Abstract
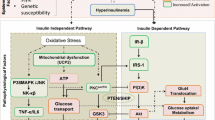
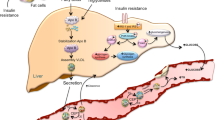
Insulin resistance (IR) is present in pathologies such as diabetes, obesity, metabolic syndrome, impaired glucose tolerance, hypertension, inflammation, cardiac disease, and dyslipidemias. Population studies show that IR is multifactorial and has genetic components, such as defects in the insulin-signaling pathway (as serine phosphorylation on insulin substrate or decreased activation of signaling molecules) and RAS/MAPK-dependent pathways. IR is connected to mitochondrial dysfunction, overproduction of oxidants, accumulation of fat, and an over-activation of the renin-angiotensin system linked to the NADPH oxidase activity. In addition, nitric oxide (NO), synthesized by nitric oxide synthases (endothelial and inducible), is also associated with IR when both impaired release and reduced bioavailability of all which lead to inflammation and hypertension. However, increased NO may promote vasculoprotection. Moreover, reduced NO release induces heat shock protein 70 kDa (HSP70) expression in IR and diabetes, mediating beneficial effects against oxidative stress injury, inflammation and apoptosis. HSP70 may be used as biomarker of the chronicity of diabetes. Hsp72 (inducible protein) is linked to vascular complications with a high-fat diet by blocking inflammation signaling (cytoprotective and anti-cytotoxicity intracellular role). Elucidating the IR signaling pathways and the roles of NO and HSPs is relevant to the application of new treatments, such as heat shock and thermal therapy, nitrosylated drugs, chemical chaperones or exercise training.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53.
Yip J, Facchini FS, Reaven GM. Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. J Clin Endocrinol Metab. 1998;83(8):2773–6.
Jeon JY, Ko SH, Kwon HS, Kim NH, Kim JH, Kim CS, et al. Prevalence of diabetes and prediabetes according to fasting plasma glucose and HbA1. Diabetes Metab J. 2013;37(5):349–57.
DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–94. This review discussed that insulin resistance appears to be a syndrome that is associated with a clustering of metabolic disorders.
Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806.
Manrique C, Lastra G, Sowers JR. New insights into insulin action and resistance in the vasculature. Ann N Y Acad Sci. 2014;1311(1):138–50.
Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Targher G, et al. Prevalence of IR in metabolic disorders: the Bruneck study. Diabetes. 1988;47(10):1643–9.
Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin N Am. 2011;95(5):875–92.
Muniyappa R, Yavuz S. Metabolic actions of angiotensin II and insulin: a microvascular endothelial balancing act. Mol Cell Endocrinol. 2012;378(1–2):59–69.
Pfeilschifter J, Eberhardt W, Huwiler A. Nitric oxide and mechanisms of redox signaling. J Am Soc Nephrol. 2003;14(8 Suppl 3):S237–40. Generation and action of free radicals such as nitric oxide, is discussed with a special focus on the renal injury.
Steinberg H, Cressman E, Wu Y, Hook G, Cronin J, Johnson A, et al. Insulin mediated nitric oxide production is impaired in insulin resistance. Diabetes. 1997;46:24A.
Morimoto RI. The heat shock response: systems biology of proteotoxic stress in aging and disease. Cold Spring Harb Symp Quant Biol. 2011;76:91–9. This review examines the properties of the stress-responsive transcription factor in the regulation of the HSR, our current understanding of the stress-sensing mechanisms that recognize and distinguish between acute stress such as heat shock and chronic proteostasis imbalance.
Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381(6583):571–80.
Macario AJL, Conway de Macario E. Sick chaperones, cellular stress, and disease. N Engl J Med. 2005;353(14):1489–501.
Tytell M, Hooper PL. Heat shock proteins: new keys to the development of cytoprotective therapies. Expert Opin Ther Targets. 2001;5(2):267–87.
Krause M, Bock PM, Takahashi HK, Homem De Bittencourt Jr PI, Newsholme P. The regulatory roles of NADPH oxidase, intra- and extra-cellular HSP70 in pancreatic islet function, dysfunction and diabetes. Clin Sci (Lond). 2015;128(11):789–803. This review describes possible mechanisms by which HSP70 participates in cell function/dysfunction, focusing on the possible role of HSPs in pancreatic islet α- and β-cell physiological function in health and Type 2 diabetes mellitus.
Hooper P. Diabetes, nitric oxide, and heat shock protein. Diabetes Care. 2003;26(3):951–2. Dr. Hooper describes that decreased Hsps linked to nitric oxide imbalance in type 1 and type 2 diabetes may be a primary factor leading to the development of diabetes and its diverse, widespread organ damage.
Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–607. The resistance to insulin-stimulated glucose uptake and hyperinsulinemia are involved in the etiology and clinical course of three major related diseases- non-insulin-dependent diabetes mellitus, hypertension, and coronary artery disease.
Faerch K, Borch-Johnsen K, Holst JJ, Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia. 2009;52(9):1714–23.
Kahn CR. Insulin resistance, Insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metab Clin Exp. 1978;27(12 Suppl 2):1893–902.
Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol Metab Clin N Am. 2004;33(2):283–303.
Groop LC, Saloranta C, Shank M, Bonadonna RC, Ferrannini E, DeFronzo RA. The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1991;72(1):96–107.
Ritz P, Berrut G. Mitochondrial function, energy expenditure, aging and insulin resistance. Diabetes Metab. 2005;31(Spec No2):5S67–73.
Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55 Suppl 2:S9–15. Recent magnetic resonance spectroscopy studies in healthy lean elderly subjects and healthy lean insulin-resistant offspring of parents with type 2 diabetes have demonstrated that reduced mitochondrial function may predispose these individuals to insulin resistance.
Dedoussis GV, Kaliora AC, Panagiotakos DB. Genes, diet and type 2 diabetes mellitus: a review. Rev Diabetes Stud. 2007;4(1):13–24.
Franks PW. Gene & environment interactions in type 2 diabetes. Curr Diabetes Rep. 2011;11(6):552–61.
Ashcroft FM, Rorsman P. Diabetes mellitus and the cell: the last ten years. Cell. 2012;148(6):1160–71.
Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic beta-cells. Nature. 2001;414(6865):807–12.
Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106(2):165–9.
Kim J, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102(4):401–14. The authors propose that mitochondrial dysfunction may be a central cause of insulin resistance and associated complications.
Zierath JR, Krook A, Wallberg-Henriksson H. Insulin action and insulin resistance in human skeletal muscle. Diabetologia. 2000;43(7):821–35.
Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7(9):353–61.
Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104(31):12587–94.
DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in non-insulin-dependent (Type II) diabetes mellitus. J Clin Invest. 1985;76(1):149–55.
Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–71.
Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–7. This review presents emerging evidence that supports the potentially unifying hypothesis that prominent features of type 2 diabetes are caused by mitochondrial dysfunction.
Jelenik T, Roden M. Mitochondrial plasticity in obesity and diabetes mellitus. Antioxid Redox Signal. 2013;19(3):258–68. The variability of baseline mitochondrial function in the main target tissue of insulin action, skeletal muscle and liver, may be attributed to inherited and acquired changes in either mitochondrial quantity or quality.
Lee HK. Evidence that the mitochondrial genome is the thrifty genome. Diabetes Res Clin Pract. 1999;45(2–3):127–35.
Crescenzo R, Bianco F, Mazzoli A, Giacco A, Liverini G, Iossa S. Mitochondrial efficiency and insulin resistance. Front Physiol. 2015;5:512. The authors present evidence suggesting that an increase in mitochondrial efficiency precede and therefore can contribute to the development of high-fat-induced insulin resistance in skeletal muscle.
Ren J, Pulakat L, Whaley-Connelland A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl). 2010;88(10):993–1001.
Murphy MP. Induction of mitochondrial ROS production by electrophilic lipids: a new pathway of redox signaling? Am J Physiol Heart Circ Physiol. 2006;290:H1754–5.
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40.
Solinas G, Karin M. JNK1 and IKKβ: molecular links between obesity and metabolic dysfunction. FASEB J. 2010;24:2596–611.
Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and b-cell dysfunction? Diabetes. 2003;52:1–8.
Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaeffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–37.
Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25.
Kim JK, Wi JK, Youn JH. Metabolic impairment precedes insulin resistance in skeletal muscle during high-fat feeding in rats. Diabetes. 1996;45:651–8.
Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–9.
Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol. 2005;25:989–94.
Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–7.
Zhang DX, Zou AP, Li PL. Ceramide-induced activation of NADPH oxidase and endothelial dysfunction in small coronary arteries. Am J Physiol Heart Circ Physiol. 2003;284:H605–12.
Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–45.
Ogihara T, Asano T, Ando K, Chiba Y, Sakoda H, Anai M, et al. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension. 2002;40(6):872–9.
Diamond-Stanic MK, Henriksen EJ. Direct inhibition by angiotensin II of insulin-dependent glucose transport activity in mammalian skeletal muscle involves a ROS-dependent mechanism. Arch Physiol Biochem. 2010;116:88–95.
Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51:993–9.
Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord. 2013;14(1):5–12.
Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev. 1991;43:109–42. Original review, which highlights the physiological, patho-physiological and, pharmacological aspects of the nitric oxide.
Poderoso JJ, Lisdero C, Schopfer F, Riobo N, Carreras MC, Cadenas E, et al. The regulation of mitochondrial oxygen uptake by redox reactions involving nitric oxide and ubiquinol. J Biol Chem. 1999;274:37709–16.
Boveris A, Valdez LB, Zaobornyj T, Bustamante J. Mitochondrial metabolic states regulate nitric oxide and hydrogen peroxide diffusion to the cytosol. Biochim Biophys Acta. 2006;1757(5–6):535–42.
Kissner R, Nauser T, Bugnon P, Lye PG, Koppenol WH. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem Res Toxicol. 1997;10:1285–92.
Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:C670–86.
Albina JE, Reichner JS. Role of nitric oxide in mediation of macrophage cytotoxicity and apoptosis. Cancer Metastasis Rev. 1998;17:39–53.
Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424.
Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev. 2009;89:27–71. The cellular and molecular mechanisms by which ROS and RNS are thought to participate in normal insulin action and in the induction of insulin resistance are mainly described.
Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84(24):9265–9.
Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615.
Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75(2):247–60.
Sansbury BE, Cummins TD, Tang Y, Hellmann J, Holden CR, Harbeson MA, et al. Overexpression of endothelial nitric oxide synthase prevents diet-induced obesity and regulates adipocyte phenotype. Circ Res. 2012;111:1176–89. Increased eNOS activity prevents the obesogenic effects of high-fat diet without affecting systemic insulin resistance, in part, by stimulating metabolic activity in adipose tissue.
Sadler CJ, Wilding JP. Reduced ventromedial hypothalamic neuronal nitric oxide synthase and increased sensitivity to NOS inhibition in dietary obese rats: further evidence of a role for nitric oxide in the regulation of energy balance. Brain Res. 2004;1016:222–8.
Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. Endocrinol Metab Clin N Am. 2008;37(3):685–711.
Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Ren Physiol. 2008;294:F1–9.
Vallés P, Manucha W. Nitric oxide in the kidney: physiological roles and regulation. In: Gimenéz MS, Gómez NN, editors. Advances in chemistry and biology of nitric oxide. Kerala: Research Signpost; 2007. p. 53–80. This review is focused on NOS isoforms distribution and regulation and on nitric oxide roles in renal physiology.
Mazzei L, Manucha W. Wt-1 expression linked to nitric oxide availability during neonatal obstructive nephropathy. Adv Urol. 2013;2013:401750.
Ushmorov A, Ratter F, Lehmann V, Dröge W, Schirrmacher V, Umansky V. Nitric oxide- induced apoptosis in human leukemic lines requires mitochondrial lipid degradation and cytochrome c release. Blood. 1999;93:2342–52.
Kim YM, Bombeck CA, Billiar TR. Nitric oxide as a bifunctional regulator of apoptosis. Circ Res. 1999;84(3):253–6.
Kim YM, Talanian RV, Billiar TR. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem. 1997;272:31138–48.
Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17(9):5317–27.
Manucha W, Vallés P. Cytoprotective role of nitric oxide associated whit Hsp70 expression in neonatal obstructive nephropathy. Nitric Oxide Biol Chem. 2008;18(3):204–15. In this original study, the authors postulated that the mechanism of apoptosis inhibition by nitric oxide would include stimulation of heat shock protein 70 expression.
Kaneki M, Shimizu N, Yamada D, Chang K. Nitrosative stress and pathogenesis of insulin resistance. Antioxid Redox Signal. 2007;9:319–29. S-nitrosylation has recently been proposed to play an important role in the pathogenesis of insulin resistance.
Wang H, Wang AX, Aylor K, Barrett EJ. Nitric oxide directly promotes vascular endothelial insulin transport. Diabetes. 2013;62:4030–42. Nitric oxide directly promotes endothelial cell insulin transport by enhancing protein S-nitrosylation.
Scherrer U, Randin D, Vollenweider P, Vollenweider L, Nicod P. Nitric oxide release accounts for insulin’s vascular effects in humans. J Clin Invest. 1994;94:2511–5.
Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101:1539–45. Receptor kinase activity is necessary to mediate production of nitric oxide through the insulin receptor. Both PI3K and Akt contribute importantly to this process.
Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser1179. J Biol Chem. 2001;276(32):30392–8.
Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28(5):463–91.
Zhang QJ, Holland WL, Wilson L, Tanner JM, Kearns D, Cahoon JM, et al. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes. 2012;61(7):1848–59.
Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284(1):R1–12.
Steinberg H, Cressman E, Wu Y, et al. Insulin mediated nitric oxide production is impaired in insulin resistance. Diabetes. 1997;46:24A.
Sansbury BE, Hill BG. Regulation of obesity and insulin resistance by nitric oxide. Free Radic Biol Med. 2014;73:383–99. This review discusses the role of nitric oxide in regulating adiposity and insulin sensitivity and places its modes of action into context with the known causes and consequences of metabolic disease.
Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Chayama K, Oshima T. Effect of obesity on endothelium-dependent, nitric oxide-mediated vasodilation in normotensive individuals and patients with essential hypertension. Am J Hypertens. 2001;14:1038–45.
Williams IL, Wheatcroft SB, Shah AM, Kearney MT. Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes Relat Metab Disord. 2002;26:754–64.
Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104:342–5. eNOS is important for the control not only of arterial pressure but also of glucose and lipid homeostasis.
Abudukadier A, Fujita Y, Obara A, Ohashi A, Fukushima T, Sato Y, et al. Tetrahydrobiopterin has a glucose-lowering effect by suppressing hepatic gluconeogenesis in an endothelial nitric oxide synthase-dependent manner in diabetic mice. Diabetes. 2013;62:3033–43.
Paneni F, Costantino S, Cosentino F. Role of oxidative stress in endothelial insulin resistance. World J Diabetes. 2015;6(2):326–32. The authors describe emerging theories concerning endothelial insulin resistance, with particular emphasis on the role oxidative stress. Complex molecular circuits including endothelial nitric oxide synthase, mitochondrial adaptor p66(Shc), and nicotinamide adenine dinucleotide phosphate-oxidase are discussed.
Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13:294–307. Improving endothelial insulin signaling may serve as a therapeutic strategy for ameliorating skeletal muscle insulin resistance.
Hasegawa Y, Saito T, Ogihara T, Ishigaki Y, Yamada T, Imai J, et al. Blockade of the nuclear factor-κB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation. 2012;125:1122–33.
Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, et al. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–8. The authors have demonstrated that during obesity induced by high-fat diet feeding, inflammation and insulin resistance develop in the vasculature well before these responses are detected in muscle, liver, or adipose tissue. This observation suggests that the vasculature is more susceptible than other tissues to the deleterious effects of nutrient overload.
Bender SB, Herrick EK, Lott ND, Klabunde RE. Diet-induced obesity and diabetes reduce coronary responses to nitric oxide due to reduced bioavailability in isolated mouse hearts. Diabetes Obes Metab. 2007;9:688–96.
Noronha BT, Li JM, Wheatcroft SB, Shah AM, Kearney MT. Inducible nitric oxide synthase has divergent effects on vascular and metabolic function in obesity. Diabetes. 2005;54:1082–9.
Fujimoto M, Shimizu N, Kunii K, Martyn JA, Ueki K, Kaneki M. A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice. Diabetes. 2005;54:1340–8. iNOS plays a role in fasting hyperglycemia and contributes to hepatic insulin resistance in ob/ob mice.
Sugita H, Fujimoto M, Yasukawa T, Shimizu N, Sugita M, Yasuhara S, et al. Inducible nitric-oxide synthase and NO donor induce insulin receptor substrate-1 degradation in skeletal muscle cells. J Biol Chem. 2005;280:14203–11.
Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23.
Turini P, Thalmann S, Jayet PY, Cook S, Mathieu C, Burcelin R, et al. Insulin resistance in mice lacking neuronal nitric oxide synthase is related to an alpha-adrenergic mechanism. Swiss Med Wkly. 2007;137:700–4.
Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–49.
Asea A. Hsp70: a chaperokine. Novartis Found Symp. 2008;291:173–9. discussion 179–183, 221–4.
Murphy ME. The HSP70 family and cancer. Carcinogenesis. 2013;34(6):1181–8.
Kang SS, Song JH, Lee MY, Kang YH, Lim SS, Ryu SY, et al. Developmental immunolocalization of heat shock protein 70 (HSP70) in epithelial cell of rat kidney. Histol Histopathol. 2011;26(11):1363–73.
Mazzei L, Docherty NG, Manucha W. Mediators and mechanisms of heat shock protein 70 based cytoprotection in obstructive nephropathy. Cell Stress Chaperones. 2015;20(6):893–906. This review summarizes our understanding of how the biological actions of Hsp70 may affect renal cytoprotection in the context of organ injury.
Vallés P, Jorro F, Carrizo L, Manucha W, Oliva J, Cuello-Carrión FD, et al. Heat shock proteins HSP27 and HSP70 in unilateral obstructed kidneys. Pediatr Nephrol. 2003;18(6):527–35.
Manucha W, Carrizo L, Ruete C, Molina H, Vallés P. Angiotensin II type I antagonist on oxidative stress and heat shock protein 70 (HSP 70) expression in obstructive nephropathy. Cell Mol Biol (Noisy-le-grand). 2005;51(6):547–55.
Carrizo LC, Ruete CM, Manucha WA, Ciocca DR, Vallés PG. Heat shock protein 70 expression is associated with inhibition of renal tubule epithelial cell apoptosis during recovery from low-protein feeding. Cell Stress Chaperones. 2006;11(4):309–24.
Borges TJ, Lopes RL, Pinho NG, Machado FD, Souza AP, Bonorino C. Extracellular Hsp70 inhibits pro-inflammatory cytokine production by IL-10 driven down-regulation of C/EBPβ and C/EBPδ. Int J Hyperth. 2013;29(5):455–63.
Rinaldi Tosi ME, Bocanegra V, Manucha W, Gil Lorenzo A, Valles PG. The Nrf2-Keap1 cellular defense pathway and heat shock protein 70 (Hsp70) response. Role in protection against oxidative stress in early neonatal unilateral ureteral obstruction (UUO). Cell Stress Chaperones. 2011;16(1):57–68.
Ferder L, Inserra F, Martinez-Maldonado M. Inflammation and the metabolic syndrome: role of angiotensin II and oxidative stress. Curr Hypertens Rep. 2006;8(3):191–8.
Garcia IM, Altamirano L, Mazzei L, Fornes M, Molina MN, Ferder L, et al. Role of mitochondria in paricalcitol-mediated cytoprotection during obstructive nephropathy. Am J Physiol Ren Physiol. 2012;302(12):F1595–605. This study evaluates the cytoprotective effects of paricalcitol, a VDR activator, at the mitochondrial level using an obstructive nephropathy model.
Garcia IM, Altamirano L, Mazzei L, Fornes M, Cuello-Carrion FD, Ferder L, et al. Vitamin D receptor modulated Hsp70/AT1 expression may protect the kidneys of SHRs at the structural and functional levels. Cell Stress Chaperones. 2014;19(4):479–91. Recent data suggest that Hsp70/AT1 modulated by VDR is involved in the mechanism by which paricalcitol provides renal protection in a hypertension model. In addition, lower AT1 expression through VDR induction could be a consequence of the heat shock response Hsp70-mediated cell protection.
Lutz W, Kohno K, Kumar R. The role of heat shock protein 70 in vitamin D receptor function. Biochem Biophys Res Commun. 2001;282(5):1211–9. Hsp70-like chaperone proteins play a role in controlling concentrations of the VDR within the cell.
Adams JS, Chen H, Chun RF, Nguyen L, Wu S, Ren SY, et al. Novel regulators of vitamin D action and metabolism: lessons learned at the Los Angeles zoo. J Cell Biochem. 2003;88(2):308–14.
Mitri J, Pittas AG. Vitamin D and diabetes. Endocrinol Metab Clin N Am. 2014;43(1):205–32. This article summarizes the current evidence from human studies that suggests but does not prove a relation between vitamin D and type 2 diabetes, and briefly reports on the potential association between vitamin D and type 1 diabetes.
Koroshi A, Idrizi A. Renoprotective effects of Vitamin D and renin-angiotensin system. Hippokratia. 2011;15(4):308–11.
Cheng Q, Boucher BJ, Leung PS. Modulation of hypovitaminosis D-induced islet dysfunction and insulin resistance through direct suppression of the pancreatic islet renin-angiotensin system in mice. Diabetologia. 2013;56(3):553–62.
Ferder M, Inserra F, Manucha W, Ferder L. The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system. Am J Physiol Cell Physiol. 2013;304(11):C1027–39. This review attempts to show that there may be a relationship between inflammatory processes induced by chronic overstimulation of the renin-angiotensin system (RAS) and the worldwide deficiency of vitamin D (VitD) and that both disorders are probably associated with environmental factors.
Soti C, Nagy E, Giricz Z, Vigh L, Csermely P, Ferdinandy P. Heat shock proteins as emerging therapeutic targets. Br J Pharmacol. 2005;146:769–80.
Pandey KB, Mishra N, Rizvi SI. Protein oxidation biomarkers in plasma of type 2 diabetic patients. Clin Biochem. 2010;43(4–5):508–11.
Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes. 2009;58:567–78.
Kondo T, Motoshima H, Igata M, Kawashima J, Matsumura T, Kai H, et al. The role of heat shock response in insulin resistance and diabetes. Diabetes Metab J. 2014;38:100–6. Physical medicine using simultaneous stimulation of heat with mild electric current activates heat shock response, thereby reducing visceral adiposity, insulin resistance, chronic inflammation and improving glucose homeostasis in mice models of T2DM, as well as in humans with MS or T2DM.
Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2011;300:E894–901.
Wei W, Liu Q, Tan Y, Liu L, Li X, Cai L. Oxidative stress, diabetes, and diabetic complications. Hemoglobin. 2009;33(5):370–7. Protective agents such as metallothionein, zinc and FGFs play an important role in preventing the development of diabetes and diabetic complications.
Marucci A, Miscio G, Padovano L, Boonyasrisawat W, Florez JC, et al. The role of HSP70 on ENPP1 expression and insulin-receptor activation. J Mol Med (Berl). 2009;87:139–44. The authors propose that type 2 diabetes results from a vicious cycle of metabolically induced inflammation, impaired insulin responsiveness, and subsequent loss of homeostatic signaling. A crucial and previously under-recognized event contributing to this loss of homeostasis is a reduction in heat shock proteins (HSPs, or stress proteins).
Hooper PL, Hooper PL. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones. 2009;14(2):113–5.
Krause M, Bock PM, Takahashi HK, Homem De Bittencourt Jr PI, Newsholme P. The regulatory roles of NADPH oxidase, intra- and extra-cellular HSP70 in pancreatic islet function, dysfunction and diabetes. Clin Sci (Lond). 2015;128(11):789–803. In this review, authors describe possible mechanisms by which HSP70 participates in cell function/dysfunction, including the activation of NADPH oxidase isoforms leading to oxidative stress, focusing on the possible role of HSPs and signaling in pancreatic islet α- and β-cell physiological function in health and Type 2 diabetes mellitus.
Kavanagh K, Zhang L, Wagner JD. Tissue-specific regulation and expression of heat shock proteins in type 2 diabetic monkeys. Cell Stress Chaperones. 2009;14(3):291–9.
Hooper PL. Insulin signaling, GSK-3, heat shock proteins and the natural history of type 2 diabetes mellitus: a hypothesis. Metab Syndr Relat Disord. 2007;5(3):220–30.
Lee JH, Gao J, Kosinski PA, Elliman SJ, Hughes TE, Gromada J, et al. Heat shock protein 90 (HSP90) inhibitors activate the heat shock factor 1 (HSF1) stress response pathway and improve glucose regulation in diabetic mice. Biochem Biophys Res Commun. 2013;430(3):1109–13.
Krause M, Heck TG, Bittencourt A, Scomazzon SP, Newsholme P, Curi R, et al. The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediat Inflamm. 2015;2015:249205. Imbalances in the HSP70 status, described by the [eHSP70]/[iHSP70] ratio, may be determinant to trigger a chronic pro-inflammatory state that leads to insulin resistance and T2DM development.
Nakhjavani M, Morteza A, Khajeali L, Esteghamati A, Khalilzadeh O, Asgarani F, et al. Increased serum HSP70 levels are associated with the duration of diabetes. Cell Stress Chaperones. 2010;15:959–64. Serum level of HSP70 is significantly higher in patients with diabetes and correlates with the duration of disease. Higher HSP70 in prolonged diabetes versus newly diagnosed diabetes may be an indicator of metabolic derangement in the course of diabetes.
Karpe PA, Tikoo K. Heat shock prevents insulin resistance-induced vascular complications by augmenting angiotensin-(1–7) signaling. Diabetes. 2014;63:1124–39. Induction of HSP72 is a novel approach to prevent insulin resistance-induced vascular complications.
Henstridge DC, Bruce CR, Drew BG, Tory K, Kolonics A, Estevez E, et al. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. 2014;63(6):1881–94. Increased oxidative metabolism associated with activation of HSP72 has potential clinical implications not only for type 2 diabetes but also for other disorders where mitochondrial function is compromised.
Chow AM, Steel R, Anderson RL. Hsp72 chaperone function is dispensable for protection against stress-induced apoptosis. Cell Stress Chaperones. 2009;14:253–63.
Chung J, Nguyen AK, Henstridge DC, Holmes AG, Stanley Chan MH, Mesa JL, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105:1739–44. HSP72 is essential in blocking inflammation and preventing insulin resistance in the context of genetic obesity or high-fat feeding.
Chae JI, Kim J, Son MY, Jeon YJ, Kim DW, Kim HE, et al. Cardioprotective molecules are enriched in beating cardiomyocytes derived from human embryonic stem cells. Int J Cardiol. 2013;165(2):341–54.
Gao H, Meng J, Xu M, Zhang S, Ghose B, Liu J, et al. Serum heat shock protein 70 concentration in relation to polycystic ovary syndrome in a non-obese Chinese population. PLoS ONE. 2013;8(6):e67727. Increased serum Hsp70 levels are associated with the combination of IR, oxidative stress and low-grade chronic inflammation in Polycystic ovary syndrome individuals, which provides supportive evidence that Hsp70 plays a key role in this pathogenesis.
Atalay M, Oksala NK, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, et al. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol (1985). 2004;97(2):605–11. Diabetic therapies that induce the stress response, whether via heat, bioactive compounds, or genetic manipulation, improve or prevent all of the morbidities and comorbidities associated with the disease.
Hooper PL, Balogh G, Rivas E, Kavanagh K, Vigh L. The importance of the cellular stress response in the pathogenesis and treatment of type 2 diabetes. Cell Stress Chaperones. 2014;19(4):447–64.
Krause M, Ludwig MS, Heck TG, Takahashi HK. Heat shock proteins and heat therapy for type 2 diabetes: pros and cons. Curr Opin Clin Nutr Metab Care. 2015;18(4):374–80. Transient increments in nitric oxide and heat shock protein 70 levels may explain the benefits of heat therapy in obesity and diabetes.
Bathaie SZ, Jafarnejad A, Hosseinkhani S, Nakhjavani M. The effect of hot-tub therapy on serum Hsp70 level and its benefit on diabetic rats: a preliminary report. Int J Hyperthermia. 2010;26(6):577–85. A decrease in complications in diabetic rats after hot-tub therapy is shown here.
Bhatia S, Shukla R, Venkata Madhu S, Kaur Gambhir J, Madhava Prabhu K. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin Biochem. 2003;36:557–62.
Henstridge DC, Whitham M, Febbraio MA. Chaperoning to the metabolic party: the emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes. Mol Metab. 2014;3:781–93. Targeted manipulation of Hsp72 or use of chemical chaperiones may have clinical utility in treating metabolic disorders such as insulin resistance and T2DM.
Prasad P, Kochhar A. Interplay of vitamin D and metabolic syndrome: a review. Diabetes Metab Syndr. 2015. March 6. The paper provides an insight into the physiology of vitamin D and relationship of vitamin D deficiency with risk factors of metabolic syndrome through observational and supplementation studies.
Rajan RS, Tsumoto K, Tokunaga M, Tokunaga H, Kita Y, Arakawa T. Chemical and pharmacological chaperones: application for recombinant protein production and protein folding diseases. Curr Med Chem. 2011;18(1):1–15.
Kashfi K. Nitric oxide-releasing hybrid drugs target cellular processes through S-nitrosylation. For Immunopathol Dis Ther. 2012;3(2):97–108.
Zaouali MA, Bejaoui M, Calvo M, Folch-Puy E, Pantazi E, Pasut G, et al. Polyethylene glycol rinse solution: an effective way to prevent ischemia-reperfusion injury. World J Gastroenterol. 2014;20(43):16203–14. The PEG-35 rinse solution prevented oxidative stress, mitochondrial damage, and liver autophagy. Further, it increased the expression of cytoprotective heat shock proteins such as HO-1 and HSP70, activated AMPK, and contributed to the restoration of cytoskeleton integrity after IRI.
McCarty MF, Barroso-Aranda J, Contreras F. Regular thermal therapy may promote insulin sensitivity while boosting expression of endothelial nitric oxide synthase-effects comparable to those of exercise training. Med Hypotheses. 2009;73(1):103–5. Regular thermal therapy has the potential to improve impaired insulin sensitivity and boost endothelial expression of the “constitutive” isoform of nitric oxide synthase-effects.
Atalay M, Oksala N, Lappalainen J, Laaksonen DE, Sen CK, Roy S. Heat shock proteins in diabetes and wound healing. Curr Protein Pept Sci. 2009;10(1):85–95.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
The animal experiments described herein were not performed by any of the authors, signifying that no animals were harmed in the course of preparing this manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Research and Technology Council of Cuyo University (SECyT), Mendoza, Argentina, and from the National Council of Scientific and Technical Research (CONICET) PIP 2010–2012, both of which were awarded to Walter Manucha. Grant no. PICT 2012–0234, BID 2777 OC/AR.
Additional information
This article is part of the Topical Collection on Hypertension and Obesity
Rights and permissions
About this article
Cite this article
Molina, M.N., Ferder, L. & Manucha, W. Emerging Role of Nitric Oxide and Heat Shock Proteins in Insulin Resistance. Curr Hypertens Rep 18, 1 (2016). https://doi.org/10.1007/s11906-015-0615-4
Published:
DOI: https://doi.org/10.1007/s11906-015-0615-4