Abstract
Background
The combined association of triglyceride-glucose (TyG) index and different systolic blood pressure (SBP) levels with all-cause and cardiovascular mortality among the general population remains unclear.
Methods
In this study, 6245 individuals were from the National Health and Nutrition Examination Survey (1999–2002). The study endpoints were all-cause and cardiovascular mortality. Multivariate Cox proportional hazards regression models were used to explore the combined association of TyG index and different SBP levels with all-cause and cardiovascular mortality.
Results
During a mean follow-up period of 66.8 months, a total of 284 all-cause deaths (331/100000 person-years) and 61 cardiovascular deaths (66/100000 person-years) were recorded. Multivariate Cox regression analysis revealed that the combination of low TyG index and low SBP (< 120 mmHg and < 130 mmHg) was associated with a reduced risk of all-cause and cardiovascular mortality than others. However, survival benefit was not observed in the combined group with the low TyG index and SBP < 140 mmHg. Furthermore, the mortality rate in the combined group of low TyG index and low SBP gradually increased with the elevation of SBP level.
Conclusion
The combination of low TyG index and low SBP (< 120 mmHg and < 130 mmHg) was associated with a lower risk of all-cause and cardiovascular mortality. However, no survival benefit was observed in the combined group of low TyG index and SBP < 140 mmHg.
Similar content being viewed by others
Background
Cardiovascular disease (CVD) and diabetes mellitus (DM) is currently the most prevalent co-morbidities worldwide, and their intrinsic association is based on sharing some common pathological pathways and risk factors [1]. The triglyceride-glucose (TyG) index was regarded as a reliable surrogate biomarker of insulin resistance (IR), first proposed by Simental-Mendía et al. in 2008 [2]. Initial studies found that the TyG index might be a valuable indicator for identifying DM and metabolic syndrome [3, 4]. Subsequently, available studies have reported that the TyG index plays an important role in a variety of CVD, including stable coronary artery disease (CAD), atherosclerosis, coronary artery calcification, acute coronary syndrome (ACS), and stent restenosis [5,6,7,8,9,10]. On the other hand, hyperglycemia would have deleterious cardiovascular effects. Especially in cancer patients, hyperglycemia could enhance the cardiotoxicity of anticancer agents and attenuate its anticancer efficacy [11]. Apart from the above research evidence, the association between the TyG index and hypertension has rarely been addressed. However, IR and hypertension may develop through a shared pathophysiological pathway that IR triggers excessive activation of the renin–angiotensin–aldosterone system and increases sympathetic nervous system activity [12].
To date, there is consensus that elevated systolic blood pressure (SBP) results in a significantly higher risk of CVD and mortality, but previous evidence suggests that different SBP levels have markedly different effects on prognosis. For example, the Systolic Blood Pressure Intervention Trial (SPRINT) showed that patients with SBP < 120 mmHg could achieve significant cardiovascular and survival benefits compared to the SBP < 140 mmHg group [13]. The recent Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients (STEP) showed that patients with SBP < 130 mmHg were associated with lower cardiovascular risk than those with SBP < 150 mmHg [14]. In addition to the clinical benefits of lowering blood pressure (BP), it is also critical to consider that the low BP level may affect specific populations. For example, hypotension may be a more significant risk factor than hypertension in patients with small disease-related dementia [15, 16]. The above evidence suggests that patients with different SBP levels would experience a markedly different risk of CVD and mortality, and these differences may significantly impact clinicians' treatment decisions [17]. From this perspective, exploring the combination of the TyG index and different levels of SBP not only fills the gaps in previous studies but also contributes to improved cardiovascular and survival benefits. However, studies on the combined association of the TyG index and different SBP levels on all-cause and cardiovascular mortality are limited.
Therefore, the present study was designed to investigate the combined effect of the TyG index and different SBP levels on mortality and further explore the prevalence of mortality in these combined groups.
Materials and methods
Study design and population
NHANES (National Health and Nutrition Examination Survey) is a survey designed to assess adults' and children's health and nutritional status in the United States and is conducted every two years. NHANES uses a complex multistage sampling design, and statistical analysis requires weighting using sample weights to correct for nonresponse and sample design [18]. Data from this study were merged from 2 cycles (1999–2000 and 2001–2002), and a 4-year fasting weight (WTSAF4YR) was applied to the data analysis according to NHANES analysis guidelines. NHANES is conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS). The NCHS Research Ethics Review Committee reviewed and approved the NHANES study protocol. All participants signed written informed consent. The NHANES data used in this study can be extracted from DataDryad (https://doi.org/10.5061/dryad.d5h62). The study sample was obtained from the NHANES database from 1999 to 2002. After excluding missing baseline data and individuals under 18 years of age, 6,245 samples entered the final analysis. The specific study population flow chart is shown in Additional file 1: Fig. S1.
Data collection and definitions
Age, gender, education, physical activity level, smoking, drinking, history of comorbid diseases (hypertension, CAD, and DM), medication history (antihypertensive, statin, and antithrombotic) were collected by questionnaire. Antihypertensive drugs included angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blocker (ARB), β-receptor blockers, calcium channel blockers, and diuretics. Antithrombotic drugs include warfarin, aspirin, and clopidogrel. All study participants were measured for height, waist circumference (WC), weight, and heart rate (HR) by trained examiners at the Mobile Examination Center (MEC). Body mass index (BMI) is calculated according to the following formula: BMI = weight (kg)/height (m2). Blood pressure (BP) was the average of at least three measurements taken by two physicians using a standard mercury sphygmomanometer [19]. Fasting venous blood samples were sent to the Lipoprotein Analytical Laboratory (Johns Hopkins University School of Medicine), and total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) concentrations were measured using Hitachi 704 Analyzer. Fasting blood glucose (FBG) concentration was measured using a complete blood count (CBC) identification procedure. Glycated hemoglobin (HbA1c) was measured by analyzing whole blood using the Primus Automated HPLC System (Primus I, Model CLC330). Serum creatinine and uric acid (UA) were detected using a Beckman automated clinical analyzer. The estimated glomerular filtration rate (eGFR) was calculated using the abbreviated MDRD formula [20].
Endpoints and follow-up
The study endpoint was all-cause and cardiovascular mortality. All-cause mortality in this study was mainly caused by heart disease (I00-I09, I11, I13, I20-I51), cerebrovascular disease (I60-I69), cancer (C00-C97) and respiratory disease (J10-J18, J40-J47). Cardiovascular mortality included deaths caused by heart disease (I00-I09, I11, I13, I20-I51) and cerebrovascular disease (I60-I69). The NHANES participants were followed up for mortality through December 31, 2006. Death data were extracted from public-use linked mortality files in the NHANSE database.
Statistical analysis
All statistical analyses in this study were performed under the guidance of the CDC guidelines (https://wwwn.cdc.gov/nchs/nhanes/tutorials/default.aspx). All statistical analyses of this study were weighted with the sample weights provided by NHANES, as recommended by NCHS. The basic characteristics of the study population are presented with unweighted and weighted samples. For unweighted samples, continuous variables were presented as mean ± standard deviation or median (interquartile range), and categorical variables were presented as proportions. For weighted samples, continuous variables were presented by survey-weighted mean (95% confidence interval (CI)), and categorical variables were presented by survey-weighted percentage (95% CI). Univariate Cox hazard regression analysis was used to explore the association of baseline variables with all-cause and cardiovascular mortality to identify risk factors. The optimal cut-point for the TyG index was determined by receiver operating characteristic (ROC) analysis. Univariate and multivariate Cox hazard regression analyses were used to analyze the combined association of TyG index and SBP with all-cause and cardiovascular mortality. Adjusted variables in multivariate Cox proportional hazards regression models were based on clinical relevance or univariate association with outcomes, and further screening was performed to determine the final regression model based on the number of available events [21]. Bar charts and trend lines were used to show all-cause and cardiovascular mortality trends in the combined variable groups, and mortality rates were calculated using the person-year method. Kaplan–Meier estimator plotted cumulative risk curves for the combined variable groups, and differences between groups were compared using the log-rank test.
All analyses were performed using R statistical software version 4.0.2, and Microsoft Excel (Microsoft, Washington, DC, USA). All P values were 2-sided with a significance level of < 0.05.
Results
Baseline characteristics
A total of 6,245 participants were included in the final analysis. The baseline characteristics of the unweighted and weighted samples are presented in Table 1. The mean (95% CI) age of the weighted sample was 42.00 years (41.28, 42.73), and 49.83% were men, of whom 58.16% were at SBP < 120 mmHg, 81.83% were at SBP < 130 mmHg, and 94.92% was at SBP < 140 mmHg). The TyG index and SBP were approximately normally distributed, and the mean (95% CI) of the TyG index and SBP were 8.56 (8.52, 8.59) and 118.64 (117.89, 119.39) mmHg, respectively (Additional file 1: Fig. S2 and S3). Pearson correlation analysis showed a significant positive correlation between the TyG index and SBP (Pearson r = 0.204; p < 0.001), as shown in Additional file 1: Table S1 and Fig. S4.
Univariate Cox regression analysis of risk factors for all-cause and cardiovascular mortality
During a mean follow-up period of 66.8 months, 284 all-cause deaths occurred, with an all-cause mortality rate of 331 /100000 person-years; 61 cardiovascular deaths occurred, with a cardiovascular mortality rate of 66 /100000 person-years. Univariate Cox regression analysis revealed that age, male, WC, SBP, HR, low education and physical activity level, smoking, drinking, DM, hypertension, CAD, FBG, HbA1c, TC, TG, UA, eGFR, and TyG index were risk factors for all-cause mortality. Meanwhile, age, SBP, low levels of education and physical activity, DM, hypertension, CAD, FBG, HbA1c, TC, UA, eGFR, and TyG index were risk factors for cardiovascular mortality, as shown in Additional file 1: Table S2.
Combined association of TyG index and different SBP levels with all-cause and cardiovascular mortality
The ROC curves analysis determined that the optimal cut-off points for the predictive value of the TyG index for all-cause and cardiovascular mortality were 8.86 and 8.55, respectively (Additional file 1: Tables S3 and S4). The multivariate-adjusted model in Tables 2, 3 and 4 showed that the low TyG index group was associated with a reduced risk of all-cause and cardiovascular mortality compared with the high TyG index group (all-cause mortality rate: 257/100000 person-years, HR: 0.55, 95% CI 0.39–0.78; cardiovascular mortality rate: 39/100000 person-years, HR: 0.58, 95% CI 0.31–0.96).
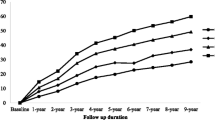
The multivariate-adjusted model in Table 2 showed that the individuals with SBP < 120 mmHg had a lower risk of all-cause and cardiovascular mortality than those with SBP ≥ 120 mmHg (all-cause mortality rate: 209/100000 person-years, HR: 0.48, 95% CI 0.34–0.67; cardiovascular mortality rate: 20/100000 person-years, HR: 0.28, 95% CI 0.14–0.55). The combined group with low TyG index and SBP < 120 mmHg had the lowest risk of all-cause and cardiovascular mortality compared with the other groups (all-cause mortality rate: 165/100000 person-years, HR: 0.29, 95% CI 0.18–0.46; cardiovascular mortality rate: 16/100000 person-years, HR: 0.16, 95% CI 0.05–0.46). In Table 3, the multivariate-adjusted model showed that the individuals with SBP < 130 mmHg were associated with a reduced risk of all-cause and cardiovascular mortality compared with those with SBP ≥ 130 mmHg (all-cause mortality rate: 276/100000 person-years, HR: 0.60, 95% CI 0.43–0.85; cardiovascular mortality rate: 41/100000 person-years, HR: 0.44, 95% CI 0.23–0.83). The combination of low TyG index and SBP ≥ 130 mmHg had the lowest risk of all-cause and cardiovascular mortality compared with the others (all-cause mortality rate: 211/100000 person-years, HR: 0.36, 95% CI 0.22–0.60; cardiovascular mortality rate: 31/100000 person-years, HR: 0.32, 95% CI 0.13–0.77). However, univariate and multivariate analysis showed that the individuals with SBP < 140 mmHg were not different from the SBP ≥ 140 mmHg group in the risk of all-cause and cardiovascular mortality in Table 4. In addition, the combination of low TyG index and SBP < 140 mmHg did not achieve any benefit in all-cause or cardiovascular mortality. During a mean follow-up period of 66.8 months, the combined group with low TyG index and low SBP levels (< 120 mmHg and < 130 mmHg) experienced a lower cumulative risk of all-cause and cardiovascular mortality compared with the other groups (Log-rank p < 0.001, Fig. 1). In addition, the all-cause and cardiovascular mortality in the different combination groups and trends was illustrated in Fig. 2. The low TyG index and SBP < 120 mmHg combination had the lowest all-cause and cardiovascular mortality compared with the other groups. Moreover, all-cause and cardiovascular mortality in the combination of low TyG index and low SBP tended to increase as the level of SBP increased.
Discussion
Our findings suggest that the TyG index and SBP are risk factors for all-cause and cardiovascular mortality based on a large representative sample of the general US population. The study results showed that low TyG index and SBP levels (< 120 mmHg and < 130 mmHg) were associated with a reduced risk of all-cause and cardiovascular mortality, respectively. More importantly, the combination of low TyG index and low SBP (< 120 mmHg and < 130 mmHg) yielded a further reduction in all-cause and cardiovascular mortality risk than the other groups, with a more significant effect on cardiovascular mortality. However, survival benefit was not observed in the combined group with the low TyG index and SBP < 140 mmHg. Moreover, the mortality rate in the combined group of low TyG index and low SBP gradually increased with the elevation of SBP level.
Currently, growing evidence suggests that the high TyG index is a risk factor for all-cause and cardiovascular mortality in patients with CVD. For example, some studies have identified the TyG index as an independent risk factor for a composite endpoint (with components such as all-cause or cardiovascular death) in patients with stable CAD [5, 22]. Some other studies have shown that the TyG index is an independent predictor of composite endpoints (with components such as all-cause or cardiovascular death) in patients with ACS [6, 23]. Likewise, several studies suggested that the TyG index is a risk factor for all-cause or cardiovascular mortality in patients with HF [10, 24]. However, the above studies only included mortality events as part of the composite endpoint and failed to directly investigate the independent relationship between the TyG index and death. A recent paper revealed a U-shaped relationship between the TyG index and death in hypertensive patients, and this specific relationship may be related to factors such as diet, aging, and low BMI [25]. It is important to note that these findings are from patients with CVD. In fact, the TyG index was calculated from glycemic and triglyceride parameters in healthy individuals [2], so the application of the TyG index in patients with CVD may be influenced by hyperlipidemia or DM, and these confounding factors cannot be eliminated [26]. Indeed, several studies failed to find an independent association between the TyG index and new-onset cardiovascular events in patients with DM or CVD [27, 28]. These results further support that the application of the TyG index in patients with CVD is influenced by hyperlipidemia and DM. The TyG index in this study was derived from healthy individuals, adhering to its initial definition, so that the TyG index can be used to its best value in our study. The findings of this study favorably support the TyG index as a risk factor for all-cause and cardiovascular mortality events in the general population.
As mentioned in the background section, the effect of changes in SBP levels on death is dramatic. The SPRINT study showed that the risk of all-cause and cardiovascular mortality was reduced by 27% and 43% in the group with SBP < 120 mmHg compared to those with SBP < 140 mmHg during a median follow-up of 3.26 years [13]. Due to the tremendous impact of the SPRINT results, the ACC/AHA guidelines subsequently redefined the hypertension diagnostic criteria to ≥ 130/80 mmHg, replacing the previous 140/90 mmHg [29]. The results of the recent STEP study suggest that the patients with SBP < 130 mmHg can make an important contribution to cardiovascular event benefits than those with SBP < 150 mmHg in older patients, but there is no difference in mortality [14]. The above studies suggest different optimal SBP levels, which also provides direction for our study to compare the effect of these SBP levels on mortality in the general population. The results of this study suggest that it is advisable to maintain SBP below 120 or 130 mmHg in the general population. In addition, we conducted a supplementary analysis that SBP at 120–130 mmHg had a significantly higher risk of all-cause and cardiovascular mortality than those with SBP ≤ 120 mmHg. However, when the SBP ≥ 130 mmHg group was used as a reference, individuals with SBP at 120–130 mmHg did not observe a reduction in the risk of all-cause and cardiovascular mortality (Additional file 1: Table S5). This may be because the difference in all-cause and cardiovascular mortality between the groups with SBP at 120–130 mmHg and > 130 mmHg was not significant.
However, long-term hypotension would affect the blood supply to all organs. Especially for vital organs such as the brain, heart, and kidney, insufficient blood supply could trigger related symptoms and organ damage [30]. The available evidence suggests that prolonged hypotension is associated with a higher risk of cognitive decline and vascular dementia [31, 32]. There are three main mechanisms regarding the impaired brain function resulting from hypotension. First, excessive BP reduction would lead to insufficient perfusion of small and medium-sized blood vessels in the brain, which would lead to local brain tissue degeneration and obstruction of cerebral venous return, disrupting the blood–brain barrier [33]. Second, hypotension would weaken the exchange of substances between the cerebrospinal fluid and the brain's interstitial fluid, which prevents the brain from removing metabolic wastes and excess proteins in a timely manner, thereby severely disrupting brain homeostasis [34]. Third, in the case of vascular aging or trauma, the structure and function of cerebral arteries would be damaged to various degrees, resulting in the decline of autoregulation capability [35]. Accordingly, controlled hypotension may also lead to a local insufficiency of blood supply to the brain, greatly increasing the possibility of brain damage. In addition, hypotension could cause insufficient blood supply to the coronary arteries [36]. Especially for patients with CVD, sudden acute episodes of hypotension might induce the occurrence of angina pectoris or even myocardial infarction [37]. Altogether, blindly antihypertensive treatment may not be suitable for all populations. The appropriate lowing BP treatment is worthwhile for the general population. However, for patients with severe cardio-cerebrovascular disease or vascular dementia, hypotension may bring them potential risks, so the blind pursuit of low BP levels requires careful consideration.
The introduction of the TyG index and SBP as combined variables into our study is based on two main aspects. On the one hand, the TyG index and SBP are risk factors for CVD and mortality [38, 39]. It seems reasonable that combining these two risk factors is supposed to generate a cumulative risk of mortality, but this hypothesis has not been confirmed in previous studies. The findings confirm our hypothesis that the combination of low TyG index and low SBP (< 120 mmHg and < 130 mmHg) resulted in an incremental effect on reducing all-cause and cardiovascular mortality risk. Furthermore, the combined TyG index and SBP can greatly facilitate the application of the TyG index in clinical treatment. A clinician will usually pay attention to BP values first in analyzing a patient's clinical data, and the TyG index does not appear directly on the laboratory test orders. This study suggests that it is advisable for clinicians to monitor adverse changes in BP, blood lipids, and FBG simultaneously during clinical treatment. Notably, the TyG index may vary with changes in BP levels in individuals. Therefore, we used Pearson correlation analysis to examine the correlation between the TyG index and SBP. The results showed that the correlation between SBP and TyG index was significant (p < 0.0001), but r < 0.3 indicated the correlation was very weak (Additional file 1: Fig. S4 and Table S1). Therefore, the bias from the interaction of the two may not influence the study results significantly.
The results of this study suggest that SBP may have a more important diagnostic value than the TyG index for all-cause and cardiovascular mortality. Therefore, we performed a ROC analysis to compare the diagnostic value of SBP and TyG index for all-cause and cardiovascular mortality. The results showed that SBP had a more important determinant value for all-cause (AUC: 0.630 vs. 0.604) and cardiovascular mortality (AUC: 0.665 vs. 0.570) than the TyG index (Additional file 1: Fig. S5). An important finding in this study was that elevated SBP (< 140 mmHg) combined with low TyG index did not yield a survival benefit. In contrast, the SBP < 140 mmHg group was only 10 mmHg higher than the SBP < 130 mmHg group. This phenomenon may be attributed to the significantly higher risk of all-cause and cardiovascular mortality in the population at 130–140 mmHg, with these increased risks offsetting the survival benefit of SBP < 140 mmHg. As shown in the Additional file 1: Table S6, the 130–140 mmHg group had the highest risk of all-cause and cardiovascular mortality, regardless of SBP < 130 or > 140 mmHg as the reference group. On the other hand, low SBP (< 140 mmHg) combined with the low TyG index group was not statistically different, but a reduction in the risk of all-cause and cardiovascular mortality was still observed. This negative result did not reach statistical significance, presumably due to the insufficient sample size of this combined group. Therefore, future studies with larger cohorts are needed to confirm these findings.
Some potential mechanisms may explain the combined association of the TyG index and SBP with all-cause and cardiovascular mortality. Long-term hypertension can cause target organ damage, including heart, brain, and kidneys complications, resulting in an increased risk of mortality due to corresponding organ failure or cardiovascular and cerebrovascular events [40, 41]. The association of the TyG index with all-cause and cardiovascular mortality is mainly mediated through cardiovascular events [42]. First, the TyG index is calculated from FBG and TG, both of which are acknowledged risk factors for CVD [43, 44]. Elevated FBG levels can have toxic effects on heart tissue and blood vessels and cause disorders of lipid metabolism and abnormal platelet function [45]. In addition, hyperglycemic states have pro-inflammatory and cardiotoxic effects, which may adversely affect cardiovascular outcomes. Interestingly, Quagliariello V et al. [46] found that SGLT-2 inhibitors may reduce the adverse effects of inflammation and apoptosis through engaging NLRP3 and MyD88-related pathways in doxorubicin-treated mice, resulting in significant improvements in cardiac functions. Higher TG levels can cause disorders of lipid metabolism and atherosclerosis, leading to the development of CVD [47]. More importantly, higher levels of the TyG index reflect IR, which is related to the pathogenesis of CVD [48]. First, IR would cause elevated blood glucose levels, triggering inflammation, oxidative stress, and dyslipidemia, which can exacerbate CVD progression [49]. Second, IR induces abnormal secretion of nitric oxide (NO) and impairs endothelial function [50, 51]. Third, IR may exacerbate platelet aggregation and adhesion, promoting the formation of thrombosis and inflammation [52]. Fourth, insulin plays an important inhibitory role in the hydrolysis of triglycerides [53]. In the case of insufficient insulin secretion or insulin action failure, triglycerides' hydrolysis will be significantly accelerated, resulting in a large number of fatty acids entering the mitochondria for oxidation to supply energy [54]. In this process, excessive reactive oxygen species (ROS) are generated that would damage the mitochondrial function and reduce its viability [55]. The above factors may reasonably explain the mechanism of the role of the TyG index on the occurrence and development of CVD.
Some limitations of this study should be mentioned. First, the results of this study are from a cohort study. Although multivariate hazard regression models were used to adjust for confounding factors, the confounding effects on the results were not completely eliminated. In addition, some study individuals were excluded due to missing data and lost follow-up, which may introduce selection bias to the study results. Second, this study is a non-randomized controlled trial (RCT), and individuals with low SBP levels may not have been treated with antihypertensive medication and therefore may have failed to consider the clinical benefits of antihypertensive medication. Further RCTs are needed to validate these findings. Third, this study excluded those samples with missing data, which might result in a potential selection bias. Lastly, the findings were drawn from healthy individuals in the United States and may not apply to other regions or diseased populations.
Conclusion
Our findings suggest that the combination of low TyG index and low SBP (< 120 mmHg and < 130 mmHg) was associated with a lower risk of all-cause and cardiovascular mortality. However, no survival benefit was observed in the combined group of low TyG index and SBP < 140 mmHg. Furthermore, the mortality rate in the combined group of low TyG index and low SBP gradually increased with the elevation of SBP level. This study's results may help clinicians identify potentially fatal risk factors as early as possible, thus effectively reducing the risk of cardiovascular events and death.
Availability of data and materials
The NHANES datasets are available on DataDryad (https://doi.org/10.5061/dryad.d5h62).
Abbreviations
- CVD:
-
Cardiovascular disease
- DM:
-
Diabetes mellitus
- IR:
-
Insulin resistance
- CAD:
-
Coronary artery disease
- ACS:
-
Acute coronary syndrome
- HF:
-
Heart failure
- TyG:
-
Triglyceride-glucose
- SBP:
-
Systolic blood pressure
- ACEI:
-
Angiotensin-converting enzyme inhibitors
- ARB:
-
Angiotensin receptor blocker
- WC:
-
Waist circumference
- HR:
-
Heart rate
- MEC:
-
Mobile examination center
- BMI:
-
Body mass index
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- FBG:
-
Fasting blood glucose
- HbA1c:
-
Glycated haemoglobin
- UA:
-
Uric acid
- eGFR:
-
Estimated glomerular filtration rate
- ROC:
-
Receiver operating characteristic
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Goshayeshi L, Akbari Rad M, Bergquist R, Allahyari A, Hashemzadeh K, Tead MC-R, Hoseini B. Demographic and clinical characteristics of severe Covid-19 infections: a cross-sectional study from Mashhad University of Medical Sciences Iran. BMC Infect Dis. 2021;21(1):656.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK, Lee JH, Yim HW, Kang MI, Lee WC, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS ONE. 2014;9(2): e90430.
Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, Fernández-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the Vascular-Metabolic CUN cohort. Prev Med. 2016;86:99–105.
Jin JL, Cao YX, Wu LG, You XD, Guo YL, Wu NQ, Zhu CG, Gao Y, Dong QT, Zhang HW, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10(11):6137–46.
Wang L, Cong HL, Zhang JX, Hu YC, Wei A, Zhang YY, Yang H, Ren LB, Qi W, Li WY, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):80.
Zhu Y, Liu K, Chen M, Liu Y, Gao A, Hu C, Li H, Zhu H, Han H, Zhang J, et al. Triglyceride-glucose index is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents. Cardiovasc Diabetol. 2021;20(1):137.
Li M, Zhan A, Huang X, Hu L, Zhou W, Wang T, Zhu L, Bao H, Cheng X. Positive association between triglyceride glucose index and arterial stiffness in hypertensive patients: the China H-type Hypertension Registry Study. Cardiovasc Diabetol. 2020;19(1):139.
Won KB, Park EJ, Han D, Lee JH, Choi SY, Chun EJ, Park SH, Han HW, Sung J, Jung HO, et al. Triglyceride glucose index is an independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc Diabetol. 2020;19(1):34.
Yang S, Du Y, Liu Z, Zhang R, Lin X, Ouyang Y, Chen H. Triglyceride-glucose index and extracellular volume fraction in patients with heart failure. Front Cardiovasc Med. 2021;8: 704462.
Quagliariello V, De Laurentiis M, Cocco S, Rea G, Bonelli A, Caronna A, Lombari MC, Conforti G, Berretta M, Botti G, et al. NLRP3 as putative marker of ipilimumab-induced cardiotoxicity in the presence of hyperglycemia in estrogen-responsive and triple-negative breast cancer cells. Int J Mol Sci. 2020;21(20):7802.
Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, Sowers J. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment–a position paper of the The Obesity Society and The American Society of Hypertension. Obesity (Silver Spring). 2013;21(1):8–24.
Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–16.
Zhang W, Zhang S, Deng Y, Wu S, Ren J, Sun G, Yang J, Jiang Y, Xu X, Wang TD, et al. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med. 2021;385(14):1268–79.
Caruso P, Signori R, Moretti R. Small vessel disease to subcortical dementia: a dynamic model, which interfaces aging, cholinergic dysregulation and the neurovascular unit. Vasc Health Risk Manag. 2019;15:259–81.
Moretti R, Caruso P. Small vessel disease-related dementia: an invalid neurovascular coupling? Int J Mol Sci. 2020;21(3):1095.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104.
Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR. National health and nutrition examination survey: analytic guideline, 1999-2010. Vital Health Stat 2. 2013;161:1–24.
Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88(5 Pt 1):2460–70.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70.
Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–35.
Gao A, Liu J, Hu C, Liu Y, Zhu Y, Han H, Zhou Y, Zhao Y. Association between the triglyceride glucose index and coronary collateralization in coronary artery disease patients with chronic total occlusion lesions. Lipids Health Dis. 2021;20(1):140.
Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, Tang C. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. 2019;18(1):150.
Guo W, Zhao L, Mo F, Peng C, Li L, Xu Y, Guo W, Sun A, Yan H, Wang L. The prognostic value of the triglyceride glucose index in patients with chronic heart failure and type 2 diabetes: a retrospective cohort study. Diabetes Res Clin Pract. 2021;177: 108786.
Zhou D, Liu XC, Kenneth L, Huang YQ, Feng YQ. A non-linear association of triglyceride glycemic index with cardiovascular and all-cause mortality among patients with hypertension. Front Cardiovasc Med. 2021;8: 778038.
Alizargar J, Bai CH, Hsieh NC, Wu SV. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. 2020;19(1):8.
Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–97.
Cho YR, Ann SH, Won KB, Park GM, Kim YG, Yang DH, Kang JW, Lim TH, Kim HK, Choe J, et al. Association between insulin resistance, hyperglycemia, and coronary artery disease according to the presence of diabetes. Sci Rep. 2019;9(1):6129.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–248.
Sulowicz W, Radziszewski A. Pathogenesis and treatment of dialysis hypotension. Kidney Int. 2006;70:S36–9.
Moretti R, Torre P, Antonello RM, Manganaro D, Vilotti C, Pizzolato G. Risk factors for vascular dementia: hypotension as a key point. Vasc Health Risk Manag. 2008;4(2):395.
Rawlings AM, Juraschek SP, Heiss G, Hughes T, Meyer ML, Selvin E, Sharrett AR, Windham BG, Gottesman RF. Association of orthostatic hypotension with incident dementia, stroke, and cognitive decline. Neurology. 2018;91(8):e759–68.
Mayer SA, Rincon F. Treatment of intracerebral haemorrhage. The Lancet Neurology. 2005;4(10):662–72.
Orešković D, Radoš M, Klarica M. Role of choroid plexus in cerebrospinal fluid hydrodynamics. Neuroscience. 2017;354:69–87.
Yang T, Sun Y, Lu Z, Leak RK, Zhang F. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res Rev. 2017;34:15–29.
Tuman KJ, McCarthy RJ, Najafi H, Ivankovich AD. Differential effects of advanced age on neurologic and cardiac risks of coronary artery operations. J Thorac Cardiovasc Surg. 1992;104(6):1510–7.
Luukinen H, Koski K, Laippala P, Airaksinen K. Orthostatic hypotension and the risk of myocardial infarction in the home-dwelling elderly. J Intern Med. 2004;255(4):486–93.
Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–67.
Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68.
Bielecka-Dabrowa A, Gluba-Brzózka A, Michalska-Kasiczak M, Misztal M, Rysz J, Banach M. The multi-biomarker approach for heart failure in patients with hypertension. Int J Mol Sci. 2015;16(5):10715–33.
Pimenta E, Oparil S. Prehypertension: epidemiology, consequences and treatment. Nat Rev Nephrol. 2010;6(1):21–30.
Piironen M, Ukkola O, Huikuri H, Havulinna AS, Koukkunen H, Mustonen J, Ketonen M, Lehto S, Airaksinen J, Antero Kesäniemi Y, et al. Trends in long-term prognosis after acute coronary syndrome. Eur J Prev Cardiol. 2017;24(3):274–80.
Jacobsen BK, Oda K, Knutsen SF, Fraser GE. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: the Adventist Health Study, 1976–88. Int J Epidemiol. 2009;38(1):245–52.
Czumaj A, Śledziński T, Carrero JJ, Stepnowski P, Sikorska-Wisniewska M, Chmielewski M, Mika A. Alterations of fatty acid profile may contribute to dyslipidemia in chronic kidney disease by influencing hepatocyte metabolism. Int J Mol Sci. 2019;20(10):2470.
Park CW, Kim HW, Lim JH, Yoo KD, Chung S, Shin SJ, Chung HW, Lee SJ, Chae CB, Kim YS, et al. Vascular endothelial growth factor inhibition by dRK6 causes endothelial apoptosis, fibrosis, and inflammation in the heart via the Akt/eNOS axis in db/db mice. Diabetes. 2009;58(11):2666–76.
Quagliariello V, De Laurentiis M, Rea D, Barbieri A, Monti MG, Carbone A, Paccone A, Altucci L, Conte M, Canale ML, et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol. 2021;20(1):150.
Guan Y, Hou X, Tian P, Ren L, Tang Y, Song A, Zhao J, Gao L, Song G. Elevated levels of apolipoprotein CIII increase the risk of postprandial hypertriglyceridemia. Front Endocrinol (Lausanne). 2021;12: 646185.
Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;10:74.
Yang Q, Vijayakumar A, Kahn BB. Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol. 2018;19(10):654–72.
Molina MN, Ferder L, Manucha W. Emerging role of nitric oxide and heat shock proteins in insulin resistance. Curr Hypertens Rep. 2016;18(1):1.
Nishikawa T, Kukidome D, Sonoda K, Fujisawa K, Matsuhisa T, Motoshima H, Matsumura T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of insulin resistance. Diabetes Res Clin Pract. 2007;77(Suppl 1):S161-164.
Gerrits AJ, Koekman CA, van Haeften TW, Akkerman JW. Platelet tissue factor synthesis in type 2 diabetic patients is resistant to inhibition by insulin. Diabetes. 2010;59(6):1487–95.
Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Investig. 2000;106(4):453–8.
Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2011;1807(6):726–34.
Baumber J. BALL BA, GRAVANCE CG, Medina V, DAVIES-MOREL MC: The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. Journal of andrology. 2000;21(6):895–902.
Acknowledgements
The authors thank to all of the NHANES participants who gave freely of their time to make this and other studies possible.
Funding
No.
Author information
Authors and Affiliations
Contributions
YY and WH participated in the study design. YY, HH, SJC, YD, XL, MG, HXN, XHC, CC, XHN participated in data cleaning. YY and MG performed the statistical analysis. YY and WH interpreted data, and drafted, then edited the report. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
NHANES is conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS). The NCHS Research Ethics Review Committee reviewed and approved the NHANES study protocol. All participants signed written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Fig. S1. Study population flow chart. Fig. S2. The mean (95% CI) of TyG index was 8.56 (8.52, 8.59). Fig. S3. The mean (95% CI) of SBP was 118.64 (117.89, 119.39) mmHg. Fig. S4. The correlation analysis between TyG index and SBP. Table S1. The correlation between TyG index and SBP was analyzed by Pearson correlation test. Table S2. Results of univariate Cox regression analysis. Table S3. ROC curve analysis determined optimal cut off thresholds for TyG and all-cause mortality. Table S4. ROC curve analysis determined optimal cut-off thresholds for TyG and cardiovascular mortality. Table S5. Univariate and multivariate Cox regression analysis of different SBP levels (<120 mmHg, 120-130 mmHg, and >130 mmHg) with all-cause and cardiovascular mortality. Fig. S5. Comparison of the predictive ability of SBP and TyG index for all-cause and cardiovascular mortality. Table S6. Univariate and multivariate Cox regression analysis of different SBP levels (<130 mmHg, 130-140 mmHg, and >140 mmHg) with all-cause and cardiovascular mortality.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, Y., Gu, M., Huang, H. et al. Combined association of triglyceride-glucose index and systolic blood pressure with all-cause and cardiovascular mortality among the general population. J Transl Med 20, 478 (2022). https://doi.org/10.1186/s12967-022-03678-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03678-z