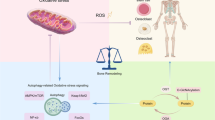
Abstract
O-linked N-acetylglucosamine (O-GlcNAc) protein modification (O-GlcNAcylation) is a critical post-translational modification (PTM) of cytoplasmic and nuclear proteins. O-GlcNAcylation levels are regulated by the activity of two enzymes, O-GlcNAc transferase (OGT) and O‑GlcNAcase (OGA). While OGT attaches O-GlcNAc to proteins, OGA removes O-GlcNAc from proteins. Since its discovery, researchers have demonstrated O-GlcNAcylation on thousands of proteins implicated in numerous different biological processes. Moreover, dysregulation of O-GlcNAcylation has been associated with several pathologies, including cancers, ischemia-reperfusion injury, and neurodegenerative diseases. In this review, we focus on progress in our understanding of the role of O-GlcNAcylation in bone pathophysiology, and we discuss the potential molecular mechanisms of O-GlcNAcylation modulation of bone-related diseases. In addition, we explore significant advances in the identification of O-GlcNAcylation-related regulators as potential therapeutic targets, providing novel therapeutic strategies for the treatment of bone-related disorders.
Similar content being viewed by others
Introduction
The functional diversity of the proteome is considerably augmented by post-translational modification through glycosylation, phosphorylation, methylation, ubiquitylation, and acetylation. In recent years, the pathophysiological roles of these post-translational modifications (PTMs) have attracted a great deal of attention [1, 2]. Glycosylation, the post-translational modification of proteins with various carbohydrates, is one of the most important classes of PTMs, with approximately half of all proteins in living organisms exhibiting this modification [3]. Glycosylation affects protein function, regulates cell signaling, and modulates a range of biological processes [4,5,6]. O-linked N-acetylglucosamine protein modification (O-GlcNAcylation) was first identified by Hart and Torres in 1984. These authors used bovine milk galactosyltransferase to couple tritiated UDP-galactose to N-acetylglucosamine (GlcNAc) residues on the surfaces of mouse lymphocytes [7]. Nowadays, after in-depth research, the number of O-GlcNAcylated proteins identified has reached more than 4000 [8].
O-GlcNAcylated proteins are typically localized in the nucleus, cytoplasm, or mitochondria [9, 10]. Extensive research has revealed that O-GlcNAcylated proteins play important roles in a variety of cellular processes, including transcription, translation, apoptosis, the cell cycle, protein transportation, mitochondrial function, and signal transduction [11,12,13]. Moreover, dysregulation of O-GlcNAcylation has been associated with several pathologies, including cancers [14], ischemia-reperfusion injury [15], and neurodegenerative diseases [16]. However, the precise role of O-GlcNAcylation in bone pathophysiology remains incompletely understood. Here, we focus on the effects of O-GlcNAcylation changes on bone physiology and pathophysiology and discuss its potential value as a target in clinical treatment.
O-GlcNAcylation and related enzymes
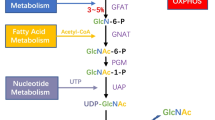
O-GlcNAcylation functions as a nutrient sensor through the hexosamine biosynthetic pathway (HBP). Because the HBP requires input from several molecules, including glucose, glutamine, UTP, and acetate, it plays a crucial role in sensing and integrating nutrients [17, 18]. Under normal conditions, a fraction (2–3%) of the glucose entering the cell is directed to the HBP [19]. In this pathway, glutamine-fructose-6-phosphate amidotransferase (GFAT) converts fructose 6-phosphate into glucosamine 6-phosphate, and this is ultimately converted into UDP-N-acetylglucosamine (UDP-GlcNAc), which is a key substrate for protein O-GlcNAcylation. The addition and removal steps of UDP-GlcNAc modification are mediated by two highly conserved enzymes, O‑GlcNAc transferase (OGT) and O‑GlcNAcase (OGA), respectively [20](Fig. 1).
The hexosamine biosynthesis pathway and O-GlcNAcylation
The hexosamine biosynthetic pathway (HBP) integrates four metabolic intermediates, a carbohydrate (glucose), an amino acid (glutamine), a lipid (Acetyl-CoA), and a nucleotide (UTP), to produce UDP-N-Acetlyglucosamine (UDP-GlcNAc). O-GlcNAcylation is cycled on and off proteins by two enzymes, O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), that catalyze the addition and removal of O-GlcNAc, respectively
Abbreviations used in this figure: HK, hexokinase; GPI, glucose-6-phosphate isomerase; GFAT, glutamine-fructose-6-phosphate amidotransferase; GNPNAT (EMeg32), glucosamine-phosphate N-acetyltransferase; PGM3/ AGM1, GlcNAc phosphomutase; UAP/ AGX1, UDP-GlcNAc pyrophosphorylase
O-GlcNAc transferase (OGT), which is encoded by a single gene on the X chromosome (OGT), was first isolated in 1992 from rat liver and subsequently cloned five years later [21,22,23]. OGT is highly conserved in mammals, and it is essential for embryonic stem cell viability and development [21, 24]. Functional OGT enzymes are composed of an N-terminal substrate recognition domain and a C-terminal catalytic domain. In the cells tested, three different transcripts are produced by alternative gene splicing, and these transcripts encode a nucleocytoplasmic isoform (ncOGT, 116 kDa), a mitochondrial isoform (mOGT, 103 kDa), and a short isoform (sOGT, 75 kDa). As shown in Fig. 2, the C-termini of these isoforms are identical, although their N-termini differ in the number of the N-terminal tetratricopeptide repeats (TPR) motifs [25]. The unique lengths of their N-terminal domains and differences in the relative positioning of the TPR motifs allow for the targeting of different subsets of protein substrates within the proteome [26]. Through this mechanism, OGT modulates almost every biological process that occurs within the cell.
Schematic representation of the OGT and OGA isoforms
Schematic representation of the OGT and OGA structural domains, with amino acids labeling the boundaries of each. While ncOGT and sOGT are both distributed in the nucleus and cytoplasm, mOGT is mainly localized in the mitochondria. These isoforms have 13.5, 9.5, and 2.5 (orange) TPRs, respectively. The N-terminal catalytic domain (N-cat) and C-terminal catalytic domain (C-cat) are the two catalytic structural domains (blue), and the intervening domain (Int-D) (green) is of unknown function. lOGA is distributed in the cytoplasm and nucleolus, while sOGA is located in the nucleus. Both isomers contain the same N-terminal catalytic structural domain (Catalytic) (orange) and stalk structural domain (Stalk) (green), but the C-terminal HAT-like structural domain (pHAT) (blue) is absent in sOGA
O‑GlcNAcase (OGA) is the only enzyme capable of hydrolyzing O-GlcNAcylation modifications from various glycoprotein substrates. OGA has two major splice isoforms, long (lOGA) and short (sOGA). Both isoforms are comprised of a glycoside hydrolase 84 (GH84) catalytic domain at the N-terminus and a helical bundle stalk-like domain. However, while lOGA possesses a pseudo-histone acetyltransferase domain (pHAT) in its C‑terminal region, sOGA lacks this pHAT-like domain [17, 27, 28]. The hydrolase activity responsible for removing O-GlcNAcylation is situated in the GH domain (Fig. 2). To date, information concerning the mechanism underlying substrate recognition by OGA is limited. Further elucidation of the structural features of OGA should uncover its substrate recognition mechanism.
In recent years, multiple studies have demonstrated that O-GlcNAcylation plays a crucial role in regulating the dynamic balance of the bone matrix, ultimately constituting a complex network involved in bone metabolism. As a consequence of this important role, O-GlcNAcylation exhibits potential for exploitation as a new molecular marker and as a target for bone therapy.
O-GlcNAcylation in bone physiology
The role of O-GlcNAcylation in BMSCs
As an important and dynamic post-translational modification in mammalian cells, O-GlcNAcylation plays a crucial role in the differentiation of cell lineages, such as bone marrow mesenchymal stromal cells (BMSCs), neural stem cells (NSCs) [29], and hematopoietic stem cells (HSCs) [30, 31]. BMSCs are a heterogeneous group of multipotent stem cells, including osteochondral and adipocyte progenitors, that exhibit niche forming and immunomodulatory capabilities [32]. BMSCs are essential for maintaining bone homeostasis, tissue regeneration, and global energy homeostasis. Glucose, fatty acids, and amino acids are known to fuel BMSC differentiation. Plasticity in energy metabolism enables BMSCs to meet the different demands of osteo-adipogenic differentiation. Targeting the metabolic pathways of BMSCs could offer a new therapeutic approach for diseases related to imbalanced BMSC differentiation [33]. Interestingly, researchers have observed changes in intracellular O-GlcNAcylation levels during the differentiation of BMSCs into adipogenic, chondrogenic, and osteogenic cells [34]. In short, O-GlcNAcylation modifies and regulates transcripts, which in turn affects the differentiation fate and niche function of early BMSCs, ultimately coordinating the early development of skeletal and hematopoietic systems.
The role of O-GlcNAcylation in osteogenic differentiation
Osteoblasts, which are bone-forming cells, play a crucial role in the modeling and remodeling of bones. Osteogenic differentiation is dependent upon multiple signaling pathways, including the Wnt, BMP (bone morphogenetic protein), TGF-β, and hedgehog (Hh) pathways [35]. However, the molecular mechanisms that underlie this process are not completely understood. Runt-related transcription factor 2 (Runx2), a master transcription factor for osteogenesis, orchestrates the differentiation of mesenchymal stem cells into osteoblasts, and these then mature into osteocytes [36]. Researchers have partially elucidated the mechanisms through which O-GlcNAcylation affects the activity of Runx2. Kim et al. first reported that O-GlcNAcylation modification of Runx2 positively regulates its transcriptional activity, resulting in increased expression of its target gene osteocalcin, which may itself indirectly participate in osteoblastic differentiation [37]. Additionally, by modifying and activating Runx2, O-GlcNAcylation promotes osteogenic differentiation of BMSCs, thus controlling niche function [38]. Inhibition of OGA also augmented the transcriptional activity of Runx2, which in turn influenced the production of alkaline phosphatase (ALP), a matrix maturation marker, during the early phases of osteoblast differentiation [39].
As mentioned above, osteogenic differentiation is also dependent upon the Wntpathway. The Wnt signaling pathway can be divided into two main pathways, the canonical pathway, and the non-canonical pathway. The canonical Wnt signaling pathway is mediated by β-catenin, which promotes osteogenesis [40]. A study by Garg et al. demonstrated that treatment with triptolide reduced the expression level of OGT and decreased O-GlcNAcylation modification of β-catenin, thus inhibiting the canonical pathway in pancreatic cancer cells [41]. OGT-mediated O-GlcNAcylation is also known to regulate embryonic neurogenesis and neuronal development in vivo and in vitro by increasing β-catenin levels [42]. Although the mechanism underlying osteogenic differentiation remains unclear, this topic still provides reliable insights for our study.
Interestingly, several studies have reported that O-GlcNAcylation has the opposite effect on osteogenic differentiation, which reveals the contradictory nature of O-GlcNAcylation regulation. For instance, diabetic patients under hyperglycemic conditions may experience an abnormal increase in O-GlcNAcylation. In C2C12 cells, an increase in O-GlcNAcylation was followed by a reduction in bone BMP2-induced osteogenic differentiation [43]. The p38 mitogen-activated protein kinase (MAPK) pathway — one of the three major MAPK signaling pathways, along with the extracellular signal-regulated kinase (ERK) pathway and the c-Jun N-terminal protein kinases (JNK) pathway — is known to be involved in inducing osteogenic differentiation [44]. Papanicolaou et al. found that decreasing O-GlcNAcylation caused activation of basal p38 phosphorylation (and downstream signaling) in the early phase of cardiac myocytes [45]. Similarly, Goldberg et al. [46]. revealed that high levels of O-GlcNAcylation promote cancer progression by downregulation of p38 MAPK activity in the tumor microenvironment (Fig. 3). This opposing effect also occurs in other types of stem cells, such as NSCs [29, 47], which suggests that O-GlcNAcylation has a diverse and multifaceted role in regulating cytogenesis. Hence, O-GlcNAcylation should be considered an important molecular target for the osteogenic differentiation of BMSCs, and further research into its role could offer a new approach to clinical therapy of bone-related diseases.
The molecular mechanism and physiological regulation roles of O-GlcNAcylation modification in bone
O-GlcNAcylation is involved in osteogenic differentiation, osteoclastic differentiation, adipogenic differentiation, and chondrogenic differentiation, therein regulating the expression of potential target genes and related signaling pathways. Dotted arrows represent a possible but not yet established effect
The role of O-GlcNAcylation in osteoclastic differentiation
Osteoclasts are tissue-specific macrophage polykaryons that degrade bone matrix, and they are known to differentiate from monocyte/ macrophage precursor cells at or near the bone surface [48]. Recent studies have demonstrated that O-GlcNAcylation is a prerequisite for osteoclast differentiation from these precursor cells, indicating that O-GlcNAcylation also plays a role in the formation of osteoclasts.
Activation of nuclear factor-kappaB (NF-κB) pathways is also required for the maturation and activation of osteoclasts [49]. Moreover, nuclear factor of activated T-cells c1 (NFATc1), a transcription factor, is known to play a crucial role in regulating the expression of osteoclast-specific downstream target genes [50]. Kim’s group found that HBP was activated during the differentiation of murine osteoclasts. Furthermore, OGT deficiency inhibited the O-GlcNAcylation of NF-κB p65 and NFATc1, preventing their transfer to the nucleus and reducing downstream transcriptional activities that control osteoclast differentiation [51]. Similarly, a genetic deletion of OGT inhibited osteoclast formation and downregulated markers related to their differentiation and function, such as α(v) integrin and cathepsin K [52].
Interleukin-6 (IL-6) is a cytokine that regulates B-cell differentiation and was first identified and cloned by Kishimoto and Hirano in 1986 [53, 54]. In a co-culture system comprised of RAW264.7 cells and the human synovial sarcoma cell line SW982, IL-6 enhanced the expression of NFATc1, leading to the induction of osteoclast differentiation [53]. Hu et al. found that the increased O-GlcNAcylation of p65 enhanced its activity and nuclear translocation, leading to the upregulation of IL-6 in the skeletal muscle of mice during exposure to cold temperatures [55]. In addition, amyloid beta (Aβ) is known to increase O-GlcNAcylation of c-Fos, a vital component of activator protein-1. As shown in Fig. 3, O-GlcNAcylation increases the stability of c‐Fos, stimulating both its interaction with c‐Jun and transcriptional activity in neuronal cells [56, 57]. This process may also be essential for osteoclast differentiation [58]. Together, the above findings should guide future research on osteoclastic differentiation of BMSCs, even if not all these mechanisms have been observed in osteoclasts.
The role of O-GlcNAcylation in adipogenic differentiation
Bone marrow adipocytes are a unique cell population originating from BM mesenchymal progenitors and marrow adipogenic lineage precursors, and they play important physiological roles in hematopoiesis and bone metabolism [59]. As discussed above, O-GlcNAcylation modification is a novel and essential post-transcriptional modulator of gene expression, and several studies have linked it to adipogenic differentiation. Zhang et al. demonstrated that O-GlcNAcylation negatively affects C/EBPβ-dependent marrow adipogenesis [38]. As an early transcription factor, C/EBPβ plays a decisive role in adipogenic differentiation of BMSCs [60, 61]. However, Ishihara et al. found that O-GlcNAcylation was drastically increased during adipogenic differentiation of 3T3-L1 preadipocytes, and that this increase was associated with C/EBPβ expression [62]. GFAT-1 is also known to regulate adipogenesis by increasing the expression levels of critical transcription factors (C/EBPβ, PPARγ), fatty acid synthase (FAS), and lipid droplet (LD) proteins in 3T3-L1 cells [63]. These results suggest that the effects of O-GlcNAcylation are cell-specific and condition-specific. Further studies are needed to elucidate these mechanisms.
Notch signaling is a critical communication pathway between cells that influences numerous cell fate decisions during development [64]. Osathanon et al. found that the Notch pathway demonstrates both inhibitory and stimulatory effects on adipogenic differentiation [65]. Hao et al. provided evidence that the expression levels of Notch signaling-activated genes, Rbpj and Hes1, were significantly induced in wild-type (Eogt+/+) CD4+ T cells but not in Eogt knockout (Eogt-/-) counterparts. Thus, loss of Eogt appears to suppress Notch signaling pathway [66]. The Hedgehog (Hh) family of signaling proteins is known to control differentiation and proliferation during development [67]. During the adipogenic differentiation of BMSCs, Hh signaling is downregulated due to the decreased expression of transcription factor Gli [68]. Interestingly, O-GlcNAcylation modification functionally increased transcriptional activity in the Hh/ Gli pathway in tumor cells (Fig. 3) [69]. Although there is no direct evidence that regulation of adipogenic differentiation by O-GlcNAcylation involves the above mechanisms, these studies provide new directions for further research on adipogenic differentiation.
The role of O-GlcNAcylation in chondrogenic differentiation
Cao et al. utilized the chondrogenic differentiation of BMSCs for treating and repairing cartilage defects [70]. In fact, some promising in vitro studies have reported the chondrogenic effects of glucosamine (GlcN), which include chondrocyte proliferation [71, 72]. However, the mechanisms regulating O-GlcNAcylation modification during this process are not fully understood.
O-GlcNAcylated protein expression is increased during insulin-induced Pre-chondrogenic ATDC5 cell differentiation, suggesting a possible link between O-GlcNAcylation and chondrocyte differentiation. In addition, accumulation of O-GlcNAcylation after inhibition of OGA leads to pre-hypertrophic chondrocyte differentiation in vitro and in vivo in newborn mice (Fig. 3) [73]. However, our current understanding of this topic is limited, and further research is needed to better understand the role of O-GlcNAcylation in regulating chondrogenic differentiation and development.
O-GlcNAcylation participates in pathological bone diseases
Rheumatoid arthritis (RA)
RA is a chronic inflammatory joint disease associated with loss of cartilage, bone damage, and disability [74]. An accumulation of immune cells and expansion of resident stromal and vascular cells in the local pathology generates an aggressive environment that leads to gradual bone mass loss [75]. However, the pathogenic cause of this disease is not yet fully understood. Establishing an early diagnosis, initiating prompt treatment, and designing novel treatment strategies to control inflammation, minimize damage, and prevent further complications are crucial.
Tumor necrosis factor alpha (TNF-α), a key mediator of inflammation in arthritis, fosters the dynamic regulation of O-GlcNAcylation during osteoclastogenesis [76]. Mechanically, TNF-α enhances osteoclastogenesis in inflammatory arthritis by increasing the expression levels of OGT and OGA. Kim et al. found that hyper-O-GlcNAcylation of p65 significantly aggravated the severity of RA in response to TNF-α. This was due to increased nuclear translocation of p65, increased binding of p65 protein to DNA, and enhanced transcriptional activity [77]. The cytokine Interleukin-1β (IL-1β) is involved in the perpetuation of the chronic inflammation state characterizing RA [78]. Umar et al. found that Penta-o-galloyl-beta-d-Glucose (PGG) could inhibit O-GlcNAcylation of transforming growth factor beta-activated kinase 1-binding protein 1 (TAB1) and reduce TGF-β-activated kinase 1 (TAK1) activation in IL-1β-stimulated human RA synovial fibroblasts (RASFs). The resulting reduction in TAK1 signaling led to a reduction in inflammation and tissue destruction [79]. Another critical inflammatory pathway in RA is the NF-kB signaling pathway [80]. Previous studies have shown that O-GlcNAc modification of NF-kB positively regulates its gene transcription functions in different cell types [81,82,83,84]. These findings may inform future treatments for RA.
Dendritic cells (DCs) are specialized cells that present antigens, and they play a crucial role in immunity and immune tolerance [85]. In RA, there is a massive infiltration of DCs in the synovium. Monocyte-derived dendritic cells (moDCs) in the RA synovium facilitate the production of pro-inflammatory cytokines [86]. Weiss’s group demonstrated that OGT-mediated O-GlcNAcylation modulates the AKT and MEK/ ERK pathways in moDCs, ultimately hindering the differentiation of monocytes into immature DCs, and impeding the maturation process in already differentiated immature moDCs [87].
Macrophages in the immune system can be divided into two types: classically activated macrophages (M1) and alternatively activated macrophages (M2), which perform pro-inflammatory and anti-inflammatory functions, respectively [88]. In RA, the imbalance between pro-inflammatory M1 and anti-inflammatory M2 macrophages leads to synovial inflammation, autoimmunity, and joint damage [89]. Yang et al. reported that OGT-mediated O-GlcNAcylation modifications suppress macrophage pro-inflammatory polarization by regulating S6 kinase beta 1 (S6K1) O-GlcNAcylation/ phosphorylation and S6K1 activation [90]. Nagai-Singer et al. demonstrated that NOD-like receptor (NLR) X1 (NLRX1) suppresses pro-inflammatory NF-κB signaling and blocks downstream pro-inflammatory factors [91]. Chen et al. found that elevated O-GlcNAcylation levels promoted NLRX1 ubiquitination, which enhanced the binding of NLRX1 and IkappaB kinase-alpha (IKK-α). This ultimately led to a reduction in the expression of the inflammatory cytokine IL-1β in M1 macrophages [92].
The pro-inflammatory function of Th17 cells, which originate from CD4+ Th cells, is enhanced in RA. Interleukin-17 A (IL-17 A) is the major cytokine that mediates the function of Th17 cells, and it plays a pivotal role in promoting inflammatory reactions [93]. Recent studies have demonstrated the critical role of O-GlcNAcylation nutrient sensing in regulating Th17 cell function. Excessive O-GlcNAcylation can lead to an increase in acetyl-CoA carboxylase 1 (ACC1) O-GlcNAcylation and alterations in the lipidome. These changes in lipid expression activate RORγt, leading to an increase in IL-17 production (Fig. 4) [94]. The above studies hint that regulation of O-GlcNAcylation metabolism in these cells may have clinical applications. However, further research is required to determine whether O-GlcNAcylation directly regulates RA and its underlying mechanisms through the above cells.
The role of O-GlcNAcylation modification in RA
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease associated with loss of cartilage, bone damage, and disability. O-GlcNAcylation may affect the progression of RA by targeting proteins or regulating signaling pathways. Dotted arrows represent a possible but not yet established effect
Osteoarthritis (OA)
O-GlcNAcylation is known to play a crucial role in regulating various cellular processes during embryonic development and during immune responses [79]. Nonetheless, the impact of O-GlcNAcylation on inflammation is still not clear. OA is a prevalent and chronic joint disease that causes progressive cartilage degradation, subchondral bone remodeling, bone marrow lesions, meniscal damage, and synovitis [95]. The classical view of the pathogenesis of OA is that subchondral sclerosis is associated with age-related joint degeneration. Notably, clinical investigations on OA patients and research using animal models suggest that there is a connection between abnormal glycosylation and the onset and advancement of OA [96]. An analysis of gene expression data revealed that glycosyltransferase expression was affected by pro-inflammatory cytokines, potentially resulting in protein degradation and an acceleration of OA pathology [97].
O-GlcNAcylation levels are also known to change significantly during the progression of OA. Glucosamine (GlcN) is a naturally occurring amino monosaccharide widely used as a dietary supplement for OA [98,99,100,101,102,103]. Someya et al. reported that GlcN exerts an anti-inflammatory effect on synovial cells in OA by suppressing the gene expression of multiple pro-inflammatory cytokines (including IL-6, IL-24, and TNF-α) via O-GlcNAc modification. Thus, GlcN exhibits a protective effect on OA [104]. Interleukin-1 (Il-1), a multifunctional monokine, plays a significant role in the destruction of articular cartilage in degenerative arthropathies [105]. Interestingly, research has shown that the concentration of IL-1a is also increased in OA joints. Moreover, IL-1a induced an accumulation of O-GlcNAcylation in cultured human OA chondrocytes (HOC), thus providing evidence for a link between OA and O-GlcNAc levels (Fig. 5) [106].
The role of O-GlcNAcylation modification in other bone-related diseases
O-GlcNAcylation also plays a role in other bone-related diseases, including OA (Osteoarthritis), osteolysis, bone metastasis and IDD (Intervertebral disc degeneration). Generally, aberrant O-GlcNAcylation modifications on diverse substrates promote or suppress bone-related disease progression
Intervertebral disc degeneration (IDD)
IDD is a common finding on spine imaging, and it becomes more prevalent with age [107]. IDD is characterized by the increased secretion of pro-inflammatory cytokines, including TNF, IL-1α, IL-1β, IL-6, and IL-17, from intervertebral disc (IVD) cells. These cytokines facilitate extracellular matrix degradation, chemokine production, and changes in IDD cell phenotype [108]. Recent studies provide evidence of a direct interaction between OGT and FAM134B protein, resulting in the stabilization of FAM134B via inhibition of ubiquitin-mediated degradation. In addition, this interaction improves the adaptive capability of nucleus pulposus (NP) cells and retards IDD progression by modulating FAM134B-mediated ER-phagy [109]. However, a study on human degenerated intervertebral discs revealed that disc degeneration was associated with increased O-GlcNAcylation of nuclear and cytoplasmic proteins in IVD cells [110]. Diabetes is known to exacerbate disc degeneration by increasing apoptosis and senescence in nucleus pulposus cells [111]. Further study claimed that glucose-induced O-GlcNAcylation of Sox9 and Runx2 altered the fate of chondro-osteogenic differentiation of cartilage endplate stem cells (CESCs), leading to faster degeneration of the intervertebral disc (Fig. 5) [112]. The different reported outcomes of O-GlcNAcylation modification in IDD demonstrate the complexity of this pathway and suggest the importance of additional research.
Osteolysis
Osteolysis is the loss of bone tissue due to a pathological process [113]. The main mechanism of osteolysis involves an immunologic response to particulate debris, leading to progressive bone loss and implant loosening [114]. To date, an osteolysis diagnosis remains challenging, and the treatments are considered controversial. Massaccesi’s group reported that Vitamin E significantly increased levels of OGT, improving the ability of osteoblasts to respond to oxidative stress and ultimately reducing osteolysis caused by oxidation (Fig. 5) [115]. This study provides new insights into preventing osteolysis and improving the longevity of total joint replacements.
Bone metastasis
Bone is one of the most common sites targeted in advanced solid tumors, and cancer cells undergo complex biochemical, morphological, pathophysiological, and genetic changes in order to colonize bone sites [116, 117]. Unfortunately, once cancer has spread to the bone, it is rarely curable, and bone cancer is associated with pain, increased risk of fracture, and hypercalcemia [118]. It has been observed that higher levels of O-GlcNAcylation and OGT in cancer patients are associated with cancer progression and a poor prognosis. By reducing OGT levels, the expression level and angiogenic potential of invasion and angiogenesis effectors (MMP-2, MMP-9, and VEGF) in PC3-ML cells can be inhibited, and this process is dependent on increased degradation of FoxM1 [119]. It is important to note that this mechanism could inhibit prostate cancer metastasis to bone (Fig. 5). This discovery sheds light on the significance of OGT-mediated O-GlcNAcylation in bone metastasis. Moreover, it suggests that OGT may be a novel therapeutic target for treating tumor-associated bone metastasis.
The promising role of O-GlcNAcylation in the treatment of bone diseases
Since the discovery of O-GlcNAcylation in the early 1980s, several studies have addressed the role of protein O-GlcNAcylation in various human diseases. Although we have explored some of its important mechanistic details, research on the treatment of bone diseases through modulation of O-GlcNAcylation is limited. Nonetheless, O-GlcNAcylation is expected to become a new molecular marker and target for the treatment of bone disease. There are currently two main strategies for regulating the O-GlcNAcylation pathway to achieve therapeutic effects [120]. The first strategy involves the targeting of O-GlcNAcylation modifying enzymes, OGT and OGA. The second strategy is more targeted, and it involves changing the O-GlcNAcylation moieties on specific target proteins. This targeted approach has obvious advantages.
Previous reports have described the effects of several small molecule inhibitors of OGT and OGA on disease states. OSMI-1 is a cell-permeable, small-molecule inhibitor of OGT that inhibits O-GlcNAcylation [121]. Zoledronic acid is a potent amino bisphosphonate that is commonly used worldwide for the treatment of primary or secondary osteoporosis and low bone mass [122]. An investigation into the mechanism by which OSMI-1 interferes with osteoclastogenesis revealed that the effects of OSMI-1 and zoledronic acid on osteoclast differentiation were synergistic [51]. Interestingly, PUGNAc and Thiamet-G, commonly used in vitro and in vivo inhibitors of OGA, have also demonstrated inhibition of osteoclast differentiation [123,124,125,126]. This result can be explained by considering the complex off-target effects of inhibitors in different cell environments under different treatment regimens. Similarly, according to a study by Riegger’s group, PUGNAc exhibits both chondroprotective effects and chondroanabolic effects after cartilage trauma [127] (Fig. 6). Thiamet-G has also been shown to evoke both chondrocyte differentiation and matrix remodeling by increasing the presence of different matrix metalloproteinases (MMPs). Despite the promising pre-clinical data from the above findings, there are still many issues that need to be addressed. Inhibitors of OGT and OGA have a broad specificity and may alter the O-GlcNAcylation of thousands of proteins. Unspecified alterations in the patterns of O-GlcNAcylation on affected proteins could lead to severe side effects or new conditions. In addition, their high toxicity, low efficiency, low water solubility and cell permeability make in vivo studies difficult [128, 129].
Strategy for targeting O-GlcNAcylation in the treatment of bone diseases
OSMI-1, PUGNAc, and Thiamet-G mediated O-GlcNacylation can inhibit osteoclast differentiation and may have potential use in the future for preventing or treating osteoclast-activated bone diseases. PUGNAc and Thiamet-G administration may offer new avenues for studying cartilage formation, metabolism, and repair, by acting on chondrocytes
To address these problems, novel approaches for the selective protein regulation of O-GlcNAc are required. The development of liquid chromatography-mass spectrometry (LC-MS) has enabled accurate and large-scale prediction of O-GlcNAcylation sites in specific proteins. Retention and slow release of small molecules drugs at the lesion site by constructing a drug delivery system improves the targeting ability of O-GlcNAcylation and reduces systemic side effects. Yang et al. reported that nanocarriers allow selective delivery of OGT inhibitors or other compounds to target cells, thereby improving the efficiency of O-GlcNAcylation targeting [129]. In addition, Zhu et al. developed a notable strategy that involves using short peptides containing glycosylation sites to competitively inhibit glycosylation in a specific protein [130]. These strategies should provide future opportunities for designing targeted drug therapies. However, the relevant research is still evolving, and there is a long way to go to take these developments through to clinical applications.
Conclusions
Researchers have extensively studied the mechanism of protein O-GlcNAcylation over the past 30 years. O-GlcNAcylation is an essential component in the integration of metabolism with transcription, translation, proteostasis, and signaling (among others). Although the significance of O-GlcNAcylation in bone is increasingly recognized, our understanding of the molecular mechanisms by which O-GlcNAcylation regulates signaling events in bone and how this alters bone pathophysiology is still superficial. Hence, these are essential areas for future research. Moreover, through the development and increased accessibility of tools for detecting O-GlcNAcylation, especially LC-MS, our understanding of O-GlcNAcylation in the proteome is constantly being increased. With a comprehensive knowledge of O-GlcNAcylation in the proteome, we will be better able to understand its complex role in biological processes and to develop its potential as a therapeutic target for various types of bone diseases.
Data availability
Not applicable.
Abbreviations
- O-GlcNAcylation:
-
O-linked N-acetylglucosamine protein modification
- PTM:
-
post translational modification
- GlcNAc:
-
N-acetylglucosamine
- HBP:
-
hexosamine biosynthetic pathway
- UDP-GlcNAc:
-
UDP N-acetyl-glucosamine
- HK:
-
hexokinase
- GPI:
-
glucose 6-phosphate isomerase
- GFAT:
-
Glutamine fructose-6-phosphate amidotransferase
- GlcN-6P:
-
glucosamine 6-phosphate
- GNPNAT, EMeg32:
-
glucosamine phosphate N-acetyltransferase
- GlcNAc-6P:
-
N-acetylglucosamine-6-phosphate
- PGM3/AGM1:
-
GlcNAc phosphomutase
- GlcNAc-1P:
-
N-acetylglucosamine-1-phosphate
- UAP/AGX1:
-
pyrophosphorylase
- TPR:
-
tetratricopeptide repeats
- PPIs:
-
protein protein interactions
- GH84:
-
84 glycoside hydrolase
- pHAT:
-
pseudo histone acetyltransferase domain
- BMSCs:
-
bone marrow mesenchymal stromal cells
- NSCs:
-
neural stem cells
- HSCs:
-
hematopoietic stem cells
- BMP:
-
bone morphogenetic protein
- Hh:
-
hedgehog
- Runx2:
-
Runt related transcription factor 2
- ALP:
-
alkaline phosphatase
- MAPK:
-
mitogen activated protein kinase
- ERK:
-
extracellular signal regulated kinase
- JNK:
-
Jun N-terminal protein kinases
- NF-κB:
-
nuclear factor kappaB; nuclear factor of activated T cells c1
- IL-6:
-
Interleukin 6
- FAS:
-
fatty acid synthase
- LD:
-
lipid droplet
- GlcN:
-
glucosamine
- RA:
-
Rheumatoid arthritis
- TNFα:
-
Tumor necrosis factor alpha
- IL-1β:
-
Interleukin 1β
- TAB1:
-
transforming growth factor beta activated kinase 1-binding protein 1
- TAK1:
-
TGFβ activated kinase 1
- RASFs:
-
RA synovial fibroblasts
- DCs:
-
Dendritic cells
- moDCs:
-
Monocyte derived dendritic cells
- M1:
-
classically activated macrophages
- M2:
-
alternatively activated macrophages
- S6K1:
-
S6 kinase beta 1
- NLRX1:
-
NOD like receptor (NLR)X1
- IL-17 A:
-
Interleukin 17 A
- ACC1:
-
acetyl-CoA carboxylase 1
- OA:
-
Osteoarthritis
- Il-1:
-
Interleukin-1
- HOC:
-
human OA chondrocytes
- IDD:
-
Intervertebral disc degeneration
- CESCs:
-
cartilage endplate stem cells
- VEGF:
-
vascular endothelial derived growth factor
- LC-MS:
-
liquid chromatography-mass spectrometry
References
Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446(7139):1017–22.
Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–58.
Hombu R, Neelamegham S, Park S. Cellular and Molecular Engineering of Glycan Sialylation in Heterologous systems. Molecules. 2021;26(19).
Eichler J. Protein glycosylation. Curr Biol. 2019;29(7):R229–31.
Wang M, Zhu J, Lubman DM, Gao C. Aberrant glycosylation and cancer biomarker discovery: a promising and thorny journey. Clin Chem Lab Med. 2019;57(4):407–16.
Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126(5):855–67.
Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259(5):3308–17.
Ma J, Hart GW. O-GlcNAc profiling: from proteins to proteomes. Clin Proteom. 2014;11(1):8.
Holt GD, Hart GW. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986;261(17):8049–57.
Hart GW. Nutrient regulation of signaling and transcription. J Biol Chem. 2019;294(7):2211–31.
Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol. 2017;18(7):452–65.
Bond MR, Hanover JA. A little sugar goes a long way: the cell biology of O-GlcNAc. J Cell Biol. 2015;208(7):869–80.
Xue Q, Yan R, Ji S, Yu S. Regulation of mitochondrial network homeostasis by O-GlcNAcylation. Mitochondrion. 2022;65:45–55.
Akella NM, Ciraku L, Reginato MJ. Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 2019;17(1):52.
Jensen RV, Andreadou I, Hausenloy DJ, Bøtker HE. The role of O-GlcNAcylation for Protection against Ischemia-Reperfusion Injury. Int J Mol Sci. 2019;20(2).
Park J, Lai MKP, Arumugam TV, Jo D-G. O-GlcNAcylation as a therapeutic target for Alzheimer’s Disease. Neuromolecular Med. 2020;22(2):171–93.
Hanover JA, Krause MW, Love DC. Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol. 2012;13(5):312–21.
Vaidyanathan K, Durning S, Wells L. Functional O-GlcNAc modifications: implications in molecular regulation and pathophysiology. Crit Rev Biochem Mol Biol. 2014;49(2):140–63.
Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci. 2010;35(10):547–55.
Nie H, Yi W. O-GlcNAcylation, a sweet link to the pathology of diseases. J Zhejiang Univ Sci B. 2019;20(5):437–48.
Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272(14):9308–15.
Haltiwanger RS, Blomberg MA, Hart GW. Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 1992;267(13):9005–13.
Lubas WA, Frank DW, Krause M, Hanover JA. O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J Biol Chem. 1997;272(14):9316–24.
Shafi R, Iyer SP, Ellies LG, O’Donnell N, Marek KW, Chui D, et al. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci U S A. 2000;97(11):5735–9.
Hanover JA, Yu S, Lubas WB, Shin SH, Ragano-Caracciola M, Kochran J, Love DC. Mitochondrial and nucleocytoplasmic isoforms of O-linked GlcNAc transferase encoded by a single mammalian gene. Arch Biochem Biophys. 2003;409(2):287–97.
Love DC, Kochan J, Cathey RL, Shin SH, Hanover JA. Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J Cell Sci. 2003;116(Pt 4):647–54.
Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J Biol Chem. 2001;276(13):9838–45.
Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J Biol Chem. 2002;277(3):1755–61.
Cheng J, Wu Y, Chen L, Li Y, Liu F, Shao J, et al. Loss of O-GlcNAc transferase in neural stem cells impairs corticogenesis. Biochem Biophys Res Commun. 2020;532(4):541–7.
Murakami K, Kurotaki D, Kawase W, Soma S, Fukuchi Y, Kunimoto H, et al. OGT regulates hematopoietic stem cell maintenance via PINK1-Dependent Mitophagy. Cell Rep. 2021;34(1):108579.
Abramowitz LK, Harly C, Das A, Bhandoola A, Hanover JA. Blocked O-GlcNAc cycling disrupts mouse hematopoeitic stem cell maintenance and early T cell development. Sci Rep. 2019;9(1):12569.
Gao Q, Wang L, Wang S, Huang B, Jing Y, Su J. Bone marrow mesenchymal stromal cells: identification, classification, and differentiation. Front Cell Dev Biology. 2021;9:787118.
Gao Q, Wang L, Wang S, Huang B, Jing Y, Su J. Bone marrow mesenchymal stromal cells: identification, classification, and differentiation. Front Cell Dev Biol. 2021;9:787118.
Sun W, Shi Y, Lee WC, Lee SY, Long F. Rictor is required for optimal bone accrual in response to anti-sclerostin therapy in the mouse. Bone. 2016;85:1–8.
Amarasekara DS, Kim S, Rho J. Regulation of osteoblast differentiation by Cytokine Networks. Int J Mol Sci. 2021;22(6).
Gomathi K, Akshaya N, Srinaath N, Moorthi A, Selvamurugan N. Regulation of Runx2 by post-translational modifications in osteoblast differentiation. Life Sci. 2020;245:117389.
Kim SH, Kim YH, Song M, An SH, Byun HY, Heo K, et al. O-GlcNAc modification modulates the expression of osteocalcin via OSE2 and Runx2. Biochem Biophys Res Commun. 2007;362(2):325–9.
Zhang Z, Huang Z, Awad M, Elsalanty M, Cray J, Ball LE et al. O-GlcNAc glycosylation orchestrates fate decision and niche function of bone marrow stromal progenitors. Elife. 2023;12.
Nagel AK, Ball LE. O-GlcNAc modification of the runt-related transcription factor 2 (Runx2) links osteogenesis and nutrient metabolism in bone marrow mesenchymal stem cells. Mol Cell Proteom. 2014;13(12):3381–95.
Maeda K, Kobayashi Y, Koide M, Uehara S, Okamoto M, Ishihara A et al. The Regulation of Bone Metabolism and disorders by wnt signaling. Int J Mol Sci. 2019;20(22).
Garg B, Giri B, Majumder K, Dudeja V, Banerjee S, Saluja A. Modulation of post-translational modifications in β-catenin and LRP6 inhibits wnt signaling pathway in pancreatic cancer. Cancer Lett. 2017;388:64–72.
Shen H, Zhao X, Chen J, Qu W, Huang X, Wang M, et al. O-GlcNAc transferase ogt regulates embryonic neuronal development through modulating Wnt/β-catenin signaling. Hum Mol Genet. 2021;31(1):57–68.
Gu H, Song M, Boonanantanasarn K, Baek K, Woo KM, Ryoo HM, Baek JH. Conditions inducing excessive O-GlcNAcylation inhibit BMP2-Induced osteogenic differentiation of C2C12 cells. Int J Mol Sci. 2018;19(1).
Zhu JH, Liao YP, Li FS, Hu Y, Li Q, Ma Y, et al. Wnt11 promotes BMP9-induced osteogenic differentiation through BMPs/Smads and p38 MAPK in mesenchymal stem cells. J Cell Biochem. 2018;119(11):9462–73.
Papanicolaou KN, Jung J, Ashok D, Zhang W, Modaressanavi A, Avila E, et al. Inhibiting O-GlcNAcylation impacts p38 and Erk1/2 signaling and perturbs cardiomyocyte hypertrophy. J Biol Chem. 2023;299(3):102907.
Goldberg H, Whiteside C, Fantus IG. O-linked β-N-acetylglucosamine supports p38 MAPK activation by high glucose in glomerular mesangial cells. Am J Physiol Endocrinol Metab. 2011;301(4):E713–26.
Chen J, Dong X, Cheng X, Zhu Q, Zhang J, Li Q, et al. Ogt controls neural stem/progenitor cell pool and adult neurogenesis through modulating notch signaling. Cell Rep. 2021;34(13):108905.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42.
Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12(1):17–25.
Kim JH, Kim K, Youn BU, Jin HM, Kim JY, Moon JB, et al. RANKL induces NFATc1 acetylation and stability via histone acetyltransferases during osteoclast differentiation. Biochem J. 2011;436(2):253–62.
Kim MJ, Kim HS, Lee S, Min KY, Choi WS, You JS. Hexosamine Biosynthetic pathway-derived O-GlcNAcylation is critical for RANKL-Mediated osteoclast differentiation. Int J Mol Sci. 2021;22(16).
Taira TM, Ramos-Junior ES, Melo PH, Costa-Silva CC, Alteen MG, Vocadlo DJ, et al. HBP/O-GlcNAcylation metabolic Axis regulates bone resorption outcome. J Dent Res. 2023;102(4):440–9.
Carvajal Alegria G, Boukhlal S, Cornec D, Devauchelle-Pensec V. The pathophysiology of polymyalgia rheumatica, small pieces of a big puzzle. Autoimmun Rev. 2020;19(11):102670.
Takeuchi T, Yoshida H, Tanaka S. Role of interleukin-6 in bone destruction and bone repair in rheumatoid arthritis. Autoimmun Rev. 2021;20(9):102884.
Hu Y, Liu Y, Yang Y, Lv H, Lian S, Xu B, Li S. OGT upregulates myogenic IL-6 by mediating O-GlcNAcylation of p65 in mouse skeletal muscle under cold exposure. J Cell Physiol. 2022;237(2):1341–52.
Matsuo K, Owens JM, Tonko M, Elliott C, Chambers TJ, Wagner EF. Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat Genet. 2000;24(2):184–7.
Choi H, Kim C, Song H, Cha MY, Cho HJ, Son SM, et al. Amyloid β-induced elevation of O-GlcNAcylated c-Fos promotes neuronal cell death. Aging Cell. 2019;18(1):e12872.
Zhao JJ, Wu ZF, Yu YH, Wang L, Cheng L. Effects of interleukin-7/interleukin-7 receptor on RANKL-mediated osteoclast differentiation and ovariectomy-induced bone loss by regulating c-Fos/c-Jun pathway. J Cell Physiol. 2018;233(9):7182–94.
Li Z, Bagchi DP, Zhu J, Bowers E, Yu H, Hardij J et al. Constitutive bone marrow adipocytes suppress local bone formation. JCI Insight. 2022;7(21).
Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5(9):1538–52.
Darlington GJ, Ross SE, MacDougald OA. The role of C/EBP genes in adipocyte differentiation. J Biol Chem. 1998;273(46):30057–60.
Ishihara K, Takahashi I, Tsuchiya Y, Hasegawa M, Kamemura K. Characteristic increase in nucleocytoplasmic protein glycosylation by O-GlcNAc in 3T3-L1 adipocyte differentiation. Biochem Biophys Res Commun. 2010;398(3):489–94.
Hsieh TJ, Lin T, Hsieh PC, Liao MC, Shin SJ. Suppression of glutamine:fructose-6-phosphate amidotransferase-1 inhibits adipogenesis in 3T3-L1 adipocytes. J Cell Physiol. 2012;227(1):108–15.
Blacklow SC. Signal Transduction: Notch catches a jagged edge. Nat Chem Biol. 2017;13(6):570–1.
Osathanon T, Subbalekha K, Sastravaha P, Pavasant P. Notch signalling inhibits the adipogenic differentiation of single-cell-derived mesenchymal stem cell clones isolated from human adipose tissue. Cell Biol Int. 2012;36(12):1161–70.
Hao X, Li Y, Wang J. Deficient O-GlcNAc Glycosylation Impairs Regulatory T Cell Differentiation and Notch Signaling in Autoimmune Hepatitis. Front Immunol. 2018;9:2089.
Neumann CJ. Hedgehogs as negative regulators of the cell cycle. Cell Cycle. 2005;4(9):1139–40.
Jia B, Jiang Y, Yao Y, Xu Y, Wang Y, Li T. Baicalin attenuates dexamethasone-induced apoptosis of bone marrow mesenchymal stem cells by activating the hedgehog signaling pathway. Chin Med J (Engl). 2023;136(15):1839–47.
Das S, Bailey SK, Metge BJ, Hanna A, Hinshaw DC, Mota M, et al. O-GlcNAcylation of GLI transcription factors in hyperglycemic conditions augments hedgehog activity. Lab Invest. 2019;99(2):260–70.
Yang L, Ren Z, Yan S, Zhao L, Liu J, Zhao L, et al. Nsun4 and Mettl3 mediated translational reprogramming of Sox9 promotes BMSC chondrogenic differentiation. Commun Biol. 2022;5(1):495.
Ma Y, Zheng W, Chen H, Shao X, Lin P, Liu X, et al. Glucosamine promotes chondrocyte proliferation via the Wnt/β–catenin signaling pathway. Int J Mol Med. 2018;42(1):61–70.
Derfoul A, Miyoshi AD, Freeman DE, Tuan RS. Glucosamine promotes chondrogenic phenotype in both chondrocytes and mesenchymal stem cells and inhibits MMP-13 expression and matrix degradation. Osteoarthritis Cartilage. 2007;15(6):646–55.
Andrés-Bergós J, Tardio L, Larranaga-Vera A, Gómez R, Herrero-Beaumont G, Largo R. The increase in O-linked N-acetylglucosamine protein modification stimulates chondrogenic differentiation both in vitro and in vivo. J Biol Chem. 2012;287(40):33615–28.
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38.
Pablos JL, Cañete JD. Immunopathology of rheumatoid arthritis. Curr Top Med Chem. 2013;13(6):705–11.
Li YN, Chen CW, Trinh-Minh T, Zhu H, Matei AE, Györfi AH, et al. Dynamic changes in O-GlcNAcylation regulate osteoclast differentiation and bone loss via nucleoporin 153. Bone Res. 2022;10(1):51.
Kim HB, Lee SW, Mun CH, Yoon JY, Pai J, Shin I, et al. O-linked N-acetylglucosamine glycosylation of p65 aggravated the inflammation in both fibroblast-like synoviocytes stimulated by tumor necrosis factor-α and mice with collagen induced arthritis. Arthritis Res Ther. 2015;17(1):248.
Jacques C, Floris I, Lejeune B. Ultra-low Dose cytokines in Rheumatoid Arthritis, three birds with One Stone as the Rationale of the 2LARTH(®) Micro-immunotherapy Treatment. Int J Mol Sci. 2021;22(13).
Umar S, Singh AK, Chourasia M, Rasmussen SM, Ruth JH, Ahmed S. Penta-o-galloyl-beta-d-Glucose (PGG) inhibits inflammation in human rheumatoid arthritis synovial fibroblasts and rat adjuvant-induced arthritis model. Front Immunol. 2022;13:928436.
Ilchovska DD, Barrow DM. An overview of the NF-kB mechanism of pathophysiology in rheumatoid arthritis, investigation of the NF-kB ligand RANKL and related nutritional interventions. Autoimmun Rev. 2021;20(2):102741.
Yang YR, Kim DH, Seo YK, Park D, Jang HJ, Choi SY, et al. Elevated O-GlcNAcylation promotes colonic inflammation and tumorigenesis by modulating NF-κB signaling. Oncotarget. 2015;6(14):12529–42.
James LR, Tang D, Ingram A, Ly H, Thai K, Cai L, Scholey JW. Flux through the hexosamine pathway is a determinant of nuclear factor kappab- dependent promoter activation. Diabetes. 2002;51(4):1146–56.
Allison DF, Wamsley JJ, Kumar M, Li D, Gray LG, Hart GW, et al. Modification of RelA by O-linked N-acetylglucosamine links glucose metabolism to NF-κB acetylation and transcription. Proc Natl Acad Sci U S A. 2012;109(42):16888–93.
Yang WH, Park SY, Nam HW, Kim DH, Kang JG, Kang ES, et al. NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci U S A. 2008;105(45):17345–50.
Khan S, Greenberg JD, Bhardwaj N. Dendritic cells as targets for therapy in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):566–71.
Suwa Y, Nagafuchi Y, Yamada S, Fujio K. The role of dendritic cells and their immunometabolism in rheumatoid arthritis. Front Immunol. 2023;14:1161148.
Weiss M, Anderluh M, Gobec M. Inhibition of O-GlcNAc Transferase alters the differentiation and maturation process of human monocyte derived dendritic cells. Cells. 2021;10(12).
Li P, Hao Z, Wu J, Ma C, Xu Y, Li J, et al. Comparative proteomic analysis of Polarized Human THP-1 and mouse RAW264.7 macrophages. Front Immunol. 2021;12:700009.
Li H, Feng Y, Zheng X, Jia M, Mei Z, Wang Y, et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Control Release. 2022;341:16–30.
Yang Y, Li X, Luan HH, Zhang B, Zhang K, Nam JH, et al. OGT suppresses S6K1-mediated macrophage inflammation and metabolic disturbance. Proc Natl Acad Sci U S A. 2020;117(28):16616–25.
Nagai-Singer MA, Morrison HA, Allen IC. NLRX1 is a multifaceted and enigmatic Regulator of Immune System function. Front Immunol. 2019;10:2419.
Chen L, Li Y, Zeng S, Duan S, Huang Z, Liang Y. The interaction of O-GlcNAc-modified NLRX1 and IKK-α modulates IL-1β expression in M1 macrophages. Vitro Cell Dev Biol Anim. 2022;58(5):408–18.
Huang N, Dong H, Luo Y, Shao B. Th17 cells in Periodontitis and its regulation by A20. Front Immunol. 2021;12:742925.
Machacek M, Saunders H, Zhang Z, Tan EP, Li J, Li T, et al. Elevated O-GlcNAcylation enhances pro-inflammatory Th17 function by altering the intracellular lipid microenvironment. J Biol Chem. 2019;294(22):8973–90.
Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: current knowledge and future perspectives. Biomed Pharmacother. 2019;109:2318–26.
Li Q, Wen Y, Wang L, Chen B, Chen J, Wang H, Chen L. Author correction: hyperglycemia-induced accumulation of advanced glycosylation end products in fibroblast-like synoviocytes promotes knee osteoarthritis. Exp Mol Med. 2022;54(6):862–5.
Yu H, Li M, Shu J, Dang L, Wu X, Wang Y, et al. Characterization of aberrant glycosylation associated with osteoarthritis based on integrated glycomics methods. Arthritis Res Ther. 2023;25(1):102.
Pavelká K, Gatterová J, Olejarová M, Machacek S, Giacovelli G, Rovati LC. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2002;162(18):2113–23.
Towheed TE. Current status of glucosamine therapy in osteoarthritis. Arthritis Rheum. 2003;49(4):601–4.
Clegg DO, Reda DJ, Harris CL, Klein MA, O’Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795–808.
Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137–62.
Reginster JY, Neuprez A, Lecart MP, Sarlet N, Bruyere O. Role of glucosamine in the treatment for osteoarthritis. Rheumatol Int. 2012;32(10):2959–67.
Henrotin Y, Chevalier X, Herrero-Beaumont G, McAlindon T, Mobasheri A, Pavelka K, et al. Physiological effects of oral glucosamine on joint health: current status and consensus on future research priorities. BMC Res Notes. 2013;6:115.
Someya A, Ikegami T, Sakamoto K, Nagaoka I. Glucosamine downregulates the IL-1β-Induced expression of Proinflammatory Cytokine genes in human synovial MH7A cells by O-GlcNAc modification-dependent and -independent mechanisms. PLoS ONE. 2016;11(10):e0165158.
Jenei-Lanzl Z, Meurer A, Zaucke F. Interleukin-1β signaling in osteoarthritis - chondrocytes in focus. Cell Signal. 2019;53:212–23.
Tardio L, Andrés-Bergós J, Zachara NE, Larrañaga-Vera A, Rodriguez-Villar C, Herrero-Beaumont G, Largo R. O-linked N-acetylglucosamine (O-GlcNAc) protein modification is increased in the cartilage of patients with knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):259–63.
Francisco V, Pino J, González-Gay M, Lago F, Karppinen J, Tervonen O, et al. A new immunometabolic perspective of intervertebral disc degeneration. Nat Rev Rheumatol. 2022;18(1):47–60.
Kutuk MO, Tufan E, Gokcen C, Kilicaslan F, Karadag M, Mutluer T, et al. Cytokine expression profiles in autism spectrum disorder: a multi-center study from Turkey. Cytokine. 2020;133:155152.
Luo R, Li G, Zhang W, Liang H, Lu S, Cheung JPY, et al. O-GlcNAc transferase regulates intervertebral disc degeneration by targeting FAM134B-mediated ER-phagy. Exp Mol Med. 2022;54(9):1472–85.
Nikolaou G, Zibis AH, Fyllos AH, Katsioulis A, Sotiriou S, Kotrotsios A, et al. Detection of O-Linked-N-Acetylglucosamine modification and its Associated enzymes in human degenerated intervertebral discs. Asian Spine J. 2017;11(6):863–9.
Jiang L, Zhang X, Zheng X, Ru A, Ni X, Wu Y, et al. Apoptosis, senescence, and autophagy in rat nucleus pulposus cells: implications for diabetic intervertebral disc degeneration. J Orthop Res. 2013;31(5):692–702.
Sun C, Lan W, Li B, Zuo R, Xing H, Liu M, et al. Glucose regulates tissue-specific chondro-osteogenic differentiation of human cartilage endplate stem cells via O-GlcNAcylation of Sox9 and Runx2. Stem Cell Res Ther. 2019;10(1):357.
Saleh KJ, Thongtrangan I, Schwarz EM. Osteolysis: medical and surgical approaches. Clin Orthop Relat Res. 2004;427:138–47.
Kurcz B, Lyons J, Sayeed Z, Anoushiravani AA, Iorio R. Osteolysis as it pertains to total hip arthroplasty. Orthop Clin North Am. 2018;49(4):419–35.
Massaccesi L, Ragone V, Papini N, Goi G, Corsi Romanelli MM, Galliera E. Effects of vitamin E-Stabilized Ultra High Molecular Weight Polyethylene on oxidative stress response and Osteoimmunological Response in Human osteoblast. Front Endocrinol (Lausanne). 2019;10:203.
Hong S, Youk T, Lee SJ, Kim KM, Vajdic CM. Bone metastasis and skeletal-related events in patients with solid cancer: a Korean nationwide health insurance database study. PLoS ONE. 2020;15(7):e0234927.
Molla MS, Katti DR, Katti KS. Mechanobiological evaluation of prostate cancer metastasis to bone using an in vitro prostate cancer testbed. J Biomech. 2021;114:110142.
Fornetti J, Welm AL, Stewart SA. Understanding the bone in Cancer Metastasis. J Bone Min Res. 2018;33(12):2099–113.
Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, Reginato MJ. Critical role of O-Linked β-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem. 2012;287(14):11070–81.
Zhu Y, Hart GW. Targeting O-GlcNAcylation to develop novel therapeutics. Mol Aspects Med. 2021;79:100885.
Ortiz-Meoz RF, Jiang J, Lazarus MB, Orman M, Janetzko J, Fan C, et al. A small molecule that inhibits OGT activity in cells. ACS Chem Biol. 2015;10(6):1392–7.
Dhillon S. Zoledronic acid (reclast(®), Aclasta(®)): a review in osteoporosis. Drugs. 2016;76(17):1683–97.
Horsch M, Hoesch L, Vasella A, Rast DM. N-acetylglucosaminono-1,5-lactone oxime and the corresponding (phenylcarbamoyl)oxime. Novel and potent inhibitors of beta-N-acetylglucosaminidase. Eur J Biochem. 1991;197(3):815–8.
Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4(8):483–90.
Takeuchi T, Horimoto Y, Oyama M, Nakatani S, Kobata K, Tamura M, et al. Osteoclast differentiation is suppressed by increased O-GlcNAcylation due to Thiamet G Treatment. Biol Pharm Bull. 2020;43(10):1501–5.
Takeuchi T, Nagasaka M, Shimizu M, Tamura M, Arata Y. N-acetylglucosamine suppresses osteoclastogenesis in part through the promotion of O-GlcNAcylation. Bone Rep. 2016;5:15–21.
Riegger J, Baumert J, Zaucke F, Brenner RE. The hexosamine Biosynthetic Pathway as a therapeutic target after cartilage trauma: modification of chondrocyte survival and metabolism by glucosamine derivatives and PUGNAc in an Ex vivo model. Int J Mol Sci. 2021;22(14).
Zhu Z, Li S, Yin X, Sun K, Song J, Ren W, et al. Review: protein O-GlcNAcylation regulates DNA damage response: a novel target for cancer therapy. Int J Biol Macromol. 2024;264(Pt 1):130351.
Yang R, Wang L, Wu Z, Yin Y, Jiang SW. How nanotechniques could vitalize the O-GlcNAcylation-Targeting Approach for Cancer Therapy. Int J Nanomed. 2022;17:1829–41.
Zhu Q, Wang H, Chai S, Xu L, Lin B, Yi W, Wu L. O-GlcNAcylation promotes tumor immune evasion by inhibiting PD-L1 lysosomal degradation. Proc Natl Acad Sci U S A. 2023;120(13):e2216796120.
Acknowledgements
We thank BioRender (https://app.biorender.com/) for its help in the creating figures. We would like to thank MogoEdit (https://www.mogoedit.com) for its English editing during the preparation of this manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No.42176096 [L.G.], 42176097 [K.Q.Z.]), the Natural Science Foundation of Shandong Province (No. ZR2021MD065 [K.Q.Z.], ZR2021MH305[J.J.Z.], ZR2022MH223[W.H.R]), the TaiShan Scholars Foundation of Shandong Province (No. tsqn202306397 [L.G.]), and the Shandong Province medical health science and technology development plan project (202208020829 [S.M.L]), Science and Technology Project of Qingdao West Coast New Area (No.2022-47 [L.G.], 2022-46 [J.J.Z.]).
Author information
Authors and Affiliations
Contributions
Xiaohan Yan: Study conception and design, data collection, writing – original draft. Jingjing Zheng: Data collection, writing – review & editing, and visualization. Wenhao Ren: Study conception, editing and visualization. Shaoming Li: Data collection and review. Shuying Yang: Supervision and visualization. Keqian Zhi: Study conception and design, supervision, and funding acquisition. Ling Gao: Supervision and funding acquisition. All authors approved the final manuscript to be published.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors state that they have no potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yan, X., Zheng, J., Ren, W. et al. O-GlcNAcylation: roles and potential therapeutic target for bone pathophysiology. Cell Commun Signal 22, 279 (2024). https://doi.org/10.1186/s12964-024-01659-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-024-01659-x