Abstract
Small cell lung cancer (SCLC) is a recalcitrant malignancy with elusive mechanism of pathogenesis and dismal prognosis. Over the past decades, platinum-based chemotherapy has been the backbone treatment for SCLC. However, subsequent chemoresistance after initial effectiveness urges researchers to explore novel therapeutic targets of SCLC. Recent years have witnessed significant improvements in targeted therapy in SCLC. New molecular candidates such as Ataxia telangiectasia and RAD3-related protein (ATR), WEE1, checkpoint kinase 1 (CHK1) and poly-ADP-ribose polymerase (PARP) have shown promising therapeutic utility in SCLC. While immune checkpoint inhibitor (ICI) has emerged as an indispensable treatment modality for SCLC, approaches to boost efficacy and reduce toxicity as well as selection of reliable biomarkers for ICI in SCLC have remained elusive and warrants our further investigation. Given the increasing importance of precision medicine in SCLC, optimal subtyping of SCLC using multi-omics have gradually applied into clinical practice, which may identify more drug targets and better tailor treatment strategies to each individual patient. The present review summarizes recent progress and future directions in SCLC. In addition to the emerging new therapeutics, we also focus on the establishment of predictive model for early detection of SCLC. More importantly, we also propose a multi-dimensional model in the prognosis of SCLC to ultimately attain the goal of accurate treatment of SCLC.
Similar content being viewed by others
Introduction
Characterized by rapid growth and high recurrence, small cell lung cancer (SCLC) is a devastating malignancy with elusive mechanism of pathogenesis [1]. Platinum-based chemotherapy and etoposide has remained the main treatment paradigm for SCLC. However, drug resistance and relapse would ultimately occur for most patients and the outcomes are heterogeneous [2, 3]. In contrast to non-small cell lung cancer (NSCLC) in which many driving mutations have been detected, druggable alterations are rare in SCLC [4, 5]. Disappointedly, many clinical trials of molecularly targeted therapies have yielded negative results [6,7,8]. The frustrations have been observed in receptor tyrosine kinase inhibitors in the clinical setting of SCLC, which might be attributed to the limited efficiency as ascribed to mono-targeting as well as the sophisticated genetic landscape of SCLC. Our deepened understanding into the mechanism of pathogenesis underlying SCLC have reignited the appeal for development of novel drugs in treating this therapeutically challenging disease. Encouragingly, DNA damage and cell cycle inhibitors have shown promising activity in in-vivo and in-vitro models and SCLC patients [9,10,11]. However, mere adoption of these inhibitors is insufficient to achieve optimized response. Optimized efficacy and promising results could only be detected in the combination treatment with other treatments in SCLC. Notably, the clinical management of SCLC is rapidly developing with the advent of immune checkpoint inhibitors (ICIs). However, several issues need to be addressed in patients with SCLC using ICI, which entails appropriate timing for usage, identification of biomarkers predicting prognosis and selection of appropriate patients for ICI, as well as the combinational treatment modality. Additionally, SCLC has entered the precision era, with subtyping of patients according to multi-omics [12,13,14,15]. Optimization of therapeutic strategies proper for each subtyping remain the priority that need to be dealt in the personalized treatment of SCLC. In summary, an in-depth understanding of the genetic, transcriptional, proteomic and metabolic profiling of SCLC may help better facilitate drug development and tailor treatment strategies to each individual patient. Since early diagnosis of SCLC may contribute to the improvement of prognosis, we therefore envision a roadmap to improve early diagnosis of SCLC. Lastly, the prospect of establishing a multi-dimensional model in the prediction of SCLC prognosis is also unraveled. In summary, in the current review, we elucidate the biology of SCLC against its multi-omics and immunological background. Particularly, we have highlighted the challenges existing in SCLC treatment against the background of multiple treatment options (Fig. 1). Ultimately, an outlook into future perspective of SCLC has been depicted.
Dilemma that are faced with SCLC currently. The dilemma could be categorized into several classifications: (1) Chemoresistance in SCLC, (2) SCLC heterogeneity, (3) Unclear mechanism of pathogenesis, (4) Little progress in targeted therapy, (5) Metastasis upon initial diagnosis, (6) Lack of precision treatment, (7) Limited ICI efficacy, (8) Few therapeutic targets in SCLC
Chemoresistance in SCLC
Platinum-based chemotherapy represents the standard treatment regimen as the first-line treatment of SCLC. Despite high response rate upon initial treatment, patients would succumb to SCLC, with almost all SCLC patients ultimately developing drug resistance [16,17,18]. To date, topotecan monotherapy has been recommended as second-line options in SCLC, whose efficacy heavily rests with the duration of response to the frontline platinum-based chemotherapy [19, 20]. Notably, the duration of a treatment-free interval (TFI) has been recognized as a caliber to identify patients proper for subsequent chemotherapies: those with a TFI shorter than 2 months are most likely to be refractory to salvage second-line chemotherapy with worse outcome. For patients undergoing relapse with more than 2 months after the first-line treatment are considered sensitive relapse. These patients tend to be responsive to subsequent chemotherapies [21] (Fig. 2A). Irinotecan monotherapy serves as an alternative in this scenario, whereas sufficient data lacks on the outcome of irinotecan. Concerning the underlying mechanisms of resistance to chemotherapy, it is proposed that the several factors may contribute to chemoresistance in SCLC, as demonstrated in Fig. 2B. The most important factor is DNA damage responses, which can potentially lead to resistance by the disruption of cell cycle after exposure to therapeutic agents, thereby promoting repair of lesions induced by drugs and shielding tumor cells from death [9, 22]. Gardner has found that in some of the SCLC cases, EZH2 mediates resistance by downregulating SLFN11 [23]. Moreover, Anish Thomas showed that inhibition of ATR sensitizes platinum-resistant SCLCs to topotecan [24]. Multidrug resistance (MDR) is another important element accounting for the incapability of chemotherapy in SCLC. MDR modulated by P-gp (MDR1) and MDR-associated proteins (MRP1 and MRP2) are common indicators of chemoresistance. Studies have revealed that patients harboring MDR gene expression are linked with worsened prognosis [25,26,27]. Yeh demonstrated fortified chemoresistance in SCLC specimens with upregulated expression of P-gp and MRP1 [28]. Cancer stem cells, a cluster of cells with highly tumorigenic and chemo-resistant properties, would survive chemotherapy and lead to tumor recurrence [29, 30]. Studies have found an increase in CD133-positive cells in tumors upon chemotherapy [31, 32]. In addition, Stewart has shown that chemoresistance in SCLC is featured by the coexistence of clusters of cells with heterogeneous gene expression that would lead to multiple, concurrent resistance mechanisms. They therefore suggest rational combination therapies for treatment-naïve SCLC tumors to optimize initial responses and counteract the heterogeneity and various resistance mechanisms [2]. Altered metabolism is also found to be associated with chemoresistance in SCLC [33]. Comparison between chemo-resistant human SCLC cell lines and their parental cells indicated metabolically disordered amino acids in the chemo-resistant specimens. Chemo-resistant cells exhibited reduced viability when they are deprived of arginine in the cell media, in comparison with their parental lines that are non-chemoresistant [34].
Chemoresistance in SCLC. A the duration of a treatment-free interval (TFI) has been recognized as a caliber to identify patients proper for subsequent chemotherapies: those with a TFI shorter than 2 months are most likely to be refractory to salvage second-line chemotherapy with worse outcome. For patients undergoing relapse with more than 2 months after the first-line treatment are considered sensitive relapse, who tend to be responsive to subsequent chemotherapies. B Factors that may contribute to chemoresistance in SCLC, which include MDR proteins, DNA repair, heterogeneity, aberrant metabolism and cancer stem cells
Elusive and complicated mechanism of SCLC pathogenesis
The cell origin of SCLC
Most SCLC have been reported to express several neuroendocrine markers featured by synaptophysin and chromogranin A, as well as transcription factors that are vitally essential in neuroendocrine differentiation. It is therefore postulated that pulmonary neuroendocrine (NE) cells serve as the progenitors of SCLC [35,36,37]. In addition, SCLC could also arise from type 2 alveolar cells (AT2) cells and club cells [38] (Fig. 3). Further studies have identified both NE and non-NE transcriptional subtypes of SCLC, as tested in cell lines, human specimens, and genetically engineered mouse models (GEMMs) [39, 40]. A transition from NE to non-NE which is stimulated by plasticity, often predicts worsened prognosis [41, 42]. There is a high possibility that SCLC could also be originated from the transformation of normal stem cells considering the similar signaling pathways that modulate both stem cells and cancer cells [43,44,45].
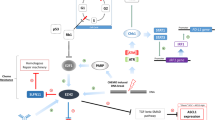
Elusive and complicated mechanism of SCLC pathogenesis. Frequently aberrant genes, dysregulated developmental pathways, receptor tyrosine kinase pathways and DNA repair pathways have been detected in SCLC. Targets associated with these pathways, such as IGF-1R, c-MET, VEGF, DLL3, TROP-2, CD-56, ATR, WEE1, CHK1, PARP1 have been proposed in SCLC. Other possible targets associated with metabolism have been also suggested
Elusive mechanism
Due to the unobvious symptoms at early stages and the amazingly rapid growth rate of SCLC, most SCLC patients have been diagnosed at advanced stages [46, 47]. Therefore, they are usually subject to cytological and tissue biopsy rather than undergoing surgical resection, thereby resulting in the insufficiency of available tumor samples thus inadequate studies on the mechanism of SCLC pathogenesis. To address this tricky issue, a variety of study models have been established such as SCLC cell lines, GEMMs, patient derived in vivo models [48,49,50]. Through these study models, we have more access to gain a better understanding of SCLC.
Genomic aberrations or altered gene expression in SCLC
Multiple genetic alterations have been found in patients with SCLC, which could be attributed to the carcinogens induced by tobacco exposure [21, 51]. These mutations could be manifested in multiple forms such as insertions, deletions, mutations, chromosomal rearrangements, and copy number alterations [52, 53]. Notably, TP53 and RB1 mutations were found in most patients with SCLC, with an approximately rate of 80–90% and 60–90%. TP73 is also frequently altered in the SCLC genome, accounting for about 13%. PTEN mutations are found to be in 4–9% of SCLC. Besides, NOTCH1 plays a tumor-suppressive role, with 25% most widely mutated in SCLC. Overexpression in NOTCH1 inhibits SCLC growth and neuroendocrine features. Other frequently mutated genes include RBL1, RBL2, CDKN2A, CCND1 and Bcl-2 [54,55,56,57,58] (Fig. 3).
Developmental pathways
Developmental signaling pathways are vital for the function of stem and progenitor cells, the dysregulation of which may lead to tumor initiation and progression. Thus, targeting these pathways have been viewed as means to inhibit tumor progression by regulation of stem cells [59, 60]. The most common developmental signaling pathway are Hedgehog, Notch and WNT pathways, as demonstrated in Fig. 3. The Hedgehog (Hh) pathway has played a pivotal role in tumorigenesis, angiogenesis and cellular differentiation. It can modulate lung embryogenesis and is instrumental in SCLC maintenance [61, 62]. Mikko has shed light on the function of Hh signaling activation in SCLC by revealing a direct interaction between Hh and BN/GRPR pathway [63]. Genomic studies on SCLC samples revealed Notch pathway could modulate neuroendocrine gene expression in SCLC. Non-neuroendocrine cells and precursors could be induced into neuroendocrine differentiation via NOTCH activation, as tested in Meder’s study [64]. Additionally, Wagner proved that samples of patients with SCLC exhibited recurrent mutations of WNT signaling, and suggested activation of WNT pathway may contribute to chemoresistance in relapsed SCLC [65].
Receptor tyrosine kinase pathways
Receptor tyrosine kinase (RTK) pathways act as a promising approach to explore novel therapeutic vulnerabilities for SCLC, since amounting evidences proved that polypeptide growth factors are vital players in SCLC cell proliferation [66, 67]. The insulin-like growth factor-I receptor (IGF-IR), c-Kit, and vascular endothelial growth factor receptor (VEGFR) have brought possibilities as potential drug therapeutic vulnerabilities in SCLC [68,69,70,71], as depicted in Fig. 3. Moreover, phosphoinositide 3-kinase (PI3K)/Akt and the mammalian target of rapamycin (mTOR), the downstream signaling of RTK pathway, could also be served as favorable candidates for SCLC [72, 73]. The function of c-Met/HGF pathway in SCLC has been proven in H69 SCLC cell line, as tested in Gautam’s study [74]. In another study led by Jagadeeswaran, it was proven that HGF/c-Met overactivation results in the reactive oxygen species production and SCLC motility [75].
The IGF-IR pathway plays a prominent role in the growth of SCLC [68, 76]. SCLC cell lines boost increased expressions of IGF-IR. Besides, the IGF-I/IGF-IR pathway acts as an autocrine loop stimulating growth in these SCLC cell lines. Additionally, SCLC patients were found to exhibit higher IGF-I levels than normal controls and the expression of IGF-I is correlated with high-risk for SCLC [77, 78]. It has been found that growth of SCLC could be achieved by the stimulation of IGF-IR through PI3K-Akt pathway [79]. Additionally, the interaction of IGF-IR with RAS/RAF/MEK/ERK cascade may exert the tumor-promoting role [80]. In summary, IGF-IR could be a possible therapeutic vulnerability in SCLC and inhibition of IGF-IR could serve as an approach to inhibit SCLC growth [81, 82].
Dysregulation of MET pathway contributes to development and progression in a variety of tumors [83, 84]. Maulik has demonstrated the involvement of c-Met/HGF pathway in SCLC via H69 cell line. They further tested the role of PI3K in the c-Met/HGF pathway [85]. SCLC has demonstrated different forms of c-Met mutations. These new gain-of-function mutations in this receptor increased cell motility and migration of SCLC cells and may be associated with a more aggressive phenotype. There are multiple approaches in the inhibition of dysregulated c-MET/HGF pathway [86, 87]. HGF, c-MET receptor inhibitors might be of clinical utility. For instance, geldanamycin, an antibiotic with many effects on tumor cells, has been shown to disrupt the c-Met/HGF axis, reduced growth and caused apoptosis in SCLC cells [74, 85].
DNA repair pathways
The high genomic aberrations rate in SCLC contributes to DNA damage accumulation and genomic instability [88, 89]. Recent years have witnessed many novel drug targets via preclinical models and patient tissues. Several molecules in DNA damage response (DDR) pathway have been identified as drug targets, including poly-ADP-ribose polymerase (PARP), checkpoint kinase 1 (CHK1), ataxia telangiectasia and Rad3-related protein (ATR), and WEE1 [90,91,92,93], as depicted in Fig. 3. In addition to the observation that these targets are elevated in SCLC, investigators also revealed the preclinical utility of inhibitors against these targets, suggesting their translational implications. Up to date, a series of DDR inhibitors have been developed or are under clinical investigation for SCLC. PARP inhibitors subject to clinical trials in SCLC include Olaparib, veliparib, talazoparib and sacituzumab Govitecan [94,95,96]. ATR is another DDR protein that is involved in the development of SCLC. In circumstances of DNA damage and genotoxic stress, ATR/CHK1 overactivation was observed [11]. Nagel demonstrated that VE-822-induced ATR inhibition combined with cisplatin also outperforms the treatment of cisplatin with etoposide in vivo [97]. Notably, NCT02487095 on ATR inhibitors among SCLC patients has been conducted [98]. ATR and CHK1 inhibitors have been developed and their combined treatments with either radiotherapy or chemotherapies in preclinical studies [99,100,101]. WEE1 exerts a vital role in modulating cell cycle and DNA damage in both normal and tumor cells [102, 103]. Thus, inhibition of WEE1 holds promise as an anti-cancer therapy in SCLC. Currently, AZD1775, the WEE1 inhibitor, has demonstrated clinical utility in a cohort of SCLC patients. It is proven that the combination of olaparib/AZD1775 is helpful in reversing disease relapse. However, the efficacy of these drugs has been detected in a fraction of SCLC patients [104]. Thus, it is important to explore underlying biomarkers that may accelerate the identification of proper SCLC patients benefiting from DDR inhibitors.
Other possible targets in SCLC
In addition to pathways associated DNA damage repair and cell cycle, inhibition of molecules in apoptotic pathway could also be an anti-cancer approach in SCLC [105]. The high levels of BCL-2, a regulator of apoptosis, indicates their possible therapeutic values in SCLC [106], as demonstrated in Fig. 3. In Choi’s study, they proved that YPN-005, a powerful cyclin-dependent kinase 7 (CDK7) inhibitor, has anticancer effects in SCLC. They further concluded that targeting CDK7 by YPN-005 in SCLC is an appealing treatment strategy for SCLC resistant to conventional therapy [107]. Metabolic reprogramming is intimately linked with tumor progression and metabolic liabilities can be exploited as therapeutic vulnerabilities [108]. For instance, Cristea has revealed the regulatory role of MEK5-ERK5 in lipid metabolism and in the promotion of SCLC growth [109]. Huang’s study demonstrated the dependence of IMPDH as a targetable vulnerability in chemoresistant MYChi SCLC [110]. Besides, Li demonstrated the pharmacological inhibition of dihydroorotate dehydrogenase (DHODH) destroyed SCLC cells in vitro and inhibited SCLC tumor growth in human patient-derived xenograft (PDX) models, indicating the potential role of DHODH in treating SCLC [111].
Vascular endothelial growth factor (VEGF) is well established as a major modulator in contributing to tumor angiogenesis [112, 113]. The amount of VEGF in the serum of patients with SCLC was reported to be associated with chemoresistance and thus inferior prognosis [114,115,116,117]. This made the VEGF pathway as an intriguing therapeutic for patients with SCLC. Clinical trials of anti-VEGF drugs have been tested in patients with SCLC. However, the results of clinical trials evaluating antiangiogenic drugs such as bevacizumab and sorafenib have been unsatisfactory with no OS benefit. For instance, the addition of bevacizumab to standard chemotherapy of cisplatin and etoposide benefited progression free survival (PFS) but did not prolong overall survival (OS). Significant toxicity and low efficacy were observed in the combined treatment of sorafenib with chemotherapy in a phase II trial [6, 118].
Due to the overexpression of Delta-like canonical Notch ligand 3 (DLL3) in SCLC, it is therefore considered a promising therapeutic vulnerability in SCLC [119, 120]. In Tanaka’s study where a total of 63 patients with SCLC were immunohistochemically stained for DLL3, DLL3-positive tumors account for 83%, and DLL3-high tumors accounts for 32% [121]. The comparison of DLL3 expression between tumors and non-tumor tissues has ushered the adoption of regimen that utilize DLL3 to specifically target SCLC cells. There have been several clinical trials being undertaken concerning drugs that use DLL3 in SCLC. Both the safety and efficacy of Rova-T, an antibody-drug conjugate targeting DLL3, has been tested in recurrent SCLC. In a phase 1 clinical trial (NCT01901653), Rova-T presented favorable antitumor property and controllable safety profile [122]. However, phase II TRINITY study demonstrated minimal clinical utility in the third-line and beyond setting of SCLC with associated toxicities [123]. Additionally, the Phase 3 TAHOE study comparing the efficacy and safety of Rova-T with topotecan as second-line therapy among SCLC patients with DLL3 high, demonstrated that an inferior OS and higher toxicity rate [124]. The fact that these Rova-T trials have ended up in vain impels us to ponder the validity and reliability of DLL3 as a therapeutic vulnerability in SCLC. Considering the toxicity profile and high incidence of treatment discontinuation rates of Rova-T, further attempts to target DLL3 as the potential therapeutic targeting of SCLC are needed.
Trophoblast cell-surface antigen 2 (TROP-2), a surface marker presented on trophoblasts, has been demonstrated to be upregulated on SCLC. In a phase II study, Gray tested Sacituzumab govitecan, an antibody against TROP-2, in a total of 50 patients with SCLC without any prior treatment. Hematologic toxicities such as neutrophilic granulocytopenia, anemia and diarrhea, fatigue have been observed. In this pretreated sub-cohort, an OS of 7.5 months was observed encouragingly. Interestingly, those patients with prior topoisomerase I-inhibiting treatment with topotecan or irinotecan, responded well to govitecan, with an OS of 8.8 months [125]. However, its adoption as first-line treatment is needed and requires further investigation.
Due to the common expression of CD56 in SCLC, an antibody-drug conjugate, lorvotuzumab mertansine (IMGN901), was developed and tested in both preclinical and clinical settings [126, 127]. The addition of IMGN901 to carboplatin/etoposide did not improve efficacy over standard carboplatin/etoposide therapy in SCLC patients at extensive stages and demonstrated enhanced toxicity such as serious infections with fatal outcomes [128]. Crossland evaluated CD56-specific ‘chimeric antigen receptor T (CAR T) cells’ in in vivo SCLC models, which can destroy CD56-positive SCLC tumor cells in vitro. Besides, CD56R-CAR + T cells were able to inhibit tumor growth in vivo, indicating the role of CD56-CARs as a potential treatment for SCLC [129].
Long non-coding RNA (lncRNA) in SCLC
The vital role of lncRNAs in the tumorigenesis of SCLC cannot be neglected. Several studies have demonstrated the modulatory role of lncRNAs in regulating cell proliferation and metastasis of SCLC. They exert their functions by sponging miRNAs to modulate target genes or binding to specific proteins, thus modulating molecules and signaling pathways associated with tumor progression and metastasis. For instance, Zeng has proven that linc00173 promoted SCLC proliferation and migration by acting as a competing endogenous RNAs (ceRNA) for miR-218 [130]. Besides, In Sun’s study, they identified that HOTTIP was associated with SCLC tumorigenesis via the ceRNA network “HOTTIP/miR-574-5p/EZH1” [131].Therefore, there is a huge potential of lncRNA in the identification of SCLC mechanisms.
Challenges of ICI in SCLC
Historical timeline of ICI in the field of SCLC
In recent decade, we have witnessed a shift in our notion on the treatment of SCLC, with the advent of immune checkpoint inhibitor that regulate the immune system. The unprecedented results have been observed in several clinical trials undertaken in SCLC, as depicted in Fig. 4A. In IMpower 133, the addition of atezolizumab, an anti-PD-L1 antibody, to carboplatin with etoposide (CE) significantly ameliorated overall survival (OS) as compared to the CE with placebo [132]. In the CheckMate032, both the function and safety of nivolumab in combination with ipilimumab in relapsed SCLC was assessed. Results showed no difference in survival improvement between nivolumab versus nivolumab plus ipilimumab [133]. In the CASPIAN study, durvalumab, another anti-PD-L1 antibody, also led to an improvement in OS [134]. The CAPSTONE-1 study, a randomized, double-blind, phase 3 trial, conducted in China, made a comparison between the efficacy and safety of adebrelimab (SHR-1316), a novel anti-PD-L1 antibody and standard chemotherapy as a first-line therapy for patients with SCLC at extensive stage. Results have shown that adebrelimab significantly improved OS and showed bearable toxicities in patients with extensive stage SCLC, confirming the addition of adebrelimab to previous chemotherapy as a new therapeutic paradigm [135]. As the KEYNOTE-028 and KEYNOTE-158 studies demonstrate, pembrolizumab demonstrated lasting therapeutic effect in a subset of patients with recurrent or metastatic SCLC in the third-line or later-line setting, without the influence of PD-L1 expression [136, 137]. In the CheckMate 451 study, OS was not greatly improved by the combined treatment of nivolumab with ipimumab as maintenance therapy as in comparison with the placebo [138]. CheckMate 331 demonstrated that nivolumab did not prolong survival versus chemotherapy in relapsed SCLC [139]. In the Keynote604 study, OS was numerically elevated by the addition of pembrolizumab to the regimen of chemotherapy whereas without statistically significant difference [140].
Challenges of ICI in SCLC. A Historical timeline of ICI in the field of SCLC. The unprecedented results have been observed in several clinical trials undertaken in SCLC. B Factors affecting ICI response in SCLC. Tumor-intrinsic factors: (1) Neoantigen depletion, (2) Defects in antigen presentation, (3) Genetic factors, (4) Aberration of IFN signaling; Tumor microenvironment: Tumor associated macrophages, MDSC, metabolites, T cells; Host: Metabolite, nutrients, gut microbiota. C Major issues facing ICI treatment. The ICI challenges include four aspects: (1) Timing, (2) Biomarker response, (3) Combinational treatment, (4) Toxicity
Factors affecting ICI response in SCLC
Our insight into the ICI resistance mechanisms is gradually advancing due to the deepening understanding of the reciprocal interplay between the intrinsic tumor, the tumor microenvironment and the host [141,142,143], as demonstrated in Fig. 4B.
Tumor-intrinsic factors
Neoantigen depletion are crucial determinants for ICI efficacy
Neoantigens at high qualities are crucial determinants for ICI efficacy, and impairment of their expressions would lead to acquired resistance [144]. For the surveillance of immune elimination, immune attack would be escaped by cancer cells due to either HLA loss of heterozygosity (LOH) or impaired expression of neoantigen. It should be realized that, for patients exhibiting potent immune infiltration and without HLA loss, they tend to be presented with more violent defects in neoantigen [145].
Defects in antigen presentation affects response to ICI
After its binding with MHC-I molecule and its expression on the surface of tumor cells, tumor antigens can then be recognized by the cytotoxic T lymphocyte. The sound antigen presentation rests on the normal operations of a cohort of molecules inclusing HLA-I and beta-2- microglobulin (B2M). Under the pressure of immune infiltration, cancer cells can evade immune attacks by virtue of antigen-presenting genes abnormalities [146, 147].
Genetic factors are associated with ICI resistance
The genetic landscape of tumor serves as primary factors affecting response to ICIs. As tumor develops, tumor cells undergo genetic mutations which results in the generation of mutated peptides. These newly generated peptides serve as neoantigens as distinct from self-antigens. The emergence of neoantigens and abnormal self-antigens within the tumor can draw T cells that would clear off tumor cells and further augment ICI-elicited anti-tumor immune responses [148, 149].
Aberration of interferon (IFN) signaling diminishes ICI efficacy
Interferon (IFN) signaling plays a prominent role in ICI treatment via a variety of means such as elevation of MHC-I levels, uplifting of PD-L1 expression and destroy of tumor cells. The biallelic JAK1/2 loss-of-function mutation results in the weakened PD-L1 expression induced by IFN signaling, leading to resistance to ICI [150, 151]. Additionally, the overactivation of IFN can also contribute to ICI resistance via several inhibitory pathways, such as the arise in IDO and other immune checkpoint ligands [152].
Tumor microenvironment
T cell receptor repertoire clonality was reported to be associated with response to PD-1 inhibition [153]. Besides, B cells and tertiary lymphoid structures also act as important elements linked with ICI responses, as shown in recent studies [154, 155]. Stromal cells including tumor associated macrophages, endothelial cells, myeloid-derived suppressor cells, fibroblasts, forms the tumor microenvironment. Their existences are essential for tumor angiogenesis, invasion into extracellular matrix (ECM). Amounting evidences suggest that these stromal cells are involved in the immune evasion and resistance to ICI [142, 156].
Host
More recently, the role of intratumoral microbes has been shown to significantly affect the responses to ICI [157, 158]. The microbes within tumors can also shape the tumor immune microenvironment. Association could be found between tumor associated microbes and immune cell immersion. Disparities in the component of the tumor microbiota have been found between responders and non-responders among a cohort of melanoma patients subject to immunotherapy [159, 160]. Other host-associated factors influencing immune responses including nutrient and metabolite, which are also intimately linked with gut microbiota within the host [161, 162]. Considering the complicated factors affecting ICI resistance, multiple measures have been adopted to overcome ICI resistance and improve ICI sensitivity. Disappointedly, trials addressing ICI resistance have been attempted and demonstrated to be in vain.
Major issues facing ICI treatment
Despite great strides made in the adoption of ICI in SCLC, major challenges still exist [5, 163]. We assume that the following major challenges should be overcome to move the field of ICI application in SCLC forward (Fig. 4C). One issue is the timing for the adoption of ICI in SCLC should be explored further. Another issue involves the exploration of reliable novel biomarkers other than PD-1/PD-L1, TMB/bTMB and TIL signature, which may contribute to a more personalized treatment of SCLC. The third one is the potentiation of ICI effects with the combination treatment modality. It is expected that combinational treatment of ICI with chemotherapy, radiotherapy, targeted therapy, anti-angiogenic therapy would enhance efficacy [164,165,166]. Lastly, the toxicities incurred by either the combinational treatment or the immunotherapy should not be neglected, which could possibly generate unbearable toxicities to patients with SCLC [167, 168]. Besides, immunotherapy may also engender autoimmune dysfunctions in patients with SCLC, demonstrated as paraneoplastic syndromes [169]. In this clinical setting, the balance between immune-associated toxicities and treatment response should be scaled. The maximization of therapeutic effects remains a challenging task for the combinational treatments, which should take factors including drug dosage, adopted timing and order into account. However, the selection of proper combinational treatments and exploration of biomarkers predicting treatment responses remains a knotty issue [170]. Due to the heterogeneous nature of SCLC, liquid biopsy could serve as an approach to monitor the tumor microenvironment of SCLC in a real-time manner [171]. The specific drugs of a combinational treatment could be determined by the immunological profiling obtained from liquid biopsies. Moreover, a comprehensive architecture encompassing genome, transcriptome, immune profiling, microbiome can be referred to select proper patients from different combinational regimens.
Novel immunotherapies in SCLC
Immunotherapies represent a major advance in the management of SCLC. Several novel targets, which have been discovered due to our deepening understanding of the immunological milieu of SCLC, include TIM-3, TIGIT and LAG3 [172,173,174], as demonstrated in Fig. 5A. Sun’s study has revealed the expression of LAG3 in SCLC tumor tissues. LAG3 expression was markedly correlated with PD-1 and PD-L1 expression. In addition, OS was significantly improved in LAG3-high patients with SCLC. Significantly, LAG3 expression was linked with immune-associated biological processes including immune response, antigen presentation, and T cell co-stimulation. They demonstrated that LAG3 is a vital immune checkpoint closely related with PD-1/PD-L1[174]. Therefore, LAG3 holds as an appealing novel immunological target for SCLC. Besides, targeting other signaling pathways, such as DNA damage repair; and co-targeting SCLC-specific tumor antigens, such as fucosyl-GM1 and DLL3 are also favorable options [175, 176].
T cells with a chimeric antigen receptor (CAR) has been viewed as an appealing approach for getting rid of tumor cells [177,178,179]. CAR T cell therapy, a form of adoptive T cell therapy that utilizes a patient’s own T cells and maneuvers them to express CARs sensibly, target cancer cells. CARs involve two parts: an intracellular T cell activation domain and an extracellular antigen-recognition domain. These two domains are bound together by a transmembrane domain connected to a hinge [180]. Reppel L demonstrated that GD2 is a promising target for CAR-T cell therapy in lung cancer. Tazemetostat could be used to uplift GD2 expression in tumor cells, and boost their susceptibility to CAR-T cell targeting [181]. In addition to CAR T cell therapy, CAR-NK cell therapy has also captured attention as a potential immunotherapeutic strategy, also demonstrated in Fig. 5B. In many types of cancer, NK cells destroy tumor cells and its infiltration indicates favorable prognosis. A merit of CAR-NK cell therapy is its capacity to be delivered to a patient with HLA mismatch, thus rendering off-the-shelf therapy that is readily available. However, the energy and expanse of NK cell expansion and manufacturing is a hurdle for CAR-NK cell treatment [182].
Personalized treatment of SCLC
In the past decade, we have gained an in-depth understanding of the molecular biology of SCLC with the advent of personalized medicine. Once viewed as a homogeneous malignancy and a single entity, SCLC has been currently classified into different molecular subtypes [50, 183]. Genomics has been the core focus in the achievement of precision medicine. George first performed a comprehensive study of somatic genomic aberrations in SCLC and revealed possible targets in SCLC [51]. As we have elaborated in our previous section, inactivation of TP53 and RB1, aberrations in TP73, RBL1/2, CDKN2A, CCND1, Bcl-2, as well as proteins in developmental pathways, receptor tyrosine kinases and their downstream effectors have been revealed as therapeutic vulnerabilities. Additionally, inter and intratumoral heterogeneity in SCLC has been detected and associated with dismal clinical outcomes [184, 185]. To deal with the heterogeneity, Rudin collected and analyzed data from a variety of models including cell lines, and patient-derived xenografts and patient samples to propose a SCLC classification dependent on the expression of dominant transcriptional factors necessary for NE and non-NE differentiation process [186, 187]. The expression of specific transcription factors provides a benchmark to differentiate different SCLC subtypes. Those with elevated ASCL1 levels is defined as SCLC-A and those with high levels of NEUROD1 is defined as SCLC-N. SCLC-P and SCLC-Y are featured by high expression of POU2F3 and YAP1[50]. More recently, Gay identified four SCLC subtypes based on the differentiated expression of transcription factors ASCL1, NEUROD1, and POU2F3 or the low expression of all three transcription factor signatures, accompanied by SCLC-I, which is an inflamed gene signature. YAP1 expression and its transcriptional targets showed higher expression in the SCLC-I group. SCLC-I was shown to be infiltrated with the most CD8 + T cells, suggesting it benefit most from ICI [12]. In addition to genomics and transcriptomics, there is also a need to integrate other types of “omics” including proteomics, immunomics, and metabolomics to provide a holistic landscape of SCLC. Since major breakthroughs have been made in the immunotherapy of SCLC, the exploration of immunological profiles in patients with SCLC would help to identify patients appropriate for immunotherapies. And novel immunotherapies are currently being explored in SCLC, such as oncolytic viruses, vaccine platforms, and adoptive cell therapy [188, 189]. The incorporation of these “omics” indicators into clinical setting would warrant an optimized procedure to integrate all these data into clinic records to achieve a more efficacious, convenient and personal-oriented clinical testing. Additionally, in a bid to lead a personalized paradigm in SCLC, exploration and screening for therapeutic vulnerabilities has also become a vitally important step in moving the precision treatment in SCLC ahead. Biomarkers-guided precision treatment has revolutionized the clinical development and administration of molecular-targeted drugs. Tailored anti-tumor drugs showed better response rate in comparison with unselected treatment [190]. The final aim of personalized treatment is patient-oriented rather than drug-centered trial according to a cohort of reliable biomarkers available. In “N-of-1” trials, drug combinations are tailored to each patient’ based on genetic, transcriptional, proteomic and metabolic features. Interestingly, associating patients with drugs according to genomics has proven to be more efficient in ameliorating survival than associating them with proteomic features. Despite the current drawbacks, protein assays may offer data in combination with genomic information. Recently, panels that incorporate immunological information are also of huge clinical utilities. Determination of efficacy in “N-of-1” trials need to evaluate the practice of allocating patients to drugs instead of treatments, which differs between each individual patient. Longitudinal follow-up might be helpful in overcoming the challenges as we obtain escalating understanding of the biology of SCLC. Molecular profiling of SCLC should be adopted upon diagnosis and in the course of SCLC, either from tumor tissues or from blood, as a means to monitor response and resistance [191, 192]. In summary, the combination of data from previous history (smoking status, history of previous treatments and responses), multi-omics profiling as well as longitudinal follow-up to make therapeutic strategies would contribute to a more personalized treatment paradigm of SCLC in general. The workflow of personalized treatment of SCLC has been demonstrated in Fig. 6.
Personalized treatment of SCLC. The combination of data from previous history (smoking status, history of previous treatments and responses), multi-omics profiling as well as longitudinal follow-up to make therapeutic strategies would contribute to a more personalized treatment paradigm of SCLC in general
Roadmap to improve early diagnosis of SCLC
The identification of subpopulation at high risk for SCLC is important as it would improve diagnosis. Fortified surveillance and appropriate interventions should be carried out for patients screened at high risk. Risk assessment platforms have been developed from many genetic and epidemiological studies and Biobanks. Earlier risk assessment approaches aimed at predicting SCLC risk with a strong emphasis on family history. For populations with a strong family history of SCLC, they are suggested to enter projects with more rigorous surveillance and interventions. In addition to the family history, some other factors may affect the risk of developing SCLC, which include indoor and outdoor air pollution, exposure to tobacco smoking, genetic landscape and lifestyle factors. After risk stratification, population with different risk stratifications are allocated into different detection approaches. There have been few studies reporting the detection of SCLC by screening. For patients at low risk, low-dose computed tomography (LDCT) and blood-based liquid biopsy are recommended. It should be noted that, compared with lung adenocarcinoma and squamous cell lung carcinoma, the sensitivity of LDCT for early-stage SCLC was significantly lower. Thomas and his colleagues demonstrated the low efficiency of LDCT to screen for SCLC and to reduce mortality. They suggest SCLC should be detected via other approaches earlier than LDCT [193]. Blood-derived liquid biopsy in the early detection of SCLC include circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), circulating free DNA (cfDNA) and mythylated DNA. ctDNA with high potential for early detection in solid tumors, is less invasive and can reflect real-time tumor burdens in SCLC in a clinically proper timeframe [194]. Fernandez-Cuesta extracted the plasma from patients with SCLC and non-cancer controls and evaluated their TP53 mutations in the cfDNA [195]. Common somatic mutations in cfDNA have been also detected in non-cancer controls, necessitating the need for the development of ctDNA screening tests. Chemi demonstrated that cfDNA methylation profiling could be included in early-detection of SCLC. Besides, it has been a common practice for oncologists to adopt ctDNA to evaluate minimal residual disease (MRD), an index reflecting the possibility of tumor relapse. The detection of MRD is vital for predicting outcome and guide further cancer therapy [196, 197]. These processes have been shown in Fig. 7A.
Establishment of a multi-dimensional model in the prediction of SCLC outcome
Establishment of prognostic models for patients with SCLC could aid risk stratification as well as subsequent treatment strategies. Most prognostic models have been established by referring to one mere omic data. Due to the limited efficacy of a single omic platform, multi-omics platforms are encouraged since they would yield more powerful results. The adoption of SCLC prognosis prediction would greatly benefit clinical management of SCLC patients. A significant development in computational approaches and great stride in artificial intelligence featured by deep learning has been made in recent years [198, 199]. The application of advanced statistical analysis and machine learning might contribute to a more accurate prediction of SCLC prognosis. In addition, the large-scale next generation sequencing and our easy access to open databases provide us with great opportunities to build more convincing and accurate models to predict SCLC prognosis more precisely.
Establishment of reliable predictive and prognostic models would guide treatment strategies, constitutes the fundamentals of precision oncology. Multi-omics tumor profiling approaches have developed at an amazingly fast speed over recent years. They depict the molecular landscapes of and demonstrate various biological characteristics within a tumor (Fig. 7B). However, challenges still exist. Many issues need to be dealt with regarding the interpretation of large datasets and translation of these information into clinical utility. Prospective clinical validations via large-scale clinical trials are warranted to transform large datasets into clinical practice. Despite the development of multi-omics at its budding, they would deepen our understanding of the biological function of SCLC and contribute to a more personalized paradigm of SCLC treatment.
Conclusion and perspectives
SCLC remains a recalcitrant disease for oncologists worldwide. No longer considered an orphan disease, SCLC has many challenges that lie ahead. These challenges need to be overcome to move the field of SCLC forward. This review is an effort to depict comprehensively the major challenges facing SCLC and the possible solutions. The means to combat chemoresistance, development of novel targeted therapies, approaches to boost the efficacy of ICI and exploration of reliable biomarkers to achieve early detection as well as establishment of prognostic prediction models. Development of multi-omics technologies such as liquid biopsy, next generation sequencing is critically important. These molecular technologies would help to determine the most suitable and optimized treatments for each patient with SCLC to contribute to a more personalized treatment paradigm. There is a high possibility that SCLC would step out of the predicament and usher in a new situation where promising therapeutic agents are developed and the OS is significantly improved for patients with SCLC.
Abbreviations
- AT2:
-
Type 2 alveolar cells
- ATR:
-
Ataxia telangiectasia and Rad3-related protein
- ATR:
-
Ataxia telangiectasia and RAD3- related protein
- CAR:
-
Chimeric antigen receptor
- CAR T:
-
Chimeric antigen receptor T
- CDK7:
-
Cyclin-dependent kinase 7
- CE:
-
Carboplatin with etoposide
- cfDNA:
-
Circulating free DNA
- CHK1:
-
Checkpoint kinase 1
- CHK1:
-
Checkpoint kinase 1
- CTCs:
-
Circulating tumor cells
- ctDNA:
-
Circulating tumor DNA
- DDR:
-
DNA damage response
- DLL3:
-
Delta-like canonical Notch ligand 3
- ECM:
-
Extracellular matrix
- GEMMs:
-
Genetically engineered mouse models
- Hh:
-
Hedgehog
- ICI:
-
Immune checkpoint inhibitor
- ICIs:
-
Immune checkpoint inhibitors
- IFN:
-
Interferon
- IGF-IR:
-
Insulin-like growth factor-I receptor
- LDCT:
-
Low-dose computed tomography
- LOH:
-
Loss of heterozygosity
- MDR:
-
Multidrug resistance
- MRD:
-
Minimal residual disease
- NE:
-
Neuroendocrine
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- OS:
-
Overall survival
- PARP:
-
Poly-ADP-ribose polymerase
- PARP:
-
Poly-ADP-ribose polymerase
- PFS:
-
Progression free survival
- PI3K/Akt:
-
Phosphoinositide 3-kinase
- SCLC:
-
Small cell lung cancer
- TFI:
-
Treatment-free interval
- TROP-2:
-
Trophoblast cell-surface antigen 2
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor
References
van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378(9804):1741–55.
Stewart CA, Gay CM, Xi Y, Sivajothi S, Sivakamasundari V, Fujimoto J, et al. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nat Cancer. 2020;1:423–36.
Herzog BH, Devarakonda S, Govindan R. Overcoming chemotherapy resistance in SCLC. J Thorac Oncol. 2021;16(12):2002–15.
Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121(5):664–72.
Remon J, Aldea M, Besse B, Planchard D, Reck M, Giaccone G, et al. Small cell lung cancer: a slightly less orphan disease after immunotherapy. Ann Oncol. 2021;32(6):698–709.
Spigel DR, Townley PM, Waterhouse DM, Fang L, Adiguzel I, Huang JE, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol. 2011;29(16):2215–22.
Baggstrom MQ, Qi Y, Koczywas M, Argiris A, Johnson EA, Millward MJ, Mayo Phase 2 Consortium, California Consortium, et al. A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. J Thorac Oncol. 2011;6(10):1757–60.
Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18(11):3163–9.
Byers LA, Wang J, Nilsson MB, Fujimoto J, Saintigny P, Yordy J, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2(9):798–811.
Sen T, Tong P, Diao L, Li L, Fan Y, Hoff J, et al. Targeting AXL and mTOR pathway overcomes primary and acquired resistance to WEE1 inhibition in small-cell Lung Cancer. Clin Cancer Res. 2017;23(20):6239–53.
Doerr F, George J, Schmitt A, Beleggia F, Rehkämper T, Hermann S, et al. Targeting a non-oncogene addiction to the ATR/CHK1 axis for the treatment of small cell lung cancer. Sci Rep. 2017;7(1):15511.
Gay CM, Stewart CA, Park EM, Diao L, Groves SM, Heeke S, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 2021;39(3):346-360e7.
Chemi F, Pearce SP, Clipson A, Hill SM, Conway AM, Richardson SA, et al. cfDNA methylome profiling for detection and subtyping of small cell lung cancers. Nat Cancer. 2022;3(10):1260–70.
Mollaoglu G, Guthrie MR, Böhm S, Brägelmann J, Can I, Ballieu PM, et al. MYC drives progression of small cell Lung Cancer to a variant neuroendocrine subtype with vulnerability to Aurora kinase inhibition. Cancer Cell. 2017;31(2):270–85.
Bebber CM, Thomas ES, Stroh J, Chen Z, Androulidaki A, Schmitt A, et al. Ferroptosis response segregates small cell lung cancer (SCLC) neuroendocrine subtypes. Nat Commun. 2021;12(1):2048.
Kim YH, Mishima M. Second-line chemotherapy for small-cell lung cancer (SCLC). Cancer Treat Rev. 2011;37(2):143–50.
Zhou J, Li Z, Li J, Gao B, Song W. Chemotherapy resistance molecular mechanism in small cell Lung Cancer. Curr Mol Med. 2019;19(3):157–63.
Wu Q, Guo J, Liu Y, Zheng Q, Li X, Wu C, et al. YAP drives fate conversion and chemoresistance of small cell lung cancer. Sci Adv. 2021;7(40): eabg1850.
Quoix E. Topotecan in the treatment of relapsed small cell lung cancer. Onco Targets Ther. 2008;1:79–86.
Evans TL, Cho BC, Udud K, Fischer JR, Shepherd FA, Martinez P, et al. Cabazitaxel versus topotecan in patients with small-cell lung cancer with progressive disease during or after first-line platinum-based chemotherapy. J Thorac Oncol. 2015;10(8):1221–8.
Tiseo M, Ardizzoni A. Current status of second-line treatment and novel therapies for small cell lung cancer. J Thorac Oncol. 2007;2(8):764–72.
Drapkin BJ, George J, Christensen CL, Mino-Kenudson M, Dries R, Sundaresan T, et al. Genomic and functional fidelity of small cell lung cancer patient-derived xenografts. Cancer Discov. 2018;8(5):600–15.
Gardner EE, Lok BH, Schneeberger VE, Desmeules P, Miles LA, Arnold PK, et al. Chemosensitive relapse in small cell lung cancer proceeds through an EZH2-SLFN11 axis. Cancer Cell. 2017;31(2):286–99.
Thomas A, Takahashi N, Rajapakse VN, Zhang X, Sun Y, Ceribelli M, et al. Therapeutic targeting of ATR yields durable regressions in small cell lung cancers with high replication stress. Cancer Cell. 2021;39(4):566-579e7.
Savaraj N, Wu CJ, Xu R, Lampidis T, Lai S, Donnelly E, et al. Multidrug-resistant gene expression in small-cell lung cancer. Am J Clin Oncol. 1997;20(4):398–403.
Liu H, Huang J, Peng J, Wu X, Zhang Y, Zhu W, et al. Upregulation of the inwardly rectifying potassium channel Kir2.1 (KCNJ2) modulates multidrug resistance of small-cell lung cancer under the regulation of miR-7 and the Ras/MAPK pathway. Mol Cancer. 2015;14:59.
Tiwari AK, Sodani K, Dai CL, Ashby CR Jr, Chen ZS. Revisiting the ABCs of multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12(4):570–94.
Yeh JJ, Hsu NY, Hsu WH, Tsai CH, Lin CC, Liang JA. Comparison of chemotherapy response with P-glycoprotein, multidrug resistance-related protein-1, and lung resistance-related protein expression in untreated small cell lung cancer. Lung. 2005;183(3):177–83.
Barbato L, Bocchetti M, Di Biase A, Regad T. Cancer Stem cells and targeting strategies. Cells. 2019;8(8): 926.
Nunes T, Hamdan D, Leboeuf C, El Bouchtaoui M, Gapihan G, Nguyen TT, et al. Targeting cancer stem cells to overcome chemoresistance. Int J Mol Sci. 2018;19(12):4036.
Xi G, Li YD, Grahovac G, Rajaram V, Wadhwani N, Pundy T, et al. Targeting CD133 improves chemotherapeutic efficacy of recurrent pediatric pilocytic astrocytoma following prolonged chemotherapy. Mol Cancer. 2017;16(1):21.
Soleimani A, Dadjoo P, Avan A, Soleimanpour S, Rajabian M, Ferns G, et al. Emerging roles of CD133 in the treatment of gastric cancer, a novel stem cell biomarker and beyond. Life Sci. 2022;293: 120050.
Guo C, Wan R, He Y, Lin SH, Cao J, Qiu Y, et al. Therapeutic targeting of the mevalonate-geranylgeranyl diphosphate pathway with statins overcomes chemotherapy resistance in small cell lung cancer. Nat Cancer. 2022;3(5):614–28.
Chalishazar MD, Wait SJ, Huang F, Ireland AS, Mukhopadhyay A, Lee Y, et al. MYC-driven small-cell lung cancer is metabolically distinct and vulnerable to arginine depletion. Clin Cancer Res. 2019;25(16):5107–21.
Drivsholm L, Paloheimo LI, Osterlind K, Chromogranin A. A significant prognostic factor in small cell lung cancer. Br J Cancer. 1999;81(4):667–71.
Jensen SM, Gazdar AF, Cuttitta F, Russell EK, Linnoila RI. A comparison of synaptophysin, chromogranin, and L-dopa decarboxylase as markers for neuroendocrine differentiation in lung cancer cell lines. Cancer Res. 1990;50(18):6068–74.
Metovic J, Barella M, Bianchi F, Hofman P, Hofman V, Remmelink M, et al. Morphologic and molecular classification of lung neuroendocrine neoplasms. Virchows Arch. 2021;478(1):5–19.
Sutherland KD, Proost N, Brouns I, Adriaensen D, Song JY, Berns A. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell. 2011;19(6):754–64.
Cho HJ, Hong SA, Ryu D, Hong SH, Kim TM. Transcriptional profiling reveals mesenchymal subtypes of small cell lung cancer with activation of the epithelial-to-mesenchymal transition and worse clinical outcomes. Cancers (Basel). 2022;14(22):5600.
Ito T, Kudoh S, Fujino K, Sanada M, Tenjin Y, Saito H, et al. Pulmonary neuroendocrine cells and small cell lung carcinoma: immunohistochemical study focusing on mechanisms of neuroendocrine differentiation. Acta Histochem Cytochem. 2022;55(3):75–83.
Groves SM, Ildefonso GV, McAtee CO, Ozawa PMM, Ireland AS, Stauffer PE, et al. Archetype tasks link intratumoral heterogeneity to plasticity and cancer hallmarks in small cell lung cancer. Cell Syst. 2022;13(9):690-710e17.
Frizziero M, Kilgour E, Simpson KL, Rothwell DG, Moore DA, Frese KK, et al. Expanding therapeutic opportunities for extrapulmonary neuroendocrine carcinoma. Clin Cancer Res. 2022;28(10):1999–2019.
Chen HJ, Poran A, Unni AM, Huang SX, Elemento O, Snoeck HW, et al. Generation of pulmonary neuroendocrine cells and SCLC-like tumors from human embryonic stem cells. J Exp Med. 2019;216(3):674–87.
Heng WS, Gosens R, Kruyt FAE. Lung cancer stem cells: origin, features, maintenance mechanisms and therapeutic targeting. Biochem Pharmacol. 2019;160:121–33.
Codony-Servat J, Verlicchi A, Rosell R. Cancer stem cells in small cell lung cancer. Transl Lung Cancer Res. 2016;5(1):16–25.
Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7(1):3.
Meijer JJ, Leonetti A, Airò G, Tiseo M, Rolfo C, Giovannetti E, et al. Small cell lung cancer: novel treatments beyond immunotherapy. Semin Cancer Biol. 2022;86(Pt 2):376–85.
Gazdar AF, Savage TK, Johnson JE, Berns A, Sage J, Linnoila RI, et al. The comparative pathology of genetically engineered mouse models for neuroendocrine carcinomas of the lung. J Thorac Oncol. 2015;10(4):553–64.
Owonikoko TK, Zhang G, Kim HS, Stinson RM, Bechara R, Zhang C, et al. Patient-derived xenografts faithfully replicated clinical outcome in a phase II co-clinical trial of arsenic trioxide in relapsed small cell lung cancer. J Transl Med. 2016;14(1):111.
Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19(5):289–97.
George J, Lim JS, Jang SJ, Cun Y, Ozretić L, Kong G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47–53.
Almodovar K, Iams WT, Meador CB, Zhao Z, York S, Horn L, et al. Longitudinal cell-free DNA analysis in patients with small cell lung cancer reveals dynamic insights into treatment efficacy and disease relapse. J Thorac Oncol. 2018;13(1):112–23.
Peifer M, Fernández-Cuesta L, Sos ML, George J, Seidel D, Kasper LH, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44(10):1104–10.
Jiang L, Huang J, Higgs BW, Hu Z, Xiao Z, Yao X, et al. Genomic landscape survey identifies SRSF1 as a key oncodriver in small cell lung cancer. PLoS Genet. 2016;12(4): e1005895.
Wang H, Wu S, Li Z, Zhang C, Shang X, Zhao C, et al. Molecular subtyping of small-cell lung cancer based on mutational signatures with different genomic features and therapeutic strategies. Cancer Sci. 2023;114(2):665–79.
Arriola E, Cañadas I, Arumí M, Rojo F, Rovira A, Albanell J. Genetic changes in small cell lung carcinoma. Clin Transl Oncol. 2008;10(4):189–97.
Salgia R, Skarin AT. Molecular abnormalities in lung cancer. J Clin Oncol. 1998;16(3):1207–17.
Jin W, Lei Z, Xu S, Fachen Z, Yixiang Z, Shilei Z, et al. Genetic mutation analysis in small cell lung cancer by a novel NGS-based targeted resequencing gene panel and relation with clinical features. Biomed Res Int. 2021;2021: 3609028.
Matsui WH. Cancer stem cell signaling pathways. Med (Baltim). 2016;95(1 Suppl 1):8-S19.
Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells—a clinical update. Nat Rev Clin Oncol. 2020;17(4):204–32.
Park KS, Martelotto LG, Peifer M, Sos ML, Karnezis AN, Mahjoub MR, et al. A crucial requirement for hedgehog signaling in small cell lung cancer. Nat Med. 2011;17(11):1504–8.
Lim S, Lim SM, Kim MJ, Park SY, Kim JH. Sonic hedgehog pathway as the prognostic marker in patients with extensive stage small cell Lung Cancer. Yonsei Med J. 2019;60(10):898–904.
Laukkanen MO, Gutkind JS, Castellone MD. Sonic hedgehog in SCLC. Aging. 2015;7(9):605–6.
Meder L, König K, Ozretić L, Schultheis AM, Ueckeroth F, Ade CP, et al. ASCL1, p53 and RB alterations define an alternative pathway driving neuroendocrine and small cell lung carcinomas. Int J Cancer. 2016;NOTCH(4):927–38.
Wagner AH, Devarakonda S, Skidmore ZL, Krysiak K, Ramu A, Trani L, et al. Recurrent WNT pathway alterations are frequent in relapsed small cell lung cancer. Nat Commun. 2018;9(1):3787.
Hamilton G, Rath B, Klameth L, Hochmair M. Receptor tyrosine kinase expression of circulating tumor cells in small cell lung cancer. Oncoscience. 2015;2(7):629–34.
Fischer B, Marinov M, Arcaro A. Targeting receptor tyrosine kinase signalling in small cell lung cancer (SCLC): what have we learned so far? Cancer Treat Rev. 2007;33(4):391–406.
Nakanishi Y, Mulshine JL, Kasprzyk PG, Natale RB, Maneckjee R, Avis I, et al. Insulin-like growth factor-I can mediate autocrine proliferation of human small cell lung cancer cell lines in vitro. J Clin Invest. 1988;82(1):354–9.
Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, et al. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 2002;62(21):6304–11.
Lund EL, Thorsen C, Pedersen MW, Junker N, Kristjansen PE. Relationship between vessel density and expression of vascular endothelial growth factor and basic fibroblast growth factor in small cell lung cancer in vivo and in vitro. Clin Cancer Res. 2000;6(11):4287–91.
Fontanini G, Faviana P, Lucchi M, Boldrini L, Mussi A, Camacci T, et al. A high vascular count and overexpression of vascular endothelial growth factor are associated with unfavourable prognosis in operated small cell lung carcinoma. Br J Cancer. 2002;86(4):558–63.
Arcaro A, Khanzada UK, Vanhaesebroeck B, Tetley TD, Waterfield MD, Seckl MJ. Two distinct phosphoinositide 3-kinases mediate polypeptide growth factor-stimulated PKB activation. EMBO J. 2002;21(19):5097–108.
Belyanskaya LL, Hopkins-Donaldson S, Kurtz S, Simões-Wüst AP, Yousefi S, Simon HU, et al. Cisplatin activates akt in small cell lung cancer cells and attenuates apoptosis by survivin upregulation. Int J Cancer. 2005;117(5):755–63.
Maulik G, Kijima T, Ma PC, Ghosh SK, Lin J, Shapiro GI, et al. Modulation of the c-Met/hepatocyte growth factor pathway in small cell lung cancer. Clin Cancer Res. 2002;8(2):620–7.
Jagadeeswaran R, Jagadeeswaran S, Bindokas VP, Salgia R. Activation of HGF/c-Met pathway contributes to the reactive oxygen species generation and motility of small cell lung cancer cells. Am J Physiol Lung Cell Mol Physiol. 2007;292(6):L1488-94.
Quinn KA, Treston AM, Unsworth EJ, Miller MJ, Vos M, Grimley C, et al. Insulin-like growth factor expression in human cancer cell lines. J Biol Chem. 1996;271(19):11477–83.
Izycki T, Chyczewska E, Naumnik W, Ossolinska M. Serum levels of IGF-I and IGFBP-3 in patients with lung cancer during chemotherapy. Oncol Res. 2006;16(1):49–54.
Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst. 1999;91(2):151–6.
Krystal GW, Sulanke G, Litz J. Inhibition of phosphatidylinositol 3-kinase-akt signaling blocks growth, promotes apoptosis, and enhances sensitivity of small cell lung cancer cells to chemotherapy. Mol Cancer Ther. 2002;1(11):913–22.
Ebi H, Corcoran RB, Singh A, Chen Z, Song Y, Lifshits E, et al. Receptor tyrosine kinases exert dominant control over PI3K signaling in human KRAS mutant colorectal cancers. J Clin Invest. 2011;121(11):4311–21.
García-Echeverría C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5(3):231–9.
Warshamana-Greene GS, Litz J, Buchdunger E, García-Echeverría C, Hofmann F, Krystal GW. The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clin Cancer Res. 2005;11(4):1563–71.
Ma PC, Maulik G, Christensen J, Salgia R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 2003;22(4):309–25.
Gao CF, Vande Woude GF. HGF/SF-Met signaling in tumor progression. Cell Res. 2005;15(1):49–51.
Maulik G, Madhiwala P, Brooks S, Ma PC, Kijima T, Tibaldi EV, et al. Activated c-Met signals through PI3K with dramatic effects on cytoskeletal functions in small cell lung cancer. J Cell Mol Med. 2002;6(4):539–53.
Ma PC, Tretiakova MS, Nallasura V, Jagadeeswaran R, Husain AN, Salgia R. Downstream signalling and specific inhibition of c-MET/HGF pathway in small cell lung cancer: implications for tumour invasion. Br J Cancer. 2007;97(3):368–77.
Garnett J, Chumbalkar V, Vaillant B, Gururaj AE, Hill KS, Latha K, et al. Regulation of HGF expression by ∆EGFR-mediated c-Met activation in glioblastoma cells. Neoplasia. 2013;15(1):73–84.
Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415 – 21.
Byun TS, Pacek M, Yee MC, Walter JC, Cimprich KA. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 2005;19(9):1040–52.
Lok BH, Gardner EE, Schneeberger VE, Ni A, Desmeules P, Rekhtman N, et al. PARP inhibitor activity correlates with SLFN11 expression and demonstrates synergy with temozolomide in small cell lung cancer. Clin Cancer Res. 2017;23(2):523–35.
Kundu K, Cardnell RJ, Zhang B, Shen L, Stewart CA, Ramkumar K, et al. SLFN11 biomarker status predicts response to lurbinectedin as a single agent and in combination with ATR inhibition in small cell lung cancer. Transl Lung Cancer Res. 2021;10(11):4095–105.
Venugopala KN. Targeting the DNA damage response machinery for lung cancer treatment. Pharmaceuticals (Basel). 2022;15(12):1475.
Taniguchi H, Caeser R, Chavan SS, Zhan YA, Chow A, Manoj P, et al. WEE1 inhibition enhances the antitumor immune response to PD-L1 blockade by the concomitant activation of STING and STAT1 pathways in SCLC. Cell Rep. 2022;39(7): 110814.
Woll P, Gaunt P, Danson S, Steele N, Ahmed S, Mulatero C, et al. Olaparib as maintenance treatment in patients with chemosensitive small cell lung cancer (STOMP): a randomised, double-blind, placebo-controlled phase II trial. Lung Cancer. 2022;171:26–33.
Owonikoko TK, Dahlberg SE, Khan SA, Gerber DE, Dowell J, Moss RA, et al. A phase 1 safety study of veliparib combined with cisplatin and etoposide in extensive stage small cell lung cancer: a trial of the ECOG-ACRIN Cancer Research Group (E2511). Lung Cancer. 2015;89(1):66–70.
Owonikoko TK, Dahlberg SE, Sica GL, Wagner LI, Wade JL 3rd, Srkalovic G, et al. Randomized phase II trial of cisplatin and etoposide in combination with veliparib or placebo for extensive-stage small-cell lung cancer: ECOG-ACRIN 2511 study. J Clin Oncol. 2019;37(3):222–9.
Nagel R, Avelar AT, Aben N, Proost N, van de Ven M, van der Vliet J, et al. Inhibition of the replication stress response is a synthetic vulnerability in SCLC that Acts synergistically in combination with cisplatin. Mol Cancer Ther. 2019;18(4):762–70.
Thomas A, Redon CE, Sciuto L, Padiernos E, Ji J, Lee MJ, et al. Phase I study of ATR inhibitor M6620 in combination with topotecan in patients with advanced solid tumors. J Clin Oncol. 2018;36(16):1594–602.
Sen T, Gay CM, Byers LA. Targeting DNA damage repair in small cell lung cancer and the biomarker landscape. Transl Lung Cancer Res. 2018;7(1):50–68.
Shapiro GI, Tibes R, Gordon MS, Wong BY, Eder JP, Borad MJ, et al. Phase I studies of CBP501, a G2 checkpoint abrogator, as monotherapy and in combination with cisplatin in patients with advanced solid tumors. Clin Cancer Res. 2011;17(10):3431–42.
Thompson R, Meuth M, Woll P, Zhu Y, Danson S. Treatment with the Chk1 inhibitor Gö6976 enhances cisplatin cytotoxicity in SCLC cells. Int J Oncol. 2012;40(1):194–202.
Bukhari AB, Chan GK, Gamper AM. Targeting the DNA damage response for cancer therapy by inhibiting the kinase Wee1. Front Oncol. 2022;12: 828684.
Ghelli Luserna di Rorà, Cerchione A, Martinelli C, Simonetti G. A WEE1 family business: regulation of mitosis, cancer progression, and therapeutic target. J Hematol Oncol. 2020;13(1):126.
Lallo A, Frese KK, Morrow CJ, Sloane R, Gulati S, Schenk MW, et al. The combination of the PARP inhibitor Olaparib and the WEE1 inhibitor AZD1775 as a new therapeutic option for small cell lung cancer. Clin Cancer Res. 2018;24(20):5153–64.
Pore MM, Hiltermann TJ, Kruyt FA. Targeting apoptosis pathways in lung cancer. Cancer Lett. 2013;332(2):359–68.
Inoue-Yamauchi A, Jeng PS, Kim K, Chen HC, Han S, Ganesan YT, et al. Targeting the differential addiction to anti-apoptotic BCL-2 family for cancer therapy. Nat Commun. 2017;8: 16078.
Choi YJ, Lee H, Kim DS, Kim DH, Kang MH, Cho YH, et al. Discovery of a novel CDK7 inhibitor YPN-005 in small cell lung cancer. Eur J Pharmacol. 2021;907: 174298.
Stine ZE, Schug ZT, Salvino JM, Dang CV. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 2022;21(2):141–62.
Cristea S, Coles GL, Hornburg D, Gershkovitz M, Arand J, Cao S, et al. The MEK5-ERK5 kinase axis controls lipid metabolism in small-cell lung cancer. Cancer Res. 2020;80(6):1293–303.
Huang F, Huffman KE, Wang Z, Wang X, Li K, Cai F, et al. Guanosine triphosphate links MYC-dependent metabolic and ribosome programs in small-cell lung cancer. J Clin Invest. 2021;131(1): e139929.
Li L, Ng SR, Colón CI, Drapkin BJ, Hsu PP, Li Z, et al. Identification of DHODH as a therapeutic target in small cell lung cancer. Sci Transl Med. 2019;11(517): eaaw7852.
Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl 3):4–10.
Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–27.
Minami T, Kijima T, Kohmo S, Arase H, Otani Y, Nagatomo I, et al. Overcoming chemoresistance of small-cell lung cancer through stepwise HER2-targeted antibody-dependent cell-mediated cytotoxicity and VEGF-targeted antiangiogenesis. Sci Rep. 2013;3: 2669.
Yao JH, Shao Y, Wang JJ, Li YL, Yang HQ, Liu J, et al. Evaluation of diagnostic and predictive values of the serum VEGF-A level and systemic immune-inflammation index in small cell lung cancer. J Cancer. 2021;12(5):1356–64.
Chen P, Zhu J, Liu DY, Li HY, Xu N, Hou M. Over-expression of survivin and VEGF in small-cell lung cancer may predict the poorer prognosis. Med Oncol. 2014;31(1):775.
Hou C, Lu L, Liu Z, Lian Y, Xiao J. Resveratrol reduces drug resistance of SCLC cells by suppressing the inflammatory microenvironment and the STAT3/VEGF pathway. FEBS Open Bio. 2021;11(8):2256–65.
Sharma N, Pennell N, Nickolich M, Halmos B, Ma P, Mekhail T, et al. Phase II trial of sorafenib in conjunction with chemotherapy and as maintenance therapy in extensive-stage small cell lung cancer. Invest New Drugs. 2014;32(2):362–8.
Owen DH, Giffin MJ, Bailis JM, Smit MD, Carbone DP, He K. DLL3: an emerging target in small cell lung cancer. J Hematol Oncol. 2019;12(1):61.
Kim JW, Ko JH, Sage J. DLL3 regulates notch signaling in small cell lung cancer. iScience. 2022;25(12):105603.
Tanaka K, Isse K, Fujihira T, Takenoyama M, Saunders L, Bheddah S, et al. Prevalence of Delta-like protein 3 expression in patients with small cell lung cancer. Lung Cancer. 2018;115:116–20.
Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, SCRX16-001 investigators, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18(1):42–51.
Morgensztern D, Besse B, Greillier L, Santana-Davila R, Ready N, Hann CL, et al. Efficacy and safety of Rovalpituzumab Tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: results from the phase II TRINITY study. Clin Cancer Res. 2019;25(23):6958–66.
Blackhall F, Jao K, Greillier L, Cho BC, Penkov K, Reguart N, et al. Efficacy and safety of Rovalpituzumab Tesirine compared with Topotecan as second-line therapy in DLL3-High SCLC: results from the phase 3 TAHOE Study. J Thorac Oncol. 2021;16(9):1547–58.
Gray JE, Heist RS, Starodub AN, Camidge DR, Kio EA, Masters GA, et al. Therapy of small cell Lung Cancer (SCLC) with a Topoisomerase-I-inhibiting antibody-drug conjugate (ADC) targeting Trop-2, Sacituzumab Govitecan. Clin Cancer Res. 2017;23(19):5711–9.
Whiteman KR, Johnson HA, Mayo MF, Audette CA, Carrigan CN, LaBelle A, et al. Lorvotuzumab mertansine, a CD56-targeting antibody-drug conjugate with potent antitumor activity against small cell lung cancer in human xenograft models. MAbs. 2014;6(2):556–66.
Shah MH, Lorigan P, O’Brien ME, Fossella FV, Moore KN, Bhatia S, et al. Phase I study of IMGN901, a CD56-targeting antibody-drug conjugate, in patients with CD56-positive solid tumors. Invest New Drugs. 2016;34(3):290–9.
Socinski MA, Kaye FJ, Spigel DR, Kudrik FJ, Ponce S, Ellis PM, et al. Phase 1/2 study of the CD56-Targeting antibody-drug Conjugate Lorvotuzumab Mertansine (IMGN901) in combination with Carboplatin/Etoposide in small-cell lung Cancer patients with extensive-stage disease. Clin Lung Cancer. 2017;18(1):68–76e2.
Crossland DL, Denning WL, Ang S, Olivares S, Mi T, Switzer K, et al. Antitumor activity of CD56-chimeric antigen receptor T cells in neuroblastoma and SCLC models. Oncogene. 2018;37(27):3686–97.
Zeng F, Wang Q, Wang S, Liang S, Huang W, Guo Y, et al. Linc00173 promotes chemoresistance and progression of small cell lung cancer by sponging miR-218 to regulate etk expression. Oncogene. 2020;39(2):293–307.
Sun Y, Zhou Y, Bai Y, Wang Q, Bao J, Luo Y, et al. A long non-coding RNA HOTTIP expression is associated with disease progression and predicts outcome in small cell lung cancer patients. Mol Cancer. 2017;16(1):162.
Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol. 2021;39(6):619–30.
Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. doi: 10.1016/S1470-2045(16)30098-5. Epub 2016 Jun 4. Erratum in: Lancet Oncol. 2016;17 (7):e270. Erratum in: Lancet Oncol. 2019;20(2):e70.
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, CASPIAN investigators, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–39.
Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, CAPSTONE-1 Study Group, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(6):739–47.
Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase ib KEYNOTE-028 study. J Clin Oncol. 2017;35(34):3823–9.
Chung HC, Piha-Paul SA, Lopez-Martin J, Schellens JHM, Kao S, Miller WH Jr, et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol. 2020;15(4):618–27.
Owonikoko TK, Park K, Govindan R, Ready N, Reck M, Peters S, et al. Nivolumab and Ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: CheckMate 451. J Clin Oncol. 2021;39(12):1349–59.
Spigel DR, Vicente D, Ciuleanu TE, Gettinger S, Peters S, Horn L, et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331☆. Ann Oncol. 2021;32(5):631–41.
Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, KEYNOTE-604 investigators, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-Blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–79.
Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell. 2020;37(4):443–55.
Genova C, Dellepiane C, Carrega P, Sommariva S, Ferlazzo G, Pronzato P, et al. Therapeutic implications of tumor microenvironment in lung cancer: focus on immune checkpoint blockade. Front Immunol. 2022;12: 799455.
Chia DKA, Gwee YX, Sundar R. Resistance to systemic immune checkpoint inhibition in the peritoneal niche. J Immunother Cancer. 2022;10(6): e004749.
Zou XL, Li XB, Ke H, Zhang GY, Tang Q, Yuan J, et al. Prognostic value of Neoantigen load in Immune checkpoint inhibitor therapy for cancer. Front Immunol. 2021;12: 689076.
McGranahan N, Rosenthal R, Hiley CT, Rowan AJ, Watkins TBK, Wilson GA, TRACERx Consortium, et al. Allele-specific HLA loss and Immune escape in lung cancer evolution. Cell. 2017;171(6):1259–1271e11.
Wang H, Liu B, Wei J. Beta2-microglobulin(B2M) in cancer immunotherapies: biological function, resistance and remedy. Cancer Lett. 2021;517:96–104.
Obermann S, Petrykowska S, Manns MP, Korangy F, Greten TF. Peptide-beta2-microglobulin-major histocompatibility complex expressing cells are potent antigen-presenting cells that can generate specific T cells. Immunology. 2007;122(1):90–7.
Long J, Wang D, Wang A, Chen P, Lin Y, Bian J, et al. A mutation-based gene set predicts survival benefit after immunotherapy across multiple cancers and reveals the immune response landscape. Genome Med. 2022;14(1):20.
Cormedi MCV, Van Allen EM, Colli LM. Predicting immunotherapy response through genomics. Curr Opin Genet Dev. 2021;66:1–9.
Darnell JE Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–21.
Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–11.
Kawakami Y, Ohta S, Sayem MA, Tsukamoto N, Yaguchi T. Immune-resistant mechanisms in cancer immunotherapy. Int J Clin Oncol. 2020;25(5):810–7.
Chen M, Chen R, Jin Y, Li J, Hu X, Zhang J, et al. Cold and heterogeneous T cell repertoire is associated with copy number aberrations and loss of immune genes in small-cell lung cancer. Nat Commun. 2021;12(1):6655.
Romero D. B cells and TLSs facilitate a response to ICI. Nat Rev Clin Oncol. 2020;17(4):195.
Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–5.
Wu SZ, Roden DL, Wang C, Holliday H, Harvey K, Cazet AS, et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020;39(19):e104063.
Liu Z, Zhang X, Zhang H, Zhang H, Yi Z, Zhang Q et al. multi-omics analysis reveals intratumor microbes as immunomodulators in colorectal cancer. Microbiol Spectr. 2023: e0503822.
Bhutiani N, Wargo JA. Gut microbes as biomarkers of ICI response—sharpening the focus. Nat Rev Clin Oncol. 2022;19(8):495–6.
Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103.
Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–8.
Luu M, Schütz B, Lauth M, Visekruna A. The impact of gut microbiota-derived metabolites on the tumor immune microenvironment. Cancers (Basel). 2023;15(5):1588.
Hayase E, Jenq RR. Role of the intestinal microbiome and microbial-derived metabolites in immune checkpoint blockade immunotherapy of cancer. Genome Med. 2021;13(1):107.
Rijavec E, Genova C, Biello F, Rossi G, Indini A, Grossi F. Current state of the art and future perspectives with immunotherapy in the management of small cell lung cancer. Expert Rev Respir Med. 2021;15(11):1427–35.
Wong SK, Iams WT. Front line applications and future directions of Immunotherapy in Small-Cell Lung Cancer. Cancers (Basel). 2021;13(3):506.
Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22(1):40.
Zhang Z, Li Y, Dong Y, Li J, Zhang B, Zhang C, et al. Successful treatment of a patient with multiple-line relapsed extensive-stage small-cell lung cancer receiving penpulimab combined with anlotinib: a case report. Front Oncol. 2022;12: 846597.
Hoffner B, Leighl NB, Davies M. Toxicity management with combination chemotherapy and programmed death 1/programmed death ligand 1 inhibitor therapy in advanced lung cancer. Cancer Treat Rev. 2020;85: 101979.
Knelson EH, Patel SA, Sands JM. PARP inhibitors in small-cell lung cancer: rational combinations to improve responses. Cancers (Basel). 2021;13(4):727.
Hardy-Werbin M, Arpí O, Taus A, Rocha P, Joseph-Pietras D, Nolan L, et al. Assessment of neuronal autoantibodies in patients with small cell lung cancer treated with chemotherapy with or without ipilimumab. Oncoimmunology. 2017;7(2):e1395125.
Iams WT, Porter J, Horn L. Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol. 2020;17(5):300–12.
Church M, Carter L, Blackhall F. Liquid biopsy in small cell lung cancer-a route to improved clinical care? Cells. 2020;9(12): 2586.
Kursunel MA, Taskiran EZ, Tavukcuoglu E, Yanik H, Demirag F, Karaosmanoglu B, et al. Small cell lung cancer stem cells display mesenchymal properties and exploit immune checkpoint pathways in activated cytotoxic T lymphocytes. Cancer Immunol Immunother. 2022;71(2):445–59.
Brazel D, Ou SI, Nagasaka M. Tiragolumab (Anti-TIGIT) in SCLC: Skyscraper-02, a towering inferno. Lung Cancer (Auckl). 2023;14:1–9.
Sun H, Dai J, Zhao L, Zhu J, Wang H, Chen P, et al. Lymphocyte activation gene-3 is associated with programmed death-ligand 1 and programmed cell death protein 1 in small cell lung cancer. Ann Transl Med. 2021;9(18):1468.
Scott SC, Hann CL. Immunotherapy for small cell lung cancer: established applications and novel approaches. Clin Adv Hematol Oncol. 2021;19(10):654–63.
Park S, Lee H, Lee B, Lee SH, Sun JM, Park WY, et al. DNA damage response and repair pathway alteration and its association with tumor mutation burden and platinum-based chemotherapy in SCLC. J Thorac Oncol. 2019;14(9):1640–50.
Hong M, Clubb JD, Chen YY. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell. 2020;38(4):473–88.
Wang Z, Wu Z, Liu Y, Han W. New development in CAR-T cell therapy. J Hematol Oncol. 2017;10(1):53.
Liu D. CAR-T “the living drugs”, immune checkpoint inhibitors, and precision medicine: a new era of cancer therapy. J Hematol Oncol. 2019;12(1):113.
Marofi F, Motavalli R, Safonov VA, Thangavelu L, Yumashev AV, Alexander M, et al. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res Ther. 2021;12(1):81.
Reppel L, Tsahouridis O, Akulian J, Davis IJ, Lee H, Fucà G, et al. Targeting disialoganglioside GD2 with chimeric antigen receptor-redirected T cells in lung cancer. J Immunother Cancer. 2022;10(1): e003897.
Gong Y, Klein Wolterink RGJ, Wang J, Bos GMJ, Germeraad WTV. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021;14(1):73.
Schwendenwein A, Megyesfalvi Z, Barany N, Valko Z, Bugyik E, Lang C, et al. Molecular profiles of small cell lung cancer subtypes: therapeutic implications. Mol Ther Oncolytics. 2021;20:470–83.
Zhang C, Li Z, Shang X, Zhao C, Wang H. Intratumor heterogeneity is associated with less CD8 + T cell infiltration and worse survival in patients with small cell lung cancer. Clin Transl Oncol. 2022. https://doi.org/10.1007/s12094-022-03010-7.
Zhou H, Hu Y, Luo R, Zhao Y, Pan H, Ji L, et al. Multi-region exome sequencing reveals the intratumoral heterogeneity of surgically resected small cell lung cancer. Nat Commun. 2021;12(1):5431.
Chan JM, Quintanal-Villalonga Á, Gao VR, Xie Y, Allaj V, Chaudhary O, et al. Signatures of plasticity, metastasis, and immunosuppression in an atlas of human small cell lung cancer. Cancer Cell. 2021;39(11):1479-1496e18.
Baine MK, Hsieh MS, Lai WV, Egger JV, Jungbluth AA, Daneshbod Y, et al. SCLC Subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J Thorac Oncol. 2020;15(12):1823–35.
Kellish P, Shabashvili D, Rahman MM, Nawab A, Guijarro MV, Zhang M, et al. Oncolytic virotherapy for small-cell lung cancer induces immune infiltration and prolongs survival. J Clin Invest. 2019;129(6):2279–92.
Li Q, Yuan D, Ma C, Liu Y, Ma L, Lv T. A new hope: the immunotherapy in small cell lung cancer. Neoplasma. 2016;63(3):342–50.
Tsimberidou AM, Fountzilas E, Nikanjam M, Kurzrock R. Review of precision cancer medicine: evolution of the treatment paradigm. Cancer Treat Rev. 2020;86: 102019.
Pich O, Bailey C, Watkins TBK, Zaccaria S, Jamal-Hanjani M, Swanton C. The translational challenges of precision oncology. Cancer Cell. 2022;40(5):458–78.
Kilgour E, Rothwell DG, Brady G, Dive C. Liquid biopsy-based biomarkers of treatment response and resistance. Cancer Cell. 2020;37(4):485–95.
Thomas A, Pattanayak P, Szabo E, Pinsky P. Characteristics and outcomes of small cell lung cancer detected by CT screening. Chest. 2018;154(6):1284–90.
Campos-Carrillo A, Weitzel JN, Sahoo P, Rockne R, Mokhnatkin JV, Murtaza M, et al. Circulating tumor DNA as an early cancer detection tool. Pharmacol Ther. 2020;207: 107458.
Fernandez-Cuesta L, Perdomo S, Avogbe PH, Leblay N, Delhomme TM, Gaborieau V, et al. Identification of circulating Tumor DNA for the early detection of small-cell lung Cancer. EBioMedicine. 2016;10:117–23.
Chin RI, Chen K, Usmani A, Chua C, Harris PK, Binkley MS, et al. Detection of solid tumor molecular residual disease (MRD) using circulating tumor DNA (ctDNA). Mol Diagn Ther. 2019;23(3):311–31.
Moding EJ, Nabet BY, Alizadeh AA, Diehn M. Detecting liquid remnants of solid tumors: circulating Tumor DNA minimal residual disease. Cancer Discov. 2021;11(12):2968–86.
Yin X, Liao H, Yun H, Lin N, Li S, Xiang Y, et al. Artificial intelligence-based prediction of clinical outcome in immunotherapy and targeted therapy of lung cancer. Semin Cancer Biol. 2022;86(Pt 2):146–59.
Skrede OJ, De Raedt S, Kleppe A, Hveem TS, Liestøl K, Maddison J, et al. Deep learning for prediction of colorectal cancer outcome: a discovery and validation study. Lancet. 2020;395(10221):350–60.
Acknowledgements
Not applicable.
Funding
This study was supported jointly by Special Funds for Taishan Scholars Project (Grant No. tsqn201812149), Academic Promotion Program of Shandong First Medical University (2019RC004), the National Natural Science Foundation of China (No. 82003996) and National Foundation for Lung Cancer Immunotherapy Research.
Author information
Authors and Affiliations
Contributions
HW put forward the topic of the review. CZ, CZ and KW prepared the figures. CZ and CZ prepared the main manuscript. All authors reviewed the manuscript and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, C., Zhang, C., Wang, K. et al. Orchestrating smart therapeutics to achieve optimal treatment in small cell lung cancer: recent progress and future directions. J Transl Med 21, 468 (2023). https://doi.org/10.1186/s12967-023-04338-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04338-6