Abstract
Copper is an important metal micronutrient, required for the balanced growth and normal physiological functions of human organism. Copper-related toxicity and dysbalanced metabolism were associated with the disruption of intracellular respiration and the development of various diseases, including cancer. Notably, copper-induced cell death was defined as cuproptosis which was also observed in malignant cells, representing an attractive anti-cancer instrument. Excess of intracellular copper leads to the aggregation of lipoylation proteins and toxic stress, ultimately resulting in the activation of cell death. Differential expression of cuproptosis-related genes was detected in normal and malignant tissues. Cuproptosis-related genes were also linked to the regulation of oxidative stress, immune cell responses, and composition of tumor microenvironment. Activation of cuproptosis was associated with increased expression of redox-metabolism-regulating genes, such as ferredoxin 1 (FDX1), lipoic acid synthetase (LIAS), lipoyltransferase 1 (LIPT1), dihydrolipoamide dehydrogenase (DLD), drolipoamide S-acetyltransferase (DLAT), pyruvate dehydrogenase E1 subunit alpha 1 (PDHA1), and pyruvate dehydrogenase E1 subunit beta (PDHB)). Accordingly, copper-activated network was suggested as an attractive target in cancer therapy. Mechanisms of cuproptosis and regulation of cuproptosis-related genes in different cancers and tumor microenvironment are discussed in this study. The analysis of current findings indicates that therapeutic regulation of copper signaling, and activation of cuproptosis-related targets may provide an effective tool for the improvement of immunotherapy regimens.
Graphical Abstract

Key messages
Facts
• Copper ions, essential components of human body, can activate a novel subtype of programmed cell death, defined as cuproptosis.
• Cuproptosis is tightly associated with the regulation of mitochondrial respiration and oxidative stress in various cells, including malignant.
• Changed concentration of copper ions was found to regulate a set of redox-metabolism-regulating genes, such as ferredoxin 1, lipoic acid synthetase, lipoyltransferase 1, dihydrolipoamide dehydrogenase, drolipoamide S-acetyltransferase, and pyruvate dehydrogenase E1 subunits alpha 1 and beta.
• Cuproptosis-related genes and copper-containing compounds were indicated as potential targets and tools for the development of novel anti-cancer therapy.
Open Questions
• How to target cuproptosis-related genes to stop cancer cell survival?
• Which copper-responsive targets and/or copper-containing compounds can be employed safely and effectively during cancer treatment?
• Which biomarkers can be reliably employed to define good response to the activation of cuproptosis in cancer cells?
• Are there others, undiscovered intracellular targets of copper which can be used for cancer prevention and treatment?
Similar content being viewed by others
Introduction
Metal element copper is required for the effective functioning of human organisms. Copper is used as a micronutrient involved in several important catalytic processes as a structural cofactor of metal-dependent enzymes [1, 2]. The presence of this element is required for the metabolic regulation of growth and functional activities of the human body [3, 4]. The normal copper concentration, which is equal to about 1 mg/L in blood plasma and 50–120 mg for the total body content in average adults, is beneficial for the maintenance of proper homeostasis, while high or low concentrations of copper may be damaging for the optimal physiological state [5, 6]. Copper deficiency has been linked to diseases including anemia [7], osteoporosis [8], obesity [9], coronary heart diseases [10, 11], and cancers [6, 12]. Disorders of copper metabolism can also cause neurological pathologies, including Wilson’s [13], Alzheimer’s [14, 15], and Parkinson’s diseases [16,17,18]. A high concentration of copper is a cytotoxic, cell death-activating factor [19, 20]. Copper-induced cell death was defined as cuproptosis (terminology suggested by Tsvetkov [21]), a novel form of programmed cell death (PCD) that is different from apoptosis, necroptosis, pyroptosis, and ferroptosis [21, 22]. For instance, apoptosis is a classical PCD type which is marked by activation of several death pathways, chromatin condensation, caspase (-1, -3 and − 8) cleavage, and release of cytochrome c (cyt c) from mitochondria. Various internal physiological factors, such as cytokines and glucocorticoid hormones, can trigger PCD gene activation in apoptosis [23, 24]. The mitochondrion is the central intracellular organelle which is responsible for the propagation of classical apoptosis. Mitochondria are also affected during ferroptosis and cuproptosis.
Unlike apoptosis, chromatin condensation and/or caspase − 3 cleavage were not reported during activation of ferroptosis or cuproptosis [25, 26]. Ferroptosis is a PCD trigged by the accumulation of iron ions, dysregulated iron metabolism, modified activation of specific iron-activated genes responsible for lipid synthesis and peroxidation [25]. Ferroptosis is accompanied by the functional failure or blockade of the glutathione-dependent/antioxidant cell defense system. Both ferroptosis and cuproptosis are activated by excessive retaining of metal ions (iron and copper, respectively), marked by the shrinking of mitochondria, disrupted mitochondrial membrane, and abnormal energy metabolism [21, 23, 27, 28]. While apoptosis is a PCD required for developmental programs in multicellular organisms, cuproptosis and ferroptosis were defined as metabolic types of PCD, which are not yet linked to tissue- and organogenesis [25, 26]. Accenting the differences, the inhibitors of ferroptosis and apoptosis do not block activation of cuproptosis. Furthermore, while ferroptosis is mainly induced by disturbances in cell defense against abnormal lipid peroxidation, cuproptosis can be directly activated by the excessive amount of intracellular copper which is tightly associated with the regulation of mitochondrial respiration. In mitochondria, copper causes the aggregation of lipoylation proteins and the loss of iron-sulfur (Fe-S) cluster proteins [27]. These processes are mediated by the binding of copper ions to the lipoylated tricarboxylic acid (TCA), resulting in increased proteotoxic stress and cell destruction [2, 21, 27]. Cuproptosis can be modulated by copper ion carriers, such as elesclomol, and copper chelators [27, 28].
The current study reviews recent advances in the understanding of cuproptosis and copper-associated signaling. We critically discuss the activation of cuproptosis-related genes and their diagnostic values for the selection of targeted cancer treatment. This study is focused on the role of cuproptosis and associated gene signaling in various gastrointestinal cancers. Analysis of recent reports indicates that cuproptosis-related genes and copper-containing compounds [29] can be used as potential targets and tools for the development of novel anti-cancer therapy. Therapy-developing insights and deciphered mechanisms of cuproptosis are presented and discussed.
Disbalanced copper homeostasis, transport, and role in tumorigenesis
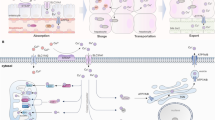
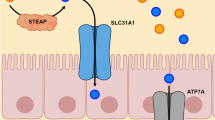
Intracellular copper concentration is very low and tightly regulated in normal cells [30]. Excessive amounts of copper trigger cytotoxicity and copper-associated death of normal cells (defined as cuproptosis) [29, 31]. Copper ions are transported by soluble carrier proteins, copper chaperones [32], which can bind a group of membrane-bound enzymes, including copper exporter ATP7A (Menkes ATPase; a proliferation-regulating effector) [33]. Ionic copper binds divalent metal transporter 1 (DMT1) which reduces Cu2+ to Cu+ in the intestinal lumen. The reduced copper can be associated with the copper transporter 1 (CTR1; also known as SLC31A1 (solute carrier family 31 member 1)) which delivers copper to the cell cytoplasm. Intracellular copper binds the copper chaperone protein antioxidant 1 (Atox1), which can form a complex with the copper transport adenosine triphosphatase (ATPase) 7B (ATP7B) in the Golgi complex [34, 35]. The chain of reactions facilitates the production of ceruloplasmin (the copper transporter) from pre-ceruloplasmin [20, 36]. Copper-binding ceruloplasmin may be targeted to increase oxidative stress and promote cancer cell death [37].
Significant changes in the concentration of intracellular copper were linked to various pathological conditions. Transformed copper metabolism was found associated with tumorigenesis, dysregulated cell proliferation, induction of tumor microenvironment (TME), metastasis, angiogenesis, and cancer immunoediting [38,39,40]. Excessive amounts of intracellular copper have been detected in various cancers, including breast [41], prostate [42], colon [43], lung [44], brain [45], liver [46], head and neck [47], and endometrial [48] malignancies. Moreover, the accumulation of copper in tumor cells was also associated with the development of drug resistance [49, 50], indicating the transformation of copper metabolism and signaling in malignant tissues. Copper storage protein metallothionein (MT), which is the established target for the anticancer drug cisplatin [51], can be used by cancer cell to safely sequester this metal ion. However, the role of MT in copper metabolism and resistance to cuproptosis remains to be confirmed.
Cancer recurrence and drug resistance represent two main impediments to the successful eradication of cancers [52]. The development of drug resistance is supported by cancer immunoediting which allows tumor cells to escape from immune surveillance [53]. Copper, as a cofactor and catalytic element in key metabolic and redox enzymes, is involved in the regulation of immune responses [39, 40, 54]. Moreover, copper was associated with the regulation of blood clotting, hormonal processing, and cellular energy metabolism. For instance, the accumulation of lipid-acylated proteins in mitochondria can be triggered by intracellular copper which impacts Fe-S cluster proteins metabolism [1, 55]. Notably, both beneficial and damaging effects were linked to copper-related signaling which is mediated by several distinct molecular mechanisms. Among the most investigated mechanisms is signaling via copper-binding enzymes. The element is required for proper functional activity of Cu, Zn-superoxide dismutases (SODs) SOD1 and SOD3 [56]. Cytochrome c oxidase (COX) and NADH deoxygenase-2 (ND2) also require copper [57].
The discovery of cuproptosis, a new type of cell death, opens new horizons for cancer therapy via targeting cuproptosis-related genes and proteins [29, 58, 59]. The cuproptosis was registered in different cancer cells by independent investigators [60,61,62,63], confirming the promising anti-cancer effect of copper. Therefore, cuproptosis-related genes were assessed to detect the association between copper-based treatment and clinical outcome, the characteristics of TME, and immune responses. It has been found that cuproptosis-related genes may serve as important predictive markers [49], although further assessment is warranted. Moreover, not only the gene expression changes should be assessed, but also the protein content and/or enzymatic activities require detailed verification in future cuproptosis studies. Genetic mechanisms of signaling, genes, and relevant signaling pathways which were found to be activated or silenced by excessive amounts of intracellular copper will be discussed below.
Cuproptosis-related genes and oxidative stress
The cell toxicity of free copper is triggered during the Fenton reaction which leads to the generation of large amounts of reactive oxygen species (ROS) [64]. Therefore, copper activates oxidative stress and different enzymes responsible for cell defense against ROS. Several recent studies assessed cuproptosis-related gene activation patterns [21, 65]. Database screening resulted in the discovery of 13 genes that are associated with cuproptosis [65]. Seven regulatory genes (ferredoxin 1 (FDX1), lipoic acid synthetase (LIAS), lipoyltransferase 1 (LIPT1), dihydrolipoamide dehydrogenase (DLD), drolipoamide S-acetyltransferase (DLAT), pyruvate dehydrogenase E1 subunit alpha 1 (PDHA1), and pyruvate dehydrogenase E1 subunit beta (PDHB)) were upregulated during cuproptosis (positive/copper-induced regulatory mechanisms). Three regulatory genes (metal-regulatory transcription factor-1 (MTF1), glutaminase (GLS), and cyclin-dependent kinase inhibitor 2 A (CDKN2A)) were silenced during cuproptosis (negative regulation pattern) [21]. Furthermore, three copper transporters were found involved in the regulation of cuproptosis, including SLC31A1, ATP7A, and ATP7B [29, 65]. The demonstrated genetic activities warrant protein-based investigations to confirm the correlation between changes in transcripts, associated targets, and end-products of the downstream enzymatic reactions.
The mechanism of cuproptosis is mediated by FDX1 (a reductase), an essential member of the redox-regulating system [66]. Reducing Cu2+, FDX1 can generate more toxic Cu+, leading to the activation of cellular stress and cuproptosis. The enzyme can promote the lipoylation of proteins in tricarboxylic acid (TCA) cycle, leading to the reduction of Fe-S cluster proteins [67]. The excessive amount of copper and/or overactivated/overexpressed FDX1 may lead to increased amount of toxic Cu + ions which promote metabolic injuries, although this process requires experimental confirmation. Moreover, the process is more complex and may involve other enzymes in mitochondria. An important component of the mitochondrial aerobic respiratory process, the PDH complex includes multiple copies of three enzymes (DLAT, PDHA1, and PDHB) which also regulate lipoylation, the highly conserved lysine posttranslational modification. PDH complex catalyzes oxidation of pyruvate and its conversion into acetyl-CoA prior to its utilization in TCA cycle; thus, connecting the anaerobic process of glycolysis and the oxidative phosphorylation. During activation of cuproptosis and proteotoxic stress, the increased amount of Cu + binds to lipoylated components of PDH (like DLAT), resulting in the aggregation of lipoylated proteins and destabilization of Fe–S protein clusters [67].
Knockout of FDX1 and/or the inhibition of lipoylation block cuproptosis [66]. An enzyme of the lipoic acid pathway, LIAS generates antioxidants in mitochondria [68]. LIPT1 and DLD also represent enzymes of the lipoic acid pathway and participate in protein lipoylation, required for activation of cuproptosis [69]. CTR1/SLC31A1 and ATP7A/B, the copper transporter, are required for intracellular transport of the element, the trigger of cytotoxicity [70]. ATP7A/B copper transporter is responsible for the regulation of normal physiological processes, such as the reabsorption of hepatic bile acids and the downregulation of intracellular concentration of copper and its toxicity (Fig. 1).
The regulation of intracellular Cu+. DMT1 reduces Cu2+ to Cu+. CTR1 (SLC31A1) deliver Cu+ to the cell cytoplasm and ATP7A/B export Cu+. CCS has got a copper binding motif and can deliver the metal element to SOD1. Intracellular copper binds the copper chaperone protein Atox1, which can form a complex with the copper transport ATP7A/B in the Golgi complex. Abbreviations: DMT1: Divalent metal transporter 1; CTR1: copper transporter 1; SLC31A1: solute carrier family 31 member 1; ATP7A/B: adenosine triphosphatase (ATPase) 7 A/B; CCS: Copper chaperone for superoxide dismutase; SOD: superoxide dismutases; Atox1: antioxidant 1
Copper ions can bind SOD1, a key defense enzyme against oxidative stress, ROS-related toxicity, membrane lipid peroxidation, and DNA damage [71]. Therefore, the excess of copper ions is associated with the increased oxidative damage. SOD enzymes transform the anion superoxide into hydrogen peroxide and are represented by three isoforms, including SOD1 (Cu/Zn dimeric form), SOD2 (mitochondrial tetrameric manganese (Mn) isoform), and SOD3 (extracellular tetrameric isoform) [72]. SOD1 expression and functioning deviate in different pathologies [73]. The activity of SOD1 is supported by a copper chaperone protein in the cell cytoplasm. Copper chaperone for superoxide dismutase (CCS) has got a copper-binding motif and delivers the metal element to SOD1. CCS also regulates the HIF-1 transcriptional complex and promotes the expression of vascular endothelial growth factor (VEGF), a tumor-stimulating effector [74]. Therefore, the role of copper in the SOD pathway is controversial and potentially may be associated with the development of cancer resistance [75].
In conclusion, the identified copper-induced genes play an important role during the activation of oxidative stress and the generation of ROS. Considering the sensitivity of various cancers to ROS-related toxicity, the identification of crosstalk targets between ROS- and copper-induced genes and their protein products in different cancers is warranted. The cuproptosis-related targets may be explored for the development of novel anti-cancer regimens. Recently, several new copper-triggered genetic targets were found activated in non-cancer pathologies. The activation of cuproptosis was reported in damaged brain cells (Alzheimer’s disease) and marked by the induction of various genes, including the gamma interferon-inducible lysosomal thiol reductase (GILT; encoded by tryptophane-tRNA ligase, cytoplasmic (IFI30) gene), phospholipase A1 member A (PLA1A), and arachidonate 5-lipoxygenase-activating protein (ALOX5AP) [14]. However, the role of copper in activation of these genes in brain cancer cells requires experimental confirmation.
TME and cancer immunoediting are influenced by cuproptosis
TME consists of two major groups of cells, including heterogenous tumor cells and normal cells, such as various immune, endothelial (vasculature), and cells of local tissues (for instance, fibroblasts). All those cells may release a diverse range of signaling molecules, products of cell metabolism, and substances required for the maintenance of extracellular matrix (for solid tumors) and/or metastasis [76]. TME is transformed and activated during tumorigenesis, tumor spreading, and the development of drug resistance [77, 78]. Notably, TME cells can release anti-cancer effectors, although cancer cells manage to adapt and reverse the apoptosis-activating signaling. The process involves the dynamic interaction of cancer cells with surrounding tissue and the immune system. The complex crosstalk and resulting transformations were defined as cancer immunoediting which often leads to the cancer-promoting modifications of the immune system [79, 80]. During immunoediting, the immune cells (such as tumor-associated macrophages (TAM), NK cells, T and B lymphocytes), the key components of TME which are supposed to kill cancer cells and control tumor growth and spreading [81], are reprogrammed to promote or neglect carcinogenesis [82]. The molecular mechanisms of this transformation are complex and have been reviewed elsewhere [79, 80, 83]. In this review, we focus on the TME effectors which are targeted by cuproptosis, including TAM and NK cells.
During normal development of immune responses, macrophages differentiate into M1 and M2 types, where M1 is mainly involved in the activation of inflammatory response (pro-inflammatory type) [84]. M1 cells are programmed to promote inflammation and anti-tumor activities of other immune cells. Type M2 cells are involved in tissue repair and can suppress inflammation. M2 effectors help tumor cells escape the immune surveillance (pro-tumoral type of macrophages) [85]. The cells also secrete growth factors that stimulate tumor growth [86]. Both M1 and M2 cells were detected in TME [87, 88].
The sensitivity of M2 macrophages to cuproptosis was regulated by serine protease inhibitor clade E member 1 (SERPINE1) as demonstrated recently in gastric cancer [89]. In glioblastoma cells, retinoic acid receptor (RAR) responder (RARRES) was involved in regulation of the macrophage infiltration and is considered as a potential therapy response marker [90]. The expression level of cyclin-dependent kinase inhibitor 2 A (CDKN2A) was linked to cuproptosis in M2 cells in pulmonary fibrosis [91], although its role in lung cancer remains to be determined. The list of the genetic effectors associated with macrophage signaling is growing and was recently appended by a group of targets in alcohol-damaged liver cells [92]. The activation of these genes in liver cancer cells and TME was not tested.
NK cells (also known as tumor-infiltrating natural killer cells (TINKs)), powerful TME effectors, play an anti-tumor role in most solid tumor tissues [93, 94]. NK cell metabolic pathway can be changed during immunoediting which is marked by reduced cytotoxicity, inhibition of T cell growth and maturation, and ineffective cancer cell elimination [95]. CD4+T cells express programmed death 1 (PD-1) receptors, while tumor cells express the ligand to these receptors, PD-L1 [96]. Advanced cancer-infiltrating and killing abilities of NK, CD8+ T cells, and neutrophils were associated with FDX1 expression [59] . However, the expression of another cuproptosis-related gene SLC31A1 was negatively correlated with dendritic and NK cell infiltration in brain cancer [40,52, 59]. The binding of PD-1 and PD-L1 was reported to stimulate T-cell exhaustion and reduce the killing capacity of NK cells. This observation was used to develop anti-PD-L1 therapies [97]. A natural NK inhibitor, anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), is expressed on the surface of regulatory T cells and can suppress the immune response of NK cells [98]. Immune checkpoint inhibitors block these effectors, exerting an anti-tumor effect (described elsewhere) [98]. Combined application of cuproptosis-activating agents and anti-PD-L1 or immune checkpoint inhibitors represent a potential area for future investigations.
Other types of immune cells are also involved in the regulation of TME responses and have the potential to influence cuproptosis. B lymphocytes can act as an anti-tumor agent and secret tumor-specific antibodies [99]. Dendritic cells orchestrate and shape diverse TME responses during carcinogenesis [100]. A handful of recent research studies indicated the activation of B [101] and dendritic cells [102] during cuproptosis. However, findings are limited and require experimental confirmation. Regulatory roles and signaling effectors in B and dendritic cells during induction of cuproptosis remain unclear and warrant future investigations.
Cuproptosis-related genes in hepatocellular carcinoma (HCC)
HCC ranks sixth in the incidence and second in the mortality of all cancers, representing a serious health burden worldwide [103]. Successful HCC treatment is complicated by limited surgical options, metastasis, and the development of chemotherapy resistance [104]. Therefore, the role of cuproptosis, as a potential new target for HCC treatment has been explored (Table 1). The analysis of cuproptosis-related gene expression indicated that FDX1, dihydrolipoamide dehydrogenase (DLD), and pyruvate dehydrogenase E1 subunit A1 (PDHA1) are positive regulators of this copper-associated PCD type in HCC [105]. Expression levels of lipoyltransferase 1 (LIPT1), dihydrolipoamide S-Acetyltransferase (DLAT), metal-regulatory transcription factor 1 (MTF1), glutaminase (GLS), and cyclin dependent kinase inhibitor 2 A (CDKN2A) genes were higher in HCC patients [105, 106]. The survival time of HCC patients with high expression of FDX1 was prolonged, while the poorer prognosis of HCC patients with low expression of PDX1 was indicated [105]. The overall survival (OS) of HCC patients with higher expression levels of GLS, DLAT, and CDKN2A was lower, compared to the patients with decreased expression levels of these genes [107]. To confirm these observations, all findings related to gene expression level should be verified at the level of protein expression.
Patients with high expression of CDKN2A demonstrated higher levels of B cells, CD4+T cells, and macrophages in the TME [108]. Cuproptosis-related risk score (CRRS) indicated that patients with a high CRRS have a poorer prognosis, lower OS, increased matrix activity and immune infiltration, and are characterized by activation of abundant cancer pathways [108]. The immune escape of the high-risk group was prevented by immunotherapy, showing the important role of immunosuppression in TME [108]. At the same time, levels of immune checkpoint effectors in the high-risk score group were high and included high expression of PDCD1 and CTLA4. Therefore, immune checkpoint inhibitor (ICI) immunotherapy was effective [107]. However, the role of these genes and their protein targets in HCC-related cuproptosis warrants further investigation.
The human liver accumulates copper deposits in the protein-bound form as part of hepatic metallothionein (MT), a cysteine-rich molecule. A low-molecular-weight protein MT has a high affinity for metals [109]. MT protects against copper toxicity via the simple retaining of copper ions [110]. The role of MT in the activation of cuproptosis in HCC remains to be tested. Controversial findings were indicated for MT in HCC. Downregulated levels of MT1 were reported in HCC [111]. Expression levels of MT may indicate cancer cell responsiveness to cuproptosis, although it was not assessed in HCC patients. Interestingly, a recent study indicated that MT expression is increased after Lenvatinib therapy and associated with lowered survival of HCC patients [112].
Cuproptosis-targeted genes in lung cancer
Lung cancer is a malignant tumor with very high incidence and mortality worldwide [113]. Early lung cancer diagnostics is poorly developed, reflecting the high heterogeneity and complexity of this type of cancer [114]. A recent study demonstrated the upregulation of seven genes (DLAT, DLD, glycine cleavage system protein H (a protein-coding gene, GCSH), LIAS, LIPT1, PDHA1, and PDHB) in lung adenocarcinoma patients (Table 1) [115]. During this study testing, three genes (ATP7B, FDX1, and SLC31A1) were found downregulated [115]. In lung cancer patients, high expression of DLD, DLAT, PHDA1, PHDB, and CDKN2A were associated with poor OS, while high expression of MTF1 was associated with longer OS [116]. In the high-risk group, immune cell infiltration was reduced [117]. Another recent study assessed the expression of cuproptosis-related genes during T-cell exhaustion [118].
In lung cancer stem cells, eight differently expressed genes (Krueppel-like transcription factor 4 (KLF4), secretoglobin family 3 A member1 (SCGB3A1), collagen type I alpha 1 chain (COL1A1), secreted phosphoprotein 1 (SPP1), Complement Component 4 Binding Protein Alpha (C4BPA), Tetraspanin 7 (TSPAN7), caveolin 2 (CAV2), and Collagen Triple Helix Repeat Containing 1 (CTHRC1); stemness gene signature) were tested and validated in vitro as markers to predict the lung cancer progression [118]. The study demonstrated that the expression of KLF4, COL1A1, SPP1, CAV2, and CTHRC1 positively correlated with the expression of immune checkpoint proteins, while TSPAN7, C4BPA, and PSMB9 showed a negative association [119]. Further validation of findings is warranted and should include the testing of relevant enzymatic activities.
Cuproptosis-related genes in gastric cancer
FDX1 gene expression was found to be upregulated in gastric cancer patients, suggesting a pro-carcinogenic role of these proteins [120]. However, in another study, gastric cancer patients with high expression of the FDX1, LIAS, SLC31A1, DLAT, and ATP7A/B genes demonstrated a better prognosis (Table 1) [121]. The study also found that gastric cancer patients with high expression of DLST had a poor prognosis and reduced survival time [121]. To optimize analysis cuproptosis-related gene (CRG) expression signatures were generated and used as CRG scores [122,123,124]. Compared with the low CRG score group, the high CRG score group was marked by a worse prognosis, shorter survival, fewer immune checkpoint targets, and higher tumor-linked immune dysfunctions [124]. In patients with gastric cancer, a high CRG score was also associated with advanced M2 (macrophage) infiltration and NK cell activity [119]. In contrast to NK cells, the lower level of mast cells, an important innate immune cell component, was linked to the poorer survival of patients from the high CRG score group [125]. Interestingly, another study confirmed that the infiltration level of various immune cells (including NK cells, neutrophils, and macrophages M2) positively correlated with the expression of SERPINE1 (also known as plasminogen activator inhibitor-1, PAI-1) during activation of cuproptosis in gastric cancer cells [89]. Higher expression of SERPINE1/PAI-1 leads to poor cancer prognosis and the development of resistance to bevacizumab (anti-VEGF-based immunotherapy) [126]. The activation of cuproptosis in mast cells remains unexplored [127], although mast cells are a very promising target in TME. The role of mast cells in the regulation of TME was recently discussed elsewhere [128].
One of the most important components of anti-cancer immune responses, CD4+ memory T cells are required for successful cancer elimination. It has been shown that CD4+T cell infiltration correlated with better survival time and prognosis in gastric cancer patients [125]. The higher activation of CD4+T cells and plasma cells in TME was reported in patients with low CRG scores. The group was also marked by a better prognosis [121]. Recent analysis indicated that expression of cuproptosis-induced FDX1 negatively correlates with numbers of CD4 + T cells and cancer-associated fibroblasts (CAFs) infiltration [129, 130]. Anti-cancer benefits of CD4 + T-cell activation during immune surveillance have been discussed elsewhere [131, 132].
Cuproptosis-related targets in breast cancer (BCa)
Expression of cuproptosis-related genes was assessed in BCa patients [133]. The analysis indicated a diversity of responses. For instance, up-regulated SLC31A1 levels correlated with poor OS, whereas high expression levels of LIPT1 and PDHA1 correlated with a better prognosis and OS [134]. In high-risk BCa patients, TME is marked by the expression of fewer immune checkpoint effectors, higher tumor stemness, and the presence of mainly resting macrophages (M2, M0) and NK cells. The profile of BCa patients with low CRG scores is characterized by abundant infiltration of immune cells, including anti-tumor lymphocytes, macrophages M1, CD8+T cells, and activated NK cells. Most of these cells expressed increased levels of PD-1, PD-L1, and CTLA4. Accordingly, the therapeutic effect of immune checkpoint inhibitors, as well as the sensitivity to immune therapy were higher in the BCa patients with increased infiltration of immune cells [133, 135, 136]. Table 1 summarizes the expression level of cuproptosis-related genes in BCa patients. However, the clinical application of cuproptosis as anti-BCa therapy tool remains to be tested.
Cuproptosis targets in kidney renal clear cell carcinoma (KIRC)
KIRC is the most common type of renal cell carcinoma (RCC) [137]. KIRC is characterized by a poor prognosis as it is often diagnosed at an advanced stage and patients develop resistance to radio- and chemotherapy [138]. Cuproptosis-related genes in patients with KIRC were analyzed and correlated with clinical parameters, diagnosis, and prognosis. KIRC patients with high expression of FDX1 demonstrated a good prognosis and high OS rate (Table 1) [139]. The low-risk CRG score positively correlated with the infiltration of macrophages, monocytes, CD8+T cells, and Tregs, whereas the high-risk CRG score negatively correlated with the infiltration of neutrophil, NK cells, and non-regulatory CD4+T cells. The immunotherapy responses and prognosis in the high-risk group were poorer than that of the low-risk group [140]. However, the expression of various cuproptosis-related genes and their targets in RCC remains to be confirmed. For instance, a recent study, which assessed levels of FDX1, found this gene highly expressed in 15 different tumors, while the gene was downregulated in 11 other tumors [141], suggesting highly differential expression of this cuproptosis marker. The same group identified FDX1-linked enrichment of genes in KIRC, including the tricarboxylic acid (TCA) cycle, NOTCH pathway, and others [141]. Many of these pathways are being explored as promising anti-RCC targets [142, 143].
We constructed Table 2 to summarize the information about current and completed cancer-targeting clinical trials which tested the role of cuproptosis.
Cuproptosis as a novel immunotherapy target
The success of anti-cancer therapy is obstructed by delayed diagnosis, recurrence, poor prognosis, and limited treatment methods. However, the development of targeted (personalized) therapy and combined immunotherapy methods delivers promising results. Malignant cells can evade the attack of immune cells and develop cancer tolerance through the transformation of immune checkpoint signaling. The most widely studied immune checkpoint effectors are cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and PD-1. Application of ICI can reverse immune tolerance and reactivate T cell-mediated cytotoxicity (anti-tumor effects) [160]. The combined regimen with ICI and bevacizumab demonstrated effectiveness in the treatment of HCC [161]. Anti-cancer effects of cuproptosis and the discovery of cuproptosis-related genes and proteins open new horizons in cancer therapy. The tumor-associated antigens, released during activation of cuproptosis, are recognized by immune system, can activate immune responses, and enhance the efficacy of current immunotherapies. Accordingly, cuproptosis-activating agents can complement the existing immunotherapy drugs and potentially provide a stronger anti-cancer response. Targeted activation of cuproptosis pathways may favor the re-activation of TME towards the eradication of cancer cells [89, 162,163,164]. However, the mechanisms of the anti-cancer effects of agents which can modulate copper metabolism and signalling in malignant tissues remain to be investigated
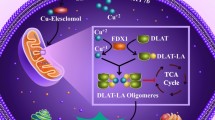
There are several methods to control copper signaling. To regulate the concentration of copper in cells, copper chelators were generated and designed to reduce the bioavailability of copper [165]. To regulate intracellular copper transport, there are copper ionophores that can be used to increase the intracellular concentration of copper ions and trigger cytotoxic stress [166]. However, these drugs lack specificity and selectivity. For instance, elesclomol (a copper ionophore) can deliver copper ions to the cytosol, increase the intracellular concentration of copper ions, promote oxidative stress, and induce cuproptosis [4, 167]. Elesclomol demonstrated promising therapeutic effects in the treatment of several diseases and cancer [167] (Fig. 2)
Interestingly, elesclomol stimulated the degradation of the copper transporter ATP7A in intestinal cancer cells and increased the concentration of copper in cancer mitochondria [168]. Suggestively, the combination of elesclomol and other chemotherapeutic drugs can improve anti-cancer efficacy. This hypothesis remains to be tested. Another copper ion carrier diethyldithiocarbamate (the active metabolite of disulfiram, an inhibitor of aldehyde dehydrogenase 1 (ALDH1) [169]) was also shown to increase intracellular copper concentrations [170]. It has been suggested that disulfiram may be used for the treatment of a variety of cancers, including colorectal and breast cancers [171]. We summarize the information about clinical trials of copper transporters in Table 2.
Copper transporter ATP7A/B has been found to mediate chemotherapeutic cancer resistance. High expression of ATP7A/B was observed in cells resistant to platinum-based chemotherapeutic drugs. Accordingly, silencing of ATP7A/B increased the sensitivity to chemotherapy [172]. As a copper transporter, ATP7A/B is an important effector of cuproptosis in cancer cells [173]. Although the mechanism of ATP7A/B signaling in tumors remains unclear, the transporter was indicated as a potential anti-cancer target and/or therapy response marker [145]. The hypothesis warrants future clinical testing.
To enhance the efficacy of cancer treatment, chemotherapy/immunotherapy regimens may be tested in combination with several copper-carriers which are in clinical trials (Table 2). Aside from immunotherapy, ferroptosis-inducing substances were found to enhance cuproptosis in liver cancer cells [174], suggesting a potential additive or synergistic effect. Whether the combination of ferroptosis and cuproptosis-targeting agents can improve the effect of cancer treatment remains to be confirmed. Notably, the application of combined multi-component therapies may be associated with higher risks. There is no reliable cancer-targeting delivery system of copper ions. It is also unclear how to estimate and maintain a less harmful level of copper in normal cells and in vital organs. Effects of copper-transporting systems in many normal cells remains under-addressed, suggesting a potential risk of copper-toxicity during combined therapies. Interdisciplinary approach should be employed to clarify the systemic toxicity of copper-transporting agents. Selective induction of cuproptosis alone and combined with other anti-cancer treatment regimens in cancer cells warrants future investigations.
Conclusions and future perspectives
Copper signaling represents an attractive therapeutic target, although both beneficial and toxic copper-induced effects were reported. Copper is an essential microelement that is required for normal physiological functions [2, 4]. However, dysbalanced copper metabolism was linked to the progression of various diseases, including cancer [175]. The discovery of cuproptosis, a new form of cell death [21, 146], uncovered a relatively new copper-associated mechanism of signaling and a new anti-cancer target.
Several recent studies reported discovery of copper-targeted genes associated with cuproptosis, including FDX1, LIAS, LIPT1, DLD, DLAT, PDHA1/B, MTF1, GLS, CDKN2A, ATP7A/B, SLC31A1 [29, 65, 66, 69, 70, 105,106,107,108]. Differential expression of these genes in malignant and normal tissues has been shown, suggesting their involvement in the regulation of carcinogenesis. Furthermore, the expression of cuproptosis-related genes correlated with TME characteristics and disease prognosis. For instance, levels of FDX1 were decreased in many cancers and reflected the level of immune cell infiltration [59,176]. However, functional implications of the differential expression of cuproptosis-related genes in normal vs. cancer cells remains to be clarified.
Successful cancer immunotherapy is obstructed by the development of immune tolerance and the escape of tumor cells from immune surveillance, defined as cancer immunoediting [177]. Cuproptosis and cuproptosis-related genes represent a novel anti-cancer target that can be also employed for the anti-cancer priming of TME [144] and re-activating of natural anti-cancer surveillance. Targeted activation of cuproptosis-related genes may be tested as a priming or contributing factors for improving current ICI immunotherapy. However, the molecular mechanism of cuproptosis and its clinical safety warrant future investigations and clinical validations.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ALOX5AP:
-
Arachidonate 5-lipoxygenase-activating protein
- Atox1:
-
Antioxidant 1
- ATP7A:
-
Adenosine triphosphatase (ATPase) 7A /Menkes ATPase
- ATP7B:
-
Adenosine triphosphatase (ATPase) 7B/ATPase7B
- BCa:
-
Breast cancer
- C4BPA:
-
Complement Component 4 Binding Protein Alpha
- CAFs:
-
Cancer-associated fibroblasts
- CAV2:
-
Caveolin 2
- CCS:
-
Copper chaperone for superoxide dismutase
- CDK4/6:
-
Cyclin dependent kinases 4 and 6
- CDKN2A:
-
Cyclin-dependent kinase inhibitor 2A
- COL1A1:
-
Collagen type I alpha 1 chain
- COX:
-
Cytochrome c oxidase
- CRG:
-
Cuproptosis-related gene
- CRRS:
-
Cuproptosis-related risk score
- CTHRC1:
-
Collagen triple helix repeat containing 1
- CTLA-4:
-
Anti-cytotoxic T lymphocyte associated antigen 4
- CTR1:
-
Copper transporter 1
- DLAT:
-
Drolipoamide S-acetyltransferase
- DLD:
-
Dihydrolipoamide dehydrogenase
- DMT1:
-
Divalent metal transporter 1
- Fe-S cluster protein:
-
Iron-sulfur cluster protein
- FDX1:
-
Ferredoxin 1
- GC:
-
Gastric cancer
- GCSH:
-
Glycine cleavage system protein H
- GILT:
-
Gamma interferon-inducible lysosomal thiol reductase
- GLS:
-
Glutaminase
- HCC:
-
Hepatocellular carcinoma
- ICI:
-
Immune checkpoint inhibitor
- KIRC:
-
Kidney renal clear cell carcinoma
- KLF4:
-
Krueppel-like transcription factor 4
- LA:
-
Lipoic acid
- LC:
-
Lung cancer
- LIAS:
-
Lipoic acid synthetase
- LIPT1:
-
Lipoyltransferase 1
- MDM2:
-
Murine double minute
- MT:
-
Metallothionein
- MTF1:
-
Metal-regulatory transcription factor-1
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- ND2:
-
NADH deoxygenase-2
- OS:
-
Overall survival
- PD-1:
-
Programmed death 1
- PDC:
-
Pyruvate dehydrogenase complex
- PDHA1:
-
Pyruvate dehydrogenase E1 subunit alpha 1
- PDHB:
-
Pyruvate dehydrogenase E1 subunit beta
- PDHC:
-
Pyruvate dehydrogenase complex
- PD-L1:
-
Programmed death 1 receptors
- PLA1A:
-
Phospholipase A1 member A
- RAR:
-
Retinoic acid receptor
- RARRES:
-
Retinoic acid receptor responder
- ROS:
-
Reactive oxygen species
- SCGB3A1:
-
Secretoglobin family 3A member1
- SERPINE1:
-
Serine protease inhibitor clade E member 1
- SLC31A1:
-
Solute carrier family 31 member 1
- SODs:
-
Superoxide dismutases
- SPP1:
-
Secreted phosphoprotein 1
- TAM:
-
Tumor associated macrophages
- TCA:
-
Tricarboxylic acid
- TINKs:
-
Tumor infiltrating natural killer cells
- TME:
-
Tumor microenvironment
- TSPAN7:
-
Tetraspanin 7
- VEGF:
-
Vascular endothelial growth factor
References
Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–85. https://doi.org/10.1038/nchembio.72.
Zhang B, Burke R. Copper homeostasis and the ubiquitin proteasome system. Metallomics. 2023;15. https://doi.org/10.1093/mtomcs/mfad010.
Ruiz LM, Libedinsky A, Elorza AA. Role of copper on mitochondrial function and metabolism. Front Mol Biosci. 2021;8:711227. https://doi.org/10.3389/fmolb.2021.711227.
Garza NM, Swaminathan AB, Maremanda KP, Zulkifli M, Gohil VM. Mitochondrial copper in human genetic disorders. Trends Endocrinol Metab. 2023;34:21–33. https://doi.org/10.1016/j.tem.2022.11.001.
Araya M, Pizarro F, Olivares M, Arredondo M, Gonzalez M, Mendez M. Understanding copper homeostasis in humans and copper effects on health. Biol Res. 2006;39:183–7. https://doi.org/10.4067/s0716-97602006000100020.
Guan D, Zhao L, Shi X, Ma X, Chen Z. Copper in cancer: from pathogenesis to therapy. Biomed Pharmacother. 2023;163:114791. https://doi.org/10.1016/j.biopha.2023.114791.
Tahir N, Ashraf A, Waqar SHB, et al. Copper deficiency, a rare but correctable cause of pancytopenia: a review of literature. Expert Rev Hematol. 2022;15:999–1008. https://doi.org/10.1080/17474086.2022.2142113.
Li D, Gao Z, Li Q, Liu X, Liu H. Cuproptosis-a potential target for the treatment of osteoporosis. Front Endocrinol (Lausanne). 2023;14:1135181. https://doi.org/10.3389/fendo.2023.1135181.
Calcaterra V, Verduci E, Milanta C, et al. Micronutrient Deficiency in children and adolescents with Obesity-A narrative review. Child (Basel). 2023;10. https://doi.org/10.3390/children10040695.
Chen X, Cai Q, Liang R, et al. Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies. Cell Death Dis. 2023;14:105. https://doi.org/10.1038/s41419-023-05639-w.
Klevay LM. Copper, coronary heart disease, and dehydroepiandrosterone. J Am Coll Cardiol. 2015;65:2151–2. https://doi.org/10.1016/j.jacc.2015.02.065.
Uauy R, Olivares M, Gonzalez M. Essentiality of copper in humans. Am J Clin Nutr. 1998;67:S952–9. https://doi.org/10.1093/ajcn/67.5.952S.
Lucena-Valera A, Ruz-Zafra P, Ampuero J. Wilson’s disease: overview. Med Clin (Barc). 2023;160:261–7. https://doi.org/10.1016/j.medcli.2022.12.016.
Zhang E, Dai F, Chen T, Liu S, Xiao C, Shen X. Diagnostic models and predictive drugs associated with cuproptosis hub genes in Alzheimer’s disease. Front Neurol. 2022;13:1064639. https://doi.org/10.3389/fneur.2022.1064639.
Ayton S, Lei P, Bush AI. Metallostasis in Alzheimer’s disease. Free Radic Biol Med. 2013;62:76–89. https://doi.org/10.1016/j.freeradbiomed.2012.10.558.
Wittung-Stafshede P. Crossroads between copper ions and amyloid formation in Parkinson’s disease. Essays Biochem. 2022;66:977–86. https://doi.org/10.1042/EBC20220043.
Gaggelli E, Kozlowski H, Valensin D, Valensin G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem Rev. 2006;106:1995–2044. https://doi.org/10.1021/cr040410w.
Bandmann O, Weiss KH, Kaler SG. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015;14:103–13. https://doi.org/10.1016/S1474-4422(14)70190-5.
Chen J, Jiang Y, Shi H, Peng Y, Fan X, Li C. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflugers Arch. 2020;472:1415–29. https://doi.org/10.1007/s00424-020-02412-2.
Xue Q, Kang R, Klionsky DJ, Tang D, Liu J, Chen X. Copper metabolism in cell death and autophagy. Autophagy. 2023;1–21. https://doi.org/10.1080/15548627.2023.2200554.
Tsvetkov P, Coy S, Petrova B, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254–61. https://doi.org/10.1126/science.abf0529.
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64. https://doi.org/10.1038/s41422-019-0164-5.
Dang Q, Sun Z, Wang Y, Wang L, Liu Z, Han X. Ferroptosis: a double-edged sword mediating immune tolerance of cancer. Cell Death Dis. 2022;13:925. https://doi.org/10.1038/s41419-022-05384-6.
Ketelut-Carneiro N, Fitzgerald KA. Apoptosis, pyroptosis, and Necroptosis-Oh my! The many ways a cell can die. J Mol Biol. 2022;434:167378. https://doi.org/10.1016/j.jmb.2021.167378.
Mao C, Wang M, Zhuang L, Gan B. Metabolic cell death in cancer: ferroptosis, cuproptosis, disulfidptosis, and beyond. Protein Cell Mar. 2024;1:pwae003. https://doi.org/10.1093/procel/pwae003.
Tong X, Tang R, Xiao M, et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15:174. https://doi.org/10.1186/s13045-022-01392-3.
Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. 2022;7:378. https://doi.org/10.1038/s41392-022-01229-y.
Li J, Cao F, Yin HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. https://doi.org/10.1038/s41419-020-2298-2.
Ji P, Wang P, Chen H, et al. Potential of copper and copper compounds for Anticancer Applications. Pharmaceuticals (Basel). 2023;16. https://doi.org/10.3390/ph16020234.
Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–8. https://doi.org/10.1126/science.284.5415.805.
Nose Y, Wood LK, Kim BE, et al. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J Biol Chem. 2010;285:32385–92. https://doi.org/10.1074/jbc.M110.143826.
Culotta VC, Yang M, O’Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta. 2006;1763:747–58. https://doi.org/10.1016/j.bbamcr.2006.05.003.
Itoh S, Kim HW, Nakagawa O, et al. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J Biol Chem. 2008;283:9157–67. https://doi.org/10.1074/jbc.M709463200.
Hatori Y, Lutsenko S. An expanding range of functions for the copper chaperone/antioxidant protein Atox1. Antioxid Redox Signal. 2013;19:945–57. https://doi.org/10.1089/ars.2012.5086.
Yang D, Xiao P, Qiu B, Yu HF, Teng CB. Copper chaperone antioxidant 1: multiple roles and a potential therapeutic target. J Mol Med (Berl). 2023;101:527–42. https://doi.org/10.1007/s00109-023-02311-w.
Festa RA, Thiele DJ. Copper: an essential metal in biology. Curr Biol. 2011;21:R877–883. https://doi.org/10.1016/j.cub.2011.09.040.
Rieber M. Cancer pro-oxidant therapy through copper Redox Cycling: Repurposing Disulfiram and Tetrathiomolybdate. Curr Pharm Des. 2020;26:4461–6. https://doi.org/10.2174/1381612826666200628022113.
Blockhuys S, Celauro E, Hildesjo C, et al. Defining the human copper proteome and analysis of its expression variation in cancers. Metallomics. 2017;9:112–23. https://doi.org/10.1039/c6mt00202a.
Cobine PA, Brady DC. Cuproptosis: Cellular and molecular mechanisms underlying copper-induced cell death. Mol Cell. 2022;82:1786–7. https://doi.org/10.1016/j.molcel.2022.05.001.
Cheng B, Tang C, Xie J, et al. Cuproptosis illustrates tumor micro-environment features and predicts prostate cancer therapeutic sensitivity and prognosis. Life Sci. 2023;325:121659. https://doi.org/10.1016/j.lfs.2023.121659.
Kuo HW, Chen SF, Wu CC, Chen DR, Lee JH. Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol Trace Elem Res. 2002;89:1–11. https://doi.org/10.1385/BTER:89:1:1.
Nayak SB, Bhat VR, Upadhyay D, Udupa SL. Copper and ceruloplasmin status in serum of prostate and colon cancer patients. Indian J Physiol Pharmacol. 2003;47:108–10.
Majumder S, Chatterjee S, Pal S, Biswas J, Efferth T, Choudhuri SK. The role of copper in drug-resistant murine and human tumors. Biometals. 2009;22:377–84. https://doi.org/10.1007/s10534-008-9174-3.
Zhang X, Yang Q. Association between serum copper levels and lung cancer risk: a meta-analysis. J Int Med Res. 2018;46:4863–73. https://doi.org/10.1177/0300060518798507.
Turecky L, Kalina P, Uhlikova E, Namerova S, Krizko J. Serum ceruloplasmin and copper levels in patients with primary brain tumors. Klin Wochenschr. 1984;62:187–9. https://doi.org/10.1007/BF01731643.
Moffett JR, Puthillathu N, Vengilote R, Jaworski DM, Namboodiri AM. Acetate revisited: a key Biomolecule at the Nexus of Metabolism, Epigenetics and Oncogenesis-Part 1: Acetyl-CoA, acetogenesis and Acyl-CoA short-chain synthetases. Front Physiol. 2020;11:580167. https://doi.org/10.3389/fphys.2020.580167.
Ressnerova A, Raudenska M, Holubova M, et al. Zinc and copper homeostasis in Head and Neck Cancer: review and Meta-analysis. Curr Med Chem. 2016;23:1304–30. https://doi.org/10.2174/0929867323666160405111543.
Atakul T, Altinkaya SO, Abas BI, Yenisey C. Serum copper and zinc levels in patients with endometrial Cancer. Biol Trace Elem Res. 2020;195:46–54. https://doi.org/10.1007/s12011-019-01844-x.
Zhang D, Lu W, Zhuo Z, Wang Y, Zhang W, Zhang M. Comprehensive analysis of a cuproptosis-related ceRNA network implicates a potential endocrine therapy resistance mechanism in ER-positive breast cancer. BMC Med Genomics. 2023;16:96. https://doi.org/10.1186/s12920-023-01511-0.
Jin J, Ma M, Shi S, et al. Copper enhances genotoxic drug resistance via ATOX1 activated DNA damage repair. Cancer Lett. 2022;536:215651. https://doi.org/10.1016/j.canlet.2022.215651.
Ghosh S. Cisplatin: the first metal based anticancer drug. Bioorg Chem. 2019;88:102925. https://doi.org/10.1016/j.bioorg.2019.102925.
Xiao C, Yang L, Jin L, et al. Prognostic and immunological role of cuproptosis-related protein FDX1 in pan-cancer. Front Genet. 2022;13:962028. https://doi.org/10.3389/fgene.2022.962028.
Nussinov R, Tsai CJ, Jang H. Anticancer drug resistance: an update and perspective. Drug Resist Updat. 2021;59:100796. https://doi.org/10.1016/j.drup.2021.100796.
Ozumi K, Sudhahar V, Kim HW, et al. Role of copper transport protein antioxidant 1 in angiotensin II-induced hypertension: a key regulator of extracellular superoxide dismutase. Hypertension. 2012;60:476–86. https://doi.org/10.1161/HYPERTENSIONAHA.111.189571.
Brancaccio D, Gallo A, Piccioli M, Novellino E, Ciofi-Baffoni S, Banci L. [4Fe-4S] Cluster Assembly in Mitochondria and its impairment by copper. J Am Chem Soc. 2017;139:719–30. https://doi.org/10.1021/jacs.6b09567.
Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–606. https://doi.org/10.1089/ars.2011.3999.
Novoselov KS, Geim AK, Morozov SV, et al. Electric field effect in atomically thin carbon films. Science. 2004;306:666–9. https://doi.org/10.1126/science.1102896.
Liu Y, Wang J, Jiang M. Copper-related genes predict prognosis and characteristics of breast cancer. Front Immunol. 2023;14:1145080. https://doi.org/10.3389/fimmu.2023.1145080.
Zhang C, Zeng Y, Guo X, et al. Pan-cancer analyses confirmed the cuproptosis-related gene FDX1 as an immunotherapy predictor and prognostic biomarker. Front Genet. 2022;13:923737. https://doi.org/10.3389/fgene.2022.923737.
Xu S, Liu D, Chang T, et al. Cuproptosis-Associated lncRNA establishes New Prognostic Profile and predicts Immunotherapy Response in Clear Cell Renal Cell Carcinoma. Front Genet. 2022;13:938259. https://doi.org/10.3389/fgene.2022.938259.
Greish K, Pittala V, Taurin S, et al. Curcumin(-)Copper complex nanoparticles for the management of Triple-negative breast Cancer. Nanomaterials (Basel). 2018;8. https://doi.org/10.3390/nano8110884.
Hu Y, Qian Y, Wei J, et al. The Disulfiram/Copper Complex induces autophagic cell death in Colorectal Cancer by Targeting ULK1. Front Pharmacol. 2021;12:752825. https://doi.org/10.3389/fphar.2021.752825.
Li H, Wang J, Wu C, Wang L, Chen ZS, Cui W. The combination of disulfiram and copper for cancer treatment. Drug Discov Today. 2020;25:1099–108. https://doi.org/10.1016/j.drudis.2020.04.003.
Aust SD, Morehouse LA, Thomas CE. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1:3–25. https://doi.org/10.1016/0748-5514(85)90025-x.
Tadini-Buoninsegni F, Smeazzetto S. Mechanisms of charge transfer in human copper ATPases ATP7A and ATP7B. IUBMB Life. 2017;69:218–25. https://doi.org/10.1002/iub.1603.
Lu J, Ling X, Sun Y, et al. FDX1 enhances endometriosis cell cuproptosis via G6PD-mediated redox homeostasis. Apoptosis. 2023;28:1128–40. https://doi.org/10.1007/s10495-023-01845-1.
Li X, Dai Z, Liu J, et al. Characterization of the functional effects of ferredoxin 1 as a cuproptosis biomarker in cancer. Front Genet. 2022;13:969856. https://doi.org/10.3389/fgene.2022.969856.
Dorsam B, Fahrer J. The disulfide compound alpha-lipoic acid and its derivatives: a novel class of anticancer agents targeting mitochondria. Cancer Lett. 2016;371:12–9. https://doi.org/10.1016/j.canlet.2015.11.019.
Tang X, Ren X, Huang T et al. (2023) Prognostic and Immunological Significance of the Molecular Subtypes and Risk Signatures Based on Cuproptosis in Hepatocellular Carcinoma. Mediators Inflamm 2023:3951940. https://doi.org/10.1155/2023/3951940.
Sharp PA. Ctr1 and its role in body copper homeostasis. Int J Biochem Cell Biol. 2003;35:288–91. https://doi.org/10.1016/s1357-2725(02)00134-6.
Inesi G. Molecular features of copper binding proteins involved in copper homeostasis. IUBMB Life. 2017;69:211–7. https://doi.org/10.1002/iub.1590.
Buettner GR. Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anticancer Agents Med Chem. 2011;11:341–6. https://doi.org/10.2174/187152011795677544.
Lewandowski L, Kepinska M, Milnerowicz H. The copper-zinc superoxide dismutase activity in selected diseases. Eur J Clin Invest. 2019;49:e13036. https://doi.org/10.1111/eci.13036.
Blockhuys S, Wittung-Stafshede P. Roles of copper-binding proteins in breast Cancer. Int J Mol Sci. 2017;18. https://doi.org/10.3390/ijms18040871.
Torrente L, Prieto-Farigua N, Falzone A, et al. Inhibition of TXNRD or SOD1 overcomes NRF2-mediated resistance to beta-lapachone. Redox Biol. 2020;30:101440. https://doi.org/10.1016/j.redox.2020.101440.
Arneth B. Tumor Microenvironment. Med (Kaunas). 2019;56. https://doi.org/10.3390/medicina56010015.
Katopodi T, Petanidis S, Charalampidis C, et al. Tumor-infiltrating dendritic cells: decisive roles in Cancer Immunosurveillance, Immunoediting, and Tumor T Cell Tolerance. Cells. 2022;11. https://doi.org/10.3390/cells11203183.
Faraj JA, Al-Athari AJH, Mohie SED, et al. Reprogramming the tumor microenvironment to improve the efficacy of cancer immunotherapies. Med Oncol. 2022;39:239. https://doi.org/10.1007/s12032-022-01842-5.
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. https://doi.org/10.1126/science.1203486.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. https://doi.org/10.1038/ni1102-991.
Del Prete A, Schioppa T, Tiberio L, Stabile H, Sozzani S. Leukocyte trafficking in tumor microenvironment. Curr Opin Pharmacol. 2017;35:40–7. https://doi.org/10.1016/j.coph.2017.05.004.
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22. https://doi.org/10.1038/ni.2703.
Perez-Romero K, Rodriguez RM, Amedei A, Barcelo-Coblijn G, Lopez DH. Immune Landscape in Tumor Microenvironment: implications for Biomarker Development and Immunotherapy. Int J Mol Sci. 2020;21. https://doi.org/10.3390/ijms21155521.
Kerneur C, Cano CE, Olive D. Major pathways involved in macrophage polarization in cancer. Front Immunol. 2022;13:1026954. https://doi.org/10.3389/fimmu.2022.1026954.
Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154:186–95. https://doi.org/10.1111/imm.12910.
Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40. https://doi.org/10.1002/jcp.26429.
Zhang J, Zhou X, Hao H. Macrophage phenotype-switching in cancer. Eur J Pharmacol. 2022;931:175229. https://doi.org/10.1016/j.ejphar.2022.175229.
Zhou K, Cheng T, Zhan J, et al. Targeting tumor-associated macrophages in the tumor microenvironment. Oncol Lett. 2020;20:234. https://doi.org/10.3892/ol.2020.12097.
Feng L, Li G, Li D, Duan G, Liu J. Cuproptosis-related gene SERPINE1 is a prognostic biomarker and correlated with immune infiltrates in gastric cancer. J Cancer Res Clin Oncol. 2023. https://doi.org/10.1007/s00432-023-04900-1.
Yan T, Yang H, Meng Y, et al. Targeting copper death genotyping associated gene RARRES2 suppresses glioblastoma progression and macrophages infiltration. Cancer Cell Int. 2023;23:105. https://doi.org/10.1186/s12935-023-02950-6.
Xu B, Yang K, Han X, Hou J. Cuproptosis-related gene CDKN2A as a molecular target for IPF diagnosis and therapeutics. Inflamm Res. 2023;72:1147–60. https://doi.org/10.1007/s00011-023-01739-7.
Hou S, Wang D, Yuan X, Yuan X, Yuan Q. Identification of biomarkers co-associated with M1 macrophages, ferroptosis and cuproptosis in alcoholic hepatitis by bioinformatics and experimental verification. Front Immunol. 2023;14:1146693. https://doi.org/10.3389/fimmu.2023.1146693.
Iyoda T, Yamasaki S, Ueda S, Shimizu K, Fujii SI. Natural killer T and natural killer cell-based immunotherapy strategies targeting Cancer. Biomolecules. 2023;13. https://doi.org/10.3390/biom13020348.
Piccinelli S, Romee R, Shapiro RM. The natural killer cell immunotherapy platform: an overview of the landscape of clinical trials in liquid and solid tumors. Semin Hematol. 2023;60:42–51. https://doi.org/10.1053/j.seminhematol.2023.02.002.
Hinshaw DC, Shevde LA. The Tumor Microenvironment innately modulates Cancer Progression. Cancer Res. 2019;79:4557–66. https://doi.org/10.1158/0008-5472.CAN-18-3962.
Wang NH, Lei Z, Yang HN, et al. Radiation-induced PD-L1 expression in tumor and its microenvironment facilitates cancer-immune escape: a narrative review. Ann Transl Med. 2022;10:1406. https://doi.org/10.21037/atm-22-6049.
Cai H, Zhang Y, Wang J, Gu J. Defects in macrophage reprogramming in Cancer Therapy: the negative impact of PD-L1/PD-1. Front Immunol. 2021;12:690869. https://doi.org/10.3389/fimmu.2021.690869.
Toor SM, Sasidharan Nair V, Decock J, Elkord E. Immune checkpoints in the tumor microenvironment. Semin Cancer Biol. 2020;65:1–12. https://doi.org/10.1016/j.semcancer.2019.06.021.
Wouters MCA, Nelson BH. Prognostic significance of Tumor-infiltrating B cells and plasma cells in Human Cancer. Clin Cancer Res. 2018;24:6125–35. https://doi.org/10.1158/1078-0432.CCR-18-1481.
Plesca I, Muller L, Bottcher JP, Medyouf H, Wehner R, Schmitz M. Tumor-associated human dendritic cell subsets: phenotype, functional orientation, and clinical relevance. Eur J Immunol. 2022;52:1750–8. https://doi.org/10.1002/eji.202149487.
Quan Y, Li W, Yan R, Cheng J, Xu H, Chen L. Tumor cuproptosis and immune infiltration improve survival of patients with hepatocellular carcinoma with a high expression of ferredoxin 1. Front Oncol. 2023;13:1168769. https://doi.org/10.3389/fonc.2023.1168769.
Zhou J, Chen D, Zhang S, Wang C, Zhang L. Identification of two molecular subtypes and a novel prognostic model of lung adenocarcinoma based on a cuproptosis-associated gene signature. Front Genet. 2022;13:1039983. https://doi.org/10.3389/fgene.2022.1039983.
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. https://doi.org/10.1002/ijc.29210.
Wang CI, Chu PM, Chen YL, Lin YH, Chen CY. Chemotherapeutic drug-regulated cytokines might Influence Therapeutic Efficacy in HCC. Int J Mol Sci. 2021;22. https://doi.org/10.3390/ijms222413627.
Li Y, Zeng X. A novel cuproptosis-related prognostic gene signature and validation of differential expression in hepatocellular carcinoma. Front Pharmacol. 2022;13:1081952. https://doi.org/10.3389/fphar.2022.1081952.
Yan C, Niu Y, Ma L, Tian L, Ma J. System analysis based on the cuproptosis-related genes identifies LIPT1 as a novel therapy target for liver hepatocellular carcinoma. J Transl Med. 2022;20:452. https://doi.org/10.1186/s12967-022-03630-1.
Zhang Z, Zeng X, Wu Y, Liu Y, Zhang X, Song Z. Cuproptosis-related risk score predicts prognosis and characterizes the Tumor Microenvironment in Hepatocellular Carcinoma. Front Immunol. 2022;13:925618. https://doi.org/10.3389/fimmu.2022.925618.
Wang G, Xiao R, Zhao S, et al. Cuproptosis regulator-mediated patterns associated with immune in fi ltration features and construction of cuproptosis-related signatures to guide immunotherapy. Front Immunol. 2022;13:945516. https://doi.org/10.3389/fimmu.2022.945516.
Lelievre P, Sancey L, Coll JL, Deniaud A, Busser B. The multifaceted roles of copper in Cancer: a Trace Metal element with Dysregulated Metabolism, but also a target or a bullet for Therapy. Cancers (Basel). 2020;12. https://doi.org/10.3390/cancers12123594.
Kurisaki E, Kuroda Y, Sato M. Copper-binding protein in acute copper poisoning. Forensic Sci Int. 1988;38:3–11. https://doi.org/10.1016/0379-0738(88)90003-5.
Datta J, Majumder S, Kutay H, et al. Metallothionein expression is suppressed in primary human hepatocellular carcinomas and is mediated through inactivation of CCAAT/enhancer binding protein alpha by phosphatidylinositol 3-kinase signaling cascade. Cancer Res. 2007;67:2736–46. https://doi.org/10.1158/0008-5472.CAN-06-4433.
Tamai Y, Iwasa M, Eguchi A, et al. Serum copper, zinc and metallothionein serve as potential biomarkers for hepatocellular carcinoma. PLoS ONE. 2020;15:e0237370. https://doi.org/10.1371/journal.pone.0237370.
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. https://doi.org/10.3322/caac.21763.
Donington JS, Colson YL. Sex and gender differences in non-small cell lung cancer. Semin Thorac Cardiovasc Surg. 2011;23:137–45. https://doi.org/10.1053/j.semtcvs.2011.07.001.
Zhang H, Shi Y, Yi Q, et al. A novel defined cuproptosis-related gene signature for predicting the prognosis of lung adenocarcinoma. Front Genet. 2022;13:975185. https://doi.org/10.3389/fgene.2022.975185.
Hu Q, Wang R, Ma H, Zhang Z, Xue Q. Cuproptosis predicts the risk and clinical outcomes of lung adenocarcinoma. Front Oncol. 2022;12:922332. https://doi.org/10.3389/fonc.2022.922332.
Wang Y, Zhang C, Ji C, et al. Molecular subtypes based on cuproptosis-related genes and immune profiles in lung adenocarcinoma. Front Genet. 2022;13:1006938. https://doi.org/10.3389/fgene.2022.1006938.
Zhu YP, Deng HT, Wang X, Rahat MA, Sun S, Zhang QZ. Cuproptosis-related molecular subtypes direct T cell exhaustion phenotypes and therapeutic strategies for patients with lung adenocarcinoma. Front Pharmacol. 2023;14:1146468. https://doi.org/10.3389/fphar.2023.1146468.
Yang J, Liu K, Yang L, et al. Identification and validation of a novel cuproptosis-related stemness signature to predict prognosis and immune landscape in lung adenocarcinoma by integrating single-cell and bulk RNA-sequencing. Front Immunol. 2023;14:1174762. https://doi.org/10.3389/fimmu.2023.1174762.
Yang L, Zhang Y, Wang Y, Jiang P, Liu F, Feng N. Ferredoxin 1 is a cuproptosis-key gene responsible for tumor immunity and drug sensitivity: a pan-cancer analysis. Front Pharmacol. 2022;13:938134. https://doi.org/10.3389/fphar.2022.938134.
Dong H, Zhao S, Zhang C, Wang X. Identification of cuproptosis related subtypes and construction of prognostic signature in gastric cancer. Front Surg. 2022;9:991624. https://doi.org/10.3389/fsurg.2022.991624.
Bao JH, Lu WC, Duan H, et al. Identification of a novel cuproptosis-related gene signature and integrative analyses in patients with lower-grade gliomas. Front Immunol. 2022;13:933973. https://doi.org/10.3389/fimmu.2022.933973.
Wang J, Qin D, Tao Z, et al. Identification of cuproptosis-related subtypes, construction of a prognosis model, and tumor microenvironment landscape in gastric cancer. Front Immunol. 2022;13:1056932. https://doi.org/10.3389/fimmu.2022.1056932.
Nie H, Wang H, Zhang M, et al. Comprehensive analysis of cuproptosis-related genes in prognosis, tumor microenvironment infiltration, and immunotherapy response in gastric cancer. J Cancer Res Clin Oncol. 2022. https://doi.org/10.1007/s00432-022-04474-4.
Ning ZK, Hu CG, Huang C, Liu J, Zhou TC, Zong Z. Molecular subtypes and CD4(+) memory T cell-based signature Associated with Clinical outcomes in gastric Cancer. Front Oncol. 2020;10:626912. https://doi.org/10.3389/fonc.2020.626912.
Yagi T, Sawada K, Miyamoto M, et al. Continuous administration of anti-VEGFA antibody upregulates PAI-1 secretion from ovarian cancer cells via mir-143-3p downregulation. Mol Cancer Res. 2023. https://doi.org/10.1158/1541-7786.MCR-23-0015.
Ma J, Gong B, Zhao Q. Pan-cancer analysis of cuproptosis-promoting gene signature from multiple perspectives. Clin Exp Med. 2023. https://doi.org/10.1007/s10238-023-01108-y.
Atiakshin D, Kostin A, Volodkin A, et al. Mast cells as a potential target of Molecular Hydrogen in regulating the local tissue microenvironment. Pharmaceuticals (Basel). 2023;16. https://doi.org/10.3390/ph16060817.
Wang L, Cao Y, Guo W, Xu J. High expression of cuproptosis-related gene FDX1 in relation to good prognosis and immune cells infiltration in colon adenocarcinoma (COAD). J Cancer Res Clin Oncol. 2023;149:15–24. https://doi.org/10.1007/s00432-022-04382-7.
Xie M, Cheng B, Yu S et al. (2022) Cuproptosis-Related MiR-21-5p/FDX1 Axis in Clear Cell Renal Cell Carcinoma and Its Potential Impact on Tumor Microenvironment. Cells 12. https://doi.org/10.3390/cells12010173.
Mao Z, Nie Y, Jia W, et al. Revealing Prognostic and Immunotherapy-sensitive characteristics of a Novel cuproptosis-related LncRNA model in Hepatocellular Carcinoma patients by Genomic Analysis. Cancers (Basel). 2023;15. https://doi.org/10.3390/cancers15020544.
Sun L, Su Y, Jiao A, Wang X, Zhang B. T cells in health and disease. Signal Transduct Target Ther. 2023;8:235. https://doi.org/10.1038/s41392-023-01471-y.
Song S, Zhang M, Xie P, Wang S, Wang Y. Comprehensive analysis of cuproptosis-related genes and tumor microenvironment infiltration characterization in breast cancer. Front Immunol. 2022;13:978909. https://doi.org/10.3389/fimmu.2022.978909.
Sha S, Si L, Wu X, et al. Prognostic analysis of cuproptosis-related gene in triple-negative breast cancer. Front Immunol. 2022;13:922780. https://doi.org/10.3389/fimmu.2022.922780.
Li Z, Zhang H, Wang X, et al. Identification of cuproptosis-related subtypes, characterization of tumor microenvironment infiltration, and development of a prognosis model in breast cancer. Front Immunol. 2022;13:996836. https://doi.org/10.3389/fimmu.2022.996836.
Li L, Li L, Sun Q. High expression of cuproptosis-related SLC31A1 gene in relation to unfavorable outcome and deregulated immune cell infiltration in breast cancer: an analysis based on public databases. BMC Bioinformatics. 2022;23:350. https://doi.org/10.1186/s12859-022-04894-6.
Sung H, Ferlay J, Siegel RL, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Makhov P, Joshi S, Ghatalia P, Kutikov A, Uzzo RG, Kolenko VM. Resistance to systemic therapies in Clear Cell Renal Cell Carcinoma: mechanisms and management strategies. Mol Cancer Ther. 2018;17:1355–64. https://doi.org/10.1158/1535-7163.MCT-17-1299.
Yuan H, Qin X, Wang J, Yang Q, Fan Y, Xu D. The cuproptosis-associated 13 gene signature as a robust predictor for outcome and response to immune- and targeted-therapies in clear cell renal cell carcinoma. Front Immunol. 2022;13:971142. https://doi.org/10.3389/fimmu.2022.971142.
Liu A, Li Y, Shen L, et al. Molecular subtypes based on cuproptosis regulators and immune infiltration in kidney renal clear cell carcinoma. Front Genet. 2022;13:983445. https://doi.org/10.3389/fgene.2022.983445.
Xu J, Hu Z, Cao H, et al. Multi-omics pan-cancer study of cuproptosis core gene FDX1 and its role in kidney renal clear cell carcinoma. Front Immunol. 2022;13:981764. https://doi.org/10.3389/fimmu.2022.981764.
Saad E, Saliby RM, Labaki C et al. (2023) Novel Immune therapies for Renal Cell Carcinoma: looking beyond the programmed cell death protein 1 and cytotoxic T-Lymphocyte-Associated protein 4 axes. Hematol Oncol Clin North Am. https://doi.org/10.1016/j.hoc.2023.05.023.
Schiavoni V, Campagna R, Pozzi V, et al. Recent advances in the management of Clear Cell Renal Cell Carcinoma: novel biomarkers and targeted therapies. Cancers (Basel). 2023;15. https://doi.org/10.3390/cancers15123207.
Xie J, Yang Y, Gao Y, He J. Cuproptosis: mechanisms and links with cancers. Mol Cancer. 2023;22:46. https://doi.org/10.1186/s12943-023-01732-y.
Petruzzelli R, Polishchuk RS. Activity and trafficking of copper-transporting ATPases in Tumor Development and Defense against Platinum-based drugs. Cells. 2019;8. https://doi.org/10.3390/cells8091080.
Fang Y, Tian S, Pan Y, et al. Pyroptosis: a new frontier in cancer. Biomed Pharmacother. 2020;121:109595. https://doi.org/10.1016/j.biopha.2019.109595.
O’Day SJ, Eggermont AM, Chiarion-Sileni V, et al. Final results of phase III SYMMETRY study: randomized, double-blind trial of elesclomol plus paclitaxel versus paclitaxel alone as treatment for chemotherapy-naive patients with advanced melanoma. J Clin Oncol. 2013;31:1211–8. https://doi.org/10.1200/JCO.2012.44.5585.
Berkenblit A, Eder JP Jr., Ryan DP, et al. Phase I clinical trial of STA-4783 in combination with paclitaxel in patients with refractory solid tumors. Clin Cancer Res. 2007;13:584–90. https://doi.org/10.1158/1078-0432.CCR-06-0964.
Monk BJ, Kauderer JT, Moxley KM, et al. A phase II evaluation of elesclomol sodium and weekly paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube or primary peritoneal cancer: an NRG oncology/gynecologic oncology group study. Gynecol Oncol. 2018;151:422–7. https://doi.org/10.1016/j.ygyno.2018.10.001.
O’Day S, Gonzalez R, Lawson D, et al. Phase II, randomized, controlled, double-blinded trial of weekly elesclomol plus paclitaxel versus paclitaxel alone for stage IV metastatic melanoma. J Clin Oncol. 2009;27:5452–8. https://doi.org/10.1200/JCO.2008.17.1579.
Huang J, Chaudhary R, Cohen AL, et al. A multicenter phase II study of temozolomide plus disulfiram and copper for recurrent temozolomide-resistant glioblastoma. J Neurooncol. 2019;142:537–44. https://doi.org/10.1007/s11060-019-03125-y.
Schweizer MT, Lin J, Blackford A, et al. Pharmacodynamic study of disulfiram in men with non-metastatic recurrent prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:357–61. https://doi.org/10.1038/pcan.2013.28.
Nechushtan H, Hamamreh Y, Nidal S, et al. A phase IIb trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. Oncologist. 2015;20:366–7. https://doi.org/10.1634/theoncologist.2014-0424.
Karamanakos PN. Possible role for furazolidone in the treatment of glioblastoma multiforme. J BUON. 2013;18:1097.
Saifi MA, Shaikh AS, Kaki VR, Godugu C. Disulfiram prevents collagen crosslinking and inhibits renal fibrosis by inhibiting lysyl oxidase enzymes. J Cell Physiol. 2022;237:2516–27. https://doi.org/10.1002/jcp.30717.
Kelley KC, Grossman KF, Brittain-Blankenship M, et al. A phase 1 dose-escalation study of disulfiram and copper gluconate in patients with advanced solid tumors involving the liver using S-glutathionylation as a biomarker. BMC Cancer. 2021;21:510. https://doi.org/10.1186/s12885-021-08242-4.
Jakola AS, Werlenius K, Mudaisi M, et al. Disulfiram repurposing combined with nutritional copper supplement as add-on to chemotherapy in recurrent glioblastoma (DIRECT): study protocol for a randomized controlled trial. F1000Res. 2018;7:1797. https://doi.org/10.12688/f1000research.16786.1.
Huang J, Campian JL, Gujar AD, et al. A phase I study to repurpose disulfiram in combination with temozolomide to treat newly diagnosed glioblastoma after chemoradiotherapy. J Neurooncol. 2016;128:259–66. https://doi.org/10.1007/s11060-016-2104-2.
Halatsch ME, Kast RE, Karpel-Massler G, et al. A phase Ib/IIa trial of 9 repurposed drugs combined with temozolomide for the treatment of recurrent glioblastoma: CUSP9v3. Neurooncol Adv. 2021;3:vdab075. https://doi.org/10.1093/noajnl/vdab075.
Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212–7. https://doi.org/10.1073/pnas.1320318110.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894–905. https://doi.org/10.1056/NEJMoa1915745.
Kang X, Jadhav S, Annaji M et al. (2023) Advancing Cancer Therapy with Copper/Disulfiram nanomedicines and Drug Delivery systems. Pharmaceutics 15. https://doi.org/10.3390/pharmaceutics15061567.
Xiong C, Ling H, Hao Q, Zhou X. Cuproptosis: p53-regulated metabolic cell death? Cell Death Differ. 2023;30:876–84. https://doi.org/10.1038/s41418-023-01125-0.
Cheng F, Peng G, Lu Y, et al. Relationship between copper and immunity: the potential role of copper in tumor immunity. Front Oncol. 2022;12:1019153. https://doi.org/10.3389/fonc.2022.1019153.
Babak MV, Ahn D. Modulation of intracellular copper levels as the mechanism of action of Anticancer Copper complexes: clinical relevance. Biomedicines. 2021;9. https://doi.org/10.3390/biomedicines9080852.
Li Y. Copper homeostasis: emerging target for cancer treatment. IUBMB Life. 2020;72:1900–8. https://doi.org/10.1002/iub.2341.
Zheng P, Zhou C, Lu L, Liu B, Ding Y. Elesclomol: a copper ionophore targeting mitochondrial metabolism for cancer therapy. J Exp Clin Cancer Res. 2022;41:271. https://doi.org/10.1186/s13046-022-02485-0.
Gao W, Huang Z, Duan J, Nice EC, Lin J, Huang C. Elesclomol induces copper-dependent ferroptosis in colorectal cancer cells via degradation of ATP7A. Mol Oncol. 2021;15:3527–44. https://doi.org/10.1002/1878-0261.13079.
Oliveri V. Selective targeting of Cancer cells by copper ionophores: an overview. Front Mol Biosci. 2022;9:841814. https://doi.org/10.3389/fmolb.2022.841814.
Lu Y, Pan Q, Gao W, et al. Leveraging disulfiram to treat cancer: mechanisms of action, delivery strategies, and treatment regimens. Biomaterials. 2022;281:121335. https://doi.org/10.1016/j.biomaterials.2021.121335.
Fasehee H, Dinarvand R, Ghavamzadeh A, et al. Delivery of disulfiram into breast cancer cells using folate-receptor-targeted PLGA-PEG nanoparticles: in vitro and in vivo investigations. J Nanobiotechnol. 2016;14:32. https://doi.org/10.1186/s12951-016-0183-z.
Li YQ, Yin JY, Liu ZQ, Li XP. Copper efflux transporters ATP7A and ATP7B: novel biomarkers for platinum drug resistance and targets for therapy. IUBMB Life. 2018;70:183–91. https://doi.org/10.1002/iub.1722.
Zhang C, Xu T, Ji K, et al. An integrative analysis reveals the prognostic value and potential functions of PSMD11 in hepatocellular carcinoma. Mol Carcinog. 2023. https://doi.org/10.1002/mc.23568.
Wang W, Lu K, Jiang X, et al. Ferroptosis inducers enhanced cuproptosis induced by copper ionophores in primary liver cancer. J Exp Clin Cancer Res. 2023;42:142. https://doi.org/10.1186/s13046-023-02720-2.
Denoyer D, Masaldan S, La Fontaine S, Cater MA. Targeting copper in cancer therapy: ‘Copper that Cancer’. Metallomics. 2015;7:1459–76. https://doi.org/10.1039/c5mt00149h.
Liu H. Pan-cancer profiles of the cuproptosis gene set. Am J Cancer Res. 2022;12:4074–81.
Desai R, Coxon AT, Dunn GP. Therapeutic applications of the cancer immunoediting hypothesis. Semin Cancer Biol. 2022;78:63–77. https://doi.org/10.1016/j.semcancer.2021.03.002.
Funding
This study was supported by the Henan Natural Science Foundation of China (No. 222300420534) and by the Ministry of Science and Higher Education of the Russian Federation within the framework of State assignments № 075-00277-24-00 (INEOS RAS).
Author information
Authors and Affiliations
Contributions
All authors contributed the manuscript conceptualization, planning, and data collection. R.Z., O.S., M.N., J.L., and H.G. wrote the original draft. E.T., M.N., Yu.Z., D.Z., S.V.M., and J.L were responsible for supervision, data investigation and validation (preparation of tables), and editing. M.N., J.L., and R.F. were involved in the funding acquisition and data curation. Yu.Z., Yu.A, and H.G. were responsible for data visualization (preparation of figures). SVM, E.T., J.L., X.Z., R.F. provided resourses and the project administration. All authors approved the submission of the final version of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, R., Sukocheva, O., Tse, E. et al. Cuproptosis, the novel type of oxidation-induced cell death in thoracic cancers: can it enhance the success of immunotherapy?. Cell Commun Signal 22, 379 (2024). https://doi.org/10.1186/s12964-024-01743-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-024-01743-2