-scale. b. Longitudinal medial scan of the suprapatellar recess in a 2 years old child, grey-scale. c. Longitudinal scan of the Achilles tendon at insertion to the calcaneus in a 5 years old child, power Doppler d. Longitudinal medial scan of the suprapatellar recess in a 5 years old child, power Doppler
Usefulness of MSUS in children
Despite several challenges, MSUS is commonly regarded as a very useful tool in children, as outlined by the results of recent national and international surveys among pediatric rheumatologists [13, 14]. For most of the respondents, MSUS owned particular relevance for the ability to detect subclinical synovitis and to improve the classification of patients in JIA subtypes, as guidance to intraarticular corticosteroid injections and for capturing early articular damage. In addition, some specific joints were considered as most suited to be studied by MSUS, specifically the midfoot, the ankle, the hip, the wrist, the small joints of hands and feet.
What is the evidence for usefulness of MSUS in JIA?
Subclinical synovitis and tenosynovitis
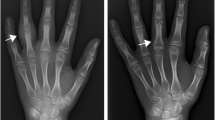
Arthritis in JIA is so far defined as swelling within a joint, or limitation in the range of joint movement with joint pain or tenderness, persistent over time, observed by a physician, and not due to primarily mechanical disorders or to other identifiable causes [15]. Currently, the definition of an oligoarticular or polyarticular involvement is based on the number of active joints. Therefore, complete joint assessment is mandatory for accurate assessment of the disease. However, in the recent years several authors documented a discrepancy between clinical and MSUS examination in detecting synovitis in JIA. In our experience, 1664 joints in 32 children with JIA were evaluated both clinically and with MSUS. A total of 104 (6.3 %) and 167 (10 %) joints had clinical and MSUS synovitis, respectively. Of the 1560 clinically normal joints, 86 (5.5 %) had synovitis on MSUS. The frequency of subclinical synovitis was greater in wrists, PIP, subtalar and foot joints [16]. Haslam et al. compared clinical and MSUS evaluation in 680 joints of 17 patients with early (<12 months) oligoarticular JIA. Six children had subclinical synovitis, more frequently detected in the small joints of hands and feet [17]. Other authors reported similar findings in the assessment of peripheral joints and the ankle [18–20]. In particular, Rooney at al. observed that the clinical examination might not be able to distinguish whether joint swelling in the ankle is due to synovitis, tenosynovitis or both (Fig. 4). In 34 JIA patients who had clinically detected swelling in 49 ankles they evaluated the prevalence of MSUS synovitis and tenosynovitis. Only 29 % of ankles had tibiotalar effusion alone, whereas tenosynovitis associated with tibiotalar synovitis and tenosynovitis alone were detected in 71 and 39 % of ankles, respectively. Concomitant tenosynovitis and tibiotalar effusion were found in 33 % of ankles [20]. In another study of the same group, 32 % of the ankles considered clinically involved did not show MSUS synovitis. In 42 % of ankles recorded as clinically normal MSUS detected involvement of medial tendons, whereas less than 50 % of the lateral tendons deemed to be clinically involved were affected on MSUS [21].
A 10 years old girl with JIA presented with mild swelling of the left ankle, without tenderness/pain on motion or limitation on motion. MSUS showed no signs of synovitis at the tibiotalar joint (a), and allowed detection of a hypo-anechoic halo around the medial tendons (b) and pathologic vascularization on power Doppler along both the posterior tibialis tendon (c) and the common flexor digiti tendon (d), indicating flourishing tenosynovitis. PTT: posterior tibialis tendon; CDT: common flexor digitorum tendon; A: posterior tibialis artery; V: posterior tibialis vein
These findings highlight that clinical examination in JIA may underestimate the extension of synovitis and sometimes cannot be precise, whereas MSUS can improve the sensitivity and the accuracy in the detection of the exact sites of inflammation in the joint. Therefore, implementation of clinical examination with MSUS in children with JIA can lead to important implications in therapeutic decisions (i.e. indication to a second line drug or biologic treatment, or exact location of intraarticular corticosteroid injections) and for monitoring treatment efficacy.
Enthesitis
Enthesitis represents the main feature of the enthesitis-related arthritis (ERA) JIA subgroup, according to the International League of Associations for Rheumatology (ILAR) classification of JIA, and is clinically defined as tenderness at the insertion of a tendon, ligament, joint capsule, or fascia to bone [15]. The clinical demonstration of enthesitis in children is challenging owing to the peculiar fat distribution, that can mask the anatomical landmarks, and the frequently insufficient cooperation of very young children. A recent study reported on MSUS sensitivity to detect enthesitis in children with JIA [22]. The authors compared physical examination and power Doppler (PD) MSUS in detecting enthesitis in five sites (the quadriceps tendon insertion, the proximal and the distal patellar ligament insertion on the tibial tuberosity, the Achilles tendon insertion on the posterior surface of the calcaneus, and the plantar fascia insertion) in 26 patients with JIA and 41 healthy children. None of the healthy children had PD MSUS evidence of enthesitis. In patients with JIA physical examination showed enthesitis at only 12.5 % of sites, whereas PD MSUS enthesitis was found at 9.4 % of the investigated sites. Clinical enthesitis was often associated with PD MSUS enthesitis. On the other hand, 50 % of the sites exhibiting PD MSUS were clinically normal. Of note, 20 % of the sites with PD MSUS enthesitis were in patients with oligoarticular JIA and 10 % in patients with polyarticular JIA.
Recent studies confirmed a higher sensitivity of MSUS in comparison with clinical examination in detecting enthesitis in different entheseal sites in patients with ERA-JIA [23, 24].
These findings overall indicate that MSUS may help to detect clinically silent peripheral enthesitis in ERA and non-ERA patients, and can contribute substantially to the correct diagnosis and classification of JIA. On the other hand, comprehensive knowledge of the ultrasonographic appearance of entheses in healthy subjects during the developmental ages is still under investigation (Fig. 5) and reliable definitions for normal and pathological MSUS findings in pediatric entheses need first to be addressed.
ILAR classification JIA subgroups
The most recent ILAR classification criteria for JIA were developed to delineate homogeneous subtypes of JIA for research purposes [15] and so far have been adopted to stratify patients and select candidates to second-line treatment or biologic therapy [25–31]. In particular, children with JIA are classified as having oligoarthritis or polyarthritis based on the number of affected joints; the presence of active arthritis in at least five joints is a requisite for the definition of a polyarticular disease course and for the eligibility to second-line or biologic agents in several health systems. As already mentioned, MSUS demonstrates higher sensitivity in detecting synovitis than the clinical examination, and, as a consequence, can lead to reclassify patients, with a trend towards a more extensive joint involvement and potential more aggressive therapy. In the author’s experience, of 32 children with JIA evaluated cross-sectionally both with clinical and MSUS examinations, five patients, classified as having mono/oligoarthritis involvement by clinical examination, showed a polyarthritis involvement based on MSUS findings [16]. Similarly, one patient out of 17 children with oligoarticular JIA was reclassified as having polyarthritis after MSUS examination by Haslam et al. [17]. Of note, MSUS could precisely identify inflammatory tendon involvement in JIA patients with ankle arthritis, as outlined by Rooney at al [20, 21]. Though tendons can be affected throughout the whole course of JIA, the current ILAR classification does not take into consideration tendon involvement. Further, MSUS enthesitis could be found in both in ERA and non-ERA-JIA patients, as mentioned above [22–24].
Overall these findings suggest that the use of MSUS may yield important insights in the location of inflammatory changes in joints and in different JIA subtypes, providing the anatomic rationale for a future refinement of the classification of childhood arthritis.
Definition of disease remission
It has been recently argued that remission of JIA defined on clinical grounds does not couple with remission defined with imaging [32–34]. However, the clinical significance and prognostic value of this finding is unclear, as the presence of MSUS abnormalities, including PD signal, in patients with clinically defined inactive disease did not predict subsequent synovitis flare [35]. This finding contrasts with observations in adults with rheumatoid arthritis (RA), in which vascularization detected by PD MSUS predicted shortterm disease flare after clinical remission [36, 37].
These puzzling MSUS features may be due to underlying immunopathological mechanism and local changes peculiar of JIA, still unknown and different to the classical autoinflammatory process in seropositive RA [38]. Future research oriented to explore this supposition and correlation of MSUS with biologic markers of disease activity [39, 40] is advisable.
Imaging guided injections
Intra-articular corticosteroid injections (IACI) are widely used in JIA to induce prompt relief of symptoms of active synovitis. Blind-method is sometimes difficult in the younger, due to the small joint size and the subcutaneous fat masking bony landmarks. Therefore MSUS may represent a fundamental tool, not only for the precise detection of the inflamed area, but also for the accurate placement of the needle tip within the different affected anatomical structures, in order to maximize the treatment efficacy and minimize local side effects (mainly subcutaneous atrophy or local skin hypopigmentation) [41] (Fig. 6). Several authors documented the correct needle placement and the efficacy of MSUS-guided injections of clinically difficult to access joints, such as the hip [42], or clinically difficult to assess joints, such as the ankle and the midfoot [43]. In particular, Laurell at al. performed MSUS-guided IACI in 85 compartments of the ankle, including twenty-one tendon sheaths and a ganglion cyst, and observed normalization or regression of MSUS-detected synovial hypertrophy in 89 % of the injected sites at 4 weeks after IACI. The same authors also reported on the efficacy of MSUS-guided IACI in 21 compartments of 15 wrists, with increasing improvement of synovial hypertrophy and synovial hyperemia at 1 week and 4 weeks post-injection [44]. Another joint that can particularly benefit from MSUS-guided IACI is the temporomadibular joint (TMJ). Efficacy and safety assessed 6–8 weeks post MSUS-guided injection of 63 TMJ in 39 children with JIA showed improvement in all symptoms and only one side effect (local scar) in one patient [45]. However, data on correct needle placement in TMJ by MSUS-guidance are scarce and controversial [46, 47], and need to be further investigated.
Cartilage damage
Joint cartilage is a known target in inflammatory arthritis. Though MSUS do not allow to visualizing cartilage in its entireness in all joints, due to its limited acoustic window, loss of MSUS detected cartilage in “key sites” of the joints may represent an early marker of damage in JIA. Spannow, et al were the first to attempt the measurement and quantification of cartilage thickness in pediatric subjects [48]. They provided normal ranges of MSUS-detected cartilage thickness in small and large joints of healthy children, and designed a complicated formula in order to calculate cartilage thickness in the clinically dominant joints for different age groups of children [49]. Cartilage was found significantly thicker in boys than in girls and diminished progressively with age in both sexes, as similarly reported later by other authors [50]. The same investigators provided data on intra- and interobserver agreement and validated MSUS measurement of cartilage thickness in several joints of healthy children by comparing MSUS with findings obtained with magnetic resonance imaging (MRI), with an overall good agreement except for the wrist [51, 52]. In a subsequent study, Spannow and coworkers measured cartilage thickness with MSUS in joints of patients with JIA, and compared the findings with those obtained in healthy children. Of note, cartilage thickness in joints of patients with JIA was significantly lower than in the healthy cohort, regardless of whether the examined joints have been previously affected by arthritis [53]. Pradsgaard, et al. from the same group of investigators, compared MSUS and MRI measurements of distal femoral cartilage thickness in children with JIA. They identified the intercondylar notch as the best site to assess cartilage thickness, because of its easier assessment and lower variability on MSUS as compared to MRI [54]. These findings may support that irreversible changes can occur in cartilage of children with JIA despite the localization of synovial inflammation. The measurement of cartilage thickness at the intercondylar notch of the knee may act as a surrogate of overall early cartilage damage in JIA patients. However, since MSUS and MRI cartilage measurements at different sites of the knee were problematic in the previously affected and unaffected knees compared to the whole cohort, this proposal is controversial [55]. Thorough studies in patients with long term inactive and active JIA and comparison with the newer MRI techniques for the biochemical evaluation of cartilage matrix composition over time [56] would give insights to address these challenging issues.
Bone erosions
Compared with conventional radiography, the capacity to assess dynamically and in real-time the joints in several planes makes MSUS a more useful tool for detecting erosions [57, 58]. The OMERACT (Outcome Measures in Rheumatology Clinical Trials) definitions of bone erosion require documenting an interruption of the intraarticular bone surface or cortical breaks with a step-off bone defect visible in at least two perpendicular planes [59]. However, children anatomy is characterized by physiological bone irregularities due to the presence of ossification centers and growth plates at the epiphyseal cartilage. Moreover, when ossified, some bones can at first appear fragmented and irregular (Fig. 7). Consequently, in children all these peculiar normal findings may potentially be misinterpreted as cortical erosions by inexperienced operator eyes. Further, due to the peculiar vascularization of the epiphysis in children, that anastomoses with metaphyseal vessels through the growth plate, an inflammation affecting the epiphyseal cartilage may spread to the ossification center, causing excessive growth, deformities, or destruction with epiphyseal erosions rather than marginal erosions, unlike RA patients. These observations advise that further validation and large-scale studies are required to determine the potential role and the accuracy of MSUS in the detection of bone damage in children.
Advances and perspectives
At present, increasing interest in the use of MSUS during daily clinical practice is spreading among pediatric rheumatologists. Nonetheless, several topics are still under investigation or need to be further addressed. First of all, the anatomical specificities of the growing skeleton in children make imaging interpretation more challenging than in adults and thorough knowledge of the sonoanatomy changes at the different growth ages is essential to distinguish physiological appearance from pathological findings. Recently, definitions for gray scale sonographic features of joints in healthy children have been proposed and validated [60]. Sonographic appearance of tendons, entheses and Doppler MSUS in healthy children was not described and requires specific additional studies. Secondly, in the recent years several investigators proposed to assess children using standard scans according to the EULAR (European League Against Rheumatism) guidelines [61]. However, this scanning approach has been developed for MSUS examination of adults, and its applicability and reliability in children were not demonstrated. In the frame of the OMERACT Ultrasound pediatric subtask force, Collado et al. set up a standardized MSUS examination method specific for the pediatric population [12]. The study showed a high quality of images obtained by all the investigators, despite the use of different sonographic equipments, indicating suited methodology. MSUS standard scans proposed were appropriate and reproducible in children regardless of their age. Further, the standardized MSUS examination, together with the MSUS definitions of normal features in pediatric joints, enabled the investigators to achieve information on physiological blood flow and on age-related changes of bones in each joint. The next step would be standardization of findings in synovitis and in abnormal vascularization detected by Doppler, and is already ongoing.
Studies in adults with RA have recommended the use of reduced and simplified joint counts for MSUS assessment of disease activity, preventing in this way too long-lasting evaluations [62, 63]. This may be particularly relevant in children, since they notoriously are less tolerant than adults of undergoing investigations. Recently, some investigators proposed a reduced joint PD MSUS assessment and provided preliminary evidence of its validity, reliability, sensitivity to change and feasibility in evaluating synovitis in JIA [64]. Nonetheless, these results should be confirmed in independent cohorts with large number of patients. Additional studies in children with JIA in order to define the pattern and number of joints to be assessed with MSUS, both with grey scale and Doppler, are warranted.
Although there is an astounding interest in the application of MSUS in children, only few pediatric rheumatologists are able to perform MSUS on their own, as outlined by recent surveys [13, 14]. On the other hand, most of the respondents to the surveys stated their willingness to embrace this imaging modality and to gain experience in performing MSUS by themselves. Therefore, access to suitable training and mentorship for MSUS in children is of primary relevance. Further educational activities are required to enhance the use of this imaging modality in pediatric rheumatologists’ practice.
Conclusions
MSUS shows great promise in the assessment and management of children with JIA, so that it is likely to play an increasing role in clinical practice of pediatric rheumatologists, as in adult rheumatology. However, several issues, including the anatomical knowledge of sonographic changes over time and the underlying immunopathophysiology, the standardization and validation of MSUS in healthy children and in patients with JIA, are still under investigation. Currently, few pediatric rheumatologists are able to perform MSUS on their own. In the future further research and educational efforts are required for expanding this imaging modality to more clinicians in their daily practice.
Key points
-
MSUS can detect inflammatory changes more frequently than the clinical examination and can visualize their exact location in joints of children with different JIA ILAR subtypes, yielding important insights in the extent, pathophysiology and classification of childhood arthritis.
-
Due to the unique features of the growing skeleton, accurate knowledge of MSUS findings over time in children is warranted. Currently, validated definitions of MSUS features in joints of healthy children are available; standardization of scanning techniques specific for the pediatric population and definitions of MSUS pathological findings in children are ongoing.
-
Correlation of MSUS findings with biologic markers, clinical features and other imaging tools should be studied prospectively to investigate the clinical meaning and prognostic value of MSUS-detected abnormalities in different joints at different time points of the disease course.
-
Despite the astounding interest in the use of MSUS in children, only few pediatric rheumatologists currently perform MSUS on their own. Further educational activities for suitable training and mentorship in MSUS in children are advisable in the future.
Abbreviations
MSUS, musculoskeletal ultrasound; JIA, juvenile idiopathic arthritis; ERA, enthesitis-related-arthritis; PD, power Doppler; ILAR, International League of Associations for Rheumatology; RA, rheumatoid arthritis; IACI, intraarticular corticosteroid injection(s); TMJ, temporo-mandibular joint(s); MRI, magnetic resonance imaging; OMERACT, Outcome Measures in Rheumatology Clinical Trials; EULAR, European League Against Rheumatism.
References
Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767–78.
Kessler EA, Becker ML. Therapeutic advancements in juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol. 2014;28:293–313.
Webb K, Wedderburn LR. Advances in the treatment of polyarticular juvenile idiopathic arthritis. Curr Opin Rheumatol. 2015;27:505–10.
Hinze C, Gohar F, Foell D. Management of juvenile idiopathic arthritis: hitting the target. Nat Rev Rheumatol. 2015;11:290–300.
Naredo E, Wakefield RJ, Iagnocco A, et al. The OMERACT ultrasound task force--status and perspectives. J Rheumatol. 2011;38:2063–7.
Bruyn GA, Naredo E, Iagnocco A, et al. OMERACT Ultrasound Task Force. The OMERACT Ultrasound Working Group 10 Years On: Update at OMERACT 12. J Rheumatol. 2015;42:2172–6.
D’Agostino MA, Wakefield RJ, Berner-Hammer H, et al. OMERACT-EULAR-Ultrasound Task Force. Value of ultrasonography as a marker of early response to abatacept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results from the APPRAISE study. Ann Rheum Dis. 2015. doi:10.1136/annrheumdis-2015-207709.
Buchmann RF, Jaramillo D. Imaging of articular disorders in children. Radiol Clin North Am. 2004;42:151–68.
Babyn P, Doria AS. Radiologic investigations of rheumatic diseases. Pediatr Clin North Am. 2005;52:373–411.
Magni-Manzoni S, Malattia C, Lanni S, Ravelli A. Advances and challenges in imaging in juvenile idiopathic arthritis. Nat Rev Rheumatol. 2012;8:329–36.
Lanni S, Wood M, Ravelli A, Magni Manzoni S, Emery P, Wakefield RJ. Towards a role of ultrasound in children with juvenile idiopathic arthritis. Rheumatology. 2013;52:413–20.
Collado P, Vojinovic J, Nieto JC, et al. Omeract Ultrasound Pediatric Group. Toward Standardized Musculoskeletal Ultrasound in Pediatric Rheumatology: Normal Age-Related Ultrasound Findings. Arthritis Care Res. 2016;68:348–56.
Magni-Manzoni S, Collado P, Jousse-Joulin S, et al. Paediatric Ultrasound Group of the OMERACT Ultrasound Task Force. Current state of musculoskeletal ultrasound in paediatric rheumatology: results of an international survey. Rheumatology. 2014;53:491–6.
Lanni S, De Lucia O, Possemato N, Malattia C, Ravagnani V, Magni-Manzoni S. Musculoskeletal-ultrasound in paediatric rheumatology: the Italian perspective. Clin Exp Rheumatol. 2016;2016:28 [Epub ahead of print].
Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: 2nd revision, Edmonton, 2001. J Rheumatol. 2004;31:390–2.
Magni-Manzoni S, Epis O, Ravelli A, et al. Comparison of clinical versus ultrasound-determined synovitis in juvenile idiophatic arthritis. Arthritis Rheum. 2009;61:1497 504.
Haslam KE, McCann LJ, Wyatt S, Wakefield RJ. The detection of subclinical synovitis by ultrasound in oligoarticular juvenile idiopathic arthritis: a pilot study. Rheumatology. 2010;49:123–7.
Breton S, Jousse-Joulin S, Cangemi C, et al. Comparison of clinical and ultrasonographic evaluations for peripheral synovitis in juvenile idiopathic arthritis. Semin Arthritis Rheum. 2011;41:272–8.
Karmazyn B, Bowyer SL, Schmidt KM, et al. US findings of metacarpophalangeal joints in children with idiopathic juvenile arthritis. Pediatr Radiol. 2007;37:475–82.
Rooney ME, McAllister C, Burns JF. Ankle disease in juvenile idiopathic arthritis: ultrasound findings in clinically swollen ankles. J Rheumatol. 2009;36:1725–9.
Pascoli L, Wright S, McAllister C, Rooney M. Prospective evaluation of clinical and ultrasound findings in ankle disease in juvenile idiopathic arthritis: importance of ankle ultrasound. J Rheumatol. 2010;37:2409–14.
Jousse-Joulin S, Breton S, Cangemi C, et al. Ultrasonography for detecting enthesitis in juvenile idiopathic arthritis. Arthritis Care Res. 2011;63:849–55.
Weiss PF, Chauvin NA, Klink AJ, et al. Detection of enthesitis in children with enthesitis- related arthritis: dolorimetry compared to ultrasonography. Arthritis Rheumatol. 2014;66:218–27.
Shenoy S, Aggarwal A. Sonologic enthesitis in children with enthesitis-related arthritis. Clin Exp Rheumatol. 2016;34:143–7.
Giannini EH, Brewer EJ, Kuzmina N, et al. Methotrexate in resistant juvenile rheumatoid arthritis. Results of the USA–USSR double-blind, placebo-controlled trial. The Pediatric Rheumatology Collaborative Study Group and The Cooperative Children’s Study Group. N Engl J Med. 1992;326:1043–9.
Lovell DJ, Giannini EH, Reiff A, et al. Pediatric Rheumatology Collaborative Study Group. Etanercept in children with polyarticular juvenile rheumatoid arthritis. N Engl J Med. 2000;342:763–9.
Lovell DJ, Ruperto N, Goodman S, et al. Pediatric Rheumatology Collaborative Study Group; Pediatric Rheumatology International Trials Organisation. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359:810–20.
Lahdenne P, Vähäsalo P, Honkanen V. Infliximab or etanercept in the treatment of children with refractory juvenile idiopathic arthritis: an open label study. Ann Rheum Dis. 2003;62:245–7.
Ruperto N, Lovell DJ, Quartier P, et al. Paediatric Rheumatology INternational Trials Organisation; Pediatric Rheumatology Collaborative Study Group. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383–91.
Brunner H, Ruperto N, Tzaribachev N, et al. A148: a multi-center, double- blind, randomized-withdrawal trial of subcutaneous golimumab in pediatric patients with active polyarticular course juvenile idiopathic arthritis despite methotrexate therapy: week 48 results. Arthritis Rheum. 2014;66:S191–2.
Brunner HI, Ruperto N, Zuber Z, et al. Paediatric Rheumatology International Trials Organisation PRINTO; Pediatric Rheumatology Collaborative Study Group (PRCSG). Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomised, double-blind withdrawal trial. Ann Rheum Dis. 2015;74:1110–7.
Rebollo-Polo M, Koujok K, Weisser C, Jurencak R, Bruns A, Roth J. Ultrasound findings on patients with juvenile idiopathic arthritis in clinical remission. Arthritis Care Res. 2011;63:1013–9.
Collado P, Gamir ML, López-Robledillo JC, Merino R, Modesto C, Monteagudo I. Detection of synovitis by ultrasonography in clinically inactive juvenile idiopathic arthritis on and off medication. Clin Exp Rheumatol. 2014;32:597–603.
Bugni Miotto e Silva V, De Freitas Tavares da Silva C, De Aguiar Vilela Mitraud S, et al. Do patients with juvenile idiopathic arthritis in remission exhibit active synovitis on joint ultrasound? Rheumatol Int. 2014;34:937–45.
Magni-Manzoni S, Sciré CA, Ravelli A, et al. Ultrasound-detected synovial abnormalities are frequent in clinically inactive juvenile idiophatic arthritis, but do not predict a flare of synovitis. Ann Rheum Dis. 2013;72:223–8.
Sciré CA, Montecucco C, Codullo V, et al. Ultrasonographic evaluation of joint involvement in early rheumatoid arthritis in clinical remission: power Doppler signal predicts short-term relapse. Rheumatology. 2009;48:1092–7.
Peluso G, Michelutti A, Bosello S, et al. Clinical and ultra- sonographic remission determines different chances of relapse in early and long standing rheumatoid arthritis. Ann Rheum Dis. 2011;70:172–5.
McGonagle D, Benjamin M. Towards a new clinic-immunopathological classification of juvenile inflammatory arthritis. J Rheumatol. 2009;36:1573–4.
Rothmund F, Gerss J, Ruperto N, et al. Paediatric Rheumatology International Trials Organisation (PRINTO). Validation of relapse risk biomarkers for routine use in patients with juvenile idiopathic arthritis. Arthritis Care Res. 2014;66:949–55.
Świdrowska J, Smolewski P, Stańczyk J, Smolewska E. Serum Angiogenesis Markers and Their Correlation with Ultrasound-Detected Synovitis in Juvenile Idiopathic Arthritis. J Immunol Res. 2015;2015:741457.
Young CM, Shiels II WE, Coley BD, et al. US-guided corticosteroid injection therapy for juvenile idiopathic arthritis: 12-year care experience. Pediatr Radiol. 2012;42:1481–9.
Tynjälä P, Honkanen V, Lahdenne P. Intra-articular steroids in radiologically confirmed tarsal and hip synovitis of juvenile idiopathic arthritis. Clin Exp Rheumatol. 2004;22:643–8.
Laurell L, Court-Payen M, Nielsen S, et al. Ultrasonography and color Doppler in juvenile idiopathic arthritis: diagnosis and follow-up of ultrasound-guided steroid injection in the ankle region. A descriptive interventional study. Pediatr Rheumatol Online J. 2011;9:4.
Laurell L, Court-Payen M, Nielsen S, et al. Ultrasonography and color Doppler in juvenile idiopathic arthritis: diagnosis and follow-up of ultrasound-guided steroid injection in the wrist region. A descriptive interventional study. Pediatr Rheumatol Online J. 2012;10:11.
Habibi S, Ellis J, Strike H, Ramanan AV. Safety and efficacy of US-guided CS injection into temporomandibular joints in children with active JIA. Rheumatology. 2012;51:874–7.
Parra DA, Chan M, Krishnamurthy G, et al. Use and accuracy of US guidance for image-guided injections of the temporomandibular joints in children with arthritis. Pediatr Radiol. 2010;40:1498–504.
Fritz J, Pereira PL, Lewin JS. Temporomandibular joint injections: interventional MR imaging demonstrates anatomical landmark approach to be inaccurate when compared to direct visualization of the injectant. Pediatr Radiol. 2010;40:1964–5.
Spannow AH, Stenboeg E, Pfeiffer-Jensen M, Herlin T. Ultrasound measurement of joint cartilage thickness in large and small joints in healthy children: a clinical pilot study assessing observer variability. Pediatr Rheumatol Online J. 2007;5:3.
Spannow AH, Pfeiffer-Jensen M, Andersen NT, Stenbøg E, Herlin T. Inter- and intraobserver variation of ultrasonographic cartilage thickness assessments in small and large joints in healthy children. Pediatr Rheumatol Online J. 2009;7:12.
Spannow AH, Pfeiffer-Jensen M, Andersen NT, Herlin T, Stenbøg E. Ultrasonographic measurements of joint cartilage thickness in healthy children: age- and sex-related standard reference values. J Rheumatol. 2010;37:2595–601.
Panghaal V, Janow G, Trinh A, Ilowite N, Levin TL. Normal epiphyseal cartilage measurements in the knee in children: an alternative sonographic approach. J Ultrasound Med. 2012;31:49–53.
Spannow AH, Stenboeg E, Pfeiffer-Jensen M, Fiirgaard B, Haislund M, Ostergaard M, et al. Ultrasound and MRI measurements of joint cartilage in healthy children: a validation study. Ultraschall Med. 2011;32 Suppl 1:S110–6.
Pradsgaard DØ, Spannow AH, Heuck C, Herlin T. Decreased cartilage thickness in juvenile idiopathic arthritis assessed by ultrasonography. J Rheumatol. 2013;40:1596–603.
Pradsgaard DØ, Fiirgaard B, Spannow AH, Heuck C, Herlin T. Cartilage thickness of the knee joint in juvenile idiopathic arthritis: comparative assessment by ultrasonography and magnetic resonance imaging. J Rheumatol. 2015;42:534–40.
Magni-Manzoni S. Ultrasound measurement of cartilage thickness in childhood arthritis--target the tissue, tailor the technique. J Rheumatol. 2015;42:360–2.
Restrepo R, Lee EY, Babyn PS. Current practical imaging assessment with emphasis on magnetic resonance imaging. Radiol Clin N Am. 2013;51:703–19.
Graham TB. Imaging in juvenile arthritis. Curr Opin Rheumatol. 2005;17:574-8.
Malattia C, Damasio MB, Magnaguagno F, et al. Magnetic resonance imaging, ultrasonography, and conventional radiography in the assessment of bone erosions in juvenile idiopathic arthritis. Arthritis Rheum. 2008;59:1764–72.
Wakefield RJ, Balint PV, Szkudlarek M, et al. OMERACT 7 Special Interest Group. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–7.
Roth J, Jousse-Joulin S, Magni-Manzoni S, et al. Definitions for the sonographic features of joints in healthy children. Arthritis Care Res. 2015;67:136–42.
Backhaus M, Burmester GR, Gerber T, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001;60:641–9.
Naredo E, Gamero F, Bonilla G, Uson J, Carmona L, Laffon A. Ultrasonographic assessment of inflammatory activity in rheumatoid arthritis: comparison of extended versus reduced joint evaluation. Clin Exp Rheumatol. 2005;23:881–4.
Backhaus M, Ohrndorf S, Kellner H, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis Rheum. 2009;61:1194–201.
Collado P, Naredo E, Calvo C, ECO-JIA Study Group, et al. Reduced joint assessment vs comprehensive assessment for ultrasound detection of synovitis in juvenile idiopathic arthritis. Rheumatology. 2013;52:1477–84.
Acknowledgements
The author is grateful to the patients' guardians/parents for their consent to include images in the present review.
Funding
No funding supported the review.
Availability of data and materials
Data presented in the review are available in the references listed below.
Authors’ contributions
Silvia Magni-Manzoni conducted the literature review, drafted and reviewed the manuscript.
Competing Interests
The author has no competing interests.
Consent for publication
Written informed consent was obtained from the patients' guardians/parents for the publication of images in the present review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Magni-Manzoni, S. Ultrasound in juvenile idiopathic arthritis. Pediatr Rheumatol 14, 33 (2016). https://doi.org/10.1186/s12969-016-0096-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12969-016-0096-2