Abstract
Background
Metastatic pancreatic cancer has a dismal prognosis, with a mean six-month progression-free survival of approximately 50% and a median survival of about 11 months. Despite intensive research, only slight improvements of clinical outcome could be achieved over the last decades. Hence, new and innovative therapeutic strategies are urgently required. ParvOryx is a drug product containing native parvovirus H-1 (H-1PV). Since H-1PV was shown to exert pronounced anti-neoplastic effects in pre-clinical models of pancreatic cancer, the drug appears to be a promising candidate for treatment of this malignancy.
Methods
ParvOryx02 is a non-controlled, single arm, open label, dose-escalating, single center trial. In total seven patients with pancreatic cancer showing at least one hepatic metastasis are to be treated with escalating doses of ParvOryx according to the following schedule: i) 40% of the total dose infused intravenously in equal fractions on four consecutive days, ii) 60% of the total dose injected on a single occasion directly into the hepatic metastasis at varying intervals after intravenous infusions. The main eligibility criteria are: age ≥ 18 years, disease progression despite first-line chemotherapy, and at least one hepatic metastasis. Since it is the second trial within the drug development program, the study primarily explores safety and tolerability after further dose escalation of ParvOryx. The secondary objectives are related to the evaluation of certain aspects of anti-tumor activity and clinical efficacy of the drug.
Discussion
This trial strongly contributes to the clinical development program of ParvOryx. The individual hazards for patients included in the current study and the environmental risks are addressed and counteracted adequately. Besides information on safety and tolerability of the treatment after further dose escalation, thorough evaluations of pharmacokinetics and intratumoral spread as well as proof-of-concept (PoC) in pancreatic cancer will be gained in the course of the trial.
Trial registration
ClinicalTrials.gov-ID: NCT02653313, Registration date: Dec. 4th, 2015.
Similar content being viewed by others
Background
According to epidemiological estimations for 40 European countries the overall incidence of pancreatic cancer in the year 2012 amounted to approximately 10.5 cases per 100,000 inhabitants [1]. The figures for mortality were only slightly lower with 10.1 cases per 100,000, indicating the limited treatment options for this disease [1, 2]. Unlike in other neoplasms, the apparent mortality from pancreatic cancer has increased gradually in the past decades and was approximately 20 to 30% higher in 2014 than in 1970. This is probably due to an improvement of diagnostic procedures with a parallel increase in the number of properly documented disease cases. Nevertheless, pancreatic cancer is the only major cancer showing nearly no improvement of therapeutic outcome over the last decades [1,2,3].
Currently, there are no modalities for early diagnosis or screening for pancreatic cancer so that the disease is typically discovered only at advanced stages. Based on the analysis of the US National Cancer Database (NCDB) performed by the American Joint Committee on Cancer (AJCC) for the period between 1992 and 1998, the following relative distribution of disease stages at the time of the initial diagnosis can be assumed: stage I 9.8, stage II 21.9, stage III: 13.0, and stage IV 55.2%. The corresponding 5-year survival rates are: 25.3, 11.6, 2.7, and 0.7%, respectively [4]. The locally advanced (stage III) and metastatic disease (stage IV) are primarily not eligible for surgical intervention and therefore associated with poor prognosis. The current standard of care for these tumor stages relies upon different chemotherapeutic regimens.
Based on the results of a randomized, controlled clinical trial comparing the therapeutic efficacy of a combination of oxaliplatin, leucovorin, irinotecan, and 5-FU (FOLFIRINOX) to monotherapy with gemcitabine, FOLFIRINOX has been established as the first-line therapy in patients with inoperable pancreatic cancer who are in good physical condition([5]). Another phase III randomized, controlled clinical trial including 861 patients compared the clinical outcome after treatment with a combination of nab-paclitaxel and gemcitabine to gemcitabine alone [6]. As the drug combination showed significant increase in overall survival with acceptable toxicity, it was approved for the first-line treatment of inoperable disease by the US Food and Drug Administration (FDA) [7]. However, neither FOLFIRINOX nor the combination of nab-paclitaxel and gemcitabine bring about any relevant advantage in terms of long-term clinical outcome [5, 6].
Trial rationale/justification
As briefly outlined above, despite intense efforts to improve treatment, the prognosis for pancreatic cancer patients is still disappointing. Therefore, all agents showing anti-tumor effects with an acceptable safety profile should undergo rapid clinical development to assess their therapeutic potential.
ParvOryx is a drug that contains parvovirus H-1 (H-1PV) as active substance. H-1PV is a small, single-stranded rodent DNA virus. The natural host is rat, but like other related parvoviruses, H-1PV is able to infect and replicate in cells of various other species including humans. Parvoviruses exert cytopathic effects mainly in neoplastic cells: they preferentially kill in-vitro-transformed and tumor-derived human and rodent cell lines, with limited-to-no cytocidal action in non-transformed cells [8]. Moreover, these viruses have been shown to have oncosuppressive properties, inhibiting the formation of spontaneous as well as chemically or virally induced tumors in laboratory animals [8, 9]. Furthermore, implants of tumor cells, including human neoplastic cells, were shown to be targets for parvoviral anti-cancer activity (oncolysis) in recipient animals [8,9,10,11,12]. Parvoviral cytotoxicity seems to be attributed to the viral nonstructural protein NS-1 [13].
H-1PV showed efficacy in preclinical, in-vitro models of pancreatic cancer. All investigated human pancreatic cancer cell lines, both of primary tumor and of metastatic origin, were susceptible to the stand-alone treatment with H-1PV, although to a varying extent [14, 15]. Synergistic increase of efficacy could be achieved by combination with valproic acid (VPA), a histone deacetylase inhibitor (HDACI) [14]. Moreover, based on the results from investigations on cellular pathways affected by H-1PV as well as by gemcitabine, synergistic effects of concomitant treatment with both agents can be anticipated [15]. Consecutive preclinical in-vivo investigations in animal models of pancreatic cancer carried out in mice and rats showed promising effects of H-1PV in the dose range between 1E09 and 2.5E09 plaque forming units (pfu). The anti-tumor effects were dose-dependent and the viral proteins were selectively expressed in the tumor as opposed to normal tissues. H-1PV virotherapy in an orthotopic pancreatic carcinoma model led to a significant delay in tumor growth and prolongation of survival, with 20% of the treated animals remaining disease-free for 16 weeks [15]. Importantly, in some cases, complete remission of pre-existing tumors was observed. Moreover, inoculation of the primary tumor with H-1PV at early stages of tumor development resulted in almost 50% suppression of distant metastases involving the visceral lymph nodes of the upper abdominal cavity and liver [15]. Also in animal models, the co-administration of VPA increased the potency of H-1PV, allowing a dose reduction by one power of ten down to 2.5E08 pfu without loss of efficacy [14].
Based on the findings described above, ParvOryx can reasonably be assumed to show efficacy against pancreatic cancer in humans. Ideally, the drug would not only be directly cytotoxic to the neoplastic cells but also induce anti-cancer vaccination by destruction of cancer cells and activation of the adaptive immune system. Based on the preclinical investigations synergistic effects with gemcitabine, a drug commonly used for treatment of pancreatic cancer, can be assumed. Thus, there is a strong rationale for treating patients suffering from pancreatic cancer with a combination regimen of ParvOryx and gemcitabine.
Design and methods
Aim
The trial aims at investigation of safety and tolerability, virus distribution and shedding as well as at evaluation of anti-tumor activity and clinical efficacy after multiple intravenous and a single intrametastatic administration of ParvOryx to patients suffering from pancreatic cancer.
Objectives
Primary objectives
The primary objectives of the trial are related to the safety and tolerability of the Investigational Medicinal Product (IMP):
-
Safety and tolerability assessed on the basis of physical examinations, chosen laboratory parameters, 12-lead electrocardiogram (ECGs), adverse events (AEs), and serious adverse events (SAEs),
-
Assessment of humoral immune response to H-1PV after intravenous infusions and intrametastatic injection (detection of anti-drug-antibodies (ADA)),
-
Investigation of the kinetics of H-1PV genomes in blood following intravenous and intrametastatic administration of the IMP by means of quantitative real-time polymerase chain reaction (qPCR),
-
Investigation of virus shedding in faeces, urine, and saliva following intravenous and intrametastatic administration of the IMP.
Secondary objectives
The secondary objectives of the study are related to the anti-tumor activity and clinical efficacy of the IMP:
-
Investigation of anti-tumor effects of ParvOryx by means of the following histo-immuno-pathological findings: i) extent of metastatis necrosis, proliferation rate and other pathological characteristics, ii) density of tumor-infiltrating immune cells,
-
Quantity of cytokines and chemokines in tumor tissue,
-
Investigation of viral replication in the tumor tissue by means of NS-1 detection in the tumor material,
-
Investigation of the cellular immune response against viral proteins and tumor antigens by means of enzyme-linked immunospot assay (ELISPOT) and fluorescence-activated cell sorting (FACS),
-
Progression-free survival (PFS) up to 6 months after the first administration of the IMP (determined by RECIST criteria),
-
Morphological changes of the liver metastasis assessed by ultrasonography,
-
Overall survival (OS) up to 6 months after the first administration of the IMP,
-
Course of the tumor marker carbohydrate antigen 19-9 (CA 19-9) up to 6 months after the first administration of the IMP.
Design
ParvOryx02 is a non-controlled, single arm, open label, dose-escalating, single center trial.
Due to an exploratory approach with regard to safety and tolerability of the IMP, no positive control is used. In face of the small size of the trial population and the intensity of collecting the biological samples, including multiple liver biopsies, no negative control (placebo) was implemented.
In a foregoing, first-in-man trial, referred to as ParvOryx01, comprehensive information on safety and tolerability of the IMP up to the total dose of 5E09 pfu administered by systemic (intravenous) as well as by local (intratumoral and intracerebral) route was obtained. Since the current trial includes further dose escalation up to the total dose of 1E10 pfu, a sequential design, including intervals of at least 28 days between treatments of consecutive subjects, is employed.
Due to the complex handling and administration of the IMP this trial is being performed in a single center with a sound experience in clinical research as well as in clinical management of patients with pancreatic cancer.
Eligibility
Histologically confirmed pancreatic ductal adenocarcinoma with at least one hepatic metastasis is a prerequisite for inclusion in this trial. Moreover, patients have to fulfill the following main inclusion criteria: i) at least 18 years of age, ii) disease progression despite first-line therapy, iii) ECOG performance scale 0 or 1, iv) adequate main organ function, including normal thyroid function, v) negative beta-HCG-test and willingness to abide by the rules of adequate contraception.
Main criteria for exclusion of patients are: i) eligibility for surgery, ii) symptomatic cerebral, pulmonary, osseous metastases and/or peritoneal carcinomatosis, (iii) liver cirrhosis, previous splenectomy and/or severe respiratory impairment, (iv) chemo- and/or radiotherapy within 2 and 6 weeks prior to trial inclusion, respectively, (v) known allergy to iodinated contrast media, (vi) presumed contact to pregnant women and/or infants within 2 months after the first administration of the IMP.
Sample size
As no confirmatory hypothesis tests are performed in this trial, the choice of sample size was not based on formal sample size calculation but on the following pragmatic considerations. The trial aims at evaluating safety and tolerability as well as proof-of-concept (PoC) regarding efficacy of ParvOryx in the treatment of pancreatic cancer. The number of seven subjects is assumed to be adequate to gain information on safety and tolerability of ParvOryx at the scheduled dose levels as required for continuation of the clinical drug development. Moreover, since PoC is mainly related to pathological and immunological parameters, the size of the trial population seems to be sufficient.
Course of the trial
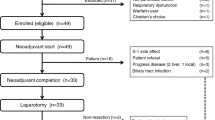
A schematic overview of the trial is given in Fig. 1. For each individual subject, the trial consists of three phases, i.e. screening, treatment (including observation until study Day 28) and follow-up phase between study Day 28 and 6 months:
-
Screening
Screening of patients, aiming at assessment of their eligibility for the trial and collection of the baseline parameters, must be carried out within 2 weeks prior to the study inclusion. Potentially eligible patients are provided with comprehensive written and verbal information. Procedures that are carried out during the screening include:
Written informed consent, demography and medical history, concomitant medication, physical examination, vital signs and 12-lead ECG, clinical chemistry, hematology and coagulation, CA19-9, ELISPOT and FACS, H-1PV-specific antibodies, serology of human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV), pregnancy test, thoracic computed tomography (CT) and abdominal magnetic resonance imaging (MRI), abdominal ultrasonography.
At the end of the screening phase the inclusion and exclusion criteria will be reviewed and the final judgment on the subject’s eligibility will be made. If eligible, the subject will enter the treatment phase and receive the study-specific intervention.
-
Treatment (main intervention)
The IMP in this trial is ParvOryx, i.e. a GMP-grade preparation of H-1PV. The administration of the IMP is carried out as follows: i) 40% of the total dose, divided into four equal fractions (10% of the total dose each) infused intravenously (i.v.) over 2 h on four consecutive days, ii) 60% of the total dose injected on a single occasion directly into the hepatic metastasis under ultrasound guidance. The timing of intrametastatic injection differs between the trial subjects. The injection is to be performed either 6 or 9 or 13 days after the first i.v. administration of the IMP (see Fig. 1). The total doses of ParvOryx are: 1E09 pfu in the first subject, 5E09 pfu in three further subjects and 1E10 pfu in the last three subjects (see Table 1). The dose escalation and maintenance at the given dose level is only allowed if ParvOryx proved safe and well-tolerated in the previously treated subjects.
The different time points for the intrametastatic injection were chosen to explore the most appropriate schedule for boosting the anticipated anti-tumor immune reaction. Furthermore, the tissue samples taken in parallel to the intrametastatic treatment will allow for assessment of pharmacokinetics and pharmacodynamics at the respective time points.
There are two chemotherapeutics defined as non-investigational medicinal products (NIMP) in this trial: i) Gemcitabine, administered at the dose of 1000 mg/m2 of body surface area (BSA) on days 1, 8 and 15 of each 28-day cycle. The administration (cycle 1, day 1) is to be commenced 27 days after the first intravenous administration of ParvOryx, ii) Nab-paclitaxel, administered at the dose of 125 mg/m2 of BSA, given on days 1, 8 and 15 of each 28-day cycle, immediately prior to gemcitabine. Nab-paclitaxel is to be introduced only in case of disease progression despite previous treatment with ParvOryx and gemcitabine. Nab-paclitaxel has emerged as a second-line treatment option in PDAC after FOLFIRINOX failure [16]. Mechanistically, preclinical data suggest that nab-paclitaxel increases gemcitabine levels by decreasing intratumoral cytidine deaminase activity [17]. In absence of preclinical data of combining ParvOryx, gemcitabine and nab-paclitaxel, a triple therapy was not feasible within this trial. Nevertheless, this treatment option should be made available to patients with disease progression.
Although the primary objective of this trial refers to the safety and tolerability of ParvOryx, the investigations related to the local anti-tumor activity and to the pharmacokinetics (PK) of H-1PV genomes are of substantial importance. In order to detect possible time-dependent differences, study days appointed for biopsies and PK sampling differ interindividually (see Fig. 2). Likewise, the thorough blood PK evaluations (PK profiles) are scheduled for different study days, i.e. they are performed either on the last day of i.v. administration (3 subjects) or on the day of intrametastatic administration (the remaining four subjects) of ParvOryx. In either case the timing of blood collection is as follows: The first sample is taken prior to the dosing of the IMP, the second sample up to 10 min after the end of the administration procedure, i.e. either at the end-of-infusion or after intrametastatic injection is completed; further samples are obtained 0.5, 1, 2, 4, 7 and 22 h thereafter. Three biopsies per subject are to be collected: i) prior to the overall first administration of the IMP, ii) 6, 9, or 13 days after the first i.v. administration of ParvOryx, directly prior to the intrametastatic administration, iii) either one or 2 months after the first i.v. administration of ParvOryx. Since cycle 1 of gemcitabine begins on day 27, effects of chemotherapy will be accounted for when interpreting biopsies taken 2 months after i.v. ParvOryx.
General overview of the course of the trial. The trial consists of three phases: Screening, aiming at verification of patients’ eligibility for the trial; Treatment, in which the IMP is administered and the chosen parameters on safety, tolerability, distribution and biological activity of ParvOryx are investigated; Follow-up, aiming at the long-term assessment of safety, tolerability, biological activity and clinical efficacy of ParvOryx. Abbreviations: i.m.: intrametastatic, i.v.: intravenous, Sub: subjects
Schedule of the main trial-specific interventions. In order to account for potential time-dependent effects, the trial-specific interventions are to be carried out at different time points. Abbreviations: BPS: biopsy of liver metastasis, LA: local (intrametastatic) administration of ParvOryx, PK: thorough pharmacokinetic investigations, Sub: subject
-
Follow-up
During the follow-up phase, which extends up to 6 months after the first administration of the IMP, delayed and/or long-term effects of ParvOryx are evaluated. If no complications occur, the subjects are to attend the study visits at months 2, 4 and 6. At each visit safety, tolerability and clinical efficacy will be assessed by the parameters described above.
-
Trial schedule and duration
ParvOryx02 is the overall second clinical trial with the IMP ParvOryx. In this trial further dose escalation is planned. Since the starting dose level equals to the concluding dose level of the previous trial (ParvOryx01), a sequential escalation design is used. The subsequent patient may only receive the first dose of ParvOryx if the treatment proved safe and well-tolerated in the previous subject, i.e. if none of the following pre-defined events occurred up to 27 days after the first i.v. administration: i) elevation of alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), alkaline phosphatase (AP), bilirubin or c-reactive protein (CRP) > 3 times the baseline ii) neutrophil count <1.0 × 1E09/L or >12E09/L, iii) hemoglobin <7.5 g/L, iv) platelet count <5E10/L, v) INR > 2.5, aPTT >50 s., vi) occurrence of neurological symptoms with no other explanation than the administration of the IMP, vii) occurrence of thromboembolic event(s), vii) serious adverse events (SAE(s)) classified as at least ‘possibly’ related to the IMP viii) deteriorations in medical monitoring parameters (laboratory values, ECG, etc.), classified as at least ‘possibly’ related to the IMP and requiring countermeasures to avert conditions fulfilling at least one of the ‘seriousness’-criteria, ix) medical necessity to interrupt or to prematurely terminate the scheduled treatment. If any of the before mentioned events occur, an independent data safety monitoring board (DSMB) will be provided with all required data and consulted regarding the trial continuation and/or implementation of any modifications.
The overall duration of the clinical trial, including completion of all follow-up visits related to the efficacy of the IMP in all subjects, is scheduled to last approximately 12 months.
Specific hygienic measures
ParvOryx contains the active, replication-competent virus. Therefore, environmental safety has to be considered as an important factor in the context of administering ParvOryx.
Based on the findings from the previous clinical trial, the risk of virus transmission from study patients to other persons is very low even after the planned dose escalation, if the general hygienic measures are observed. Thus, there is no need for isolation of the patients treated with ParvOryx. However, to discover and appropriately meet the very unlike case of uncontrolled viral replication after administration of ParvOryx, certain measures have been implemented. The in-patient stay is to be continued until first occurrence of H-1PV-specific antibodies in serum or until all shedding samples (feces, urine, and saliva) are tested negative for H-1PV genomes. During each follow-up visit the presence of specific antibodies will be determined. If the antibodies fall below the detection limit, the subject has to be re-admitted to the trial unit and the extent of virus shedding is to be determined. If no viral genomes are shed in any matrix, no further measures are required and the subject may be discharged. Otherwise the subject remains in-patient until re-occurrence of H-1PV-specific antibodies or until all shedding samples are tested negative for viral genomes.
The only a-priori planned safety measure related to environmental safety is that subjects should entirely avoid contact with pregnant women and infants up to 2 months after the first administration of ParvOryx.
Benefit/risk assessment
As discussed above, no satisfying therapeutic options exist for treatment of locally advanced and metastatic pancreatic cancer. The prognosis is dismal with a six-month progression-free survival of approximately 50% and a median survival of about 11 months [5].
The first open, non-randomized clinical trial with ParvOryx, referred to as ParvOryx01, evaluated safety and tolerability as well as antitumor activity and clinical efficacy in patients with progressive primary or recurrent glioblastoma multiforme (GBM). ParvOryx01 was completed in May 2015 [18]. The investigated doses ranged between 1E06 and 5E09 pfu. They were administered as combination of either multiple intravenous infusions or a single intratumoral injection and multifocal intracerebral injections at the end of tumor resection surgery. As in the current trial, the intravenous dose was divided into equal fractions which were given on consecutive days. The interval between the first administration and surgery with subsequent intracerebral injections of the drug was 10 days. In general, ParvOryx was safe and well-tolerated with only one potential serious adverse reaction observed after a combination of direct glioblastoma administration and intracerebral injection at the end of surgery. The clinical symptoms of the above reaction (mainly hydrocephalus and reduced level of consciousness) were strictly confined to the central nervous system, i.e. there was no causal link to the systemically available virus. As the potential underlying pathomechanisms remained unclear, the causal relationship to ParvOryx can neither be confirmed nor excluded. Of note, no comparable clinical events occurred in any of the other patients treated by the same route and with the same dose of ParvOryx. Since neither of the above routes of administration is used in the current study, there is no risk of similar adverse reactions.
The single intravenous dose at the initial level equals to that investigated at the highest level in ParvOryx01. Since the intravenous administrations are to be performed on four, instead of five consecutive days, the total intravenous dose is reduced to 80% of the dose investigated previously. The starting local, i.e. intrametastatic dose is more than four times lower than the highest doses injected intratumorally and intracerebrally in the foregoing trial. Since the metastatic/hepatic tissue is presumably far less susceptible to any kind of injury than the neuronal tissue of the brain, the chosen approach appears acceptable. Owing to the overall higher dose range investigated in the current study as well as to the planned co-treatment with gemcitabine, the steps of dose-escalation were chosen more conservatively than in the previous trial.
As in the foregoing trial, the treatment of a consecutive subject is only allowed if the treatment was well-tolerated in the previous subject who, as per protocol, underwent a close medical monitoring during the treatment and up to 3 weeks thereafter. This sequential schedule of enrolment minimizes the individual risks for the included patients.
Although H-1PV is non-pathogenic in man, strong preventive hygienic measures were implemented in the ParvOryx01 study [18]. Amongst others, the investigation of virus distribution and excretion belonged to the main objectives of the trial. Considering the fact that no active virus could ever be detected in any body fluid (faeces, urine, and saliva) and taking into account the rapid formation of virus-specific antibodies, the transmission of H-1PV from the trial patients is considered as highly unlikely. Thus, it is well justifiable to omit the strict isolation conditions applied previously and to rely on the general hygienic standards which are routinely applied at the trial center. In case of a surface contamination with ParvOryx, an adequate disinfection is to be carried out. Since H-1PV was shown to have some embryo- and fetotoxic effects in rodents [19, 20], the trial subjects are obliged to strictly avoid contact with pregnant women and newborn infants for the period of 2 months after beginning of the treatment with ParvOryx. This is to be considered an additional safety measure, i.e. there are currently no indications for a predisposition of pregnant women or infants for an infection with H-1PV.
The risk of trial-specific procedural complications related to the intrametastatic administration of ParvOryx, collection of tissue and blood samples is generally very low.
Taken together, the current protocol of ParvOryx02 trial is well justifiable and may be associated with individual benefits for the included patients. Moreover, the trial will yield important information required for further clinical development of ParvOryx, which may have important implications for the general population of patients with pancreatic cancer. Taken together, the individual hazards for study subjects and the environmental risks are well predictable and acceptable.
Statistical analysis
-
Analysis variables
-
Safety and tolerability will be assessed on the basis of the following parameters:
AEs and SAEs; physical examinations, vital signs, 12-lead ECGs, and chosen laboratory parameters (clinical chemistry, hematology, coagulation); viremia and virus shedding (H-1PV genomes (Vg) and active virus (H-1PV) in body fluids); virus-specific antibodies.
-
Efficacy will be assessed on the basis of the following parameters:
Investigation of the metastatic tissue: findings in general pathological examination, detection of H-1PV by FISH and qPCR, assessment of tumor infiltration with immune cells, determination of quantity and distribution of cytokines and chemokines, determination of H-1PV protein expression (NS-1).
Parameters derived from blood: determination of absolute and relative abundance of distinct immune cell subsets as determined by FACS, investigation of cellular anti-viral and anti-tumor immunity by ELISPOT.
Clinical Parameters: progression-free survival (PFS) and overall survival (OS) assessed by RECIST-criteria.
-
-
Statistical methods
-
Safety analysis: AEs will be summarized by MedDRA system organ class and preferred term. Separate tabulations will be produced for all treatment-emergent AEs, treatment-related AEs (those considered by the Investigator as at least possibly IMP-related), SAEs, and discontinuations due to AEs. Summary tables and by-patient listings will be provided for AEs, SAEs, events leading to discontinuation of treatment, and deaths. Summary tables and by-patient listings will be provided for clinical laboratory data and vital signs data, presented as both actual values and changes from baseline relative to each on-study evaluation. Details of any abnormalities will be included in patient listings.
-
Efficacy analysis: No confirmatory statistical analyses will be performed. All recorded variables (see above) will be analyzed descriptively by providing by-patient listings as well as calculating appropriate summary measures as mean, standard deviation, median, minimum and maximum or absolute and relative frequencies, respectively. If appropriate, changes from baseline relative to each on-study evaluation will be considered. For time-to-event endpoints (progression-free survival and overall survival), Kaplan-Meier estimates and summary measures of the survival function will be provided. Additionally, analyses will be performed separately for each particular dose level. The course of variables over time will be depicted for the total analysis population as well as for each subject.
-
Discussion
Among other emerging biopharmaceuticals, the clinical use of oncolytic viruses appears to be a promising treatment option for various malignancies. Currently, there is a range of mainly genetically modified oncolytic viruses at different stages of clinical development [21,22,23,24,25,26,27,28,29,30,31]. Recently, talimogene laherparepvec (T-vec) received market authorization by the U.S. Food and Drug Administration (FDA) for treatment of melanoma patients with injectable but non-resectable skin and/or lymphatic lesions [32]. In general the tolerability of oncolytic viruses after systemic and/or local administration is very good with none or only mild unspecific adverse reactions such as fatigue, chills or slight fever. There are no indications for major organ toxicities, local tissue damage or induction of adverse immune effects. The oncolytic viruses are used either as monotherapies or in combination with established chemotherapeutics and/or targeted therapies. There are strong indications for anti-tumor activity and clinical efficacy in connection with either approach. However, in most cases the optimum mode of administration, including dosing schedule and type as well as timing of concomitant treatments still needs to be specified. Interestingly, concomitant therapy with oncolytic viruses and checkpoint inhibitors seems not to influence the safety and tolerability of either treatment [33]. This is of high relevance as the combination may enhance individual anti-tumor immune responses with an improvement of clinical outcome.
Recently, ParvOryx was clinically investigated for its safety and tolerability, anti-tumor activity, immunological effects, and clinical efficacy in patients with GBM [18]. In this study the virus was administered intravenously and into the tumor or in the tumor bed directly after resection. The drug was safe and well-tolerated and showed a promising profile of anti-tumor effects and signs of clinical efficacy, i.e. prolonged survival. However, the optimum dose as well as the most appropriate route and schedule of administration have to be further investigated. The current, second trial with ParvOryx, addresses these questions. Since the total dose of the drug will be further escalated, the primary objective is to evaluate the safety and tolerability of the treatment. At the beginning of the trial, i.e. at the first level, the total dose of ParvOryx equals to the highest total dose in the previous trial. Since a relevant part of the total dose has to be administered directly into the liver metastasis, it is indicated to include one up-front patient at this level, in order to obtain first information on the local tolerability of the drug after direct injection in the liver. Considering the fact that in the foregoing study the local tolerability in the neuronal tissue was very good, no safety-related issues are expected in this context. Since the potential hazards for the consecutive patients have to be minimized as far as possible, a sequential, dose escalation design with extended intervals between enrollment of consecutive subjects and rather conservative dose escalation steps was chosen. As in the previous trial, a broad range of different investigational parameters was implemented. Apart from extended safety tests, various measurements enabling insights into the mode and extent of action of ParvOryx, including local virus availability in the tumor, triggering of changes at the tissue level and induction of virus- and PDAC-specific immune responses were included into the current protocol. Moreover, in order to account for presumable time-dependency of pharmacokinetic characteristics, virus disposition in the tumor tissue and related pharmacodynamics, varying intervals were intercalated between the intravenous administrations on the one hand and biopsies of liver metastases, thorough PK-profiles as well as local administrations of ParvOryx on the other hand.
The IMP contains an active, replication-competent parvovirus H-1PV. Although H-1PV is non-pathogenic in humans, biosafety is still considered a relevant issue in the context of administration of ParvOryx. Based on the results from the previous trial, a transmission of H-1PV from trial patients to others is highly unlikely, since general hygienic measures applicable to the handling of chemotherapeutics, consumables and nursing of patients are implemented. Owing to the pre-clinical findings showing an embryo- and fetotoxicity of H-1PV in rodents, patients’ contact with pregnant women and infants is restricted as an additional precaution.
In summary, the current trial will provide further crucial information within the clinical development program of ParvOryx. Since there were pronounced anti-tumor effects of the drug in various preclinical in-vitro and in-vivo models of pancreatic cancer, the trial will hopefully bring clinical benefits for study patients and, in consequence, for the general patient population.
Abbreviations
- (N)IMP:
-
(Non-) Investigational Medicinal Product
- (S)AE:
-
(Serious) Adverse Event
- ADA:
-
Anti-drug Antibodies
- AJCC:
-
American Joint Committee on Cancer
- ALAT:
-
Alanine aminotransferase
- aPTT:
-
activated Partial Thromboplastin Time
- ASAT:
-
Aspartate aminotransferase
- Beta-HCG:
-
Beta-human Chorionic Gonadotropin
- BSA:
-
Body surface area
- CA19–9:
-
Carbohydrate antigen 19–9
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- DSMB:
-
Data safety monitoring board
- ECG:
-
Electrocardiogram
- ECOG:
-
Eastern Cooperative Oncology Group
- ELISPOT:
-
Enzyme-linked immunospot assay
- FACS:
-
Fluorescence-activated cell sorting
- FDA:
-
US Food and Drug Administration
- FISH:
-
Fluorescence in-situ Hybridization
- GBM:
-
Glioblastoma multiforme
- H-1PV:
-
Parvovirus H-1
- HDACI(s):
-
Histone deacetylase inhibitor(s)
- INR:
-
International Normalized Ratio
- MedDRA:
-
Medical Dictionary for Regulatory Activities
- NCDB:
-
US National Cancer Database
- NS-1:
-
Non-structural Protein 1
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- Pfu:
-
Plaque forming units
- PK:
-
Pharmacokinetics
- PoC:
-
Proof-of-concept
- qPCR:
-
quantitative real-time polymerase chain reaction
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- VPA:
-
Valproic acid
References
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–403.
Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2014. Ann Oncol. 2014;25(8):1650–6.
Quaresma M, Coleman MP, Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971-2011: a population-based study. Lancet. 2015;385(9974):1206–18.
Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110(4):738–44.
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
Saif MW. U.S. Food and Drug Administration approves paclitaxel protein-bound particles (Abraxane(R)) in combination with gemcitabine as first-line treatment of patients with metastatic pancreatic cancer. JOP. 2013;14(6):686–8.
Rommelaere J, Cornelis JJ. Antineoplastic activity of parvoviruses. J Virol Methods. 1991;33(3):233–51.
Geletneky K, Herrero YC, Rommelaere J, Schlehofer JR. Oncolytic potential of rodent parvoviruses for cancer therapy in humans: a brief review. J Vet Med B Infect Dis Vet Public Health. 2005;52(7–8):327–30.
Dupressoir T, Vanacker JM, Cornelis JJ, Duponchel N, Rommelaere J. Inhibition by parvovirus H-1 of the formation of tumors in nude mice and colonies in vitro by transformed human mammary epithelial cells. Cancer Res. 1989;49(12):3203–8.
Faisst S, Schlehofer JR, zur Hausen HA. Transformation of human cells by oncogenic viruses supports permissiveness for parvovirus H-1 propagation. J Virol. 1989;63(5):2152–8.
Guetta E, Graziani Y, Tal J. Suppression of Ehrlich ascites tumors in mice by minute virus of mice. J Natl Cancer Inst. 1986;76(6):1177–80.
Nuesch JP, Rommelaere J. Tumor suppressing properties of rodent parvovirus NS1 proteins and their derivatives. Adv Exp Med Biol. 2014;818:99–124.
Angelova AL, Aprahamian M, Grekova SP, Hajri A, Leuchs B, Giese NA, et al. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H-1PV. Clin Cancer Res. 2009;15(2):511–9.
Angelova AL, Grekova SP, Heller A, Kuhlmann O, Soyka E, Giese T, et al. Complementary induction of immunogenic cell death by oncolytic parvovirus H-1PV and gemcitabine in pancreatic cancer. J Virol. 2014;88(10):5263–76.
Portal A, Pernot S, Tougeron D, Arbaud C, Bidault AT, De La Fouchardière C, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer. 2015;113(7):989–95.
Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2(3):260–9.
Geletneky K, Huesing J, Rommelaere J, Schlehofer JR, Leuchs B, Dahm M, et al. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer. 2012;12:99.
FERM VH, Kilham L. CONGENITAL ANOMALIES INDUCED IN HAMSTER EMBRYOS WITH H-1 VIRUS. Science. 1964;145:510–1.
FERM VH, Kilham L. HISTOPATHOLOGIC BASIC OF THE TERATOGENIC EFFECTS OF H-1 VIRUS ON HAMSTER EMBRYOS. J Embryol Exp Morphol. 1965;13:151–8.
Philip PA, Benedetti J, Corless CL, Wong R, O'Reilly EM, Flynn PJ, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28(22):3605–10.
Nakao A, Kasuya H, Sahin TT, Nomura N, Kanzaki A, Misawa M, et al. A phase I dose-escalation clinical trial of intraoperative direct intratumoral injection of HF10 oncolytic virus in non-resectable patients with advanced pancreatic cancer. Cancer Gene Ther. 2011;18(3):167–75.
Hamano S, Mori Y, Aoyama M, Kataoka H, Tanaka M, Ebi M, et al. Oncolytic reovirus combined with trastuzumab enhances antitumor efficacy through TRAIL signaling in human HER2-positive gastric cancer cells. Cancer Lett. 2015;356(2 Pt B):846–54.
Zeh HJ, Downs-Canner S, McCart JA, Guo ZS, Rao UN, Ramalingam L, et al. First-in-man study of western reserve strain oncolytic vaccinia virus: safety, systemic spread, and antitumor activity. Mol Ther. 2015;23(1):202–14.
Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10(5):958–66.
Eisenberg DP, Adusumilli PS, Hendershott KJ, Yu Z, Mullerad M, Chan MK, et al. 5-fluorouracil and gemcitabine potentiate the efficacy of oncolytic herpes viral gene therapy in the treatment of pancreatic cancer. J Gastrointest Surg. 2005;9(8):1068–77.
Himeno Y, Etoh T, Matsumoto T, Ohta M, Nishizono A, Kitano S. Efficacy of oncolytic reovirus against liver metastasis from pancreatic cancer in immunocompetent models. Int J Oncol. 2005;27(4):901–6.
Galanis E, Carlson SK, Foster NR, Lowe V, Quevedo F, McWilliams RR, et al. Phase I trial of a pathotropic retroviral vector expressing a cytocidal cyclin G1 construct (Rexin-G) in patients with advanced pancreatic cancer. Mol Ther. 2008;16(5):979–84.
Kaufman HL, Kim-Schulze S, Manson K, DeRaffele G, Mitcham J, Seo KS, et al. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med. 2007;5:60.
Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9(2):555–61.
Galanis E, Atherton PJ, Maurer MJ, Knutson KL, Dowdy SC, Cliby WA, et al. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res. 2015;75(1):22–30.
Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology. 2016;5(1):e1115641.
Pusonov I, Milhem M, Andtabacka R, Minor D, Hamid O, Li A, et al. Survival, safety, and response patterns in a phase 1b multicenter trial of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma. J Clin Oncol. 2016;33(15_suppl (May 20 Supplement)):Abstract 9063.
Acknowledgements
None
Funding
The ParvOryx02 trial is funded by Oryx GmbH & Co KG, Baldham, Germany. The company was involved in the discussion of the trial design and in the preparation of the current study protocol.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
JH made major contributions to the study design, edited the trial documents, interacted with regulatory bodies and drafted the manuscript, ML made contributions to the study design, reviewed the trial documents and proofread the manuscript. OK made contributions to the study design, reviewed the trial documents and proofread the manuscript. MK was responsible for the statistical concept of the trial and proofread the manuscript. KG made contributions to the study design and reviewed the manuscript. DJ made contributions to the study design and reviewed the manuscript. MD made contributions to the study design and reviewed the manuscript. BH made contributions to the study design and reviewed the manuscript. TS planned the handling of the trial medication and reviewed the manuscript. OS contributed to the implementation of radiological methods and reviewed the manuscript. AS planned and described pathological methodology and reviewed the manuscript. NH planned and described method of immunological tissue investigations and reviewed the manuscript. VD planned and described immunological investigations and reviewed the manuscript. BL planned and described methods of virus determinations in blood and tissue, provided contributions to the trial documents and reviewed the manuscript. AA planned and described methods of virus determinations in tissue, provided contributions to the trial documents and reviewed the manuscript. JR planned and described methods of virus determinations in blood and tissue, provided contributions to the trial documents and reviewed the manuscript. CEE made contributions to the study design, reviewed the trial documents and proofread the manuscript. CS made major contributions to the study design and drafted the manuscript. GU made major contributions to the study design and drafted the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The reported trial, including all written information given to patients, has been approved by the Ethics Committee of the Medical Faculty Heidelberg prior to the inclusion of the first subject. The written consent for study participation has to be obtained in written form from each subject prior to the first study-specific procedure.
Date of approval: September 29, 2015.
Ethics Committee’s reference ID: AFmo-207/2015.
Consent for publication
Not applicable.
Competing interests
The ParvOryx02 trial is funded by Oryx GmbH & Co KG, Baldham, Germany.
JH and ML declare no competing interests. OK receives salary from Oryx GmbH & Co KG. MK declares no competing interests. KG holds patents related to H-1PV. DJ declares no competing interests. MD and BH receive salary from Oryx GmbH & Co KG. TS, OS, AS, NH, VD, BL and AA declare no competing interests. JR holds patents related to H-1PV. CE, CS and GU declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hajda, J., Lehmann, M., Krebs, O. et al. A non-controlled, single arm, open label, phase II study of intravenous and intratumoral administration of ParvOryx in patients with metastatic, inoperable pancreatic cancer: ParvOryx02 protocol. BMC Cancer 17, 576 (2017). https://doi.org/10.1186/s12885-017-3604-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-017-3604-y