Abstract
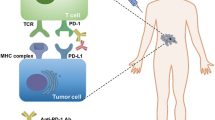
Although immune checkpoint inhibitors (anti-PD-1 antibody, anti-PD-L1 antibody, and anti-CTLA-4 antibody) have displayed considerable success in the treatment of malignant tumors, the therapeutic effect is still unsatisfactory for a portion of patients. Therefore, it is imperative to develop strategies to enhance the effect of these ICIs. Increasing evidence strongly suggests that the key to this issue is to transform the tumor immune microenvironment from a state of no or low immune infiltration to a state of high immune infiltration and enhance the tumor cell-killing effect of T cells. Therefore, some combination strategies have been proposed and this review appraise a summary of 39 strategies aiming at enhancing the effectiveness of ICIs, which comprise combining 10 clinical approaches and 29 foundational research strategies. Moreover, this review improves the comprehensive understanding of combination therapy with ICIs and inspires novel ideas for tumor immunotherapy.
Similar content being viewed by others
Introduction
Immunotherapy with checkpoint inhibitors has profoundly changed the landscape of treatment in oncology over the last decade as it has provided long-lasting responses and potential long-term remissions in numerous patients [1]. Increasing evidences has demonstrated that anti-programmed cell death 1 (PD-1) antibody, anti-programmed death ligand 1 (PD-L1) antibody, and anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibody, have revolutionized cancer treatment for various malignancies, enabling some patients to achieve long-term remission and survival [2,3,4,5,6,7].
However, only a small number of cancer patients could benefit from it due to low tumor mutation burden, mutations in critical anti-tumor pathways, immunosuppressive state of the tumor immune microenvironment, and the expression level of PD-L1. Therefore, it is necessary to develop a combination therapy strategy to overcome ICIs resistance and improve the efficacy of immunotherapy [8, 9].
Currently some combination strategies have been proposed to enhance the therapeutic efficacy of ICIs. In clinical practice, ICIs combined with chemotherapy, radiotherapy, antiangiogenic agents, epidermal growth factor receptor‑tyrosine kinase inhibitor (EGFR-TKI), other ICIs, vitamin E, ablation techniques, natural killer (NK) cells infusion, oncolytic virus therapy and interleukin(IL)-2 have been proved to be effective treatment strategies [10,11,12,13,14,15,16,17,18,19,20,21]. Furthermore, several methods are currently under development for combination therapy with ICIs including cytokines, cyclin dependent kinases inhibitors (CDKs), targeted signaling pathway inhibitors, ablation techniques, photothermal therapy (PTT), photodynamic therapy (PDT), focused ultrasound, vitamin C supplementation, antihistamines, metformin, nanoparticle-based therapies, modulation of the intestinal microbiome, alternative immunotherapies such as cancer vaccines and adoptive cell transfer therapy (ACT) and more [22,23,24,25,26,27,28,29,30,31,32,33,34,35].
Other potential strategies to enhance the effectiveness of ICIs include targeting innate immune pathways, induction of non-apoptotic regulated cell death (RCD) mechanisms, delivery of nitric oxide (NO), regulation of metabolic pathways, modulation of immune cell function, targeting hormone receptors, and intratumoral MgCl2 injection therapy [36,37,38,39,40,41,42]. Additionally, this review also discusses the potential benefits of targeting co-stimulatory and co-inhibitory receptors, fasting-mimicking and ketogenic diets (KD), epigenetic modulations and DNA damage response (DDR) regulators, tumor treating fields (TTFields), sonodynamic therapy (SDT), application of fucoidans, and radiation-nuclide guided local release of ICIs [43,44,45,46,47,48,49,50,51].
As a growing number of clinical and preclinical studies concerning the efficacy of combination therapy with ICIs, in order to explore these combination strategies systematically, a more comprehensive review on such studies is warranted. Therefore, this review would provide theoretical guidance for more effective individualized treatment strategies and inspires novel ideas in tumor immunotherapy, so as to improve the prognosis of patients.
Combined treatment strategies which have been applied in clinical practice
ICIs combined with chemotherapy
Chemotherapy not only inhibits DNA replication and synthesis in tumor cells but also enhances the efficacy of ICIs through immune mechanisms such as immunogenic cell death (ICD) and anti-angiogenesis [52,53,54]. Additionally, chemotherapy enhances antigen presentation, induces recruitment and differentiation of CD8+ T cells, and reduces the number of marrow derived suppressor cells (MDSCs) and regulatory T cells (Tregs) [55, 56].
Therefore, ICIs combined with chemotherapy is currently applied in many types of cancers such as breast cancer, colorectal cancer (CRC), gastric cancer or esophageal cancer, lung cancer, urothelial cancer, etc. [11, 53, 57,58,59,60,61]. Notably, in a randomized phase III study, the addition of nivolumab to carboplatin, paclitaxel, and bevacizumab significantly prolonged progression-free survival (PFS) in patients with untreated stage IIIB/IV or recurrent non-squamous non-small cell lung cancer (NSCLC) [12.1 months versus 8.1 months; hazard ratio (HR), 0.56; 96.4% confidence interval (CI) 0.43–0.71; P < 0.0001] [62]. Similarly, it was reported that adding a PD-L1 blocker to standard platinum plus etoposide is more effective than chemotherapy alone in the first-line treatment of small-cell lung cancer (SCLC) [63, 64]. Moreover, the atezolizumab plus nab-paclitaxel group achieved a longer median overall survival (OS) (7.2 months versus 8.1 months; 95% CI 0.69–0.92; P = 0.002) in the treatment of triple-negative breast cancer (TNBC) than the placebo plus nab-paclitaxel group [61]. Atezolizumab or pembrolizumab combined with platinum-based chemotherapy achieved better efficacy than monotherapy in patients with metastatic urothelial cancer [65,66,67]. Therefore, ICIs combined with chemotherapy significantly improved patient prognosis, and this combination therapy modality will be approved for more cancers and entry into first-line therapy.
ICIs combined with radiotherapy
Radiation therapy induces the release of new antigens from tumor cells that up-regulate the immunogenicity of the tumor microenvironment (TME) and promotes effector CD8+ T cell-mediated tumor cell killing, enhancing the efficacy of ICIs [68,69,70]. Additionally, radiation-damaged DNA released from tumor cells modulates adaptive immune responses by inducing interferon (IFN)-γ release from dendritic cells (DCs) [71].
The combination of radiotherapy and ICIs has achieved excellent progress in cancer treatment. It was showed that combined low-dose graded radiotherapy and anti-PD-1 antibody led to complete tumor regression in over 70% of colon cancer-bearing mice [72]. Furthermore, radiotherapy combination with anti-PD-1 antibody significantly prolonged OS and PFS, and increased objective response rate (ORR) compared with monotherapy in patients with NSCLC [13]. Radiotherapy also overcomes the immune resistance of ICIs by inducing inflammatory immune response and intratumor infiltration of CD8+ T cells in hepatocellular carcinoma (HCC) patients [73]. Similar synergistic effects have also been demonstrated in breast cancer, melanoma, nasopharyngeal carcinoma, CRC, renal cell carcinoma (RCC), recurrent diffuse intrinsic pontine glioma and brain metastases [74,75,76,77,78,79].
Hypofractionated radiotherapy may induce a stronger immune response than conventional fractionated radiotherapy [80]. As a type of hypofractionation high-dose radiotherapy, stereotactic body radiation therapy (SBRT) is particularly beneficial to the anticancer effect [81]. A phase II trial for NSCLC showed that patients treated with SBRT and pembrolizumab achieved relatively longer median PFS and median OS than those treated with pembrolizumab alone [82]. Besides, SBRT combined with ICIs has been shown to be effective and safe in the treatment of tumors such as HCC, cholangiocarcinoma and head and neck squamous cell carcinoma [83,84,85].
In conclusion, although there are still unresolved issues such as radiotherapy dose and segmentation modalities, sequencing of combination therapy, and selection of ICIs, ICIs combined with radiotherapy still holds considerable promise for application in cancer treatment. This combination therapy strategy has the potential to be widely used in clinical practice, provided that more preclinical and clinical trials are needed to offer definitive evidence and address the challenges described above.
ICIs combined with epidermal growth factor receptor‑tyrosine kinase inhibitor
Epidermal growth factor receptor (EGFR) belongs to the erythroblastic oncogene B family and plays an important role in the occurrence and development of a variety of cancers [86]. Multiple EGFR-targeted agents improve major histocompatibility complex (MHC)-I expression, enhance DCs antigen presentation, and could initiate T cells and promote NK cells activation even in the absence of additional immune stimulatory signals [87, 88].
EGFR-TKI upregulates PD-L1 expression while initiating T cells to enhance the efficacy of immune checkpoint therapy in EGFR mutant cancer patients [89, 90]. Treatment with EGFR-TKIs enhances PD-L1 expression, and in clinical practice, the combination of EGFR-TKI and anti-PD-1 antibody in NSCLC patients with high PD-L1 expression has a longer median PFS (7.1 months versus 1.7 months; P = 0.0033) compared to NSCLC patients with low PD-L1 expression [16]. Furthermore, in a phase I trial, nivolumab plus erlotinib not only achieved durable anti-tumor responses but also tolerable adverse effects in the treatment of patients with EGFR-mutant advanced NSCLC [91].
Therefore, the combination of EGFR-TKIs with ICIs is a promising strategy, and the development of new biomarkers will allow combination of ICIs with EGFR-TKIs more effectively. Besides, it is necessary to investigate the effectiveness of this combination therapy for other types of cancer.
ICIs combined with antiangiogenics
Antiangiogenic drugs work by blocking the vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) signaling pathway, leading to reduced blood supply to tumor tissues, hypoxia, and a decrease in nutrient supply to cancer tissues [92,93,94]. Furthermore, normalized blood vessels could regulate TME by promoting CD8+ T cells infiltration, inducing M1 tumor associated macrophages (TAM), and reducing the accumulation of Tregs and MDSCs, thus enhancing the effect of ICIs [95, 96].
The combination of antiangiogenic drugs with ICIs has shown promising effect in HCC, NSCLC, RCC, etc. [92, 97,98,99]. In a clinical trial of pembrolizumab in combination with axitinib for the treatment of advanced RCC, after a median follow-up of 12.8 months, the estimated percentage of patients alive at 12 months was 89.9% in the pembrolizumab plus axitinib group and 78.3% in the sunitinib group (HR, 0.53; 95% CI 0.38–0.74; P < 0.0001) [14]. Pembrolizumab plus axitinib also achieved a longer median PFS (15.1 months versus 11.1 months; HR, 0.69; 95% CI 0.57–0.84; P < 0.001) and higher ORR [59.3% (95% CI 54.5–63.9) versus 35.7% (95% CI 31.1–40.4); P < 0.001] than the sunitinib group [14]. Based on the results of this trial, pembrolizumab in combination with axitinib was approved by the U.S. Food and Drug Administration (FDA) as a first-line treatment option for advanced RCC in 2019 [100]. Besides, based on the results of the CheckMate-9ER and JAVELIN Renal 101 trials, nivolumab plus cabozantinib and avelumab plus axitinib were also approved by the FDA as first-line treatment options for RCC [101, 102]. Moreover, the FDA approved atezolizumab in combination with bevacizumab for the treatment of advanced HCC based on trial results from IMbrave150 [103]. Furthermore, pembrolizumab plus lenvatinib has been approved for the treatment of advanced endometrial cancer [104].
Tumor vascular normalization promotes the aggregation of immune cells and enhances immune function [92]. In turn, immune cell activation promotes vascular normalization [105]. Therefore, the combination of antiangiogenic drugs and ICIs is complementary in tumor therapy and could be regarded as a promising strategy to enhance the therapeutic effect of ICIs. However, further studies are needed to determine the sequence and dose of administration, as well as more precise biomarkers to select for benefit populations to optimize the efficacy, resistance, and adverse effects of combination therapy.
ICIs combined with ICIs
Recent research has demonstrated that dual ICIs therapy is more effective than monotherapy, albeit with an increased incidence of toxicity [106]. Table 1 presents clinical studies of ICIs combine with ICIs.
The FDA has currently approved the combination of dual ICIs for treating various solid tumors such as HCC, melanoma, RCC, microsatellite instability-high/mismatch repair-deficient metastatic CRC (MSI-H/dMMR mCRC), NSCLC, etc. [12, 15, 107,108,109]. A study on advanced melanoma showed that the median OS was greater than 60 months (95% CI 38.2 to not reached) in the nivolumab plus ipilimumab group versus 36.9 months (95% CI 28.2–58.7) in the nivolumab group or 19.9 months (95% CI 16.8–24.6) in the ipilimumab group [15]. Furthermore, nivolumab plus ipilimumab group demonstrated a longer median PFS compared to the nivolumab group and the ipilimumab group [11.5 months (95% CI 8.7–19.3) versus 6.9 months (95% CI 5.1–10.2) versus 2.9 months (95% CI 2.8–3.2)] in treating advanced melanoma [15]. Notably, lower-dose ipilimumab plus nivolumab resulted in a higher OS rate and 12-months PFS rate than nivolumab or ipilimumab alone (OS rate: 85% versus 73% versus 72%; 12-months PFS rate:71% versus 50% versus 34%) in MSI-H/dMMR mCRC patients [108]. Besides, durvalumab plus tremelimumab has demonstrated better PFS [3.8 months (95% CI 3.7–5.3) versus 3.7 months (95% CI 3.2–3.8)], and ORR (20.1% versus 17.0%) compared to durvalumab monotherapy in the treatment of HCC [110]. Moreover, a meta-analysis on ICIs combination therapy for NSCLC revealed that a combination of anti-CTLA-4 antibody and anti-PD-1 antibody significantly improved OS (HR, 0.63; 95% CI 0.44–0.82; P < 0.001), PFS (HR, 0.56; 95% CI 0.44–0.69; P = 0.002), and ORR (HR, 1.31; 95% CI 0.92–1.71; P < 0.001) compared to monotherapy [111].
Lymphocyte activation gene-3 (LAG-3) is an inhibitory receptor expressed on T cells, it coregulates T-cell function with PD-1, which suggests that combined anti-LAG-3 antibody and anti-PD-1 antibody therapy may be effective [112, 113]. In patients with previously untreated metastatic or unresectable melanoma, the combination of relatlimab and nivolumab demonstrated superior median PFS [10.1 months (95% CI 6.4–15.7) versus 4.6 months (95% CI 3.4–5.6)] and 12-month PFS rate [47.7% (95% CI 41.8–53.2) versus 36.0% (95% CI 30.5–41.6)] compared to the monotherapy group treated with nivolumab alone [44]. In another study, a soluble LAG-3 protein (eftilagimod α) combined with pembrolizumab was observed to achieve an ORR of 33% in melanoma patients [114].
Notably, the sequence of ICIs administration may impact their efficacy in the combination strategy of ICIs [115]. A melanoma clinical trial indicated that patients who received anti-CTLA-4 antibody followed by anti-PD-1 antibody exhibited significantly higher survival rates than those treated with other regimens [116].
Using dual ICIs simultaneously or different orders of use could further enhance efficacy and prolong OS, but they could also result in toxic side effects. Currently, more combinations have been developed with fewer toxic side effects, which is a promising treatment strategy.
ICIs combined with vitamin E
Vitamin E is a fat-soluble antioxidant known to enhance human immune responses by modulating various immune cells such as macrophages, NK cells, DCs, T cells and B cells [117]. Specifically, vitamin E could enter DCs and bind to SHP1 to inhibit its protein activity, thereby restoring the function of DCs in initiating T cells responses to enhance the therapeutic effect of ICIs [23]. In a study of melanoma patients receiving anti-PD-1 antibody or anti-PD-L1 antibody, those who also took vitamin E showed significant improvement in OS (HR, 0.7; 95% CI 0.53–0.92; P < 0.05) [23]. These findings suggest that the use of vitamin E as a dietary supplement to improve the efficacy of ICIs is a promising strategy, but the mechanisms involved need to be further investigated. Moreover, such studies on other type of tumors need to be further observed.
ICIs combined with NK cells infusion
NK cells are innate lymphocytes that could identify and eliminate virus-infected or tumor cells [118]. In a clinical trial for advanced NSCLC, the combination therapy of pembrolizumab and NK cells demonstrated longer median OS (15.5 months versus 13.3 months; P < 0.05) and median PFS (6.5 months versus 4.3 months; P < 0.05) compared to pembrolizumab monotherapy [40]. Similarly, the combined treatment of ex vivo activated and expanded NK cell therapy (SNK01) and pembrolizumab demonstrated higher ORR (41.7% versus 0%) and median PFS (6.2 months versus 1.6 months; P = 0.001) compared to pembrolizumab monotherapy in treating NSCLC [19]. NK cells infusion is a promising treatment strategy, but more clinical trials are needed to clarify the efficacy and safety of this strategy for various tumors.
ICIs combine with oncolytic virotherapy
Oncolytic virus is a kind of virus that preferentially infects and proliferates in tumor cells, eventually leading to tumor cell lysis and death [119]. Oncolytic virus prepared by transgenic approach could also transform TME into an immune activated state, thus enhancing the therapeutic effect of ICIs [120].
Oncolytic viruses also changed the suppressive state of TME and increased CD8+ T cells infiltration to enhance the therapeutic efficacy of ICIs [121]. MJX-594 is an oncolytic vaccinia virus that targets GM-CSF and induces intratumoral invasion of CD8+ T cells by intratumoral injection [122]. In a mouse model of spontaneous breast cancer, triple immunotherapy wit MJX-594, anti-PD-1 antibody and anti-CTLA-4 antibody significantly reduced the overall tumor burden by 48.1%, resulting in more effective anti-cancer immunotherapy [35]. Similarly, the combination of JX and anti-PD-1 antibody or anti-PD-L1 antibody not only inhibited the occurrence of colonic peritoneal carcinoma, but also increased the tumor growth inhibition rate of anti-PD-1 antibody or anti-PD-L1 antibody alone from 15.9% to 86.3% [123]. Intratumoral injection of an engineered oncolytic virus (talimogene laherparepvecz) in combination with anti-PD-1 antibody resulted in favorable ORR [61.9% (95% CI 38.4–81.9%)] and complete response rate [33.3% (95% CI 14.6–57.0%)] in patients with advanced melanoma reported by a phase Ib trial [20]. Besides, ICIs combined with oncolytic viruses have shown success in bladder cancer, prostate cancer, pancreatic ductal adenocarcinoma (PDAC), and CRC in current clinical trials [124,125,126,127].
Consequently, oncolytic viruses promote anti-tumor immune responses by inducing chemokines and cytokines to turn “cold” tumors into “hot” tumors and further enhance the efficacy of immunotherapy. Oncolytic virus therapy for malignant tumors has abundant preclinical and clinical evidence, and its development prospects are broad, especially in combination with ICIs.
ICIs combined with ablation
Thermal ablation, such as radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation, induce tissue damage to kill tumor cells by creating extreme temperatures within the tissue [128,129,130]. In addition, irreversible electroporation (IRE) is an ablation technique that uses electrical pulses to destroy tumor cells [131].
Similar to other immunotherapy combinations, the addition of ablation to ICIs appeared to elicit a more effective immune response than ICIs alone [132,133,134,135]. RFA enhanced T cells infiltration and improved the survival of mice with CRC when combined with anti-PD-1 antibody [24]. Furthermore, tremelimumab plus RFA recruited more CD8+ T cells in patients with advanced HCC [18]. In addition, the combination of MWA and ICIs enhanced CD8+ T cells infiltration and significantly increased the survival rate of 4T1 (breast cancer cell lines) tumor-bearing mice [134]. Notably, cryoablation cools tumor tissues to approximately – 160 ℃, causing intracellular ice crystal formation, dehydration and rupture of tumor cells, destruction of small blood vessels within tumors [130]. The combination of cryoablation and PD-1 inhibitor polarized macrophages from M2 phenotype to M1 phenotype and increased the proportion of CD4+ T cells in patients with advanced solid cancers, which enhanced anti-tumor immunity [135]. Besides, treatment with low-dose anti-CTLA-4 antibody and cryoablation reduced mortality and inhibited distal tumor growth in mice with prostate cancer [133]. Moreover, IRE combined with low-dose anti-CTLA-4 antibody treatment resulted in complete tumor regression in 46% of the mice, compared with the 15.3% observed with anti-CTLA-4 antibody alone in a mouse model of prostate cancer [136].
Consequently, the synergistic effect of combining ablation with ICIs has been shown to enhance the immune response, effectively suppress tumor recurrence and metastasis, and additionally eradicate residual tumor tissue following ablation. However, more studies are needed to observe the feasibility of the combination and its efficacy in other tumor types. In addition, how to effectively combine therapeutic modalities to obtain optimal efficacy and minimize side effects is a clinical problem that needs to be addressed urgently.
ICIs combined with interleukin-2
IL-2 is a γ-chain cytokine that plays a significant role in proliferating T cells [137]. Combination therapy with anti-PD-1 antibody and IL-2 has been demonstrated to result in increased numbers of CD8+ T cells and greater secretion of IFN-γ and tumor necrosis factors-α [17]. A super mutant IL-2 could enhance the tumor control effect of anti-PD-L1 antibody in melanoma [138]. In addition, combination treatment of IL-2 with anti-PD-1 antibody and anti-CTLA-4 antibody recruited large numbers of CD8+ T cells in a mouse model of breast cancer [139]. Besides, high-dose IL-2 combined with pembrolizumab achieved a high ORR [70% (95% CI 0.50–0.86)] in the treatment of metastatic clear cell RCC [140]. However, it is a future endeavor to develop novel IL-2 formulations that are stable and target CD8+ T cells, and their dosage selection is an issue that needs to be further investigated in therapy, as natural IL-2 has a shorter half-life and is more inclined to activate Tregs. Meanwhile, such studies of this combination for other types of tumors need to be further conducted.
Combined treatment strategies which are under preclinical investigation
ICIs combined with photothermal therapy
PTT generates a thermal ablation effect on tumor cells through the use of nanoparticle-based photoabsorbents and wavelength-matched light sources to generate heat, resulting in targeted and controllable cytotoxicity and long-lasting immunogenicity [141, 142].
PTT could transform the TME into an immune-activated state, thereby sensitizing tumors to ICIs and producing synergistic anti-tumor effects [143]. Recombinant mouse programmed death receptor 1 protein combined with PTT has been shown to have therapeutic effects on mice inoculated with CT26 (colon cancer cell lines) or 4T1 tumors and therefore inhibit the growth of lung metastases after reinoculation of the same tumor cells [25]. Artificially controlling near infrared radiation to regulate the release of anti-PD-L1 antibody and induce the invasion of tumor infiltrating lymphocyte (TILs) could inhibit not only the progression of primary tumor but also the growth of distal tumor in 4T1 tumor-bearing mouse model [143]. Therefore, PTT combined with ICIs has a synergistic anti-tumor effect, but due to the limited penetration of light into human tissues, the efficacy of this combination for deep tumors may be affected [144]. Moreover, more studies are needed to prove the efficacy and safety of the combination in the treatment of other types of tumors. Notably, achieving a perfect temperature-response relationship is crucial for PTT and could indirectly impact the therapeutic efficacy of ICIs.
ICIs combined with photodynamic therapy
PDT uses light-activated photosensitive drugs to irradiating the tumor tissue area to produce reactive oxygen species, which directly produces cytotoxicity and enhances immunogenicity [145, 146]. Additionally, PDT could enhance immune response by releasing inflammatory mediators [147].
PDT induced tumor associated antigen to produce and promote immune cell infiltration, make cancer cells more sensitive to ICIs [147]. PD-L1 blockers combined with visible light-triggered prodrug nanoparticles have been shown to greatly inhibit CRC growth, recurrence, and lung metastasis by initiating a robust anti-tumor immune response in mice [148]. Besides, in a mouse model of E0771 (a bone marrow breast cancer cell lines) tumors, an implantable microfiber device with both anti-PD-1 antibody or anti-CTLA-4 antibody delivery and photodynamic therapy induced massive CD8+ T cells infiltration and cured all mice within 60 days while also measuring tumor impedance [149]. In addition, the combination of PDT and ICIs has yielded better results in the treatment of breast cancer, melanoma, CRC and RCC in mice [150,151,152,153].
It is concluded that the cytotoxicity induced by PDT reprograms TME, and the combined treatment strategy with ICIs not only treats the primary tumor but also inhibits distant metastasis. However, due to oxygen is necessary for PDT, the combined treatment strategy in hypoxic tumor response rates may be lower [144]. Furthermore, local delivery of PDT via nanoparticles allows precise control of dose and delivery site to further enhance therapeutic efficacy.
ICIs combined with cyclin-dependent kinase inhibitors
CDKs are a family of serine/threonine kinases that regulate cell cycle progression and other cellular processes [154, 155]. Abnormal activation of CDKs has been found to be intimately linked to tumor formation and progression, so CDKs may serve as potential targets for cancer therapy [156,157,158]. CDK4/6 inhibitor abemaciclib was reported to induce immune infiltration of CD8+ T cells and B cells, and recruit more lymphocytes [159]. In a mouse model of NSCLC, combination therapy with CDK4/6 inhibitor THZ1 and anti-PD-1 antibody significantly reduced tumor burden compared to treatment with either the CDK4/6 inhibitor or the anti-PD-1 antibody alone [22]. CDK4/6 inhibitors have also been shown to be justified in combination with anti-PD-1 antibody or anti-PD-L1 antibody for cancer treatment in preclinical studies of melanoma and CRC [160, 161].
Consequently, CDK inhibitors provide a promising approach to enhance anti-tumor immunity in vivo by recruiting and activating CD8+ T cells, thus alleviating resistance to ICIs. But the combination of side effects, such as lung toxicity and kidney toxicity and neutropenia may limit the clinical application of the composite [162]. However, whether CDKs inhibitors could be used as a way to enhance the effect of ICIs needs to be verified in in more preclinical and clinical studies in the future.
ICIs combined with focused ultrasound
Thermal high intensity focused ultrasound destroys tumor tissue by generating a thermal effect from acoustic energy [163]. However, pulsed high-intensity focused ultrasound (P-HIFU) and mechanical high-intensity focused ultrasound (M-HIFU) cause cell death by generating cavitation bubbles, resulting in mechanical effects that destroy the tumor tissue [164, 165].
HIFU treatment increases the infiltration and activation of CD4+ and CD8+ T cells, which may enhance the anti-tumor immune response [166]. In a mouse model of orthotopic pancreatic, P-HIFU plus ICIs activates CD8+ T cells to kill tumor cells and extended survival in mice compared to untreated subjects or P-HIFU or ICIs alone [167]. M-HIFU was reported to induces repolarization of TAM to M1 phenotype, infiltration of CD4+ T cells and CD8+ T cells [26]. When combined with anti-PD-L1 antibody, it enhances systemic anti-tumor immune response and inhibits distant metastasis of breast cancer in mice [26]. Besides, ultrasonic-targeted microbubble destruction is an emerging and effective technique that uses ultrasonic cavitation to destroy tumor blood vessels while impeding tumor angiogenesis [168]. Low-intensity focused ultrasound-targeted microbubble destruction not only reduced tumor tissue blood perfusion but also induced ICD of tumor cells, infiltration of CD8+ T cells and DCs [169]. It significantly inhibited tumor growth in combination with anti-PD-L1 antibody in a 4T1 tumor mouse model [169].
Therefore, focused ultrasound improves the therapeutic effect of ICIs by activating anti-tumor immunity and combining with ICIs, which has significant therapeutic value and application potential. Moreover, further studies on the effects of combining focused ultrasound with ICIs need to be conducted in a wider range of tumors.
ICIs combined with vaccination
Cancer vaccines primarily aim to enhance the immune response of tumor-specific CD8+ T cells [170, 171]. This approach compensates for the insufficient number of CD8+ T cells and improves therapeutic efficacy, making it a promising therapeutic strategy.
Predefined shared antigen vaccines are composed of antigens that are co-expressed in a large number of patients and could be used directly on patients who express this antigen [172]. In a mouse model expressing P1A, ChAdOx1/MVA MAGE vaccine targeted MAGE-type tumor shared antigen, enhanced the infiltration level of CD8+ T cells, significantly reduced mastocytoma growth and with a longer duration of survival when combined with anti-PD-1 antibody [173]. In addition, it was showed that durvalumab plus folate receptor alpha vaccine (TPIV200) increased T-cell response (P < 0.0001) and safety in patients with advanced ovarian cancer [28]. Therefore, predefined shared antigen vaccines not only enhance the immune recognition and attack of tumor cells in patients, but also further enhanced the efficacy of ICIs.
Vitro antigen vaccines are extracted from tumor tissues or cells, further processed into a more antigenic form, and then injected into the body as a vaccine and co-localized with antigen presenting cells (APC) to improve antigen presentation [172]. Researchers developed a tumor vaccine fused with autologous myeloma cells and DCs, and combination therapy with anti-PD-1 antibody further enhanced tumor vaccine-induced cytotoxic T lymphocytes (CTLs) activation in myeloma patients [174].
In situ antigen vaccines stimulate the body’s APC to present tumor antigens by intratumoral administration, causing systemic immune responses that enhance systemic anti-tumor effects [172]. Riboxxim is an immunostimulant, encapsulated in poly(lactic-co-glycolic acid) particles with antigens that acts synergistically with anti-CTLA-4 antibody to enhance enhances tumor-specific CD8+ T cells responses and prolong the survival of thymoma mice [175]. Additionally, the use of heterologous priming and boosting vaccines targeting CD4+ T cell epitopes primarily induced tumor-specific TH1 responses, increased infiltration of CD8+ T cells, and enhanced anti-tumor immune responses, which were further activated by administering anti-PD-L1 antibody [176].
The combination of cancer vaccines and ICIs activates antigen presentation and generates a stronger systemic anti-tumor immune response, resulting in a synergistic effect and improved therapeutic efficacy. Moreover, it is necessary to pay further attention to the enhancement effect of vaccination on ICIs in more tumor types.
ICIs combined with vitamin C
The anti-tumor effect of vitamin C is reflected in various aspects, including the regulation of the immune, metabolic, hypoxic, and microbial microenvironments [177]. Vitamin C could increase T cells infiltration in the TME, induce M2 TAM apoptosis, inhibit epithelial-mesenchymal transition (EMT), and regulates epigenetic mechanisms [178,179,180]. Additionally, vitamin C regulates energy metabolism in tumor cells and adjusts mechanical signals from stromal cells and the extracellular matrix to inhibit tumor invasion and metastasis [181, 182]. Furthermore, vitamin C regulates the composition and metabolites of intestinal microbiota and enhances the immunogenicity of tumors [183]. It is important to note that high doses of vitamin C not only inhibit angiogenesis but also promote oxidative stress leading to tumor cell death [184, 185].
In a lymphoma mouse model, high-dose vitamin C significantly enhanced the infiltration of CD8+ T cells and macrophages while synergistically acting with ICIs significantly inhibited tumor growth compared to monotherapy [27]. Similarly, triple therapy with vitamin C plus anti-PD-1 antibody plus anti-CTLA-4 antibody inhibited tumor growth and further enhanced tumor aggressive CD8+ T cells and anti-tumor immunity according to PDAC and breast cancer mouse models [178].
Consequently, vitamin C improves the efficacy of ICIs by improving the immunosuppressive state of TME. Vitamin C has great potential as an adjuvant for ICIs due to its low cost and lack of toxicity [185]. However, the mechanism and clinical benefits of vitamin C in immunotherapy need to be observed in different tumor types.
ICIs combined with antihistamines
Histamine is a histidine metabolite that is released by mast cells in response to inflammation, allergic reactions and tissue damage [186]. Besides, histamine is often elevated in cancer patients as a result of upregulation of the enzyme l-histidine decarboxylase [187].
Targeting histamine receptor H1 with antihistamines is associated with the infiltration of CD8+ T cells, induces the polarization of TAM to M1 phenotype [188]. Moreover, antihistamines combined with anti-PD-1 antibody or anti-CTLA-4 antibody significantly inhibited breast cancer and melanoma tumor growth in mice [188]. Notably, histamine dihydrochloride is used to inhibit NADPH oxidase, which further inhibits the aggregation of MDSCs in tumors [29]. This inhibition contributes to the enhanced anti-tumor efficacy of both anti-PD-1 antibody and anti-PD-L1 antibody in the EL4 (lymphoma cell lines) and MC38 (colon cancer cell lines) tumor-bearing mouse models [29].
Therefore, antihistamines could be used as promising therapeutic strategies to restore T-cell dysfunction and enhance immunotherapeutic response. In future, more studies are needed to determine the efficacy and safety of antihistamines as adjuvant therapy.
ICIs combined with metformin
Metformin could enhance the efficacy of ICIs by regulating intestinal microorganisms and their metabolites, inducing the production and activation of T cells, reducing the expression level of PD-L1, and exerting direct anti-tumor effects [189]. Besides, metformin ameliorated the metabolic dysfunction of CD8+ T cells caused by non-alcoholic steatohepatitis (NASH) and restored the therapeutic effect of anti-PD-1 antibody in NASH-induced HCC [190]. Moreover, metformin in combination with pembrolizumab resulted in an increase in CD8+ T cells and reduced the volume of STK1-mutant lung cancer [30].
Therefore, metformin shows potential therapeutic effects against tumors and has the potential to be a strategy to enhance the efficacy of ICIs therapy. However, it is important to identify the population that will benefit from metformin combined with ICIs. Moreover, the therapeutic value of the combination of metformin and ICIs needs to be clarified in a wider range of cancer types and in larger prospective clinical trials.
ICIs combined with adoptive cell transfer therapy
ACT is an infusion of autologous immunologic effector cells that are activated and amplified in vitro and rely on highly active tumor-specific CD8+ T cells, including chimeric antigen receptor (CAR)-T cell therapy and T cell receptor (TCR)-T cell therapy [191].
CAR-T cells
CAR-T therapy takes T cells from the patient, genetically modifying them to express CARs, then proliferating and transfusing them back into the patient, which binding to target cells and ultimately destroying them [192].
The combination of ICIs and CAR-T therapy has been shown to be more effective than monotherapy in solid tumors [32, 193]. Implantation of a human chondroitin sulfate proteoglycan 4 CAR-T cells conjugated with an anti-PD-L1 antibody into the residual cavity of melanoma mice after tumor resection inhibited tumor recurrence in situ and distant tumor growth [32]. In addition, the addition of hyaluronidase on the surface of CAR-T cells to assist anti-PD-L1 antibody to penetrate lymphoma and improve the efficacy of anti-tumor therapy [193].
Therefore, the combination of ICIs and CAR-T therapy could achieve synergistic anti-tumor effects. However, the efficacy of combined ICIs needs to be confirmed in more tumor types, and the toxicity of the treatment should be taken into account to determine the maximum safe dose of the treatment. In addition to CAR-T, CAR-NK is also a highly promising therapeutic modality, and several studies of CAR-NK cells are underway.
TCR-T
TCR-T cells are engineered TCRs that activate anti-tumor immunity to enhance the therapeutic efficacy of ICIs by recognizing extracellular or intracellular tumor-specific antigens presented by MHC [194]. In a P815 (mastocytoma cell lines) mouse model expressing P1A antigen, P1A tumor antigen-specific TCR-T cells producing IL-7/C–C chemokine ligand (CCL) 9 suppressed PD-1 expression and overcame CD8+ T cells depletion in TME, and the combination therapy with anti-PD-1 antibody further induced tumor regression and durable immune memory [195].
Although TCR-T, which recognizes intracellular antigens, is more advantageous than CAR-T in the treatment of solid tumors, evidence of the efficacy of combination therapies with TCR-T and ICIs in other tumor types is still lacking. Since TCR-T combined with ICIs brings new hope to cancer patients, it is necessary to conduct more studies on TCR-T combined with ICIs in a variety of tumors in the future.
ICIs combine with nanoparticle
Nanoparticles have great application potential in cancer treatment, which could be combined with chemotherapy, PTT, radiotherapy and other treatment methods or drugs to construct treatment strategies or models, enhance the therapeutic effect and reduce side effects [196, 197]. Table 2 presents preclinical studies of ICIs combine with nanotherapy. Therefore, nanotechnology could be used as a multifunctional platform in cancer therapy to supplement the deficiencies of various therapies. In addition, the combination of various therapies based on nanotechnology platforms with ICIs therapy not only effectively eliminates the primary tumor, but also has an excellent inhibitory effect on distant metastasis and prevent recurrence.
Targeting cancer cells
Nanoparticle-based chemical or physical ICD inducers are superior to free drugs in terms of anti-tumor efficiency, and their combination with ICIs could achieve better tumor treatment effect [196]. High-density lipoprotein mimicking nanosheets loaded with the doxorubicin triggered ICD in cancer cells and combined with anti-PD-1 antibody resulted in complete tumor cure in 80%–88% of CT26 and MC38-bearing animals [31]. Besides, a radioimmunostimulator nanomaterial (IPI549@HMP) could achieve the reduction of hypoxic, and IPI549@HMP-augmented radiotherapy increase the sensitivity of anti-PD-L1 antibody treatment of CRC in mice [198]. In addition, treatment with anti-PD-1 antibody alone in mice with breast cancer had almost negligible effects, whereas the addition of photothermal therapy-based nanocatalysis showed significant tumor suppression [199]. Furthermore, magnetic nanoclusters with responsive anti-PD-1 antibody enable the combination of ACT and nanotherapy with superior efficacy and manageable side effects in the treatment of solid tumors [200]. Moreover, nanotherapy based on ultrasound-guided therapy combined with ICIs treatment improved the median survival time of breast cancer-bearing mice by 76% and further inhibited primary tumor growth and distant metastasis compared with monotherapy [201].
Therefore, nanoparticle-based chemical or physical ICD inducers not only enhance the therapeutic efficacy of ICIs but also enable more precise local delivery and reduce toxic side effects.
Targeting the tumor immune microenvironment
Nano-drugs regulating TME are designed to relieve the immunosuppression in TME and enhance the infiltration and activation of effecting immune cells, thus improving the effect of immunotherapy [202]. PD-L1/Toll-like receptors (TLR) 7 dual-targeting nanobody-drug conjugate composed of anti-PD-L1 nanobody and TLR7 agonists reshaped tumor immune microenvironment, increase CD8+ T cells infiltration and stimulated NK cells activation [203]. Nanovesicles derived from M1 macrophages induced M2 TAM polarization to M1 phenotype, and combined with anti-PD-L1 antibody, the tumor growth was significantly inhibited and the drug resistance of anti-PD-L1 antibody treatment was alleviated in a CT26 tumor-bearing mouse model [204]. Moreover, gadofullerene nanoparticles also induced the polarization of TAM to M1 phenotype, overcame CD8+ T cells depletion in TME and showed synergistic anti-tumor effect with anti-PD-L1 antibody in a 4T1 tumor mouse model [205]. In conclusion, this strategy has the potential to be an effective strategy for collaborative ICIs. However, the combination of nanotherapeutics and ICIs for more types of tumor treatment still needs to be further investigated.
Targeting the peripheral immune system
Nano vaccine mediated immunotherapy could activate the immune response in the body through the delivery of exogenous antigens, and protect the antigens from degradation to achieve tumor treatment, and play a better role in immune activation [206]. Bi-adjuvant neoantigen nanovaccine has been developed to enhance neoantigen immunogenicity and antigen presentation, and combined treatment with anti-PD-1 antibody allowed complete tumor regression of 70% of CRC mice [207]. Moreover, OVAPEP-SLNP@CpG is another nanovaccine that enhances antigen presentation, induces DCs maturation and CD8+ T cells activation, and has significant anti-tumor efficacy and prevents tumor recurrence in combination with anti-PD-1 therapy in E.G7-OVA (T lymphoma cell lines) tumor-bearing mice [208]. However, more such studies are needed to validate the effectiveness of the combination.
ICIs combine with cytokines
In the TME, NK cells and macrophages release various cytokines that promote immune response, anti-tumor growth, and tumor cell apoptosis [209, 210]. And many cytokine drugs have been developed for cancer treatment [211]. However, both ICIs and cytokine monotherapy still face significant limitations, and combination therapy is becoming more and more important to improve the application rate of both. Therefore, cytokine therapy could be applied as a way to unlock the potential of ICIs and help patients with drug resistance to benefit from it.
Interleukin
Interleukins are lymphokines that interact between leukocytes or immune cells and play an important role in the activation and regulation of immune cells, mediating T and B cell activation, proliferation and differentiation, and the inflammatory response [212]. IL-6 is an essential cytokine for the differentiation of primitive CD4+ T cells into Th17 cells [213]. In a CT26 tumor mouse model, the addition of an IL-6 blocker to anti-CTLA-4 antibody therapy resulted in significant tumor shrinkage, with a cure rate of 32% in mice treated with anti-CTLA-4 antibody monotherapy versus 48% in mice treated with combination therapy [214]. In addition, similar results were observed in PDAC, CRC and HCC mouse models [215,216,217].
IL-4 not only inhibits the activity of CD8+ T cells, but also acts directly on tumor cells to promote tumor growth and metastasis [218]. In a phase Ib trial, one of six NSCLC patients treated with an IL-4Rα blocking antibody dupilumab in combination with an anti-PD-L1 antibody achieved a positive outcome [21]. The researchers found that this synergistic effect was mediated by an increase in multiple chemokines and cytokines that promote the recruitment and expansion of CD8+ T cells and a decrease in circulating monocytes [21]. Therefore, IL-4Rα blocking antibody combined with ICIs may be an effective combination therapy strategy, but there is still a lack of large-sample clinical studies to determine the potential benefits of this combination in more tumor types, which is worth further exploration in the future.
IL-15 partially shares IL-2 receptors β (CD122) and Cγ (CD132) and induces only the activation of effector cells but does not induce CD8+ T cells depletion, Tregs activation, and cell death [219]. Moreover, researchers fused IL-15-IL-15Rα with anti-PD1 antibody to construct anti-PD1-IL15-R, which reduced toxic side-effects caused by exogenous use of IL-15 while enhancing anti-tumor immunity [220]. However, the evidence for the effect of combination therapy is still insufficient, and more studies are needed to observe the effect of this combination therapy on various tumor types. In addition, toxic effects are also a problem that cannot be ignored in combination therapy.
Interferon-α
IFN-α is a cytokine with various immunomodulatory functions [209]. In treating HCC, pegylated IFN-α combined with anti-PD-1 antibody enhanced T cell infiltration, resulting in improved duration of survival for mice compared to anti-PD-1 antibody monotherapy [221]. Furthermore, targeted delivery of IFN-α using induced pluripotent stem cells in combination with anti-PD-L1 antibody further enhanced anti-tumor immunity and fostered long-term immune memory [222]. These evidences suggest that IFN-α and ICIs have synergistic effects in anti-tumor immunotherapy, but the mechanism of immune resistance induced by IFN-α inducers needs to be further investigated to achieve better therapeutic efficacy in a wide range of tumors.
Transforming growth factor-β
The transforming growth factor-β (TGF-β) pathway inhibits epithelial growth and tumor cell proliferation in early tumors [223]. However, at advanced stages, it has a tumor-promoting effect by regulating genomic instability, EMT, new angiogenesis, immune evasion, cell movement, and metastasis [224]. A TGF-β inhibitor enhanced the effect of anti-PD-1 therapy and anti-PD-L1 therapy by improving the activation and infiltration of T cells in the tumor microenvironment in a human microsatellite-stable CRC mouse model [225]. Moreover, bintrafusp, which simultaneously targets PD-L1 and TGF-β, has shown significant inhibition of tumor growth in breast cancer and colon cancer mouse models [226]. Therefore, the combination of TGF-β and ICIs is a promising strategy for future cancer immunotherapy, but more such experiments are needed to prove the therapeutic effect of this combination in various tumor types.
Chemokine
Chemokine receptors play an important role in various cancer development processes such as angiogenesis, immune cell migration, cancer cell proliferation, and invasion, which could affect patient disease progression and treatment effects [227].
The anti-C–C chemokine receptor (CCR) 4 antibody mogamulizumab is a humanized IgG1 monoclonal antibody that eliminates regulatory T cells through antibody-dependent cytotoxicity [228]. Nivolumab in combination with mogamulizumab induced CD8+ T cells infiltration and Tregs reduction, enhancing the anti-tumor effect of monotherapy [229]. CCR5 promotes tumor cells metastasis and invasion of myeloid cells such as Tregs, MDSCs, and TAMs [230].
Drugs that target CCR8 enhance anti-tumor immunity by depleting tumor-infiltrating forkhead box P3 plus CCR8 plus Tregs or by blocking the CCL1/CCR8 pathway [231]. Fc-optimized anti-CCR8 antibody in combination with anti-PD-1 antibody, has been shown to eliminate regulatory T cells, increase infiltration of CD8+ T cells, and inhibit the growth of murine bladder cancer, breast cancer, and CRC [232].
Chemokine receptor inhibitors have been used to modulate the tumor microenvironment, and their combination with ICIs has optimized the immune response in patients with satisfactory results, which is a promising combination strategy. Besides, for different tumor types need to determine the appropriate chemokines targets, to guarantee the best antitumor effect and avoid treatment side effects.
ICIs combine with regulation of the intestinal microbiome
The intestinal microbiome induces a systemic immune response dominated by CTLs and Th1 cells [233]. Additionally, gut microbes play a role in inhibiting intestinal toxicity caused by ICIs and reducing the risk of colitis [234]. Therefore, promising approaches such as fecal microbiota transplantation (FMT) or dietary therapeutic interventions could restore the microbiota in the gut, enhance ICIs promotion, reduce tumor-related immune suppression, and overcome ICIs resistance in cancer patients.
Fecal microbiota transplantation
FMT involves transferring the gut microbiota from a healthy donor to a recipient through various methods such as oral administration of fresh, frozen, freeze-dried, and encapsulated preparations or through nasointestinal tube, colonoscopy, or enema procedures [235, 236].
The researchers discovered that oral administration of Bifidobacterium could enhance the anti-tumor effect of PD-L1 blockade by increasing infiltration of peritumoral CTLs and intratumoral CD8+ T cells in melanoma mouse models [34]. Moreover, Bacillus fragile has also been proven to promote the anti-tumor effect of anti-CTLA-4 antibody by inducing a TH1 immune response [237]. Treatment with anti-CTLA-4 antibody promote intestinal proliferation of Bacillus fragile, thereby enhancing the efficacy of anti-CTLA-4 antibody and reducing intestinal complications [237]. Furthermore, analysis of stool samples from RCC and NSCLC patients treated with PD-1 blockade revealed that clinical outcomes were associated with the abundance of Akkermansia muciniphila [238]. Oral supplementation with Akkermansia muciniphila restored the efficacy of PD-1 blockade in non-responders [238]. Therefore, modulation of the gut microbiome is a feasible strategy to overcome immunoresistance to ICIs, but the specific mechanism and its effect in other tumors need to be investigated. In addition, it is essential to analyze the baseline microbiota composition and its characteristics of potential recipients of FMT in combination with ICIs and to stratify recipients to improve combination therapy efficacy.
Probiotics
Probiotics consist of carefully selected live microbial strains that provide health benefits when administered in sufficient quantities [239]. Dietary supplementation with exopolysaccharide produced by Lactobacillus delbrueckii subsp. bulgaricus OLL1073R-1 (EPS-R1) induced CCR6+ CD8+ T cells infiltration [240]. Simultaneous administration of anti-CTLA-4 antibody or anti-PD-1 antibody along with oral EPS-R1 demonstrated stronger anti-tumor effects compared to monotherapy in a 4T1 tumor mouse model [240]. Similarly, Bifidobacterium bifidum combined with anti-PD-1 antibody attenuates tumor load in mice with NSCLC [241]. Therefore, probiotics as a kind of safe and effective probiotics, in tumor immunotherapy for intervention in intestinal flora has great development potential, but still need to further explore the mechanism of combination therapy. Moreover, assessment of efficacy of this combination for more tumor types is essential to determine whether it should be applied in the clinic.
Prebiotics
Prebiotics are substrates that are selectively utilized by host microbes and provide health benefits [242]. Pectin, as a prebiotic, promoted butyrate production and enhanced CD8+ T cells infiltration. In combination with anti-PD-1 antibody, it showed tumor growth inhibition in a mouse model of CRC [243]. Another series of studies discovered that Bifidum pseudolonidum produces the metabolite adenosine through T cell-specific inosine A2A receptor signaling, which inhibits tumor growth and enhances anti-tumor immunity when administered together with anti-CTLA-4 antibody, inosine, and CpG [244]. In conclusion, prebiotics play an important role in the regulation of TME, suggesting that it could be used as a potential strategy to enhance the effect of ICIs, but its effect still needs to be verified in more tumor types.
Dietary intervention
Diet influences the composition and behavioral changes of gut microbes to varying degrees, which further affects host metabolism and immunity [245, 246]. Typically, antibiotics disrupt normal gut microbial homeostasis, leading to primary resistance to ICIs treatment [238, 247]. However, it was reported that administration of antibiotics before or 30 days after the initiation of ICIs therapy for HCC improves the efficacy of ICIs [248].
In addition, a high dietary fiber diet slowed tumor growth in melanoma mice after resistance to anti-PD-1 antibody treatment [249]. This effect may be attributed to an increase in gut bacteria such as Ruminococcaceae that produce high levels of short-chain fatty acids with anti-tumor properties [249]. Moreover, the Mediterranean diet rich in vegetables, fruits, grains, nuts and legumes also promotes the growth of gut bacteria that produce short-chain fatty acids [250]. The Mediterranean diet was found to be positively associated with ORR and 12-months PFS in melanoma patients treated with ICIs [251]. Therefore, dietary interventions such as high dietary fiber diet and antibiotics could change the composition and structure of gut microbiota, further transform TME into an immune activated state and improve the therapeutic effect of ICIs. This combination strategy had shown great potential in cancer treatment with high safety. Therefore, it is necessary to focus on the combination of other dietary interventions with ICIs for the treatment of various types of tumors in future studies.
ICIs combined with signaling pathway inhibitor
COX-2/PGE2 pathway inhibitor
COX-2/PGE2 pathway inhibitors, including non-steroidal anti-inflammatory drugs and steroidal anti-inflammatory drugs, may promote infiltration of CTLs, which enhances the response to ICIs [252]. Celecoxib combined with anti-PD-1 antibody significantly inhibited tumor growth and promoted tumor regression in mice with melanoma [253]. Moreover, the addition of celecoxib or glucocorticoids to anti-PD-1 antibody promoted complete tumor regression and prolonged survival in mice with CRC [252]. The researchers found that acute interferon response program was induced in mouse tumors that responded early after receiving the combination therapy, resulting in an enhanced IFN-γ response and an increase in the accumulation of effector T cells within the tumor [252]. However, it has been shown that glucocorticoid therapy leads to worse PFS and OS in patients with solid tumors treated with ICIs [254, 255]. Therefore, non-steroidal anti-inflammatory drugs have great potential to modulate TME and improve the efficacy of ICIs, but glucocorticoids need to be further investigated as to whether glucocorticoid enhance or decrease the efficacy of ICIs.
PI3K-AKT-mTOR pathway inhibitor
PI3K-AKT-mTOR pathway include stimulation of cancer cell proliferation, metastasis, metabolic reprogramming and inhibition of autophagy and senescence [256]. The PI3K-AKT-mTOR pathway also regulates TME, inhibits the aggregation and function of T cells, increases the recruitment of MDSCs and Tregs in tumors, and secretes inhibitory cytokines [257, 258].
The efficacy of combinations of ICIs with PI3K-AKT-mTOR pathway inhibitors has been demonstrated in current preclinical studies [259, 260]. In a mouse model of bladder cancer, treatment with low-dose everolimus combined with anti-PD-1 antibody enhanced the infiltration of CD8+ T cells and inhibits bladder tumor growth [259]. Moreover, the combination of PI3Kγ inhibitor and anti-PD-1 antibody or anti-CTLA4 antibody overcomes the resistance of anti-PD-1 antibody or anti-CTLA4 antibody and significantly inhibits the growth of breast cancer tissue in mice [260]. In conclusion, the prospect of combining PI3K-AKT-mTOR signaling pathway inhibitors with ICIs appears very appealing. Therefore, it is necessary to investigate the immunomodulatory effects of PI3K-AKT-mTOR signaling pathway inhibitors, and to further evaluate the therapeutic effect of this combination in more tumor types.
Mitogen-activated protein kinase pathway inhibitor
The MAPK pathway regulates a variety of cellular processes including proliferation, differentiation, apoptosis, and stress response, of which the RAS-RAF-MAPK (MEK)-ERK pathway is the most important signaling cascade controlling survival and development of tumor cells [261].
Inhibition of BRAF and MEK combined with anti-PD-1 antibody or anti-PD-L1 antibody enhances tumor immune infiltration in a CD8+ T cell-dependent manner [262]. In a BRAF-mutant melanoma model with anti-PD-1 antibody resistance, concurrent administration of darafanib and trametinib increased CD8+ cytotoxicity and CD4+ T helper cells infiltration, strongly suppressed tumor growth, and further enhanced the efficacy of anti-PD-1 antibody or anti-PD-L1 antibody [263]. The sequential treatment of anti-PD-1 antibody followed by targeted inhibitors has yielded longer durations of treatment response compared to using anti-PD-1 antibody alone or using targeted inhibitors first followed by anti-PD-1 antibody in a mouse model of BRAF-mutant melanoma [264]. In addition, the combination of ERK inhibitor and anti-PD-1 antibody also induced massive CD8+ T cells infiltration and prolonged OS in PDAC mice [265].
Although the combination of MAPK pathway inhibitors and ICIs has opened up new avenues for cancer treatment, future studies are needed to optimize treatment strategies and identify appropriate biomarker for patient subgroups. In addition, it is important to evaluate the efficacy of this combination in more types of tumors.
RAS inhibitor
The RAS (KRAS, NRAS and HRAS) family is the most frequently mutated in cancer cells, and when activated, it could trigger downstream pathways such as MAPK and PI3K-AKT [266]. Activation of RAS inhibits CD8+ T cells infiltration and upregulation of MDSCs and Tregs and induces infiltration of multiple immunosuppressive cytokines in the TME [267].
Targeting RAS could relieve the immunosuppression mediated by MAPK and PI3K-AKT pathways and has a synergistic effect with ICIs [268]. Notably, rigosertib is a non-ATP-competitive small molecule RAS mimetic, which blocked both MAPK and PI3K-AKT signaling pathways [269]. Rigosertib plus anti-PD-1 antibody or anti-CTLA-4 antibody increased CD8+ T cells infiltration, increased the median survival time of mice with leukemia from 11 to 22.5 days of anti-PD-1 antibody or anti-CTLA-4 antibody alone, and about 70% of tumors showed growth inhibition [270].
Therefore, targeting RAS in combination with ICIs is a promising strategy. However, the development of specific inhibitors for mutant RAS alleles and personalization of drugs according to the mutant gene are important for combination therapy. Moreover, the efficacy and safety of this combination for various tumor types warrant further investigation.
Wnt/β-catenin inhibitor
The Wnt/β-catenin signaling pathway controls a variety of cellular processes and is closely associated with cancer development [271]. The Wnt ligand inhibitors promote the transformation of the TME in a direction conducive to the function of ICIs [272]. In a bone marrow-derived cancer-associated fibroblasts-rich tumor model, Wnt/β-catenin signaling inhibitors inhibited the expression of PD-L1 and enhanced the therapeutic effect of anti-PD-L1 antibody [273]. RX-5902, a novel β-catenin modulator, in combination with nivolumab increased TILs infiltration and activation, granzyme B production, and exhibited favorable tumor control in a mouse model of 4T1 tumor [274].
Therefore, inhibition of Wnt/β-catenin signaling pathway is also a therapeutic strategy worthy of investigation to improve the efficacy of ICIs. However, this combination has been less studied in other tumor types.
ICIs combined with targeting innate immune pathways
Anti-tumor effects could be induced through activation of pattern recognition receptors (PRRs) within the patient’s innate immune pathways, such as TLRs, retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), and stimulator of interferon genes (STING) [275]. Targeting these treatment-related targets in the innate immune pathway helps prevent tumor escape and appears to offer additional possibilities for eradication when combined with ICIs.
TLRs
TLRs are considered as crucial PRRs that participate in innate immunity and serve as a bridge between innate and adaptive immunity [276]. TLR agonists lead to tumor regression by increasing the infiltration of NK cells, CTLs within the tumor [36]. Vidutolimod (CMP-001), a TLR9 agonist, has shown to increase CD8+ T cells expression in melanoma patients, resulting in a higher response rate when combined with anti-PD-1 antibody compared to monotherapy [277]. Additionally, PD-L1/TLR7 dual-targeted nanoantibody-drug conjugates have been found to induce CD8+ T cells and NK cells infiltration in the TME [203]. However, the therapeutic efficacy of TLRs agonists in combination with ICIs for other tumor types is currently unclear.
RLRs
RLRs belong to the family of DExD/H box RNA helicases that not only trigger cancer cell death but also enhance CD8+ T cell-mediated anti-tumor immune responses [275]. RIG-I activation enhances CD8+ T cells activation and infiltration in the TME [278]. Moreover, high RIG-I expression in melanoma patients receiving anti-CTLA-4 antibody treatment further activates systemic anti-tumor immunity [279]. Thus, activation or high expression of RIG could enhance the anti-tumor immune effect of ICIs by activating the intrinsic immune pathway. Moreover, it is necessary to validate the therapeutic effect of this combination in more studies.
STING signaling pathway
Activation of STING through the binding of tumor-derived DNA fragments to GMP-AMP (cGAS) stimulates the production of IFNs and activates CD8+ T cells [280]. In a B16F10 (melanoma cell lines) tumor mouse model with lung metastasis, STING agonist (STING-LNP) combined with anti-PD-1 antibody has been found to exert a synergistic anti-tumor immune effect [281]. Targeting the STING signaling pathway is crucial in cancer immunotherapy and could enhance the efficacy of checkpoint inhibitor-based immunotherapy. However, more studies are needed to observe the therapeutic effect of this combination on other types of tumors.
ICIs combined with non-apoptotic regulated cell death
RCD plays a crucial role in maintaining homeostasis and disease development and could be divided into two categories: apoptotic RCD and non-apoptotic RCD [282]. At present, induction of non-apoptotic RCD is an emerging cancer treatment modality, including autophagy, ferroptosis, pyroptosis, and necroptosis [283]. Moreover, non-apoptotic RCD profoundly affects the response of immune cells infiltrating the TME.
Autophagy
Autophagy is a regulatory mechanism that removes unnecessary or dysfunctional cellular components and recycles metabolic substrates [283]. It affects tumor progression, immunity and therapy by changing the autophagy pathway of tumor cells and immune cells in response to stress signals in TME [284]. And it is generally considered to be an important mechanism of drug resistance in cancer therapy, but it could also exert anti-tumor effects by enhancing tumor immunogenicity [285]. Autophagy inhibitors are classified into early inhibitors that target ULK1/ULK2 or VPS34, such as SBI-0206965, 3MA and wortmannin, and late inhibitors that target lysosomes, such as chloroquine, hydroxychloroquine, bafilomycin A1 and monensin [286]. The combination of chloroquine with anti-PD-L1 antibody and anti-CTLA-4 antibody induced the expression of CD8+ T cells and MHC-I molecules in PDAC in mice, and improved the anti-tumor effect [37]. Moreover, the lysosomal protein palmitoyl protein 40 thioesterase 1 inhibitor hydroxychloroquine, used in combination with anti-PD-1 antibody, induced TAM polarization from M2 phenotype to M1 phenotype, reduced MDSCs infiltration, enhanced the killing effect of T cells, and finally inhibited tumor growth and prolonged the survival of melanoma mice [287]. Autophagy inhibitors appear to be an increasingly promising combination therapy strategy for sensitizing tumor cells to ICIs. In the future, more preclinical and clinical studies of this combination should be conducted to evaluate its efficacy and safety, so that more cancer patients could benefit from it.
Ferroptosis
Ferroptosis is a regulatory cell death caused by iron-dependent lipid peroxidation, and three key features of ferroptosis have been cracked: membrane lipid peroxidation, intracellular iron availability, and loss of antioxidant defenses [288]. In addition, ferroptosis plays an important role in T cell-mediated anti-tumor immunity and affects the efficacy of immunotherapy, and direct or indirect induction of ferroptosis, such as radiotherapy and targeted therapy, is a promising combination to improve anti-PD-1 or anti-PD-L1 immunotherapy [289]. In the xenograft model of diffuse large B-cell lymphoma, the dual PI3K/HDAC inhibitor, BBT-908, induced ferroptosis of tumor cells, and the increased ferroptosis signal further stimulates MHC-I expression of tumor cells, enhancing immunogenicity, and in combination with anti-PD-1 antibody some mice survive for a long time after treatment and develop anti-tumor immune memory in vivo [290]. Further study of the specific regulatory mechanisms of ferroptosis in TME will help to design ferroptosis inducers targeting cancer therapies, and provide new options for overcoming ICIs resistance in the clinic by triggering immune responses through ferroptosis.
Pyroptosis
Pyroptosis is mainly induced by gasdermin D in gasdermin family members, and involves the inflammatory caspase-1 or caspase-4/5 pathway in the main pathway, and the most important alternative pathway is the caspase-3 pathway induced by gasdermin E [291]. A variety of therapeutic approaches such as chemotherapy drugs including platinum, paclitaxel, 5-FU, and radiation therapy induce pyroptosis in tumor cells through the gasdermin D pathway, which further activates the infiltration and activation of CTLs, and consequently eliminates tumor cells [292]. Immunomodulative photodynamic MRC nanoparticles were used for pyroptosis-mediated immunotherapy, and combined with anti-PD-1 antibody showed significant tumor suppression effect, prolonged survival time of 4T1-Luc tumor-bearing mice, and inhibited metastasis [293]. Pyroptosis inducer may improve the therapeutic effect of ICIs in tumor patients, but more studies in a wider range of tumors are needed to verify the efficacy of this combination. In addition, it is essential to develop novel cell pyroptosis inducers targeting cancer cells to obtain the best therapeutic effect and the lowest side effects.
Necroptosis
Effectors in necroptosis such as RIPK1 and RIPK3 directly regulate immune cell function [283]. RIPK1-mediated cell death induced activation of CD8+ T cells and NK cells in treating soft-tissue sarcoma, enhancing the therapeutic effect of anti-PD-1 antibody and anti-CTLA-4 antibody [294]. However, there are few studies relevant to this combination, and the effect in other tumors is not clear.
ICIs combined with nitric oxide
NO has the potential to play an anti-tumor role by promoting macrophage polarization towards the M1 phenotype and CD8+ T cells infiltration [295]. Researchers utilized nanotechnology to deliver NO to the tumor site, inducing CD8+ T cells infiltration and synergistic interaction with anti-PD-1 antibody, resulting in the inhibition of breast cancer growth and metastasis in mice [296]. Additionally, a NO delivery platform using dendritic mesoporous silica nanoparticles modified with S-nitrosothiol has been developed by researchers, which enhances NO levels in macrophages, induces TAM polarization to the M1 phenotype, and improves anti-tumor immunity [38]. Although the combination has achieved favorable therapeutic effects, more studies are needed to observe the therapeutic effects of this combination on other types of tumors.
In conclusion, NO have immunomodulatory activity and could modulate TME to an immunologically activated state. This provides a new approach to enhance the therapeutic efficacy of ICIs, but drug concentration and precise local delivery are issues that need to be thoroughly investigated for this therapeutic approach. Moreover, it is necessary to observe the efficacy of this combination in other tumor types.
ICIs combined with targeting metabolic pathways
In the TME, the nutritional competition and coordination between tumor cells and immune cells is the key to the effectiveness of anti-tumor immune response, and metabolites could also affect the metabolic process of T cells [297]. Table 3 presents preclinical studies of ICIs combined with targeting metabolic pathways. Blocking agents that target metabolic pathways could alter the behavior of other immune cells in the tumor or TME, thereby activating a killing response against the tumor and having greater tumor suppression effects in combination with ICIs.
Glycolysis
Glycolysis improves CD8+ T cell-mediated anti-tumor immune response by increased expression of phosphoenolpyruvate in T cells, deletion of VHL protein to relieve hypoxia caused by HIF, NF-κB induced kinase (NIK) to prevent autophagy degradation of glycolytic ring-limiting enzyme HK2, inhibition of PI3K-AKT-mTOR signaling pathway [298,299,300]. In a mouse model of B16F10 tumors, compared with anti-PD-1 antibody alone, glucose metabolism inhibitor (PFK-015) combined with anti-PD-1 antibody significantly reduced tumor volume [301].
In addition, inhibiting lactate transporter MCT1 expression by Tregs in intrahepatic tumors reduce lactate content in TME, which further reduces PD-1 expression in Tregs and enhances the effect of anti-PD-1 antibody [302]. Moreover, lactate enhances anti-tumor immunity via CD8+ T cells, and lactate combined with anti-PD-1 antibody enhances the efficacy of single-agent therapy in MC38 and B16F10 tumor mouse models by inhibiting tumor growth and prolonging the survival time of mice [303]. In conclusion, the regulation of glucose metabolism provides new insights into enhancing the therapeutic effects of ICIs. Therefore, future research should not only delve deeper into the underlying mechanisms but also conduct further studies on the efficacy of combination therapy in other types of tumors.
Lipid metabolism
Fatty acids could enhance anti-PD-1 antibody-mediated anti-tumor immunity by enhancing fatty acid oxidation (FAO) in CD8+ T cells [304]. They also regulate the cytotoxicity of NKT cells, promote infiltration of CD8+ central memory T cells, increase the number of NK cells, increase levels of activating receptors and effector proteins, enhance the anti-tumor activity of CTLs in a DC-dependent manner, and eventually inhibit tumor growth [304]. However, accumulation of FAs reduces the activity of effector T cells and antigen presentation function of DCs and also induced the immunosuppression of Tregs [305,306,307].
Peroxisome proliferator-activated receptor γ (PPARγ) coactivator-1α (PGC-1α) / transcription factor complexes agonist combined with anti-PD-1 antibody increased the infiltration and improved the function of CTL in CRC in mice, and activate mitochondrial respiratory function and FAO in CD8+ T cells [308]. In mouse models of melanoma and CRC, inhibiting the expression of CD36 could inhibit absorption of FAs, down-regulate intratumoral invasion by Tregs, and play a synergistic role with anti-PD-1 antibody in anti-tumor immunity [309]. Furthermore, adenosine 5ʹmonophosphate-activated protein kinase (AMPK) inhibits the synthesis of fatty acids [39]. It also inhibits abundance of PD-L1 protein and increases expression of type 1 IFN and antigen presenting genes [39]. The combination of AMPK agonist and anti-CTLA-4 antibody significantly increases CD8+ T cells and cytokines while prolonging OS in CT26 tumor-bearing mice [39]. Moreover, other drugs that regulate lipid metabolism, like stearoyl co-adesaturase1 inhibitors, fatty acid synthase inhibitors and drugs targeting carnitine palmitoyltransferase I have shown promising anti-tumor effects [310,311,312].
High cholesterol levels could deplete CD8+ T cells, weakening the body’s anti-tumor immune response [313]. Statins could regulate cholesterol metabolism by inhibiting the conversion of 3-hydroxy-3-methyl-glutaryl coenzyme a to methylglutaric acid, reducing the risk of cancer and enhancing anti-tumor immunity [314]. In a mouse harboring Lewis cells model, the combination of lovastatin and anti-PD-1 antibody resulted in a significant reduction in tumor weight and volume compared with anti-PD-1 antibody alone [315]. Besides, the addition of statins improved survival rate for NSCLC patients under 75 years old receiving anti-PD-1 antibody therapy [315]. In addition, statins also improved the prognosis of NSCLC patients treated with ICIs monotherapy, prolong the OS of NSCLC patients who have received anti-PD-1 antibody treatment, but have no effect on PFS [316]. Therefore, researches on targeting lipid metabolism have provided new modalities for cancer treatment, advancing combination therapy approaches to improve ICIs treatment. However, it is still necessary to verify the effectiveness of this combination for cancer treatment in future.
Extracellular adenosine
Extracellular adenosine (eADO) usually suppresses anti-tumor immune responses [317]. Targeted inhibition of cell-surface ectonucleotidases CD73, CD39 and eADO-specific receptors (A2A or A2B) could induce activation and proliferation of T cells, depletion of Tregs, maturation and functional restoration of NK cells [318]. Anti-CD73 antibody enhances the anti-tumor activity of anti-PD-1 antibody and anti-CTLA-4 antibody in colon cancer and prostate cancer mice in an IFN-γ and CD8+ T cell-dependent manner [319]. CPI-444 is a small molecule inhibitor of A2A that reduces the expression of PD-1 on CD8+ T cells, enhances the function of CD8+ T cells, and significantly improves the survival rate of CRC mice when combined with anti-PD-1 antibody [320]. Therefore, targeting the adenosine pathway has significant potential to enhance the therapeutic effects of ICIs in cancer. However, more preclinical studies are needed to determine the timing and intensity of A2A blockade to obtain better therapeutic effects to promote the clinical translation of this combination.
Tryptophan catabolism pathway
The first step in free tryptophan degradation metabolism is catalyzed by indoleamine-2,3 dioxygenase (IDO) 1, IDO2 or tryptophan-2,3 dioxygenase (TDO) to eventually produce kynurenine [321]. IDO1 mediates its immunosuppressive function by inducing formation of Tregs and MDSCs while inhibiting proliferation of CD3+ T cells, CD8+ T cells and NK cells [322]. Anti-PD-1 antibody or anti-CTLA-4 antibody in combination with IDO1 inhibitors increased production of CD8+ T cells and IL-2, resulting in better therapeutic efficacy on melanoma in mice compared with anti-PD-1 antibody or anti-CTLA-4 antibody alone [323]. Besides, TDO inhibitor improves the anti-tumor effect of anti-CTLA-4 antibody in TDO-expressing CRC mice (P < 0.0004) [324]. However, only patients with tumors expressing tryptophan catabolic enzymes benefit. Therefore, it is highly necessary to stratify patients before targeting tryptophan catabolism in combination with ICIs therapy. In addition, exploring the safety and efficacy of this combination in more tumor types is also an indispensable process to promote the clinical transformation of this combination.
Polyamine metabolism
Polyamines have been shown to enhance the generation and activation of B and T cells for anti-tumor immunity [325]. However, they are mostly responsible for immunosuppression by polarizing macrophages towards the M2 phenotype in the TME [325]. In mouse models with metastatic 4T1 and B16F10 tumors, polyamine blocking therapy consisting of α-difluoromethylornithine and polyamine transport inhibitors combined with anti-PD-1 antibody resulted in a reduction in the levels of MDSCs and M2 TAMs as well as a four-fold decrease in tumor volume compared to anti-PD-1 antibody alone [326]. Due to the metabolism of tumor cells and immune cells exist obvious heterogeneity and plasticity, so a better understanding of tumor immune escape mechanism of polyamine metabolism, may help to overcome the resistance of ICIs treatment. Moreover, the clinical value of ICIs combined with targeting polyamine pathways in more tumor types needs to be investigated in further studies.
Methionine metabolism
Methionine is an essential amino acid that could restore the function of CD8+ T cells [327, 328]. However, a methionine-restricted diet (MRD) could also enhance anti-tumor immunity by increasing the infiltration of CD8+ T cells [327, 328]. In mouse models with CT26 and B16F10 tumors, methionine inhibited tumor growth, increased infiltration of CD8+ T cells, decreased apoptosis, and showed a synergistic effect when combined with anti-PD-L1 antibody [329]. Moreover, the effectiveness of MRD and anti-PD-1 antibody has been demonstrated in mouse models of CT26 and MC38 [330]. In conclusion, targeting methionine metabolism may be a potential new strategy to enhance ICIs, but evidence for the efficacy of this combination in cancer treatment is still lacking.
ICIs combined with targeting sex hormone receptors
Androgen receptor (AR)-mediated signaling pathway is not conducive to the maintenance of stem-like CD8+ T cells, leading to the depletion of anti-tumor CD8+ T cells more easily in male patients [331]. Meanwhile, female patients exhibit lower levels of AR expression in CD8+ T cells and lower levels of androgen, allowing them to achieve a better anti-tumor immune effect [331, 332]. In male prostate cancer mice, a combination of androgen deprivation therapy plus AR inhibitor enzalutamide plus anti-PD-1 antibody promoted tumor regression and improved survival [333].
On the other hand, estrogens inhibit anti-tumor immunity by regulating the polarization of TAMs towards M2 phenotype and increasing the infiltration of MDSCs [334, 335]. Fulvestrant is a selective estrogen receptor downregulator that could inhibit estrogen receptor α (ERα), reduce tumor-promoting effect of estrogen and enhance the anti-tumor effect of ICIs in a mouse model of melanoma [41]. Thus, AR inhibitors provide a theoretical basis for reversing immunosuppression and improving ICIs efficacy in male patients while ERα inhibitors provide this benefit for female patients. Moreover, G protein-coupled estrogen receptor-induced c-myc depletion restored expression of antigen-presenting human leukocyte antigen (HLA)/MHC proteins [336]. Selective agonist G-1 combined with anti-PD-1 antibody prolonged the survival time of melanoma mice and produced immune memory [336].
In conclusion, incorporating sex into the treatment of ICIs may mitigate the effect of sex on cancer treatment. Targeting sex hormone receptors could be a promising strategy to enhance ICIs, but more studies are needed to further investigate the mechanisms involved and effectiveness in various tumor types.
ICIs combined with injection of magnesium
Low dietary magnesium intake and hypomagnesemia have been linked to various diseases, including infections and cancer [337]. It has been previously reported that feeding mice with a magnesium ion-deficient diet accelerated the spread and metastasis of cancer cells [337]. This is because magnesium is essential for the function of leukocyte function-associated antigen 1 (LFA-1), a costimulatory molecule protein on the surface of T cells, and CD8+ T cells require a sufficient concentration of magnesium to function effectively [42]. Magnesium ions promote the activation of effector memory CD8+ T cells through LFA-1 action to enhance the therapeutic effect of ICIs [42]. Therefore, intratumoral injection of MgCl2 has been shown to increase CD8+ T cells infiltration, inhibit tumor growth and improve tumor control in mice when combined with PD-1 inhibitors in a MC38-OVA tumor mouse model [42].
Consequently, a low serum magnesium level is associated with poor prognosis in cancer immunotherapy. Appropriate supplementation of magnesium ions in cancer patients may enhance T cell immunity and improve the efficacy of anti-PD-1 antibody. However, the efficacy and feasibility of this combination in other tumor types need to be further verified in more studies.
ICIs combined with targeting co-stimulatory receptors
Co-stimulatory receptors are located on the surface of T cells and could activate and proliferate T cells by activating the TCR [338]. These co-stimulatory signals play a critical role in regulating T cell activation, differentiation, effector function, and survival [338]. Co-stimulatory receptors are usually divided into tumor necrosis factor receptor superfamily (TNFRSF) which includes glucocorticoid induced tumor necrosis factor receptor (GITR), OX40, CD40 and 4-1BB, and immunoglobulin superfamily (IgSF) which includes CD28 and induced T cells co-stimulation (ICOS) [339]. Currently, there are no co-stimulatory receptor inhibitor has been approved for clinical practice, so despite the satisfactory therapeutic effects of these combination strategies in preclinical studies, it will take a long time for these combination strategies to be translated to the clinic. As the understanding of the mechanism of combination therapy improves, determining the optimal drug dose and order of administration is critical to obtaining the best combination therapy outcome. Table 4 presents preclinical studies that combine ICIs with co-stimulatory receptors.
GITR
GITR is a co-stimulatory molecule in the TNFRSF, and its binding to ligands could regulate the NF-κB and MAPK pathways [340, 341]. Activating the GITR signaling pathway leads to T cell activation, proliferation, and survival while inhibiting the inhibitory activity of Tregs [340, 341]. A bispecial antibody composed of multimeric GITR ligand and anti-PD-1 antibody increased CD8+ T cells infiltration and inhibited tumor growth in CT26, EMT6 (breast cancer cell lines), and JC (breast cancer cell lines) tumor-bearing mice, with a greater anti-tumor effect than GITR agonist combined with anti-PD-1 antibody [43]. Moreover, the evaluation of the efficacy of such studies in different tumor types is critical.
OX40
OX40 is a co-stimulatory molecule that is transiently expressed on activated human T cells, belongs to TNFRSF, and plays a role in effector T cells activation, expansion, differentiation, generation, and maintenance of memory T cells [342]. The combination treatment of an anti-OX40 agonist antibody and an anti-PD-1 antibody in treating PDAC increased infiltration of CD4+ T cells and CD8+ T cells, promoted tumor regression and prolonged the survival of mice compared with anti-OX40 agonist antibody or anti-PD-1 antibody monotherapy [343]. In addition, sequential therapy with an anti-OX40 agonist antibody followed by an anti-PD-1 antibody is also an effective strategy to improve the efficacy in tumor therapy [344]. In conclusion, anti-OX40 agonist antibody has been shown to improve the efficacy of ICIs, but it needs to be evaluated in more tumor types.
4-1BB
4-1BB belongs to TNFRSF9, which is activated and could induce the expression of co-stimulatory receptors on T cells and NK cells to enhance the cell killing ability of T cells and the cytotoxicity induced by NK cells [345]. In 3LL (lung cancer cell lines) and 4T1 tumor mouse models, combined therapy improved survival and inducing tumor regression in tumor-bearing mice compared to anti-PD-L1 antibody or anti-4-1BB antibody alone [346]. Meanwhile, there is evidence that changing the administration sequence based on TME further enhances the efficacy of combination therapy with anti-PD-1 antibody and anti-4-1BB antibody [347]. Thus, the synergistic anti-tumor effects of ICIs and 4-1BB agonists provide an effective therapeutic modality for cancer patients. In addition, the therapeutic effect of this combination on more tumor types still needs further attention.
ICOS
ICOS is paradoxical in anti-tumor immunity because it could both enhance the immune response of CD8+ T cells against tumor growth as well as maintain the function of Tregs to promote tumor development [348]. In a mouse model of highly aggressive melanoma, ICOS bi-specific agonistic aptamer restored the tumor inhibitory effect of anti-CTLA-4 antibody [349]. Therefore, ICOS agonist combined with ICIs is a potential therapeutic strategy, but more relevant studies are needed to evaluate the efficacy of this combination.
CD40
CD40, a member of TNFRSF, is widely expressed in hematopoietic and non-hematopoietic tissues [350]. CD40 ligand is expressed on CD4+ T cells and binding with CD40 could activate CD4+ T cells while enhancing the response and immune memory of CD8+ T cells induced by DCs [351]. Combination therapy of anti-PD-1 antibody with a CD40 agonist demonstrated enhanced efficacy in inhibiting tumor growth and improving survival rate and OS in mice with intrahepatic cholangiocarcinoma [352]. In conclusion, several preclinical and clinical studies have suggested that CD40 agonist are a promising strategy to improve the efficacy of ICIs treatment, but more clinical studies are needed to confirm the effectiveness of this combination for cancer treatment in future.
ICIs combined with targeting co-inhibitory receptors
Slow activation of co-stimulatory receptors and TCR signals may lead to inhibition of T cells activation and function [353]. Inhibited T cells may cause up-regulation of multiple inhibitory receptors, such as CTLA-4 and PD-1, LAG-3, T cell immunoglobulin and mucin domain-containing protein 3 (TIM3), T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT), and B and T lymphocyte attenuator (BTLA) [339]. Table 5 presents preclinical studies of ICIs combine with co-inhibitory receptors. Antagonists targeting these upregulated co-inhibitory receptors to further enhance the efficacy of ICIs are promising combination therapy strategies. Personalized treatment by selecting the best target to obtain the best effect is also a valuable research direction in the future.
TIM3
TIM3 belongs to the TIM family and participates in autoimmune and cancer immune regulation through various cells such as IFN-γ, CD4+ T cells, CD8+ T cells, Tregs, and NK cells [354]. In a B16F10 tumor mouse model, anti-TIM3 antibody combined with anti-PD-1 antibody and anti-CTLA-4 antibody further inhibited tumor growth compared with anti-TIM3 antibody, anti-PD-1 antibody and anti-CTLA-4 antibody monotherapy, resulting in survival benefit of mice [355]. In conclusion, ICIs have synergistic effects with anti-TIM3 antibody and represent a promising combined therapeutic strategy. However, the efficacy of this combination in cancer treatment needs to be further validated.
TIGIT
TIGIT is a suppressor receptor belonging to the PVR-like family, which could bind with CD155 on tumor cells or APCs to inhibit the function of NK cells, CD4+ T cells, and CD8+ T cells while enhancing the immunosuppressive function of Tregs [356]. Anti-TIGIT antibody enhanced the anti-tumor immune response activated by anti-PD-1 antibody by inducing stronger infiltration of CD4+ T cells and CD8+ T cells in a MC38 tumor mouse model [357]. The combination of ICIs with anti-TIGIT antibody may be the key to improving survival in cancer patients treated with ICIs alone. And it is necessary to investigate the therapeutic effect of this combination in cancer treatment in further researches.
BTLA
BTLA belongs to the CD28 superfamily and has similar structure and function to PD-1 and CTLA-4, which could inhibit the activation of B cells and T cells to play an immunosuppressive function [358]. In glioblastoma mice models, anti-PD-1 antibody combined with anti-BTLA antibody increased the overall long-term survival rate of anti-PD-1 antibody monotherapy from 20 to 60% while increasing the expression of CD4+ IFN-γ and CD8+ IFN-γ [359]. ICIs combined with anti-BTLA antibody provides a new direction for anti-tumor immunotherapy, but the efficacy of this combination needs to be validated in cancer treatment in future.
ICIs combined with regulation of dietary mode
Fasting-mimicking and ketogenic diets may provide potential benefits to cancer patients, as they improve metabolic, enhance anti-tumor ability, and increase effector T cells [39, 45]. These feasible dietary interventions may be used in conjunction with standard treatments and could possibly serve as adjunctive treatments to enhance the effects of ICIs.
Fasting-mimicking
Caloric restriction intervention is safe to use in combination with standard treatment and leads to a decrease in blood glucose levels, growth factor concentrations, immunosuppressive cells in peripheral blood, and enhanced T cell infiltration in tumors [360]. This helps regulate cancer patient metabolism and enhances anti-tumor immunity [360]. In particular, a fasting-mimicking diet combined with anti-PD-L1 antibody treatment has been shown to enhance the anti-tumor immune response and reduced tumor volume of two different low-immunogenicity TNBC subtypes (4T1 and TS/A) mice by increasing CD8+ T cells and CD4+ T cells infiltration [45]. In conclusion, fasting-mimicking is a safe, inexpensive, and accessible dietary intervention that shows exciting promise in enhancing anti-tumor immunity. However, only a small number of such studies have been conducted, and there is a need to assess the effectiveness of fasting-mimicking for adjuvant therapy based on ICIs for cancer treatment in the future.
Ketogenic-diet
The KD is a formulated diet that contains high amounts of fat, low amounts of carbohydrates, suitable protein levels, and other nutrients [361]. KD enhances innate and adaptive immune responses by increasing CD8+ T cells and CD4+ T cells while decreasing Tregs [362]. Moreover, it down-regulates the expression of PD-L1 and CTLA-4 in T cells [362]. When combined with anti-CTLA-4 antibody treatment in homogenous mice with CT26 tumors compared to single therapy it delayed tumor growth and prolonged survival rates [39]. Therefore, the ketogenic diet has the advantages of low cost, high patient compliance, and few side effects, making it an attractive strategy to enhance the efficacy of ICIs. Moreover, it is important to investigate the effect of this combination on more tumor types.
ICIs combined with epigenetic modulations
Epigenetic modification controls transcription through histone modification, DNA methylation, and regulation of non-coding RNA levels [363]. Currently, several epigenetic drugs have been developed, including histone deacetylase inhibitors (HDACi), histone methyltransferase inhibitors (HMTi), DNA methyltransferase inhibitors (DNMTi), and non-coding RNAs inhibitors, which have anti-tumor immune effects [364,365,366]. However, drugs for other epigenetic mechanisms, such as lncRNA, miRNA, and histone phosphorylation have not yet been used for clinical treatment [367].
There are two major problems that limit the efficacy of existing ICIs are low immunogenicity and drug resistance, and epigenetics will be involved through various regulatory effects, especially the regulation of the immunogenicity of cancer cells and the exhaustion of T cells, making it a promising strategy to enhance the efficacy of ICIs [368]. Nevertheless, there is still a need for extensive research to discover more epigenetic modification mechanisms and therapeutic targets, to develop more effective epigenetic drugs, and to achieve the optimal combination of epigenetic drugs and ICIs in combination therapy.
Histone acetylation
Histone acetylation is the addition of acetyl groups to multiple lysine residues in the histone tail, promoting chromatin opening and enhancing transcriptional activity [369]. The level of histone acetylation is affected by histone deacetylases (HDAC) and histone acetyltransferase [370]. HDAC removes acetyl groups from histones during regulation of cell cycle and mitosis, and it also inhibits DNA damage, maintains protein stability, participates in angiogenesis and IFN signaling [371, 372]. HDACi could inhibit these changes while inducing cell apoptosis [371, 372]. Furthermore, HDACi has shown significant therapeutic effects on various lung tumor mouse models by inducing chemokines leading to enhanced T cell recruitment along with inhibition of tumor growth in combination with anti-PD-1 antibody [46]. Moreover, the therapeutic efficacy of the combination on other types of tumors needs to be further investigated.
Histone methylation
Histone methylation, which methylates lysine or arginine residues, is another histone modifications, in which the methylation of H3K4, H3K36, and H3K79 promotes the transcriptional activation of genes, while the methylation of H3K9, H3K27 and H4K20 promotes transcriptional inhibition [373]. Histone methyltransferase (HMT) is a key enzyme involved in histone methylation, regulating cell cycle and growth [374]. Furthermore, genes such as DOT1L (lysine 79), G9a, and EZH2 encode for HMTs involved in the process of histone methylation [375].
Inhibition of EZH2 combined with anti-PD-1 antibody promoted CD8+ T cells recruitment in head and neck squamous cell carcinoma of mice, enhanced tumor destruction and reduce the resistance of anti-PD-1 antibody treatment [376]. SD1 is the first identified histone demethylation enzyme that removes methyl from histone H3K4 to regulate transcription for multiple genes [377, 378]. In a TNBC mouse model, the combination of an LSD1 inhibitor and anti-PD-1 antibody increased CD8+ T cells infiltration and inhibited tumor growth and metastasis [379]. Additionally, G9a inhibition enhances checkpoint inhibitor-blocked anti-tumor activity by modulating autophagy and IFN signaling leading to tumor regression in a B16F10 tumor mouse model [380]. Thus, regulation of histone methylation in combination with ICIs is a promising strategy for cancer treatment, the effects of this combination on a variety of tumors need to be further explored.
DNA methylation
DNA methylation transfers a methyl group to cytosine forming 5-methylcytosine as an epigenetic mechanism widely studied for its role in cancer development and occurrence [381]. DNA methyltransferases (DNMTs) induce DNA methylation, and both hypomethylation and hypermethylation promote cancer occurrence and development [364, 382]. Low-dose DNMT inhibitors enhance anti-CTLA-4 antibody anti-tumor immune responses in melanoma mice, showing a potential therapeutic advantage [383]. In conclusion, whereas DNA methylation combined with anti-CTLA-4 antibody has demonstrated achieving better tumor control, the killing effect of this combination on multiple tumors needs to be further evaluated.
Circular RNA
Circular RNA (CircRNAs) are covalently closed molecules that regulate transcription and shearing when interacting with proteins, thereby affecting tumor growth, occurrence and metastasis [384, 385]. In a NSCLC mouse model, hsa_circ_0003222 inhibitor in combination with anti-PD-L1 antibody reduced tumor volume and inhibited tumor metastasis [386]. However, although there are few reports on such studies, the combination of circRNAs inhibitors with ICIs is still a promising strategy.
Nuclide image-guided immune checkpoint therapy
Radio-labeled antibody probes based on single photon emission computed tomography (SPECT) and positron emission tomography have been utilized in both clinical and preclinical studies to observe the distribution and metabolism of ICIs in vivo [387, 388]. 99mTc-MY1523, a nanoscale probe targeting PD-L1, combined with SPECT/computed tomography (CT)-guided PD-L1 blocking therapy in combination with SPECT/CT-guided PD-L1 blocking therapy, inhibited tumor growth and improved survival time and rate in MC38, A20 (lymphoma cell lines), and 4T1 tumor-bearing mouse models [389]. Another anti-PD-L1 antibody labeled with radioiodine (I131-αPD-L1) was also found to delay tumor growth and prolonged survival in MC38 and CT26 tumor mice compared to anti-PD-L1 antibody monotherapy [47]. The use of nuclide image-guided immune checkpoint therapy is an attractive strategy to enable more precise and efficient release of ICIs by using a variety of molecular probes. However, the efficacy of this combination regimen for tumor treatment still needs to be verified in the future.
ICIs combined with DNA damage response
The DDR pathway is designed to protect the integrity of the cell genome and to monitor and repair foreign or endogenous DNA damage [390]. The DDR pathway plays an important role in the development of immune-activated TME, which makes the DDR-related therapeutic combination ICIs an attractive option for tumor treatment which including PARP inhibitor, ATM/ATR/CHK1 pathway inhibitor and WEE1 inhibitor. In the future, it is necessary to further determine the optimal dose and optimal combination of DDR inhibitors in combination with ICIs, and the study of the characteristics of DDR components in each tumor will contribute to the personalized treatment of cancer patients. Moreover, changes in DDR could be used as predictive biomarkers to identify specific subsets of patient that will respond to combination therapy with ICIs and DDR inhibitors, which is also critical to whether patients will benefit from combination therapy [391].
PARP inhibitor
PARP could be involved in DNA damage repair by adding poly (ADP-ribose) (PAR) or mono-ADP-ribose to itself and other target proteins [392]. Activation of the cGAS-STING pathway enhances anti-tumor immunity through the production of type 1 IFN [393]. PARP inhibitors inhibit base excision repair and induce systemic anti-tumor immunity through cGAS-STING pathway [394]. PARP inhibitor combined with anti-PD-L1 antibody further activated T cells killing compared with anti-PD-L1 antibody monotherapy [48]. Moreover, pamiparib as a PARP inhibitor combined with anti-PD-L1 antibody also inhibited tumor cell proliferation in a PDAC mouse model [392]. However, there is a need for further validation of the efficacy of this combination in the treatment of a wider range of tumors.
ATM/ATR/CHK1 pathway inhibitor
The ATM/ATR/CHK1 pathway is involved in regulating the cell cycle and could repair cellular damage [391]. ATR inhibitors regulate TME by producing type 1 IFN through activation of cGAS-STING pathway to improve anti-tumor immunity [395]. ATR inhibitors also enhance the killing effect of T cells on tumor cells by down-regulating PD-L1 levels [396]. ATR inhibitor combined with anti-PD-L1 antibody prolonged survival time and induced effective anti-tumor immunity in mice in the RM-1-BM syngeneic prostatic cancer model [397]. Moreover, ATM reduced tumor growth and enhanced the efficacy of anti-PD-1 antibody by activating the cGAS-STING pathway and promoting cytoplasmic leakage of mitochondrial DNA, which was confirmed in the B16F10 tumor mouse model with ATM gene knockout [398]. Furthermore, CHK1 inhibitors enhanced the expression of type I IFN, release of chemokines such as chemokine (C-X-C motif) ligand 10 and CCL5, and infiltration of CD8+ T cells by activating the STING-TBK1-IRF3 pathway [391]. Besides, prexasertib plus anti-PD-L1 antibody induced CD8+ T cells infiltration and Tregs depletion, leading to significant tumor regression according to a immunoactive SCLC mouse model [399]. In addition, it is necessary to evaluate the efficacy of this combination in more types of tumors.
WEE1 inhibitor
WEE1 is a tyrosine kinase that inhibits CDK1/2, which could prevent cell mitosis during DNA damage by activating G2-M cell cycle checkpoint [400]. WEE1 inhibitor blocks the activation of G2-M cell cycle checkpoint, resulting in abnormal cell mitosis and death [401]. In genetically engineered mouse model (GEMM) of SCLC, WEE1 inhibitor combined with anti-PD-L1 antibody enhanced type 1 and type 2 IFN pathway activation and recruitment of CD8+ T cells, and led to significant inhibition of tumor growth [402]. Therefore, ICIs combined with WEE1 inhibitors could be an attractive strategy for cancer treatment. Moreover, it is necessary to verify the effectiveness of this combination in more types of tumors in the next phase of the study.
ICIs combined with tumor treating fields
TTFields are a non-invasive therapy that uses low-intensity and medium-frequency alternating electric fields to disrupt the mitosis process of tumor cells selectively [403]. TTFields enhance ICD, induce DCs maturation, and leukocyte infiltration and reduce the tumor volume in a lung cancer mouse model when combined with anti-PD-1 treatment [49]. Furthermore, they induce CD8+ T cells infiltration and significant tumor regression in a CT26 tumor mouse model [49]. Moreover, combined treatment with TTFields and anti-PD-1 or anti-CTLA-4 antibodies also demonstrated significant tumor control effects and anti-tumor immune responses in an NSCLC mouse model [404]. Consequently, the combination of TTFields with ICIs is a feasible therapeutic strategy to enhance the effect of anti-tumor immunotherapy. However, TTFields still have a significant potential for development, and more studies are needed to observe the therapeutic effect of this combination on other types of tumors.
ICIs combined with sonodynamic therapy
SDT is a combination of low-intensity ultrasound and ultrasound sensitizers to produce reactive oxygen species (ROS), which enhances cytotoxicity and leads to cell death [405]. The combination of SDT and anti-PD-1 antibody increased the recruitment of CD8+ T cells and CD4+ T cells and enhanced the tumor control of anti-PD-1 antibody monotherapy according to a mouse model of pancreatic cancer [406]. The sPD-1/Ce6-NBs is a nanobubbles with both PD-1 blocking activity and SDT effect, which leads to ICD of HCC cells in mice [50]. Therefore, the combination of SDT and ICIs is a promising therapeutic strategy in cancer treatment. In the future, the development of sonosensitizers with stronger ROS generation ability is the next stage of SDT research, and the study of the efficacy of combination therapy on other tumor types is also critical.
ICIs combined with fucoidan
Fucoidan, a sulfated polysaccharide derived from brown algae, has been shown to act as immunomodulators, exhibiting both pro- and anti-inflammatory effects, as well as having anti-tumor properties in vivo and in vitro [407]. When combined with anti-PD-1 antibody treatment, dietary fucoidan was found to induce an increase in CD8+ T cells, NK cells and TILs and inhibit tumor growth in melanoma mice [408]. In mouse models of B16F10 and CT26 tumors, intranasal Ecklonia cava-extracted fucoidan in combination with anti-PD-L1 antibody similarly induced CTLs and NK cells activation, and inhibiting the growth of metastatic lung cancer tissues [51]. Similarly, combination of fucoidan and anti-PD-L1 antibody treatment extended survival rates beyond the use of monotherapy in a mouse harboring Lewis cells model [409].
Therefore, fucoidans are often used as dietary modulators to regulate anti-tumor immunity. Mucosal immunostimulants have also been developed. Overall, the synergistic effect of combining fucoidan with ICIs treatment enhances the anti-tumor effect, but the effect on other tumors needs to be further evaluated.
Others
In addition to the methods mentioned above, there are several other approaches to enhance the efficacy of ICIs. Hypoxia-inducible factor-1α (HIF-1α) promotes EMT and reduces TILs by enhancing tumor cell invasion ability [410]. Combining HIF-1α inhibitor PX-478 with anti-PD-1 antibody increases CD8+ T cells infiltration and granulocyte B release, further inhibit tumor growth and prolong survival compared with anti-PD-1 antibody monotherapy in the treatment of NSCLC, thus improving immunotherapy response [411]. In addition, P21-activated kinase 4 inhibitor KPT-9274 improves anti-PD-1 antibody efficacy against melanoma by increasing CD8+ T cells [412]. Fluorinated mitochondria-disrupting helical polypeptides release danger-associated molecular patterns, induce ICD, enhance CD4+ T cell activation, CD8+ T cells activation, APC activation, and anti-tumor immunity, and combined with anti-PD-L1 antibody could eliminate breast cancer and inhibit metastasis in mice [413]. Cowpea mosaic virus (CPMV) is a plant virus, inoculation with CPMV could promote the secretion of various cytokines and increase the infiltration of CTLs [414, 415]. CPMV combined with anti-PD-1 antibody prolonged survival and inhibited tumor growth in ovarian cancer mice, and the combination of CPMV with agonistic OX40-specific antibody produces similar effects in B16F10 and CT26 tumor mouse models [416]. Anti-PD-1 antibody plus anti-PD-L1 antibody had a poor response in the mouse model of late-stage metastatic orthotopic CRC, but the addition of camptothecin induced ICD and significant CTLs responses resulting in tumor regression and metastasis inhibition, and prolonged survival of the mice [417]. The receptor activator of NF-κB ligand/receptor activator of NF-κB axis regulates bone remodeling, promotes tumor growth and metastasis, and increases TAM recruitment to TME [418, 419]. In CRC, blocking METTL3 expression inhibits MDSCs aggregation in TME, promoting CD4+ T cells proliferation and CD8+ T cells infiltration [420]. Combining ICIs with METTL3 knockdown achieves complete tumor regression in 60% of mice according to a mouse model of the human immune system [420].
In conclusion, the combination of these strategies with ICIs to improve ICIs efficacy is promising. In the future, more combination therapies need to be explored to open up new avenues for tumor treatment.
Ongoing clinical trails
A large number of clinical trials on the combination strategy of ICIs, involving a wide range of tumors, but mainly focusing on the combination of chemotherapy, radiotherapy, targeted therapy and other ICIs. In addition, combinations with oncolytic viruses, tumor vaccines, TTFields, DDR, and epigenetic drugs are also hot spots in clinical trials. There are 1718 ongoing clinical trials of combination therapy with ICIs. The largest number of clinical trials were for lung cancer with 400, followed by melanoma with 241, and head and neck squamous cell carcinoma is also a hot research topic with 156. The summary of the number of clinical trial projects for other tumors is as follows: 155 for breast cancer, 128 for RCC, 124 for liver cancer, 122 for CRC, 93 for gastric cancer, 91 for cervical cancer, 86 for ovarian cancer, 77 for esophageal cancer, 57 for pancreatic cancer, 35 for glioma, 31 for Hodgkin lymphoma, 31 for metastatic urothelial carcinoma, 26 for bile duct cancer, 25 for nasopharyngeal cancer, 15 for thyroid cancer, 14 for peritoneal carcinoma, and 9 for multiple myeloma. Most of the combination regimens were double or triple therapy, and the end points were the efficacy and safety of the combination strategy. Therefore, these ongoing trials will provide more strategies to enhance the therapeutic efficacy of anti-PD-1 antibody, anti-PD-L1 antibody and anti-CTLA-4 antibody in cancer therapy.
Conclusion
ICIs, particularly anti-PD-1 antibodies, anti-PD-L1 antibodies, and anti-CTLA-4 antibodies, have revolutionized immunotherapy in clinical practice, providing survival benefits for numerous cancer patients. However, the efficacy of ICIs is hindered by immune drug resistance. Consequently, combination therapy has emerged as an effective strategy to address these challenges (Fig. 1).
A summary of therapeutic strategies to enhance the effect of anti-PD-1antibody, anti-PD-L1 antibody and anti-CTLA-4 antibody. These strategies are divided into those that have been applied in clinical practice and those that are under preclinical investigation. EGFR-TKI: epidermal growth factor receptor‑tyrosine kinase inhibitor; ICIs: immune checkpoint inhibitors; CDKs inhibitors: cyclin dependent kinases inhibitors; PDT: photodynamic therapy; PTT: photothermal therapy; ACT: adoptive cell transfer therapy; NO: nitric oxide; non-apoptotic RCD: non-apoptotic regulated cell death; SDT: sonodynamic therapy; TTFields: tumor treating fields
The killing of tumor by ICIs is a complex process, which is affected by many factors, so the mechanism of drug resistance is also complex and diverse [421]. Low tumor mutation burden means that the tumor expresses fewer tumor-associated neoantigens, which leads to diminished effector T-cell recognition and killing [422,423,424,425]. In addition, the absence or low expression of PD-L1 makes patients more susceptible to immune resistance to anti-PD-1 antibody and anti-PD-L1 antibody [426]. Moreover, alterations in key anti-tumor pathways such as activation of the MAPK signaling pathway, deletion of the PTEN gene, mutation or deletion of the IFN-γ signaling pathway and related genes could lead to the development of immune resistance in tumor cells [427,428,429,430,431]. Besides, β-2 microglobulin gene mutations, target antigen modifications, and secondary alterations in HLA class I molecular structure may disrupt antigen presentation, resulting in reduced CD8+ T cell recognition [432,433,434]. Furthermore, the expression of other immune checkpoints, such as LAG-3, TIGIT, V-domain Ig suppressor of T cell activation and TIM-3, may be increased after treatment with ICIs, leading to immune resistance [435,436,437,438].
ICIs combined with alternative immune checkpoint blockade could be a way to overcome ICIs resistance [439]. In addition, the TME could be reprogrammed to an ICIs-responsive state by inducing ICD through radiotherapy and chemotherapy as well as by altering antigen presentation and initiating immune responses such as lysosomal viruses, CAR-T cell therapies, cancer vaccines, adoptive transfer and activation of NK cells,and use of TLR agonists and type I interferons [440]. In addition, inhibitors of oncogenic signaling pathways including MAPK signaling pathway, Wnt/β-catenin signaling pathway and others are also a critical part of overcoming ICIs resistance [440,441,442]. Therefore, the development of immune resistance in cancer patients following ICIs treatment is a multifaceted issue, and how to solve this problem is a systematic project that needs to be further elucidated in subsequent studies.
Currently, the approved combinations involve ICIs with chemotherapy or antiangiogenic agents and the use of dual ICIs. Other treatments such as radiotherapy, EGFR-TKI, vitamin E, IL-2, NK cell infusion, oncolytic virus and ablation combined with ICIs have shown positive therapeutic effects in clinical and preclinical studies. Tumor vaccines, ACTS, cytokines, targeting co-stimulatory receptors, targeting co-inhibitory receptors, and targeting innate immune pathways are promising immunotherapy strategies that can induce TME in a state of immune activation and enhance the efficacy of ICIs treatment. Nanotechnology serves as a multifunctional platform that could complement various therapies by enhancing both the effectiveness of nanoparticle optimized therapy and the immunogenicity of ICIs to further strengthen the anti-tumor immune response. Regulation of non-apoptotic RCD, epigenetic mechanisms, and DDR are also potential strategies to enhance the therapeutic effects of ICIs. Furthermore, other physiotherapy methods including PTT, PDT, focused ultrasound, SDT, TTFileds, and radionuclide image-guided local release of ICIs have also improved the efficiency of ICIs treatment. Moreover, the effects of antihistamines, metformin, vitamin C, local delivery of NO, regulation of the intestinal microbiome, injection of magnesium, regulation of dietary mode, signaling pathway inhibitors, targeting metabolic pathways, targeting sex hormone receptors, and application of fucoidans in combination with ICIs have been demonstrated in various mouse tumor models. Notably, determining the appropriate dose, timing, and sequence of administration are challenges when developing a combination therapy strategy. Besides, identifying suitable biomarkers that could predict efficacy is crucial for patients to select the most suitable combination therapy. As clinical and preclinical studies continue to advance, more strategies will be proposed and validated to achieve better efficacy and higher safety of ICIs.
Availability of data and materials
Not applicable.
Abbreviations
- ICIs:
-
Immune checkpoint inhibitors
- PD-1:
-
Programmed cell death 1
- PD-L1:
-
Programmed death ligand 1
- CTLA4:
-
Cytotoxic T-lymphocyte-associated protein 4
- NK:
-
Natural killer
- DCs:
-
Dendritic cells
- MDSCs:
-
Marrow derived suppressor cells
- Tregs:
-
Regulatory T cells
- TME:
-
Tumor microenvironment
- EGFR-TKI:
-
Epidermal growth factor receptor‑tyrosine kinase inhibitor
- CDKs:
-
Cyclin dependent kinases inhibitors
- PTT:
-
Photothermal therapy
- PDT:
-
Photodynamic therapy
- ACT:
-
Adoptive cell transfer therapy
- RCD:
-
Regulated cell death
- NO:
-
Nitric oxide
- KD:
-
Ketogenic diets
- DDR:
-
DNA damage response
- TTFields:
-
Tumor treating fields
- SDT:
-
Sonodynamic therapy
- ICD:
-
Immunogenic cell death
- CRC:
-
Colorectal cancer
- PFS:
-
Progression-free survival
- NSCLC:
-
Non-small cell lung cancer
- SCLC:
-
Small-cell lung cancer
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- OS:
-
Overall survival
- TNBC:
-
Triple-negative breast cancer
- IFN-γ:
-
Interferon-γ
- ORR:
-
Objective response rate
- HCC:
-
Hepatocellular carcinoma
- RCC:
-
Renal cell carcinoma
- SBRT:
-
Stereotactic body radiation therapy
- EGFR:
-
Epidermal growth factor receptor
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
VEGF receptor
- TAM:
-
Tumor associated macrophages
- FDA:
-
Food and Drug Administration
- MSI-H/dMMR mCRC:
-
Microsatellite instability-high/mismatch repair-deficient metastatic CRC
- PDAC:
-
Pancreatic ductal adenocarcinoma
- RFA:
-
Radiofrequency ablation
- MWA:
-
Microwave ablation
- IRE:
-
Irreversible electroporation
- IL:
-
Interleukin
- TILs:
-
Tumor infiltrating lymphocyte
- P-HIFU:
-
Pulsed high-intensity focused ultrasound
- M-HIFU:
-
Mechanical high-intensity focused ultrasound
- APCs:
-
Antigen presenting cells
- EMT:
-
Epithelial-mesenchymal transition
- NASH:
-
Non-alcoholic steatohepatitis
- CAR:
-
Chimeric antigen receptor
- TCR:
-
T cell receptor
- CCL:
-
C–C chemokine ligand
- TLR:
-
Toll-like receptors
- IFN-α:
-
Interferon-α
- TGF-β:
-
Transforming growth factor-β
- FMT:
-
Fecal microbiota transplantation
- CTLs:
-
Cytotoxic T lymphocytes
- MAPK:
-
Mitogen-activated protein kinase
- PRRs:
-
Pattern recognition receptors
- RIG-I:
-
Retinoic acid-inducible gene-I
- RLRs:
-
RIG-I-like receptors
- STING:
-
Stimulator of interferon genes
- FAO:
-
Fatty acid oxidation
- AMPK:
-
Adenosine 5ʹmonophosphate-activated protein kinase
- eADO:
-
Extracellular adenosine
- IDO:
-
Indoleamine-2,3 dioxygenase
- TDO:
-
Tryptophan-2,3 dioxygenase
- MRD:
-
Methionine-restricted diet
- AR:
-
Androgen receptor
- ERα:
-
Estrogen receptor α
- LFA-1:
-
Leukocyte function-associated antigen 1
- TNFRSF:
-
Tumor necrosis factor receptor superfamily
- GITR:
-
Glucocorticoid induced tumor necrosis factor receptor
- IgSF:
-
Immunoglobulin superfamily
- ICOS:
-
Induced T cells co-stimulation
- TIM3:
-
T cell immunoglobulin and mucin domain-containing protein 3
- TIGIT:
-
T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain
- BTLA:
-
B and T lymphocyte attenuator
- HMTi:
-
Histone methyltransferase inhibitors
- HDACi:
-
Histone deacetylase inhibitors
- DNMTi:
-
DNA methyltransferase inhibitors
- HDAC:
-
Histone deacetylases
- HMT:
-
Histone methyltransferase
- DNMTs:
-
DNA methyltransferases
- CircRNAs:
-
Circular RNA
- SPECT:
-
Single photon emission computed tomography
- CT:
-
Computed tomography
- CPMV:
-
Cowpea mosaic virus
References
Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. 2022;21(7):509–28.
Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D, et al. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol. 2021;14(1):156.
Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. 2021;398(10304):1002–14.
Tang S, Qin C, Hu H, Liu T, He Y, Guo H, et al. Immune checkpoint inhibitors in non-small cell lung cancer: progress, challenges, and prospects. Cells. 2022;11(3):320.
Thana M, Wood L. Immune checkpoint inhibitors in genitourinary malignancies. Curr Oncol. 2020;27(Suppl 2):S69-s77.
Lavacchi D, Pellegrini E, Palmieri VE, Doni L, Mela MM, Di Maida F, et al. Immune checkpoint inhibitors in the treatment of renal cancer: current state and future perspective. Int J Mol Sci. 2020;21(13):4691.
Donisi C, Puzzoni M, Ziranu P, Lai E, Mariani S, Saba G, et al. Immune Checkpoint Inhibitors in the Treatment of HCC. Front Oncol. 2020;10:601240.
Zhu Y, Qin LX. Strategies for improving the efficacy of immunotherapy in hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2022;21(5):420–9.
Gerard CL, Delyon J, Wicky A, Homicsko K, Cuendet MA, Michielin O. Turning tumors from cold to inflamed to improve immunotherapy response. Cancer Treat Rev. 2021;101:102227.
Piñero F, Dirchwolf M, Pessôa MG. Biomarkers in hepatocellular carcinoma: diagnosis, prognosis and treatment response assessment. Cells. 2020;9(6):1370.
Xue Y, Gao S, Gou J, Yin T, He H, Wang Y, et al. Platinum-based chemotherapy in combination with PD-1/PD-L1 inhibitors: preclinical and clinical studies and mechanism of action. Expert Opin Drug Deliv. 2021;18(2):187–203.
Gao X, McDermott DF. Ipilimumab in combination with nivolumab for the treatment of renal cell carcinoma. Expert Opin Biol Ther. 2018;18(9):947–57.
Geng Y, Zhang Q, Feng S, Li C, Wang L, Zhao X, et al. Safety and Efficacy of PD-1/PD-L1 inhibitors combined with radiotherapy in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Cancer Med. 2021;10(4):1222–39.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–27.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–46.
Isomoto K, Haratani K, Hayashi H, Shimizu S, Tomida S, Niwa T, et al. Impact of EGFR-TKI treatment on the tumor immune microenvironment in EGFR mutation-positive non-small cell lung cancer. Clin Cancer Res. 2020;26(8):2037–46.
Hashimoto M, Araki K, Cardenas MA, Li P, Jadhav RR, Kissick HT, et al. PD-1 combination therapy with IL-2 modifies CD8(+) T cell exhaustion program. Nature. 2022;610(7930):173–81.
Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66(3):545–51.
Kim EJ, Cho YH, Kim DH, Ko DH, Do EJ, Kim SY, et al. A phase I/IIa randomized trial evaluating the safety and efficacy of SNK01 Plus pembrolizumab in patients with stage IV non-small cell lung cancer. Cancer Res Treat. 2022;54(4):1005–16.
Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109-19.e10.
LaMarche NM, Hegde S, Park MD, Maier BB, Troncoso L, Le Berichel J, et al. An IL-4 signalling axis in bone marrow drives pro-tumorigenic myelopoiesis. Nature. 2024;625(7993):166–74.
Wang J, Zhang R, Lin Z, Zhang S, Chen Y, Tang J, et al. CDK7 inhibitor THZ1 enhances antiPD-1 therapy efficacy via the p38alpha/MYC/PD-L1 signaling in non-small cell lung cancer. J Hematol Oncol. 2020;13(1):99.
Yuan X, Duan Y, Xiao Y, Sun K, Qi Y, Zhang Y, et al. Vitamin E enhances cancer immunotherapy by reinvigorating dendritic cells via targeting checkpoint SHP1. Cancer Discov. 2022;12(7):1742–59.
Shi L, Chen L, Wu C, Zhu Y, Xu B, Zheng X, et al. PD-1 blockade boosts radiofrequency ablation-elicited adaptive immune responses against tumor. Clin Cancer Res. 2016;22(5):1173–84.
Hwang J, An EK, Zhang W, Park HB, Kim SJ, Yadav D, et al. Recombinant programmed cell death protein 1 functions as an immune check point blockade and enhances anti-cancer immunity. Biomaterials. 2022;285:121550.
Abe S, Nagata H, Crosby EJ, Inoue Y, Kaneko K, Liu CX, et al. Combination of ultrasound-based mechanical disruption of tumor with immune checkpoint blockade modifies tumor microenvironment and augments systemic antitumor immunity. J Immunother Cancer. 2022;10(1): e003717.
Luchtel RA, Bhagat T, Pradhan K, Jacobs WR Jr, Levine M, Verma A, et al. High-dose ascorbic acid synergizes with anti-PD1 in a lymphoma mouse model. Proc Natl Acad Sci USA. 2020;117(3):1666–77.
Zamarin D, Walderich S, Holland A, Zhou Q, Iasonos AE, Torrisi JM, et al. Safety, immunogenicity, and clinical efficacy of durvalumab in combination with folate receptor alpha vaccine TPIV200 in patients with advanced ovarian cancer: a phase II trial. J Immunother Cancer. 2020;8(1): e000829.
Grauers Wiktorin H, Nilsson MS, Kiffin R, Sander FE, Lenox B, Rydström A, et al. Histamine targets myeloid-derived suppressor cells and improves the anti-tumor efficacy of PD-1/PD-L1 checkpoint blockade. Cancer Immunol Immunother. 2019;68(2):163–74.
Wang Z, Lu C, Zhang K, Lin C, Wu F, Tang X, et al. Metformin combining PD-1 inhibitor enhanced anti-tumor efficacy in STK11 mutant lung cancer through AXIN-1-dependent inhibition of STING ubiquitination. Front Mol Biosci. 2022;9:780200.
Kuai R, Yuan W, Son S, Nam J, Xu Y, Fan Y, et al. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci Adv. 2018;4(4): eaao1736.
Hu Q, Li H, Archibong E, Chen Q, Ruan H, Ahn S, et al. Inhibition of post-surgery tumour recurrence via a hydrogel releasing CAR-T cells and anti-PDL1-conjugated platelets. Nat Biomed Eng. 2021;5(9):1038–47.
Ngiow SF, Young A, Blake SJ, Hill GR, Yagita H, Teng MW, et al. Agonistic CD40 mAb-driven IL12 reverses resistance to anti-PD1 in a T-cell-rich tumor. Cancer Res. 2016;76(21):6266–77.
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–9.
Chon HJ, Lee WS, Yang H, Kong SJ, Lee NK, Moon ES, et al. Tumor microenvironment remodeling by intratumoral oncolytic vaccinia virus enhances the efficacy of immune-checkpoint blockade. Clin Cancer Res. 2019;25(5):1612–23.
Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9(1):57–63.
Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581(7806):100–5.
Theivendran S, Gu Z, Tang J, Yang Y, Song H, Yang Y, et al. Nanostructured organosilica nitric oxide donors intrinsically regulate macrophage polarization with antitumor effect. ACS Nano. 2022. https://doi.org/10.1021/acsnano.2c03348.
Dai X, Bu X, Gao Y, Guo J, Hu J, Jiang C, et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol Cell. 2021;81(11):2317-31.e6.
Lin M, Luo H, Liang S, Chen J, Liu A, Niu L, et al. Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J Clin Invest. 2020;130(5):2560–9.
Chakraborty B, Byemerwa J, Shepherd J, Haines CN, Baldi R, Gong W, et al. Inhibition of estrogen signaling in myeloid cells increases tumor immunity in melanoma. J Clin Invest. 2021;131(23): e151347.
Lötscher J, Martí ILAA, Kirchhammer N, Cribioli E, Giordano Attianese GMP, Trefny MP, et al. Magnesium sensing via LFA-1 regulates CD8(+) T cell effector function. Cell. 2022;185(4):585-602.e29.
Chan S, Belmar N, Ho S, Rogers B, Stickler M, Graham M, et al. An anti-PD-1-GITR-L bispecific agonist induces GITR clustering-mediated T cell activation for cancer immunotherapy. Nat Cancer. 2022;3(3):337–54.
Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24–34.
Cortellino S, Raveane A, Chiodoni C, Delfanti G, Pisati F, Spagnolo V, et al. Fasting renders immunotherapy effective against low-immunogenic breast cancer while reducing side effects. Cell Rep. 2022;40(8):111256.
Zheng H, Zhao W, Yan C, Watson CC, Massengill M, Xie M, et al. HDAC inhibitors enhance T-cell chemokine expression and augment response to PD-1 immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2016;22(16):4119–32.
Wen X, Zeng X, Cheng X, Zeng X, Liu J, Zhang Y, et al. PD-L1-targeted radionuclide therapy combined with αPD-L1 antibody immunotherapy synergistically improves the antitumor effect. Mol Pharm. 2022;19(10):3612–22.
Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23(14):3711–20.
Voloshin T, Kaynan N, Davidi S, Porat Y, Shteingauz A, Schneiderman RS, et al. Tumor-treating fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy. Cancer Immunol Immunother. 2020;69(7):1191–204.
Tan Y, Yang S, Ma Y, Li J, Xie Q, Liu C, et al. Nanobubbles containing sPD-1 and Ce6 mediate combination immunotherapy and suppress hepatocellular carcinoma in mice. Int J Nanomedicine. 2021;16:3241–54.
Zhang W, Hwang J, Yadav D, An EK, Kwak M, Lee PC, et al. Enhancement of immune checkpoint inhibitor-mediated anti-cancer immunity by intranasal treatment of ecklonia cava fucoidan against metastatic lung cancer. Int J Mol Sci. 2021;22(17):9125.
Salas-Benito D, Perez-Gracia JL, Ponz-Sarvise M, Rodriguez-Ruiz ME, Martinez-Forero I, Castanon E, et al. Paradigms on immunotherapy combinations with chemotherapy. Cancer Discov. 2021;11(6):1353–67.
Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17(12):725–41.
Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17(5):286–301.
Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–61.
Wang W, Kryczek I, Dostál L, Lin H, Tan L, Zhao L, et al. Effector T cells abrogate stroma-mediated chemoresistance in ovarian cancer. Cell. 2016;165(5):1092–105.
Burtness B, Rischin D, Greil R, Soulieres D, Tahara M, de Castro G, Jr, et al. Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048: subgroup analysis by programmed death ligand-1 combined positive score. J Clin Oncol. 2022;40(21):2321–32.
Zheng Y, Wang S, Cai J, Ke A, Fan J. The progress of immune checkpoint therapy in primary liver cancer. Biochim Biophys Acta Rev Cancer. 2021;1876(2):188638.
Noori M, Mahjoubfar A, Azizi S, Fayyaz F, Rezaei N. Immune checkpoint inhibitors plus chemotherapy versus chemotherapy alone as first-line therapy for advanced gastric and esophageal cancers: a systematic review and meta-analysis. Int Immunopharmacol. 2022;113(Pt A):109317.
Oliveira AF, Bretes L, Furtado I. Review of PD-1/PD-L1 inhibitors in metastatic dMMR/MSI-H colorectal cancer. Front Oncol. 2019;9:396.
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21.
Sugawara S, Lee JS, Kang JH, Kim HR, Inui N, Hida T, et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2021;32(9):1137–47.
Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–9.
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. The Lancet. 2019;394(10212):1929–39.
Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of > 2 years of follow-up. Ann Oncol. 2019;30(6):970–6.
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–26.
Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. The Lancet. 2020;395(10236):1547–57.
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–9.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10.
Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–71.
Storozynsky Q, Hitt MM. The impact of radiation-induced DNA damage on cGAS-STING-mediated immune responses to cancer. Int J Mol Sci. 2020;21(22):8877.
Dovedi SJ, Cheadle EJ, Popple AL, Poon E, Morrow M, Stewart R, et al. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res. 2017;23(18):5514–26.
Lee YH, Tai D, Yip C, Choo SP, Chew V. Combinational immunotherapy for hepatocellular carcinoma: radiotherapy, immune checkpoint blockade and beyond. Front Immunol. 2020;11:568759.
Masterson L, Howard J, Gonzalez-Cruz J, Jackson C, Barnett C, Overton L, et al. Immune checkpoint inhibitors in advanced nasopharyngeal carcinoma: beyond an era of chemoradiation? Int J Cancer. 2020;146(8):2305–14.
Jungles KM, Holcomb EA, Pearson AN, Jungles KR, Bishop CR, Pierce LJ, et al. Updates in combined approaches of radiotherapy and immune checkpoint inhibitors for the treatment of breast cancer. Front Oncol. 2022;12:1022542.
Ben Shimol J, Guzman-Prado Y, Karlinskaya M, Davidson T. Effectiveness and safety of immune checkpoint inhibitors in combination with palliative radiotherapy in advanced melanoma: a systematic review. Crit Rev Oncol Hematol. 2021;167:103499.
Kline C, Liu SJ, Duriseti S, Banerjee A, Nicolaides T, Raber S, et al. Reirradiation and PD-1 inhibition with nivolumab for the treatment of recurrent diffuse intrinsic pontine glioma: a single-institution experience. J Neurooncol. 2018;140(3):629–38.
Wu C, Shao Y, Gu W. Immunotherapy combined with radiotherapy to reverse immunosuppression in microsatellite stable colorectal cancer. Clin Transl Oncol. 2023;25(7):1916–28.
Mo DC, Huang JF, Luo PH, Huang SX, Wang HL. Combination therapy with immune checkpoint inhibitors in advanced renal cell carcinoma: a meta-analysis of randomized controlled trials. Clin Immunol. 2021;232:108876.
Donlon NE, Power R, Hayes C, Reynolds JV, Lysaght J. Radiotherapy, immunotherapy, and the tumour microenvironment: Turning an immunosuppressive milieu into a therapeutic opportunity. Cancer Lett. 2021;502:84–96.
Chen Y, Gao M, Huang Z, Yu J, Meng X. SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: a focus on the mechanisms, advances, and future challenges. J Hematol Oncol. 2020;13(1):105.
Theelen W, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts J, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1276–82.
Jiang J, Diaz DA, Nuguru SP, Mittra A, Manne A. Stereotactic body radiation therapy (SBRT) plus immune checkpoint inhibitors (ICI) in hepatocellular carcinoma and cholangiocarcinoma. Cancers. 2022;15(1):50.
Peng J, Lalani AK, Swaminath A. Cytoreductive stereotactic body radiotherapy (SBRT) and combination SBRT with immune checkpoint inhibitors in metastatic renal cell carcinoma. Can Urol Assoc J. 2021;15(8):281–6.
Hui C, Chau B, Gan G, Stokes W, Karam SD, Amini A. Overcoming resistance to immunotherapy in head and neck cancer using radiation: a review. Front Oncol. 2021;11:592319.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37.
Lizotte PH, Hong RL, Luster TA, Cavanaugh ME, Taus LJ, Wang S, et al. A high-throughput immune-oncology screen identifies EGFR inhibitors as potent enhancers of antigen-specific cytotoxic T-lymphocyte tumor cell killing. Cancer Immunol Res. 2018;6(12):1511–23.
Mallmann-Gottschalk N, Sax Y, Kimmig R, Lang S, Brandau S. EGFR-specific tyrosine kinase inhibitor modifies NK cell-mediated antitumoral activity against ovarian cancer cells. Int J Mol Sci. 2019;20(19):4693.
Sugiyama E, Togashi Y, Takeuchi Y, Shinya S, Tada Y, Kataoka K, et al. Blockade of EGFR improves responsiveness to PD-1 blockade in EGFR-mutated non-small cell lung cancer. Sci Immunol. 2020;5(43): eaav3937.
Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. 2021;14(1):10.
Gettinger S, Hellmann MD, Chow LQM, Borghaei H, Antonia S, Brahmer JR, et al. Nivolumab plus erlotinib in patients with EGFR-mutant advanced NSCLC. J Thorac Oncol. 2018;13(9):1363–72.
Song Y, Fu Y, Xie Q, Zhu B, Wang J, Zhang B. Anti-angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Front Immunol. 2020;11:1956.
Hack SP, Zhu AX, Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: challenges and opportunities. Front Immunol. 2020;11:598877.
Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020;52(9):1475–85.
Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18(1):60.
Ren S, Xiong X, You H, Shen J, Zhou P. The combination of immune checkpoint blockade and angiogenesis inhibitors in the treatment of advanced non-small cell lung cancer. Front Immunol. 2021;12:689132.
Matsumoto K, Shiroyama T, Kuge T, Miyake K, Yamamoto Y, Yoneda M, et al. Impact of treatment timing and sequence of immune checkpoint inhibitors and anti-angiogenic agents for advanced non-small cell lung cancer: a systematic review and meta-analysis. Lung Cancer. 2021;162:175–84.
Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72(2):307–19.
Wang Y, Wei B, Gao J, Cai X, Xu L, Zhong H, et al. Combination of fruquintinib and anti-PD-1 for the treatment of colorectal cancer. J Immunol. 2020;205(10):2905–15.
Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–73.
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–41.
Motzer RJ, Robbins PB, Powles T, Albiges L, Haanen JB, Larkin J, et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat Med. 2020;26(11):1733–41.
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905.
Makker V, Rasco D, Vogelzang NJ, Brose MS, Cohn AL, Mier J, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):711–8.
Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544(7649):250–4.
Kähler KC, Hassel JC, Heinzerling L, Loquai C, Thoms KM, Ugurel S, et al. Side effect management during immune checkpoint blockade using CTLA-4 and PD-1 antibodies for metastatic melanoma—an update. J Dtsch Dermatol Ges. 2020;18(6):582–609.
Wong JSL, Kwok GGW, Tang V, Li BCW, Leung R, Chiu J, et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J Immunother Cancer. 2021;9(2): e001945.
Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–9.
Reck M, Borghaei H, O’Byrne KJ. Nivolumab plus ipilimumab in non-small-cell lung cancer. Future Oncol. 2019;15(19):2287–302.
Kudo M. Durvalumab plus tremelimumab in unresectable hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2022;11(4):592–6.
Mo DC, Huang JF, Luo PH, Huang SX, Wang HL. The efficacy and safety of combination therapy with immune checkpoint inhibitors in non-small cell lung cancer: a meta-analysis. Int Immunopharmacol. 2021;96:107594.
Guy C, Mitrea DM, Chou PC, Temirov J, Vignali KM, Liu X, et al. LAG3 associates with TCR-CD3 complexes and suppresses signaling by driving co-receptor-Lck dissociation. Nat Immunol. 2022;23(5):757–67.
Wei Y, Li Z. LAG3-PD-1 combo overcome the disadvantage of drug resistance. Front Oncol. 2022;12:831407.
Atkinson V, Khattak A, Haydon A, Eastgate M, Roy A, Prithviraj P, et al. Eftilagimod alpha, a soluble lymphocyte activation gene-3 (LAG-3) protein plus pembrolizumab in patients with metastatic melanoma. J Immunother Cancer. 2020;8(2): e001681.
Tarhini A, Benedict A, McDermott D, Rao S, Ambavane A, Gupte-Singh K, et al. Sequential treatment approaches in the management of BRAF wild-type advanced melanoma: a cost-effectiveness analysis. Immunotherapy. 2018;10(14):1241–52.
Eschweiler S, Clarke J, Ramírez-Suástegui C, Panwar B, Madrigal A, Chee SJ, et al. Intratumoral follicular regulatory T cells curtail anti-PD-1 treatment efficacy. Nat Immunol. 2021;22(8):1052–63.
Lee GY, Han SN. The role of vitamin E in immunity. Nutrients. 2018;10(11):1614.
Laskowski TJ, Biederstädt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer. 2022;22(10):557–75.
Jhawar SR, Thandoni A, Bommareddy PK, Hassan S, Kohlhapp FJ, Goyal S, et al. Oncolytic viruses-natural and genetically engineered cancer immunotherapies. Front Oncol. 2017;7:202.
Harrington K, Freeman DJ, Kelly B, Harper J, Soria JC. Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov. 2019;18(9):689–706.
Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3(6):841–9.
Cripe TP, Ngo MC, Geller JI, Louis CU, Currier MA, Racadio JM, et al. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol Ther. 2015;23(3):602–8.
Lee YS, Lee WS, Kim CW, Lee SJ, Yang H, Kong SJ, et al. Oncolytic vaccinia virus reinvigorates peritoneal immunity and cooperates with immune checkpoint inhibitor to suppress peritoneal carcinomatosis in colon cancer. J Immunother Cancer. 2020;8(2): e000857.
Nakao S, Arai Y, Tasaki M, Yamashita M, Murakami R, Kawase T, et al. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Sci Transl Med. 2020;12(526): eaax7992.
Smelser WW, Wang J, Ogden KM, Chang SS, Kirschner AN. Intravesical oncolytic virotherapy and immunotherapy for non-muscle-invasive bladder cancer mouse model. BJU Int. 2023;132(3):298–306.
Panagioti E, Kurokawa C, Viker K, Ammayappan A, Anderson SK, Sotiriou S, et al. Immunostimulatory bacterial antigen-armed oncolytic measles virotherapy significantly increases the potency of anti-PD1 checkpoint therapy. J Clin Invest. 2021;131(13): e141614.
Veinalde R, Pidelaserra-Martí G, Moulin C, Tan CL, Schäfer TE, Kang N, et al. Virotherapy combined with anti-PD-1 transiently reshapes the tumor immune environment and induces anti-tumor immunity in a preclinical PDAC model. Front Immunol. 2022;13:1096162.
Rangamuwa K, Leong T, Weeden C, Asselin-Labat ML, Bozinovski S, Christie M, et al. Thermal ablation in non-small cell lung cancer: a review of treatment modalities and the evidence for combination with immune checkpoint inhibitors. Transl Lung Cancer Res. 2021;10(6):2842–57.
Lubner MG, Brace CL, Hinshaw JL, Lee FT Jr. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21(8 Suppl):S192-203.
Erinjeri JP, Clark TW. Cryoablation: mechanism of action and devices. J Vasc Interv Radiol. 2010;21(8 Suppl):S187–91.
Wagstaff PG, Buijs M, van den Bos W, de Bruin DM, Zondervan PJ, de la Rosette JJ, et al. Irreversible electroporation: state of the art. Onco Targets Ther. 2016;9:2437–46.
McArthur HL, Diab A, Page DB, Yuan J, Solomon SB, Sacchini V, et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin Cancer Res. 2016;22(23):5729–37.
Benzon B, Glavaris SA, Simons BW, Hughes RM, Ghabili K, Mullane P, et al. Combining immune check-point blockade and cryoablation in an immunocompetent hormone sensitive murine model of prostate cancer. Prostate Cancer Prostatic Dis. 2018;21(1):126–36.
Zhu J, Yu M, Chen L, Kong P, Li L, Ma G, et al. Enhanced antitumor efficacy through microwave ablation in combination with immune checkpoints blockade in breast cancer: a pre-clinical study in a murine model. Diagn Interv Imaging. 2018;99(3):135–42.
Wei F, Guo R, Yan Y, Lin R, Chen J, Lin Z. Investigation of the efficacy and safety of cryoablation and intra-arterial PD-1 inhibitor in patients with advanced disease not responding to checkpoint inhibitors: an exploratory study. Front Immunol. 2022;13:990224.
Burbach BJ, O’Flanagan SD, Shao Q, Young KM, Slaughter JR, Rollins MR, et al. Irreversible electroporation augments checkpoint immunotherapy in prostate cancer and promotes tumor antigen-specific tissue-resident memory CD8+ T cells. Nat Commun. 2021;12(1):3862.
Xue D, Hsu E, Fu YX, Peng H. Next-generation cytokines for cancer immunotherapy. Antib Ther. 2021;4(2):123–33.
Sun Z, Ren Z, Yang K, Liu Z, Cao S, Deng S, et al. A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8(+) T-cell response and effective tumor control. Nat Commun. 2019;10(1):3874.
Kaptein P, Jacoberger-Foissac C, Dimitriadis P, Voabil P, de Bruijn M, Brokamp S, et al. Addition of interleukin-2 overcomes resistance to neoadjuvant CTLA4 and PD1 blockade in ex vivo patient tumors. Sci Transl Med. 2022;14(642): eabj9779.
Chatzkel J, Schell MJ, Chahoud J, Zhang J, Jain R, Swank J, et al. Coordinated pembrolizumab and high dose IL-2 (5-in-a-row schedule) for therapy of metastatic clear cell renal cancer. Clin Genitourin Cancer. 2022;20(3):252–9.
Balakrishnan PB, Sweeney EE, Ramanujam AS, Fernandes R. Photothermal therapies to improve immune checkpoint blockade for cancer. Int J Hyperthermia. 2020;37(3):34–49.
Zhi D, Yang T, O’Hagan J, Zhang S, Donnelly RF. Photothermal therapy. J Control Release. 2020;325:52–71.
Huang L, Li Y, Du Y, Zhang Y, Wang X, Ding Y, et al. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat Commun. 2019;10(1):4871.
Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657–74.
Rkein AM, Ozog DM. Photodynamic therapy. Dermatol Clin. 2014;32(3):415–25.
Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250–81.
Cramer GM, Moon EK, Cengel KA, Busch TM. Photodynamic therapy and immune checkpoint blockade(dagger). Photochem Photobiol. 2020;96(5):954–61.
Choi J, Shim MK, Yang S, Hwang HS, Cho H, Kim J, et al. Visible-light-triggered prodrug nanoparticles combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. ACS Nano. 2021;15(7):12086–98.
Chin AL, Jiang S, Jang E, Niu L, Li L, Jia X, et al. Implantable optical fibers for immunotherapeutics delivery and tumor impedance measurement. Nat Commun. 2021;12(1):5138.
O’Shaughnessy MJ, Murray KS, La Rosa SP, Budhu S, Merghoub T, Somma A, et al. Systemic antitumor immunity by PD-1/PD-L1 inhibition is potentiated by vascular-targeted photodynamic therapy of primary tumors. Clin Cancer Res. 2018;24(3):592–9.
Hwang HS, Cherukula K, Bang YJ, Vijayan V, Moon MJ, Thiruppathi J, et al. Combination of photodynamic therapy and a flagellin-adjuvanted cancer vaccine potentiated the anti-PD-1-mediated melanoma suppression. Cells. 2020;9(11):2432.
Lobo CS, Mendes MIP, Pereira DA, Gomes-da-Silva LC, Arnaut LG. Photodynamic therapy changes tumour immunogenicity and promotes immune-checkpoint blockade response, particularly when combined with micromechanical priming. Sci Rep. 2023;13(1):11667.
Zhang L, Jing D, Wang L, Sun Y, Li JJ, Hill B, et al. Unique photochemo-immuno-nanoplatform against orthotopic xenograft oral cancer and metastatic syngeneic breast cancer. Nano Lett. 2018;18(11):7092–103.
Petroni G, Buque A, Zitvogel L, Kroemer G, Galluzzi L. Immunomodulation by targeted anticancer agents. Cancer Cell. 2021;39(3):310–45.
Esteva FJ, Hubbard-Lucey VM, Tang J, Pusztai L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 2019;20(3):e175–86.
Shan W, Yuan J, Hu Z, Jiang J, Wang Y, Loo N, et al. Systematic characterization of recurrent genomic alterations in cyclin-dependent kinases reveals potential therapeutic strategies for cancer treatment. Cell Rep. 2020;32(2):107884.
Petroni G, Formenti SC, Chen-Kiang S, Galluzzi L. Immunomodulation by anticancer cell cycle inhibitors. Nat Rev Immunol. 2020;20(11):669–79.
Klein ME, Kovatcheva M, Davis LE, Tap WD, Koff A. CDK4/6 inhibitors: the mechanism of action may not be as simple as once thought. Cancer Cell. 2018;34(1):9–20.
Zhang QF, Li J, Jiang K, Wang R, Ge JL, Yang H, et al. CDK4/6 inhibition promotes immune infiltration in ovarian cancer and synergizes with PD-1 blockade in a B cell-dependent manner. Theranostics. 2020;10(23):10619–33.
Schaer DA, Beckmann RP, Dempsey JA, Huber L, Forest A, Amaladas N, et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 2018;22(11):2978–94.
Yu J, Yan J, Guo Q, Chi Z, Tang B, Zheng B, et al. Genetic aberrations in the CDK4 pathway are associated with innate resistance to PD-1 blockade in chinese patients with non-cutaneous melanoma. Clin Cancer Res. 2019;25(21):6511–23.
Ettl T, Schulz D, Bauer RJ. The renaissance of cyclin dependent kinase inhibitors. Cancers. 2022;14(2):293.
Fite BZ, Wang J, Kare AJ, Ilovitsh A, Chavez M, Ilovitsh T, et al. Immune modulation resulting from MR-guided high intensity focused ultrasound in a model of murine breast cancer. Sci Rep. 2021;11(1):927.
Haar GT, Coussios C. High intensity focused ultrasound: past, present and future. Int J Hyperthermia. 2007;23(2):85–7.
Hu Z, Yang XY, Liu Y, Sankin GN, Pua EC, Morse MA, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med. 2007;5:34.
van den Bijgaart RJ, Eikelenboom DC, Hoogenboom M, Fütterer JJ, den Brok MH, Adema GJ. Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunother. 2017;66(2):247–58.
Mouratidis PXE, Costa M, Rivens I, Repasky EE, Ter Haar G. Pulsed focused ultrasound can improve the anti-cancer effects of immune checkpoint inhibitors in murine pancreatic cancer. J R Soc Interface. 2021;18(180):20210266.
Han Y, Sun J, Wei H, Hao J, Liu W, Wang X. Ultrasound-targeted microbubble destruction: modulation in the tumor microenvironment and application in tumor immunotherapy. Front Immunol. 2022;13:937344.
Wu N, Cao Y, Liu Y, Zhou Y, He H, Tang R, et al. Low-intensity focused ultrasound targeted microbubble destruction reduces tumor blood supply and sensitizes anti-PD-L1 immunotherapy. Front Bioeng Biotechnol. 2023;11:1173381.
Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12(8):557–69.
Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(1):67–73.
Lin MJ, Svensson-Arvelund J, Lubitz GS, Marabelle A, Melero I, Brown BD, et al. Cancer vaccines: the next immunotherapy frontier. Nat Cancer. 2022;3(8):911–26.
McAuliffe J, Chan HF, Noblecourt L, Ramirez-Valdez RA, Pereira-Almeida V, Zhou Y, et al. Heterologous prime-boost vaccination targeting MAGE-type antigens promotes tumor T-cell infiltration and improves checkpoint blockade therapy. J Immunother Cancer. 2021;9(9): e003218.
Rosenblatt J, Glotzbecker B, Mills H, Vasir B, Tzachanis D, Levine JD, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011;34(5):409–18.
Koerner J, Horvath D, Herrmann VL, MacKerracher A, Gander B, Yagita H, et al. PLGA-particle vaccine carrying TLR3/RIG-I ligand Riboxxim synergizes with immune checkpoint blockade for effective anti-cancer immunotherapy. Nat Commun. 2021;12(1):2935.
Xiao M, Xie L, Cao G, Lei S, Wang P, Wei Z, et al. CD4(+) T-cell epitope-based heterologous prime-boost vaccination potentiates anti-tumor immunity and PD-1/PD-L1 immunotherapy. J Immunother Cancer. 2022;10(5): e004022.
Li WN, Zhang SJ, Feng JQ, Jin WL. Repurposing vitamin C for cancer treatment: focus on targeting the tumor microenvironment. Cancers. 2022;14(11):2608.
Magrì A, Germano G, Lorenzato A, Lamba S, Chilà R, Montone M, et al. High-dose vitamin C enhances cancer immunotherapy. Sci Transl Med. 2020;12(532): eaay8707.
Xu Y, Guo X, Wang G, Zhou C. Vitamin C inhibits metastasis of peritoneal tumors by preventing spheroid formation in ID8 murine epithelial peritoneal cancer model. Front Pharmacol. 2020;11:645.
Xu YP, Lv L, Liu Y, Smith MD, Li WC, Tan XM, et al. Tumor suppressor TET2 promotes cancer immunity and immunotherapy efficacy. J Clin Invest. 2019;129(10):4316–31.
Long Y, Qiu J, Zhang B, He P, Shi X, He Q, et al. Pharmacological vitamin C treatment impedes the growth of endogenous glutamine-dependent cancers by targeting glutamine synthetase. Front Pharmacol. 2021;12:671902.
Kuo SM, Burl LR, Hu Z. Cellular phenotype-dependent and -independent effects of vitamin C on the renewal and gene expression of mouse embryonic fibroblasts. PLoS ONE. 2012;7(3): e32957.
Pham VT, Fehlbaum S, Seifert N, Richard N, Bruins MJ, Sybesma W, et al. Effects of colon-targeted vitamins on the composition and metabolic activity of the human gut microbiome- a pilot study. Gut Microbes. 2021;13(1):1–20.
Nakanishi K, Hiramoto K, Ooi K. High-dose vitamin c exerts its anti-cancer effects in a xenograft model of colon cancer by suppressing angiogenesis. Biol Pharm Bull. 2021;44(6):884–7.
Bedhiafi T, Inchakalody VP, Fernandes Q, Mestiri S, Billa N, Uddin S, et al. The potential role of vitamin C in empowering cancer immunotherapy. Biomed Pharmacother. 2022;146:112553.
Lieberman P. The basics of histamine biology. Ann Allergy Asthma Immunol. 2011;106(2 Suppl):S2-5.
Massari NA, Nicoud MB, Medina VA. Histamine receptors and cancer pharmacology: an update. Br J Pharmacol. 2020;177(3):516–38.
Li H, Xiao Y, Li Q, Yao J, Yuan X, Zhang Y, et al. The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1. Cancer Cell. 2022;40(1):36-52.e9.
Liu W, Wang Y, Luo J, Liu M, Luo Z. Pleiotropic effects of metformin on the antitumor efficiency of immune checkpoint inhibitors. Front Immunol. 2020;11:586760.
Wabitsch S, McCallen JD, Kamenyeva O, Ruf B, McVey JC, Kabat J, et al. Metformin treatment rescues CD8(+) T-cell response to immune checkpoint inhibitor therapy in mice with NAFLD. J Hepatol. 2022;77(3):748–60.
Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3(9):666–75.
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69.
Zhao Y, Dong Y, Yang S, Tu Y, Wang C, Li J, et al. Bioorthogonal equipping CAR-T cells with hyaluronidase and checkpoint blocking antibody for enhanced solid tumor immunotherapy. ACS Cent Sci. 2022;8(5):603–14.
Zhao Q, Jiang Y, Xiang S, Kaboli PJ, Shen J, Zhao Y, et al. Engineered TCR-T cell immunotherapy in anticancer precision medicine: pros and cons. Front Immunol. 2021;12:658753.
Tokunaga Y, Sasaki T, Goto S, Adachi K, Sakoda Y, Tamada K. Enhanced antitumor responses of tumor antigen-specific TCR T cells genetically engineered to produce IL7 and CCL19. Mol Cancer Ther. 2022;21(1):138–48.
Qi J, Jin F, Xu X, Du Y. Combination cancer immunotherapy of nanoparticle-based immunogenic cell death inducers and immune checkpoint inhibitors. Int J Nanomedicine. 2021;16:1435–56.
Shim MK, Song SK, Jeon SI, Hwang KY, Kim K. Nano-sized drug delivery systems to potentiate the immune checkpoint blockade therapy. Expert Opin Drug Deliv. 2022;19(6):641–52.
Guan X, Sun L, Shen Y, Jin F, Bo X, Zhu C, et al. Nanoparticle-enhanced radiotherapy synergizes with PD-L1 blockade to limit post-surgical cancer recurrence and metastasis. Nat Commun. 2022;13(1):2834.
Yu W, Sun J, Wang X, Yu S, Yan M, Wang F, et al. Boosting cancer immunotherapy via the convenient A2AR inhibition using a tunable nanocatalyst with light-enhanced activity. Adv Mater. 2022;34(8): e2106967.
Nie W, Wei W, Zuo L, Lv C, Zhang F, Lu GH, et al. Magnetic nanoclusters armed with responsive PD-1 antibody synergistically improved adoptive T-cell therapy for solid tumors. ACS Nano. 2019;13(2):1469–78.
Li X, Khorsandi S, Wang Y, Santelli J, Huntoon K, Nguyen N, et al. Cancer immunotherapy based on image-guided STING activation by nucleotide nanocomplex-decorated ultrasound microbubbles. Nat Nanotechnol. 2022;17(8):891–9.
Shi Y, Lammers T. Combining nanomedicine and immunotherapy. Acc Chem Res. 2019;52(6):1543–54.
Yu X, Long Y, Chen B, Tong Y, Shan M, Jia X, et al. PD-L1/TLR7 dual-targeting nanobody-drug conjugate mediates potent tumor regression via elevating tumor immunogenicity in a host-expressed PD-L1 bias-dependent way. J Immunother Cancer. 2022;10(10): e004590.
Choo YW, Kang M, Kim HY, Han J, Kang S, Lee JR, et al. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano. 2018;12(9):8977–93.
Li L, Zhen M, Wang H, Sun Z, Jia W, Zhao Z, et al. Functional gadofullerene nanoparticles trigger robust cancer immunotherapy based on rebuilding an immunosuppressive tumor microenvironment. Nano Lett. 2020;20(6):4487–96.
Aikins ME, Xu C, Moon JJ. Engineered nanoparticles for cancer vaccination and immunotherapy. Acc Chem Res. 2020;53(10):2094–105.
Ni Q, Zhang F, Liu Y, Wang Z, Yu G, Liang B, et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci Adv. 2020;6(12): eaaw6071.
Kim Y, Kang S, Shin H, Kim T, Yu B, Kim J, et al. Sequential and timely combination of a cancer nanovaccine with immune checkpoint blockade effectively inhibits tumor growth and relapse. Angew Chem Int Ed Engl. 2020;59(34):14628–38.
Rahimi Kalateh Shah Mohammad G, Ghahremanloo A, Soltani A, Fathi E, Hashemy SI. Cytokines as potential combination agents with PD-1/PD-L1 blockade for cancer treatment. J Cell Physiol. 2020;235(7–8):5449–60.
Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. 2018;10(12): a028472.
Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022;19(4):237–53.
Huang Y, Li D, Qin DY, Gou HF, Wei W, Wang YS, et al. Interleukin-armed chimeric antigen receptor-modified T cells for cancer immunotherapy. Gene Ther. 2018;25(3):192–7.
Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–98.
Hailemichael Y, Johnson DH, Abdel-Wahab N, Foo WC, Bentebibel SE, Daher M, et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell. 2022;40(5):509-23.e6.
Mace TA, Shakya R, Pitarresi JR, Swanson B, McQuinn CW, Loftus S, et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018;67(2):320–32.
Li J, Xu J, Yan X, Jin K, Li W, Zhang R. Targeting interleukin-6 (IL-6) sensitizes anti-PD-L1 treatment in a colorectal cancer preclinical model. Med Sci Monit. 2018;24:5501–8.
Liu H, Shen J, Lu K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun. 2017;486(2):239–44.
Li Z, Chen L, Qin Z. Paradoxical roles of IL-4 in tumor immunity. Cell Mol Immunol. 2009;6(6):415–22.
Waldmann TA, Dubois S, Miljkovic MD, Conlon KC. IL-15 in the combination immunotherapy of cancer. Front Immunol. 2020;11:868.
Shen J, Zou Z, Guo J, Cai Y, Xue D, Liang Y, et al. An engineered concealed IL-15-R elicits tumor-specific CD8+T cell responses through PD-1-cis delivery. J Exp Med. 2022;219(12): e20220745.
Zhu Y, Chen M, Xu D, Li TE, Zhang Z, Li JH, et al. The combination of PD-1 blockade with interferon-α has a synergistic effect on hepatocellular carcinoma. Cell Mol Immunol. 2022;19(6):726–37.
Fairchild PJ, Davies TJ. Boosting antitumour immunity through targeted delivery of interferon-alpha. Trends Mol Med. 2019;25(11):935–7.
Syed V. TGF-β signaling in cancer. J Cell Biochem. 2016;117(6):1279–87.
Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L, et al. TGF-beta: duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst. 2014;106(2): djt369.
Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554(7693):538–43.
Lind H, Gameiro SR, Jochems C, Donahue RN, Strauss J, Gulley JM, et al. Dual targeting of TGF-beta and PD-L1 via a bifunctional anti-PD-L1/TGF-betaRII agent: status of preclinical and clinical advances. J Immunother Cancer. 2020;8(1): e000337.
Bule P, Aguiar SI, Aires-Da-Silva F, Dias JNR. Chemokine-directed tumor microenvironment modulation in cancer immunotherapy. Int J Mol Sci. 2021;22(18):9804.
Niwa R, Sakurada M, Kobayashi Y, Uehara A, Matsushima K, Ueda R, et al. Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density. Clin Cancer Res. 2005;11(6):2327–36.
Doi T, Muro K, Ishii H, Kato T, Tsushima T, Takenoyama M, et al. A phase I study of the anti-CC chemokine receptor 4 antibody, mogamulizumab, in combination with nivolumab in patients with advanced or metastatic solid tumors. Clin Cancer Res. 2019;25(22):6614–22.
González-Martín A, Mira E, Mañes S. CCR5 in cancer immunotherapy: more than an “attractive” receptor for T cells. Oncoimmunology. 2012;1(1):106–8.
Karin N. Chemokines and cancer: new immune checkpoints for cancer therapy. Curr Opin Immunol. 2018;51:140–5.
Campbell JR, McDonald BR, Mesko PB, Siemers NO, Singh PB, Selby M, et al. Fc-optimized anti-CCR8 antibody depletes regulatory T cells in human tumor models. Cancer Res. 2021;81(11):2983–94.
Hayase E, Jenq RR. Role of the intestinal microbiome and microbial-derived metabolites in immune checkpoint blockade immunotherapy of cancer. Genome Med. 2021;13(1):107.
Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17(5):271–85.
Bhutiani N, Schucht JE, Miller KR, McClave SA. Technical aspects of fecal microbial transplantation (FMT). Curr Gastroenterol Rep. 2018;20(7):30.
Antushevich H. Fecal microbiota transplantation in disease therapy. Clin Chim Acta. 2020;503:90–8.
Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–84.
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14.
Kawanabe-Matsuda H, Takeda K, Nakamura M, Makino S, Karasaki T, Kakimi K, et al. Dietary lactobacillus-derived exopolysaccharide enhances immune-checkpoint blockade therapy. Cancer Discov. 2022;12(5):1336–55.
Lee SH, Cho SY, Yoon Y, Park C, Sohn J, Jeong JJ, et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat Microbiol. 2021;6(3):277–88.
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502.
Ma Z, Li S, Wang Y, Zhang J, Zeng X. Upregulation of a novel LncRNA AC1049582 stabilized by PCBP.2 promotes proliferation and microvascular invasion in hepatocellular carcinoma. Exp Cell Res. 2021;407(1):112791.
Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369(6510):1481–9.
Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1–24.
Wu J, Wang K, Wang X, Pang Y, Jiang C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell. 2021;12(5):360–73.
Huang XZ, Gao P, Song YX, Xu Y, Sun JX, Chen XW, et al. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients. Oncoimmunology. 2019;8(12): e1665973.
Fessas P, Naeem M, Pinter M, Marron TU, Szafron D, Balcar L, et al. Early antibiotic exposure is not detrimental to therapeutic effect from immunotherapy in hepatocellular carcinoma. Liver Cancer. 2021;10(6):583–92.
Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374(6575):1632–40.
Szczyrek M, Bitkowska P, Chunowski P, Czuchryta P, Krawczyk P, Milanowski J. Diet, microbiome, and cancer immunotherapy-a comprehensive review. Nutrients. 2021;13(7):2217.
Bolte LA, Lee KA, Björk JR, Leeming ER, Campmans-Kuijpers MJE, de Haan JJ, et al. Association of a mediterranean diet with outcomes for patients treated with immune checkpoint blockade for advanced melanoma. JAMA Oncol. 2023;9(5):705–9.
Pelly VS, Moeini A, Roelofsen LM, Bonavita E, Bell CR, Hutton C, et al. Anti-inflammatory drugs remodel the tumor immune environment to enhance immune checkpoint blockade efficacy. Cancer Discov. 2021;11(10):2602–19.
Zelenay S, van der Veen AG, Böttcher JP, Snelgrove KJ, Rogers N, Acton SE, et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162(6):1257–70.
Wang Y, Yang M, Tao M, Liu P, Kong C, Li H, et al. Corticosteroid administration for cancer-related indications is an unfavorable prognostic factor in solid cancer patients receiving immune checkpoint inhibitor treatment. Int Immunopharmacol. 2021;99:108031.
Li N, Zheng X, Gan J, Zhuo T, Li X, Yang C, et al. Effects of glucocorticoid use on survival of advanced non-small-cell lung cancer patients treated with immune checkpoint inhibitors. Chin Med J. 2023;136(21):2562–72.
Aoki M, Fujishita T. Oncogenic roles of the PI3K/AKT/mTOR axis. Curr Top Microbiol Immunol. 2017;407:153–89.
Guerrero-Zotano A, Mayer IA, Arteaga CL. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016;35(4):515–24.
Zhang Z, Richmond A. The role of PI3K inhibition in the treatment of breast cancer, alone or combined with immune checkpoint inhibitors. Front Mol Biosci. 2021;8:648663.
Xia W, Zhang S, Duan H, Wang C, Qian S, Shen H. The combination therapy of Everolimus and anti-PD-1 improves the antitumor effect by regulating CD8(+) T cells in bladder cancer. Med Oncol. 2022;39(3):37.
De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature. 2016;539(7629):443–7.
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19(3):1997–2007.
Subbiah V, Baik C, Kirkwood JM. Clinical development of BRAF plus MEK inhibitor combinations. Trends Cancer. 2020;6(9):797–810.
Homet Moreno B, Mok S, Comin-Anduix B, Hu-Lieskovan S, Ribas A. Combined treatment with dabrafenib and trametinib with immune-stimulating antibodies for BRAF mutant melanoma. Oncoimmunology. 2016;5(7): e1052212.
Phadke MS, Chen Z, Li J, Mohamed E, Davies MA, Smalley I, et al. Targeted therapy given after anti-PD-1 leads to prolonged responses in mouse melanoma models through sustained antitumor immunity. Cancer Immunol Res. 2021;9(5):554–67.
Henry KE, Mack KN, Nagle VL, Cornejo M, Michel AO, Fox IL, et al. ERK inhibition improves anti-PD-L1 immune checkpoint blockade in preclinical pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2021;20(10):2026–34.
Moore AR, Rosenberg SC, McCormick F, Malek S. RAS-targeted therapies: is the undruggable drugged? Nat Rev Drug Discov. 2020;19(8):533–52.
Nishida N. Role of oncogenic pathways on the cancer immunosuppressive microenvironment and its clinical implications in hepatocellular carcinoma. Cancers. 2021;13(15):3666.
Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21(1):28.
Hyoda T, Tsujioka T, Nakahara T, Suemori S, Okamoto S, Kataoka M, et al. Rigosertib induces cell death of a myelodysplastic syndrome-derived cell line by DNA damage-induced G2/M arrest. Cancer Sci. 2015;106(3):287–93.
Yan C, Saleh N, Yang J, Nebhan CA, Vilgelm AE, Reddy EP, et al. Novel induction of CD40 expression by tumor cells with RAS/RAF/PI3K pathway inhibition augments response to checkpoint blockade. Mol Cancer. 2021;20(1):85.
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3.
DeVito NC, Sturdivant M, Thievanthiran B, Xiao C, Plebanek MP, Salama AKS, et al. Pharmacological Wnt ligand inhibition overcomes key tumor-mediated resistance pathways to anti-PD-1 immunotherapy. Cell Rep. 2021;35(5):109071.
Huang T, Li F, Cheng X, Wang J, Zhang W, Zhang B, et al. Wnt inhibition sensitizes PD-L1 blockade therapy by overcoming bone marrow-derived myofibroblasts-mediated immune resistance in tumors. Front Immunol. 2021;12:619209.
Tentler JJ, Lang J, Capasso A, Kim DJ, Benaim E, Lee YB, et al. RX-5902, a novel β-catenin modulator, potentiates the efficacy of immune checkpoint inhibitors in preclinical models of triple-negative breast cancer. BMC Cancer. 2020;20(1):1063.
Li K, Qu S, Chen X, Wu Q, Shi M. Promising targets for cancer immunotherapy: TLRs, RLRs, and STING-mediated innate immune pathways. Int J Mol Sci. 2017;18(2):404.
Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17(1):1–14.
Ribas A, Medina T, Kirkwood JM, Zakharia Y, Gonzalez R, Davar D, et al. Overcoming PD-1 blockade resistance with CpG-A toll-like receptor 9 agonist vidutolimod in patients with metastatic melanoma. Cancer Discov. 2021;11(12):2998–3007.
Jiang Y, Zhang H, Wang J, Chen J, Guo Z, Liu Y, et al. Exploiting RIG-I-like receptor pathway for cancer immunotherapy. J Hematol Oncol. 2023;16(1):8.
Heidegger S, Wintges A, Stritzke F, Bek S, Steiger K, Koenig PA, et al. RIG-I activation is critical for responsiveness to checkpoint blockade. Sci Immunol. 2019;4(39): eaau8943.
Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–42.
Nakamura T, Sato T, Endo R, Sasaki S, Takahashi N, Sato Y, et al. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J Immunother Cancer. 2021;9(7): e002852.
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29(5):347–64.
Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7(1):196.
Ganzleben I, Neurath MF, Becker C. Autophagy in cancer therapy-molecular mechanisms and current clinical advances. Cancers. 2021;13(21):5575.
Xia H, Green DR, Zou W. Autophagy in tumour immunity and therapy. Nat Rev Cancer. 2021;21(5):281–97.
Kocaturk NM, Akkoc Y, Kig C, Bayraktar O, Gozuacik D, Kutlu O. Autophagy as a molecular target for cancer treatment. Eur J Pharm Sci. 2019;134:116–37.
Sharma G, Ojha R, Noguera-Ortega E, Rebecca VW, Attanasio J, Liu S, et al. PPT1 inhibition enhances the antitumor activity of anti-PD-1 antibody in melanoma. JCI Insight. 2020;5(17): e133225.
Stockwell BR, Jiang X. The chemistry and biology of ferroptosis. Cell Chem Biol. 2020;27(4):365–75.
Sun LL, Linghu DL, Hung MC. Ferroptosis: a promising target for cancer immunotherapy. Am J Cancer Res. 2021;11(12):5856–63.
Fan F, Liu P, Bao R, Chen J, Zhou M, Mo Z, et al. A dual PI3K/HDAC inhibitor induces immunogenic ferroptosis to potentiate cancer immune checkpoint therapy. Cancer Res. 2021;81(24):6233–45.
Loveless R, Bloomquist R, Teng Y. Pyroptosis at the forefront of anticancer immunity. J Exp Clin Cancer Res. 2021;40(1):264.
Jia Y, Wang X, Deng Y, Li S, Xu X, Qin Y, et al. Pyroptosis provides new strategies for the treatment of cancer. J Cancer. 2023;14(1):140–51.
Dong Y, Zuo D, Qiu YJ, Cao JY, Wang HZ, Yu LY, et al. Preoperative prediction of microvascular invasion (MVI) in hepatocellular carcinoma based on kupffer phase radiomics features of sonazoid contrast-enhanced ultrasound (SCEUS): a prospective study. Clin Hemorheol Microcirc. 2022;81(1):97–107.
Smith HG, Jamal K, Dayal JH, Tenev T, Kyula-Currie J, Guppy N, et al. RIPK1-mediated immunogenic cell death promotes anti-tumour immunity against soft-tissue sarcoma. EMBO Mol Med. 2020;12(6): e10979.
García-Ortiz A, Serrador JM. Nitric oxide signaling in T cell-mediated immunity. Trends Mol Med. 2018;24(4):412–27.
Jiang W, Dong W, Li M, Guo Z, Wang Q, Liu Y, et al. Nitric oxide induces immunogenic cell death and potentiates cancer immunotherapy. ACS Nano. 2022;16(3):3881–94.
Bader JE, Voss K, Rathmell JC. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell. 2020;78(6):1019–33.
Guo D, Tong Y, Jiang X, Meng Y, Jiang H, Du L, et al. Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of IkappaBalpha. Cell Metab. 2022;34(9):1312-24.e6.
Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. 2021;591(7851):645–51.
Siska PJ, Beckermann KE, Mason FM, Andrejeva G, Greenplate AR, Sendor AB, et al. Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight. 2017;2(12): e93411.
Zheng JB, Wong CW, Liu J, Luo XJ, Zhou WY, Chen YX, et al. Glucose metabolism inhibitor PFK-015 combined with immune checkpoint inhibitor is an effective treatment regimen in cancer. Oncoimmunology. 2022;11(1):2079182.
Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. 2022;40(2):201-18.e9.
Feng Q, Liu Z, Yu X, Huang T, Chen J, Wang J, et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat Commun. 2022;13(1):4981.
Yu W, Lei Q, Yang L, Qin G, Liu S, Wang D, et al. Contradictory roles of lipid metabolism in immune response within the tumor microenvironment. J Hematol Oncol. 2021;14(1):187.
Zhang C, Yue C, Herrmann A, Song J, Egelston C, Wang T, et al. STAT3 activation-induced fatty acid oxidation in CD8(+) T effector cells is critical for obesity-promoted breast tumor growth. Cell Metab. 2020;31(1):148-61.e5.
Pacella I, Procaccini C, Focaccetti C, Miacci S, Timperi E, Faicchia D, et al. Fatty acid metabolism complements glycolysis in the selective regulatory T cell expansion during tumor growth. Proc Natl Acad Sci USA. 2018;115(28):E6546–55.
Veglia F, Tyurin VA, Mohammadyani D, Blasi M, Duperret EK, Donthireddy L, et al. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat Commun. 2017;8(1):2122.
Chowdhury PS, Chamoto K, Kumar A, Honjo T. PPAR-induced fatty acid oxidation in T cells increases the number of tumor-reactive CD8(+) T cells and facilitates anti-PD-1 therapy. Cancer Immunol Res. 2018;6(11):1375–87.
Wang H, Franco F, Tsui YC, Xie X, Trefny MP, Zappasodi R, et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat Immunol. 2020;21(3):298–308.
Liu Y, Liu X, Wang H, Ding P, Wang C. Agrimonolide inhibits cancer progression and induces ferroptosis and apoptosis by targeting SCD1 in ovarian cancer cells. Phytomedicine. 2022;101:154102.
Alwarawrah Y, Hughes P, Loiselle D, Carlson DA, Darr DB, Jordan JL, et al. Fasnall, a selective FASN inhibitor, shows potent anti-tumor activity in the MMTV-Neu model of HER2(+) breast cancer. Cell Chem Biol. 2016;23(6):678–88.
Yang H, Deng Q, Ni T, Liu Y, Lu L, Dai H, et al. Targeted Inhibition of LPL/FABP4/CPT1 fatty acid metabolic axis can effectively prevent the progression of nonalcoholic steatohepatitis to liver cancer. Int J Biol Sci. 2021;17(15):4207–22.
King RJ, Singh PK, Mehla K. The cholesterol pathway: impact on immunity and cancer. Trends Immunol. 2022;43(1):78–92.
Zhu PF, Wang MX, Chen ZL, Yang L. Targeting the tumor microenvironment: a literature review of the novel anti-tumor mechanism of statins. Front Oncol. 2021;11:761107.
Mao W, Cai Y, Chen D, Jiang G, Xu Y, Chen R, et al. Statin shapes inflamed tumor microenvironment and enhances immune checkpoint blockade in non-small cell lung cancer. JCI Insight. 2022;7(18): e161940.
Takada K, Shimokawa M, Takamori S, Shimamatsu S, Hirai F, Tagawa T, et al. A propensity score-matched analysis of the impact of statin therapy on the outcomes of patients with non-small-cell lung cancer receiving anti-PD-1 monotherapy: a multicenter retrospective study. BMC Cancer. 2022;22(1):503.
Hoskin DW, Mader JS, Furlong SJ, Conrad DM, Blay J. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (review). Int J Oncol. 2008;32(3):527–35.
Allard B, Allard D, Buisseret L, Stagg J. The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol. 2020;17(10):611–29.
Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res. 2013;19(20):5626–35.
Leone RD, Sun IM, Oh MH, Sun IH, Wen J, Englert J, et al. Inhibition of the adenosine A2a receptor modulates expression of T cell coinhibitory receptors and improves effector function for enhanced checkpoint blockade and ACT in murine cancer models. Cancer Immunol Immunother. 2018;67(8):1271–84.
Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18(5):379–401.
Opitz CA, Somarribas Patterson LF, Mohapatra SR, Dewi DL, Sadik A, Platten M, et al. The therapeutic potential of targeting tryptophan catabolism in cancer. Br J Cancer. 2020;122(1):30–44.
Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer. 2014;2:3.
Schramme F, Crosignani S, Frederix K, Hoffmann D, Pilotte L, Stroobant V, et al. Inhibition of tryptophan-dioxygenase activity increases the antitumor efficacy of immune checkpoint inhibitors. Cancer Immunol Res. 2020;8(1):32–45.
Holbert CE, Cullen MT, Casero RA Jr, Stewart TM. Polyamines in cancer: integrating organismal metabolism and antitumour immunity. Nat Rev Cancer. 2022;22(8):467–80.
Alexander ET, Mariner K, Donnelly J, Phanstiel O, Gilmour SK. Polyamine blocking therapy decreases survival of tumor-infiltrating immunosuppressive myeloid cells and enhances the antitumor efficacy of PD-1 blockade. Mol Cancer Ther. 2020;19(10):2012–22.
Hung MH, Lee JS, Ma C, Diggs LP, Heinrich S, Chang CW, et al. Tumor methionine metabolism drives T-cell exhaustion in hepatocellular carcinoma. Nat Commun. 2021;12(1):1455.
Gao X, Sanderson SM, Dai Z, Reid MA, Cooper DE, Lu M, et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature. 2019;572(7769):397–401.
Bian Y, Li W, Kremer DM, Sajjakulnukit P, Li S, Crespo J, et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature. 2020;585(7824):277–82.
Li T, Tan YT, Chen YX, Zheng XJ, Wang W, Liao K, et al. Methionine deficiency facilitates antitumour immunity by altering m(6)A methylation of immune checkpoint transcripts. Gut. 2022. https://doi.org/10.1136/gutjnl-2022-326928.
Yang C, Jin J, Yang Y, Sun H, Wu L, Shen M, et al. Androgen receptor-mediated CD8(+) T cell stemness programs drive sex differences in antitumor immunity. Immunity. 2022;55(7):1268-83.e9.
Kwon H, Schafer JM, Song NJ, Kaneko S, Li A, Xiao T, et al. Androgen conspires with the CD8(+) T cell exhaustion program and contributes to sex bias in cancer. Sci Immunol. 2022;7(73): eabq2630.
Guan X, Polesso F, Wang C, Sehrawat A, Hawkins RM, Murray SE, et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature. 2022;606(7915):791–6.
Yang W, Lu Y, Xu Y, Xu L, Zheng W, Wu Y, et al. Estrogen represses hepatocellular carcinoma (HCC) growth via inhibiting alternative activation of tumor-associated macrophages (TAMs). J Biol Chem. 2012;287(48):40140–9.
Welte T, Zhang XH, Rosen JM. Repurposing antiestrogens for tumor immunotherapy. Cancer Discov. 2017;7(1):17–9.
Natale CA, Li J, Zhang J, Dahal A, Dentchev T, Stanger BZ, et al. Activation of G protein-coupled estrogen receptor signaling inhibits melanoma and improves response to immune checkpoint blockade. Elife. 2018;7: e31770.
Gile J, Ruan G, Abeykoon J, McMahon MM, Witzig T. Magnesium: the overlooked electrolyte in blood cancers? Blood Rev. 2020;44:100676.
Jeong S, Park SH. Co-stimulatory receptors in cancers and their implications for cancer immunotherapy. Immune Netw. 2020;20(1): e3.
Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol. 2022;19(1):37–50.
Knee DA, Hewes B, Brogdon JL. Rationale for anti-GITR cancer immunotherapy. Eur J Cancer. 2016;67:1–10.
Tian J, Zhang B, Rui K, Wang S. The role of GITR/GITRL interaction in autoimmune diseases. Front Immunol. 2020;11:588682.
Lu X. OX40 and OX40L interaction in cancer. Curr Med Chem. 2021;28(28):5659–73.
Ma Y, Li J, Wang H, Chiu Y, Kingsley CV, Fry D, et al. Combination of PD-1 inhibitor and OX40 agonist induces tumor rejection and immune memory in mouse models of pancreatic cancer. Gastroenterology. 2020;159(1):306-19.e12.
Messenheimer DJ, Jensen SM, Afentoulis ME, Wegmann KW, Feng Z, Friedman DJ, et al. Timing of PD-1 blockade is critical to effective combination immunotherapy with anti-OX40. Clin Cancer Res. 2017;23(20):6165–77.
Etxeberria I, Glez-Vaz J, Teijeira Á, Melero I. New emerging targets in cancer immunotherapy: CD137/4-1BB costimulatory axis. ESMO Open. 2020;4(Suppl 3): e000733.
Qu QX, Zhu XY, Du WW, Wang HB, Shen Y, Zhu YB, et al. 4–1BB agonism combined with PD-L1 blockade increases the number of tissue-resident CD8+ T cells and facilitates tumor abrogation. Front Immunol. 2020;11:577.
Chester C, Sanmamed MF, Wang J, Melero I. Immunotherapy targeting 4–1BB: mechanistic rationale, clinical results, and future strategies. Blood. 2018;131(1):49–57.
Amatore F, Gorvel L, Olive D. Role of inducible co-stimulator (ICOS) in cancer immunotherapy. Expert Opin Biol Ther. 2020;20(2):141–50.
Soldevilla MM, Villanueva H, Meraviglia-Crivelli D, Menon AP, Ruiz M, Cebollero J, et al. ICOS costimulation at the tumor site in combination with CTLA-4 blockade therapy elicits strong tumor immunity. Mol Ther. 2019;27(11):1878–91.
Karnell JL, Rieder SA, Ettinger R, Kolbeck R. Targeting the CD40-CD40L pathway in autoimmune diseases: humoral immunity and beyond. Adv Drug Deliv Rev. 2019;141:92–103.
Bullock TNJ. CD40 stimulation as a molecular adjuvant for cancer vaccines and other immunotherapies. Cell Mol Immunol. 2022;19(1):14–22.
Diggs LP, Ruf B, Ma C, Heinrich B, Cui L, Zhang Q, et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J Hepatol. 2021;74(5):1145–54.
Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–42.
Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol. 2020;20(3):173–85.
Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71(10):3540–51.
Harjunpaa H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin Exp Immunol. 2020;200(2):108–19.
Dixon KO, Schorer M, Nevin J, Etminan Y, Amoozgar Z, Kondo T, et al. Functional anti-TIGIT antibodies regulate development of autoimmunity and antitumor immunity. J Immunol. 2018;200(8):3000–7.
Ning Z, Liu K, Xiong H. Roles of BTLA in immunity and immune disorders. Front Immunol. 2021;12:654960.
Choi J, Medikonda R, Saleh L, Kim T, Pant A, Srivastava S, et al. Combination checkpoint therapy with anti-PD-1 and anti-BTLA results in a synergistic therapeutic effect against murine glioblastoma. Oncoimmunology. 2021;10(1):1956142.
Vernieri C, Fuca G, Ligorio F, Huber V, Vingiani A, Iannelli F, et al. Fasting-mimicking diet is safe and reshapes metabolism and antitumor immunity in patients with cancer. Cancer Discov. 2022;12(1):90–107.
Freeman JM, Kossoff EH, Hartman AL. The ketogenic diet: one decade later. Pediatrics. 2007;119(3):535–43.
Lussier DM, Woolf EC, Johnson JL, Brooks KS, Blattman JN, Scheck AC. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer. 2016;16:310.
Ma X, Wu J, Wang B, Liu C, Liu L, Sun C. Epigenetic modifications: critical participants of the PDL1 regulatory mechanism in solid tumors (review). Int J Oncol. 2022;61(5):1–8.
Gomez S, Tabernacki T, Kobyra J, Roberts P, Chiappinelli KB. Combining epigenetic and immune therapy to overcome cancer resistance. Semin Cancer Biol. 2020;65:99–113.
Yang F, Li J, Ge Q, Zhang Y, Zhang M, Zhou J, et al. Non-coding RNAs: emerging roles in the characterization of immune microenvironment and immunotherapy of prostate cancer. Biochem Pharmacol. 2023;214:115669.
Matsui M, Corey DR. Non-coding RNAs as drug targets. Nat Rev Drug Discov. 2017;16(3):167–79.
Chen X, Pan X, Zhang W, Guo H, Cheng S, He Q, et al. Epigenetic strategies synergize with PD-L1/PD-1 targeted cancer immunotherapies to enhance antitumor responses. Acta Pharm Sin B. 2020;10(5):723–33.
Micevic G, Bosenberg MW, Yan Q. The crossroads of cancer epigenetics and immune checkpoint therapy. Clin Cancer Res. 2023;29(7):1173–82.
Audia JE, Campbell RM. Histone modifications and cancer. Cold Spring Harb Perspect Biol. 2016;8(4): a019521.
Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004;14(14):R546–51.
Deng S, Hu Q, Zhang H, Yang F, Peng C, Huang C. HDAC3 inhibition upregulates PD-L1 expression in B-cell lymphomas and augments the efficacy of anti-PD-L1 therapy. Mol Cancer Ther. 2019;18(5):900–8.
New M, Olzscha H, La Thangue NB. HDAC inhibitor-based therapies: can we interpret the code? Mol Oncol. 2012;6(6):637–56.
Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–37.
Saleh R, Toor SM, Sasidharan Nair V, Elkord E. Role of epigenetic modifications in inhibitory immune checkpoints in cancer development and progression. Front Immunol. 2020;11:1469.
Baylin SB, Jones PA. Epigenetic determinants of cancer. Cold Spring Harb Perspect Biol. 2016;8(9): a019505.
Zhou L, Mudianto T, Ma X, Riley R, Uppaluri R. Targeting EZH2 enhances antigen presentation, antitumor immunity, and circumvents anti-PD-1 resistance in head and neck cancer. Clin Cancer Res. 2020;26(1):290–300.
Wang J, Scully K, Zhu X, Cai L, Zhang J, Prefontaine GG, et al. Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature. 2007;446(7138):882–7.
Metzger E, Wissmann M, Yin N, Müller JM, Schneider R, Peters AH, et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437(7057):436–9.
Qin Y, Vasilatos SN, Chen L, Wu H, Cao Z, Fu Y, et al. Inhibition of histone lysine-specific demethylase 1 elicits breast tumor immunity and enhances antitumor efficacy of immune checkpoint blockade. Oncogene. 2019;38(3):390–405.
Kelly GM, Al-Ejeh F, McCuaig R, Casciello F, Ahmad Kamal N, Ferguson B, et al. G9a inhibition enhances checkpoint inhibitor blockade response in melanoma. Clin Cancer Res. 2021;27(9):2624–35.
Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38(1):23–38.
Kulis M, Esteller M. DNA methylation and cancer. Adv Genet. 2010;70:27–56.
Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell. 2015;162(5):974–86.
Li Z, Ruan Y, Zhang H, Shen Y, Li T, Xiao B. Tumor-suppressive circular RNAs: mechanisms underlying their suppression of tumor occurrence and use as therapeutic targets. Cancer Sci. 2019;110(12):3630–8.
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–91.
Chen L, Shan G. CircRNA in cancer: fundamental mechanism and clinical potential. Cancer Lett. 2021;505:49–57.
England CG, Jiang D, Ehlerding EB, Rekoske BT, Ellison PA, Hernandez R, et al. (89)Zr-labeled nivolumab for imaging of T-cell infiltration in a humanized murine model of lung cancer. Eur J Nucl Med Mol Imaging. 2018;45(1):110–20.
Hettich M, Braun F, Bartholomä MD, Schirmbeck R, Niedermann G. High-resolution PET imaging with therapeutic antibody-based PD-1/PD-L1 checkpoint tracers. Theranostics. 2016;6(10):1629–40.
Gao H, Wu Y, Shi J, Zhang X, Liu T, Hu B, et al. Nuclear imaging-guided PD-L1 blockade therapy increases effectiveness of cancer immunotherapy. J Immunother Cancer. 2020;8(2): e001156.
Jiang M, Jia K, Wang L, Li W, Chen B, Liu Y, et al. Alterations of DNA damage response pathway: Biomarker and therapeutic strategy for cancer immunotherapy. Acta Pharm Sin B. 2021;11(10):2983–94.
Shi C, Qin K, Lin A, Jiang A, Cheng Q, Liu Z, et al. The role of DNA damage repair (DDR) system in response to immune checkpoint inhibitor (ICI) therapy. J Exp Clin Cancer Res. 2022;41(1):268.
Wang Y, Zheng K, Xiong H, Huang Y, Chen X, Zhou Y, et al. PARP inhibitor upregulates PD-L1 expression and provides a new combination therapy in pancreatic cancer. Front Immunol. 2021;12:762989.
Wang Y, Luo J, Alu A, Han X, Wei Y, Wei X. cGAS-STING pathway in cancer biotherapy. Mol Cancer. 2020;19(1):136.
Lee EK, Konstantinopoulos PA. Combined PARP and immune checkpoint inhibition in ovarian cancer. Trends in Cancer. 2019;5(9):524–8.
Ngoi NYL, Peng G, Yap TA. A tale of two checkpoints: ATR inhibition and PD-(L)1 blockade. Annu Rev Med. 2022;73:231–50.
Sun LL, Yang RY, Li CW, Chen MK, Shao B, Hsu JM, et al. Inhibition of ATR downregulates PD-L1 and sensitizes tumor cells to T cell-mediated killing. Am J Cancer Res. 2018;8(7):1307–16.
Tang Z, Pilie PG, Geng C, Manyam GC, Yang G, Park S, et al. ATR inhibition induces CDK1-SPOP signaling and enhances Anti-PD-L1 cytotoxicity in prostate cancer. Clin Cancer Res. 2021;27(17):4898–909.
Hu M, Zhou M, Bao X, Pan D, Jiao M, Liu X, et al. ATM inhibition enhances cancer immunotherapy by promoting mtDNA leakage and cGAS/STING activation. J Clin Invest. 2021;131(3): e139333.
Drapkin BJ, Farago AF. Unexpected synergy reveals new therapeutic strategy in SCLC. Trends Pharmacol Sci. 2019;40(5):295–7.
Pilie PG, Tang C, Mills GB, Yap TA. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat Rev Clin Oncol. 2019;16(2):81–104.
Matheson CJ, Backos DS, Reigan P. Targeting WEE1 kinase in cancer. Trends Pharmacol Sci. 2016;37(10):872–81.
Taniguchi H, Caeser R, Chavan SS, Zhan YA, Chow A, Manoj P, et al. WEE1 inhibition enhances the antitumor immune response to PD-L1 blockade by the concomitant activation of STING and STAT1 pathways in SCLC. Cell Rep. 2022;39(7):110814.
Mun EJ, Babiker HM, Weinberg U, Kirson ED, Von Hoff DD. Tumor-treating fields: a fourth modality in cancer treatment. Clin Cancer Res. 2018;24(2):266–75.
Barsheshet Y, Voloshin T, Brant B, Cohen G, Koren L, Blatt R, et al. Tumor treating fields (TTFields) concomitant with immune checkpoint inhibitors are therapeutically effective in non-small cell lung cancer (NSCLC) in vivo model. Int J Mol Sci. 2022;23(22):14073.
Son S, Kim JH, Wang X, Zhang C, Yoon SA, Shin J, et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem Soc Rev. 2020;49(11):3244–61.
Nesbitt H, Logan K, Thomas K, Callan B, Gao J, McKaig T, et al. Sonodynamic therapy complements PD-L1 immune checkpoint inhibition in a murine model of pancreatic cancer. Cancer Lett. 2021;517:88–95.
Park AY, Nafia I, Stringer DN, Karpiniec SS, Fitton JH. Fucoidan independently enhances activity in human immune cells and has a cytostatic effect on prostate cancer cells in the presence of nivolumab. Mar Drugs. 2021;20(1):12.
Yang J, Yang X, Pan W, Wang M, Lu Y, Zhang J, et al. Fucoidan-supplemented diet potentiates immune checkpoint blockage by enhancing antitumor immunity. Front Cell Dev Biol. 2021;9:733246.
An EK, Hwang J, Kim SJ, Park HB, Zhang W, Ryu JH, et al. Comparison of the immune activation capacities of fucoidan and laminarin extracted from Laminaria japonica. Int J Biol Macromol. 2022;208:230–42.
Dongre A, Rashidian M, Eaton EN, Reinhardt F, Thiru P, Zagorulya M, et al. Direct and indirect regulators of epithelial-mesenchymal transition-mediated immunosuppression in breast carcinomas. Cancer Discov. 2021;11(5):1286–305.
Luo F, Lu FT, Cao JX, Ma WJ, Xia ZF, Zhan JH, et al. HIF-1α inhibition promotes the efficacy of immune checkpoint blockade in the treatment of non-small cell lung cancer. Cancer Lett. 2022;531:39–56.
Abril-Rodriguez G, Torrejon DY, Liu W, Zaretsky JM, Nowicki TS, Tsoi J, et al. PAK4 inhibition improves PD-1 blockade immunotherapy. Nat Cancer. 2020;1(1):46–58.
Jeong SD, Jung BK, Ahn HM, Lee D, Ha J, Noh I, et al. Immunogenic cell death inducing fluorinated mitochondria-disrupting helical polypeptide synergizes with PD-L1 immune checkpoint blockade. Adv Sci. 2021;8(7):2001308.
Wang C, Fiering SN, Steinmetz NF. Cowpea mosaic virus promotes anti-tumor activity and immune memory in a mouse ovarian tumor model. Adv Ther. 2019;2(5):1900003.
Murray AA, Wang C, Fiering S, Steinmetz NF. In situ vaccination with cowpea vs tobacco mosaic virus against melanoma. Mol Pharm. 2018;15(9):3700–16.
Wang C, Steinmetz NF. A combination of cowpea mosaic virus and immune checkpoint therapy synergistically improves therapeutic efficacy in three tumor models. Adv Funct Mater. 2020;30(27):2002299.
Wang Z, Li W, Park J, Gonzalez KM, Scott AJ, Lu J. Camptothesome elicits immunogenic cell death to boost colorectal cancer immune checkpoint blockade. J Control Release. 2022;349:929–39.
Guo S, Deng CX. Effect of stromal cells in tumor microenvironment on metastasis initiation. Int J Biol Sci. 2018;14(14):2083–93.
Honma M, Ikebuchi Y, Suzuki H. RANKL as a key figure in bridging between the bone and immune system: its physiological functions and potential as a pharmacological target. Pharmacol Ther. 2021;218:107682.
Chen H, Pan Y, Zhou Q, Liang C, Wong CC, Zhou Y, et al. METTL3 inhibits antitumor immunity by targeting m(6)A-BHLHE41-CXCL1/CXCR2 axis to promote colorectal cancer. Gastroenterology. 2022;163(4):891–907.
Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49.
Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34.
Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598–608.
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8.
Martin AM, Nirschl TR, Nirschl CJ, Francica BJ, Kochel CM, van Bokhoven A, et al. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 2015;18(4):325–32.
Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2019;37(4):318–27.
Du W, Menjivar RE, Donahue KL, Kadiyala P, Velez-Delgado A, Brown KL, et al. WNT signaling in the tumor microenvironment promotes immunosuppression in murine pancreatic cancer. J Exp Med. 2023;220(1): e20220503.
Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res. 2013;19(2):393–403.
Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6(2):202–16.
Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol. 2018;9:847.
Ni L, Lu J. Interferon gamma in cancer immunotherapy. Cancer Med. 2018;7(9):4509–16.
Thibaut R, Bost P, Milo I, Cazaux M, Lemaître F, Garcia Z, et al. Bystander IFN-γ activity promotes widespread and sustained cytokine signaling altering the tumor microenvironment. Nat Cancer. 2020;1(3):302–14.
Wang H, Liu B, Wei J. Beta2-microglobulin(B2M) in cancer immunotherapies: biological function, resistance and remedy. Cancer Lett. 2021;517:96–104.
Sidaway P. Immunotherapy: HLA-1 genotype influences response to checkpoint inhibitors. Nat Rev Clin Oncol. 2018;15(2):66.
Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
Huang RY, Francois A, McGray AR, Miliotto A, Odunsi K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology. 2017;6(1): e1249561.
Blando J, Sharma A, Higa MG, Zhao H, Vence L, Yadav SS, et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc Natl Acad Sci USA. 2019;116(5):1692–7.
Bi J, Zhang Q, Liang D, Xiong L, Wei H, Sun R, et al. T-cell Ig and ITIM domain regulates natural killer cell activation in murine acute viral hepatitis. Hepatology. 2014;59(5):1715–25.
Coschi CH, Juergens RA. Overcoming resistance mechanisms to immune checkpoint inhibitors: leveraging the anti-tumor immune response. Curr Oncol. 2023;31(1):1–23.
Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20(1):25–39.
Griffin M, Scotto D, Josephs DH, Mele S, Crescioli S, Bax HJ, et al. BRAF inhibitors: resistance and the promise of combination treatments for melanoma. Oncotarget. 2017;8(44):78174–92.
Muto S, Enta A, Maruya Y, Inomata S, Yamaguchi H, Mine H, et al. Wnt/β-catenin signaling and resistance to immune checkpoint inhibitors: from non-small-cell lung cancer to other cancers. Biomedicines. 2023;11(1):190.
Acknowledgements
Not applicable.
Funding
This work was supported by Natural Science Foundation of Gansu Province (Grant No.: 23JRRA1313), Natural Science Foundation for Young Scientists and the Science & Technology Planning Project of Gansu Province (Grant No.: 18JR3RA058), Gansu Province Youth Innovative Talents Project (Grant No.: 2021LQGR15), Health Industry Scientific Research Program of Gansu Province (Grant No.: GSWSKY2020-06), and Research Projects of Gansu Provincial Hospital (Grant No.: 19SYPYB-7, 18GSSY3-1).
Author information
Authors and Affiliations
Contributions
Writing and editing by Xin Su, Jian Li, Xiao Xu, Youbao Ye, Cailiu Wang, Guanglong Pang, Wenxiu Liu, Ang Liu, Changchun Zhao; figures designed by Xin Su, Li Jian, Xiao Xu; tables designed by Xin Su, Li Jian, Xiao Xu; supervision by Xiangyong Hao. All authors have read and agreed to the version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Su, X., Li, J., Xu, X. et al. Strategies to enhance the therapeutic efficacy of anti-PD-1 antibody, anti-PD-L1 antibody and anti-CTLA-4 antibody in cancer therapy. J Transl Med 22, 751 (2024). https://doi.org/10.1186/s12967-024-05552-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-05552-6