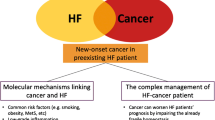
Abstract
Several large cohort studies in cardiovascular disease (CVD) patients have shown an increased incidence of cancer. Previous studies in a myocardial infarction (MI) mouse model reported increased colon, breast, and lung cancer growth. The potential mechanisms could be due to secreted cardiokines and micro-RNAs from pathological hearts and immune cell reprogramming. A study in a MI-induced heart failure (HF) mouse demonstrated an increase in cardiac expression of SerpinA3, resulting in an enhanced proliferation of colon cancer cells. In MI-induced HF mice with lung cancer, the attenuation of tumor sensitivity to ferroptosis via the secretion of miR-22-3p from cardiomyocytes was demonstrated. In MI mice with breast cancer, immune cell reprogramming toward the immunosuppressive state was shown. However, a study in mice with renal cancer reported no impact of MI on tumor growth. In addition to MI, cardiac hypertrophy was shown to promote the growth of breast and lung cancer. The cardiokine potentially involved, periostin, was increased in the cardiac tissue and serum of a cardiac hypertrophy model, and was reported to increase breast cancer cell proliferation. Since the concept that CVD could influence the initiation and progression of several types of cancer is quite new and challenging regarding future therapeutic and preventive strategies, further studies are needed to elucidate the potential underlying mechanisms which will enable more effective risk stratification and development of potential therapeutic interventions to prevent cancer in CVD patients.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is one of the leading causes of death worldwide. Over the past two decades, its prevalence has almost doubled from 271 to 523 million, and mortality rates continue to increase [1]. Cancer prevalence is also on the rise globally with an expected 28 million cases in 2040, nearly a 50% increase from 2020, and remains the leading cause of death [2]. Since the advancement of cancer treatment has led to more cancer survivors, there is increasing recognition of the devastating cardiovascular (CV) complications from cancer treatment. Cardio-oncology has emerged as a new field in an effort to mitigate the CV toxicity consequential to cancer therapy [3]. Interestingly, there is also accumulating evidence of a reverse relationship between CVD and cancer, termed “reverse cardio-oncology” [4]. Large cohort studies have demonstrated that patients with CVD have an increased risk of cancer development during follow-up [5,6,7]. In heart failure (HF) patients, non-CV death accounts for 15–30% of all deaths [8]. Cancer has been shown to be the leading cause of non-CV death in this population, contributing to approximately 40% [8].
Several cohort studies have demonstrated an increased risk of cancer in both HF and myocardial infarction (MI) patients [5,6,7, 9,10,11,12,13]. Additionally, a prospective cohort study in MI patients identified a subgroup that subsequently developed HF had an increased risk of cancer during follow-up [5]. Conversely, one retrospective study using the data from a Physicial Health Study (PHS) trial reported no association between HF and cancer among male physicians [14]. This discrepancy could be due the use of different study populations. These clinical reports are comprehensively summarized in Table 1.
The nature of these clinical studies is evidently limited by their ability to establish a causal relationship between CVD and cancer, for example the increased incidence of cancer in CVD patients could be partly explained by shared risk factors, including obesity, diabetes mellitus, hypertension (HT), smoking, and inflammation [15]. However, emerging evidence particularly from in vivo studies suggests a plausible direct effect of CVD on the enhancement of tumor growth and metastasis. In this review, we aimed to comprehensively summarize the contemporary evidence on this reverse cardio-oncology concept and highlight the potential direct mechanism of CVD on the enhancement of tumor growth and metastasis from both in vitro and in vivo studies.
Potential mechanisms of the effect of CVD on the enhancement of tumor proliferation and invasiveness: Evidence from in vitro and in vivo studies
Effects of MI on tumor growth and metastases
In an in vivo study using APCmin mice, a genetically susceptible mouse strain prone to developing colonic adenomas, MI-induced HF in these mice led to left ventricular (LV) systolic dysfunction, hypertrophy and fibrosis. It was found that these mice had enhanced colon cancer growth [16]. To exclude the potential effects of hemodynamic disturbance, the study used a heterotopic heart transplant from an MI rat into other APCmin mice, which also resulted in increased colon cancer growth in the recipient mice [16]. Likewise, a study using MI mice with orthotopic breast cancer showed that MI enhanced breast cancer growth [17]. MI also enhanced breast cancer growth and metastasis in MMTV-PyMT mice, which was a transgenic mice model of spontaneous breast cancer [17]. In MI-induced HF mice with a xenograft Lewis lung carcinoma (LLC) model, it was shown that these mice also had enhanced lung cancer growth [18]. However, in a study using MI-induced HF mice with orthotopic renal cancer it was found that there was no effect on renal cancer growth and metastasis, despite the presence of LV systolic dysfunction, hypertrophy and fibrosis as in other studies [19]. These in vivo studies suggested there is a direct effect of MI on cancer growth, and the effects on tumors were potentially cancer-type specific.
Regarding the potential mechanisms, the effects of MI on tumor growth could be due to the cardiokines and mi-RNAs secreted from pathologic hearts [16, 18]. A study in an MI-induced HF model showed increased expression by cardiac mRNA of SerpinA3, SerpinA1, fibronectin (FN), ceruloplasmin (CP), and paraoxonase 1 (PON1) [16]. However, it was demonstrated that heterotopic transplantation of an MI heart resulted in only SerpinA3, FN and PON1 having increased expression in cardiac tissues [16]. To emphasize the importance of these cardiokines, an in vitro study demonstrated that incubation of colon cancer cells with SerpinA3 10 ng/mL or SerpinA1 50 ng/mL resulted in an enhanced proliferation of colon cancer cells. However, exposure to FN 20 mcg/mL, CP 0.1 mcM, or PON1 10 mM had no effect on colon cancer cell proliferation [16]. These findings indicated that the enhanced tumor growth in the MI-induced HF model could be due to secreted cardiokines, including SerpinA3 and SerpinA1. Unfortunately, the level of SerpinA3 in plasma and tumor tissues were not reported in that study [16].
A study in MI-induced HF mice with xenograft LLC demonstrated that the MI-induced HF condition mitigated the tumor sensitivity to ferroptotic cell death [18]. Ferroptosis is a regulated cell death pathway characterized by iron-dependent lipid peroxidation with a distinct morphological form of cell death [20, 21]. Growing evidence suggests the importance of ferroptosis in tumor biology in terms of its role in tumorigenesis, tumor progression, metastasis and therapeutic resistance, as a consequence of ferroptosis evasion [20, 21]. Additionally, ferroptosis is being recognized as a target of cancer vulnerability to cancer therapy, as it is a form of cell death observed in response to various cancer treatments [20, 21]. A study in xenograft LLC mice model showed that MI-induced HF in mice attenuated the effect of ferroptosis inducer, erastin and imidazole ketone erastin (IKE), on tumor growth [18]. The ferroptosis markers including prostaglandin-endoperoxide synthase 2 (PTGS2) and acyl-CoA synthase long-chain family member 4 (ACSL4) were upregulated, whereas glutathione peroxidase 4 (GPX4) was downregulated with erastin and IKE. These effects were attenuated in an MI-induced HF condition [18]. Interestingly, injection of isolated exosomes from MI mice also attenuated the effect of ferroptosis inducer on tumor growth in xenograft LLC mice [18]. An in vitro study exposed isolated exosomes from MI mice to a lung cancer cell line (LLC) and an osteosarcoma cell line (K7M2) demonstrated that those exosomes mitigated the erastin-induced ferroptosis in those cancer cells [18]. Moreover, the inhibitory effect of ferroptosis inducer on tumor cell invasion and migration was also attenuated by exosomes from MI mice [18]. In support of those findings on the roles of ferroptosis in tumor progression, Ferrostatin-1, a ferroptosis inhibitor, was shown to effectively reverse the inhibitory effect of erastin on tumor cell invasion and migration, which are further reversed by exosomes from MI mice [18].
The micro-RNAs (mi-RNAs) are short, non-coding RNA segments that regulate gene expression [22]. Further analysis revealed that in an MI-induced HF mouse with xenograft LLC model, the mice had increased levels of miR-22-3p in both the cardiac tissues and tumor and also in plasma [18]. However, precursor miR-22 (pre-miR-22) was only increased in cardiac tissues but not in tumor, indicating that miR-22-3p was released from the pathologic heart [18]. The potential effects of miR-22-3p were further evaluated in both in vitro and in vivo studies. An in vitro study using transfecting LLC cells with miR-22-3p showed cellular resistance to erastin-induced ferroptosis, whereas blocking the action of miR-22-3p further promoted ferroptosis in those cells [18]. Consistent with these findings, inhibition of cardiac-specific miR-22-3p in MI-induced HF mice with xenograft LLC treated with erastin effectively attenuated the effect of MI-induced HF on enhanced tumor growth [18]. These findings suggest that miR-22-3p secreted in the MI-induced HF model could play a role in promoting tumor growth by reducing the cellular sensitivity to ferroptosis, as well as potentially modulating tumor response to cancer therapy.
In addition to the potential effect of secreted cardiokines and mi-RNAs from a pathologic heart in enhancing tumor growth, immune cell reprogramming has also been proposed [17]. MI mice with an orthotopic breast cancer model showed an increase in Ly6Chi monocytes in both plasma and tumor, as well as a decrease in T cells. However, the proportion of regulatory T cells in the tumor microenvironment was increased [17]. MMTV-PyMT mice with MI also had increased numbers of Ly6Chi monocytes in the tumors [17]. In an adoptive transfer experiment, MI induced Ly6Chi monocyte recruitment into the tumor was demonstrated. MI was induced in CCR2-diphtheria toxin receptor mice, which had depleted monocytes and showed decreased effects on tumor growth, decreased tumor Ly6Chi monocytes, and a decrease in the proportion of regulatory T cells, as well as an increased proportion of activated T cells (granzyme B+) [17]. A bone marrow transplant from a donor MI mouse with tumor implantation into the wild type mice and implanted with a tumor also showed enhanced tumor growth and an increase in circulating Ly6Chi monocytes [17]. These results indicated that the effect of MI on breast cancer growth and metastasis was through immune cell reprogramming and resulted in an immunosuppressive state within the tumor microenvironment [17].
In conclusion, current evidence from in vitro and in vivo studies demonstrated that MI could enhance tumor growth and metastasis as a consequence of secreted cardiokines, and mi-RNAs and immune cell reprogramming. Interestingly, the effect of MI on tumor growth was shown to be cancer-type specific. These reports are comprehensively summarized in Fig. 1, Tables 2 and 3.
Potential direct effects of myocardial infarction (MI) on tumor growth and metastasis. An MI model was shown to promote colon, lung, and breast cancer growth. The MI condition also increased metastasis of breast cancer. Cardiac expression of SerpinA3, SerpinA1, and miR-22-3p were increased in MI models. The miR-22-3p was also increased in plasma and tumor tissue, resulting in attenuated breast cancer cell sensitivity to erastin-induced ferroptosis. MI also increased Ly6Chi monocytes in plasma, tumor, and bone marrow, which led to an increased proportion of regulatory T-cells in the tumor microenvironment. Blue arrows indicate the changes of potential cardiokines and micro-RNAs. Red arrows indicate the effect of MI on tumor growth. The purple arrow indicates the change in ferroptotic cell death sensitivity. Figure created with BioRender.com
Cardiac hypertrophy
A study using transverse aortic constriction (TAC) to induce pressure overload in mice resulted in LV hypertrophy and systolic dysfunction, leading to increased tumor growth in both orthotopic breast cancer (PyMT) and LLC models [23]. When breast and lung cancer cells were injected into TAC-operated mice, it was shown that cardiac remodeling also enhanced metastasis of both cancer types [23]. In a separate study using an immunodeficient mouse model lacking lymphoid cells and dysfunctional myeloid cells, TAC-operated NOD/SCID (nonobese diabetic/severe combined immunodeficiency) mice also demonstrated an increase in breast cancer growth [23]. However, there were no effects on tumor growth in the breast cancer model in TAC-operated maladaptive cardiac remodeling-resistant (MCRR) mice, which were resistant to cardiac remodeling and did not develop any cardiac remodeling [23].
Transgenic mice with overexpression of activating transcription factor 3 (ATF3) have been shown to develop cardiac remodeling, including LV hypertrophy and systolic dysfunction, and showed enhanced tumor growth in orthotopic breast cancer and xenograft lung cancer models [24]. Injection of breast cancer cells into ATF3-transgenic mice also increased breast cancer metastasis [24]. Inhibition of ATF3 expression caused by supplementation with doxycycline after cardiac remodeling had already occurred did not have any impact on tumor growth or metastasis, indicating that the enhanced tumor effect was due to cardiac remodeling and was independent of ATF3 expression [24]. Low-dose phenylephrine (PE)-induced HT in mice, which induced LV hypertrophy without systolic dysfunction, also enhanced orthotopic breast cancer growth [25]. These in vivo studies suggested that cardiac remodeling induced by TAC and genetic modification could potentially enhance tumor growth.
Several potential biomarkers have been found and reported on in models of cardiac hypertrophy in association with tumor progression. TAC-operated mice with orthotopic breast and lung cancer had shown enhanced tumor growth and it was found that cardiac expression and plasma levels of periostin and connective tissue growth factor (CTGF) were increased [23]. Similarly, in TAC-operated mice injected with breast cancer cells, increased cardiac and plasma levels of periostin and CTGF were observed, along with enhanced metastasis [23]. However, in TAC-operated MCRR mice with an orthotopic breast cancer model, which did not develop any cardiac remodeling and with no affect on tumor growth, there was no increase in cardiac or plasma levels of periostin and CTGF [23]. When serum from TAC-operated mice with or without breast and lung cancer was applied to breast cancer cells (PyMT) and lung cancer cells (LLC), increased cancer cell proliferation in both cell types was demonstrated [23]. In the same study, periostin at 2000 and 4000 ng/mL was shown to enhance the proliferation of both breast and lung cancer cells, whereas periostin-deprived serum from a TAC-operated mouse with a breast cancer model had no effect on cancer cell proliferation [23].
ATF3-transgenic mice with orthotopic breast cancer, which showed enhanced tumor growth, exhibited increased cardiac expression of periostin, CTGF, FN, SerpinA3, and CP [24]. The expression of CTGF and FN was also increased in breast cancer tissues, whereas the plasma levels of CTGF and FN remained unchanged [24]. When the serum from ATF3-transgenic mice was applied to breast and lung cancer cells, it was shown to enhance both breast and lung cancer cell proliferation [24]. In the low-dose PE-induced LV hypertrophy with orthotopic breast cancer model which resulted in increased the tumor growth, increased cardiac expression of periostin and FN was demonstrated [25]. The plasma levels of periostin, FN, and CTGF were also increased. However, in the tumor only the expression of CTGF was increased, while periostin and FN were unchanged. The SerpinA3, PON1, and CP expression also remained unchanged in both the cardiac tissues and tumor in this model. Consistently, serum from low-dose PE-infused mice was shown to enhance the proliferation of breast cancer cells [25].
All of these findings indicated that cardiac remodeling from various models could increase tumor growth and metastasis, which could be due to the secretion of factors including periostin, FN and CTGF. The reports regarding the potential mechanisms of the effect of CVD on cancer growth and metastasis from both in vivo and in vitro studies are comprehensively summarized in Fig. 2, and Tables 2 and 3.
Potential direct effects of cardiac hypertrophy on tumor growth and metastasis. Cardiac hypertrophy was shown to enhance lung and breast cancer growth and metastasis. It has been reported that cardiac expression and plasma levels of periostin increase in a cardiac hypertrophy model. It has also been shown that expression of connective tissue growth factor (CTGF) and fibronectin (FN) increases in cardiac and tumor tissues and in plasma. Blue arrows indicate the changes of potential cardiokines. Red arrows indicate the effect of cardiac hypertrophy on tumor growth. Figure created with BioRender.com
Potential cardiokines and mi-RNAs as potential links between CVD and cancer
Growing evidence suggests that several cardiokines and mi-RNAs could be responsible for the promotion of tumor proliferation and invasiveness in CVD models. These include SerpinA3, SerpinA1, periostin, miR-21, and miR-22. The effects and expression of those cardiokines and mi-RNAs in CVD and cancer based on in vitro, in vivo and clinical studies are comprehensively summarized in Tables 4 and 5.
SerpinA3
SerpinA3, also known as anti-chymotrypsin, is a member of the Serpin superfamily that functions as a serine proteinase inhibitor and plays a role in the function and homeostasis of various organs throughout the body including regulation of blood pressure, insulin sensitivity and inflammatory response [26].
SerpinA3 in pathological hearts
An in vitro study using an atherosclerosis model demonstrated an increase in SerpinA3 expression in aortic smooth muscle cells exposed to oxidized LDL [27]. In MI and LV hypertrophy in ATF3-transgenic mice models with tumors, an enhancement in tumor growth and an increase in cardiac expression of SerpinA3 were observed [16, 24]. However, in mice with low-dose PE-induced LV hypertrophy without LV systolic dysfunction, no change in cardiac SerpinA3 expression was found [25]. In clinical studies, increased expression of SerpinA3 has been reported in various cardiac conditions and was associated with poor clinical outcome. Levels of SerpinA3 in the plasma were increased in coronary artery disease (CAD) patients and correlated with the extension of atherosclerosis [27]. In MI patients, plasma levels of SerpinA3 were increased and predicted major adverse cardiac events (MACE) [28]. In HF with reduced ejection fraction (HFrEF), levels of plasma SerpinA3 were also found to increase [29, 30]. In dilated cardiomyopathy (DCM) patients, cardiac expression and plasma levels of SerpinA3 were increased and correlated with poor outcomes [29]. The increased levels of SerpinA3 in DCM patients with LV assist devices (LVADs) were reduced after offloading LVADs [31]. Furthermore, in cases of calcific aortic stenosis, both cardiac and plasma levels of SerpinA3 were also increased [32].
SerpinA3 in cancer
SerpinA3 has been implicated in cancer proliferation and invasiveness. SerpinA3 expression was increased in various cancer cell lines including colon cancer and lung adenocarcinoma cell lines [33, 34]. The enhanced expression of SerpinA3 has been shown to promote tumor cell invasion and migration in colon cancer, breast cancer, and glioblastoma cell lines [34,35,36]. SerpinA3 also promoted the proliferation of colon cancer cells after in vitro exposure [16]. SerpinA3 was also associated with tumor invasiveness as a consequence of remodeling the extracellular matrix, as shown in studies using melanoma and glioblastoma [36, 37]. In mice injected with downregulated SerpinA3 colon cancer cells, a decrease in liver metastasis was demonstrated [34]. In clinical studies, SerpinA3 expression was increased in various tumor tissues, including colon, lung adenocarcinoma, breast and glioma [33,34,35,36]. Increased SerpinA3 expression in colon cancer tissues was associated with higher metastasis [34]. Increased expression of SerpinA3 by tumors was also associated with larger tumor size and poor survival in lung adenocarcinoma patients [33]. In addition, high SerpinA3 expression in glioma tissues was shown to be associated with poor survival [36]. Plasma levels of SerpinA3 were elevated in lung cancer patients and were also associated with metastasis [38].
SerpinA3 as a link between CVD and cancer
In summary, it has been reported that SerpinA3 is upregulated in association with various types of CVD and enhanced tumor proliferation and invasiveness in several types of cancer. In an MI mouse model which showed enhanced tumor growth, the SerpinA3 expression was increased in cardiac tissue, and an in vitro study demonstrated its role in increasing the growth of colon cancer cells [16]. An ATF3-transgenic mouse model that had enhanced tumor growth was also shown to have increased levels of expression of SerpinA3 in cardiac tissues but not in tumor cells [24]. This evidence suggests that SerpinA3 from CVD could be a cardiokine responsible for the enhanced tumor growth. These reports on SerpinA3 are comprehensively summarized in Tables 4 and 5.
SerpinA1
SerpinA1, also known as antitrypsin, is also a member of the Serpin superfamily [26].
SerpinA1 in pathological hearts
Unlike SerpinA3, the evidence surrounding the expression of SerpinA1 in CVD models is still limited. In a mouse model of MI, cardiac SepinA1 expression was found to be increased [16]. In clinical studies, plasma levels of SerpinA1 were also elevated in MI and HFrEF patients [39, 40]. More studies are needed to validate these findings regarding the level of SerpinA1 in association with CVD.
SerpinA1 in cancer
SerpinA1 has been reported to promote and be associated with outcomes in various types of cancer. It has been shown to promote cell proliferation in colon cancer and non-small cell lung cancer (NSCLC) [16, 41]. Additionally, upregulation of SerpinA1 has also been found to promote tumor migration and invasion in various cancer cell lines, including gastric, colon, breast, ovarian and lung cancer cells [41,42,43,44,45]. In mice, upregulated SerpinA1 was shown to promote metastasis of lung adenocarcinoma [43]. In clinical studies, increased expression of SerpinA1 in tumor tissues was associated with larger tumor size, metastasis and poor survival in colorectal and gastric cancer patients [42, 44]. In lung cancer patients, increased SerpinA1 expression was associated with metastasis and poor survival [41, 43, 45]. Additionally, SerpinA1 has been shown to promote lung cancer metastasis through regulation of expression of FN [43, 45].
SerpinA1 as a link between CVD and cancer
As mentioned earlier, it has been demonstrated that SerpinA1 promotes growth in various types of tumor, however, the evidence in CVD models is still very limited. Only one report has demonstrated the potential effect of CVD through SerpinA1 on the enhancement of tumor growth. In an MI-induced HF mouse model in which enhanced tumor growth was found, increased cardiac SerpinA1 expression was demonstrated [16]. However, there was no change in cardiac SerpinA1 expression following the heterotopic heart transplant of an MI model, which also reported enhanced tumor growth [16]. Further studies are needed to warrant the potential role of SerpinA1 in this setting. These reports are comprehensively summarized in Tables 4 and 5.
Periostin
Periostin is a secreted protein that serves as a component of the extracellular matrix and plays a crucial role in cell–matrix interactions [46]. It is associated with transforming growth factor-β (TGF-β) and regulates fibroblast function, contributing to collagen fibrillogenesis, which is involved in cardiac remodeling [46].
Periostin in pathological hearts
An in vitro study demonstrated that angiotensin II (Ang II) promoted the expression of periostin in adult rat cardiac fibroblasts [47]. Mice treated with an Ang II infusion developed LV hypertrophy and fibrosis, and an increase in expression of periostin by cardiac tissue was reported [47]. In high salt-induced HT rats, increased cardiac expression of periostin along with cardiac fibrosis was shown [48]. Cardiac expression of periostin was also increased in both MI and hypertensive-induced HF models [49,50,51,52]. Studies demonstrated enhanced tumor growth in cardiac remodeling models including TAC-operated mice, low-dose PE-induced HT mice, and ATF3-transgenic mice also reported increased periostin expression in cardiac tissues [23,24,25]. In clinical studies, increased cardiac expression of periostin was observed in patients with MI and HFrEF [49, 52, 53]. Additionally, levels of periostin were found to be elevated in the plasma in ST-elevation MI (STEMI) patients and were associated with increased CV events and declining LV systolic function [54].
Periostin in cancer
As a component of the tumor microenvironment, periostin is one of the matricellular proteins, a group of non-structural matrix components that plays a critical role in tumorigenesis and metastasis [55]. Periostin has been shown to interact with tumor cells and promote cell proliferation, migration, survival, epithelial-mesenchymal transition, and contribute to distant metastasis [55]. An in vitro study showed that incubation with periostin enhanced the proliferation of breast and lung cancer cells [23]. Upregulation of periostin was shown to stimulate lung cancer cell proliferation and migration, promote tumor angiogenesis in breast cancer cells, and promote colon cancer cell survival under stress conditions [56,57,58]. In mice, upregulation of periostin in breast and colon cancer cells promoted tumor growth and metastasis, respectively [57, 58]. In cancer patients, periostin expression was increased in various types of cancer tissues, including colon, prostate, NSCLC and breast cancer, and was associated with poor survival [57,58,59,60,61]. Increased tumor expression of periostin was also associated with advanced stages in colon, prostate, NSCLC and hepatocellular carcinoma (HCC) patients [56, 58, 59, 62, 63]. Plasma periostin levels were elevated in multiple types of cancer patients, including colon, NSCLC and HCC patients, and were correlated with poor survival [56, 58, 59, 64, 65]. In breast cancer patients, elevated plasma periostin levels were associated with bone metastasis [66].
Periostin as a link between CVD and cancer
Evidence demonstrated that expression of periostin in both cardiac tissue and plasma were increased in various CVD models and play a role in tumorigenesis and progression. In a cardiac remodeling mouse model that showed enhanced tumor growth, both cardiac tissues and plasma levels of periostin were reported to be increased, and an in vitro study demonstrated its role in tumor cell proliferation [23,24,25]. These findings suggest that periostin could be chemokine potentially responsible for enhanced tumor growth in CVD models. The reports on periostin in CVD and cancer are comprehensively summarized in Tables 4 and 5.
miR-21
miR-21 in pathological hearts
The miR-21 was found to be expressed by both cardiomyocytes and cardiac fibroblasts [67]. Previous in vitro studies using neonatal rat cardiomyocytes reported an increased miR-21 expression after exposure to hypertrophic stimuli including PE and Ang II [68, 69]. In various HF mouse models, including β1-adrenergic receptor transgenic mice, TAC-induced HF mice, and isoproterenol-induced HF mice, cardiac expression of miR-21 was increased [70]. In cardiac ischemic/reperfusion (I/R) injury mice, miR-21 expression was increased in the infarct region, particularly with regards to cardiac fibroblasts [71]. In an MI rat model, miR-21 expression was upregulated at the border zone but was downregulated in the infarct area during early post-MI, specifically within the first 24 h [72]. Between 3 days to 2 weeks, miR-21 expression, however, was upregulated in both the border and infarct zones, especially in the infarct area in MI mice [73]. In clinical studies, cardiac expression of miR-21 was also increased in end-stage HF patients [70]. Plasma levels of miR-21 were found to be elevated in acute coronary syndrome (ACS), CAD and HFrEF patients [74, 75]. Elevated plasma levels of miR-21 were also associated with a decline in LV ejection fraction and increased NYHA functional status in HFrEF patients [74].
miR-21 in cancer
The miR-21 is one of the most closely cancer-related mi-RNAs and is frequently upregulated in a wide range of solid tumors and hematologic malignancies [76, 77]. Several mechanisms have been identified through which miR-21 promoted cancer cell proliferation and migration [76, 77]. Upregulated miR-21 enhanced tumor cell proliferation in various cancer cell lines, including colorectal, NSCLC and gastric cancer [78,79,80]. Additionally, miR-21 expression enhanced tumor cell migration and invasiveness in colorectal, breast, NSCLC, gastric, glioblastoma and HCC [78,79,80,81,82,83,84,85,86]. Previous in vivo studies using mouse models showed that miR-21 promoted growth of colorectal and breast cancer [78, 83]. In clinical studies, it has been observed that expression of miR-21 is increased in various cancer tissues including colorectal, breast, lung, gastric, glioma, prostate, HCC and diffuse large B-cell lymphoma [64, 79, 80, 82, 85,86,87,88]. High expression of miR-21 by the tumor has been associated with metastasis in colorectal, breast, NSCLC and gastric cancer patients [64, 79, 80, 82]. In colon cancer patients, high tumor miR-21 expression was correlated with poor survival [89]. Elevated plasma miR-21 levels have been observed in breast, NSCLC, prostate, and gastric cancer patients [83, 90,91,92]. In NSCLC patients, high plasma miR-21 levels have been associated with lymph node metastasis and poor survival [90]. High plasma miR-21 levels have also been associated with advanced disease in prostate cancer patients [92].
miR-21 as a link between CVD and cancer
Overall, there is extensive evidence that there is increased expression of miR-21in CVD and that it is linked to tumorigenesis and cancer progression. Despite this evidence, there have still been no studies verifying the causal effect of miR-21 in tumor enhancement in CVD models. Further studies are required to illustrate the mechanistic link and determine the potential role of miR-21 in reverse cardio-oncology. These reports on miR-21 in CVD and cancer are comprehensively summarized in Tables 4 and 5.
miR-22
miR-22 in pathological hearts
It has been demonstrated that miR-22 is related to cardiac remodeling and LV hypertrophy [93]. Previous in vitro studies using neonatal rat cardiomyocytes demonstrated an increase in miR-22 expression after exposure to hypertrophy stimuli, including PE and Ang II [69, 94]. An in vivo study in TAC-induced cardiac hypertrophy mice also demonstrated increased cardiac expression of miR-22 [94]. In clinical studies, both cardiac expression and plasma levels of miR-22 were elevated in HFrEF patients [95, 96]. Elevated plasma miR-22 levels were also associated with an increased risk of CV death [96]. The miR-22-3p is the mi-RNA derived from the 3’ arm of miR-22 [22]. An in vivo study using MI-induced HF mice reported increased cardiac expression and plasma levels of miR-22-3p [18]. A study in HF patients, including both HFpEF and HFrEF, showed that an increased plasma miR-22-3p level was associated with a lower risk of CV events [97]. It has also been reported that plasma miR-22-3p was increased in CAD patients [98, 99]. Conversely, an earlier study reported decreased plasma miR-22 levels in CAD patients [100]. These discrepancies in the expression levels could be explained by differences in patient subgroups and specific type of miR-22.
miR-22 in cancer
The mechanistic role of miR-22 in cancer is variable and depends on specific cancer types [101]. It has been shown to have a tumor suppressor role by inhibiting tumor proliferation, invasion, and metastasis in various types of cancer [101]. Previous in vitro studies showed decreased expression of miR-22 in various cancer cell lines including NSCLC, HCC and colorectal cancer and overexpression of miR-22 suppressed cancer cell proliferation and migration in these cancer cell lines [102,103,104]. In NSCLC, overexpression of miR-22-3p also inhibited cell proliferation [105]. Similarly, in triple negative breast cancer cells, decreased expression of miR-22-3p was observed, and that the overexpression of miR-22-3p could suppress cancer cell proliferation and migration [106]. Previous in vivo studies have also demonstrated the tumor suppressing effect of miR-22 and miR-22-3p in lung, colorectal, HCC and breast cancer mice models [102,103,104, 106]. Conversely, miR-22 has been reported to promote tumor progression and metastasis in some cancers [101]. Higher miR-22 expression was observed in highly metastatic breast cancer cell lines, and also enhanced cell migration and invasion [107]. Similarly, prostate cancer cells also had increased miR-22 expression [108]. In an orthotopic breast cancer mouse model with miR-22 overexpression and an miR-22 transgenic mice model enhanced breast cancer metastasis was reported [107]. Overexpression of miR-22 also enhanced prostate cancer growth in a mouse model [108].
In clinical studies, decreased tumor expression of miR-22-3p has been reported in various cancers including lung, HCC, and breast cancer [102, 103, 106]. Low miR-22-3p expression was associated with poor survival in HCC patients [103]. Tumor miR-22 expression also decreased in colorectal cancer patients, and low tumor miR-22 expression was associated with liver metastasis and poor survival [104, 109]. However, another study in colon cancer patients reported increased expression of miR-22-3p by the tumor [110]. Conversely, elevation of tumor miR-22 has been reported in prostate cancer [108]. In breast cancer, elevated tumor miR-22 expression was associated with poor survival [107]. In advanced NSCLC patients, plasma miR-22 has been reported to be increased [111].
miR-22 as a link between CVD and cancer
A recent study demonstrated that secreted miR-22-3p from pathologic hearts of MI-induced HF mice mitigate the sensitivity of lung cancer to ferroptosis which may be responsible for tumor growth and possible cancer therapy resistance [18]. However, investigation into miR-22 is still very limited. Further studies are needed to better understand the role of decreased ferroptosis sensitivity of cancer induced by miR-22-3p in the pathological heart model. These reports on miR-22 in CVD and cancer are comprehensive summarized in Table 4 and 5, respectively.
Future perspective and conclusion
There is an increasing body of evidence from both in vitro and in vivo studies to demonstrate possible mechanisms by which CVD directly promotes cancer growth and metastasis. Currently, the effect of CVD on the promotion of tumor growth and proliferation are cancer-type specific and may be mediated via the secretion of several cardiokines, and mi-RNAs and immune cell reprogramming. An MI mouse model showed increased tumor growth and metastasis of colon, lung and breast cancer via possible cardiokines including SerpinA3, and mi-RNAs, and also as a consequence of immune cell reprogramming into an immunosuppressive tumor microenvironment. The mi-RNAs including miR-22-3p in an MI-induced HF model also attenuated the tumor sensitivity to ferroptosis in a lung cancer mouse model. Cardiac hypertrophy also enhanced breast and lung cancer growth and metastasis, which could be mediated by several cardiokines including periostin. The schematic diagram summarizing the current evidence is shown in Fig. 3. Nevertheless, there are still a limited number of studies dedicated to investigating and verifying a causal relationship between CVD and tumor progression. Furthermore, while many cardiokines and mi-RNAs have been shown to be involved in both CVD and cancer, studies that examine their causal relationship remain limited. Further studies for potential cardiokines and mi-RNAs secreted from pathological heart tissues are required. Moreover, the possible mechanisms involved in the systemic disturbance from CVD and the secreting factors from other organs as a result of hemodynamic changes or neurohormonal responses could also play a role in cancer exacerbation and require further investigations. A better understanding of the pathophysiology of reverse cardio-oncology could contribute to future risk stratification and therapeutic prevention for subsequent cancer progression in CVD patients.
A schematic diagram summarizes the potential direct effects of cardiovascular disease on tumor growth and metastasis. An MI model was shown to promote colon, lung, breast cancer growth and metastasis of breast cancer, with potential cardiokines including SerpinA3, mi-RNAs, and immune cell reprogramming. Cardiac hypertrophy was demonstrated to enhance the growth and metastasis of lung and breast cancer through potential cardiokines, including periostin. Figure created with BioRender.com
Availability of data and materials
Not applicable.
References
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Lyon AR, Lopez-Fernandez T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229–361.
Aboumsallem JP, Moslehi J, de Boer RA. Reverse cardio-oncology: cancer development in patients with cardiovascular disease. J Am Heart Assoc. 2020;9: e013754.
Hasin T, Gerber Y, Weston SA, Jiang R, Killian JM, Manemann SM, Cerhan JR, Roger VL. Heart failure after myocardial infarction is associated with increased risk of cancer. J Am Coll Cardiol. 2016;68:265–71.
Hasin T, Gerber Y, McNallan SM, Weston SA, Kushwaha SS, Nelson TJ, Cerhan JR, Roger VL. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol. 2013;62:881–6.
Banke A, Schou M, Videbaek L, Moller JE, Torp-Pedersen C, Gustafsson F, Dahl JS, Kober L, Hildebrandt PR, Gislason GH. Incidence of cancer in patients with chronic heart failure: a long-term follow-up study. Eur J Heart Fail. 2016;18:260–6.
de Boer RA, Meijers WC, van der Meer P, van Veldhuisen DJ. Cancer and heart disease: associations and relations. Eur J Heart Fail. 2019;21:1515–25.
Rinde LB, Smabrekke B, Hald EM, Brodin EE, Njolstad I, Mathiesen EB, Lochen ML, Wilsgaard T, Braekkan SK, Vik A, Hansen JB. Myocardial infarction and future risk of cancer in the general population-the Tromso Study. Eur J Epidemiol. 2017;32:193–201.
Bertero E, Robusto F, Rulli E, D’Ettorre A, Bisceglia L, Staszewsky L, Maack C, Lepore V, Latini R, Ameri P. Cancer incidence and mortality according to pre-existing heart failure in a community-based cohort. JACC CardioOncol. 2022;4:98–109.
Roderburg C, Loosen SH, Jahn JK, Gansbacher J, Luedde T, Kostev K, Luedde M. Heart failure is associated with an increased incidence of cancer diagnoses. ESC Heart Fail. 2021;8:3628–33.
Malmborg M, Christiansen CB, Schmiegelow MD, Torp-Pedersen C, Gislason G, Schou M. Incidence of new onset cancer in patients with a myocardial infarction - a nationwide cohort study. BMC Cardiovasc Disord. 2018;18:198.
Dreyer L, Olsen JH. Cancer risk of patients discharged with acute myocardial infarct. Epidemiology. 1998;9:178–83.
Selvaraj S, Bhatt DL, Claggett B, Djousse L, Shah SJ, Chen J, Imran TF, Qazi S, Sesso HD, Gaziano JM, Schrag D. Lack of association between heart failure and incident cancer. J Am Coll Cardiol. 2018;71:1501–10.
Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–14.
Meijers WC, Maglione M, Bakker SJL, Oberhuber R, Kieneker LM, de Jong S, Haubner BJ, Nagengast WB, Lyon AR, van der Vegt B, et al. Heart failure stimulates tumor growth by circulating factors. Circulation. 2018;138:678–91.
Koelwyn GJ, Newman AAC, Afonso MS, van Solingen C, Corr EM, Brown EJ, Albers KB, Yamaguchi N, Narke D, Schlegel M, et al. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat Med. 2020;26:1452–8.
Yuan Y, Mei Z, Qu Z, Li G, Yu S, Liu Y, Liu K, Shen Z, Pu J, Wang Y, et al. Exosomes secreted from cardiomyocytes suppress the sensitivity of tumor ferroptosis in ischemic heart failure. Signal Transduct Target Ther. 2023;8:121.
Shi C, Aboumsallem JP, de Wit S, Schouten EM, Bracun V, Meijers WC, Sillje HHW, de Boer RA. Evaluation of renal cancer progression in a mouse model of heart failure. Cancer Commun (Lond). 2021;41:796–9.
Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22:381–96.
Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer. 2019;19:405–14.
Romaine SP, Tomaszewski M, Condorelli G, Samani NJ. MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart. 2015;101:921–8.
Avraham S, Abu-Sharki S, Shofti R, Haas T, Korin B, Kalfon R, Friedman T, Shiran A, Saliba W, Shaked Y, Aronheim A. Early cardiac remodeling promotes tumor growth and metastasis. Circulation. 2020;142:670–83.
Awwad L, Aronheim A. Cardiac dysfunction promotes cancer progression via multiple secreted factors. Cancer Res. 2022;82:1753–61.
Awwad L, Goldenberg T, Langier-Goncalves I, Aronheim A. Cardiac remodeling in the absence of cardiac contractile dysfunction is sufficient to promote cancer progression. Cells. 2022;11:1108.
Sanchez-Navarro A, Gonzalez-Soria I, Caldino-Bohn R, Bobadilla NA. An integrative view of serpins in health and disease: the contribution of SerpinA3. Am J Physiol Cell Physiol. 2021;320:C106–18.
Li B, Lei Z, Wu Y, Li B, Zhai M, Zhong Y, Ju P, Kou W, Shi Y, Zhang X, Peng W. The association and pathogenesis of SERPINA3 in coronary artery disease. Front Cardiovasc Med. 2021;8: 756889.
Zhao L, Zheng M, Guo Z, Li K, Liu Y, Chen M, Yang X. Circulating Serpina3 levels predict the major adverse cardiac events in patients with myocardial infarction. Int J Cardiol. 2020;300:34–8.
Delrue L, Vanderheyden M, Beles M, Paolisso P, Di Gioia G, Dierckx R, Verstreken S, Goethals M, Heggermont W, Bartunek J. Circulating SERPINA3 improves prognostic stratification in patients with a de novo or worsened heart failure. ESC Heart Fail. 2021;8:4780–90.
Lok SI, Lok DJ, van der Weide P, Winkens B, Bruggink-Andre de la Porte PW, Doevendans PA, de Weger RA, van der Meer P, de Jonge N. Plasma levels of alpha-1-antichymotrypsin are elevated in patients with chronic heart failure, but are of limited prognostic value. Neth Heart J. 2014;22:391–5.
Lok SI, van Mil A, Bovenschen N, van der Weide P, van Kuik J, van Wichen D, Peeters T, Siera E, Winkens B, Sluijter JP, et al. Post-transcriptional regulation of alpha-1-antichymotrypsin by microRNA-137 in chronic heart failure and mechanical support. Circ Heart Fail. 2013;6:853–61.
Martin-Rojas T, Mourino-Alvarez L, Gil-Dones F, de la Cuesta F, Rosello-Lleti E, Laborde CM, Rivera M, Lopez-Almodovar LF, Lopez JA, Akerstrom F, et al. A clinical perspective on the utility of alpha 1 antichymotrypsin for the early diagnosis of calcific aortic stenosis. Clin Proteomics. 2017;14:12.
Higashiyama M, Doi O, Yokouchi H, Kodama K, Nakamori S, Tateishi R. Alpha-1-antichymotrypsin expression in lung adenocarcinoma and its possible association with tumor progression. Cancer. 1995;76:1368–76.
Cao LL, Pei XF, Qiao X, Yu J, Ye H, Xi CL, Wang PY, Gong ZL. SERPINA3 silencing inhibits the migration, invasion, and liver metastasis of colon cancer cells. Dig Dis Sci. 2018;63:2309–19.
Zhang Y, Tian J, Qu C, Peng Y, Lei J, Li K, Zong B, Sun L, Liu S. Overexpression of SERPINA3 promotes tumor invasion and migration, epithelial-mesenchymal-transition in triple-negative breast cancer cells. Breast Cancer. 2021;28:859–73.
Li Y, Dong X, Cai J, Yin S, Sun Y, Yang D, Jiang C. SERPINA3 induced by astroglia/microglia co-culture facilitates glioblastoma stem-like cell invasion. Oncol Lett. 2018;15:285–91.
Kulesza DW, Ramji K, Maleszewska M, Mieczkowski J, Dabrowski M, Chouaib S, Kaminska B. Search for novel STAT3-dependent genes reveals SERPINA3 as a new STAT3 target that regulates invasion of human melanoma cells. Lab Invest. 2019;99:1607–21.
Zelvyte I, Wallmark A, Piitulainen E, Westin U, Janciauskiene S. Increased plasma levels of serine proteinase inhibitors in lung cancer patients. Anticancer Res. 2004;24:241–7.
Gilutz H, Siegel Y, Paran E, Cristal N, Quastel MR. Alpha 1-antitrypsin in acute myocardial infarction. Br Heart J. 1983;49:26–9.
Lubrano V, Vergaro G, Maltinti M, Ghionzoli N, Emdin M, Papa A. alpha-1 Antitrypsin as a potential biomarker in chronic heart failure. J Cardiovasc Med (Hagerstown). 2020;21:209–15.
Ercetin E, Richtmann S, Delgado BM, Gomez-Mariano G, Wrenger S, Korenbaum E, Liu B, DeLuca D, Kuhnel MP, Jonigk D, et al. Clinical significance of SERPINA1 gene and its encoded alpha1-antitrypsin protein in NSCLC. Cancers (Basel). 2019;11:1306.
Kwon CH, Park HJ, Choi JH, Lee JR, Kim HK, Jo HJ, Kim HS, Oh N, Song GA, Park DY. Snail and serpinA1 promote tumor progression and predict prognosis in colorectal cancer. Oncotarget. 2015;6:20312–26.
Chang YH, Lee SH, Liao IC, Huang SH, Cheng HC, Liao PC. Secretomic analysis identifies alpha-1 antitrypsin (A1AT) as a required protein in cancer cell migration, invasion, and pericellular fibronectin assembly for facilitating lung colonization of lung adenocarcinoma cells. Mol Cell Proteomics. 2012;11:1320–39.
Kwon CH, Park HJ, Lee JR, Kim HK, Jeon TY, Jo HJ, Kim DH, Kim GH, Park DY. Serpin peptidase inhibitor clade A member 1 is a biomarker of poor prognosis in gastric cancer. Br J Cancer. 2014;111:1993–2002.
Li Y, Miao L, Yu M, Shi M, Wang Y, Yang J, Xiao Y, Cai H. alpha1-antitrypsin promotes lung adenocarcinoma metastasis through upregulating fibronectin expression. Int J Oncol. 2017;50:1955–64.
Landry NM, Cohen S, Dixon IMC. Periostin in cardiovascular disease and development: a tale of two distinct roles. Basic Res Cardiol. 2018;113:1.
Li L, Fan D, Wang C, Wang JY, Cui XB, Wu D, Zhou Y, Wu LL. Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-beta1 pathways in cardiac fibroblasts. Cardiovasc Res. 2011;91:80–9.
Wu H, Chen L, Xie J, Li R, Li GN, Chen QH, Zhang XL, Kang LN, Xu B. Periostin expression induced by oxidative stress contributes to myocardial fibrosis in a rat model of high salt-induced hypertension. Mol Med Rep. 2016;14:776–82.
Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, et al. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205:295–303.
Gil H, Goldshtein M, Etzion S, Elyagon S, Hadad U, Etzion Y, Cohen S. Defining the timeline of periostin upregulation in cardiac fibrosis following acute myocardial infarction in mice. Sci Rep. 2022;12:21863.
Hu Y, Wang X, Ding F, Liu C, Wang S, Feng T, Meng S. Periostin renders cardiomyocytes vulnerable to acute myocardial infarction via pro-apoptosis. ESC Heart Fail. 2022;9:977–87.
Stansfield WE, Andersen NM, Tang RH, Selzman CH. Periostin is a novel factor in cardiac remodeling after experimental and clinical unloading of the failing heart. Ann Thorac Surg. 2009;88:1916–21.
Zhao S, Wu H, Xia W, Chen X, Zhu S, Zhang S, Shao Y, Ma W, Yang D, Zhang J. Periostin expression is upregulated and associated with myocardial fibrosis in human failing hearts. J Cardiol. 2014;63:373–8.
Ling L, Cheng Y, Ding L, Yang X. Association of serum periostin with cardiac function and short-term prognosis in acute myocardial infarction patients. PLoS ONE. 2014;9: e88755.
Gonzalez-Gonzalez L, Alonso J. Periostin: A matricellular protein with multiple functions in cancer development and progression. Front Oncol. 2018;8:225.
Hong L, Sun H, Lv X, Yang D, Zhang J, Shi Y. Expression of periostin in the serum of NSCLC and its function on proliferation and migration of human lung adenocarcinoma cell line (A549) in vitro. Mol Biol Rep. 2010;37:2285–93.
Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, Gishizky ML, Marks JR, Wang XF. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol. 2004;24:3992–4003.
Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329–39.
Ben QW, Zhao Z, Ge SF, Zhou J, Yuan F, Yuan YZ. Circulating levels of periostin may help identify patients with more aggressive colorectal cancer. Int J Oncol. 2009;34:821–8.
Ratajczak-Wielgomas K, Grzegrzolka J, Piotrowska A, Gomulkiewicz A, Witkiewicz W, Dziegiel P. Periostin expression in cancer-associated fibroblasts of invasive ductal breast carcinoma. Oncol Rep. 2016;36:2745–54.
Nuzzo PV, Rubagotti A, Zinoli L, Ricci F, Salvi S, Boccardo S, Boccardo F. Prognostic value of stromal and epithelial periostin expression in human prostate cancer: correlation with clinical pathological features and the risk of biochemical relapse or death. BMC Cancer. 2012;12:625.
Jang SY, Park SY, Lee HW, Choi YK, Park KG, Yoon GS, Tak WY, Kweon YO, Hur K, Lee WK. The combination of periostin overexpression and microvascular invasion is related to a poor prognosis for hepatocellular carcinoma. Gut Liver. 2016;10:948–54.
Tischler V, Fritzsche FR, Wild PJ, Stephan C, Seifert HH, Riener MO, Hermanns T, Mortezavi A, Gerhardt J, Schraml P, et al. Periostin is up-regulated in high grade and high stage prostate cancer. BMC Cancer. 2010;10:273.
Xu CH, Wang W, Lin Y, Qian LH, Zhang XW, Wang QB, Yu LK. Diagnostic and prognostic value of serum periostin in patients with non-small cell lung cancer. Oncotarget. 2017;8:18746–53.
Lv Y, Wang W, Jia WD, Sun QK, Huang M, Zhou HC, Xia HH, Liu WB, Chen H, Sun SN, Xu GL. High preoparative levels of serum periostin are associated with poor prognosis in patients with hepatocellular carcinoma after hepatectomy. Eur J Surg Oncol. 2013;39:1129–35.
Sasaki H, Yu CY, Dai M, Tam C, Loda M, Auclair D, Chen LB, Elias A. Elevated serum periostin levels in patients with bone metastases from breast but not lung cancer. Breast Cancer Res Treat. 2003;77:245–52.
Cheng Y, Zhang C. MicroRNA-21 in cardiovascular disease. J Cardiovasc Transl Res. 2010;3:251–5.
Tatsuguchi M, Seok HY, Callis TE, Thomson JM, Chen JF, Newman M, Rojas M, Hammond SM, Wang DZ. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2007;42:1137–41.
Tu Y, Wan L, Bu L, Zhao D, Dong D, Huang T, Cheng Z, Shen B. MicroRNA-22 downregulation by atorvastatin in a mouse model of cardiac hypertrophy: a new mechanism for antihypertrophic intervention. Cell Physiol Biochem. 2013;31:997–1008.
Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–4.
Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–9.
Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–25.
Yuan J, Chen H, Ge D, Xu Y, Xu H, Yang Y, Gu M, Zhou Y, Zhu J, Ge T, et al. Mir-21 promotes cardiac fibrosis after myocardial infarction via targeting Smad7. Cell Physiol Biochem. 2017;42:2207–19.
Zhang J, Xing Q, Zhou X, Li J, Li Y, Zhang L, Zhou Q, Tang B. Circulating miRNA-21 is a promising biomarker for heart failure. Mol Med Rep. 2017;16:7766–74.
Kumar D, Narang R, Sreenivas V, Rastogi V, Bhatia J, Saluja D, Srivastava K. Circulatory miR-133b and miR-21 as novel biomarkers in early prediction and diagnosis of coronary artery disease. Genes (Basel). 2020;11:164.
Feng YH, Tsao CJ. Emerging role of microRNA-21 in cancer. Biomed Rep. 2016;5:395–402.
Bautista-Sanchez D, Arriaga-Canon C, Pedroza-Torres A, De La Rosa-Velazquez IA, Gonzalez-Barrios R, Contreras-Espinosa L, Montiel-Manriquez R, Castro-Hernandez C, Fragoso-Ontiveros V, Alvarez-Gomez RM, Herrera LA. The promising role of miR-21 as a cancer biomarker and its importance in RNA-based therapeutics. Mol Ther Nucleic Acids. 2020;20:409–20.
Wu Y, Song Y, Xiong Y, Wang X, Xu K, Han B, Bai Y, Li L, Zhang Y, Zhou L. MicroRNA-21 (Mir-21) promotes cell growth and invasion by repressing tumor suppressor PTEN in colorectal cancer. Cell Physiol Biochem. 2017;43:945–58.
Liu ZL, Wang H, Liu J, Wang ZX. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol Cell Biochem. 2013;372:35–45.
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY, Yan M. microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol Rep. 2012;27:1019–26.
Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–36.
Song B, Wang C, Liu J, Wang X, Lv L, Wei L, Xie L, Zheng Y, Song X. MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. J Exp Clin Cancer Res. 2010;29:29.
Han M, Wang F, Gu Y, Pei X, Guo G, Yu C, Li L, Zhu M, Xiong Y, Wang Y. MicroRNA-21 induces breast cancer cell invasion and migration by suppressing smad7 via EGF and TGF-beta pathways. Oncol Rep. 2016;35:73–80.
Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–33.
Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, Krichevsky AM. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008;28:5369–80.
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–58.
Song J, Shao Q, Li C, Liu H, Li J, Wang Y, Song W, Li L, Wang G, Shao Z, Fu R. Effects of microRNA-21 on apoptosis by regulating the expression of PTEN in diffuse large B-cell lymphoma. Medicine (Baltimore). 2017;96: e7952.
Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61.
Nielsen BS, Jorgensen S, Fog JU, Sokilde R, Christensen IJ, Hansen U, Brunner N, Baker A, Moller S, Nielsen HJ. High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clin Exp Metastasis. 2011;28:27–38.
Wang ZX, Bian HB, Wang JR, Cheng ZX, Wang KM, De W. Prognostic significance of serum miRNA-21 expression in human non-small cell lung cancer. J Surg Oncol. 2011;104:847–51.
Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi K, Fujiwara H, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102:1174–9.
Yaman Agaoglu F, Kovancilar M, Dizdar Y, Darendeliler E, Holdenrieder S, Dalay N, Gezer U. Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumour Biol. 2011;32:583–8.
Huang ZP, Wang DZ. miR-22 in cardiac remodeling and disease. Trends Cardiovasc Med. 2014;24:267–72.
Huang ZP, Chen J, Seok HY, Zhang Z, Kataoka M, Hu X, Wang DZ. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ Res. 2013;112:1234–43.
Matkovich SJ, Van Booven DJ, Youker KA, Torre-Amione G, Diwan A, Eschenbacher WH, Dorn LE, Watson MA, Margulies KB, Dorn GW 2nd. Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation. 2009;119:1263–71.
Gupta SK, Foinquinos A, Thum S, Remke J, Zimmer K, Bauters C, de Groote P, Boon RA, de Windt LJ, Preissl S, et al. Preclinical development of a MicroRNA-based therapy for elderly patients with myocardial infarction. J Am Coll Cardiol. 2016;68:1557–71.
van Boven N, Akkerhuis KM, Anroedh SS, Rizopoulos D, Pinto Y, Battes LC, Hillege HL, Caliskan KC, Germans T, Manintveld OC, et al. Serially measured circulating miR-22-3p is a biomarker for adverse clinical outcome in patients with chronic heart failure: the Bio-SHiFT study. Int J Cardiol. 2017;235:124–32.
Zhong Z, Zhong W, Zhang Q, Zhang Q, Yu Z, Wu H. Circulating microRNA expression profiling and bioinformatics analysis of patients with coronary artery disease by RNA sequencing. J Clin Lab Anal. 2020;34: e23020.
Zhang M, Hu Y, Li H, Guo X, Zhong J, He S. miR-22-3p as a potential biomarker for coronary artery disease based on integrated bioinformatics analysis. Front Genet. 2022;13: 936937.
Chen B, Luo L, Zhu W, Wei X, Li S, Huang Y, Liu M, Lin X. miR-22 contributes to the pathogenesis of patients with coronary artery disease by targeting MCP-1: an observational study. Medicine (Baltimore). 2016;95: e4418.
Wang J, Li Y, Ding M, Zhang H, Xu X, Tang J. Molecular mechanisms and clinical applications of miR-22 in regulating malignant progression in human cancer (Review). Int J Oncol. 2017;50:345–55.
Ling B, Wang GX, Long G, Qiu JH, Hu ZL. Tumor suppressor miR-22 suppresses lung cancer cell progression through post-transcriptional regulation of ErbB3. J Cancer Res Clin Oncol. 2012;138:1355–61.
Zhang J, Yang Y, Yang T, Liu Y, Li A, Fu S, Wu M, Pan Z, Zhou W. microRNA-22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity. Br J Cancer. 2010;103:1215–20.
Liu Y, Chen X, Cheng R, Yang F, Yu M, Wang C, Cui S, Hong Y, Liang H, Liu M, et al. The Jun/miR-22/HuR regulatory axis contributes to tumourigenesis in colorectal cancer. Mol Cancer. 2018;17:11.
Yang X, Su W, Li Y, Zhou Z, Zhou Y, Shan H, Han X, Zhang M, Zhang Q, Bai Y, et al. MiR-22-3p suppresses cell growth via MET/STAT3 signaling in lung cancer. Am J Transl Res. 2021;13:1221–32.
Gorur A, Bayraktar R, Ivan C, Mokhlis HA, Bayraktar E, Kahraman N, Karakas D, Karamil S, Kabil NN, Kanlikilicer P, et al. ncRNA therapy with miRNA-22-3p suppresses the growth of triple-negative breast cancer. Mol Ther Nucleic Acids. 2021;23:930–43.
Pandey AK, Zhang Y, Zhang S, Li Y, Tucker-Kellogg G, Yang H, Jha S. TIP60-miR-22 axis as a prognostic marker of breast cancer progression. Oncotarget. 2015;6:41290–306.
Poliseno L, Salmena L, Riccardi L, Fornari A, Song MS, Hobbs RM, Sportoletti P, Varmeh S, Egia A, Fedele G, et al. Identification of the miR-106b~25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci Signal. 2010;3:ra29.
Zhang G, Xia S, Tian H, Liu Z, Zhou T. Clinical significance of miR-22 expression in patients with colorectal cancer. Med Oncol. 2012;29:3108–12.
Schee K, Lorenz S, Worren MM, Gunther CC, Holden M, Hovig E, Fodstad O, Meza-Zepeda LA, Flatmark K. Deep sequencing the MicroRNA transcriptome in colorectal cancer. PLoS ONE. 2013;8: e66165.
Franchina T, Amodeo V, Bronte G, Savio G, Ricciardi GR, Picciotto M, Russo A, Giordano A, Adamo V. Circulating miR-22, miR-24 and miR-34a as novel predictive biomarkers to pemetrexed-based chemotherapy in advanced non-small cell lung cancer. J Cell Physiol. 2014;229:97–9.
Song SJ, Poliseno L, Song MS, Ala U, Webster K, Ng C, Beringer G, Brikbak NJ, Yuan X, Cantley LC, et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell. 2013;154:311–24.
Long F, Tian L, Chai Z, Li J, Tang Y, Liu M. Identification of stage-associated exosome miRNAs in colorectal cancer by improved robust and corroborative approach embedded miRNA-target network. Front Med (Lausanne). 2022;9: 881788.
Hussein NA, Kholy ZA, Anwar MM, Ahmad MA, Ahmad SM. Plasma miR-22-3p, miR-642b-3p and miR-885-5p as diagnostic biomarkers for pancreatic cancer. J Cancer Res Clin Oncol. 2017;143:83–93.
Acknowledgements
Not applicable.
Funding
This work was supported by a NSTDA Research Chair grant from the National Science and Technology Development Agency Thailand and the National Research Council of Thailand (NC), the Distinguished Research Professor grant (N42A660301) from the National Research Council of Thailand (SCC), and the Chiang Mai University Center of Excellence Award (NC).
Author information
Authors and Affiliations
Contributions
TA, SCC, and NC participated in the conception and designed the review. TA, SCC, and NC wrote the manuscript. TA, SCC, and NC revised the whole writing process. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethic approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Attachaipanich, T., Chattipakorn, S.C. & Chattipakorn, N. Current evidence regarding the cellular mechanisms associated with cancer progression due to cardiovascular diseases. J Transl Med 22, 105 (2024). https://doi.org/10.1186/s12967-023-04803-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04803-2