Abstract
Since ancient times, plants have been an extensive reservoir of bioactive compounds with therapeutic interest for new drug development and clinical application. Cucurbitacins are a compelling example of these drug leads, primarily present in the plant kingdom, especially in the Cucurbitaceae family. However, these natural compounds are also known in several genera within other plant families. Beyond the Cucurbitaceae family, they are also present in other plant families, as well as in some fungi and one shell-less marine mollusc. Despite the natural abundance of cucurbitacins in different natural species, their obtaining and isolation is limited, as a result, an increase in their chemical synthesis has been developed by researchers. Data on cucurbitacins and their anticancer activities were collected from databases such as PubMed/MedLine, TRIP database, Web of Science, Google Scholar, and ScienceDirect and the information was arranged sequentially for a better understanding of the antitumor potential. The results of the studies showed that cucurbitacins have significant biological activities, such as anti-inflammatory, antioxidant, antimalarial, antimicrobial, hepatoprotective and antitumor potential. In conclusion, there are several studies, both in vitro and in vivo reporting this important anticancer/chemopreventive potential; hence a comprehensive review on this topic is recommended for future clinical research.
Similar content being viewed by others
Introduction
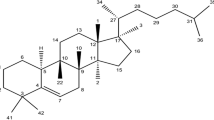
Cancer is a worldwide public health problem that develops following an abnormal growth of potentially invasive cells, capable of infinite replication and metastasis [1,2,3,4,5,6]. It maintains a poor prognosis, with the yearly incidence and death rate steadily increasing over time, discovering new therapeutic molecules and strategies an ever-present necessity [7,8,9,10,11,12]. Opportunely, several biologically active natural molecules have been researched and applied for cancer treatment, including cucurbitacins and their derivatives which, are mainly organized in the groups: A, B, C, D, E, I, H, Q, R, and dihydrocucurbitacin B [13]. From the chemical point of view, cucurbitacins are cucurbitane-type tetracyclic triterpenoid saponins with 30 carbons atoms on their basic skeleton (the detailed chemical data can be found in Figures S1, S2 and S3 and Table 1, and the synthesis of cucurbitacins in Figures S4 to S12, in the Additional file 1).
Cucurbitacin B isolated from Trichosanthes cucumerina (snake gourd) is the greatest source of cucurbitacins, which ensued the attention of scientists because of its several anticancer mechanisms [14, 15]. There are numerous health benefits displayed by the entire Cucurbitaceae family, each vegetable or fruit with a unique effect on human health [16]. For example, the well-known vegetable Cucumis sativus (cucumber) rich in cucurbitacins A, B, C, D, and E, has been reported to alleviate symptoms of indigestion and constipation, to help with skin problems and to promote hair growth [17]. Cucurbits have been also to stimulate the central nervous system and to treat dizziness. Furthermore, their seeds have also been used in the treatment of depression since their content in l-tryptophan increases serotonin levels, more commonly known as the “happy hormone” in the brain [18]. In this updated and comprehensive review, the latest data on the anticancer activity of cucurbitacins, targets and molecular mechanisms of action, and perspectives in chemotherapeutic therapy by association with other agents are synthesized. In the light of the encouraging results obtained from preclinical pharmacological studies, new windows are opening for the generation of new ideas and strategies for cancer chemotherapy.
Review methodology
Data on the anticancer effects of cucurbitacins, necessary to carry out the current review were obtained by analyzing the following specialized databases PubMed/MedLine, TRIP database, Web of Science, Google Scholar, and ScienceDirect using the following MesH terms: “Antineoplastic Agents, Phytogenic/pharmacology”, “Animals”, “Apoptosis/drug effects”, “Cell Line, Tumor”, “Cell Transformation, Neoplastic/drug effects”, “Cucurbitacins/chemistry”, “Cucurbitacins/pharmacology”, “Cucurbitacins/therapeutic use”, “Drug Design”, “Drug Therapy/Combination” “Humans”, “Molecular Targeted Therapy”, “Neoplasms/drug therapy”, “Neoplasm Invasiveness”, “Neoplasms/pathology”, “Phytotherapy”, “Signal Transduction/drug effects”. The study included the published papers with full text that highlighted the mechanisms and anticancer signaling pathways of cucurbitacins resulting from preclinical pharmacological studies. Papers with duplicate titles, abstracts and pharmacological experiments that included homoeopathic preparations associated with cucurbitacins were excluded. The scientific names of plant species were validated following WorldFloraOnline [19] and chemical formulas according to ChemSpider [20]. The most representative data were summarized in tables and figures.
Cucurbitacins: origin, traditional uses and therapeutic potential
Among living organisms, some of them exhibit secondary metabolites unique to some species being considered chemotaxonomic markers because they reveal an evolutionary relationship [21]. Nevertheless, some other metabolites occur in organisms without taxonomic similarity, which is related to specific roles in nature. Hence, environment-mediated stimuli probably lead to the emergence of metabolites in non-related species [22]. Cucurbitacins belong to the latter classification, representing an intriguing convergence between plants, fungi and even some animals. These stimuli can be authenticated with an analogous ecological role—their intensive bitterness is toxic to a wide range of herbivores, microorganisms, insects, and parasites, thus protecting their hosts from predators [23]. Cucurbitacins are more commonly present within the plant kingdom, with the vast majority being found in the Cucurbitaceae family, although they can also exist in several other taxonomically distant families. Nonetheless, some studies have also reported the presence of cucurbitacins in both the fungi and the animal kingdom. Therefore, establishing the distributions of secondary metabolites in this family is of utmost importance.
Cucurbitacins are mainly found in the Cucurbitaceae family, which consists of 965 plant species in 120 genera. Most of them are consumed in vegetables or in fruits, but they also show medicinal value, the reason why they have been used for traditional medicine in China [24]. Cucurbitacins within this family are more commonly present in Cucumis sativus (cucumber), Cucurbita moschata (pumpkin), Citrullus lanatus (watermelon), Cucumis melo (melon), Cucurbita pepo (squash), Lagenaria siceraria (bottle gourd), Luffa acutangula (ridge gourd), and Bryonia dioica (red/white bryony). Furthermore, a special kind of cucurbitacins from the Cucurbitaceae family called momordicosides can be found in the Momordica charantia (bitter melon or African cucumber). Cucurbitacins have diverse therapeutically important and attractive bioactivities, including in cancer chemoprevention/chemotherapy, and numerous preclinical studies have proven these properties (Table 1) [25,26,27].
The extract from Cucumis sativus flowers was isolated and tested for their anticancer potential against liver cancer HepG2 cell line. With a LD50 of 103.7 µg/mL, this extract induced apoptosis in the HepG2 cell line [28].
Hou et al. evaluated the anticancer potential of Cucurbita moschata in an in vitro model, using K562 human leukaemia cells, B16 murine melanoma cells, and A549 lung adenocarcinoma cells. They confirmed that isolated compounds from this vegetable inhibit cell tumour growth by working like ribosome-inactivating proteins [17, 29, 30]. Cucumis melo is well known for its beneficial pharmacological assets, such as analgesic, anti-inflammatory, antioxidant, antiulcer, anticancer, antimicrobial, diuretic, antidiabetic, hepatoprotective, and immunomodulator [31]. Vella et al. have recently established the significance of seeds and peels from Cucurmis melo, which until then were only considered waste. They concluded that the Cucumis melo by-products are a source of polyphenols and tannins, responsible for antioxidant and anticarcinogenic properties, respectively. Hence, the valorization of this entire vegetable is extremely important because it can reduce waste, as well as its environmental impact and the economic costs associated with its disposal. Besides, it can also be a source of active compounds for cosmetics, and pharmaceutical products [17, 32]. Citrullus lanatus has a potential use in the treatment of diabetes mellitus being investigated in vivo with obese and diabetic-induced rats. Watermelon extracts seems to play an important role in the prevention of diabetes’s complications, through attenuation of specific parameters in the kidneys and liver of diabetic animals [33].
Cucurbita pepo has been used in traditional medicine in several countries, treating patients infected with worms and parasites. In Europe, it has helped in the treatment of prostate enlargements, as well as irritable bladders. Moreover, it has also been used due to its antioxidant, anti-carcinogenic, anti-inflammatory, antiviral, antimicrobial, and analgesic properties. The oil obtained by cold pressure from Cucurbita pepo seeds is rich in antioxidant and antimicrobial components. Thus, it can be useful in some cosmetic formulations as it protects against dermatological wounds [17, 34]. There have been some reports regarding the cytotoxicity activity of Cucurbita pepo. Wang et al. detected a dose-dependent inhibitory effect between Cucurbita pepo fruit extracts and HeLa and HepG2 cell growth [35]. Martinez et al. observed in HL60 tumour cells, that it inhibits significantly the H2O2-induced damage and presents anti-proliferative and pro-apoptotic properties [36]. Besides the Cucurbitaceae family, cucurbitacins are also present in other plant families and some fungi and animals. Below follows some of these examples.
One of the most extensive plant families distributed worldwide is Brassicaceae, which comprises almost 400 genera and 4000 species. Interestingly, this family used to be known as Cruciferae owing to their cruciform appearance and flower shape [37]. A vast majority of the several Brassicaceae species are vegetables, which are commonly identified by their functional properties, like their phytochemical composition. The phytochemicals are categorised as micronutrients, macronutrients, and secondary metabolites. There are currently some reports linking a wide spectrum of bioactivity of these secondary metabolites to the prevention and treatment of several chronic diseases, such as obesity, type 2 diabetes, cardiovascular diseases, cancer, and osteoporosis. Furthermore, antioxidant activity, as well as antimicrobial capacity have also been detailed. This phenomenon is mainly on account of the synergistic effect of glucosinolates, polyphenols, and triterpenes (specific cucurbitacins), the main constituents in cruciferous plants [38]. Khayyal et al. evaluated the anti-inflammatory and antioxidant properties of Iberis amara extracts (rich in cucurbitacins) in rats, making use of both acute and chronic experimental models of inflammation. A dose-dependent reduction in inflammation was observed in both models, reflecting the extract's potent anti-inflammatory properties. Furthermore, other biological roles have also been assigned to Iberis amara (due to the presence of cucurbitacins B, E, and I), such as antifeedant activity, protecting many brassicaceous species [39, 40].
Picria fel-terrae, Neopicrorhiza scrophulariiflora and Gratiola officinalis belong to the family Scrophulariaceae, and have been used in traditional medicine, due to their cucurbitacins' content. [14, 41, 42]. Coutarea hexandra, from the Rubiaceae family, has been used traditionally in the treatment of malaria, inflammation, and diabetes. Olmedo et al. performed, with cucurbitacins isolated from the ethanolic extract (80%) of this plant, sulphorhodamine B assay in the following cancer cell lines: breast (MCF-7), lung (H-460) and central nervous system (SF-268), observing moderate cytotoxicity [15]. Wu et al. isolated some cucurbitacins from Begonia nantoensis (Begoniaceae family) such as cucurbitacin B, dihydrocucurbitacin B, cucurbitacin E, dihydrocucurbitacin E, and cucurbitacin I and evaluated their cytotoxicity. These compounds presented strong cytotoxic effects against the following cancer cell lines: gastric (NUGC-3), nasopharyngeal (HONE-1), breast (MCF-7) and lung (A549) [43].
Stems and leaves of Kageneckia oblonga (Rosaceae family) containing cucurbitacin’s derivatives have analgesic, antipyretic, and anti-inflammatory activity [44, 45]. East Asian countries commonly use Rubus chingii s unripe fruits to treat several diseases, especially those related to kidney deficiencies. Pharmacological studies validated the anti-ageing, anticancer, antioxidant, anti-inflammatory and antidiabetic properties due to its content in cucurbitacins [46]. Aquilaria agallocha (Thymelaeaceae family) is one of the largest producers of agarwood used throughout history in religious ceremonies, herbal medicines and perfumes, and whose composition is rich in cucurbitacins particularly E and I) [47]. Cucurbitacins have also been isolated from a few genera of mushrooms, including Hebeloma vinosophyllum, Russula lepida, and Leucopaxillus gentianeus. Several studies reported that cucurbitacins exert a protective role against mushroom predators, such as microorganisms and insects, due to their cytotoxicity. For example, the reason why the Hebeloma vinosophyllum is a poisonous mushroom is believed to be due to the presence of hebevinosides I–XI, together with hydroxyhebevinogenin and methoxyhebevinogenin, some derivatives from cucurbitacins [48, 49]. Russula lepida (Russulaceae family) has already been exploited throughout the years as both food and medicinal agents in China due to its content in seco-cucurbitacins—at least three seco-cucurbitacins were already isolated. Specifically, the extracts of Russula lepida’s fruiting bodies exhibited antitumour activity [50]. Furthermore, they also demonstrated a protein tyrosine phosphatase 1B (PTP1B) inhibitory activity—a compound manipulated as a negative regulator in the signal transduction via insulin and leptin pathways—without showing cytotoxicity [51, 52].
The mushroom Leucopaxillus gentianeus, to protect itself from external predators, first accumulates inactive fatty acid esters, such as 16-oleyl, 16-linoleyl, and 16-palmityl esters in its tissues. Then, after injury, some lipases cleave these esters, transforming them into a more active compound—the bioactive metabolite cucurbitacin B, warding off external attacks due to its toxicity [23, 53]. The shell-less marine molluscs (Dorid nudibranchs) are particularly susceptible to predators and as a chemical defence mechanism, they release terpenoid metabolites—particularly cucurbitacins. Interestingly, these cucurbitacins showed modest in vitro cytotoxicity against human ovarian carcinoma (HEY) and human glioblastoma/astrocytoma (U373) cell lines [54].
Cucurbitacins in anticancer pharmacological research: molecular mechanisms of action and efficacy
One of the fundamental characteristics of cancer cells is probably the ability to permanently stimulate their growth and proliferation [55]. Cancer cells have a low dependence on external proliferative stimuli and often do not need stimulation to multiply; through the mutations of some oncogenes, these cells acquire a proliferative autonomy, producing their mitogenic signals [2, 8, 56]. The major strategies used by tumor cells to achieve their proliferative independence are the following: the production of their growth factors; dysregulation of growth factor receptors, which transduce proliferative signals inside the cell; alteration of components of cytoplasmic signaling pathways, which produce a flow of mitogenic signaling without their stimulation by receptors; for example, the MAP-kinase (mitogen-activated protein-kinase) pathway, consisting of the proteins: RAS, RAF, MEK, MAPK (mitogen-activated protein kinase), ERK (extracellular signal-regulated kinase), FOS, plays a central role in human cancers [9, 57, 58]. The different cucurbitacins have been studied in experimental preclinical pharmacological studies and found to possess anticancer properties for a myriad of cancer types (Table 2).
Cucurbitacin B
The anticancer activity of cucurbitacin B, obtained from the dried rhizome powder of Corallocarpus epigaeus, was determined to induce the apoptotic machinery in the A375 melanoma cell line, by targeting the MAPK pathway and suppressing proliferation. This cytotoxic effect was confirmed in vivo using a xenograft melanoma model, in male NOD-SCID mice induced by A375 cells, with cucurbitacin B substantially reducing tumour growth when compared with untreated mice [59]. Similarly, for gastric cancer, cucurbitacin B reversed the multi-drug resistance of the SGC7901/DDP gastric cancer cells by downregulating the drug-resistant protein HIF-1a and P-pg. It also promoted apoptosis and autophagy via modifications of the CIP2A/PP2A/mTORC1 signaling axis. In detail, cucurbitacin B induced apoptosis and autophagy by inhibiting mTORC1. This inhibition is dependent on PP2A activity, and this study showed that the triterpenoid may target CIP2A to reactivate PP2A [60].
Cucurbitacin B was also found to suppress cell invasion and migration induced by 12-O-tetradecanoylphorbol 13-acetate (TPA) of the hepatoma human cell lines HepG2 and BEL-7402. The therapeutic molecule additionally inhibited the metabolic activity of TPA-induced MMP-9, by inactivating the extracellular signal-regulated kinase (ERK) 1/2, p38, and the Akt signaling pathway [61].
For non-small cell lung cancer (NSCLC), another study reported that treatment with cucurbitacin B led to inhibition of histone deacetylase and DNA methyltransferase in the H1299 cells, and this inhibition in turn allows for epigenetic modifications, such as the upregulation of important tumour suppressor genes and downregulation of key oncogenes. Specifically, the triterpenoid induced gene expression of CDKN1A and CDKN2A and downregulated the tumour promoter gene, hindering cellular growth and promoting apoptosis. In vivo treatment with cucurbitacin B lowered tumour mass and incidence in a lung tumourigenesis model induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, decreasing tumour angiogenesis in a dose-dependent manner [62]. The same authors also found that cucurbitacin B can inhibit the expression and nuclear translocation of β-catenin, a key participator in cancer development and metastasis, by suppressing the expression of Wnt3 and Wnt3a ligands, commonly overexpressed in NSCLC [63].
On human cholangiocarcinoma cells, cucurbitacin B inhibited the growth and replication of KKU-100 cells, preventing colony formation. This antiproliferative ability is linked to cell cycle arrest at the G2/M phase, downregulation of cyclins A and D1, and upregulation of p21 and p53. In addition, the study found that cucurbitacin B inhibits the activation of folate adhesion kinase (FAK) in KKU-100 cells by possibly targeting the expression of EGFR and HER2, frequently found overexpressed in the disease, and thus acts as a modulator of the FAK/PI3K/PDK1/AKT and FAK/p53 signaling pathways [64].
Particularly regarding breast cancer, the anticancer potential of cucurbitacin B has been heavily documented, in both in vitro and in vivo studies. Of note, Cucurbitacin B was able to suppress breast cancer growth in four different cell lines, namely MDA-MB-231, SKBR3- MCF-7 and 4T-1, by reducing the expression of HER2 and EGFR in a dose-dependent pattern. The HER2 inhibition was higher in the cell lines where the ILK1/YB-1/Twist signalling axis, identified as a regulator of HER2 expression, was also inhibited. Furthermore, when compared with other integrins, the triterpenoid compound significantly suppressed ITGA6 and ITGB4 expression, commonly overexpressed in breast cancer, in a time-dependent way. In vivo testing using MDA-MB-231 injected orthotopically in female athymic nude mice showed up to 50% tumour volume reduction following cucurbitacin B treatment when compared with untreated mice. In the 4T-1 orthotopic model, injected in BALB/c mice, the treated group had about 40% less tumour volume than the control mice [65]. A different study found that cucurbitacin B disrupts the cytoskeletal network of MDA-MB-231 cells, resulting in fast morphologic changes and irregular polymerization of the microtubule network. These alterations lead to apoptosis increase and cause arrest at the G2/M cell cycle phase. Additionally, cucurbitacin B treatment significantly reduced the c-Myc and nucleophosmin/B23 expression, with an increased translocation of the latter phosphoprotein from the nucleolus to the nucleoplasm, thereby preventing its cytoplasmic shuttling and subsequent role in cell proliferation [66]. Furthermore, cucurbitacin B acts as a mediator in the distribution and reorganization of cytoskeletal proteins via RAC1/CDC42/RhoA signaling, altering the mechanical properties of MDA‐MB‐231 and SKBR‐3 breast cancer cells. Cucurbitacin B treatment inhibited FAK and vinculin expression, affecting the cytoskeleton’s cell adhesion and contractility. It also interfered with the activity of the GTPases RAC1 and CDC42, which are important regulators of cell tension, thereby impacting cell migration in vitro and in vivo breast cancer models. Further in vivo testing on BALB/c nude mice bearing SKBR‐3 cells suggest that cucurbitacin B suppresses breast cancer metastasis to the lungs and liver [67].
For prostate cancer, cucurbitacin B showed anticancer activity, associated with apoptosis induction. This is evidenced by a substantial increase in the activity of Caspase 3/7 in the human prostate cancer cell lines LNCaP and PC-3, as opposed to normal prostate epithelial cells (PrEC), an increase in the Sub-G0/G1 phase, but also cleavage of Poly (ADP-ribose) polymerase, involved in DNA repair. In vivo, cucurbitacin B pre-treatment inhibited considerably PC-3 xenograft growth in athymic mice compared to controls after 31 days. The authors proposed cucurbitacin’s dose-dependent inhibition of ATP citrate lyase, or ACYL, as the anticancer mechanism of cucurbitacin, in in vitro and in vivo prostate tumour models [68].
On the human glioblastoma cell line U87, cucurbitacin B isolated from the leaves of Ecballium elaterium exhibited integrin-associated anticancer activity. In-depth, the article states that the molecule conditioned U87 cells from migrating and adhering to fibronectin dose-dependently, by targeting the α5β1 glioma protein and disrupting its activity, resulting in decreased motility and directional persistence of the tumour cells. This particular integrin plays a role in tumour formation and angiogenesis, and cucurbitacin B was able to inhibit angiogenesis in human microvascular endothelial cells, dose-dependently, in concentrations as low as 100 nM [69].
Cucurbitacin D
For cervical cancer, cucurbitacin D was able to decrease tumour cell growth and metastasis, dose-dependently, in the CaSki and SiHa cell lines by inducing apoptosis, measured by increased Annexin V staining and PARP protein cleavage and causing cell cycle arrest at the G1/S phase by interfering with the cell cycle regulatory proteins CDK4 and cyclin D1, as well as preventing RB protein phosphorylation. Furthermore, this study suggests that cucurbitacin D acts as an inhibitor of the signalling pathway PI3K/AKT by targeting HPV E6, STAT3 and its downstream targets MMP9 and c-Myc. In vivo testing in athymic nude mice bearing CaSki cells determined that administering cucurbitacin D intratumorally inhibited the growth of the orthotopic xenograft tumours [70].
For pancreatic cancer, treatment with cucurbitacin D caused cell cycle arrest at the G2/M phase, apoptosis induction, and intracellular ROS production in the AsPC-1 and Capan-1 cell lines. Cucurbitacin D suppressed cyclin B1, phospho-cdc2, and phospho-cdc25c, while upregulating the expression of p21, a cyclin/CDK complex inhibitor. By activating caspases-7 and -8 and cleaving PARP, the molecule induced apoptosis. Furthermore, the generation of ROS was linked to phosphorylation of p38 and c-Jun. Together, the article proposes that these anticancer mechanisms were caused via the ROS/p38 pathway [71].
Regarding NSCLC, cucurbitacin D proved to be cytostatic on NSCLC-N6 cells, an action associated with cell cycle arrest at the G1 phase. Treatment with the molecule led to an overexpression of CDK1 mRNA, which accumulates during the G1 phase, increasing the activity of CDK1 over the intermediate level necessary for the G1/S transition. Additionally, the cell line used in the study has mutated and inactive p53, a protein that negatively regulates CDK1 transcription [72].
Cucurbitacin E
The anticancer potential of cucurbitacin E against glioblastoma was investigated. The study demonstrates the compound’s ability to hinder cell proliferation and survival on the GBM8401 and U-87-MG cell lines, in a concentration-dependent manner. Cucurbitacin E treatment also led to an accumulation of cells at the G2/M phase of the cell cycle, therefore delaying mitosis. The cell cycle arrest was achieved by downregulating the expression of CDC2 and cyclin B1 proteins, disassociating the CDC2/cyclin B1 complex through GADD45β upregulation [73].
A more recent article found that cucurbitacin E was able to induce apoptosis and cause cell cycle arrest on NSCLC cells through the EGFR/MAPK pathway. More precisely, following cucurbitacin E treatment, the A549 cells exhibited an increase in the cleaved caspases-3 and -9, both apoptosis regulators, while also decreasing the expression of the apoptosis inhibitor protein survivin and the levels of phosphorylated STAT3. Cucurbitacin E reduced cyclin A2, cyclin B1 and cyclin E1 levels, resulting in an accumulation of cells at the G1/G0 phase of the cell cycle. Finally, the article showed that the derivative increased the phosphorylation of EGFR, altering the phosphorylation levels of the downstream members ERK1/2 and MEK1/2, found upregulated and downregulated, respectively [74].
The antiproliferative activity of cucurbitacin E on the brain in the malignant glioma GBM8401 human cell line was evaluated. Cucurbitacin E treatment inhibited cell growth by inducing cell cycle arrest at the G2/M phase, linked to GADD45γ upregulation and dissociation of the complex cyclin B1/CDC2 in a dose-dependent manner [75].
Cucurbitacin I
For ovarian and pancreatic cancers, the antitumour action of cucurbitacin was researched. Li’s work demonstrated that, in SKOV3 ovarian cells and PANC-1 pancreatic cells, treatment with the compound induced cells’ apoptosis associated with endoplasmic reticulum stress or ERS. Cucurbitacin I raised the levels of ERS through activation of enzyme IRE1α and the kinase PERK, leading to apoptosis induction dependent on the activation of the caspase-12 and CHOP pathways, and dependent on Bax increase. The excessive ERS levels additionally prompted autophagic cell death [76].
The antitumour potential of cucurbitacin I on colon cancer was the focal point of Kim and colleagues’ research published in 2014. The article states that, following cucurbitacin I treatment, the SW480 cells suffered cell viability and proliferation decrease in a dose-dependent pattern, presenting minimal effect on the normal cell line CCD-18Co. Following other derivatives, cucurbitacin I induced cell cycle arrest at the G2/M phase, associated with a decrease in the expression of cyclin A, cyclin B1, CDC25C, and CDK1 and subsequent inhibition of the CDK1/cyclin B1 complex. As for apoptosis induction, cucurbitacin I treatment was linked to an increase in the cleavage of caspases-3, -7, -8, -9. In vivo, the derivative repressed the growth and proliferation of a syngeneic transplanted CT-26 BALB/c mice tumour model, and as observed in vitro, an increase in the apoptosis-related proteins and a decrease in the expression of G2/M phase cell cycle-related proteins was detected [77].
Cucurbitacin II
The anticancer mechanisms of cucurbitacin IIa against NSCLC, namely its ability to induce apoptosis and cell cycle arrest, were investigated on the A549 cell line. The researchers found that cucurbitacin IIa acts as a tyrosine kinase inhibitor of the EGFR, and treatment with the molecule altered the expression of genes in the EGFR/MAPK pathway, namely downregulating the expression of cyclinB1, ERK1, MEK1, and MEK2, and upregulating the survivin, BRAF, Raf1, ERK2, and STAT3 genes. Notably, the expressions of ERK1 and MEK1 were significantly downregulated when compared with non-treated cells [78]. In 2021, a different group achieved corresponding results with cucurbitacin IIb in NSCLC research, namely acting as a tyrosine kinase inhibitor and CuIIb, inducing apoptosis via the STAT3 pathway and causing G2/M phase cell cycle arrest through the suppression of the EGFR/MAPK pathway [79]. Similarly, cucurbitacin IIb isolated from Ibervillea sonorae was tested as an antitumoural treatment option for NSCLC in the A549 cell line, reducing cell growth and inducing apoptosis at low concentrations and in a dose-dependent manner, while also inhibiting STAT3 expression and suppressing the activity of EGFR and/or of its downstream proteins [80]. Table 2 and Fig. 1 summarize the most representative anticancer mechanisms of cucurbitacins in different cancer modern experimental models.
Summarized scheme with the most representative anticancer molecular mechanisms of cucurbitacins. ↑ increase, ↓decrease; Bax: Bcl-2-associated X protein; Bcl-XL: B-cell lymphoma-extra large; Bcl-2: B-cell lymphoma 2 protein; mTOR: mammalian target of rapamycin protein; VEGFR: vascular endothelial growth factor receptor; VEGF: vascular endothelial growth factor; DNAMT: mitochondrial DNA
Synergistic anticancer effects of cucurbitacin B in combination with chemotherapeutic agents
Despite the numerous advantages of natural compounds in the treatment of several diseases, including cancer, some reports have found that combining these bioactive compounds with other clinically used chemotherapy drugs/small molecules strengthens the anticancer effect and reduces drug toxicity [81,82,83]. This combination therapy shows a promising treatment for cancer in both preclinical pharmacological studies in vitro and in vivo [84, 85]. In this section, an emphasis on the synergistic activity of the most used cucurbitacin—cucurbitacin B—with other compounds in several types of cancers is going to be detailed and the most important data are summarized in Table 3.
Breast cancer
Despite recent advances in cancer therapies, breast cancer remains the second leading cause of death among women [94,95,96,97]. The combination of chemotherapeutic agents with non-chemotherapeutic agents is clinically interesting and plays a significant role in breast cancer management [83, 98]. By treating MCF-7 breast cancer cells with cucurbitacin B alone or in combination with imatinib mesylate, it was discovered that the combination treatment synergistically inhibited cell proliferation and induced apoptosis [86]. Similarly, combining gemcitabine or docetaxel with cucurbitacin B increases apoptosis, inhibiting the proliferation of MDA-MB-231 breast cancer cells in vitro. When analyzing these same compounds in vivo, the authors observed that the combination of either one of the drugs with cucurbitacin B significantly reduced tumour volume without any signs of increased toxicity [87]. Furthermore, cucurbitacin B combined with ionizing radiation blocks cancer cells in the G2/M phase and promotes apoptosis, inhibiting cancer cells. Additionally, this combination decreases the content of molecules such as p-STAT3, c-Myc, Bcl-2, and Bcl-xL and increases the content of apoptosis-related molecules such as caspase-9, p21 and p53 [88].
Pancreatic cancer
In Western societies, pancreatic cancer is the fourth most common cause of cancer death, with a mortality rate of 95% within 5 years. This high mortality rate is primarily due to the insensitivity of pancreatic cancer to most chemotherapy and radiotherapy treatments. Zhou et al. combined cucurbitacin B and SCH772984, an ERK inhibitor, to inhibit pancreatic cancer cell growth and apoptosis by inhibiting the EGFR and its downstream signaling pathways, including PI3K/Akt/mTOR and STAT3. Additionally, they increased the pro-apoptotic protein Bim and reduced the anti-apoptotic proteins McL-1, Bcl-2, Bcl-xl, and survivin. By studying the synergistic combination of these two molecules in vivo, it was possible to observe a significant delay in tumour growth, both in the reduction of its volume and weight. Thus, the combined therapy of cucurbitacin B and SCH772984 resulted in critical growth inhibition of pancreatic cancer mice HPAC xenograft models. SCH772984 increased significantly cucurbitacin B sensitivity in pancreatic cancer cells but not in normal pancreatic ductal epithelial cells (used as a control) [89].
Colorectal cancer
Colorectal cancer is the fourth most common cancer-related mortality in men and women worldwide [99,100,101] and associated bacterial and fungal infections can worsen the prognosis [102,103,104,105]. It is characterized by overexpression of EGFR and its downstream signaling pathways, such as Janus kinase/signal transducer and activator of transcription (JAK/STAT) [106, 107]. The combination of these two molecules results in significant apoptotic and anti-proliferative effects on HT-29 and HCT-116 colorectal cancer cell lines compared with cucurbitacin B alone [90]. By treating SW480 colorectal cancer cells with cucurbitacin B alone or in combination with imatinib mesylate, it was discovered that the combination treatment synergistically inhibited cell proliferation and induced apoptosis. Additionally, cucurbitacin B can also increase the inhibitory effect of imatinib mesylate on matrix metalloproteinase-2 (MMP-2) expression, a member of the MMP family, which is responsible for the degradation of extracellular matrix, in a dose-dependent manner [86].
Ovarian cancer
One of the deadliest types of gynaecological cancer is ovarian cancer [108, 109]. In the first instance, platinum-based drugs, such as cisplatin, are used to treat the disease. Most patients, however, experience tumour recurrences that are resistant to cisplatin. Thus, co-administering chemotherapeutic agents with natural products may offer a synergistic effect [85]. El-Senduny et al. observed that cucurbitacin B exhibited cytotoxicity against the ovarian cancer cell line A2780, and pretreatment of cisplatin-resistant cell line A2780CP with this natural compound led to a significant increase in the cytotoxicity of cisplatin [91]. When investigating the effect of cucurbitacin B on human paclitaxel-resistant ovarian cancer A2780/Taxol cells, Qu et al. detected a dose- and time-dependent cytotoxicity. Furthermore, these cells were also blocked in the G2/M phase of the cell cycle, by several molecular mechanisms, such as upregulation of p53 and p21, downregulation of Bcl-2, activation of caspase-3 and suppression of P-gp [92].
Osteosarcoma
Human osteosarcoma is the most common malignant bone tumour occurring in children and teenagers [8]. The estimated incidence rate worldwide is 4 million per year, with a peak incidence at the age of 15–19 years [110]. Owing to its numerous metastasis and high recurrence rate, its treatment requires several simultaneous approaches, such as surgery, radiotherapy, and chemotherapy. Thus, the combination of different compounds in the osteosarcoma treatment can be a potentially beneficial approach. Lee et al. showed that treatment with low doses of cucurbitacin B and methotrexate synergistically inhibit the AKT and mTOR signaling pathways in human osteosarcoma cells both in vivo and in vitro improving the tumour suppression rate [93].
Limitation of the evidence
Cancer is a complex and dynamic disease, and its treatment requires substances with multiple actions, such as: detoxifying the body of harmful compounds (xenobiotics and endotoxins) with carcinogenic action; reducing oxidative stress; maintaining normal cell division; induction of malignant cell apoptosis; inhibition of angiogenesis; improving the antitumor immune response [5, 111,112,113,114,115]. The therapeutic anticancer potential of cucurbitacins depends on the quality and quantity of the bioactive compounds contained, the soil in which the plants are grown and the geographical location of these species. Adjuvant therapy must generally use purified and standardized extracts of active constituents that have a targeted pharmacological activity [116]. The standardization of cucurbitacins is necessary to ensure the identification, isolation and reproducibility of the active compounds in different pharmaceutical formulations. Another important limitation is the weak and variable absorption of cucurbitacins; as a result, new nano-formulations are needed to increase absorption, bioavailability and transport to the target in tumor cells [117, 118]. Also, the control of the therapeutic actions, the safety and the efficiency of the administration of the products must be ensured compared to the use of the whole plant or only some parts of the plant.
The main therapeutic limitation derives from the concerns associated with cancer treatment due to the aggressive nature of certain cancers and tumour resistance to chemotherapeutic drugs [119]. As previously mentioned, cucurbitacins have antiproliferative properties on several tumour cells, indicating their potential as anticancer agents. Furthermore, cucurbitacins synergistically enhance the efficacy of many other small-molecule drugs in cancer treatment. However, since most of the reported studies were performed in vitro, further in vivo work either on model animals or human clinical trials needs to be executed. All the findings presented in this comprehensive review suggest that cucurbitacins are potential candidates for use in combination therapy with clinical anticancer drugs.
Conclusion and future perspectives
Cucurbitacins are structurally diverse triterpenes found in a wide range of plant families, especially the Cucurbitaceae and present several biological properties. Future research may benefit from chemical modifications of specific functional groups to improve pharmacokinetic and pharmacodynamic issues. Their anticancer potential has received increasing attention, as they can prevent the proliferation of different tumour cells by inducing apoptosis, cell cycle arrest, autophagy, and cytoskeletal disruption. These processes happen both in vivo and in vitro through multiple targets. Additionally, cucurbitacins exert strong synergistic anticancer effects when combined with clinically used chemotherapeutic drugs. New nanotechnological formulations must be developed to increase the bioavailability of cucurbitacins. Translational pharmacological studies are also necessary to establish the exact dose in humans and the best way of their administration. Further clinical studies on cucurbitacins should be performed to confirm their potential as drug candidates using these molecules from nature as new effective chemotherapeutics.
Availability of data and materials
Not applicable.
References
GRB 2019 Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:627–47.
Ali ES, Akter S, Ramproshad S, Mondal B, Riaz TA, Islam MT, Khan IN, Docea AO, Calina D, Sharifi-Rad J, Cho WC. Targeting Ras-ERK cascade by bioactive natural products for potential treatment of cancer: an updated overview. Cancer Cell Int. 2022;22:246.
Jain D, Chaudhary P, Varshney N, Bin Razzak KS, Verma D, Zahra TRK, Janmeda P, Sharifi-Rad J, Dastan SD, Mahmud S, et al. Tobacco smoking and liver cancer risk: potential avenues for carcinogenesis. J Oncol. 2021;2021:5905357.
Iqbal MJ, Javed Z, Herrera-Bravo J, Sadia H, Anum F, Raza S, Tahir A, Shahwani MN, Sharifi-Rad J, Calina D, Cho WC. Biosensing chips for cancer diagnosis and treatment: a new wave towards clinical innovation. Cancer Cell Int. 2022;22:354.
Ianoși SL, Batani A, Ilie MA, Tampa M, Georgescu SR, Zurac S, Boda D, Ianosi NG, Neagoe D, Calina D, et al. Non-invasive imaging techniques for the in vivo diagnosis of Bowen’s disease: three case reports. Oncol Lett. 2019;17:4094–101.
Pietrobon V. Cancer metabolism. J Transl Med. 2021;19:87.
Quispe C, Herrera-Bravo J, Javed Z, Khan K, Raza S, Gulsunoglu-Konuskan Z, Daştan SD, Sytar O, Martorell M, Sharifi-Rad J, Calina D. Therapeutic applications of curcumin in diabetes: a review and perspective. Biomed Res Int. 2022;2022:1375892.
Asgharian P, Tazekand AP, Hosseini K, Forouhandeh H, Ghasemnejad T, Ranjbar M, Hasan M, Kumar M, Beirami SM, Tarhriz V, et al. Potential mechanisms of quercetin in cancer prevention: focus on cellular and molecular targets. Cancer Cell Int. 2022;22:257.
Javed Z, Khan K, Herrera-Bravo J, Naeem S, Iqbal MJ, Raza Q, Sadia H, Raza S, Bhinder M, Calina D, et al. Myricetin: targeting signaling networks in cancer and its implication in chemotherapy. Cancer Cell Int. 2022;22:239.
Dhyani P, Quispe C, Sharma E, Bahukhandi A, Sati P, Attri DC, Szopa A, Sharifi-Rad J, Docea AO, Mardare I, et al. Anticancer potential of alkaloids: a key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022;22:206.
Sharifi-Rad J, Herrera-Bravo J, Kamiloglu S, Petroni K, Mishra AP, Monserrat-Mesquida M, Sureda A, Martorell M, Aidarbekovna DS, Yessimsiitova Z, et al. Recent advances in the therapeutic potential of emodin for human health. Biomed Pharmacother. 2022;154:113555.
Liu X, Yao L, Qu J, Liu L, Lu N, Wang J, Zhang J. Cancer-associated fibroblast infiltration in gastric cancer: the discrepancy in subtypes pathways and immunosuppression. J Transl Med. 2021;19:325.
Alghasham AA. Cucurbitacins—a promising target for cancer therapy. Int J Health Sci (Qassim). 2013;7:77–89.
Kaya G, Melzig M. Quantitative determination of cucurbitacin E and cucurbitacin I in homoeopathic mother tincture of Gratiola officinalis L. by HPLC. Die Pharmazie-Int J Pharm Sci. 2008;63:851–3.
Olmedo D, Rodríguez N, Vásquez Y, Solis P, Lopez-Perez J, Feliciano AS, Gupta M. A new coumarin from the fruits of Coutarea hexandra. Nat Prod Res. 2007;21:625–31.
Salehi B, Sharifi-Rad J, Capanoglu E, Adrar N, Catalkaya G, Shaheen S, Jaffer M, Giri L, Suyal R, Jugran AK, et al. Cucurbita plants: from farm to industry. Appl Sci-Basel. 2019;9:21.
Rolnik A, Olas B. Vegetables from the Cucurbitaceae family and their products: positive effect on human health. Nutrition. 2020;78:110788.
Salehi B, Capanoglu E, Adrar N, Catalkaya G, Shaheen S, Jaffer M, Giri L, Suyal R, Jugran AK, Calina D, et al. Cucurbits plants: a key emphasis to its pharmacological potential. Molecules. 2019;24:23.
WFO The World Flora Online. http://www.worldfloraonline.org/.
Chemspider. http://www.chemspider.com/.
Painuli S, Quispe C, Herrera-Bravo J, Semwal P, Martorell M, Almarhoon ZM, Seilkhan A, Ydyrys A, Rad JS, Alshehri MM, et al. Nutraceutical profiling, bioactive composition, and biological applications of Lepidium sativum L. Oxid Med Cell Longev. 2022;2022:2910411.
Hossain R, Quispe C, Saikat ASM, Jain D, Habib A, Janmeda P, Islam MT, Radha S, Daştan SD, Kumar M, et al. Biosynthesis of secondary metabolites based on the regulation of microRNAs. Biomed Res Int. 2022;2022:9349897.
Clericuzio M, Mella M, Vita-Finzi P, Zema M, Vidari G. Cucurbitane triterpenoids from Leucopaxillus gentianeus. J Nat Prod. 2004;67:1823–8.
Islam MT, Quispe C, El-Kersh DM, Shill MC, Bhardwaj K, Bhardwaj P, Sharifi-Rad J, Martorell M, Hossain R, Al-Harrasi A, et al. A literature-based update on Benincasa hispida (Thunb.) Cogn.: traditional uses, nutraceutical, and phytopharmacological profiles. Oxid Med Cell Longev. 2021;2021:6349041.
Ul Haq F, Ali A, Khan MN, Shah SMZ, Kandel RC, Aziz N, Adhikari A, Choudhary MI, El-Seedi HR, Musharraf SG. Metabolite profiling and quantitation of cucurbitacins in Cucurbitaceae plants by liquid chromatography coupled to tandem mass spectrometry. Sci Rep. 2019;9:1–11.
Kaushik U, Aeri V, Mir SR. Cucurbitacins—an insight into medicinal leads from nature. Pharmacogn Rev. 2015;9:12–8.
Omokhua-Uyi AG, Van Staden J. Phytomedicinal relevance of South African Cucurbitaceae species and their safety assessment: a review. J Ethnopharmacol. 2020;259:112967.
Muruganantham N, Solomon S, Senthamilselvi M. Anti-cancer activity of Cucumis sativus (cucumber) flowers against human liver cancer. Int J Pharm Clin Res. 2016;8:39–41.
Rajasree R, Sibi P, Francis F, William H. Phytochemicals of Cucurbitaceae family—a review. Int J Pharmacogn Phytochem Res. 2016;8:113–23.
Hou X, Meehan EJ, Xie J, Huang M, Chen M, Chen L. Atomic resolution structure of cucurmosin, a novel type 1 ribosome-inactivating protein from the sarcocarp of Cucurbita moschata. J Struct Biol. 2008;164:81–7.
Manchali S, Chidambara Murthy KN, Vishnuvardana S, Patil BS. Nutritional composition and health benefits of various botanical types of melon (Cucumis melo L.). Plants (Basel). 2021;10:1755.
Vella FM, Cautela D, Laratta B. Characterization of polyphenolic compounds in cantaloupe melon by-products. Foods. 2019;8:196.
Huerta-Reyes M, Tavera-Hernández R, Alvarado-Sansininea JJ, Jiménez-Estrada M. Selected species of the Cucurbitaceae family used in Mexico for the treatment of diabetes mellitus. Molecules. 2022;27:3440.
Bardaa S, Ben Halima N, Aloui F, Ben Mansour R, Jabeur H, Bouaziz M, Sahnoun Z. Oil from pumpkin (Cucurbita pepo L.) seeds: evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016;15:1–12.
Wang D-C, Pan H-Y, Deng X-M, Xiang H, Gao H-Y, Cai H, Wu L-J. Cucurbitane and hexanorcucurbitane glycosides from the fruits of Cucurbita pepo cv dayangua. J Asian Nat Prod Res. 2007;9:525–9.
Martínez-Valdivieso D, Font R, Fernández-Bedmar Z, Merinas-Amo T, Gómez P, Alonso-Moraga Á, del Río-Celestino M. Role of zucchini and its distinctive components in the modulation of degenerative processes: genotoxicity, anti-genotoxicity, cytotoxicity and apoptotic effects. Nutrients. 2017;9:755.
Melim C, Lauro MR, Pires IM, Oliveira PJ, Cabral C. The role of glucosinolates from cruciferous vegetables (Brassicaceae) in gastrointestinal cancers: from prevention to therapeutics. Pharmaceutics. 2022;14:190.
Favela-González KM, Hernández-Almanza AY, De la Fuente-Salcido NM. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: a review. J Food Biochem. 2020;44:e13414.
Dong L, Almeida A, Pollier J, Khakimov B, Bassard J-E, Miettinen K, Stærk D, Mehran R, Olsen CE, Motawia MS. An independent evolutionary origin for insect deterrent cucurbitacins in Iberis amara. Mol Biol Evol. 2021;38:4659–73.
Khayyal MT, Agha AM, Zaki HF, El-Sahar A, Abdel-Aziz H. Mechanisms involved in the anti-inflammatory and vascular effects of Iberis amara extract. Planta Med. 2015;81:1097–102.
Zou J-M, Wang L-S, Ma X-M, Guo Y-J, Shi R-B. A new cucurbitacin from Picria fel-terrae: Note. J Asian Nat Prod Res. 2006;8:367–71.
Kim IH, Uchiyama N, Kawahara N, Goda Y. Iridoid glycosides and cucurbitacin glycoside from Neopicrorhiza scrophulariiflora. Phytochemistry. 2006;67:2691–6.
Wu P-L, Lin F-W, Wu T-S, Kuoh C-S, Lee K-H, Lee S-J. Cytotoxic and anti-HIV principles from the rhizomes of Begonia nantoensis. Chem Pharm Bull. 2004;52:345–9.
Muñoz O, Delporte C, Backhouse N, Erazo S, Negrete R, Maldonado S, López-Pérez JL, San Feliciano A. A new cucurbitacin glycoside from Kageneckia oblonga (Rosaceae). Zeitschrift für Naturforschung C. 2000;55:141–5.
Delporte C, Munozb O, Rojas J, Ferrándiz M, Payá M, Erazo S, Negrete R, Maldonado S, San Feliciano A, Backhouse N. Pharmaco-toxicological study of Kageneckia oblonga, Rosaceae. Zeitschrift für Naturforschung C. 2002;57:100–8.
Wan J, Wang X-J, Guo N, Wu X-Y, Xiong J, Zang Y, Jiang C-X, Han B, Li J, Hu J-F. Highly oxygenated triterpenoids and diterpenoids from Fructus Rubi (Rubus chingii Hu) and their NF-kappa B inhibitory effects. Molecules. 1911;2021:26.
Chen C-H, Kuo TC-Y, Yang M-H, Chien T-Y, Chu M-J, Huang L-C, Chen C-Y, Lo H-F, Jeng S-T, Chen L-FO. Identification of cucurbitacins and assembly of a draft genome for Aquilaria agallocha. BMC Genomics. 2014;15:1–11.
Fujimoto H, Hagiwara H, Suzuki K, Yamazaki M. New toxic metabolites from a mushroom, Hebeloma vinosophyllum. II. isolation and structures of hebevinosides VI, VII, VIII, IX, X, and XI. Chem Pharm Bull. 1987;35:2254–60.
Fujimoto H, Suzuki K, Hagiwara H, Yamazaki M. New Toxic Metabolites from a Mushroom, Hebeloma vinosophyllum. I.: Structures of hebevinosides I, II, III, IV, and V. Chem Pharm Bull. 1986;34:88–99.
Jian-Wen T, Ze-Jun D, Ji-Kai L. New terpenoids from basidiomycetes Russula lepida. Helv Chim Acta. 2000;83:3191–7.
Lee J-S, Maarisit W, Abdjul DB, Yamazaki H, Takahashi O, Kirikoshi R, Kanno S-I, Namikoshi M. Structures and biological activities of triterpenes and sesquiterpenes obtained from Russula lepida. Phytochemistry. 2016;127:63–8.
Maarisit W, Yamazaki H, Kanno S-I, Tomizawa A, Lee J-S, Namikoshi M. Protein tyrosine phosphatase 1B inhibitory properties of seco-cucurbitane triterpenes obtained from fruiting bodies of Russula lepida. J Nat Med. 2017;71:334–7.
Clericuzio M, Tabasso S, Bianco MA, Pratesi G, Beretta G, Tinelli S, Zunino F, Vidari G. Cucurbitane triterpenes from the fruiting bodies and cultivated mycelia of Leucopaxillus gentianeus. J Nat Prod. 2006;69:1796–9.
Graziani EI, Allen TM, Andersen RJ. Lovenone, a cytotoxic degraded triterpenoid isolated from skin extracts of the North Sea dorid nudibranch Adalaria loveni. Tetrahedron Lett. 1995;36:1763–6.
Dhyani P, Sati P, Sharma E, Attri DC, Bahukhandi A, Tynybekov B, Szopa A, Sharifi-Rad J, Calina D, Suleria HAR, Cho WC. Sesquiterpenoid lactones as potential anti-cancer agents: an update on molecular mechanisms and recent studies. Cancer Cell Int. 2022;22:305.
Ali ES, Mitra K, Akter S, Ramproshad S, Mondal B, Khan IN, Islam MT, Sharifi-Rad J, Calina D, Cho WC. Recent advances and limitations of mTOR inhibitors in the treatment of cancer. Cancer Cell Int. 2022;22:284.
Irfan M, Javed Z, Khan K, Khan N, Docea AO, Calina D, Sharifi-Rad J, Cho WC. Apoptosis evasion via long non-coding RNAs in colorectal cancer. Cancer Cell Int. 2022;22:280.
Sharifi-Rad J, Herrera-Bravo J, Semwal P, Painuli S, Badoni H, Ezzat SM, Farid MM, Merghany RM, Aborehab NM, Salem MA, et al. Artemisia spp.: an update on its chemical composition, pharmacological and toxicological profiles. Oxid Med Cell Longev. 2022;2022:5628601.
Aiswarya SUD, Vikas G, Haritha NH, Liju VB, Shabna A, Swetha M, Rayginia TP, Keerthana CK, Nath LR, Reshma MV, et al. Cucurbitacin B, purified and characterized from the rhizome of corallocarpus epigaeus exhibits anti-melanoma potential. Front Oncol. 2022;12:903832.
Liu X, Duan C, Ji J, Zhang T, Yuan X, Zhang Y, Ma W, Yang J, Yang L, Jiang Z, et al. Cucurbitacin B induces autophagy and apoptosis by suppressing CIP2A/PP2A/mTORC1 signaling axis in human cisplatin resistant gastric cancer cells. Oncol Rep. 2017;38:271–8.
Zhou X, Yang J, Wang Y, Li W, Li-Ling J, Deng Y, Zhang M. Cucurbitacin B inhibits 12-O-tetradecanoylphorbol 13-acetate-induced invasion and migration of human hepatoma cells through inactivating mitogen-activated protein kinase and PI3K/Akt signal transduction pathways. Hepatol Res. 2012;42:401–11.
Shukla S, Khan S, Kumar S, Sinha S, Farhan M, Bora HK, Maurya R, Meeran SM. Cucurbitacin B alters the expression of tumor-related genes by epigenetic modifications in NSCLC and inhibits NNK-induced lung tumorigenesis. Cancer Prev Res (Phila). 2015;8:552–62.
Shukla S, Sinha S, Khan S, Kumar S, Singh K, Mitra K, Maurya R, Meeran SM. Cucurbitacin B inhibits the stemness and metastatic abilities of NSCLC via downregulation of canonical Wnt/beta-catenin signaling axis. Sci Rep. 2016;6:21860.
Klungsaeng S, Kukongviriyapan V, Prawan A, Kongpetch S, Senggunprai L. Targeted modulation of FAK/PI3K/PDK1/AKT and FAK/p53 pathways by cucurbitacin B for the antiproliferation effect against human cholangiocarcinoma cells. Am J Chin Med. 2020;48:1475–89.
Gupta P, Srivastava SK. Inhibition of Integrin-HER2 signaling by Cucurbitacin B leads to in vitro and in vivo breast tumor growth suppression. Oncotarget. 2014;5:1812–28.
Duangmano S, Sae-Lim P, Suksamrarn A, Domann FE, Patmasiriwat P. Cucurbitacin B inhibits human breast cancer cell proliferation through disruption of microtubule polymerization and nucleophosmin/B23 translocation. BMC Complement Altern Med. 2012;12:185.
Liang J, Zhang XL, Yuan JW, Zhang HR, Liu D, Hao J, Ji W, Wu XZ, Chen D. Cucurbitacin B inhibits the migration and invasion of breast cancer cells by altering the biomechanical properties of cells. Phytother Res. 2019;33:618–30.
Gao Y, Islam MS, Tian J, Lui VW, Xiao D. Inactivation of ATP citrate lyase by cucurbitacin B: a bioactive compound from cucumber, inhibits prostate cancer growth. Cancer Lett. 2014;349:15–25.
Touihri-Barakati I, Kallech-Ziri O, Ayadi W, Kovacic H, Hanchi B, Hosni K, Luis J. Cucurbitacin B purified from Ecballium elaterium (L.) A. Rich from Tunisia inhibits alpha5beta1 integrin-mediated adhesion, migration, proliferation of human glioblastoma cell line and angiogenesis. Eur J Pharmacol. 2017;797:153–61.
Sikander M, Hafeez BB, Malik S, Alsayari A, Halaweish FT, Yallapu MM, Chauhan SC, Jaggi M. Cucurbitacin D exhibits potent anti-cancer activity in cervical cancer. Sci Rep. 2016;6:36594.
Kim MS, Lee K, Ku JM, Choi YJ, Mok K, Kim D, Cheon C, Ko SG. Cucurbitacin D induces G2/M phase arrest and apoptosis via the ROS/p38 pathway in Capan-1 pancreatic cancer cell line. Evid Based Complement Alternat Med. 2020;2020:6571674.
Jacquot C, Rousseau B, Carbonnelle D, Chinou I, Malleter M, Tomasoni C, Roussakis C. Cucurbitacin-D-induced CDK1 mRNA up-regulation causes proliferation arrest of a non-small cell lung carcinoma cell line (NSCLC-N6). Anticancer Res. 2014;34:4797–806.
Cheng AC, Hsu YC, Tsai CC. The effects of cucurbitacin E on GADD45β-trigger G2/M arrest and JNK-independent pathway in brain cancer cells. J Cell Mol Med. 2019;23:3512–9.
Jing SY, Wu ZD, Zhang TH, Zhang J, Wei ZY. In vitro antitumor effect of cucurbitacin E on human lung cancer cell line and its molecular mechanism. Chin J Nat Med. 2020;18:483–90.
Hsu YC, Chen MJ, Huang TY. Inducement of mitosis delay by cucurbitacin E, a novel tetracyclic triterpene from climbing stem of Cucumis melo L., through GADD45gamma in human brain malignant glioma (GBM) 8401 cells. Cell Death Dis. 2014;5:e1087.
Li H, Chen H, Li R, Xin J, Wu S, Lan J, Xue K, Li X, Zuo C, Jiang W, Zhu L. Cucurbitacin I induces cancer cell death through the endoplasmic reticulum stress pathway. J Cell Biochem. 2018;120:2391–403.
Kim HJ, Park JH, Kim JK. Cucurbitacin-I, a natural cell-permeable triterpenoid isolated from Cucurbitaceae, exerts potent anticancer effect in colon cancer. Chem Biol Interact. 2014;219:1–8.
Zhang J, Song Y, Liang Y, Zou H, Zuo P, Yan M, Jing S, Li T, Wang Y, Li D, et al. Cucurbitacin IIa interferes with EGFR-MAPK signaling pathway leads to proliferation inhibition in A549cells. Food Chem Toxicol. 2019;132: 110654.
Liang Y, Zhang T, Ren L, Jing S, Li Z, Zuo P, Li T, Wang Y, Zhang J, Wei Z. Cucurbitacin IIb induces apoptosis and cell cycle arrest through regulating EGFR/MAPK pathway. Environ Toxicol Pharmacol. 2021;81:103542.
Torres-Moreno H, Marcotullio MC, Velazquez C, Ianni F, Garibay-Escobar A, Robles-Zepeda RE. Cucurbitacin IIb, a steroidal triterpene from Ibervillea sonorae induces antiproliferative and apoptotic effects on cervical and lung cancer cells. Steroids. 2020;157: 108597.
Sharifi-Rad J, Quispe C, Patra JK, Singh YD, Panda MK, Das G, Adetunji CO, Michael OS, Sytar O, Polito L, et al. Paclitaxel: application in modern oncology and nanomedicine-based cancer therapy. Oxid Med Cell Longev. 2021;2021:3687700.
Sharifi-Rad J, Quispe C, Butnariu M, Rotariu LS, Sytar O, Sestito S, Rapposelli S, Akram M, Iqbal M, Krishna A, et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021;21:318–318.
Salehi B, Prakash Mishra A, Nigam M, Karazhan N, Shukla I, Kiełtyka-Dadasiewicz A, Sawicka B, Głowacka A, Abu-Darwish MS, Hussein Tarawneh A, et al. Ficus plants: state of the art from a phytochemical, pharmacological, and toxicological perspective. Phytother Res. 2021;35:1187–217.
Sharifi-Rad J, Kamiloglu S, Yeskaliyeva B, Beyatli A, Alfred MA, Salehi B, Calina D, Docea AO, Imran M, Kumar NVA, et al. Pharmacological activities of psoralidin: a comprehensive review of the molecular mechanisms of action. Front Pharmacol. 2020;11:11.
Sharifi-Rad J, Quispe C, Imran M, Rauf A, Nadeem M, Gondal TA, Ahmad B, Atif M, Mubarak MS, Sytar O, et al. Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Oxid Med Cell Longev. 2021;2021:3268136.
Bakar F. Cucurbitacin B enhances the anticancer effect of imatinib mesylate through inhibition of MMP-2 expression in MCF-7 and SW480 tumor cell lines. Anti-Cancer Agents Med Chem. 2016;16:747–54.
Aribi A, Gery S, Lee DH, Thoennissen NH, Thoennissen GB, Alvarez R, Ho Q, Lee K, Doan NB, Chan KT. The triterpenoid cucurbitacin B augments the antiproliferative activity of chemotherapy in human breast cancer. Int J Cancer. 2013;132:2730–7.
Zhang M, Yin L, Yang S, Hong J, Chen C, Han D, Hou Y, Zhang B, Huang L, Zhang A. The synergistic effect of Cucurbitacin B and radiation treatment. Cancer Res. 2012;72:5728–5728.
Zhou J, Zhao T, Ma L, Liang M, Guo Y-J, Zhao L-M. Cucurbitacin B and SCH772984 exhibit synergistic anti-pancreatic cancer activities by suppressing EGFR, PI3K/Akt/mTOR, STAT3 and ERK signaling. Oncotarget. 2017;8: 103167.
Yar Saglam A, Alp E, Elmazoglu Z, Menevse S. Treatment with cucurbitacin B alone and in combination with gefitinib induces cell cycle inhibition and apoptosis via EGFR and JAK/STAT pathway in human colorectal cancer cell lines. Hum Exp Toxicol. 2016;35:526–43.
El-Senduny FF, Badria FA, El-Waseef AM, Chauhan SC, Halaweish F. Approach for chemosensitization of cisplatin-resistant ovarian cancer by cucurbitacin B. Tumor Biol. 2016;37:685–98.
Qu Y, Cong P, Lin C, Deng Y, Li-Ling J, Zhang M. Inhibition of paclitaxel resistance and apoptosis induction by cucurbitacin B in ovarian carcinoma cells. Oncol Lett. 2017;14:145–52.
Lee DH, Thoennissen NH, Goff C, Iwanski GB, Forscher C, Doan NB, Said JW, Koeffler HP. Synergistic effect of low-dose cucurbitacin B and low-dose methotrexate for treatment of human osteosarcoma. Cancer Lett. 2011;306:161–70.
Hossain R, Ray P, Sarkar C, Islam MS, Khan RA, Khalipha ABR, Islam MT, Cho WC, Martorell M, Sharifi-Rad J, et al. Natural compounds or their derivatives against breast cancer: a computational study. Biomed Res Int. 2022;2022:5886269.
Amir S, Shah STA, Mamoulakis C, Docea AO, Kalantzi O-I, Zachariou A, Calina D, Carvalho F, Sofikitis N, Makrigiannakis A, Tsatsakis A. Endocrine disruptors acting on estrogen and androgen pathways cause reproductive disorders through multiple mechanisms: a review. Int J Environ Res Public Health. 2021;18:1464.
Chainitikun S, Saleem S, Lim B, Valero V, Ueno NT. Update on systemic treatment for newly diagnosed inflammatory breast cancer. J Adv Res. 2021;29:1–12.
Luo S, Wang H, Bai L, Chen Y, Chen S, Gao K, Wang H, Wu S, Song H, Ma K, et al. Activation of TMEM16A Ca2+-activated Cl− channels by ROCK1/moesin promotes breast cancer metastasis. J Adv Res. 2021;33:253–64.
Quetglas-Llabrés MM, Quispe C, Herrera-Bravo J, Catarino MD, Pereira OR, Cardoso SM, Dua K, Chellappan DK, Pabreja K, Satija S, et al. Pharmacological properties of bergapten: mechanistic and therapeutic aspects. Oxid Med Cell Longev. 2022;2022:8615242.
Mitrut P, Docea AO, Kamal AM, Mitrut R, Calina D, Gofita E, Padureanu V, Gruia C, Streba L. Colorectal cancer and inflammatory bowel disease. 2016.
Sharifi-Rad J, Rodrigues CF, Stojanovic-Radic Z, Dimitrijevic M, Aleksic A, Neffe-Skocinska K, Zielinska D, Kolozyn-Krajewska D, Salehi B, Prabu SM, et al. Probiotics: versatile bioactive components in promoting human health. Medicina-Lithuania. 2020;56:30.
Zlatian OM, Comanescu MV, Rosu AF, Rosu L, Cruce M, Gaman AE, Calina CD, Sfredel V. Histochemical and immunohistochemical evidence of tumor heterogeneity in colorectal cancer. Rom J Morphol Embryol. 2015;56:175–81.
Tanase A, Colita A, Ianosi G, Neagoe D, Branisteanu DE, Calina D, Docea AO, Tsatsakis A, Ianosi SL. Rare case of disseminated fusariosis in a young patient with graft vs host disease following an allogeneic transplant. Exp Ther Med. 2016;12:2078–82.
Calina D, Rosu L, Rosu AF, Ianosi G, Ianosi S, Zlatian O, Mitrut R, Docea AO, Rogoveanu O, Mitrut P, et al. Etiological diagnosis and pharmacotherapeutic management of parapneumonic pleurisy. Farmacia. 2016;64:946–52.
Ungureanu A, Zlatian O, Mitroi G, Drocas A, Tirca T, Calina D, Dehelean C, Docea AO, Izotov BN, Rakitskii VN, et al. Staphylococcus aureus colonisation in patients from a primary regional hospital. Mol Med Rep. 2017;16:8771–80.
Zlatian O, Balasoiu AT, Balasoiu M, Cristea O, Docea AO, Mitrut R, Spandidos DA, Tsatsakis AM, Bancescu G, Calina D. Antimicrobial resistance in bacterial pathogens among hospitalised patients with severe invasive infections. Exp Ther Med. 2018;16:4499–510.
Kitic D, Miladinovic B, Randjelovic M, Szopa A, Sharifi-Rad J, Calina D, Seidel V. Anticancer potential and other pharmacological properties of Prunus armeniaca L.: an updated overview. Plants. 2022;11:1885.
Sharifi-Rad J, Dey A, Koirala N, Shaheen S, El Omari N, Salehi B, Goloshvili T, Cirone Silva NC, Bouyahya A, Vitalini S, et al. Cinnamomum species: bridging phytochemistry knowledge, pharmacological properties and toxicological safety for health benefits. Front Pharmacol. 2021;12:600139–600139.
Boda D, Docea AO, Calina D, Ilie MA, Caruntu C, Zurac S, Neagu M, Constantin C, Branisteanu DE, Voiculescu V, et al. Human papilloma virus: apprehending the link with carcinogenesis and unveiling new research avenues (Review). Int J Oncol. 2018;52:637–55.
Sharifi-Rad J, Quispe C, Rahavian A, Pereira Carneiro JN, Rocha JE, Alves Borges Leal AL, Bezerra Morais Braga MF, Melo Coutinho HD, Ansari Djafari A, Alarcón-Zapata P, et al. Bioactive compounds as potential agents for sexually transmitted diseases management: a review to explore molecular mechanisms of action. Front Pharmacol. 2021;12:1886.
Cole S, Gianferante DM, Zhu B, Mirabello L. Osteosarcoma: a surveillance, epidemiology, and end results program-based analysis from 1975 to 2017. Cancer. 2022;128:2107–18.
Sani TA, Mohammadpour E, Mohammadi A, Memariani T, Yazdi MV, Rezaee R, Calina D, Docea AO, Goumenou M, Etemad L, Shahsavand S. Cytotoxic and apoptogenic properties of Dracocephalum Kotschyi aerial part different fractions on calu-6 and mehr-80 lung cancer cell lines. Farmacia. 2017;65:189–99.
Buga AM, Docea AO, Albu C, Malin RD, Branisteanu DE, Ianosi G, Ianosi SL, Iordache A, Calina D. Molecular and cellular stratagem of brain metastases associated with melanoma. Oncol Lett. 2019;17:4170–5.
Sharifi-Rad J, Quispe C, Durazzo A, Lucarini M, Souto EB, Santini A, Imran M, Moussa AY, Mostafa NM, El-Shazly M, et al. Resveratrol’ biotechnological applications: enlightening its antimicrobial and antioxidant properties. J Herb Med. 2022;32:100550.
Hu C, Iwasaki M, Liu Z, Wang B, Li X, Lin H, Li J, Li JV, Lian Q, Ma D. Lung but not brain cancer cell malignancy inhibited by commonly used anesthetic propofol during surgery: implication of reducing cancer recurrence risk. J Adv Res. 2021;31:1–12.
Bauer C, Herwig R, Lienhard M, Prasse P, Scheffer T, Schuchhardt J. Large-scale literature mining to assess the relation between anti-cancer drugs and cancer types. J Transl Med. 2021;19:274.
Kirdeeva Y, Fedorova O, Daks A, Barlev N, Shuvalov O. How should the worldwide knowledge of traditional cancer healing be integrated with herbs and mushrooms into modern molecular pharmacology? Pharmaceuticals (Basel). 2022;15:868.
Quispe C, Herrera-Bravo J, Khan K, Javed Z, Semwal P, Painuli S, Kamiloglu S, Martorell M, Calina D, Sharifi-Rad J. Therapeutic applications of curcumin nanomedicine formulations in cystic fibrosis. Progr Biomater. 2022;11:321–9.
Salehi B, Calina D, Docea AO, Koirala N, Aryal S, Lombardo D, Pasqua L, Taheri Y, Castillo CMS, Martorell M, et al. Curcumin’s Nanomedicine formulations for therapeutic application in neurological diseases. J Clin Med. 2020;9:35.
Musat G, Evsei A, Calina D, Docea AO, Doukas SG, Vageli DP, Nepka C, Spandidos DA, Mitroi M. Rare amyloidoma of the tongue base: a case report and review of the literature. Mol Clin Oncol. 2020;12:258–62.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the review. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Cucurbitacins chemical characterization: identity, physical and chemical properties, isolation,synthesis of cucurbitacins and their derivatives.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Varela, C., Melim, C., Neves, B.G. et al. Cucurbitacins as potential anticancer agents: new insights on molecular mechanisms. J Transl Med 20, 630 (2022). https://doi.org/10.1186/s12967-022-03828-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03828-3