Abstract
Background
Oncolytic virotherapy (OVT) is a promising anti-tumor modality that utilizes oncolytic viruses (OVs) to preferentially attack cancers rather than normal tissues. With the understanding particularly in the characteristics of viruses and tumor cells, numerous innovative OVs have been engineered to conquer cancers, such as Talimogene Laherparepvec (T-VEC) and tasadenoturev (DNX-2401). However, the therapeutic safety and efficacy must be further optimized and balanced to ensure the superior safe and efficient OVT in clinics, and reasonable combination therapy strategies are also important challenges worthy to be explored.
Main body
Here we provided a critical review of the development history and status of OVT, emphasizing the mechanisms of enhancing both safety and efficacy. We propose that oncolytic virotherapy has evolved into the fourth generation as tumor immunotherapy. Particularly, to arouse T cells by designing OVs expressing bi-specific T cell activator (BiTA) is a promising strategy of killing two birds with one stone. Amazing combination of therapeutic strategies of OVs and immune cells confers immense potential for managing cancers. Moreover, the attractive preclinical OVT addressed recently, and the OVT in clinical trials were systematically reviewed.
Conclusion
OVs, which are advancing into clinical trials, are being envisioned as the frontier clinical anti-tumor agents coming soon.
Similar content being viewed by others
Introduction
Cancer is still a serious threat to human health and a major cause of death worldwide, even among adolescents and young adults [1, 2]. The scientists have been pursuing the ideal tumor prevention and treatment strategies all the time. Numerous promising tactics have been well developed, such as immunotherapy, photodynamic therapy and oncolytic virotherapy (OVT) [3,4,5,6].
OVT has its unique advantages and prospects, because oncolytic viruses (OVs) preferentially infect and replicate in tumor cells and destroy them, while leaving healthy cells largely untouched [7]. With increasingly high therapeutic efficacy being achieved recent years and owing to the unique features such as specific tumor tropism, low cytotoxicity against normal cells, OVT has been inviting a great attention as an ideal weapon against cancers.
OVT has a long development history. Originally, viruses were known as the cause of human diseases, including some cancers [8]. It was not until early 1950s that the potential of viruses as anti-cancer agents had been recognized and applied [7, 9]. At that time, the application of tumor treatment with the spontaneous viruses or wild type viruses which quite often being scavenged by immune system, merely induces a subtle inhibition to tumor progression in patients. Meanwhile, these non-engineered viruses sometimes inevitably infect and spread to normal tissues, indiscriminately killed both tumor and normal cells, causing a series of unpredicted side effects. Therefore, safety and efficacy were the greatest challenges for the development of OVT. With the leap of gene cloning in the molecular virology, the scientists focus on improvement of their antitumor specificity and efficiency by manipulating the viral genomes. As shown in Fig. 1, we propose that OVT can be divided into the following four phases of development. The viruses originally used for treatment are usually spontaneous viruses. The first generation (G1) of engineered OVs mainly focus on manipulating within virus genome. By the genetic recombination the viruses were conferred with high specificity against tumor cells without targeting normal tissues. The first application of virotherapy with the engineered thymidine kinase (TK)-deficient herpes simplex viruses (HSV) was initiated in 1991 [10]. The second generation (G2) of engineered OVs armed with viral and/or non-viral genes. A series of chimeric viruses strategies, such as transductional targeting, transcriptional targeting, micro-RNA targeting and DNA shuffling approaches have been developed for restricting virus infection and toxicity in off-target tissues [11,12,13]. For example, Myb34.5, a second-generation replication-conditional HSV-1, has been exploited to target and dampen the pancreatic tumors [13]. Moreover, HSV engineered in gH of a scFv targeting the cancer-specific HER2 receptor, scFv-HER2-gH chimera, can enter, replicate and kill cancer cells efficiently [14]. The third generation (G3) OVs were engineered with multiple coordinated viral and non-viral genes for tumor immunotherapy. Rivadeneira et al. demonstrates that intratumoral delivery of leptin by a VV can metabolically enhance tumor-infiltrating lymphocytes (TILs) effector and memory functions through improved mitochondrial oxidative phosphorylation, thereby potentiating therapeutic efficacy [15]. Anthony et al. engineered the vaccinia virus to express a nonsignaling, truncated CD19 (CD19t) protein for tumor-selective delivery, enabling targeting by CD19-CAR T cells [16]. Keeping stringency on tumor specificity and normal tissues safety usually hampers replicative fitness of viruses in target tissues. Thus, scientists keep pursuing ideal OVs that are highly tumor-specific without an attenuated clinical efficacy. In the first place, OVs have been designed to eliminate infected cancer cells by taking advantage of some of the most important properties of viruses or immune responses, including direct oncolysis, antitumor immunity, vascular-disrupting effects and bystander killing effect [17]. Secondly, along with the improvement of the tumor specificity, scientists are also constantly boosting the potency of OVT via prodrug activation, radiosensitization, immunostimulation and so on [18,19,20,21,22,23]. Worth to be noted, among these designs a second-generation oncolytic HSVs expressing TNF-α are being developed for cancer therapy and exerting its high efficacy for cancer therapy [24].
Until now, more than twenty different virus families have been engineered for cancer therapy, including but not limited to HSV, adenovirus (Ad), measles virus (MV), Newcastle disease virus (NDV), vaccinia virus (VV), reovirus, myxomavirus, poliovirus, poxviruses, vesicular stomatitis virus (VSV) [7, 19, 25,26,27]. These engineered viruses usually focused on targeting replication of OVs in the tumor bed, initiation of an immune-stimulating or immune-recruiting inflammatory response and exposure of tumor-associated antigens that can be targeted by the immune system [28]. Moreover, the safety and efficacy of OVs in combination with other treatments have been explored continuously [28,29,30]. Arming strategies that combine chemo-, radio- and immuno-therapies with OVT will be strengthened by greater viruses replication and spread [19, 30,31,32,33,34,35,36]. In this review, the summary of the knowledge on the OVT, including the development history, the applications of preclinical studies, the mechanism of enhancing the safety and efficacy, and clinical trials were provided. In addition, the most important attractive schemes of genetic modifications and combinatorial regimens with OVs were highlighted.
OVs in preclinical development
As a promising cancer therapy strategy, OVT has immeasurable application potential, bringing a bright future to cancer patients. Many natural and genetically engineered OVs have been developed and underwent pre-clinical research stages (Table 1). Although the idea of using viruses to treat cancer originated in 1950s and has been around for more than 70 years, the modern era of OVT can be traced back to a 1991 cornerstone study, in which a TK gene was deleted in HSV with attenuated neurovirulence was shown to be active in a murine glioblastoma model [10]. Subsequently, the OVT upsurged globally and made great advance. The researchers began immersing themselves in manipulating various modifications with different types of viruses and testing them in animal models.
Recently, Lin et al. developed a novel immunotherapeutic HSV-1 (OVH-aMPD-1) expressing a scFv against PD-1, which releases damage-associated molecular patterns (DAMPs), promoting antigen cross-presentation by DCs, and enhancing the infiltration of activated T cells; these modifications resulted in activation of antitumor T-cell that led to reduced tumor burdens in a mouse model of liver cancer [29]. In addition to awaken T cell response, activating other types of immune cells is also a wise option. The combination of EGFR-CAR NK-92 cells with oHSV-1 resulted in more efficient killing of MDA-MB-231 breast tumor cells and significantly longer survival of tumor-bearing mice when compared to monotherapies [36]. A UV light-inactivated HSV-1 (UV-HSV-1) potently activates human peripheral blood mononuclear cells (PBMCs) to lyse leukemic cell lines and primary AML samples, but not healthy allogeneic lymphocytes. The data suggested that UV-HSV-1 synergizes with IL-15 and IL-2 in inducing activation and cytolytic activity of NK cells [37]. Moreover, to reduce toxicity and enhance oncolysis to destroy glioma, Delwar et al. replaced the HSV ICP4 promoter with the survivin promoter and introduced the 5’UTR of rat FGF-2, and 5 copies of the miRNA 124 target sequence 3’UTR into the ICP4 gene. The intratumorally injected oHSV-1 was demonstrated to be effective in mice bearing human glioma U87 tumors, whereas viral DNA was almost undetectable in normal organs [38]. To evade antiviral defense response, arming oHSV with antiangiogenic N-terminal cleavage fragment of brain angiogenesis inhibitor (Vstat120) shields oHSV-Vstat120 from inflammatory macrophage antiviral response, without reducing safety [39]. oHSV-Vstat120 treated mice harboring renal adenocarcinoma and melanoma tumors presented increased infiltration of tumor-associated macrophages (TAMs), NK cells, and tumor-infiltrating lymphocytes [40].
Activating the host immune system seems to be a popular route for potentiating anti-tumor effect of OVs. Polysialic acid (polySia) is expressed on several malignant tumors of neuroendocrine origin. PolySia-dependent systemic infection in vivo facilitated effective uptake of viruses in subcutaneous polySia-expressing human tumors, whereas hepatic viral load and hepatotoxicity were significantly reduced. Enhanced tumor regression and prolonged survival was only observed in immunocompetent mice, but not in T-cell-deficient mice, suggesting that a polySia-retargeted oAd elicits an effective tumor-directed T-cell response after systemic virus delivery and facilitates therapy of disseminated lung cancer [41]. DNX-2401 (Delta-24-RGD; tasadenoturev) is a tumor-selective, replication-competent oAds, which is proven to be safe in mice and results in a pronounced increase in survival in immunodeficient and immunocompetent models of high-grade pediatric glioma and diffuse intrinsic pontine gliomas [42]. The Ad was engineered to express an EGFR-targeting BiTA (cBiTA) antibody under the control of the major late promoter, leading to generation of ICOVIR-15 K-cBiTA, which bound specifically to both CD3 + and EGFR + cells. Intra-tumor (IT) injection of this cBiTA-expressing Ad increased the accumulation and persistence of tumor-infiltrating T cells and the antitumor efficacy in vivo [43]. Actually, as MSCs present tropism for tumors, the use of MSCs to transport OVs to tumor sites is a promising alternative to IT administration [40]. The data suggested that treatment with oAd-MSCs significantly reduced tumor volumes by 50% and induced a pro-inflammatory TME. In a veterinary dog trial with dCelyvir (canine MSCs infected with an oAd ICOCAV17) in 27 canine patients, Cejalvo et al. observed an excellent toxicity profile as well as a clinical benefit in 74% of patients, including 14.8% showing complete remissions [44]. Actually, it is a very promising attempt to arouse T cells by designing BiTAs OVs [45, 46]. Particularly, together with T cells a VV encoding a secretory BiTA consisting of two scFvs specific for CD3 and EphA2 (EphA2-TEA-VV) had potent antitumor activity in comparison with control VVs plus T cells in a lung cancer xenograft model [47]. In vivo, the therapeutic efficacy of MVs targeted to HER2/neu and EpCAM by designing ankyrin repeat proteins (DARPins), was confirmed in an orthotopic ovarian carcinoma model revealing an effective reduction of tumor mass [48]. Overall, these successful preclinical results have made a decisive contribution to further investigation in the clinics.
Safety of oncolytic virotherapy
Therapeutic safety remains a paramount concern during OVT while the tumor targeting/tropism is a highly desirable characteristic for OVs. Generally, tumor-specific and natural receptors were responsible for tumor selectivity and cell entry. To achieve cancer cell specificity in different OVs, a few viruses, e.g., parvovirus and NDVs, own a naturally tumor tendency. Many, if not most, such as MVs, Ads, VSVs, VVs and HSVs exhibit no preference for cancer cells. Thus, the viruses from these families need to be designed to preferentially target cancers rather than normal tissues.. Genetically engineered viruses can be exploited in several aspects, such as tumor cell receptor targeting, driving the expression of certain viral replication genes by promoters and enhancers, translational targeting, engineered microRNA target sequences, immunogenic tumor-associated antigen targeting, etc. (Table 2 and Fig. 2) [19, 49]. Taking HSV, one of the most widespread and widely used OVs, as an example, to improve its safety, various engineering and modifications have been carried out on its genome [24]. Mutants of HSV-1 with deletion of ICP34.5 and ICP47 genes (such as T-VEC) have been successfully harnessed as attenuated oncolytic vectors [50, 51]. For HSV-based OVT, the detargeting-retargeting strategies so far were based on genetic manipulations of glycoprotein (g) D, gB and/or gH [52]. In particular, to enhance the tumor tropism and safety of HSV, a novel ligand in gH was designed to confer tumor cells entry [14]. To re-target the virus tropism to the HER2- and GCN4R-positive cells, the HER2 binding peptide was inserted in gB and GCN4 peptide in gD or gB [53, 54]. A safe and effective therapeutic oncolytic HSV-2 (deletion of ICP47 and ICP35.4) was also be used in combination with doxorubicin for breast cancer treatment [55]. Similarly, arming the miR-122a complimentary sequences to HSVs have shown high specificity to target hepatocellular carcinoma cells [12]. Engineering miRNA target sequences into viruses’ genomes was thereby inhibiting spread in tissues expressing cognate miRNAs. Tumor-specific translational regulation presents an attractive possibility for generating oncoselective therapies. Villanueva et al. reported the insertion of CPE regulatory sequences in the 3’-UTR of the E1A gene that confers translational E1A expression regulation, resulted in tumor-specific AdCPE viruses [56]. It is demonstrated that neurotoxicity was most profoundly reduced in a virus carrying four tandem copies of a neuronal miR-125 target sequence inserted in the 3′-UTR of the VSV polymerase gene [57]. Alexander Muik et al. have engineered a chimeric VSV, an oncolytic virus called rVSV (GP) devoid of natural neurotoxicity with undetectable immunogenicity and enhanced oncolytic potency [58].
The tumor specificity of oncolytic virotherapy. IA: Deletion of the required genes for virus replication in normal cells. IB: Deletion or inactivation of the required genes for virus replication and insertion of killer genes. IC: Transcriptional targeting. IIA: Retargeting strategies based on genetic manipulations of glycoproteins. IIB: T-cell activator. III: Translational targeting. IV: Hypoxic or drug induction. V: Intracellular restrictions by miRNA targeting
Here we summarized the virulence and tumor specificity mechanisms of different virus families in recent years (Table 2). Among them, the selection of tumor-specific antigens is a leader in increasing the safety of OVs. The detargeting-retargeting strategies were based on genetic manipulations of glycoprotein of different types of viruses, such as antigens of HER2, EGFR, GCN4, EpCAM have been sucessfully applied in HSV, VSV and MV etc. To date, OVT have been evaluated for safety by both localized and systemic administration. The most common adverse effects are fever and general flu-like symptoms. Moreover, no transmission of OVs from treated patients to others has been reported [19]. However, therapeutic safety concerns must be scrupulously addressed to ensure the safety of patients and other people who may have contact with the patients. The development of OVT were greatly benefited from the studies on structures and characteristics of virus particles [59,60,61,62]. More engineered OVs for particular tumor treatment will be safely applied in clinical trials and approved protocols.
Efficacy of oncolytic virotherapy
Although safety concern is a paramount priority, high efficacy to eliminate tumors is the goal of OVT. OVs can destruct cancer cells in many ways, including direct oncolysis, antitumor immunity, vascular-disrupting effect, bystander killing effect [17]. Therefore, to pursue the ideal therapeutic effect, we may start from following aspects. First, the importance of tumor targeting in improving therapeutic effect is out of question. Due to the rapid replication and cell lysis properties of some virus families, with a wide range of tissue tendencies, it is necessary to continue rational optimization of these viruses to efficient kill specific types of cancer. For example, the natural neurotropism of HSVs has made it attractive as vectors for the development of OVs for application in the nervous system [63, 64]. Moreover, retargeted OVs infected only cells that expressed the targeted TAAs, such as EGFR, HER-2, PSMA, GCN4R (Fig. 3A and Table 3). Second, suitable doses and delivery system of OVs in administration, such as intratumor (I.T.), intra-vein (I.V.) and intra-muscle (I.M.) injection, are required [65,66,67]. Third, to elicit the bystander immune response is a preeminent blueprint [68, 69]. Fourth, arming the viruses with destruction/immunostimulatory genes, innovative combination with other therapies are promising strategies gaining momentum [70]. Herein, the arming mechanisms of OVs were summarized (Table 3 and Fig. 3) and discussed below.
The optimization on virus spread and delivery of OVs play a crucial role directing therapeutic efficacy. There are several host barriers hampering the potency of OVT in patients. If the OVs is not administrated I.T., I.V. and I.M. injection of OVs was usually hindered by antibodies and complements in the blood stream. Thus, it is essential to develop strategies to escape antibody and complement neutralization in the blood stream. To limit the neutralization of OVs, there are several classical oncolytic vector shielding strategies, including envelope protein exchange within a virus species or families, multiple epitope replacements, devising cell carriers, and chemical modifications [19, 49, 71, 72].
To restrict antibody-mediated HSV neutralization, the antibodies targeting functional epitopes on HSV glycoproteins can mediate neutralization directly. For example, epitopes modification on HSV have been well-defined and characterized in humans [73,74,75]. MVs-based shielded oncolytic vectors to circumvent antibody neutralization have been developed by exchanging the envelope glycoproteins, hemagglutinin (H) and fusion (F) protein, with those from the non-cross-reactive Tupaia paramyxovirus [76]. In genital disease, HSV-2 vaccination with human papillomavirus vectors expressing HSV glycoprotein antigens was developed successfully for eliciting anti-viral response [77]. Cristian et al. demonstrated that Ads coated with MHC-I tumor epitopes increase the antitumor immunity and efficacy against melanoma [78]. Cell carriers, such as cytokine-induced killer cells, mesenchymal stem cells (MSCs), neural stem cells (NSCs), and stromal vascular fraction cells (SVFs), are capable of accelerating the OVs delivery to tumors and in the same time protecting OVs from antibody neutralization [7, 79,80,81]. Multiple studies have demonstrated that MSCs or NSCs allow for safe and efficient ferrying of OVs to tumor foci to trigger immune response [65, 71, 79, 82,83,84]. Specifically, a TK-positive oVV ACAM2000, delivered by autologous adipose SVF cells, fostered such treatment in the patients with advanced solid tumors or acute myelocytic leukemia (AML) in a great safety and accessibility. The clinical data revealed that the viral DNA could be readily detected in all patients’ blood samples immediately after treatment [80]. Certainly, chemical or other modifications are also good OVs shielding option. Reoviruses and HSVs have been armed with cyclophosphamide, an immune modulator, to combat the antibody neutralization, thereby enhancing the virus infection [85]. Since copper in serum prevents replication of HSV-1, when armed the oHSV with a copper chelator ATN-224, significantly enhanced its therapeutic efficacy by increasing serum stability and systemic delivery of oHSV [86]. Rommelfanger et al. have demonstrated that the combination of VSVs and LPS generated significantly enhanced therapy of melanoma B16ova tumors upon direct I.T. administration [87]. Besides, the modification of the fiber knob and an arginine-grafted biodecomposible polymer arming were proved to be a feasible strategy to dodge antibody neutralization during systemic administration [88]. When measured just before the second treatment cycle, serum neutralizing antibodies titers differed in 83% of patients, suggesting that even minor changes in the fiber knob would able to circumvent host antibody neutralization [88]. Another example of modification is that the NDVs armed with regulators of complement activity CD46 and CD55 could enhance the efficient complement evasion [89]. Some complement inhibitors, such as CP40, have been shown to abolish host antibody neutralization and augment the dose of infectious oVVs ferried to tumor sites [90].
Once high doses of the viruses were maintained in the tumor microenvironment (TME), the therapeutic efficacy will be ultimately determined by the potency of OVs. As shown in Figs. 3 and 4, to reinforce the antitumor activity of OVs, eliciting bystander cell killing, introduction of pro-apoptotic or toxin genes and innovative combination therapy strategies were developed. OVs could use oncolysis to kill the infected tumor cells directly in TME. Except tumor cells, OVs can target several other components including cancer-associated fibroblasts (CAFs) and vascular endothelial cells (ECs). Then OV infection and the lysed cells causes the release of cytokines or neo-antigens, as well as the OV-armed immuno-stimulation genes, including GM-CSF, INF-γ, to initiate anti-viral immune priming by stimulating immune cells, including T cells, NK cells. The recruitment and maturation of innate immune cells which can cross-present TAAs to CD8 T cells, thus generating populations of TAA-specific CTLs. The generation of an OV infection-mediated anti-tumor immune response also counteracts the immunosuppression associated with myeloid derived suppressor cells and Tregs. In addition, the various destructive genes (such as pro-apoptotic genes, toxin genes) that are engineered within the OVs will take effects in TME. It is effective to mediate T and/or NK cell bystander killing of uninfected tumor cells in TME by engineering BiTA, CiTA, TriKA etc. (Fig. 4). Thus, OV infection acts on both the innate and adaptive immune system, which work together to kill cancers. The promising methods to create the bystander killing were prodrug activations, radiosensitization and immunostimulation [19]. For example, the purine nucleoside phosphorylase (PNP), one of convertase enzymes expressed in infected cells could convert prodrugs within the TME into toxic metabolites which eventually diffuse into and destruct adjacent uninfected tumor cells [19]. The sodium-iodide symporter (NIS) concentrates radioactive ions in infected cells, which triggers radiation poisoning of uninfected bystander tumor cells [19, 91, 92]. The clinical study demonstrated that oMV therapy can function as an antigen agnostic vaccine, increasing cytotoxic T-lymphocyte responses against TAAs in patients with multiple myelomas [92]. Of course, the most exciting strategy is the clinical application of OVs immunotherapy. The successful introduction of the granulocyte macrophage colony-stimulating factor (GM-CSF) gene into oHSVs represents a great breakthrough of immunostimulation. Such oHSVs, including T-VEC, CG0070, JX594, JX963, etc., have been shown in clinical trials to stimulate granulocytes and monocytes to elicit impressive anti-tumor immunity [21, 30, 93,94,95]. T-VEC, which produce GM-CSF, can efficiently treat the patients with metastatic melanoma, pancreatic carcinoma etc. [18, 21, 30, 96,97,98]. The phase III trial proved that local intralesional injections with T-VEC in advanced malignant melanoma patients can not only suppress the growth of injected tumors but also act systemically and prolong overall survival (OS) [30, 99]. Besides of immune stimulatory cytokines GM-CSF, IFNα, IL-12, IL-15 etc., immune checkpoint inhibitors (ICIs), bispecific T-cell activators (BiTA), some pro-apoptotic or toxin genes and shRNAs (targeting Bcl-2, Survivin, COX-2 or STAT3) were also engaged in OVT [22, 29, 100,101,102,103,104]. The redirecting of T cells to the tumor by arming oVVs with BiTA (EphA2-TEA-VV) has the potential to boost the antitumor activity of oncolytic VVs [47]. An HSV-2 based OV can actively recruit T effector cells to the site of infection, suggesting that oHSV-2-based virotherapy can be armed with adoptive T-cell therapy to advance its therapeutic effect against solid tumors [105]. Expression of cytokines together with BiTAs has shown to mediate T cell bystander killing of uninfected tumor cells not only in vitro, but also in vivo [47, 100, 101, 106]. A combination of trans-genes encoding BiTAs, ICIs and APC enhancers will remove suppressive hurdles in the TME and allow for optimal antitumor efficacy of armed OVs [22]. The antibodies against immune checkpoint receptors have been exploited to conquer cancer by inducing T cell response, such as the antibodies against CTLA4, PD-1, PDL-1 and some alternative antibody formats (scFvs, Fabs, scAbs and VHHs) [22, 29, 107]. Zamarin et al. boosted the efficacy of systemic immune checkpoint blockade and avoided additional systemic toxicity by engineering a recombinant ICOS ligand-expressing NDV (NDV-ICOSL) [108]. Antibodies against immune checkpoint receptors, such as anti-CTLA4 and anti-PD-1, has clearly proven the therapeutic potential of antigen presentation and T-cell response against cancer [22, 29]. Moreover, the larger natural antibodies are not easy to eliminate and penetrate into solid tumors, the alternate antibody forms such as scFvs, Fabs, scAbs and VHHs have been increasingly exploited and applied [22, 29].
Maria et al. engineered a specific oncolytic Ads expressing a scFv of an antibody against PD-L1 to combine blockage of PD-1/PD-L1interaction with the antitumoral activity of Ad5 [109, 110]. They also armed Ads expression of an Anti-PD-L1-scFv improves anti-tumoral efficacy in a melanoma mouse model [109] Anthony et al. engineered the OVs to express a nonsignaling truncated CD19 (CD19t) protein tumor-selectively, enabling CD19-CAR T cells to target, and showing effective anti-tumor effect [16]. A recent report by Rivadeneira et al. demonstrated that OVs engineered to express the adipokine leptin boosted T cell metabolic function in the TME, and thereby allowed a superior antitumor response [15]. Dendritic cells played important role in oncolytic virotherapy. Cytopathogenic infection of neoplastic cells releases the proteome and exposes pathogen- and damage-associated molecular patterns. At the same time, sublethal infection of antigen-presenting cells, such as dendritic cells and macrophages, yields potent, sustained type I interferon-dominant activation in an immunosuppressed microenvironment and promotes the development of tumor antigen-specific T cell responses in vitro and antitumor immunity in vivo [111]. The recombinant poliovirus/rhinovirus chimera oncolytic virus PVSRIPO’s immune adjuvancy stimulates canonical innate anti-pathogen inflammatory responses within the TME that culminate in dendritic cell and T cell infiltration. The findings provide mechanistic evidence that PVSRIPO functions as a potent intratumor immune adjuvant and generates tumor antigen-specific cytotoxic T lymphocyte responses [111]. T-Vec results in a rapid eradication of malignant cells and leads to interferon pathway activation and early influx of natural killer cells, monocytes, and dendritic cells. These events are followed by enrichment in cytotoxic T cells and a decrease of regulatory T cells in injected and noninjected lesions [112]. High-grade serous ovarian cancers (HGSOCs) exhibit limited response to immune checkpoint blockade. In a new study in Cancer Cell, Duraiswamy et al. highlighted that intratumoral CD28 co-stimulation by myeloid-antigen-presenting cells as a key mechanism was required for activation of programmed cell death receptor 1 (PD-1)+ tumor-infiltrating T lymphocytes during PD-1 blockade in HGSOC [113, 114].
The destructive genes, e.g. pro-apoptotic and toxin genes, have been engineered with OVs successfully. For example, arming OVs with a secretable and self-multimerizing apoptosis inducer is a approachable strategy to enhance the potency of OVT. Loya et al. armed HSV with a secreted form of an Her2 single chain antibody linked to the Fas ligand extracellular domain (Her2-COL-sFasL), which improved the bystander effect of OVT effectively [17]. Arming human MSCs with oHSV and its pro-apoptotic variant, oHSV-TRAIL, proved to be efficient in treatment for malignant glioblastoma multiforme [115]. Therapy of experimentally induced lung melanoma in mice with IL-15-carrying myxomavirus delivered by MSCs led to marked regression of lesions and with increased animal survival, suggesting that it allowed for safe and effective delivery of OVs to pulmonary melanoma lesions triggering immune responses [83]. HSV1716 administration led to marked tumor shrinkage in primary mammary tumors and a decrease in metastases by reprograming tumor-associated macrophage to a less immunosuppressive phenotype. This was associated with a significant increase in the recruitment/activation of cytotoxic T cells [116]. A pro-apoptotic gene p53 has been engineered in Ads to treat hepatocellular carcinoma (HCC) and could prolong the survival time of the patients [117]. Dual silencing of Bcl-2 and Survivin with oHSV-1 was also a promising tool for improving the antitumor efficacy [118]. A toxin gene, staphylococcal enterotoxin A, is also a potential useful anti-tumor agent in arming Ads [119]. A virulence factor, helicobacter pylori neutrophil-activating protein (HP-NAP), can mediate antitumor effects by recruiting neutrophils and inducing Th1-type differentiation in the TME. Thus, Ads armed with HP-NAP gene provoked antitumor immune response and enhanced the therapeutic effect against neuroendocrine tumors [120]. The study demonstrated that the cancer-associated fibroblasts (CAFs) induced high levels of fibroblast growth factor 2 (FGF2), which enhanced the susceptibility of the cancer cells to OV infection and improved therapeutic efficacy [121]. Telomelysin, a telomerase-specific replication-competent Ads with hTERT promoter, has been proven to have a strong antitumor effect on a variety of cancers and applied in combination treatment for head and neck squamous cell carcinoma [122]. The control of exogenous gene expression can also improve OVT. Jochen Stritzker et al. has characterized a doxycycline-inducible promoter system in oVVs, which was proven to be beneficial to OVT [123]. Therefore, determination of the structure and characteristics of various viruses and tumor cells will be greatly beneficial for the development of efficient OVT.
Overall, in addition to edit the viruses and exogenous genes, to excavate the reasonable combinatorial modalities are regarded as an excellent strategy to improve efficiency, especially ICIs [124,125,126,127] (Tables 3 and 4). For example, T-VEC with ipilimumab (a CTLA-4 inhibitor) had a tolerable safety profile, and the combination appeared to have greater efficacy than either T-VEC or ipilimumab monotherapy [30, 35]. The combination of intratumoral G47Δ and systemic anti-CTLA-4 antibody was shown to recruit effector T cells into the tumor efficiently while decreasing regulatory T cells [128]. Viral replication and the creation of new T-cell clones have been detected during treatment with reovirus pelareorep combined with a PD-1 inhibitor pembrolizumab [129]. While anti-PD-1 antibody monotherapy moderately improved tumor survival, when co-administered with oncolytic Zika virus (ZIKV), survival extended [27].
OVs in clinical trials
Although the pre-clinical trials so far have established the safety and efficacy of those approaches, the challenge now is to achieve safety and efficacy in clinics. Many promising OVs, such as oHSVs, oAds, and oVVs, have been applied in clinic trials successfully (Table 4).
T-VEC, a recombinant oHSV, which is administered by direct I.T. injection to patients with metastatic malignant melanoma led to lesion regressions of [30, 34, 35, 51, 96, 99, 130]. As an example, the biodistribution, shedding, and potential transmission of T-VEC have been systematically evaluated during and after completion of therapy in adults with advanced melanoma [131]. The data demonstrated that T-VEC improved longer-term efficacy versus GM-CSF and maintained well tolerated. The final planned OPTiM analysis suggested that the median OS was 23.3 months (95% confidence interval [CI] 19.5–29.6) and 18.9 months (95% CI 16.0–23.7) in the T-VEC and GM-CSF arms, respectively [130]. A phase II study evaluated patients with unresectable stage IIIB-IVM1c malignant melanoma who received T-VEC plus ipilimumab or ipilimumab alone. The results showed that 39% (n = 38/98) in the combination arm and 18% (n = 18/100) in the ipilimumab arm had an objective response. Eight responders (combination, n = 7 [18.4%]; ipilimumab, n = 1 [5.6%]) had pseudo-progression; most occurred by week 12 and were caused by an increase in existing lesions [30]. In addition, to determine the safety of administering HSV1716 (Seprehvir) systemically, Streby et al. conducted the phase I trial of intravenous (I.V.) injection in young patients with relapsed or refractory extra-cranial solid cancers [132]. They did not observe any dose-limiting toxicities. All five HSV-1 seronegative patients seroconverted by day 28. Four out of nine patients had detectable HSV-1 genomes in peripheral blood on day + 4, which is consistent with de novo virus replication. A phase I/IIa trial of intrapleural administration of HSV1716 with malignant pleural mesothelioma patients demonstrated that viral replication/persistence in pleural fluid in seven of the twelve patients. Induction of Th1 cytokine responses to HSV1716 treatment was achieved in eight patients and four patients developed novel anti-tumor IgG [133]. However, it is also suggested that the efficacy of T-VEC therapy in patients with in-transit melanoma metastasis diminished with increasing lesion size [134]. Of 27 patients, an objective response was observed in 11 (40.7%), including one patient with partial response (3.7%) and 10 with complete response (37.0%). Logistic regression demonstrated each millimeter increase in maximum lesion diameter predicted decreased ORR (odds ratio [OR] 0.866, 95% CI 0.753–0.995; p = 0.04) [134]. Todo et al. have been reported the results of a phase I/II trial using triple-mutated oHSV-1 G47Δ in Japanese patients with recurrent or progressive glioblastoma [135, 136]. G47Δ caused immediate infiltration of lymphocytes that seemingly directed towards tumor cells, which was reflected on image studies with features characteristic to G47Δ therapy. Long-term survival (> 46 months) was observed in 3 of 13 patients, which may be due to the delayed effect of G47Δ via antitumor immunity [136].
Since that first approve of the human p53 adenovirus (Gendicine), a steady stream of new oAds entering the clinical arena [137, 138]. Clinical studies demonstrated that DNX-2401 is safe and tolerable after injection into the cerebellar peduncle in pediatric patients with diffuse intrinsic pontine gliomas and can induce a direct oncolytic effect followed by an antitumor immune response [68]. ICOVIR5 was derived from the oAd DNX-2401. The clinical results in 12 patients treated with a single dose up to 1 × 1013 viral particles showed that ICOVIR5 was able to reach melanoma metastatic lesions after infusion but failed to induce tumor regressions [139]. The homing capacity of MSCs to tumors makes them excellent carriers of anticancer therapeutics [40, 44]. Autologous MSCs may allow an increasing amount of ICOVIR5 by repeated administration, avoiding or minimizing emergent toxicities [82]. Evidence have been reported that MSCs successfully delivered an oAd CRAd-S-pK7 with fiber modification of seven lysine residues to diffuse intrinsic pontine glioma [71]. Similarly, it is shown to protect CRAd-S-pK7 from neutralizing antibodies within patient ascites fluid and to enhance delivery of CRAd-S-pK7 by NSCs for treatment of metastatic ovarian cancer [84]. Recently, the safety and feasibility of NSC-CRAd-S-pk7 in patients with newly diagnosed high-grade glioma have been examined, and the results showed that the median progression-free survival was 91 months (95% CI 85-not reached) and median OS was 184 months [65]. In addition, Pascual-Pasto et al. confirmed that the oAd VCN-01 provided targeted therapeutic activity against even chemo- resistant retinoblastoma. The phase I data in patients showed the feasibility of the administration of intravitreous VCN-01 and resulted in antitumor activity in retinoblastoma vitreous seeds and evidence of viral replication markers in tumor cells [140]. In another phase I study of gene-mediated cytotoxic immunotherapy using aglatimagene besadenovec (AdV-tk), an adenoviral vector expressing the HSV-tk gene, followed by valacyclovir, 3 patients in a dose of level 2 (3 × 1011 vp) survived more than 24 months after treatment, and 2 remain alive without progression at 37.3 and 47.7 months after AdV-tk injection [141]. Enadenotucirev is a tumor selective oAd, which can be administrated intravenously in patients undergoing primary tumor resection [142]. Additionally, the EVOLVE (Evaluating Oncolytic Vaccine Efficacy) study of the enadenotucirev, administered intravenously to patients with epithelial solid tumors, showed that enadenotucirev monotherapy can be administered in a single cycle or repeated cycles with manageable tolerability [67]. Recent clinic trial confirmed that enadenotucirev is a radiosensitizer in chemoradiation therapy of locally advanced rectal cancers [143]. Intravenously dosed enadenotucirev plus paclitaxel demonstrated manageable tolerability and increased tumor immune-cell infiltration in phase 1 studies in platinum-resistant ovarian cancer [144].
An oVV, Pexa-Vec (pexastimogene devacirepvec, JX-594), engineered to express GM-CSF, was administered IT and IV to patients with HCC and colorectal cancer, respectively [94, 145, 146]. No dose-limiting toxicity (DLT) was reported, and the maximum tolerated dose was not reached in phase Ib trial of biweekly IV of Pexa-Vec. Moreover, the most common adverse events were grade 1/2 flu-like symptoms, generally lasting less than 24 h [146]. TG4023 is a modified vaccinia virus Ankara (MVA), the first-in-human study demonstrated that IT injections of TG4023 were feasible and well tolerated, and the maximum tolerated dose (MTD) was defined as 4 × 108 p.f.u. [147]. The safety of oVV GL-ONC1 have been determined when delivered intravenously with chemoradiotherapy to patients with primary, nonmetastatic head and neck cancer [148]. Moreover, the study showed that GL-ONC1 was well tolerated when administered into the peritoneal cavity of patients with advanced stage peritoneal carcinomatosis. Importantly, in 8 of 9 studied patients, effective peritoneal infections, in-patient replication of GL-ONC1, and subsequent oncolysis were detected [149]. ACAM2000, a TK-positive strain of oVV, is the current smallpox vaccine in the US. The phase I clinical trial confirmed that ACAM2000/SVF can safely be administered in patients with advanced metastatic solid tumors or advanced AML [80].
In addition to the above described oHSVs, oAds, and oVVs, an oMV engineered to express the human thyroidal natrium iodine symporter (MV-NIS) monitors localization of viral gene expression and successfully used in clinical trials against multiple myelomas and ovarian cancers [92, 150, 151]. Packiriswamy et al. conformed that MV-NIS treatment significantly (P < 0.05) increased cytotoxic T-lymphocyte responses against TAAs in patients with MM [92]. An oncolytic parvovirus ParvOryx containing native parvovirus H-1 (H-1PV) have been shown to be a promising candidate for treatment of patients with recurrent glioblastoma and metastatic, inoperable pancreatic cancers [152, 153]. Pelareorep, an oncolytic reovirus, in combination with chemotherapy and pembrolizumab in patients with advanced, pre-treated pancreatic ductal adenocarcinoma (PDAC) was well-tolerated and showed prolonged efficacy in 3 of 11 patients (27.3%) [129].
Despite the confirmed safety and antitumor efficacy of OVs, additional challenges have been gained from the ongoing and completed clinical trials. A first insight is that the predictive values including safety and efficacy profile are limited by the relatively small sample size of patients and short follow-up. A second awareness is that the antigenic specificity of the T cell response to these OVs has not been determined, and whether the treatment expands the appearance of new antigen specific T cell lineages; further research is required to monitor/determine any relationship between virus persistence and the TME. Third, the role of adaptive immunity in restricting the benefits of repeated administrations of viruses is unknown. In addition, it is not clear which administrations of OVs is better, injecting the tumor intratumorally, intravenously, or orally, which may vary depending on the individual tumors, viruses, patients, and combination therapy regimen.
Significantly, Gendicine is the first OV approved for clinical OVT in the world in 2003 [137, 138], which was approved for head and neck carcinoma by China FDA and T-VEC is the second OV approved for clinical OVT in the world in 2015 [154], which was approved for melanoma by the US FDA. Many promising OVT clinical trials are under way but there is still a long way off to improve their safety and efficiency.
Conclusions
OVT is an amazingly versatile and malleable class of cancer therapy, which has the unique advantages when compared with that in conventional therapies. OVs can attack tumor cells selectively, and then trigger the cell death by multiple approaches, including direct oncolytic effects, targeting blood vessel endothelial cells, delivery of the therapeutic genes within tumors, synergistic effects with traditional and immunotherapies, resulting in systemic anticancer effects. The toxicity of OVs has been self-limiting flu-like illness and fever etc. Until now, OVT has become a realistic therapeutic candidate, and has been evaluated for safety by both localized and systemic administration in clinics. From the previous studies, we conclude that the status of OVs potencies including: (i) induces systemic tumor-specific immunity, (ii) synergistic effects with other therapies, (iii) different tumor sites and patients showed varying response to different viruses, (iv) neutralizing antibody is not a barrier to successful therapy; and (v) anti-tumor T cell (BiTAs, checkpoint inhibitory T-cell-activators/CiTAs) or NK cell (trispecific killer activators, TriKAs) responses augment antitumor efficacy by OVTs.
Oral, I.V., I.T., intrapleural, intraperitoneal (IP), aerosol and limb injections are the common delivery routes for OVs. However, these methods still have their own disadvantages. To be specific, oral administration is most convenient and most unavailable. I.V. and I.T. injections are easy to be neutralized in blood stream of patients. Besides, not all patients can be adapted to I.T. injection. Intrapleural injection should be utilized by using an indwelling intrapleural catheter. To avoid uncontrolled adverse events and long-term complications of OVs, the patients need to orchestrate the appropriate time and delivery routes in clinics.
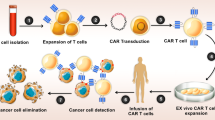
We believe OVT has a bright future and requires continue efforts working for its safety and efficiency. It is wise to explore the key factors affecting the efficacy of OVs from three aspects: virus, tumor and patient. This include reconstructing the viruses for better efficiency with more safety, utilizing intrinsic tumor-associated genes for target specificity, invoking immune responses from host for enhanced tumoricidal effect. To further avoid host immunity to viruses or enhance tumor specific immunity induced by OVs in the future, the potential novel investigations should be focusing on the following aspects: (i) sequential harness of two different OVs, (ii) choreographed combination of OVs and antibody therapies (anti-PD-1/PDL-1, anti-CTLA-4), or cell therapies (adoptive cell transfer therapy, DC, Car-T), and (iii) improve the efficacy of administration and delivery by excellent cell carriers (MSCs, NSCs, etc.).
Availability of data and materials
Not applicable.
Abbreviations
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PKR:
-
Double-stranded RNA-dependent protein kinase
- CPEBs:
-
Cytoplasmic polyadenylation element-binding proteins
- ANTXR1:
-
Anthrax toxin receptor 1
- ICOS:
-
Inducible co-stimulator
- TIL:
-
Tumor infiltrated leukocyte
- TAA:
-
Tumor associated antigen
- scFv:
-
Single-chain antibodies
- CPEB:
-
Cytoplasmic polyadenylation element-binding protein
- HCC:
-
Hepatocellular carcinoma
- EGFR:
-
Epidermal growth factor receptor
- FR:
-
Folate receptor
- PSMA:
-
Prostate membrane-specific antigen
- HIF:
-
Hypoxia-inducible factor
- NSCLC:
-
Non-small cell lung cancer
- MOIs:
-
Multiplicities of infection
- NDV:
-
Newcastle disease virus
- SVV:
-
Seneca Valley virus
- TME:
-
Tumor microenvironment
- VSV:
-
Vesicular stomatitis virus
- ZIKV:
-
Zika virus
- Nabs:
-
Neutralizing antibodies
- RCA:
-
Regulators of complement activity
- TPMV:
-
Tupaia paramyxovirus
- CAFs:
-
Cancer-associated fibroblasts
- FGF2:
-
Fibroblast growth factor 2
- MSC:
-
Human mesenchymal stem cells
- sECM:
-
Synthetic extracellular matrix
- EnAd:
-
Oncolytic group B adenovirus EnAdenotucirev
- BiTA:
-
Bispecific T-cell activator
- DARPins:
-
Designed ankyrin repeat proteins
- CSC:
-
Cancer stem cell
- MSCs:
-
Mesenchymal stem cells
- NIS:
-
Human thyroidal sodium-iodide symporter
- RT3D:
-
Reovirus serotype 3 Dearing
- RUX:
-
Ruxolitinib
- MPNSTs:
-
Malignant peripheral nerve sheath tumors
- PARPi:
-
Poly(ADP-ribose) polymerase inhibitors
- HR:
-
Homologous recombination
- TGF-β:
-
Transforming growth factor beta
- LPS:
-
Lipopolysaccharide
- HP-NAP:
-
Helicobacter pylori neutrophil-activating protein
- EnAd:
-
EnAdenotucirev
- BAI1:
-
Brain Angiogenesis Inhibitor 1
- hTERT:
-
Human telomerase reverse transcriptase
- cBiTA:
-
EGFR-targeting BiTA
- G47Δ-mIL12:
-
OHSV G47Δ expressing murine IL-12
- ICOS:
-
Inducible co-stimulator
- NDV:
-
Newcastle disease virus
- NDV-ICOSL:
-
NDV-expressing ICOS ligand
- AE:
-
Adverse events
- PGE2:
-
Prostaglandin E2
- HPGD:
-
Hydroxyprostaglandin dehydrogenase
- VV:
-
Vaccinia virus
- SCLC:
-
Small cell lung cancer
- IP:
-
Intraperitoneal
- BiTA:
-
Bispecific T-cell activator
- UV-HSV-1:
-
UV light-inactivated HSV-1
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- IT:
-
Intratumoral
- IV:
-
Intravenous
- oAd-MSCs:
-
Oncolytic adenovirus dlE102
- MM:
-
Multiple myeloma
- T-VEC:
-
Talimogene laherparepvec
- MTD:
-
Maximum tolerated dose
- PoC:
-
Proof-of-concept
- DLT:
-
Dose-limiting toxicities
- NMIBC:
-
Non-muscle invasivebladder cancer
- PC:
-
Peritoneal carcinomatosis
- PM:
-
Peritoneal mesothelioma
- IT:
-
Intratumoral
- pfu:
-
Plaque-forming units
- PPR:
-
Progression prior to response
- MPM:
-
Malignant pleural mesothelioma
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70(6):443–59.
Wang X, Yang Y, Cai WQ, Lu Y. The relationship of sphingosine kinase 1 with pyroptosis provides a new strategy for tumor therapy. Front Immunol. 2020;11:574990.
Wang Y, Xiang Y, Xin VW, Wang XW, Peng XC, Liu XQ, Wang D, Li N, Cheng JT, Lyv YN, et al. Dendritic cell biology and its role in tumor immunotherapy. J Hematol Oncol. 2020;13(1):107.
Li Y, Xu Y, Peng X, Huang J, Yang M, Wang X. A novel photosensitizer Znln(2)S(4) mediated photodynamic therapy induced-HepG2 cell apoptosis. Radiat Res. 2019;192(4):422–30.
Hemminki O, Dos Santos JM, Hemminki A. Oncolytic viruses for cancer immunotherapy. J Hematol Oncol. 2020;13(1):84.
Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–70.
Alemany R. Viruses in cancer treatment. Clin Transl Oncol. 2012;15(3):182–8.
Moore AE. The destructive effects of viruses on transplantable mouse tumors. Acta Unio Int Contra Cancrum. 1951;7(2):279–81.
Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252(5007):854–6.
Foka P, Pourchet A, Hernandez-Alcoceba R, Doumba PP, Pissas G, Kouvatsis V, Dalagiorgou G, Kazazi D, Marconi P, Foschini M, et al. Novel tumour-specific promoters for transcriptional targeting of hepatocellular carcinoma by herpes simplex virus vectors. J Gene Med. 2010;12(12):956–67.
Fu X, Rivera A, Tao L, De Geest B, Zhang X. Construction of an oncolytic herpes simplex virus that precisely targets hepatocellular carcinoma cells. Mol Ther. 2012;20(2):339–46.
Gayral M, Lulka H, Hanoun N, Biollay C, Selves J, Vignolle-Vidoni A, Berthomme H, Trempat P, Epstein AL, Buscail L, et al. Targeted oncolytic herpes simplex virus type 1 eradicates experimental pancreatic tumors. Hum Gene Ther. 2015;26(2):104–13.
Gatta V, Petrovic B, Campadelli-Fiume G. The engineering of a novel ligand in gH confers to HSV an expanded tropism independent of gD activation by its receptors. PLoS Pathog. 2015;11(5): e1004907.
Rivadeneira DB, DePeaux K, Wang Y, Kulkarni A, Tabib T, Menk AV, Sampath P, Lafyatis R, Ferris RL, Sarkar SN, et al. Oncolytic viruses engineered to enforce leptin expression reprogram tumor-infiltrating T cell metabolism and promote tumor clearance. Immunity. 2019;51(3):548-560.e544.
Park AK, Fong Y, Kim SI, Yang J, Murad JP, Lu J, Jeang B, Chang WC, Chen NG, Thomas SH, et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci Transl Med. 2020;12(559):eaaz1863.
Loya SM, Zhang X. Enhancing the bystander killing effect of an oncolytic HSV by arming it with a secretable apoptosis activator. Gene Ther. 2015;22(3):237–46.
Johnson DB, Puzanov I, Kelley MC. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma. Immunotherapy. 2015;7(6):611–9.
Miest TS, Cattaneo R. New viruses for cancer therapy: meeting clinical needs. Nat Rev Microbiol. 2014;12(1):23–34.
Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64.
Kaufman HL, Ruby CE, Hughes T, Slingluff CL. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J Immunother Cancer. 2014;2:11.
de Gruijl TD, Janssen AB, van Beusechem VW. Arming oncolytic viruses to leverage antitumor immunity. Exp Opin Biol Ther. 2015;15(7):959–71.
Ishino R, Kawase Y, Kitawaki T, Sugimoto N, Oku M, Uchida S, Imataki O, Matsuoka A, Taoka T, Kawakami K, et al. Oncolytic virus therapy with HSV-1 for hematological malignancies. Mol Ther. 2021;29(2):762–74.
Peters C, Rabkin SD. Designing herpes viruses as oncolytics. Mol Ther oncolytics. 2015;2:15010.
Spiesschaert B, McFadden G, Hermans K, Nauwynck H, Van de Walle GR. The current status and future directions of myxoma virus, a master in immune evasion. Vet Res. 2011;42:76.
Brown MC, Dobrikova EY, Dobrikov MI, Walton RW, Gemberling SL, Nair SK, Desjardins A, Sampson JH, Friedman HS, Friedman AH, et al. Oncolytic polio virotherapy of cancer. Cancer. 2014;120(21):3277–86.
Nair S, Mazzoccoli L, Jash A, Govero J, Bais SS, Hu T, Fontes-Garfias CR, Shan C, Okada H, Shresta S, et al. Zika virus oncolytic activity requires CD8+ T cells and is boosted by immune checkpoint blockade. JCI Insight. 2021;6(1): e144619.
Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14(8):559–67.
Lin C, Ren W, Luo Y, Li S, Chang Y, Li L, Xiong D, Huang X, Xu Z, Yu Z, et al. Intratumoral delivery of a PD-1-blocking scFv encoded in oncolytic HSV-1 promotes antitumor immunity and synergizes with TIGIT blockade. Cancer Immunol Res. 2020;8(5):632–47.
Chesney J, Puzanov I, Collichio F, Milhem MM, Hauschild A, Chen L, Sharma A, Garbe C, Singh P, Mehnert JM. Patterns of response with talimogene laherparepvec in combination with ipilimumab or ipilimumab alone in metastatic unresectable melanoma. Br J Cancer. 2019;121(5):417–20.
Ghonime MG, Cassady KA. Combination therapy using ruxolitinib and oncolytic HSV renders resistant MPNSTs susceptible to virotherapy. Cancer Immunol Res. 2018;6(12):1499–510.
Saha D, Martuz RL, Rabkin SD. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell. 2017;32(2):253-267.e255.
Ning J, Wakimoto H, Peters C, Martuza RL, Rabkin SD. Rad51 degradation: role in oncolytic virus-poly (ADP-ribose) polymerase inhibitor combination therapy in glioblastoma. J Natl Cancer Inst. 2017;109(3):1–13.
Chesney JPI, Collichio F, Singh P, Milhem MM, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, Logan TF, Hauschild A, Lebbé C, Chen L, Kim JJ, Gansert J, Andtbacka RHI, Kaufman HL. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. 2018;36(17):1658–67.
Puzanov I, Milhem MM, Minor D, Hamid O, Li A, Chen L, Chastain M, Gorski KS, Anderson A, Chou J, et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol. 2016;34(22):2619–26.
Chen X, Han J, Chu J, Zhang L, Zhang J, Chen C, Chen L, Wang Y, Wang H, Yi L, et al. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget. 2016;7(19):27764–77.
Samudio I, Rezvani K, Shaim H, Hofs E, Ngom M, Bu L, Liu G, Lee JT, Imren S, Lam V, et al. UV-inactivated HSV-1 potently activates NK cell killing of leukemic cells. Blood. 2016;127(21):2575–86.
Delwar ZM, Liu G, Kuo Y, Lee C, Bu L, Rennie PS, Jia WW. Tumour-specific triple-regulated oncolytic herpes virus to target glioma. Oncotarget. 2016;7(19):28658–69.
Bolyard C, Meisen WH, Banasavadi-Siddegowda Y, Hardcastle J, Yoo JY, Wohleb ES, Wojton J, Yu JG, Dubin S, Khosla M, et al. BAI1 orchestrates macrophage inflammatory response to HSV infection-implications for oncolytic viral therapy. Clin Cancer Res. 2017;23(7):1809–19.
Morales-Molina A, Rodríguez-Milla M, Gimenez-Sanchez A, Perisé-Barrios AJ, García-Castro J. Cellular virotherapy increases tumor-infiltrating lymphocytes (TIL) and decreases their PD-1 (+) subsets in mouse immunocompetent models. Cancers (Basel). 2020;12(7):1920.
Kloos A, Woller N, Gurlevik E, Ureche CI, Niemann J, Armbrecht N, Martin NT, Geffers R, Manns MP, Gerardy-Schahn R, et al. PolySia-specific retargeting of oncolytic viruses triggers tumor-specific immune responses and facilitates therapy of disseminated lung cancer. Cancer Immunol Res. 2015;3(7):751–63.
Martínez-Vélez N, Garcia-Moure M, Marigil M, González-Huarriz M, Puigdelloses M, Gallego Pérez-Larraya J, Zalacaín M, Marrodán L, Varela-Guruceaga M, Laspidea V, et al. The oncolytic virus Delta-24-RGD elicits an antitumor effect in pediatric glioma and DIPG mouse models. Nat Commun. 2019;10(1):2235.
Fajardo CA, Guedan S, Rojas LA, Moreno R, Arias-Badia M, de Sostoa J, June CH, Alemany R. Oncolytic adenoviral delivery of an EGFR-targeting T-cell engager improves antitumor efficacy. Cancer Res. 2017;77(8):2052–63.
Cejalvo T, Perisé-Barrios AJ, Del Portillo I, Laborda E, Rodriguez-Milla MA, Cubillo I, Vázquez F, Sardón D, Ramirez M, Alemany R, et al. Remission of spontaneous canine tumors after systemic cellular viroimmunotherapy. Cancer Res. 2018;78(17):4891–901.
Guo ZS, Lotze MT, Zhu Z, Storkus WJ, Song XT. Bi- and tri-specific t cell engager-armed oncolytic viruses: next-generation cancer immunotherapy. Biomedicines. 2020;8(7):204.
Huang Q, Cai WQ, Han ZW, Wang MY, Zhou Y, Cheng JT, Zhang Y, Wang YY, Xin Q, Wang XW, et al. Bispecific T cell engagers and their synergistic tumor immunotherapy with oncolytic viruses. Am J Cancer Res. 2021;11(6):2430–55.
Yu F, Wang X, Guo ZS, Bartlett DL, Gottschalk SM, Song XT. T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy. Mol Ther. 2014;22(1):102–11.
Hanauer JR, Gottschlich L, Riehl D, Rusch T, Koch V, Friedrich K, Hutzler S, Prufer S, Friedel T, Hanschmann KM, et al. Enhanced lysis by bispecific oncolytic measles viruses simultaneously using HER2/neu or EpCAM as target receptors. Mol Ther Oncolytics. 2016;3:16003.
Dehaven BC, Gupta K, Isaacs SN. The vaccinia virus A56 protein: a multifunctional transmembrane glycoprotein that anchors two secreted viral proteins. J Gen Virol. 2011;92(Pt 9):1971–80.
Lim F, Khalique H, Ventosa M, Baldo A. Biosafety of gene therapy vectors derived from herpes simplex virus type 1. Curr Gene Ther. 2013;13(6):478–91.
Chesney J, Awasthi S, Curti B, Hutchins L, Linette G, Triozzi P, Tan MCB, Brown RE, Nemunaitis J, Whitman E, et al. Phase IIIb safety results from an expanded-access protocol of talimogene laherparepvec for patients with unresected, stage IIIB-IVM1c melanoma. Melanoma Res. 2018;28(1):44–51.
Shi F, Xin VW, Liu XQ, Wang YY, Zhang Y, Cheng JT, Cai WQ, Xiang Y, Peng XC, Wang X, et al. Identification of 22 novel motifs of the cell entry fusion glycoprotein B of oncolytic herpes simplex viruses: sequence analysis and literature review. Front Oncol. 2020;10:1386.
Petrovic B, Leoni V, Gatta V, Zaghini A, Vannini A, Campadelli-Fiume G. Dual ligand insertion in gB and gD of oncolytic herpes simplex viruses for retargeting to a producer vero cell line and to cancer cells. J Virol. 2018;92(6):10.
Leoni V, Petrovic B, Gianni T, Gatta V, Campadelli-Fiume G. Simultaneous insertion of two ligands in gD for cultivation of oncolytic herpes simplex viruses in noncancer cells and retargeting to cancer receptors. J Virol. 2018;92(6):10.
Zhao Q, Zhang W, Ning Z, Zhuang X, Lu H, Liang J, Li J, Zhang Y, Dong Y, Zhang Y, et al. A novel oncolytic herpes simplex virus type 2 has potent anti-tumor activity. PLoS ONE. 2014;9(3): e93103.
Villanueva E, Navarro P, Rovira-Rigau M, Sibilio A, Mendez R, Fillat C. Translational reprogramming in tumour cells can generate oncoselectivity in viral therapies. Nat Commun. 2017;8:14833.
Kelly EJ, Nace R, Barber GN, Russell SJ. Attenuation of vesicular stomatitis virus encephalitis through microRNA targeting. J Virol. 2010;84(3):1550–62.
Muik A, Stubbert LJ, Jahedi RZ, Geibeta Y, Kimpel J, Dold C, Tober R, Volk A, Klein S, Dietrich U, et al. Re-engineering vesicular stomatitis virus to abrogate neurotoxicity, circumvent humoral immunity, and enhance oncolytic potency. Cancer Res. 2014;74(13):3567–78.
Laine RF, Albecka A, van de Linde S, Rees EJ, Crump CM, Kaminski CF. Structural analysis of herpes simplex virus by optical super-resolution imaging. Nat Commun. 2015;6:5980.
Lee CC, Lin LL, Chan WE, Ko TP, Lai JS, Wang AH. Structural basis for the antibody neutralization of herpes simplex virus. Acta Crystallogr Sec D Biol crystallogr. 2013;69(Pt 10):1935–45.
Kumru OS, Joshi SB, Thapa P, Pheasey N, Bullock PS, Bashiri H, Siska CS, Kerwin BA, He F, Volkin DB, et al. Characterization of an oncolytic herpes simplex virus drug candidate. J Pharm Sci. 2015;104(2):485–94.
Jacobsen K, Pilyugin SS. Analysis of a mathematical model for tumor therapy with a fusogenic oncolytic virus. Math Biosci. 2015;270(Pt B):169–82.
Ning J, Wakimoto H. Oncolytic herpes simplex virus-based strategies toward a breakthrough in glioblastoma therapy. Front Microbiol. 2014;5:303.
Grandi P, Peruzzi P, Reinhart B, Cohen JB, Chiocca EA, Glorioso JC. Design and application of oncolytic HSV vectors for glioblastoma therapy. Expert Rev Neurother. 2009;9(4):505–17.
Fares J, Ahmed AU, Ulasov IV, Sonabend AM, Miska J, Lee-Chang C, Balyasnikova IV, Chandler JP, Portnow J, Tate MC, et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 2021;22(8):1103–14.
Li L, Liu S, Han D, Tang B, Ma J. Delivery and biosafety of oncolytic virotherapy. Front Oncol. 2020;10:457.
Machiels JP, Salazar R, Rottey S, Duran I, Dirix L, Geboes K, Wilkinson-Blanc C, Pover G, Alvis S, Champion B, et al. A phase 1 dose escalation study of the oncolytic adenovirus enadenotucirev, administered intravenously to patients with epithelial solid tumors (EVOLVE). J Immunother Cancer. 2019;7(1):20.
Tejada S, Alonso M, Patiño A, Fueyo J, Gomez-Manzano C, Diez-Valle R. Phase I trial of DNX-2401 for diffuse intrinsic pontine glioma newly diagnosed in pediatric patients. Neurosurgery. 2018;83(5):1050–6.
Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, Prabhu SS, Rao G, Fuller GN, Aldape KD, et al. Phase I study of DNX-2401 (delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36(14):1419–27.
Crunkhorn S. Delivering CARs with oncolytic viruses. Nat Rev Drug Discov. 2020;19(11):756.
Chastkofsky MI, Pituch KC, Katagi H, Zannikou M, Ilut L, Xiao T, Han Y, Sonabend AM, Curiel DT, Bonner ER, et al. Mesenchymal stem cells successfully deliver oncolytic virotherapy to diffuse intrinsic pontine glioma. Clin Cancer Res. 2021;27(6):1766–77.
Du W, Seah I, Bougazzoul O, Choi G, Meeth K, Bosenberg MW, Wakimoto H, Fisher D, Shah K. Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proc Natl Acad Sci USA. 2017;114(30):E6157-e6165.
Cairns TM, Huang ZY, Whitbeck JC, Ponce de Leon M, Lou H, Wald A, Krummenacher C, Eisenberg RJ, Cohen GH. Dissection of the antibody response against herpes simplex virus glycoproteins in naturally infected humans. J Virol. 2014;88(21):12612–22.
Cairns TM, Huang ZY, Gallagher JR, Lin Y, Lou H, Whitbeck JC, Wald A, Cohen GH, Eisenberg RJ. Patient-specific neutralizing antibody responses to herpes simplex virus are attributed to epitopes on gD, gB, or both and can be type specific. J Virol. 2015;89(18):9213–31.
Liu XQ, Xin HY, Lyu YN, Ma ZW, Peng XC, Xiang Y, Wang YY, Wu ZJ, Cheng JT, Ji JF, et al. Oncolytic herpes simplex virus tumor targeting and neutralization escape by engineering viral envelope glycoproteins. Drug Deliv. 2018;25(1):1950–62.
Hudacek AW, Navaratnarajah CK, Cattaneo R. Development of measles virus-based shielded oncolytic vectors: suitability of other paramyxovirus glycoproteins. Cancer Gene Ther. 2013;20(2):109–16.
Çuburu N, Wang K, Goodman KN, Pang YY, Thompson CD, Lowy DR, Cohen JI, Schiller JT. Topical herpes simplex virus 2 (HSV-2) vaccination with human papillomavirus vectors expressing gB/gD ectodomains induces genital-tissue-resident memory CD8+T cells and reduces genital disease and viral shedding after HSV-2 challenge. J Virol. 2015;89(1):83–96.
Capasso C, Hirvinen M, Garofalo M, Romaniuk D, Kuryk L, Sarvela T, Vitale A, Antopolsky M, Magarkar A, Viitala T, et al. Oncolytic adenoviruses coated with MHC-I tumor epitopes increase the antitumor immunity and efficacy against melanoma. Oncoimmunology. 2016;5(4): e1105429.
Mader EK, Maeyama Y, Lin Y, Butler GW, Russell HM, Galanis E, Russell SJ, Dietz AB, Peng KW. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res. 2009;15(23):7246–55.
Minev BR, Lander E, Feller JF, Berman M, Greenwood BM, Minev I, Santidrian AF, Nguyen D, Draganov D, Killinc MO, et al. First-in-human study of TK-positive oncolytic vaccinia virus delivered by adipose stromal vascular fraction cells. J Transl Med. 2019;17(1):271.
Shimizu Y, Gumin J, Gao F, Hossain A, Shpall EJ, Kondo A, Kerrigan BC, Yang J, Ledbetter D, Fueyo J, et al. Characterization of patient-derived bone marrow human mesenchymal stem cells as oncolytic virus carriers for the treatment of glioblastoma. J Neurosurg. 2022;136(3):757–67.
Ruano D, López-Martín JA, Moreno L, Lassaletta Á, Bautista F, Andión M, Hernández C, González-Murillo Á, Melen G, Alemany R, et al. First-in-human, first-in-child trial of autologous MSCs carrying the oncolytic virus Icovir-5 in patients with advanced tumors. Mol Ther. 2020;28(4):1033–42.
Jazowiecka-Rakus J, Sochanik A, Rusin A, Hadryś A, Fidyk W, Villa N, Rahman MM, Chmielik E, Franco LS, McFadden G. Myxoma virus-loaded mesenchymal stem cells in experimental oncolytic therapy of murine pulmonary melanoma. Mol Ther Oncolytics. 2020;18:335–50.
Mooney R, Majid AA, Batalla-Covello J, Machado D, Liu X, Gonzaga J, Tirughana R, Hammad M, Dellinger TH, Lesniak MS, Curiel DT, et al. Enhanced delivery of oncolytic adenovirus by neural stem cells for treatment of metastatic ovarian cancer. Mol Ther Oncolytics. 2019;12:79–92.
Roulstone V, Khan K, Pandha HS, Rudman S, Coffey M, Gill GM, Melcher AA, Vile R, Harrington KJ, De Bono J, et al. Phase I trial of cyclophosphamide as an immune modulator for optimizing oncolytic reovirus delivery to solid tumors. Clin Cancer Res. 2015;21(6):1305–12.
Yoo JY, Pradarelli J, Haseley A, Wojton J, Kaka A, Bratasz A, Alvarez-Breckenridge CA, Yu JG, Powell K, Mazar AP, et al. Copper chelation enhances antitumor efficacy and systemic delivery of oncolytic HSV. Clin Cancer Res. 2012;18(18):4931–41.
Rommelfanger DM, Grau MC, Diaz RM, Ilett E, Alvarez-Vallina L, Thompson JM, Kottke TJ, Melcher A, Vile RG. The efficacy versus toxicity profile of combination virotherapy and TLR immunotherapy highlights the danger of administering TLR agonists to oncolytic virus-treated mice. Mol Ther. 2013;21(2):348–57.
Raki M, Sarkioja M, Escutenaire S, Kangasniemi L, Haavisto E, Kanerva A, Cerullo V, Joensuu T, Oksanen M, Pesonen S, et al. Switching the fiber knob of oncolytic adenoviruses to avoid neutralizing antibodies in human cancer patients. J Gene Med. 2011;13(5):253–61.
Biswas M, Johnson JB, Kumar SR, Parks GD, Subbiah E. Incorporation of host complement regulatory proteins into Newcastle disease virus enhances complement evasion. J Virol. 2012;86(23):12708–16.
Evgin L, Acuna SA, De Souza CT, Marguerie M, Lemay CG, Ilkow CS, Findlay CS, Falls T, Parato KA, Hanwell D, et al. Complement inhibition prevents oncolytic vaccinia virus neutralization in immune humans and cynomolgus macaques. Mol Ther. 2015;23(6):1066–76.
Wu ZJ, Tang FR, Ma ZW, Peng XC, Xiang Y, Zhang Y, Kang J, Ji J, Liu XQ, Wang XW, et al. Oncolytic viruses for tumor precision imaging and radiotherapy. Hum Gene Ther. 2018;29(2):204–22.
Packiriswamy N, Upreti D, Zhou Y, Khan R, Miller A, Diaz RM, Rooney CM, Dispenzieri A, Peng KW, Russell SJ. Oncolytic measles virus therapy enhances tumor antigen-specific T-cell responses in patients with multiple myeloma. Leukemia. 2020;34(12):3310–22.
Packiam VT, Lamm DL, Barocas DA, Trainer A, Fand B, Davis RL III, Clark W, Kroeger M, Dumbadze I, Chamie K, et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: interim results. Urol Oncol. 2018;36(10):440–7.
Breitbach CJ, Moon A, Burke J, Hwang TH, Kirn DH. A phase 2, open-label, randomized study of Pexa-Vec (JX-594) administered by intratumoral injection in patients with unresectable primary hepatocellular carcinoma. Methods Mol Biol. 2015;1317:343–57.
Toulmonde M, Cousin S, Kind M, Guegan JP, Bessede A, Le Loarer F, Perret R, Cantarel C, Bellera C, Italiano A. Randomized phase 2 trial of intravenous oncolytic virus JX-594 combined with low-dose cyclophosphamide in patients with advanced soft-tissue sarcoma. J Hematol Oncol. 2022;15(1):149.
Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–8.
Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Nemunaitis J, Chesney J, Puzanov I, Harrington K, Zhang Y, Chen L, et al. Durable complete responses (CRs) in patients (pts) with stage IIIB-IV melanoma treated with talimogene laherparepvec (T-VEC) in OPTiM. Ann Surg Oncol. 2016;23:S31–2.
Liu H, Yuan SJ, Chen YT, Xie YB, Cui L, Yang WZ, Yang DX, Tian YT. Preclinical evaluation of herpes simplex virus armed with granulocyte-macrophage colony-stimulating factor in pancreatic carcinoma. World J Gastroenterol. 2013;19(31):5138–43.
Andtbacka RH, Ross M, Puzanov I, Milhem M, Collichio F, Delman KA, Amatruda T, Zager JS, Cranmer L, Hsueh E, et al. Patterns of clinical response with talimogene laherparepvec (T-VEC) in patients with melanoma treated in the OPTiM Phase III clinical trial. Ann Surg Oncol. 2016;23(13):4169–77.
Speck T, Heidbuechel JP, Veinalde R, Jaeger D, Von Kalle C, Ball CR, Ungerechts G, Engeland CE. Targeted BiTE expression by an oncolytic vector augments therapeutic efficacy against solid tumors. Clin Cancer Res. 2018;24(9):2128–37.
Freedman JD, Hagel J, Scott EM, Psallidas I, Gupta A, Spiers L, Miller P, Kanellakis N, Ashfield R, Fisher KD, et al. Oncolytic adenovirus expressing bispecific antibody targets T-cell cytotoxicity in cancer biopsies. EMBO Mol Med. 2017;9(8):1067–87.
Chaurasiya S, Fong Y, Warner SG. Optimizing oncolytic viral design to enhance antitumor efficacy: progress and challenges. Cancers (Basel). 2020;12(6):1699.
Heidbuechel JPW, Engeland CE. Oncolytic viruses encoding bispecific T cell engagers: a blueprint for emerging immunovirotherapies. J Hematol Oncol. 2021;14(1):63.
Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RH, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2018;174(4):1031–2.
Fu X, Rivera A, Tao L, Zhang X. An HSV-2 based oncolytic virus can function as an attractant to guide migration of adoptively transferred T cells to tumor sites. Oncotarget. 2015;6(2):13.
Albelda SM, Thorne SH. Giving oncolytic vaccinia virus more BiTE. Mol Ther. 2014;22(1):6–8.
Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RH, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–19.
Zamarin D, Holmgaard RB, Ricca J, Plitt T, Palese P, Sharma P, Merghoub T, Wolchok JD, Allison JP. Intratumoral modulation of the inducible co-stimulator ICOS by recombinant oncolytic virus promotes systemic anti-tumour immunity. Nat Commun. 2017;8:14340.
Vitale M, Scialò F, Passariello M, Leggiero E, D’Agostino A, Tripodi L, Gentile L, Bianco A, Castaldo G, Cerullo V, et al. Oncolytic adenoviral vector-mediated expression of an anti-PD-L1-scFv improves anti-tumoral efficacy in a melanoma mouse model. Front Oncol. 2022;12:902190.
Feola S, Capasso C, Fusciello M, Martins B, Tähtinen S, Medeot M, Carpi S, Frascaro F, Ylosmäki E, Peltonen K, et al. Oncolytic vaccines increase the response to PD-L1 blockade in immunogenic and poorly immunogenic tumors. Oncoimmunology. 2018;7(8): e1457596.
Brown MC, Holl EK, Boczkowski D, Dobrikova E, Mosaheb M, Chandramohan V, Bigner DD, Gromeier M, Nair Sk. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci Transl Med. 2017;9(408):4220.
Ramelyte E, Tastanova A, Balázs Z, Ignatova D, Turko P, Menzel U, Guenova E, Beisel C, Krauthammer M, Levesque MP, et al. Oncolytic virotherapy-mediated anti-tumor response: a single-cell perspective. Cancer Cell. 2021;39(3):394-406.e394.
Uhlitz F, Zamarin D. Rejuvenating dysfunctional T cells in ovarian cancer: CD28 is the license to kill. Cancer Cell. 2021. https://doi.org/10.1016/j.ccell.2021.10.011.
Duraiswamy J, Turrini R, Minasyan A, Barras D, Crespo I, Grimm AJ, Casado J, Genolet R, Benedetti F, Wicky A, et al. Myeloid antigen-presenting cell niches sustain antitumor T cells and license PD-1 blockade via CD28 costimulation. Cancer Cell. 2021;39(12):1623-1642.e1620.
Duebgen M, Martinez-Quintanilla J, Tamura K, Hingtgen S, Redjal N, Wakimoto H, Shah K. Stem cells loaded with multimechanistic oncolytic herpes simplex virus variants for brain tumor therapy. J Natl Cancer Instit. 2014;106(6):dju090.
Kwan A, Winder N, Atkinson E, Al-Janabi H, Allen RJ, Hughes R, Moamin M, Louie R, Evans D, Hutchinson M, et al. Macrophages mediate the antitumor effects of the oncolytic virus HSV1716 in mammary tumors. Mol Cancer Ther. 2021;20(3):589–601.
Chen S, Chen J, Xi W, Xu W, Yin G. Clinical therapeutic effect and biological monitoring of p53 gene in advanced hepatocellular carcinoma. Am J Clin Oncol. 2014;37(1):24–9.
Chen X, Zhou Y, Wang J, Yang J, Zhai Y, Li B. Dual silencing of Bcl-2 and Survivin by HSV-1 vector shows better antitumor efficacy in higher PKR phosphorylation tumor cells in vitro and in vivo. Cancer Gene Ther. 2015;22(8):380–6.
Zhang PY, Hao L, Zhang ZG, Dong BZ, Yang D, Wang XL, Xuan XJ, Yan Z, Qing L, Shi ZD, et al. Construction of conditionally replicating adenovirus expressing staphylococcal enterotoxin A gene: potential usefulness for anti-tumor therapies. Eur Rev Med Pharmacol Sci. 2014;18(16):2258–63.
Ramachandran M, Yu D, Wanders A, Essand M, Eriksson F. An infection-enhanced oncolytic adenovirus secreting H. pylori neutrophil-activating protein with therapeutic effects on neuroendocrine tumors. Mol Ther. 2013;21(11):2008–18.
Ilkow CS, Marguerie M, Batenchuk C, Mayer J, Ben Neriah D, Cousineau S, Falls T, Jennings VA, Boileau M, Bellamy D, Bastin D, et al. Reciprocal cellular cross-talk within the tumor microenvironment promotes oncolytic virus activity. Nat Med. 2015;21(5):530–6.
Sakakibara A, Tsukuda M, Kondo N, Ishiguro Y, Kimura M, Fujita K, Takahashi H, Matsuda H. Examination of the optimal condition on the in vitro sensitivity to telomelysin in head and neck cancer cell lines. Auris Nasus Larynx. 2011;38(5):589–99.
Stritzker J, Huppertz S, Zhang Q, Geissinger U, Härtl B, Gentschev I, Szalay AA. Inducible gene expression in tumors colonized by modified oncolytic vaccinia virus strains. J Virol. 2014;88(19):11556–67.
Zhang B, Cheng P. Improving antitumor efficacy via combinatorial regimens of oncolytic virotherapy. Mol Cancer. 2020;19(1):158.
Watanabe N, McKenna MK, Shaw AR, Suzuki M. Clinical CAR-T cell and oncolytic virotherapy for cancer treatment. Mol Ther. 2021;29(2):505–20.
Mahalingam D, Fountzilas C, Moseley J, Noronha N, Tran H, Chakrabarty R, Selvaggi G, Coffey M, Thompson B, Sarantopoulos J. A phase II study of REOLYSIN(®) (pelareorep) in combination with carboplatin and paclitaxel for patients with advanced malignant melanoma. Cancer Chemother Pharmacol. 2017;79(4):697–703.
Melcher A, Harrington K, Vile R. Oncolytic virotherapy as immunotherapy. Science. 2021;374(6573):1325–6.
Sugawara K, Iwai M, Ito H, Tanaka M, Seto Y, Todo T. Oncolytic herpes virus G47Δ works synergistically with CTLA-4 inhibition via dynamic intratumoral immune modulation. Mol Ther Oncolytics. 2021;22:129–42.
Mahalingam D, Wilkinson GA, Eng KH, Fields P, Raber P, Moseley JL, Cheetham K, Coffey M, Nuovo G, Kalinski P, et al. Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic adenocarcinoma: a phase Ib study. Clin Cancer Res. 2020;26(1):71–81.
Andtbacka RH, Collichio F, Harrington KJ, Middleton MR, Downey G, Ӧhrling K, Kaufman HL. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J Immunother Cancer. 2019;7(1):145.
Andtbacka RH, Amatruda T, Nemunaitis J, Zager JS, Walker J, Chesney JA, Liu K, Hsu CP, Pickett CA, Mehnert JM. Biodistribution, shedding, and transmissibility of the oncolytic virus talimogene laherparepvec in patients with melanoma. EBioMedicine. 2019;47:89–97.
Streby KA, Currier MA, Triplet M, Ott K, Dishman DJ, Vaughan MR, Ranalli MA, Setty B, Skeens MA, Whiteside S, et al. First-in-human intravenous seprehvir in young cancer patients: a phase 1 clinical trial. Mol Ther. 2019;27(11):1930–8.
Danson SJ, Conner J, Edwards JG, Blyth KG, Fisher PM, Muthana M, Salawu A, Taylor F, Hodgkinson E, Joyce P, et al. Oncolytic herpesvirus therapy for mesothelioma—a phase I/IIa trial of intrapleural administration of HSV1716. Lung Cancer. 2020;150:145–51.
Masoud SJ, Hu JB, Beasley GM, Stewart JH, Mosca PJ. Efficacy of talimogene laherparepvec (T-VEC) therapy in patients with in-transit melanoma metastasis decreases with increasing lesion size. Ann Surg Oncol. 2019;26(13):4633–41.
Todo T, Ito H, Ino Y, Ohtsu H, Ota Y, Shibahara J, Tanaka M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial. Nat Med. 2022;28(8):1630–9.
Todo T, Ino Y, Ohtsu H, Shibahara J, Tanaka M. A phase I/II study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma. Nat Commun. 2022;13(1):4119.
Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005;16(9):1016–27.
China SFaDAo. Guidance for human gene therapy research and its products (State biological products standardization commission of the people’s republic of China, SFDA, Beijing, China). 2003.
Garcia M, Moreno R, Gil-Martin M, Cascallo M, de Olza MO, Cuadra C, Piulats JM, Navarro V, Domenech M, Alemany R, et al. A phase 1 trial of oncolytic adenovirus ICOVIR-5 administered intravenously to cutaneous and uveal melanoma patients. Hum Gene Ther. 2019;30(3):352–64.
Pascual-Pasto G, Bazan-Peregrino M, Olaciregui NG, Restrepo-Perdomo CA, Mato-Berciano A, Ottaviani D, Weber K, Correa G, Paco S, Vila-Ubach M, et al. Therapeutic targeting of the RB1 pathway in retinoblastoma with the oncolytic adenovirus VCN-01. Sci Transl Med. 2019;11(476): eaat9321.
Kieran MW, Goumnerova L, Manley P, Chi SN, Marcus KJ, Manzanera AG, Polanco ML, Guzik BW, Aguilar-Cordova E, Diaz-Montero CM, et al. Phase I study of gene-mediated cytotoxic immunotherapy with AdV-tk as adjuvant to surgery and radiation for pediatric malignant glioma and recurrent ependymoma. Neuro Oncol. 2019;21(4):537–46.
Garcia-Carbonero R, Salazar R, Duran I, Osman-Garcia I, Paz-Ares L, Bozada JM, Boni V, Blanc C, Seymour L, Beadle J, et al. Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J Immunother Cancer. 2017;5(1):71.
O’Cathail SM, Davis S, Holmes J, Brown R, Fisher K, Seymour L, Adams R, Good J, Sebag-Montefiore D, Maughan T, et al. A phase 1 trial of the safety, tolerability and biological effects of intravenous Enadenotucirev, a novel oncolytic virus, in combination with chemoradiotherapy in locally advanced rectal cancer (CEDAR). Radiat Oncol. 2020;15(1):151.
Moreno V, Barretina-Ginesta MP, García-Donas J, Jayson GC, Roxburgh P, Vázquez RM, Michael A, Antón-Torres A, Brown R, Krige D, et al. Safety and efficacy of the tumor-selective adenovirus enadenotucirev with or without paclitaxel in platinum-resistant ovarian cancer: a phase 1 clinical trial. J Immunother Cancer. 2021;9(12): e003645.
Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, Cho M, Lim HY, Chung HC, Kim CW, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19(3):329–36.
Park SH, Breitbach CJ, Lee J, Park JO, Lim HY, Kang WK, Moon A, Mun JH, Sommermann EM, Avidal LM, et al. Phase 1b trial of biweekly intravenous Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus in colorectal cancer. Mol Ther. 2015;23(9):1532–40.
Husseini F, Delord JP, Fournel-Federico C, Guitton J, Erbs P, Homerin M, Halluard C, Jemming C, Orange C, Limacher JM, et al. Vectorized gene therapy of liver tumors: proof-of-concept of TG4023 (MVA-FCU1) in combination with flucytosine. Ann Oncol. 2017;28(1):169–74.
Mell LK, Brumund KT, Daniels GA, Advani SJ, Zakeri K, Wright ME, Onyeama SJ, Weisman RA, Sanghvi PR, Martin PJ, et al. Phase I trial of intravenous oncolytic vaccinia virus (GL-ONC1) with cisplatin and radiotherapy in patients with locoregionally advanced head and neck carcinoma. Clin Cancer Res. 2017;23(19):5696–702.
Lauer UM, Schell M, Beil J, Berchtold S, Koppenhöfer U, Glatzle J, Königsrainer A, Möhle R, Nann D, Fend F, et al. Phase I study of oncolytic vaccinia virus GL-ONC1 in patients with peritoneal carcinomatosis. Clin Cancer Res. 2018;24(18):4388–98.
Dispenzieri A, Tong C, LaPlant B, Lacy MQ, Laumann K, Dingli D, Zhou Y, Federspiel MJ, Gertz MA, Hayman S, et al. Phase I trial of systemic administration of Edmonston strain of measles virus genetically engineered to express the sodium iodide symporter in patients with recurrent or refractory multiple myeloma. Leukemia. 2017;31(12):2791–8.
Galanis E, Atherton PJ, Maurer MJ, Knutson KL, Dowdy SC, Cliby WA, Haluska P Jr, Long HJ, Oberg A, Aderca I, et al. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res. 2015;75(1):22–30.
Geletneky K, Hajda J, Angelova AL, Leuchs B, Capper D, Bartsch AJ, Neumann JO, Schöning T, Hüsing J, Beelte B, et al. Oncolytic H-1 Parvovirus shows safety and signs of immunogenic activity in a first phase I/IIa glioblastoma trial. Mol Ther. 2017;25(12):2620–34.
Hajda J, Lehmann M, Krebs O, Kieser M, Geletneky K, Jäger D, Dahm M, Huber B, Schöning T, Sedlaczek O, et al. A non-controlled, single arm, open label, phase II study of intravenous and intratumoral administration of ParvOryx in patients with metastatic, inoperable pancreatic cancer: ParvOryx02 protocol. BMC Cancer. 2017;17(1):576.
Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology. 2016;5(1): e1115641.
Yoo JY, Jaime-Ramirez AC, Bolyard C, Dai H, Nallanagulagari T, Wojton J, Hurwitz BS, Relation T, Lee TJ, Lotze MT, et al. Bortezomib treatment sensitizes oncolytic HSV-1-treated tumors to NK cell immunotherapy. Clin Cancer Res. 2016;22(21):5265–76.
Esaki S, Nigim F, Moon E, Luk S, Kiyokawa J, Curry W Jr, Cahill DP, Chi AS, Iafrate AJ, Martuza RL, et al. Blockade of transforming growth factor-beta signaling enhances oncolytic herpes simplex virus efficacy in patient-derived recurrent glioblastoma models. Int J Cancer. 2017;141(11):2348–58.
Crespo-Rodriguez E, Bergerhoff K, Bozhanova G, Foo S, Patin EC, Whittock H, Buus R, Haider S, Muirhead G, Thway K, et al. Combining BRAF inhibition with oncolytic herpes simplex virus enhances the immune-mediated antitumor therapy of BRAF-mutant thyroid cancer. J Immunother Cancer. 2020;8(2): e000698.
Jahan N, Lee JM, Shah K, Wakimoto H. Therapeutic targeting of chemoresistant and recurrent glioblastoma stem cells with a proapoptotic variant of oncolytic herpes simplex virus. Int J Cancer. 2017;141(8):1671–81.
Nigim F, Esaki SI, Hood M, Lelic N, James MF, Ramesh V, Stemmer-Rachamimov A, Cahill DP, Brastianos PK, Rabkin SD, et al. A new patient-derived orthotopic malignant meningioma model treated with oncolytic herpes simplex virus. Neuro Oncol. 2016;18(9):1278–87.
Thakur S, Ruan Y, Zhang C, Lun X, Jayanthan A, Narendran A. Human SNF5 arming of double-deleted vaccinia virus shows oncolytic and cytostatic activity against central nervous system atypical teratoid/rhabdoid tumor cells. Cancer Gene Ther. 2020;28(7–8):739–44.
Kalkavan H, Sharma P, Kasper S, Helfrich I, Pandyra AA, Gassa A, Virchow I, Flatz L, Brandenburg T, Namineni S, et al. Spatiotemporally restricted arenavirus replication induces immune surveillance and type I interferon-dependent tumour regression. Nat Commun. 2017;8:14447.
Le Boeuf F, Selman M, Son HH, Bergeron A, Chen A, Tsang J, Butterwick D, Arulanandam R, Forbes NE, Tzelepis F, et al. Oncolytic Maraba virus MG1 as a treatment for sarcoma. Int J Cancer. 2017;141(6):1257–64.
Müller LM, Holmes M, Michael JL, Scott GB, West EJ, Scott KJ, Parrish C, Hall K, Stäble S, Jennings VA, et al. Plasmacytoid dendritic cells orchestrate innate and adaptive anti-tumor immunity induced by oncolytic coxsackievirus A21. J Immunother Cancer. 2019;7(1):164.
Marchica V, Franceschi V, Vescovini R, Storti P, Vicario E, Toscani D, Zorzoli A, Airoldi I, Dalla Palma B, Campanini N, et al. Bovine pestivirus is a new alternative virus for multiple myeloma oncolytic virotherapy. J Hematol Oncol. 2020;13(1):89.
Pan W, Bodempudi V, Esfandyari T, Farassati F. Utilizing ras signaling pathway to direct selective replication of herpes simplex virus-1. PLoS ONE. 2009;4(8): e6514.
Yao F, Murakami N, Bleiziffer O, Zhang P, Akhrameyeva NV, Xu X, Brans R. Development of a regulatable oncolytic herpes simplex virus type 1 recombinant virus for tumor therapy. J Virol. 2010;84(16):8163–71.
Post DE, Sandberg EM, Kyle MM, Devi NS, Brat DJ, Xu Z, Tighiouart M, Van Meir EG. Targeted cancer gene therapy using a hypoxia inducible factor dependent oncolytic adenovirus armed with interleukin-4. Cancer Res. 2007;67(14):6872–81.
Ayala-Breton C, Barber GN, Russell SJ, Peng KW. Retargeting vesicular stomatitis virus using measles virus envelope glycoproteins. Hum Gene Ther. 2012;23(5):484–91.
Petrovic B, Gianni T, Gatta V, Campadelli-Fiume G. Insertion of a ligand to HER2 in gB retargets HSV tropism and obviates the need for activation of the other entry glycoproteins. PLoS Pathog. 2017;13(4): e1006352.
Miles LA, Burga LN, Gardner EE, Bostina M, Poirier JT, Rudin CM. Anthrax toxin receptor 1 is the cellular receptor for Seneca Valley virus. J Clin Investig. 2017;127(8):2957–67.
Jayawardena N, Burga LN, Easingwood RA, Takizawa Y, Wolf M, Bostina M. Structural basis for anthrax toxin receptor 1 recognition by Seneca Valley Virus. Proc Natl Acad Sci USA. 2018;115(46):E10934-e10940.
Zhu Z, Gorman MJ, McKenzie LD, Chai JN, Hubert CG, Prager BC, Fernandez E, Richner JM, Zhang R, Shan C, et al. Zika virus has oncolytic activity against glioblastoma stem cells. J Exp Med. 2017;214(10):2843–57.
Hou W, Sampath P, Rojas JJ, Thorne SH. Oncolytic virus-mediated targeting of PGE2 in the tumor alters the immune status and sensitizes established and resistant tumors to immunotherapy. Cancer Cell. 2016;30(1):108–19.
Leoni V, Vannini A, Gatta V, Rambaldi J, Sanapo M, Barboni C, Zaghini A, Nanni P, Lollini PL, Casiraghi C, Campadelli-Fiume G. A fully-virulent retargeted oncolytic HSV armed with IL-12 elicits local immunity and vaccine therapy towards distant tumors. PLoS Pathog. 2018;14(8): e1007209.
Liu Z, Ravindranathan R, Kalinski P, Guo ZS, Bartlett DL. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat Commun. 2017;8:14754.
Fu X, Rivera A, Tao L, Zhang X. An HSV-2 based oncolytic virus can function as an attractant to guide migration of adoptively transferred T cells to tumor sites. Oncotarget. 2015;6(2):902–14.
Xu B, Ma R, Russell L, Yoo JY. An oncolytic herpesvirus expressing E-cadherin improves survival in mouse models of glioblastoma. Nat Biotechnol. 2018. https://doi.org/10.1038/nbt0119-102c.
Streby KA, Geller JI, Currier MA, Warren PS, Racadio JM, Towbin AJ, Vaughan MR, Triplet M, Ott-Napier K, Dishman DJ, et al. Intratumoral injection of HSV1716, an oncolytic herpes virus, is safe and shows evidence of immune response and viral replication in young cancer patients. Clin Cancer Res. 2017;23(14):3566–74.
Hirooka Y, Kasuya H, Ishikawa T, Kawashima H, Ohno E, Villalobos IB, Naoe Y, Ichinose T, Koyama N, Tanaka M, et al. A Phase I clinical trial of EUS-guided intratumoral injection of the oncolytic virus, HF10 for unresectable locally advanced pancreatic cancer. BMC Cancer. 2018;18(1):596.
Cui C, Wang X, Lian B, Ji Q, Zhou L, Chi Z, Si L, Sheng X, Kong Y, Yu J, et al. OrienX010, an oncolytic virus, in patients with unresectable stage IIIC-IV melanoma: a phase Ib study. J Immunother Cancer. 2022;10(4): e004307.
Gállego Pérez-Larraya J, Garcia-Moure M, Labiano S, Patiño-García A, Dobbs J, Gonzalez-Huarriz M, Zalacain M, Marrodan L, Martinez-Velez N, Puigdelloses M, et al. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic pontine glioma. N Engl J Med. 2022;386(26):2471–81.
Van Putten EH, Kleijn A, Van Beusechem VW, Noske D, Lamers CH, De Goede AL, Idema S, Hoefnagel D, Kloezeman JJ, Fueyo J, et al. Convection enhanced delivery of the oncolytic adenovirus delta24-RGD in patients with recurrent gbm: a phase I clinical trial including correlative studies. Clin Cancer Res. 2022;28(8):1572–85.
Manyam M, Stephens AJ, Kennard JA, LeBlanc J, Ahmad S, Kendrick JE, Holloway RW. A phase 1b study of intraperitoneal oncolytic viral immunotherapy in platinum-resistant or refractory ovarian cancer. Gynecol Oncol. 2021;163(3):481–9.
Kurokawa C, Iankov ID, Anderson SK, Aderca I, Leontovich AA, Maurer MJ, Oberg AL, Schroeder MA, Giannini C, Greiner SM, et al. Constitutive interferon pathway activation in tumors as an efficacy determinant following oncolytic virotherapy. J Natl Cancer Instit. 2018;110(10):1123–32.
Bernstein V, Ellard SL, Dent SF, Tu D, Mates M, Dhesy-Thind SK, Panasci L, Gelmon KA, Salim M, Song X, et al. A randomized phase II study of weekly paclitaxel with or without pelareorep in patients with metastatic breast cancer: final analysis of Canadian Cancer Trials Group IND.213. Breast Cancer Res Treat. 2018;167(2):485–93.
Andtbacka RH, Curti B, Daniels GA, Hallmeyer S, Whitman ED, Lutzky J, Spitler LE, Zhou K, Bommareddy PK, Grose M, et al. Clinical responses of oncolytic coxsackievirus A21 (V937) in patients with unresectable melanoma. J Clin Oncol. 2021;39(34):3829–38.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31700736, 81872412), The Central Government guides local funds for scientific and Technological Development (XZ202201YD0024C), Key R & D Program of Hubei Province (2021BGD010), Hubei Province Scientific and Technological Research Project (D20201306), Hubei Province Key Project of Research and Development Plan (to XW Wang), Hubei Province Health Research Project (WJ2019-01), Hubei Medical Youth Tip-Top Talent (to XW Wang), Leading Talent Program of Yangtze Talent Project (to XW Wang) and the College Students Innovative Entrepreneurial Training Program in Yangtze University (YZ2021296 and YZ2022307). We thank members of our lab for helpful comments and advice.
Funding
This work was supported by grants from the National Natural Science Foundation of China (31700736, 81872412), The Central Government guides local funds for scientific and Technological Development (XZ202201YD0024C), Key R & D Program of Hubei Province (2021BGD010), Hubei Province Scientific and Technological Research Project (D20201306), Hubei Province Key Project of Research and Development Plan (to XW Wang), Hubei Province Health Research Project (WJ2019-01), Hubei Medical Youth Tip-Top Talent (to XW Wang), Leading Talent Program of Yangtze Talent Project (to XW Wang) and the College Students Innovative Entrepreneurial Training Program in Yangtze University (YZ2021296 and YZ2022307).
Author information
Authors and Affiliations
Contributions
XWW and HWX designed the manuscript. XWW drafted the manuscript. XWW, YHS, XXW, XQH, WQC, ZJW, QX and XQL completed the figures and tables. XWW, JGG, HYX and HWX revised and edited the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors read the fnal version and approved it.
Competing interests
The authors do not have competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, X., Shen, Y., Wan, X. et al. Oncolytic virotherapy evolved into the fourth generation as tumor immunotherapy. J Transl Med 21, 500 (2023). https://doi.org/10.1186/s12967-023-04360-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04360-8