Abstract
Akkermansia muciniphila (A. muciniphila) is present in the human gut microbiota from infancy and gradually increases in adulthood. The potential impact of the abundance of A. muciniphila has been studied in major cardiovascular diseases including elevated blood pressure or hypertension (HTN). HTN is a major factor in premature death worldwide, and approximately 1.28 billion adults aged 30–79 years have hypertension. A. muciniphila is being considered a next-generation probiotic and though numerous studies had highlighted the positive role of A. muciniphila in lowering/controlling the HTN, however, few studies had highlighted the negative impact of increased abundance of A. muciniphila in the management of HTN. Thus, in the review, we aimed to discuss the current facts, evidence, and controversy about the role of A. muciniphila in the pathophysiology of HTN and its potential effect on HTN management/regulation, which could be beneficial in identifying the drug target for the management of HTN.
Similar content being viewed by others
Introduction
Akkermansia spp., belong to the Verrucomicrobia family, with only two species identified so far, namely Akkermansia muciniphila (A. muciniphila) and Akkermansia glycaniphila [1, 2]. Both species are considered intestinal mucin-degrading bacterium; the former was initially isolated from the fecal samples of healthy Caucasian females in 2004, whereas the latter was isolated from fecal samples of reticulated python, Malayopython reticulatus in 2016 [1, 2]. A. muciniphila is an oval-shaped, non-motile, and gram-negative anaerobic bacteria, that grows well with an optimum temperature of 37 °C and pH of 6.5 [1], and it is present in wild animals, mice, hamsters, and humans [3]. Caputo et al. successfully sequenced A. muciniphila, directly from human stool samples using the whole-genome assembly method [4], and the abundance level of A. muciniphila in the human feces sample is approximately 3–4%. In rare conditions, the abundance level can increase up to 5% [5]. A. muciniphila is considered a potential probiotic due to its nature that can effectively use the gastrointestinal tract (GI) mucin [6] and possesses a unique survival mechanism, that is, the degradation of gastrointestinal mucin from the host causing the release of carbon and nitrogen sources for its survival [1, 7]. Also, its abundance level is modulated by dietary patterns and other changes in the mucin level [8]. In addition, it promotes the growth of other bacteria through a cross-feeding mechanism, mainly releasing amino acids and sugars during the GI mucin degradation process [8]. Its role has been studied in major diseases, such as diabetes mellitus, obesity, cardiovascular diseases, immune disorders, pregnancy complications, cancer, tumor, brain disorders, liver diseases, and kidney diseases. Its abundance level is very critical for normal physiological functions and any abnormality in its level is closely associated with the pathophysiology of these diseases [9,10,11,12,13]. Moreover, the drugs that we use to treat these diseases also impact A. muciniphila’s abundance level, mainly metformin [14], gemcitabine [15], paclitaxel [16], anti-PDI therapy [17], and some phytochemicals, such as andrographolide [18], puerarin [19], Bofutsushosan or Kampo [20] and resveratrol [21]. Additionally, A. muciniphila augments the drugs’ beneficial effects, mainly cisplatin—an anticancer drug- in lung cancer mice [21] and anti-PDI therapy through the production of CD4+T cells [17], metformin for glucose tolerance and glucose metabolism via secretion of glucose-regulating peptides and short-chain fatty acids (SCFAs) production in mice and humans [14, 22,23,24]. Thus, its interaction with the drugs is bidirectional. Interestingly, few recent studies from preclinical and clinical settings have shown that gut microbial dysbiosis can cause hypertension (HTN) [25, 26]. A Coronary Artery Risk Development in Young Adults (CARDIA) study conducted by Sun et al. found that both systolic and diastolic blood pressure (BP) is negatively correlated with the abundance of A. muciniphila [27]. Though it has been widely believed that A. muciniphila could play an unprecedented role in controlling the HTN, its role in the control of BP remains sparse. The current literature review suggests that there is no mechanistic evidence that unearths the direct role of A. muciniphila on the regulation of BP. Still, there is available indirect evidence that makes A. muciniphila a strong potential probiotic candidate for the control of BP. However, there are few contrary reports which highlight the negative role of A. muciniphila in controlling BP, especially in conditions where a higher inflammatory response is observed. Therefore, in this review, we aim to address and discuss the current knowledge on the potential roles of A. muciniphila in controlling/managing HTN.
Mechanisms of gut microbiota-mediated HTN
So far, scientists worldwide have collected enormous data and made substantial progress in understanding the pathophysiology of HTN. Homeostasis of BP is regulated by numerous factors, such as the sympathetic nervous system through the release of vasoactive peptides, mainly dopamine, serotonin, and norepinephrine [25, 28, 29], renin–angiotensin–aldosterone (RAA) system [30], chronic kidney diseases (CKDs) [31], genetics [32], and various environmental and lifestyle factors [33, 34]. Recently the gut microbiota has been included in this long list, and it changed the paradigm of understanding the etiology of HTN [35, 36]. Scientists are now considering the microbiome as a central regulator of human health [37]. Gut microbiota dysbiosis is defined as the imbalance in the gut microbial composition that is linked to the disease state [38] can lead to HTN through the modulation of several factors, including short-chain fatty acids (SCFA)-mediated role in the RAAS system and immune system, sympathetic nervous system, trimethylamine-N-oxide (TMAO) pathway, nitric oxide pathway, energy homeostasis pathway, chronic inflammation pathway, microbiota-derived hydrogen-sulfide pathway, bile-acids, and uremic toxin pathway [39, 40]. Thus, gut microbiota plays a crucial role through a variety of checkpoints for the regulation of HTN. Humans contain trillions of microbiota, and each microbe in a group or alone plays a specific role in human physiology, especially probiotics such as genera Lactobacillus and Bifidobacterium [41]. Based on recent results from various animal and human studies, a new microbe—A. muciniphila has been included in the list of probiotics [6, 41, 42], which controls many metabolites, especially SCFA, lipopolysaccharide, TMAO, and hydrogen sulfide [43].
Role of A. muciniphila in SCFAs regulated HTN
The SCFAs are produced from the diet mainly via saccharolytic fermentation of undigested carbohydrates, by anaerobic gut bacteria [43], and highly glycosylated mucin-degrading microbes, including A. muciniphila [5]. Humans produce approximately 500–600 mmol of SCFAs every day. Still, it strictly depends upon the amount of diet, especially the macronutrient content, such as carbohydrates, fibers, protein, and fat, that we consume [44]. The primary role of A. muciniphila is to degrade the mucin in the intestinal epithelial cells and supply energy sources (carbon and nitrogen) to goblet cells to the secretion of mucin again. This cycle should be in homeostasis [43]. In the secreted mucin, SCFAs such as acetate, propionate, butyrate, formate, isobutyrate, valerate, and isovalerate are produced in different concentrations, with acetate, propionate, and butyrate being the most abundant [45]. A. muciniphila mainly secretes acetate and propionate, which strengthen the gut barrier integrity through the regulation of tight junction proteins, such as occludin, claudins, and zona occludens [46]. Furthermore, the various clinical and preclinical studies using the 16s rRNA sequencing data have suggested that a decrease in the abundance of A. muciniphila could lead to the development of HTN [25, 28, 47]. Thus, all these results are based on association or correlation analysis. Still, to date, no direct studies (in-vivo, in-vitro, and clinical) use A. muciniphila to control BP. One of the major mechanisms of A. muciniphila in controlling BP could be the release of SCFAs from mucin [1, 7]. In the early days, SCFAs were thought to regulate BP through G-protein coupled receptors (GPR41 or FFAR3 and GPR43 or FFAR2) in humans, and Olfr78 in mice. In addition to this, presently, SCFAs regulate BP through GPR109a (also called HCA2 or NIACR1 or PUMA-G or HM47a) and OR51E2 in humans. In humans and mice, all three dominant SCFAs—propionate, acetate, butyrate, and subdominant SCFA—lactate regulate BP through these receptors, with propionate being the strongest ligand [48,49,50,51,52,53] to influence BP in humans via OR51E2.
Additionally, β-ionone—a volatile compound present in fruits, vegetables, and plants and cleaved from beta-carotene by an enzyme called carotenoid cleavage dioxygenase [54] influences BP in humans via OR51E2 [55, 56], but not through Olfr78 in mice [57]. The SCFAs – acetate and propionate [58,59,60], bind with the GPR41/GPR43 receptors, and promote the secretion of GLP-1/GLP-2 in the enteroendocrine L-cells of ileum and large intestine [1, 61]. In different diabetic and non-diabetic animal models, activation of GLP-1R (both exogenous and endogenous) has been demonstrated to lower BP by reducing sympathetic activity in the carotid body [62, 63]. Also, propionate involves in the release of a fat hormone–leptin, which alters the normotensive to hypertensive conditions. Zhao et al. have found that supplementation of A. muciniphila in chow diet-fed mice, reduced chronic low-grade inflammation by reducing plasma leptin and LPS [64], indicating that A. muciniphila might be playing more complex roles than what we knew so far. Besides, previous studies using GPR41−/− mice, have demonstrated that the acetate and propionate-promoted release of leptin is mediated with the activation of the GPR43 receptor only, not through the GPR41 receptor [65, 66]. These data suggest that A. muciniphila supplementation might have profound effects on BP regulation through the GPR41 receptor rather than the GPR43 receptor, but this hypothesis needs to be further studied.
Lipopolysaccharides synthesis
Gut barrier integrity plays an important role in preventing the leaking of synthesized lipopolysaccharides (LPS) and inflammatory markers into circulation, thereby regulating BP [67]. LPS is a group of lipopolysaccharide chemical complexes produced by the bacteria, especially from the outer membrane of the gram-negative bacteria, and it acts as a permeability barrier for the bacteria [68]. Also, LPS is a pathogen-associated molecular pattern (PAMP) molecule known to stimulate the Toll-Like Receptors (TLRs)—a class of pattern recognition receptors that potentiate the innate immune response. In particular, the TLR4 induces myddosome, which consists of adaptor proteins, such as MyD88, TIRAP, and serine-threonine kinases from the IRAK family, which receive the signals from TLR4, leading to the activation of inflammatory gene expression through NF-kB and AP-1 activation [69,70,71]. Disruption in the gut barrier integrity is closely associated with HTN due to increased intestinal inflammation and permeability [67, 72]. Kim et al. have demonstrated that hypertensive subjects had increased plasma LPS, intestinal fatty acid-binding protein (I-FABP), proinflammatory Th17 cells, and zonulin levels [72]. Supplementation of A. muciniphila improves the gut epithelial barrier integrity possibly through the reduction of circulatory LPS, inflammatory markers such as TNF-α, IL-6, CD36, SR-A1), IL-2, IFN-γ, IL-12p40, and MCP-1, and potentiating the expression of tight junction proteins (occludin, zonal occludens, and claudin-3, claudin-5) [73–76]. In addition, Chelakkot et al. have shown that Akkermansia-derived extracellular vesicles–Amuc_1100 reduce circulatory LPS levels [76] and activation of the TLR2 pathway [77], thereby promoting an anti-inflammatory effect. On the contrary, few studies have reported a higher level of A. muciniphila during disease progression [78, 79]. In our previous two studies, we found that a higher abundance of A. muciniphila was linked with the activation of LPS in CKD mice than in the control mice [80], and insufficient glycemic control in pediatric subjects with T1DM is linked to the higher level of A. muciniphila [81]. Thus, the level of A. muciniphila is critical for the pathophysiology of certain diseases where there is activation of an inflammatory-mediated pathway. Increased abundance of A. muciniphila degrades more mucus in the gut which damages the mucosal barrier, leading to the leak of inflammatory markers, LPS, and the activation of an inflammatory response [82]. Ganesh et al. have reported that A. muciniphila could differentially affect the gut ecosystem in relevance to the level of inflammation. In brief, they showed the presence of A. muciniphila in Salmonella enterica Typhimurium-infected gnotobiotic mice exacerbates the gut inflammatory response by increasing the mRNA levels of IFN-γ, IP-10, TNF-α, IL-2, IL-17, and IL-6 in the cecal and colonic tissue compared to the gnotobiotic controls mice [83], which explains A. muciniphila’s ability to disturb the host mucus homeostasis through the excessive mucin degradation process and eventually disturbs the gut ecosystem. Li et al. have demonstrated that the supplementation of A. muciniphila prevents the severity of atherosclerotic lesions by reducing metabolic endotoxemia [10]. To support this hypothesis, a recent study by Dan et al. on the Chinese population has demonstrated that the abundance of A. muciniphila is greatly elevated in hypertensive subjects [84]. Also, Cekanaviciute et al. have reported that A. muciniphila promotes Th1 lymphocyte differentiation [85]. Further, a study on the Brazilian population conducted by Silveira-Nunes et al. found a higher abundance of Akkermansia along with the increased TNF-α/IFN-γ ratio in the hypertensive subjects [86], suggesting the differential role of Akkermansia in mediating HTN.
So, activation of the LPS pathway might be detrimental in inciting hypertension through various mechanisms, such as endothelial dysfunction through the release of inducible nitric oxide, activating TLR4, and vasculature inflammation through the release of NADPH oxidase-dependent free radicals [87–89]. On the other hand, Akkermansia supplementation reduced low-grade inflammation by suppressing circulatory LPS in HFD-diet mice [64].
Trimethylamine-N-oxide
Several studies demonstrated that trimethylamine-N-oxide (TMAO) is a pathogenic metabolite formed by the gut microbiota and act as an independent risk factor for cardiovascular diseases, including HTN [90,91,92,93]. TMAO is formed by the oxidation of the intermediate metabolite TMA from the dietary L-carnitine, phosphatidylcholine, lecithin, and γ-butyrobetaine (γ-BB) via flavin monooxygenase 3 (FMO3) enzyme from gut microbes [94]. TMAO is involved in the development of hypertension through the elevation of vascular oxidative stress and endothelial dysfunction [95]. Xu et al. reported that the supplementation of A. muciniphila reverses the high expression of FMO3 in obesity conditions [76]. Also, recently Zhou et al. studied the relationship between circulating plasma TMAO levels and HTN. They found that TMAO levels are higher in patients with HTN than those without HTN [96]. Hsu et al. reported that a higher TMA level is associated with high BP load and abnormal 24-h ambulatory BP monitoring profile, along with the reduced abundance of genus Akkermansia [92]. Plovier et al. demonstrated that the supplementation of A. muciniphila significantly decreased plasma levels of TMAO and TMA and a two-fold reduction of FMO3 expression in mice [97]. Luo et al. demonstrated that cold exposure modulated the A. muciniphila abundance level in rats, thereby causing excessive secretion of TMAO which elevated the susceptibility to atrial fibrillation (AF) condition mainly through the enhanced infiltration of M1 macrophages in atria and increased protein expression of Casp1-p20 and cleaved GSDMD. The oral supplementation of A. muciniphila in rats significantly ameliorated the pro-AF property induced by cold exposure [98]. These results indicate a positive correlation between a higher level of TMAO or TMA with the HTN and a negative association with the abundance of the genus Akkermansia.
Hydrogen sulfide
Another potential mechanism by which the A. muciniphila regulates BP could be the release of endogenous hydrogen sulfide (H2S), as it is involved in the sulfate reduction process [99]. Rosario et al. have demonstrated that A. muciniphila produced H2S together with Escherichia spp., which has been considered a potential regulator of vascular homeostasis, possibly through the regulation of vascular tone and inflammation, antioxidant mechanism, vascular cell proliferation, and apoptosis [100, 101]. Several studies have pointed out that the H2S levels are inversely associated with hypertensive disease severity [102,103,104]. Supplementation of H2S reduces BP in experimental animal models [105, 106]. On the contrary, H2S has been implied in activating proinflammatory response [107, 108], and its higher level could cause inflammation in the gut [99]. Interestingly, A. muciniphila has been proposed to use the H2S for the production of cysteine [43], and cysteine is well-known for its anti-hypertensive effect [109], thereby it could control BP regulation. In addition, when there is an activation of the inflammatory response, the relation between A. muciniphila and H2S might turn on pathologic response rather than the protective response, because both entities have been found to have a differential role. There is a possibility that a high level of A. muciniphila can produce a high amount of H2S, which might elevate the BP due to the activation of an inflammatory response [99]. This is a mere hypothesis and must be proved experimentally, but the evidence strongly encourages this concept. Recently, pangenomic analysis of A. muciniphila revealed that the phylotype AmI contains genes required for the assimilatory sulfate reduction (ASR) process and thereby possibly increasing H2S level.
Role of A. muciniphila on risk factors that cause HTN
Chronic kidney disease (CKD)
CKD is closely associated with the pathogenesis of HTN, and the basic mechanisms behind the development of CKD-associated HTN are salt retention, endothelial dysfunction, sympathetic overactivity, volume overload, and abnormal hormonal level [110]. A classic manifestation of CKD is chronic inflammation, which causes a leaky gut, results in translocation of endotoxin, bacterial fragments, and uremic toxins in the circulation, and eventually leads to the accumulation of gut microbiota-derived uremic toxins in the circulation [111]. Gut microbiota-derived uremic toxins are mainly indoles (indole-3-acetic acid, indoxyl glucuronide, indoxyl sulfate (IS), kynurenine, kynurenic acid, melatonin, and quinolinic acid), phenols (hydroquinone, p-cresyl glucuronide, p-cresyl sulfate (PCS), phenol, and phenylacetic acid) [112]. The general effects of these uremic toxins are vascular dysfunction, such as increased vasoconstriction and decreased vasorelaxation, decrease in NO production, induction of oxidative stress, promotion of vascular cell apoptosis, and vascular remodeling [113]. Also, IS and PCS have been reported to promote vascular calcification [114]. Collectively all these detrimental effects are significantly associated with the pathogenesis of HTN. The role of A. muciniphila in gut-microbiota-derived toxins mediated vascular dysfunction leading to the HTN is controversial because of its differential role and abundance level in renal HTN. Li et al. have reported A. muciniphila level is reduced in association with elevated proinflammatory cytokines, such as IL-6, IL-8, and IFN-γ, and decreased anti-inflammatory cytokines, such as IL-4 and IL-10 in the CKD subjects in comparison with the HC subjects [79]. But, several studies have shown that significant elevation in the abundance of A. muciniphila level was observed in CKD animal models and human subjects [12, 80, 115, 116] along with the increase of IS and PCS [80]. So, the precise role of A. muciniphila in the context of renal HTN caused by CKD is still unclear, but it seems that A. muciniphila might augment the development of renal HTN rather than prevent it because A. muciniphila promotes inflammation, and in renal HTN a higher level of the inflammatory response has been reported [117] which might potentiate the level of A. muciniphila.
Renin-angiotensin system
The role and the activation of the renin-angiotensin (RAS) pathway in HTN has been extensively studied [118] and there is a possibility that a greater connection between A. muciniphila and the RAS pathway will be unveiled by the following studies: Buford et al. have reported that oral administration of the probiotic Lactobacillus paracasei increased the expression of the Angiotensin (1-7), an Angiotensin-I vasopeptide, and significantly enriched the abundance of A. muciniphila [119]. Roshanravan et al. also have concluded that the protective effects of sodium butyrate and inulin supplementation on type 2 diabetes mellitus can act via the enrichment of A. muciniphila on the angiotensin signaling pathway [120]. Duan et al. pointed out that the level of A. muciniphila was less in the ACE2−/y mice than in the ACE−/y-Akita mice and it showed a reduced number of myeloid angiogenic cells (MACs) without a significant increase in inflammatory monocytes. Furthermore, the administration (exogenous) of MACs restored gut barrier integrity and altered the gut microbiota [121]. Recently, a study conducted by Suzuki et al. showed that the altitude variation approximately ranging from 33 to 4000 m from the sea level, greatly affects the composition of gut microbiota in wild-type mice [122]. They found that the relative abundance of genus A. muciniphila was the most negatively correlated with the increasing altitude [122].
Furthermore, their predicted functional metagenome analysis indicated that the KEGG pathway—RAS was positively correlated with the increasing altitude, suggesting a strong association between A. muciniphila and the RAS pathway [122]. In addition, Robles-Vera et al. demonstrated that 5 weeks of treatment of renin-angiotensin-II blocker—losartan on spontaneously hypertensive rats (SHR) significantly reduced the gut dysbiosis and sympathetic drive in the gut, which possibly can contribute to the reduction of BP by modulating the immune system in the gut [123]. Also, they reported that losartan treatment significantly restored the abundance level of genus Akkermansia along with the other genera, such as Pedobacter and Lactobacillus in SHR [123]. Thus, these studies have pointed out the potential link between RAS and A. muciniphila, and establishing these two entities warrants further mechanistic studies.
Endothelial dysfunction/endothelial-derived nitric oxide pathway
Endothelial dysfunction is a type of non-obstructive chronic artery disease (CAD) in which blood vessel constricts instead to relax, leading to chronic chest pain [124]. The molecular mechanism for the occurrence of endothelial dysfunction is an imbalance between endothelial-derived relaxing factors (endothelin-1, angiotensin-II, thromboxane A2, and reactive oxygen species), and endothelium-derived hyperpolarizing factors (NO and prostacyclin) [125, 126]. Among them, endothelium-derived NO is considered as a potent vasodilator, and its implication in the regulation of BP through various mechanisms, such as inhibition of vasoconstriction, platelet aggregation, angiogenesis, oxidative stress, inflammation, and atherogenesis, and its reduced level would have opposite effects on vascular smooth muscle cells are well-established [127, 128]. Gut microbiota is closely linked to vascular dysfunction regulation [129]. Wang et al. reported that berberine improves vascular dysfunction via the modulation of gut microbiota possibly through the inhibition of TMAO production in angiotensin II-induced hypertensive mice [130]. Oral supplementation of A. muciniphila in Apolipoprotein E knockout mice (ApoE−/−) model to study the endothelial dysfunction along with atherogenesis, has shown the reduction in the metabolic endotoxemia-induced inflammatory chemokines, such as ICAM-1, MCP-1, and TNF-α on the endothelium [10]. Also, inulin-type fructans supplementation improved the endothelial dysfunction in ApoE−/− through the eNOS-NO pathway, along with the increment in the abundance of A. muciniphila [131]. Lee et al. have found a significant correlation between vascular dysfunction and the abundance of A. muciniphila levels, and dapagliflozin, an antidiabetic drug, improves the vascular dysfunction along with the increase of A. muciniphila level in the T2DM [132]. Haywood et al. have demonstrated that endothelial dysfunction is reversed with the overexpression of endothelial cell insulin-like growth factor 1 receptor (ECIGF-1R), along with the increase of A. muciniphila abundance in obesity conditions [133]. Thus, it is evident that A. muciniphila has a role in endothelial function through NO release and inhibition of proinflammatory chemokines, and the improvement in vascular/endothelial dysfunction might restore elevated BP. Surprisingly, Neyrinck et al. have reported that endothelial dysfunction was improved with the reduction of A. muciniphila abundance in ApoE−/− mice and suggested the reason for the negative role of A. muciniphila could be due to an individual’s health and microbiota pattern [134].
Epigenetic mechanisms
The epigenetic mechanisms are a set of post-translational modifications that can regulate the gene expression transiently without affecting the DNA sequence and involve chemical modification of DNA. Still, the changes are reversible in influencing the phenotype [135]. It results from an interplay between DNA and environmental factors [136]. The most common epigenetic mediators are DNA methylation and histone modifications, and recently long-coding RNA has been identified as another epigenetic mediator [136]. Richard et al. have found that methylation at cg08035323 influenced BP, and BP influenced methylation at different sites, such as cg00533891, cg00574958, and cg02711608 [137]. Also, DNA methylation and histone modifications (methylation and acetylation) with various drug treatments (valproic acid, decitabine, and 5-aza-2ʹ-deoxycytidine) have been associated with BP reduction in animal models [138,139,140,141]. Cardinale et al. have reported that histone deacetylase (HDAC) inhibition attenuates hypertension in Spontaneous Hypertensive Rats (SHR) [140], whereas A. muciniphila was found to potentiate HDAC3 and HDAC5 in improving the host lipid metabolism condition [61]. Furthermore, Jabs et al. have identified that A. muciniphila greatly affects the N6-methyladenosine modifications in mRNA in mono-associated mice [142]. Thus, the clarity on epigenetic mechanisms by A. muciniphila requires further studies as we have minimal available data.
The DASH diet, Mediterranean diet, and weight-loss intervention program on A. muciniphila to control BP
Diet is an essential modulator of gut microbiota [143,144,145], and the specific dietary pattern called the dietary approach to stop hypertension (DASH) has been proposed to control or lower BP [146]. The DASH diet promotes a diet that is adjusted to be lower in sodium and rich in potassium, magnesium, and calcium nutrients [146], which causes a reduction in high BP [147]. Maifeld et al. demonstrated that fasting followed by the modified DASH diet reduced the BP in patients with metabolic syndrome (MetS) through an altered gut microbiome, especially increasing the abundance of A. muciniphila, which in turn, increases SCFAs either locally or systemically [148]. Also, beta-glucan supplementation for four weeks in patients with MetS had a higher outcome in patients with a higher baseline A. muciniphila abundance [149]. Another weight-loss intervention study in patients with MetS had improved BP by enhancing the abundance of A. muciniphila and F. parusnitzii [150]. American Heart Association (AHA) recommends the Mediterranean diet (MD) as a healthy eating style; it improves both systolic and diastolic BP, and arterial stiffness [151,152,153,154]. Also, MD has been reported to increase the relative abundance of A. muciniphila in the stool samples from participants with different body weight compositions [155]. Thus, A. muciniphila could be a potential entity in lowering the BP in patients with MetS.
Interaction between anti-hypertensive drugs and A. muciniphila
Most hypertensive drugs, such as ARB, ACE inhibitors, beta-blockers (BBs), and calcium channel blockers (CCBs), are administered orally. After the administration by oral route, it is metabolized into pharmacologically active metabolites or toxic based on the molecule's structure [156]. These active molecules can be further metabolized by the rich enzyme repositories of the gut microbiome [157, 158]. The interaction between the drug and gut microbiome is bidirectional. Thus, identifying gut bacteria and their communities, which produce drug-metabolizing microbial proteins, can contribute to improving the drug’s effect and can be converted into new drugs and reduce the adverse effect. Understanding the relationship between the gut microbiome and HTN drug bioavailability can help us better understand individual variation, which can be useful in precision and personalized medicine. Unfortunately, the literature review indicates the need to study these two entities (anti-hypertensive drugs vs A. muciniphila) as we have very little evidence. For example, Li et al. reported that amlodipine aspartate and amlodipine besylate, a CCBs, improved the NFALD condition in mice with HTN through the enhancement of A. muciniphila, which potentiates the intestinal expression of antimicrobial peptide Reg3g [159]. As mentioned earlier, losartan treatment significantly improved the hypertensive condition in SHR rats possibly through the restoration of gut dysbiosis and modulation of immune response, along with the increased the abundance of A. muciniphila [123]. Furthermore, minocycline, an age-old antibiotic, is demonstrated to lower BP, and administration of minocycline in an SHR animal model decreased BP with an increase of A. muciniphila abundance [28]. Also, A. muciniphila secreted proteins induces the uptake of Ca++ through Ryanodine Receptors (RYr), a family of Calcium channel, in an enteroendocrine cell line and increase the synthesis of reactive oxygen species (ROS) [160]. Nonetheless, more research is needed to fully understand the possible bidirectional role of A. muciniphila and anti-hypertensive drugs.
Can A. muciniphila be considered in the field of pharmacomicrobiomics?
Numerous studies have highlighted the presence of microbiota in the gut as they play various crucial roles in improving health, including lowering BP through complex mechanisms. Pharmacomicrobiomics describes the differential effect of the microbiome on the pharmacokinetic profile of the drugs [161] with a focus on the microbiota-drug interaction. Akkermansia could be one of the potential regulators in the field of pharmacomicrobiomics as they potentiate drugs’ effects, such as PD-1/PD-L1A blockers, metformin, rifaximin, resveratrol, vancomycin, and vitamin-D in variety of disease conditions. A cross-sectional study using atorvastatin in hypercholesterolemic patients focused on understanding the impact of the gut microbiota revealed that untreated patients had an increase in inflammatory microbes, such as Collinsella, and Streptococcus, and treated patients had an increase in anti-inflammatory microbes such as A. muciniphila and F. parusnitzii [162]. A study among Brazilian hypertensive participants revealed that Akkermansia was highly abundant in addition to Lactobacillus and Eggerthella than in the normotensive group. Thus, we can speculate that A. muciniphila may play a role in HTN, by promoting the proinflammatory environment in the host. Considering all these available data, it suggests that A. muciniphila can be a potential candidate to be considered in the field of pharmacomicrobiomics.
Pangenome of A. muciniphila and its role in lowering BP
Pangenome is a set of entire genes in a collection of an organism's genomes [71]. It consists of a core genome shared by about 99% of the strains and a dispensable genome. Cluster analysis of microbial species genomes can offer insight into multiple fields, including taxonomy, evolutionary dynamics, strain diversity, strain identification, and reverse vaccinology [163, 164]. A comparative study to explore the population structure, genomic and functional diversity of A. mucinphila by comparing 39 genomes of its isolates showed that it contains 5644 unique proteins, and 106 new A. muciniphila metagenome-assembled genomes (MAGs) of human, pig, and mouse gut microbiomes. Focusing on the functional diversity analysis revealed that its pangenome consists of 198 CAZymes coding genes higher than other members of gut microbiota [165]. Becken et al. reported that human (obese) isolates of A. muciniphila have four distinct phylotypes (AmI to AmIV) and genotypic and phenotypic diversity [166]. These phylotypes of A. muciniphila have more affinity toward amino sugar and nucleotide sugar metabolism, fructose and mannose metabolism, and carbon metabolism pathways [166]. They reported that several phylogroup-specific phenotypes impacted colonization of the GI tract and modulated several host functions, such as oxygen tolerance, adherence to epithelial cells, and iron and sulfur metabolism. They also found that phylotype AmI was the most prominent in these populations, and this phylotype AmI contains more genes required for ASR, interestingly it was absent in the phylotypes AmII and AmIV. Due to a higher number of ASR genes present in the phylotype AmI, more sulfate is converted into H2S in the canonical ASR pathway, which increases the synthesis of sulfur-containing anti-hypertensive amino acids, such as cysteine and methionine. Thus, it shows that the pangenome of A. muciniphila, especially the phylotype AmI could be a potential target in lowering BP.
Obstacles and future perspectives
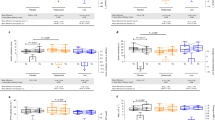
Even though numerous studies have revealed the gut microbial composition and metabolic roles in various diseases, it is still unclear how this knowledge can be applied to clinical medicine. For a better understanding of the gut microbiota and its dynamic nature during the onset, progression, and response to the pharmacological treatment, the application of longitudinal and interventional studies is essential to descriptive studies which only show dysbiosis. To improve drug efficacy, the microbiome can be considered a vital factor to use in non-responsive and variable-responsive patients. Subsequently, standardized strategies for reprogramming the gut microbiota should be implemented. Furthermore, other secondary metabolites produced by the gut microbiota from xenobiotics and dietary molecules should be taken into account, which will also interact with drugs [167]. Furthermore, currently available, a limited number of public data (16s rDNA sequencing) from studies that involved both humans and small rodents did not provide conclusive evidence as it shows a moderate but not a significant decrease in the abundance of A. muciniphila in hypertensive conditions (Table 1 and 2), which highlight the need of more studies on A. muciniphila in hypertension. Also, the literature review indicates that there is enormous scope in elucidating the potential role of A. muciniphila on different pathways, such as the H2S pathway, RAS pathway, and epigenetic mechanisms. As currently, we have limited information on these potential regulatory pathways. Recently, Liu et al. reported that the isolation of miR-30d from feces of multiple sclerosis (MS) patients and its administration significantly improved the MS-like symptoms by expanding the abundance of A. muciniphila [168]. This fascinating finding opens up a new path in the field of microbiome research, and it has the potential to impact disease treatment in the near future enormously. Advanced multi-omics approaches, such as metabolomics, lipidomics, and genomics with microbiomics, as well as the integration of these data, can help to dissect the mechanisms through which the gut microbiota modulates the host-drug response [169].
Conclusions
According to current evidence from clinical and preclinical studies, the gut microbiota plays an essential role in hypertension. A. muciniphila, in particular, is regarded as an important beneficial microbe because it regulates several molecular pathways, chemical entities, and dietary metabolites involved in BP regulation (Fig. 1). Our knowledge of these interactions and their effects on HTN is limited. Through well-controlled studies, researchers will better understand the molecular mechanisms by which the gut microbiota regulates HTN and the host response to drugs, could result in improved clinical outcomes and bring a step closer to precision medicine.
Schematic representation of the possible mechanism of action of A. muciniphila on the control of BP. The potential possible mechanisms of A. muciniphila to control the BP are (1) the degradation of mucin to secrete the SCFAs, especially acetate and propionate that reduces plasma leptin secretion and sympathetic activity through the secretion of GLP-1/GLP-2 in enteroendocrine L-cells via G-protein-coupled receptors; (2) maintenance of gut barrier integrity through the regulation of the tight junctions proteins (occludins, claudins, zona occludens), and activation of TLR2 pathway through the reduction of LPS synthesis and inflammatory markers; (3) utilization of H2S to produce cysteine which improves the BP; (4) direct action (possibly) on the renin-angiotensin-II pathway, and (5) reduction of oxidative stress induced by TMAO through the dietary L-carnitine metabolite
Availability of data and materials
Not applicable.
Abbreviations
- A. muciniphila :
-
Akkermansia muciniphila
- ACE:
-
Angiotensin convertingangiotensin-converting angiotensin-converting enzyme
- ACE2:
-
Angiotensin-converting enzyme 2
- AP-1:
-
Activator protein 1
- ApoE:
-
Apolipoprotein E
- ASR:
-
Assimilatory sulfur reduction
- BP:
-
Blood pressure
- CD36:
-
Cluster of differentiation 36
- CKD:
-
Chronic kidney diseases
- DASH:
-
Dietary approach to stop hypertension
- ECIGF-1R:
-
Endothelial cell insulin-like growth factor 1 receptor
- ECIGF-1R:
-
Endothelial cell insulin-like growth factor 1 receptor
- eNOS:
-
endothelial nitric-oxide synthase
- FFAR2:
-
Free fatty acid receptor 2
- FFAR3:
-
Free fatty acid receptor 3
- GI tract:
-
Gastrointestinal tract
- GLP1:
-
Glucagon-like peptide-1
- GLP2:
-
Glucagon-like peptide-2
- GPR:
-
G-protein coupled receptor
- GPR109a:
-
G-protein coupled receptor 109a
- GPR41:
-
G-protein coupled receptor 41
- GPR43:
-
G-protein coupled receptor 43
- H2S:
-
Hydrogen sulfide
- HC:
-
Healthy control
- HFD:
-
High-fat diet
- HTN:
-
Hypertension
- I-FABP:
-
Intestinal fatty acid-binding protein
- IFN-γ:
-
Interferon-gamma
- IL-2:
-
Interleukin 2
- IL-4:
-
Interleukin 4
- IL-6:
-
Interleukin 6
- IL-10:
-
Interleukin 10
- IP-10:
-
Interferon γ-induced protein 10
- IRAK:
-
Interleukin-1 receptor-associated kinase
- IS:
-
Indoxyl sulfate
- LPS:
-
Lipopolysaccharide synthesis
- MAG:
-
Metagenome-assembled genomes
- MCP-1:
-
Monocyte chemoattractant protein-1
- MD:
-
Mediterranean diet
- MetS:
-
Metabolic syndrome
- MyD88:
-
Myeloid differentiation primary response 88
- NFALD:
-
Non-alcoholic fatty liver disease
- NF-кB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NO:
-
Nitric oxide
- Olfr78:
-
Olfactory receptor 78
- OR51E2:
-
Olfactory receptor family 51 subfamily E member 2
- PCS:
-
P-cresyl sulfate
- PD-1:
-
Programmed cell death protein 1
- PDL1A:
-
Programmed cell death ligand 1A
- RAA system:
-
Renin–angiotensin–aldosterone system
- RAS pathway:
-
Renin-angiotensin system pathway
- ROS:
-
Reactive oxygen species
- RYr:
-
Ryanodin receptors
- SCFAs:
-
Short-chain fatty acids
- SHR:
-
Spontaneously hypertensive rats
- SR-A1:
-
Scavenging receptor A1
- T1DM:
-
Type 1 diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- Th1:
-
T-helper 1
- Th17:
-
T-helper 17
- TIRAP:
-
Toll/interleukin-1 receptor domain-containing adapter protein
- TLR2:
-
Toll-like receptor 2
- TLR4:
-
Toll-like receptor 4
- TLRs:
-
Toll-like receptors
- TMA:
-
Trimethylamine
- TMAO:
-
Trimethylamine-N-oxide
- TNF-α:
-
Tumor necrosis factor-alpha
References
Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469–76.
Ouwerkerk JP, Aalvink S, Belzer C, de Vos WM. Akkermansia glycaniphila sp. nov., an anaerobic mucin-degrading bacterium isolated from reticulated python faeces. Int J Syst Evol Microbiol. 2016;66:4614–20.
Karcher N, Nigro E, Puncochar M, Blanco-Miguez A, Ciciani M, Manghi P, Zolfo M, Cumbo F, Manara S, Golzato D, et al. Genomic diversity and ecology of human-associated Akkermansia species in the gut microbiome revealed by extensive metagenomic assembly. Genome Biol. 2021;22:209.
Caputo A, Dubourg G, Croce O, Gupta S, Robert C, Papazian L, Rolain JM, Raoult D. Whole-genome assembly of Akkermansia muciniphila sequenced directly from human stool. Biol Direct. 2015;10:5.
Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol. 2008;74:1646–8.
Zhang T, Li Q, Cheng L, Buch H, Zhang F. Akkermansia muciniphila is a promising probiotic. Microb Biotechnol. 2019;12:1109–25.
Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog. 2017;106:171–81.
Liu X, Zhao F, Liu H, Xie Y, Zhao D, Li C. Transcriptomics and metabolomics reveal the adaption of Akkermansia muciniphila to high mucin by regulating energy homeostasis. Sci Rep. 2021;11:9073.
Hanninen A, Toivonen R, Poysti S, Belzer C, Plovier H, Ouwerkerk JP, Emani R, Cani PD, De Vos WM. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut. 2018;67:1445–53.
Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia Muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− Mice. Circulation. 2016;133:2434–46.
Kim S, Lee Y, Kim Y, Seo Y, Lee H, Ha J, Lee J, Choi Y, Oh H, Yoon Y. Akkermansia muciniphila prevents fatty liver disease, decreases serum triglycerides, and maintains gut homeostasis. Appl Environ Microbiol. 2020;86:e03004-19
Lau WL, Vaziri ND, Nunes ACF, Comeau AM, Langille MGI, England W, Khazaeli M, Suematsu Y, Phan J, Whiteson K. The phosphate binder ferric citrate alters the gut microbiome in rats with chronic kidney disease. J Pharmacol Exp Ther. 2018;367:452–60.
Si J, Kang H, You HJ, Ko G. Revisiting the role of Akkermansia muciniphila as a therapeutic bacterium. Gut Microbes. 2022;14:2078619.
de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velasquez-Mejia EP, Carmona JA, Abad JM, Escobar JS. Metformin Is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40:54–62.
Panebianco C, Adamberg K, Jaagura M, Copetti M, Fontana A, Adamberg S, Kolk K, Vilu R, Andriulli A, Pazienza V. Influence of gemcitabine chemotherapy on the microbiota of pancreatic cancer xenografted mice. Cancer Chemother Pharmacol. 2018;81:773–82.
Ramakrishna C, Corleto J, Ruegger PM, Logan GD, Peacock BB, Mendonca S, Yamaki S, Adamson T, Ermel R, McKemy D, et al. Dominant role of the gut microbiota in chemotherapy induced neuropathic pain. Sci Rep. 2019;9:20324.
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–7.
Su H, Mo J, Ni J, Ke H, Bao T, Xie J, Xu Y, Xie L, Chen W. Andrographolide exerts antihyperglycemic effect through strengthening intestinal barrier function and increasing microbial composition of Akkermansia muciniphila. Oxid Med Cell Longev. 2020;2020:6538930.
Wang L, Wu Y, Zhuang L, Chen X, Min H, Song S, Liang Q, Li AD, Gao Q. Puerarin prevents high-fat diet-induced obesity by enriching Akkermansia muciniphila in the gut microbiota of mice. PLoS ONE. 2019;14:e0218490.
Fujisaka S, Usui I, Nawaz A, Igarashi Y, Okabe K, Furusawa Y, Watanabe S, Yamamoto S, Sasahara M, Watanabe Y, et al. Bofutsushosan improves gut barrier function with a bloom of Akkermansia muciniphila and improves glucose metabolism in mice with diet-induced obesity. Sci Rep. 2020;10:5544.
Chen M, Hou P, Zhou M, Ren Q, Wang X, Huang L, Hui S, Yi L, Mi M. Resveratrol attenuates high-fat diet-induced non-alcoholic steatohepatitis by maintaining gut barrier integrity and inhibiting gut inflammation through regulation of the endocannabinoid system. Clin Nutr. 2020;39:1264–75.
Wahlstrom A, Sayin SI, Marschall HU, Backhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50.
Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–35.
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066–71.
Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14.
Canale MP, Noce A, Di Lauro M, Marrone G, Cantelmo M, Cardillo C, Federici M, Di Daniele N, Tesauro M. Gut dysbiosis and western diet in the pathogenesis of essential arterial hypertension: a narrative review. Nutrients. 2021;13:1162.
Sun S, Lulla A, Sioda M, Winglee K, Wu MC, Jacobs DR Jr, Shikany JM, Lloyd-Jones DM, Launer LJ, Fodor AA, Meyer KA. Gut microbiota composition and blood pressure. Hypertension. 2019;73:998–1006.
Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–40.
Afsar B, Vaziri ND, Aslan G, Tarim K, Kanbay M. Gut hormones and gut microbiota: implications for kidney function and hypertension. J Am Soc Hypertens. 2016;10:954–61.
Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res. 2015;116:960–75.
Travers A, Farber HW, Sarnak MJ. Pulmonary hypertension in chronic kidney disease. Cardiol Clin. 2021;39:427–34.
Patel RS, Masi S, Taddei S. Understanding the role of genetics in hypertension. Eur Heart J. 2017;38:2309–12.
Habeeb E, Aldosari S, Saghir SA, Cheema M, Momenah T, Husain K, Omidi Y, Rizvi SAA, Akram M, Ansari RA. Role of environmental toxicants in the development of hypertensive and cardiovascular diseases. Toxicol Rep. 2022;9:521–33.
Verma N, Rastogi S, Chia YC, Siddique S, Turana Y, Cheng HM, Sogunuru GP, Tay JC, Teo BW, Wang TD, et al. Non-pharmacological management of hypertension. J Clin Hypertens. 2021;23:1275–83.
Vallianou NG, Geladari E, Kounatidis D. Microbiome and hypertension: where are we now? J Cardiovasc Med. 2020;21:83–8.
Cook KL, Chappell MC. Gut dysbiosis and hypertension: is it cause or effect? J Hypertens. 2021;39:1768–70.
Ding RX, Goh WR, Wu RN, Yue XQ, Luo X, Khine WWT, Wu JR, Lee YK. Revisit gut microbiota and its impact on human health and disease. J Food Drug Anal. 2019;27:623–31.
Belizario JE, Faintuch J. Microbiome and gut dysbiosis. Exp Suppl. 2018;109:459–76.
Al Khodor S, Reichert B, Shatat IF. The microbiome and blood pressure: can microbes regulate our blood pressure? Front Pediatr. 2017;5:138.
Chen X, Li HY, Hu XM, Zhang Y, Zhang SY. Current understanding of gut microbiota alterations and related therapeutic intervention strategies in heart failure. Chin Med J. 2019;132:1843–55.
Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Author correction: probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16:642.
Cheng D, Xie MZ. A review of a potential and promising probiotic candidate—Akkermansia muciniphila. J Appl Microbiol. 2021;130:1813–22.
Ottman N, Geerlings SY, Aalvink S, de Vos WM, Belzer C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract Res Clin Gastroenterol. 2017;31:637–42.
Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. 2020;11:25.
Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72.
Liu P, Wang Y, Yang G, Zhang Q, Meng L, Xin Y, Jiang X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol Res. 2021;165:105420.
Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mahler A, Balogh A, Marko L, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–9.
Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–9.
Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–9.
Pluznick JL. Microbial Short-chain fatty acids and blood pressure regulation. Curr Hypertens Rep. 2017;19:25.
Chang AJ, Ortega FE, Riegler J, Madison DV, Krasnow MA. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature. 2015;527:240–4.
Zhou T, Chien MS, Kaleem S, Matsunami H. Single cell transcriptome analysis of mouse carotid body glomus cells. J Physiol. 2016;594:4225–51.
Aisenberg WH, Huang J, Zhu W, Rajkumar P, Cruz R, Santhanam L, Natarajan N, Yong HM, De Santiago B, Oh JJ, et al. Defining an olfactory receptor function in airway smooth muscle cells. Sci Rep. 2016;6:38231.
Paparella A, Shaltiel-Harpaza L, Ibdah M. Beta-ionone: its occurrence and biological function and metabolic engineering. Plants. 2021;10:754.
Neuhaus EM, Zhang W, Gelis L, Deng Y, Noldus J, Hatt H. Activation of an olfactory receptor inhibits proliferation of prostate cancer cells. J Biol Chem. 2009;284:16218–25.
Gelis L, Jovancevic N, Veitinger S, Mandal B, Arndt HD, Neuhaus EM, Hatt H. Functional characterization of the odorant receptor 51E2 in human melanocytes. J Biol Chem. 2016;291:17772–86.
Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. 2013;110:4410–5.
Verhoog S, Taneri PE, Diaz ZMR, Marques-Vidal P, Troup JP, Bally L, Franco OH, Glisic M, Muka T. Dietary factors and modulation of bacteria strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: a systematic review. Nutrients. 2019;11:1565.
Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–36.
Remely M, Hippe B, Geretschlaeger I, Stegmayer S, Hoefinger I, Haslberger A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: a pilot study. Wien Klin Wochenschr. 2015;127:394–8.
Lukovac S, Belzer C, Pellis L, Keijser BJ, de Vos WM, Montijn RC, Roeselers G. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio. 2014;5:e01438–14.
Martins FL, Bailey MA, Girardi ACC. Endogenous activation of glucagon-like peptide-1 receptor contributes to blood pressure control: role of proximal tubule Na(+)/H(+) exchanger isoform 3, renal angiotensin II, and insulin sensitivity. Hypertension. 2020;76:839–48.
Pauza AG, Thakkar P, Tasic T, Felippe I, Bishop P, Greenwood MP, Rysevaite-Kyguoliene K, Ast J, Broichhagen J, Hodson DJ, et al. GLP1R attenuates sympathetic response to high glucose via carotid body inhibition. Circ Res. 2022;130:694–707.
Zhao S, Liu W, Wang J, Shi J, Sun Y, Wang W, Ning G, Liu R, Hong J. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J Mol Endocrinol. 2017;58:1–14.
Zaibi MS, Stocker CJ, O’Dowd J, Davies A, Bellahcene M, Cawthorne MA, Brown AJ, Smith DM, Arch JR. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010;584:2381–6.
Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–9.
Li C, Xiao P, Lin D, Zhong HJ, Zhang R, Zhao ZG, He XX. Risk factors for intestinal barrier impairment in patients with essential hypertension. Front Med. 2020;7:543698.
Sperandeo P, Martorana AM, Polissi A. Lipopolysaccharide biogenesis and transport at the outer membrane of Gram-negative bacteria. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:1451–60.
Bertani B, Ruiz N. Function and biogenesis of lipopolysaccharides. EcoSal Plus. 2018;8:10.1128/ecosalplus.ESP-0001-2018.
Carpenter TS, Parkin J, Khalid S. The free energy of small solute permeation through the escherichia coli outer membrane has a distinctly asymmetric profile. J Phys Chem Lett. 2016;7:3446–51.
Rosadini CV, Kagan JC. Early innate immune responses to bacterial LPS. Curr Opin Immunol. 2017;44:14–9.
Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, Mohammed M, Handberg EM, Richards EM, Pepine CJ, Raizada MK. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci. 2018;132:701–18.
Anhe FF, Jensen BAH, Perazza LR, Tchernof A, Schertzer JD, Marette A. Bacterial postbiotics as promising tools to mitigate cardiometabolic diseases. J Lipid Atheroscler. 2021;10:123–9.
Raftar SKA, Ashrafian F, Abdollahiyan S, Yadegar A, Moradi HR, Masoumi M, Vaziri F, Moshiri A, Siadat SD, Zali MR. The anti-inflammatory effects of Akkermansia muciniphila and its derivates in HFD/CCL4-induced murine model of liver injury. Sci Rep. 2022;12:2453.
Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, Jeon J, Kim MS, Jee YK, Gho YS, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50:e450.
Xu Y, Wang N, Tan HY, Li S, Zhang C, Feng Y. Function of Akkermansia muciniphila in obesity: interactions with lipid metabolism, immune response and gut systems. Front Microbiol. 2020;11:219.
Ottman N, Reunanen J, Meijerink M, Pietila TE, Kainulainen V, Klievink J, Huuskonen L, Aalvink S, Skurnik M, Boeren S, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE. 2017;12:e0173004.
Shin J, Noh JR, Chang DH, Kim YH, Kim MH, Lee ES, Cho S, Ku BJ, Rhee MS, Kim BC, et al. Elucidation of Akkermansia muciniphila probiotic traits driven by mucin depletion. Front Microbiol. 2019;10:1137.
Li F, Wang M, Wang J, Li R, Zhang Y. Alterations to the gut microbiota and their correlation with inflammatory factors in chronic kidney disease. Front Cell Infect Microbiol. 2019;9:206.
Lakshmanan AP, Al Za’abi M, Ali BH, Terranegra A. The influence of the prebiotic gum acacia on the intestinal microbiome composition in rats with experimental chronic kidney disease. Biomed Pharmacother. 2021;133:110992.
Lakshmanan AP, Kohil A, El Assadi F, Al Zaidan S, Al Abduljabbar S, Bangarusamy DK, Al Khalaf F, Petrovski G, Terranegra A. Akkermansia, a possible microbial marker for poor glycemic control in qataris children consuming arabic diet-A pilot study on pediatric T1DM in Qatar. Nutrients. 2021;13:836.
Ghaffari S, Abbasi A, Somi MH, Moaddab SY, Nikniaz L, Kafil HS, Leylabadlo HE. Akkermansia muciniphila: from its critical role in human health to strategies for promoting its abundance in human gut microbiome. Crit Rev Food Sci Nutr. 2022;1–21.
Ganesh BP, Klopfleisch R, Loh G, Blaut M. Commensal Akkermansia muciniphila exacerbates gut inflammation in salmonella typhimurium-infected gnotobiotic mice. PLoS ONE. 2013;8:e74963.
Dan X, Mushi Z, Baili W, Han L, Enqi W, Huanhu Z, Shuchun L. Differential analysis of hypertension-associated intestinal microbiota. Int J Med Sci. 2019;16:872–81.
Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA. 2017;114:10713–8.
Silveira-Nunes G, Durso DF, de Oliveira LRA Jr, Cunha EHM, Maioli TU, Vieira AT, Speziali E, Correa-Oliveira R, Martins-Filho OA, Teixeira-Carvalho A, et al. Hypertension is associated with intestinal microbiota dysbiosis and inflammation in a brazilian population. Front Pharmacol. 2020;11:258.
Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, Fortes ZB, Webb RC, Carvalho MH. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci. 2012;122:535–43.
Toral M, Gomez-Guzman M, Jimenez R, Romero M, Sanchez M, Utrilla MP, Garrido-Mesa N, Rodriguez-Cabezas ME, Olivares M, Galvez J, Duarte J. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clin Sci. 2014;127:33–45.
Grylls A, Seidler K, Neil J. Link between microbiota and hypertension: focus on LPS/TLR4 pathway in endothelial dysfunction and vascular inflammation, and therapeutic implication of probiotics. Biomed Pharmacother. 2021;137:111334.
Liu Y, Dai M. Trimethylamine N-oxide generated by the gut microbiota is associated with vascular inflammation: new insights into atherosclerosis. Mediators Inflamm. 2020;2020:4634172.
Zhu Y, Li Q, Jiang H. Gut microbiota in atherosclerosis: focus on Trimethylamine N-oxide. APMIS. 2020;128:353–66.
Zhang WQ, Wang YJ, Zhang A, Ding YJ, Zhang XN, Jia QJ, Zhu YP, Li YY, Lv SC, Zhang JP. TMA/TMAO in hypertension: novel horizons and potential therapies. J Cardiovasc Transl Res. 2021;14:1117–24.
Roncal C, Martinez-Aguilar E, Orbe J, Ravassa S, Fernandez-Montero A, Saenz-Pipaon G, Ugarte A, de Estella-HermosoMendoza A, Rodriguez JA, Fernandez-Alonso S, et al. Trimethylamine-N-Oxide (TMAO) Predicts Cardiovascular Mortality in Peripheral Artery Disease. Sci Rep. 2019;9:15580.
Koeth RA, Levison BS, Culley MK, Buffa JA, Wang Z, Gregory JC, Org E, Wu Y, Li L, Smith JD, et al. gamma-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20:799–812.
Brunt VE, Gioscia-Ryan RA, Casso AG, VanDongen NS, Ziemba BP, Sapinsley ZJ, Richey JJ, Zigler MC, Neilson AP, Davy KP, Seals DR. Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension. 2020;76:101–12.
Zhou J, Wang D, Li B, Li X, Lai X, Lei S, Li N, Zhang X. Relationship between plasma Trimethylamine N-oxide levels and renal dysfunction in patients with hypertension. Kidney Blood Press Res. 2021;46:421–32.
Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107–13.
Luo Y, Zhang Y, Han X, Yuan Y, Zhou Y, Gao Y, Yu H, Zhang J, Shi Y, Duan Y, et al. Akkermansia muciniphila prevents cold-related atrial fibrillation in rats by modulation of TMAO induced cardiac pyroptosis. EBioMedicine. 2022;82:104087.
Dordevic D, Jancikova S, Vitezova M, Kushkevych I. Hydrogen sulfide toxicity in the gut environment: Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J Adv Res. 2021;27:55–69.
Lv B, Chen S, Tang C, Jin H, Du J, Huang Y. Hydrogen sulfide and vascular regulation—An update. J Adv Res. 2021;27:85–97.
Rosario D, Benfeitas R, Bidkhori G, Zhang C, Uhlen M, Shoaie S, Mardinoglu A. Understanding the representative gut microbiota dysbiosis in metformin-treated type 2 diabetes patients using genome-scale metabolic modeling. Front Physiol. 2018;9:775.
Sun NL, Xi Y, Yang SN, Ma Z, Tang CS. Plasma hydrogen sulfide and homocysteine levels in hypertensive patients with different blood pressure levels and complications. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:1145–8.
Shi YX, Chen Y, Zhu YZ, Huang GY, Moore PK, Huang SH, Yao T, Zhu YC. Chronic sodium hydrosulfide treatment decreases medial thickening of intramyocardial coronary arterioles, interstitial fibrosis, and ROS production in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;293:H2093-2100.
Wang C, Han J, Xiao L, Jin CE, Li DJ, Yang Z. Role of hydrogen sulfide in portal hypertension and esophagogastric junction vascular disease. World J Gastroenterol. 2014;20:1079–87.
Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–7.
Zhao X, Zhang LK, Zhang CY, Zeng XJ, Yan H, Jin HF, Tang CS, Du JB. Regulatory effect of hydrogen sulfide on vascular collagen content in spontaneously hypertensive rats. Hypertens Res. 2008;31:1619–30.
Li L, Bhatia M, Moore PK. Hydrogen sulphide–a novel mediator of inflammation? Curr Opin Pharmacol. 2006;6:125–9.
Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–20.
Vasdev S, Singal P, Gill V. The antihypertensive effect of cysteine. Int J Angiol. 2009;18:7–21.
Ku E, Lee BJ, Wei J, Weir MR. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis. 2019;74:120–31.
Lau WL, Kalantar-Zadeh K, Vaziri ND. The gut as a source of inflammation in chronic kidney disease. Nephron. 2015;130:92–8.
Graboski AL, Redinbo MR. Gut-derived protein-bound uremic toxins. Toxins. 2020;12:590.
Six I, Flissi N, Lenglet G, Louvet L, Kamel S, Gallet M, Massy ZA, Liabeuf S. Uremic toxins and vascular dysfunction. Toxins. 2020;12:404.
Filipska I, Winiarska A, Knysak M, Stompor T. Contribution of gut microbiota-derived uremic toxins to the cardiovascular system mineralization. Toxins. 2021;13:274.
Ren Z, Fan Y, Li A, Shen Q, Wu J, Ren L, Lu H, Ding S, Ren H, Liu C, et al. Alterations of the human gut microbiome in chronic kidney disease. Adv Sci. 2020;7:2001936.
Chen H, Wang MC, Chen YY, Chen L, Wang YN, Vaziri ND, Miao H, Zhao YY. Alisol B 23-acetate attenuates CKD progression by regulating the renin-angiotensin system and gut-kidney axis. Ther Adv Chronic Dis. 2020;11:2040622320920025.
Rodriguez-Iturbe B, Johnson RJ. The role of renal microvascular disease and interstitial inflammation in salt-sensitive hypertension. Hypertens Res. 2010;33:975–80.
Su C, Xue J, Ye C, Chen A. Role of the central reninangiotensin system in hypertension (review). Int J Mol Med. 2021;47:95.
Buford TW, Sun Y, Roberts LM, Banerjee A, Peramsetty S, Knighton A, Verma A, Morgan D, Torres GE, Li Q, Carter CS. Angiotensin (1–7) delivered orally via probiotic, but not subcutaneously, benefits the gut-brain axis in older rats. Geroscience. 2020;42:1307–21.
Roshanravan N, Mahdavi R, Alizadeh E, Ghavami A, Rahbar Saadat Y, Mesri Alamdari N, Alipour S, Dastouri MR, Ostadrahimi A. The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of Akkermansia muciniphila abundance in type 2 diabetes; a randomized, double-blind, placebo-controlled trial. J Cardiovasc Thorac Res. 2017;9:183–90.
Duan Y, Prasad R, Feng D, Beli E, Li Calzi S, Longhini ALF, Lamendella R, Floyd JL, Dupont M, Noothi SK, et al. Bone marrow-derived cells restore functional integrity of the gut epithelial and vascular barriers in a model of diabetes and ACE2 deficiency. Circ Res. 2019;125:969–88.
Suzuki TA, Martins FM, Nachman MW. Altitudinal variation of the gut microbiota in wild house mice. Mol Ecol. 2019;28:2378–90.
Robles-Vera I, Toral M, de la Visitacion N, Sanchez M, Gomez-Guzman M, Munoz R, Algieri F, Vezza T, Jimenez R, Galvez J, et al. Changes to the gut microbiota induced by losartan contributes to its antihypertensive effects. Br J Pharmacol. 2020;177:2006–23.
Bonetti PO, Lerman LO, Lerman A: Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 2003, 23:168-175.
Dawes MG, Bartlett G, Coats AJ, Juszczak E. Comparing the effects of white coat hypertension and sustained hypertension on mortality in a UK primary care setting. Ann Fam Med. 2008;6:390–6.
Cahill PA, Redmond EM. Vascular endothelium–gatekeeper of vessel health. Atherosclerosis. 2016;248:97–109.
Konukoglu D, Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. 2017;956:511–40.
Levine AB, Punihaole D, Levine TB. Characterization of the role of nitric oxide and its clinical applications. Cardiology. 2012;122:55–68.
Rovella V, Rodia G, Di Daniele F, Cardillo C, Campia U, Noce A, Candi E, Della-Morte D, Tesauro M. Association of gut hormones and microbiota with vascular dysfunction in obesity. Nutrients. 2021;13:613.
Wang Z, Wu F, Zhou Q, Qiu Y, Zhang J, Tu Q, Zhou Z, Shao Y, Xu S, Wang Y, Tao J. Berberine improves vascular dysfunction by inhibiting Trimethylamine-N-oxide via regulating the gut microbiota in angiotensin II-induced hypertensive mice. Front Microbiol. 2022;13:814855.
Catry E, Bindels LB, Tailleux A, Lestavel S, Neyrinck AM, Goossens JF, Lobysheva I, Plovier H, Essaghir A, Demoulin JB, et al. Targeting the gut microbiota with inulin-type fructans: preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut. 2018;67:271–83.
Lee DM, Battson ML, Jarrell DK, Hou S, Ecton KE, Weir TL, Gentile CL. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol. 2018;17:62.
Haywood NJ, Luk C, Bridge KI, Drozd M, Makava N, Skromna A, Maccannell A, Ozber CH, Warmke N, Wilkinson CG, et al. Endothelial IGF-1 receptor mediates crosstalk with the gut wall to regulate microbiota in obesity. EMBO Rep. 2021;22:e50767.
Neyrinck AM, Catry E, Taminiau B, Cani PD, Bindels LB, Daube G, Dessy C, Delzenne NM. Chitin-glucan and pomegranate polyphenols improve endothelial dysfunction. Sci Rep. 2019;9:14150.
D’Addario C, Maccarrone M. Alcohol and epigentic modulations. Cambridge: Academic Press; 2016;261–273.
Liang M. Epigenetic mechanisms and hypertension. Hypertension. 2018;72:1244–54.
Richard MA, Huan T, Ligthart S, Gondalia R, Jhun MA, Brody JA, Irvin MR, Marioni R, Shen J, Tsai PC, et al. DNA Methylation analysis identifies loci for blood pressure regulation. Am J Hum Genet. 2017;101:888–902.
Nanduri J, Peng YJ, Wang N, Khan SA, Semenza GL, Kumar GK, Prabhakar NR. Epigenetic regulation of redox state mediates persistent cardiorespiratory abnormalities after long-term intermittent hypoxia. J Physiol. 2017;595:63–77.
Pushpakumar S, Kundu S, Narayanan N, Sen U. DNA hypermethylation in hyperhomocysteinemia contributes to abnormal extracellular matrix metabolism in the kidney. FASEB J. 2015;29:4713–25.
Cardinale JP, Sriramula S, Pariaut R, Guggilam A, Mariappan N, Elks CM, Francis J. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension. 2010;56:437–44.
Won KJ, Jung SH, Jung SH, Lee KP, Lee HM, Lee DY, Park ES, Kim J, Kim B. DJ-1/park7 modulates vasorelaxation and blood pressure via epigenetic modification of endothelial nitric oxide synthase. Cardiovasc Res. 2014;101:473–81.
Jabs S, Biton A, Becavin C, Nahori MA, Ghozlane A, Pagliuso A, Spano G, Guerineau V, Touboul D, Giai Gianetto Q, et al. Impact of the gut microbiota on the m(6)A epitranscriptome of mouse cecum and liver. Nat Commun. 2020;11:1344.
Beam A, Clinger E, Hao L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients. 2021;13:2795.
Mentella MC, Scaldaferri F, Pizzoferrato M, Gasbarrini A, Miggiano GAD. Nutrition, IBD and gut microbiota: a review. Nutrients. 2020;12:944.
Leeming ER, Johnson AJ, Spector TD, Le Roy CI. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients. 2019;11:2862.
Filippou CD, Tsioufis CP, Thomopoulos CG, Mihas CC, Dimitriadis KS, Sotiropoulou LI, Chrysochoou CA, Nihoyannopoulos PI, Tousoulis DM. Dietary approaches to stop hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11:1150–60.
Ozemek C, Laddu DR, Arena R, Lavie CJ. The role of diet for prevention and management of hypertension. Curr Opin Cardiol. 2018;33:388–93.
Maifeld A, Bartolomaeus H, Lober U, Avery EG, Steckhan N, Marko L, Wilck N, Hamad I, Susnjar U, Mahler A, et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat Commun. 1970;2021:12.
Velikonja A, Lipoglavsek L, Zorec M, Orel R, Avgustin G. Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley beta glucans in patients with high risk for metabolic syndrome development. Anaerobe. 2019;55:67–77.
Guevara-Cruz M, Flores-Lopez AG, Aguilar-Lopez M, Sanchez-Tapia M, Medina-Vera I, Diaz D, Tovar AR, Torres N. Improvement of lipoprotein profile and metabolic endotoxemia by a lifestyle intervention that modifies the gut microbiota in subjects with metabolic syndrome. J Am Heart Assoc. 2019;8:e012401.
Psaltopoulou T, Naska A, Orfanos P, Trichopoulos D, Mountokalakis T, Trichopoulou A. Olive oil, the Mediterranean diet, and arterial blood pressure: the Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am J Clin Nutr. 2004;80:1012–8.
Nunez-Cordoba JM, Valencia-Serrano F, Toledo E, Alonso A, Martinez-Gonzalez MA. The Mediterranean diet and incidence of hypertension: the Seguimiento Universidad de Navarra (SUN) study. Am J Epidemiol. 2009;169:339–46.
Tzima N, Pitsavos C, Panagiotakos DB, Skoumas J, Zampelas A, Chrysohoou C, Stefanadis C. Mediterranean diet and insulin sensitivity, lipid profile and blood pressure levels, in overweight and obese people; the Attica study. Lipids Health Dis. 2007;6:22.
De Pergola G, D’Alessandro A. Influence of mediterranean diet on blood pressure. Nutrients. 2018;10:1700.
Tagliamonte S, Laiola M, Ferracane R, Vitale M, Gallo MA, Meslier V, Pons N, Ercolini D, Vitaglione P. Mediterranean diet consumption affects the endocannabinoid system in overweight and obese subjects: possible links with gut microbiome, insulin resistance and inflammation. Eur J Nutr. 2021;60:3703–16.
Obach RS. Pharmacologically active drug metabolites: impact on drug discovery and pharmacotherapy. Pharmacol Rev. 2013;65:578–640.
Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. 2019;16:137–54.
Weersma RK, Zhernakova A, Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69:1510–9.
Li Y, Zhao D, Qian M, Liu J, Pan C, Zhang X, Duan X, Zhang Y, Jia W, Wang L. Amlodipine, an anti-hypertensive drug, alleviates non-alcoholic fatty liver disease by modulating gut microbiota. Br J Pharmacol. 2022;179:2054–77.
Neto DPA, Bosque BP, de Godoy JVP, Rodrigues PV, Meneses DD, Tostes K, Costa Tonoli CC, de Carvalho HF, Gonzalez-Billault C, de Castro FM. Akkermansia muciniphila induces mitochondrial calcium overload and alpha-synuclein aggregation in an enteroendocrine cell line. iScience. 2022;25:103908.
Chen HQ, Gong JY, Xing K, Liu MZ, Ren H, Luo JQ. Pharmacomicrobiomics: exploiting the drug-microbiota interactions in antihypertensive treatment. Front Med. 2021;8:742394.
Khan TJ, Ahmed YM, Zamzami MA, Siddiqui AM, Khan I, Baothman OAS, Mehanna MG, Kuerban A, Kaleemuddin M, Yasir M. Atorvastatin treatment modulates the gut microbiota of the hypercholesterolemic patients. OMICS. 2018;22:154–63.
Anani H, Zgheib R, Hasni I, Raoult D, Fournier PE. Interest of bacterial pangenome analyses in clinical microbiology. Microb Pathog. 2020;149:104275.
Bukhari SAR, Irfan M, Ahmad I, Chen L. Comparative genomics and pan-genome driven prediction of a reduced genome of Akkermansia muciniphila. Microorganisms. 2022;10:1350.
Guo X, Li S, Zhang J, Wu F, Li X, Wu D, Zhang M, Ou Z, Jie Z, Yan Q, et al. Genome sequencing of 39 Akkermansia muciniphila isolates reveals its population structure, genomic and functional diverisity, and global distribution in mammalian gut microbiotas. BMC Genomics. 2017;18:800.
Becken B, Davey L, Middleton DR, Mueller KD, Sharma A, Holmes ZC, Dallow E, Remick B, Barton GM, David LA, et al. Genotypic and phenotypic diversity among human isolates of Akkermansia muciniphila. mBio. 2021;12:e00478–21.
Carmody RN, Turnbaugh PJ. Host-microbial interactions in the metabolism of therapeutic and diet-derived xenobiotics. J Clin Invest. 2014;124:4173–81.
Liu S, Rezende RM, Moreira TG, Tankou SK, Cox LM, Wu M, Song A, Dhang FH, Wei Z, Costamagna G, Weiner HL. Oral administration of miR-30d from feces of MS patients suppresses MS-like symptoms in mice by expanding Akkermansia muciniphila. Cell Host Microbe. 2019;26(779–794):e778.
Sharma A, Buschmann MM, Gilbert JA. Pharmacomicrobiomics: the holy grail to variability in drug response? Clin Pharmacol Ther. 2019;106:317–28.
Ding R, Xiao Z, Jiang Y, Yang Y, Ji Y, Bao X, Xing K, Zhou X, Zhu S. Calcitriol ameliorates damage in high-salt diet-induced hypertension: Evidence of communication with the gut-kidney axis. Exp Biol Med (Maywood) 2022, 247:624-40
Leng Y, Yi M, Fan J, Bai Y, Ge Q, Yao G. Effects of acute intra-abdominal hypertension on multiple intestinal barrier functions in rats. Sci Rep 2016, 6:22814.
16s RNA sequencing of gut microbiota in Intra-abdominal hypertension model rats. The First Affiliated Hospital of Anhui Medical University. https://www.ebi.ac.uk/ena/browser/view/PRJNA781404?show=reads. Accessed date: 25-04-2022
Abboud FM, Cicha MZ, Ericsson A, Chapleau MW, Singh MV. Altering Early Life Gut Microbiota Has Long-Term Effect on Immune System and Hypertension in Spontaneously Hypertensive Rats. Front Physiol 2021, 12:752924.
Singh A, Zapata RC, Pezeshki A, Workentine ML, Chelikani PK. Host genetics and diet composition interact to modulate gut microbiota and predisposition to metabolic syndrome in spontaneously hypertensive stroke-prone rats. FASEB J 2019, 33:6748-66.
Li P, Cai X, Xiao N, Ma X, Zeng L, Zhang LH, Xie L, Du B. Sacha inchi (Plukenetia volubilis L.) shell extract alleviates hypertension in association with the regulation of gut microbiota. Food Funct 2020, 11:8051-67.
Wu D, Ding L, Tang X, Wang W, Chen Y, Zhang T. Baicalin Protects Against Hypertension-Associated Intestinal Barrier Impairment in Part Through Enhanced Microbial Production of Short-Chain Fatty Acids. Front Pharmacol 2019, 10:1271.
Thomaz FS, Altemani F, Panchal SK, Worrall S, Dekker Nitert M. The influence of wasabi on the gut microbiota of high-carbohydrate, high-fat diet-induced hypertensive Wistar rats. J Hum Hypertens 2021, 35:170-80.
de Araujo Henriques Ferreira G, Magnani M, Cabral L, Brandao LR, Noronha MF, de Campos Cruz J, de Souza EL, de Brito Alves JL. Potentially Probiotic Limosilactobacillus fermentum Fruit-Derived Strains Alleviate Cardiometabolic Disorders and Gut Microbiota Impairment in Male Rats Fed a High-Fat Diet. Probiotics Antimicrob Proteins 2022, 14:349-59.
McGavigan AK, Henseler ZM, Garibay D, Butler SD, Jayasinghe S, Ley RE, Davisson RL, Cummings BP. Vertical sleeve gastrectomy reduces blood pressure and hypothalamic endoplasmic reticulum stress in mice. Dis Model Mech 2017, 10:235-43.
Nakai M, Ribeiro RV, Stevens BR, Gill P, Muralitharan RR, Yiallourou S, Muir J, Carrington M, Head GA, Kaye DM, Marques FZ. Essential Hypertension Is Associated With Changes in Gut Microbial Metabolic Pathways: A Multisite Analysis of Ambulatory Blood Pressure. HTN 2021, 78:804-15.
Calderon-Perez L, Gosalbes MJ, Yuste S, Valls RM, Pedret A, Llaurado E, Jimenez-Hernandez N, Artacho A, Pla-Paga L, Companys J, et al. Gut metagenomic and short chain fatty acids signature in hypertension: a cross-sectional study. Sci Rep 2020, 10:6436.
Fei N, Bernabe BP, Lie L, Baghdan D, Bedu-Addo K, Plange-Rhule J, Forrester TE, Lambert EV, Bovet P, Gottel N, et al. The human microbiota is associated with cardiometabolic risk across the epidemiologic transition. PLoS One 2019, 14:e0215262.
hypertension microbial diversity. Hunan University of Chinese Medicine. https://www.ebi.ac.uk/ena/browser/view/PRJNA765574?show=reads. Accessed date: 25-04-2022.
Zhong HJ, Zeng HL, Cai YL, Zhuang YP, Liou YL, Wu Q, He XX. Washed Microbiota transplantation Lowers Blood Pressure in Patients With Hypertension. Front Cell Infect Microbiol 2021, 11:679624.
Acknowledgements
We would like to acknowledge Sidra Medicine for funding this study.
Funding
This study was funded by Sidra Internal Research Fund (SDR200077), Doha, Qatar.
Author information
Authors and Affiliations
Contributions
APL and SM designed the concept and wrote the manuscript. SAK and AT reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed and approved this submission.
Competing interests
The authors listed in this manuscript, Dr. Souhaila Al Khodor serve as an Editorial Board member and Dr. Selvasankar Murugesan serve as Associate Editor in one of the JTRM Journal’s sections—Translational Metagenomics. Other than this, the author(s) declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lakshmanan, A.P., Murugesan, S., Al Khodor, S. et al. The potential impact of a probiotic: Akkermansia muciniphila in the regulation of blood pressure—the current facts and evidence. J Transl Med 20, 430 (2022). https://doi.org/10.1186/s12967-022-03631-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03631-0