Abstract
Combined chemotherapy is a treatment method based on the simultaneous use of two or more therapeutic agents; it is frequently necessary to produce a more effective treatment for cancer patients. Such combined treatments often improve the outcomes over that of the monotherapy approach, as the drugs synergistically target critical cell signaling pathways or work independently at different oncostatic sites. A better prognosis has been reported in patients treated with combination therapy than in patients treated with single drug chemotherapy. In recent decades, 5-fluorouracil (5-FU) has become one of the most widely used chemotherapy agents in cancer treatment. This medication, which is soluble in water, is used as the first line of anti-neoplastic agent in the treatment of several cancer types including breast, head and neck, stomach and colon cancer. Within the last three decades, many studies have investigated melatonin as an anti-cancer agent; this molecule exhibits various functions in controlling the behavior of cancer cells, such as inhibiting cell growth, inducing apoptosis, and inhibiting invasion. The aim of this review is to comprehensively evaluate the role of melatonin as a complementary agent with 5-FU-based chemotherapy for cancers. Additionally, we identify the potential common signaling pathways by which melatonin and 5-FU interact to enhance the efficacy of the combined therapy.
Graphic abstract

Video abstract
Similar content being viewed by others
Introduction
Cancer is a general term used to describe a group of chronic diseases that result from abnormal proliferation of cells and can occur in all organs [1]. Pre-cancerous cells transform into malignant tumor cells in a progressive process. Cancer is the leading cause of death worldwide and has been a major burden for the World Health Organization for many decades. There were 23.6 million diagnosed cancer cases in 2019 and approximately 10 million deaths (one in six total deaths) due to cancer in 2020 alone. Cancer is a multifactorial disease in which genetics plays an important role and the incidence of cancer increases dramatically with age. There are more than 100 types of cancer; the most common types worldwide are: breast cancer, lung cancer, colon and rectal cancer, prostate cancer, skin cancer and stomach cancer [1, 2].
Since cancer has such a major impact on human health, it is important to identify effective and efficient treatments. Correct diagnosis of cancer is a prerequisite for optimal treatment, since each cancer type requires specific treatment. Surgery, radiotherapy, chemotherapy and hormone therapy are common means to treat cancer. In addition to the type of cancer, the patient's situation is an important factor in choosing the best treatment option. Chemotherapy has been one of the most common cancer therapies since the 1940s and significant progress in identifying more effective anti-cancer agents. Chemotherapy involves the administration of cytotoxic drugs to kill abnormal cells and destroy malignant tissue [3]. Usually, chemotherapeutic drugs are administered intravenously to maintain high levels of the drug and to improve safety. In some cases, the cytotoxic drug is injected directly into the affected tissue for better efficacy and fewer side effects.
Chemotherapeutic agents are categorized into several groups based on their mode of action. Alkylators and alkylator-related agents, as well as platinum agents, bind to intracellular macromolecules (such as DNA) and interfere with their function. Antimetabolites resemble essential molecules of the cell and interfere with the function of DNA and RNA, by mimicking them. Topoisomerase inhibitors suppress enzymes related to DNA transcription, i.e., topoisomerase I and II. Agents interacting on microtubules disrupt and destroy the protein skeleton of the cell. Fludarabine and cladribine resemble antimetabolites and induce programmed cell death (apoptosis) [4].
Multi-drug resistance (MDR) is common limitation in chemotherapy. Several mechanisms of drug resistance have been proposed. Many of these mechanisms involve an action at the cellular level to neutralize chemotherapeutic agents; increased metabolism and the excretion of drugs are other causes of drug resistance. Also, poorly vascularized tumors often exhibit drug resistance. One means to resolve or reduce drug resistance may be the use of combination chemotherapies; these treatments involve the combination of different types of drugs that either act on different metabolic pathways, or the combination of chemotherapies include pro-drug agents to increase the efficacy of primary chemotherapy. Combination chemotherapy was first introduced in 1960s; this was the MOPP regimen used to treat Hodgkin's lymphoma. While combination chemotherapies sometimes result in more efficient treatment strategies, they often lead to more side effects [5].
Melatonin is an endogenous molecule produced by many cells. The pineal gland, an outgrowth of the epithalamus, is the main source of circulating melatonin. The secretion of melatonin is mainly regulated by the light–dark cycle; thus, melatonin is one of the most important regulators of circadian rhythms of all organs. Besides, the production of melatonin by many extrapineal tissues and the presence of its receptors in various tissues have been identified. Further evidence demonstrated that the extrapineal melatonin is a multifunctional molecule and performs many other functions [6]. For example, it modulates the immune system and regulates T/B cells function and cytokine production. Many studies have documented the anti-inflammatory effects and potent antioxidant actions of this ubiquitously-acting molecule. Melatonin is also associated with bone homeostasis and has been used to treat osteoporosis. Over the last several decades, the anti-proliferative effects of melatonin against cancer cells have been extensively examined [7]. Melatonin is a cell-protective agent and is considered an anticancer agent due to its anti-proliferative, antioxidant, and immunomodulatory actions. In this review, we examine the current findings and the results of studies that show that melatonin may act as a worthy combination therapy when used with 5FU-based treatment. We also highlight the clinical roles of melatonin that may provide enhanced antitumor effects in combination with chemotherapy drugs.
Melatonin
Melatonin (N-acetyl-5-methoxytryptamine), an indolamine neurohormone, is derived from serotonin, an intermediate compound, that synthetized from amino acid tryptophan, through intracellular enzymes such as tryptophan hydroxylase, aromatic amino acid decarboxylase, serotonin N-acetyltransferase, and hydroxyindole-O-methyltransferase [8]. Melatonin is considered as a highly preserved molecule produced by various spectrum of living organisms from bacteria to mammals [9, 10]. Melatonin is well known as a photosensitive hormone and important regulator of the circadian rhythm that produced by pineal gland [9]. However, accumulating studies revealed that many other tissues and organs, such as retina, gastrointestinal tract, lymphocytes of the skin, platelets, bone marrow, liver, heart, kidney, thymus, and cerebellum, are able to produce melatonin, called as extrapineal melatonin [6], which can play various essential roles in cellular processes via intracrine, autocrine and paracrine functions [11].
Melatonin synthesized in cells other than the pineal gland is not released in the blood circulation. It is release from the mitochondria into the cytosol and acts on receptors on the mitochondrial membrane [12]. The anti -oxidant activity of melatonin in the mitochondria of normal cells is mediated by two mechanisms: [1] a direct scavenger of free radicals and reactive oxygen species (ROS) and [2] an indirect function by increasing the expression and activity of endogenous antioxidant enzymes [13, 14]. The key role of melatonin in detoxification of free radicals during the cellular processes and metabolism and decreasing the oxidative stress is well describe in all organisms [15]. Additionally, melatonin plays an important role in promoting the immune system and postponing cell aging [16,17,18]. For example, it has been reported that melatonin that produced by thymus could regulate the production of some thymic peptides and prevents apoptosis of thymocytes [19, 20]. Also, it should be noted that melatonin can exert either a pro- or anti-inflammatory regulatory function in non-tumor cells under different conditions that is poorly understood. The stimulatory roles of melatonin in inflammation could be stimulated by induction of pro-inflammatory cytokines and other mediators. In contrast, melatonin can suppress inflammation by inhibiting NO release, inactivating toll-like receptor-4 and mTOR signaling, inhibiting pro-inflammatory cytokine release, enhancing release of the anti-inflammatory cytokines such as IL-4 and IL-10, and promoting the production of macrophage M2 that has anti-inflammatory action [21].
Recently, growing evidence identified that melatonin could act as an anti-cancer agent through suppressing cancer cell growth, proliferation, inducing apoptosis in cancer cells, and modulating the immune responses. Melatonin causes a deterioration in the T-cell populations in tumor microenvironment, with increased the CD8 + lymphocytes and decreased the T-reg numbers [22, 23]. Moreover, in cancer cells, melatonin shows pro-oxidant/cytotoxic effects, which depends on the pro-apoptotic signaling pathways such as NF-κB activation, leading to destroying the cancer cells [24,25,26].
Antitumor activity of melatonin
Melatonin's anticancer activities have been confirmed by increasing evidence from experimental investigation. These anticancer function of melatonin, as well as the biochemical pathways involved, have been comprehensively demonstrated on tumor initiation, growth, and development [38, 39]. Melatonin is shown to suppress tumor growth and cancerous cells proliferation by prompting apoptosis in cancer cells, thus decreases the probability that healthy cells may develop neoplasms. It also promotes cellular turnover and the replacement of tumor cells with healthy cells via apoptosis [40, 41]. Melatonin is a potent antioxidant that prevents ROS-induced activation of Akt and extracellular-regulated protein kinase (ERK) pathways that are participated in the survival of tumor cells. Suppression of ROS-dependent Akt signalling pathway leads to reduction of cyclin D1 and Bcl-2 and elevation of Bax in cancerous cells [42]. Furthermore, melatonin affects the expression of MDM2, an E3 ubiquitin ligase that negatively regulates the p53 tumor suppressor, which results in the induction of apoptosis. According to the studies, melatonin through decreasing MDM2 expression promotes upregulation of caspase-3 and -9 activity and thus induces apoptosis [43, 44]. Additionally, melatonin causes apoptosis by simultaneously suppressing the p300/NF-B, COX-2/prostaglandin E 2 (PGE2), and PI3K/Akt signaling pathways. When these pathways are inhibited, Apaf-1 expression is increased, which causes the releasing of cytochrome c as well as the activation of caspase-3 and -9 [45]. Numerous investigations have shown that melatonin has anticancer effects in leukemia and solid tumors, with particular effectiveness in lymphoproliferative malignancies [46,47,48]. Bejarano et al. showed melatonin has proapoptotic and/or oncostatic action in HL-60 human myeloid cells by activating proapoptotic proteins such as Bax and Bid [47].
Several studies have shown that melatonin's antiproliferative effects are achieved via inhibiting or halting the cell cycle [49]. El Missiri et al. investigated the effect of melatonin on Ehrlich ascites carcinoma (EAC) cells. In this study, it was discovered that melatonin can cause the death of EAC tumor cells in addition to prolonging the survival of experimental animals [50]. Melatonin by influencing cell cycle-related proteins, such as decreasing the expression of cyclin D1, causes the inhibition of the proliferation and induces cell cycle arrest of HepG2 and Hep3B cell line of hepatocellular carcinoma (HCC) [51].
It has been demonstrated that melatonin modulates the angiogenesis process [52]. Under hypoxic situations in prostate cancer cells, melatonin suppresses the generation of HIF-1 by preventing the production of ROS and the sphingosine kinase 1 (SPHK1) pathway [53]. Melatonin also inhibits the growth of gastric cancer cells by regulating the TGF-1 signalling pathway, which is crucial in the regulation of tumor cell growth [54]. Melatonin inhibits the expression of endothelin-1 (ET-1) mRNA in colon cancer cells. ET-1 is a peptide that stimulates cell proliferation and angiogenesis in colon cancer. Melatonin inhibits the activity of the ET-1 promoter and thus decreasing ET-1 expression and production, which is regulated by NF-kb and FoxO1 [55]. Melatonin function as a natural antioxidant against oxidative stress has been demonstrated in various in vitro and in vivo research [56]. Melatonin detoxification of oxidants is caused by numerous processes. Melatonin not only neutralizes oxidants by scavenging free radicals, but it also promotes oxidative content reduction through a variety of mechanisms including activating antioxidant enzymes and inhibiting pro-oxidative enzymes. In addition, by stabilizing the mitochondrial inner membrane, the integrity of the mitochondria is preserved, and as a result, electron leakage and ROS production are reduced [57, 58].
Melatonin, an antioxidant, can elevate ROS production in specific types of cancerous cells because of the distinct differences between the metabolism of tumor cells and healthy cells (particularly glucose metabolism and cellular respiration) [59]. There are suggested two potential explanations for how melatonin enhance the level of ROS in cancer cells: First, melatonin directly acts as a stimulant ROS; this process happens when melatonin stimulates electron transport chain (ETC) complexes, causing them to generate free radicals and ROS by ETC complexes (I and III) [60, 61], and second, by reducing the number of antioxidant molecules that destroy ROS, melatonin disrupts the balance of oxidant and antioxidant compounds and cellular redox and thereby increases oxidative stress. According to research findings, melatonin decrease antioxidant enzymes and also rises lipid peroxidation in various types of cancer cells [62,63,64] whereas it reduces oxidative stress damage and enhances levels of activities of antioxidant enzymes in non-tumor cells [65].
Autophagy is modulated by melatonin in a variety of cell types and physiological situations. Melatonin affects oxidative stress and inflammation to modulate autophagy [66]. Melatonin increases the efficacy of cisplatin and radiotherapy in treating head and neck squamous cell carcinoma; this effects is caused by extreme mitochondrial induction that results in an excess of ROS and the consequent stimulation of autophagy and apoptosis [67]. Administering melatonin in mice model with colitis-associated colon carcinogenesis (CACC), by improving inflammation and oxidative stress, and on the other hand modulating autophagy and Nrf2 signaling pathways, reduced the process of autophagy and the progression of CACC [68].
5FU as a chemotherapeutic drug: from history to mechanisms of action
5-fluorouracil (5-FU) is a pyrimidine derivative used as an antimetabolite and is one of the most commonly used chemotherapeutic agents since the 1960s. Antimetabolites enter cells and mimic molecules critical for cell growth. They are naturally cytotoxic and disrupt the normal mechanisms of the cells by injuring the genetic material: DNA and RNA [69]. 5-FU is the major drug used in chemotherapy regimens for a variety of carcinomas such as gastrointestinal, breast, head and neck cancer, and skin cancer. Antibacterial (against S. aureus and S. epidermidis) and antiviral effects of 5-FU have also been observed in several studies [70].
5-FU is an analogue of uracil and differs structurally from uracil by having an additional fluorine atom attached to carbon 5 instead of a hydrogen atom. At room temperature, 5-FU is a colorless in a solution buffered with NaOH and it is administered intravenously [71]. Dihydropyrimidine dehydrogenase (DPD) is the main enzyme that degrades FU and is mainly present in the liver. 80% of 5-FU is immediately converted to inactive metabolites and 5–20% is excreted by the kidneys. Only 1–3% of administered 5-FU produces its clinical effects. Due to its short half-life (less than 20 min), 5-FU rapidly enters into cells after administration. Like uracil, 5-FU is transported into cells by the human nucleoside transporter (hNT). The human nucleoside transporter includes three concentrative constituents (hCNT 1,2,3) and four equilibrated transporters (hENT 1,2,3,4). 5-FU is transported only by hENT 1 and 2. In the cell, 5-FU is converted by phosphorylation into three major active metabolites: fluorouridine triphosphate (FUTP), fluorodeoxyuridine triphosphate (FdUTP), and fluorodeoxyuridine monophosphate (FdUMP). These active metabolites induce the anticancer effects of 5-FU via different pathways. The next section explains the proliferative inhibitory effects of 5-FU at the molecular level [72].
5-FU metabolites and DNA damage
Fluorodeoxyuridine monophosphate (FdUMP) impairs DNA repair and replication by inhibiting thymidylate synthase (TS) and thymidine formation. Thymidine is a crucial molecule for DNA repair and replication. Thymidylate synthase (TS) is an important enzyme for thymidine production. TS converts deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP) using 5, 10-methylenetetrahydrofolate (CH2THF) as a methyl donor. Thus, it functions optimally as a complex with dUMP and CH2THF. FdUMP interferes with this complex by binding to TS in the nucleotide site (the site of dUMP) and impedes dTMP production. This leads to an imbalance of the deoxynucleotide pool with low dTTP and high dUTP concentrations, which eventually causes DNA repair cessation. Fluorodeoxyuridine triphosphate (FdUTP) (another active metabolite of 5-FU with anti-proliferative activity) is a substrate of DNA polymerase. FdUTP mis-incorporates with dUTP into DNA and damages its structure [69, 72, 73].
5-FU Effects on RNA
Initially, researchers believed that the main anti-cancer effects of 5-FU came from its destructive effects on DNA, but recent studies have shown otherwise. A study on the effects of 5-FU on U2snRNA in vivo found that metabolites of 5-FU are incorporated into U2 snRNA at pseudouridylation sites, inhibiting pre-mRNA splicing and pseudouridation of U2 snRNA. Several studies have demonstrated the inhibitory effect of 5-FU on the conversion of uridine to pseudouridine, resulting in defective and nonfunctional substance of pseudouridine-containing tRNA, mRNA, and snRNA. Fluorouridine triphosphate (FUTP) (active metabolite of 5-FU) can also interfere with RNA function. It mislocalizes to the rRNA precursor and inhibits proper transcription and maturation of rRNA. Thus, these studies demonstrate that the major proliferation inhibitory effects of 5-FU result from its incorporation into various RNA species [72, 74].
5-FU was approved as a drug by the FDA in 1962 and has since been widely used in various chemotherapy regimens, alone or in combination with other drugs. Several studies reported a marked effect of 5-FU especially in colorectal carcinoma, and the use of 5-FU in a combined chemotherapy regimen may have an efficacy rate of up to 40–60%. Despite the remarkable effect of 5-FU in cancer treatment over the past 60 years, drug resistance is a major limitation for the use of 5-FU, while the administration of 5-FU solely as chemotherapeutic regimen, showed a low response rate of 10–15% [73, 75].
Many studies have tried to identify mechanisms to overcome drug resistance of tumors. Chemoresistant cancer cells (CCCs) are a subclass of tumor cells that are not susceptible to chemotherapeutic agents. It has been suggested that CCCs develop from cancer stem cells and become refractory to 5-FU after altering some basic properties such as metabolism and proliferation [76]. To prevent and reduce 5-FU resistance, various modulations in chemotherapy have been proposed. Leucovorin (LV) or folinic acid is an analog of folic acid and is used to reduce the toxicity of metotroxate. LV increases the intracellular concentration of CH2THF by converting to it and enhances the ternary complex of CH2THF, thymidylate synthase, and FdUMP. As a result, the toxicity and proliferation inhibitory effects of 5-FU increase. Dihydropyrimidine dehydrogenase (DPD) is the main enzyme that degrades pyrimidines, uracil and thymine, and it is also responsible for the degradation of almost 80% of 5-FU immediately after its administration. The combination of uracil and the pro-drug 5-FU (ftorafur) as UFT (uracil/ftorafur; in a 4/1 ratio) is one of the strategies used to reduce the effects of DPD on 5-FU. Capecitabine is a chemotherapy drug used to treat breast cancer, gastric cancer, and colorectal cancer. It is administered orally and converts to 5'-deoxy-5-fluorouridine (5'DFUR) in gastrointestinal wall cells. 5'DFUR eventually converts to 5-FU, which actively kills the tumor cells [69, 72, 73].
Melatonin and 5-FU combination chemotherapy in cancer
The efficacy and clinical application of 5-FU is limited by the development of drug resistance, which is common in cancer chemotherapy [77, 78]. The combination of 5-FU, as the main chemotherapeutics agent, with naturally occurring bioactive compounds, has recently gained attention as a means to reduce the effective dose, and thereby adverse side effects [79]. Regarding melatonin, a pleiotropic molecule with various biological activities, has increasingly emerged as an effective adjuvant agent to conventional anticancer therapy for a variety of cancer types [80, 81]. Reduction in drug resistance to antineoplastic agents and chemotherapy-induced toxicity are other benefits of melatonin application in clinical oncology as has been a greater therapeutic response [82,83,84,85]. In addition to the diverse range of proven biological functions of melatonin in oxidative stress (anti-oxidative effects) [65], and immunomodulation (anti-inflammatory effects) [55, 86], the involvement of melatonin in anticancer and oncostatic processes including apoptosis induction, as well as inhibition of cell proliferation, tumor growth, and metastases has been repeatedly confirmed [87, 88].
Induction apoptosis via pro-oxidants activity
As a follow-up investigation, an experimental study conducted by Uguz et al. investigated the augmenting effects of melatonin on the efficacy of chemotherapeutic drugs, which included 5-FU. Their results demonstrated that melatonin and 5-FU co-treatment in AR42J cells of rat pancreatic cancer model, resulted in increased apoptosis via induction the mitochondrial membrane depolarization and ROS production in the cancer cells, leading to an enhancement of the chemotherapy-induced cytotoxicity effects, compared to treatment with 5-FU alone [85]. Previously, the findings of several studies suggested that melatonin may enhance the efficacy of other current chemotherapeutic drugs in terms of tumor regression via potentiating their chemotherapy-mediated cytotoxicity [90, 91] and promoting apoptosis [92, 93], which led to an increased survival rate of cancer-bearing humans [94]. An elevated intracellular ROS production and the subsequent loss of mitochondrial membrane potential; this resulted in the activation of mitochondrial apoptogenic factors, such as cytochrome c release, with the stimulation of the caspase cascades which are fundamental mechanisms of the intrinsic apoptosis pathway [95, 96].
Mihanfar et al. [97] reported that melatonin in combination with 5-FU significantly decrease the IC50 value of 5-FU from 50 to 100 μM as well as improved the cytotoxic efficacy of 5-FU inSW-480 CRC cell line of human CRC. Regarding the underlying mechanism, melatonin negatively regulated the resistance of cancer cells to apoptosis through targeting of oxidative stress, X-linked IAP (XIAP) and survivin in CRC cells, hence decreasing the cell proliferation. Indeed, the intracellular levels of ROS increased, and in contrast, the antioxidant enzymatic activities, and the expression levels of XIAP and survivin were downregulated after combination therapy of melatonin and 5-FU [97]. Selective apoptosis induction in CRC cells is an important anti-neoplastic function of 5-FU [98, 99]. Moreover, dysregulation of apoptotic pathways was proven to be potently related to failure of cancer treatment and to drug resistance [100,101,102]. XIAP and survivin are recognized as major members of the inhibitor of apoptosis proteins (IAPs) family, which are involved in anti-apoptotic processes through suppression of caspase activity [103,104,105]. Invariably, the essential function of XIAP and survivin in determining the resistance of cancer cells to apoptosis and subsequently to anti-neoplastic agents were demonstrated in various human malignancies; thus, it appears that downregulation of IAPs may contribute to successful treatment via improving of the sensitivity of cancer cells to chemotherapy and/or radiotherapy mediated by regulation of apoptosis [103, 106,107,108,109]. In this regard, a recent report noted that double knockdown of survivin and XIAP enhanced the sensitivity of human CRC to radiation therapy and also mediated a reduction in the cancer cells migration [110]. Additionally, melatonin in combination with 5-FU elevated Bax expression as well as Bax/Bcl-2 ratio in CRC cells [97] and the phosphorylation of Bcl-2 and Bax; as members of the Bcl-2 family they participate in promoting the p53-mediated apoptosis in cancer [111, 112]. The effect of the combination of melatonin and 5-FU for improvement of 5-FU potency for CRC treatment may be in part mediated by ROS overproduction and reduction of antioxidant levels in cancer cells [97]. Certainly, recent evidence indicates that the anti-cancer effects and adjuvant actions of melatonin as a co-treatment with other chemotherapeutic drugs involves ROS overproduction and a reduction of antioxidant enzymes, leading to increased apoptosis [64, 113]. It is known that ROS play dual roles in cancer; while at low levels, ROS can exert oncogenic activity [114], at high levels they provide an intracellular oxidizing environment leaing to induction of apoptosis [115]. One report documented that melatonin intensifies the anti-cancer effects of 5-FU and cisplatin in human colorectal adenocarcinoma HT–29 cells, by moderating the chemo-sensitivity and proapoptotic processes. This study revealed that in the presence of melatonin further increased caspase-3 and caspase-9 activation following FU-5 application. Also, melatonin promoted 5-FU-evoked ROS production, and thereby enhanced mitochondrial apoptosis of cancer HT–29 cells [113].
Induction apoptosis via PI3K/Akt and Erk signaling pathway
In similar studies, combined treatment with melatonin and puromycin, a chemotherapeutic agent, caused a dysregulation of the cell cycle and promoted the pro-apoptotic activities of puromycin by enhancing the downregulation of the anti-apoptotic proteins, such as Bcl-2 and Bcl-xL, activation of caspase-3, poly-(ADP-ribose) polymerase (PARP), and 5′-adenosine monophosphate-activated kinase (AMPK) [116]. Moreover, several studies indicate that melatonin in combination with conventional anti-cancer drugs significantly increases apoptosis via several mechanisms including activation of caspase-mediated and inactivation of Erk/p90RSK/HSP27 cascades in cancer, without any measurable changes in normal non-cancer cells [93, 117]. Indeed, it has been repeatedly demonstrated that melatonin has a cytoprotective effects in normal cells against chemotherapy-induced cytotoxicity, apoptosis, and genomic damage [90, 118, 119], actions that may be mediated by its antioxidant properties [57].
Considering the findings mentioned in the previous paragraph, it was not surprising when similar results were reported in which melatonin notably enhanced 5-FU-mediated inhibition of cell proliferation, migration and invasion of SW620 and LOVO cells in mouse model of human CRC. Further mechanistic investigations revealed that melatonin synergized with 5-FU efficacy by simultaneous inhibiting multiple signaling pathways including activation of caspase/PARP-dependent apoptosis, induction the cell cycle arrest, suppression of the PI3K/Akt and NF-κB/iNOS signaling pathways, inhibition of MMP9 expression, and on the contrary promotion of E-cadherin expression [120]. NF‑κB, a transcription factor, exerts an important role in regulation of anti-apoptotic proteins and growth factors, and thereby promotes tumorigenesis by elevating the expression of nitric oxide synthase (iNOS) [121, 122]. In this sense, accumulating evidence indicates that inducible iNOS is markedly upregulated in certain inflammatory and cancerous tissues [123,124,125], and also capable to promote tumor development and progression [126, 127]. Moreover, the anti-tumor activity of melatonin is likely mediated in part by downregulation of iNOS expression [128, 129]. The PI3K/Akt signal transduction cascade possess anti-apoptotic activity; hence, inhibition of this pathway participates in apoptosis induction [130]. The PI3K/Akt signaling is also a target of melatonin [131, 132]. The results of a recent study indicated that survival of melanoma cells after co-treatment with melatonin was suppressed through regulation of the PI3K/Akt/mTOR pathway [133]. Moreover, increasing the expression of E-cadherin and reducing the expression of MMP9 contribute to the development of chemotherapeutic drug resistance, metastasis and cancer invasion [134, 135].
As with other investigators, Lu et al. [136]. assessed the impact of co-treatment with melatonin on the sensitivity to 5-FU in esophageal squamous cell carcinoma (ESCC) in a mouse model. They concluded that melatonin improved the suppression of cell proliferation, migration, invasion, and promoted apoptosis in a mitochondria-dependent manner in ESCC cells in vitro, as well as inhibition of tumor growth in vivo [136]. A probable underlying mechanism of melatonin relates to its association with the regulation of extracellular regulated protein kinase (Erk) and protein kinase B (PKB or Akt). Indeed, co-treatment with melatonin reverses the impact of 5-FU on the of activation MEK/Erk and GSK3β/Akt signaling pathway, which effectively leads to increased sensitivity to 5-FU in ESCC cells, as well as the cytotoxicity of 5-FU in ESCC [136]. Akt, activated by PI3K, enhances tumorigenesis via regulation of phosphorylation of several downstream target genes including GSK3β and mTOR [137, 138]. Phosphorylation-mediated activated Akt is over-expressed in various type tumor, which is associated with poor prognosis [138,139,140]. The genetic abnormalities in the PI3K/Akt signaling pathway is common in human cancer [137]. Also, genetic variations in PI3K/PTEN/Akt/mTOR axis were found to be predict the elevated recurrence risk of esophageal cancer after chemoradiotherapy [141]. Conversely, the overexpression of an activated Erk pathway and its role in cell proliferation and progression of head and neck squamous carcinoma, such as in ESCC cells, was detected [142, 143].
It has been reported that Erk and Akt can cooperatively modulate downstream gene expression to cell proliferation and cell cycle progression [144]. Regarding the relationship between PI3K/Akt and the Erk pathway, applying PI3K inhibitors cause MEK/Erk pathway suppression in breast cancer cells. Also, inhibition of both Akt and Erk pathways are necessary for optimal antitumoral effects [145]. Finally, it has been documented that suppression of Erk and Akt pathways are, in part, responsible for tumor-suppressive functions of melatonin in breast cancer [45].
Stimulation of MT3 receptor
Pariente et al. [146] documented that melatonin potently enhanced the cytotoxic and apoptotic effect of chemotherapeutic agents including cisplatin and 5-FU in human CRC colon HT-29 cells and cervical cancer HeLa cells; this was particularly obvious in the 5-FU-challenged cells. Indeed, melatonin can to be effective in terms of promoting the tumor cell sensitivity of 5-FU through the signal transduction elicited by MT3 receptor stimulation [146]. In addition, concomitant treatments with melatonin and 5-FU cause further increased caspase-3 activation, thereby evoking the 5-FU-mediated apoptotic cell death, which also is mediated by MT3 receptor trigger [146]. MT3 is a melatonin receptor that is present in the retina, liver, heart, intestine, kidney, muscle, and fat and acts as a fluid pressure regulator and detoxification enzyme. It may have neuroprotective and oncostatic effects. [28, 29].
Regulate cancer stem cells
Investigations conducted by Lee et al. [147] revealed that the combination of 5-FU and melatonin inhibits proliferation, promotes apoptosis and autophagy of colon cancer stem cells (CSCs), thereby suppressing the CRC progression and tumor-mediated angiogenesis; this involves targeting the PrPC-Oct4-HSPA1L axis. 5-FU and melatonin suppressed the expression of human CSC markers, particularly Oct4, by downregulating cellular prion protein (PrPC) expression, Also, PrPC and Oct4 expressions were found to be associated with human CRC metastasis [147]. The findings of this study also suggested that PrPC prevents Oct4 degradation by enhancing the binding of heat shock protein family A member 1-like (HSPA1L) to octamer-binding transcription factor 4 (Oct4) [147]. CSCs, which are capable of self-renewing, account for tumor development and maintenance, as well as treatment failure [148]. Oct4 acts is an essential transcriptional factor for self-renewal, pluripotency, and survival of CSCs, and reprogramming [149], through a potential Oct4-AKT-ABCG2 regulatory circuit [150]. Also, Oct4 gene knockdown induces apoptosis of CSCs, leading to suppressed tumor growth [151], and weakened tumorigenicity of drug-resistant cancer cells [152]. For example, pancreatic cancer cells resistant to 5-FU overexpressed the Oct4 gene [153]. The metastatic and angiogenic roles of Oct4 have also been described [154]. Growing evidence indicates that PrPC takes part in behaviors of cancer cells such as, invasion, metastasis, cell migration, proliferation and apoptosis and finally tumor survival and progression [155,156,157]. In addition, PrPC participates in cancer cell self-renewal [158]. In addition, PrPC found to be associated with chemoresistance [159]. The expression of heat shock proteins (HSPs), which are correlated to tumor cell proliferation and the inhibition of apoptosis [160], recently have been found to be associated with Oct4 and PrPC expression in tumor cells [161,162,163]. Melatonin administration promotes senescence, autophagy, and apoptosis in human CRC cells [164]. An earlier study indicated that melatonin advances apoptosis in oxaliplatin-resistant CRC cells through inhibition of PrPC [165].
Regulate immune response
A clinical trial, the results of which were published in 1995, reported that low-dose interleukin-2 (IL-2) and melatonin prolonged the one-year survival and improved the quality of life in patients with metastatic colorectal cancer (CRC), who progressed despite initial response to 5-FU [89]. In 2018, Akyuz et al. [166]. observed that treatment with melatonin improved healing following colonic anastomosis and attenuated the adverse effects of pre-operative chemotherapy with 5-FU. Histopathological examination exhibited remarkable reduction in inflammation and necrosis formation in the rat model group treated with melatonin [166]. This study also showed that serum TNF-α and IL-1β levels were significantly reduced, leading to decreased the activation and infiltration of neutrophils, which have a main role in inflammatory tissue damage, after melatonin treatment [166]. It is known that TNF-α and IL-1β play a major regulatory roles in the inflammatory response and their cytotoxic effects [167]. Under inflammatory and trauma conditions, neutrophils are stimulated and release ROS and cytokines, such as TNF-α and IL-1β [168].
Epigenic regulation
Importantly, an evaluation of the effect of melatonin on molecular mechanisms in 5-FU resistant human CRC cells provided significant results. Significant additive effects of conjugation of FU-5 therapy with melatonin led to inhibition of the cell growth, promotion of apoptosis, enhancement of 5-FU-mediated cytotoxicity and sensitivity of 5-FU resistant CRC cells. As an explanation of the underlying mechanism, these effects were manifested through depressing thymidylate synthase (TYMS) transcription and expression, which were mediated by upregulation of miR-215-5p. Hence, TYMS serves as the key direct downstream target for miR-215-5p [169]. TYMS, a substantial target for chemotherapeutic agents and a known folate-dependent catalytic enzyme, exhibits substantial effects on the production of intracellular thymidine, a fundamental precursor for DNA biosynthesis [170, 171]. Accumulating evidence has demonstrated that TYMS expression inversely associates with 5-FU sensitivity and efficacy in cancer cells. Therefore, TYMS is suggested as an important determinant for therapeutic responsiveness to 5-FU and mechanistic driver of 5-FU resistance [172,173,174]. Moreover, high expression of TYMS in tumor tissues indicates poor responsiveness to 5-FU, and thereby worse overall prognosis [175,176,177]. Previously, it was reported that TYMS mRNA includes several miRNAs, including miR-433 and miR-203 that negatively regulate TYMS expression; this in turn, promotes 5-FU chemosensitivity of cancer cells [178, 179]. Melatonin was also found to exert antitumor and anti-oxidant effects by regulating the expression of several miRNAs [180,181,182]. Additionally, the tumor inhibitory function of miR-215 was found to target NOB1 in ovarian cancer [183] and by targeting AKT serine/threonine kinase 1in breast cancer [184]. Also, miR-215 is involved in the cellular 5-FU response via regulation of the TYMS expression in tumor cells [185, 186].
Circadian changes
Baldueva et al. [187] demonstrated that oral melatonin supplementation improved the efficacy and the antitumor response to chemotherapy regimens including cyclophosphamide, adriamycin, 5-FU, and docetaxel, in HER2/neu transgenic mice model with spontaneous mammary adenocarcinomas; these therapies had been shown to be ineffective perhaps related to the inapropriate time of administration. Also, melatonin supplementation reduced toxicity and provided further improvement of cancer stabilization rates [187]. Involvement of periodic cellular gene expression by the circadian system is controlled by chronobiotic agents such as melatonin; these coordinate cellular physiology with diurnal alterations in the environment [188]. The circadian changes in cell biology influence the cellular processes (including metabolism, signaling networks) as well as have an impact on the molecular processes such as DNA repair and cell cycle in response to different treatments [189]. Since, circadian rhythms are disturbed in patients suffering from cancer, a chronobiotic such as melatonin can influence tumor responsiveness to chemotherapy drugs as well as modulating the chronopharmacokinetics and chronopharmacodynamics of anticancer agents [190,191,192,193]. Also, recent findings revealed that tumor glucose metabolism may be switched from cytosolic glycolysis to mitochondrial oxidative phosphorylation by the nighttime rise in circulating melatonin [194].
Inhibition of oncogenic factors
Similar results were obtained by Zhang et al. [195] in which the synergistic effects of melatonin and 5‑FU in combination therapy for human esophageal cancer (EC) were apparent. In this case, melatonin improved the sensitivity to 5-FU and diminished the IC50 of 5‑FU in EC cells [195]. The 5‑FU‑mediated inhibition of the cell proliferation, migration, and invasion, as well as activation of cell apoptosis in EC‑9706 and EC‑109 cells was strongly enhanced when melatonin was added with 5-FU; hence, melatonin sensitizes EC cells to 5-FU [195]. The data collected from this study also showed that melatonin and 5-FU co-treatment exerted an anti-proliferative and pro-apoptotic function through downregulating of histone‑lysine N‑methyltransferase EZH2 (EZH2) expression. In addition, it is known that EZH2 participates in EC via activation of the JAK2/STAT3 signaling pathway [195]. The expression of EZH2, an enzymatic subunit of polycomb repressive complex 2 (PRC2), is upregulated in various types of cancer cells, and also is under the influence of multiple oncogenic factors. Therefore, EZH2 plays a critical oncogenic role in numerous related processes including cancer initiation, differentiation, development, progression, and metastasis [196,197,198]. Accumulating evidence has revealed the key contributing role of EZH2 in increasing the resistance of numerous cancer cells to chemotherapeutic drugs such as 5-FU [199,200,201]. The results of earlier studies also reported that melatonin may act as a tumor inhibitor molecule in glioblastoma stem-like cells by recruiting the EZH2-related axis, which could involve multiple pathways including EZH2‑NOTCH1 [202] and AKT‑EZH2‑STAT3 signaling [203].
Conclusions
Research has shown that combination therapies may not only reduce drug resistance, but also can simultaneously provide improved anti-cancer effects such as by reducing cell proliferation, limiting tumor growth, promoting cell cycle arrest, and increasing apoptosis in cancer cells. It is noted, however, that these effects are not always apparent and this approach may only increase cancer cell toxicity, without having a substantial effect on controlling the behavior of cancer cells.
5-FU is a representative of the category of antitumor drugs and widely used chemotherapy in the management and control of cancer. In recent years, the potential of melatonin in the treatment and management of some disorders has increased because of its multiple actions as an antioxidant and as an anti-inflammatory molecule. Also, the antitumor effects of melatonin and its effects in increasing the efficiency of other therapeutic procedures such as chemotherapy, have been reported. Herein, we describe the extensive role of melatonin in combination with 5-FU in cancer treatment. The studies reviewed in this report show that melatonin may be an important cornerstone treatment for a variety of cancers, although in each case the specific mechanism have not been elucidated. Since conventional chemotherapies often have severe side effects and negatively influences the quality and outcome of life. Melatonin has also proven its benefits as an adjuvant to reduce the side effects of these toxic drugs; this makes melatonin a highly suitable ancillary treatment option. The combination treatment of 5FU with melatonin in both in vitro and in vivo cancer models increases the hope for a brighter future for the common use of combined chemotherapies. Overall, melatonin in combination with 5FU as a chemotherapy drug may improve its clinical application in cancer treatment and play a significant role as an adjunct for a variety of different tumors (Table 1 and Fig. 1). It is noted that the current evidence was mainly obtained in preclinical models of cancer, and for this reason, more studies are needed to better understand the therapeutic potential and the underlying mechanisms of melatonin combined chemotherapy. It is also necessary to further investigate the effects of melatonin in different cell signaling pathways to better define the mechanisms of these combined therapies.
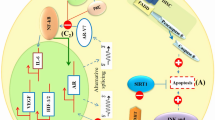
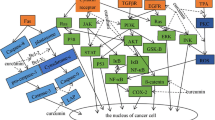
A schematic view of the combination therapy of melatonin and 5-FU in regulating the signaling pathways of cancer cells. The combination therapy of melatonin and 5-FU can lead to effects such as induction of apoptosis, increasing the sensitivity of cells to 5-FU and inhibition of cell growth through different signaling pathways. Red blocked-end arrow illustrate inhibition and black arrow show stimulation and/or activation. Blue down arrow: decrease, blue up arrow: increase. Abbreviations: ROS, Reactive oxygen species; XIAP, X-linked inhibitor of apoptosis protein; GSK3β, Glycogen synthase kinase 3β; NF-κB, Nuclear factor κB; iNOS, Inducible nitric oxide synthase; Oct4, Octamer-binding transcription factor 4 TYMS, Thymidylate synthetase
Availability of data and materials
Not applicable.
References
Hornberg JJ, Bruggeman FJ, Westerhoff HV, Lankelma J. Cancer: a systems biology disease. Biosystems. 2006;83(2–3):81–90.
Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, et al. Cancer incidence, mortality, Years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022;8(3):420–44.
Chabner BA, Roberts TG. Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5(1):65–72.
Nygren P. What is cancer chemotherapy? Acta Oncol. 2001;40(2–3):166–74.
Schirrmacher V. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment. Int J Oncol. 2019;54(2):407–19.
Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71(16):2997–3025.
Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, et al. Melatonin: pharmacology, functions and therapeutic benefits. Curr Neuropharmacol. 2017;15(3):434–43.
Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12(2):151–80.
Hardeland R. Melatonin, hormone of darkness and more–occurrence, control mechanisms, actions and bioactive metabolites. Cell Mol Life Sci. 2008;65(13):2001–18.
Hardeland R, Poeggeler B. Non-vertebrate melatonin. J Pineal Res. 2003;34(4):233–41.
Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, et al. Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res. 2003;34(1):75–8.
Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J, et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc Natl Acad Sci U S A. 2017;114(38):E7997-e8006.
Reiter RJ, Sharma R, Rosales-Corral S, de Campos Zuccari DAP, de Almeida Chuffa LG. Melatonin: a mitochondrial resident with a diverse skill set. Life Sci. 2022;301: 120612.
Tomás-Zapico C, Coto-Montes A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J Pineal Res. 2005;39(2):99–104.
Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, et al. Melatonin synthesis and function: evolutionary history in animals and plants. Front Endocrinol (Lausanne). 2019;10:249.
Bondy SC, Campbell A. Mechanisms underlying tumor suppressive properties of melatonin. Int J Mol Sci. 2018;19(8):2205.
Carrillo-Vico A, Reiter RJ, Lardone PJ, Herrera JL, Fernández-Montesinos R, Guerrero JM, et al. The modulatory role of melatonin on immune responsiveness. Curr Opin Investig Drugs. 2006;7(5):423.
Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36(1):1–9.
Sainz RM, Mayo JC, Uría H, Kotler M, Antolfn I, Rodriguez C, et al. The pineal neurohormone melatonin prevents in vivo and in vitro apoptosis in thymocytes. J Pineal Res. 1995;19(4):178–88.
Naranjo M, Guerrero J, Rubio A, Lardone P, Carrillo-Vico A, Carrascosa-Salmoral M, et al. Melatonin biosynthesis in the thymus of humans and rats. Cell Mol Life Sci. 2007;64(6):781–90.
Hardeland R. Aging, melatonin, and the pro-and anti-inflammatory networks. Int J Mol Sci. 2019;20(5):1223.
Liu S, Madu CO, Lu Y. The role of melatonin in cancer development. Oncomedicine. 2018;3(1):37–47.
Moradkhani F, Moloudizargari M, Fallah M, Asghari N, Heidari Khoei H, Asghari MH. Immunoregulatory role of melatonin in cancer. J Cell Physiol. 2020;235(2):745–57.
González-González A, Mediavilla MD, Sánchez-Barceló EJ. Melatonin: a molecule for reducing breast cancer risk. Molecules (Basel, Switzerland). 2018;23(2):336.
Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L, et al. Melatonin: an inhibitor of breast cancer. Endocr Relat Cancer. 2015;22(3):R183–204.
Cristofanon S, Uguccioni F, Cerella C, Radogna F, Dicato M, Ghibelli L, et al. Intracellular prooxidant activity of melatonin induces a survival pathway involving NF-κB activation. Ann N Y Acad Sci. 2009;1171(1):472–8.
Tobeiha M, Mirazimi MA, Dashti F, Amiri A, Khan P, Asemi Z, et al. Evidence for the benefits of melatonin in cardiovascular disease. Frontiers in cardiovascular medicine. 2022:1409.
Conway S, Drew JE, Mowat ES, Barrett P, Delagrange P, Morgan PJ. Chimeric melatonin mt1 and melatonin-related receptors: Identification of domains and residues participating in ligand binding and receptor activation of the melatonin mt1receptor. J Biol Chem. 2000;275(27):20602–9. https://doi.org/10.3389/fcvm.2022.888319.
Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351(2):152–66.
Lissoni P, Rovelli F, Malugani F, Bucovec R, Conti A, Maestroni G. Anti-angiogenic activity of melatonin in advanced cancer patients. Neuroendocrinol Lett. 2001;22(1):45–8.
Chottanapund S, Van Duursen MB, Navasumrit P, Hunsonti P, Timtavorn S, Ruchirawat M, et al. Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol In Vitro. 2014;28(7):1215–21.
Proietti S, Cucina A, D’Anselmi F, Dinicola S, Pasqualato A, Lisi E, et al. Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFβ-1-dependent growth inhibition of breast cancer cells. J Pineal Res. 2011;50(2):150–8.
Sadoughi F, Maleki Dana P, Homayoonfal M, Sharifi M, Asemi Z. Molecular basis of melatonin protective effects in metastasis: a novel target of melatonin. Biochimie. 2022;202:15–25.
Targhazeh N, Reiter RJ, Rahimi M, Qujeq D, Yousefi T, Shahavi MH, et al. Oncostatic activities of melatonin: Roles in cell cycle, apoptosis, and autophagy. Biochimie. 2022;200:44–59.
Ordoñez R, Fernández A, Prieto-Domínguez N, Martínez L, García-Ruiz C, Fernández-Checa JC, et al. Ceramide metabolism regulates autophagy and apoptotic cell death induced by melatonin in liver cancer cells. J Pineal Res. 2015;59(2):178–89.
Stevens RG. Electric power use and breast cancer: a hyptothesis. Am J Epidemiol;(United States). 1987;125(4):556-61.
Menéndez-Menéndez J, Martínez-Campa C. Melatonin: an anti-tumor agent in hormone-dependent cancers. Int J Endocrinol. 2018;2018:3271948.
Suwanjang W, Phansuwan-Pujito P, Govitrapong P, Chetsawang B. The protective effect of melatonin on methamphetamine-induced calpain-dependent death pathway in human neuroblastoma SH-SY5Y cultured cells. J Pineal Res. 2010;48(2):94–101.
Proietti S, Cucina A, Reiter RJ, Bizzarri M. Molecular mechanisms of melatonin’s inhibitory actions on breast cancers. Cell Mol Life Sci. 2013;70(12):2139–57.
Ferreira C da S, Maganhin CC, Simões R dos S, Girão MJ, Baracat EC, Soares JM, Jr. Melatonin: cell death modulator. Rev da Assoc Med Bras (1992). 2010;56(6):715–8.
Sánchez-Hidalgo M, Guerrero JM, Villegas I, Packham G, de la Lastra CA. Melatonin, a natural programmed cell death inducer in cancer. Curr Med Chem. 2012;19(22):3805–21.
Liu R, Wang HL, Deng MJ, Wen XJ, Mo YY, Chen FM, et al. Melatonin inhibits reactive oxygen species-driven proliferation, epithelial-mesenchymal transition, and vasculogenic mimicry in oral. Cancer. 2018;2018:3510970.
Proietti S, Cucina A, Dobrowolny G, D’Anselmi F, Dinicola S, Masiello MG, et al. Melatonin down-regulates MDM 2 gene expression and enhances p53 acetylation in MCF-7 cells. J Pineal Res. 2014;57(1):120–9.
Song J, Ma SJ, Luo JH, Liu H, Li L, Zhang ZG, et al. Downregulation of AKT and MDM2, melatonin induces apoptosis in AGS and MGC803 Cells. Anat Rec. 2019;302(9):1544–51.
Wang J, Xiao X, Zhang Y, Shi D, Chen W, Fu L, et al. Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J Pineal Res. 2012;53(1):77–90.
Sánchez-Hidalgo M, Lee M, de la Lastra CA, Guerrero JM, Packham G. Melatonin inhibits cell proliferation and induces caspase activation and apoptosis in human malignant lymphoid cell lines. J Pineal Res. 2012;53(4):366–73.
Bejarano I, Redondo PC, Espino J, Rosado JA, Paredes SD, Barriga C, et al. Melatonin induces mitochondrial-mediated apoptosis in human myeloid HL-60 cells. J Pineal Res. 2009;46(4):392–400.
Trubiani O, Recchioni R, Moroni F, Pizzicannella J, Caputi S, Di Primio R. Melatonin provokes cell death in human B-lymphoma cells by mitochondrial-dependent apoptotic pathway activation. J Pineal Res. 2005;39(4):425–31.
Long F, Dong C, Jiang K, Xu Y, Chi X, Sun D, et al. Melatonin enhances the anti-tumor effect of sorafenib via AKT/p27-mediated cell cycle arrest in hepatocarcinoma cell lines. RSC Adv. 2017;7(34):21342–51.
El-Missiry MA, Abd El-Aziz AF. Influence of melatonin on proliferation and antioxidant system in Ehrlich ascites carcinoma cells. Cancer Lett. 2000;151(2):119–25.
Mi L, Kuang H. Melatonin regulates cisplatin resistance and glucose metabolism through hippo signaling in hepatocellular carcinoma cells. Cancer Manag Res. 2020;12:1863–74.
Ma Q, Reiter RJ, Chen Y. Role of melatonin in controlling angiogenesis under physiological and pathological conditions. Angiogenesis. 2020;23(2):91–104.
Cho SY, Lee HJ, Jeong SJ, Lee HJ, Kim HS, Chen CY, et al. Sphingosine kinase 1 pathway is involved in melatonin-induced HIF-1α inactivation in hypoxic PC-3 prostate cancer cells. J Pineal Res. 2011;51(1):87–93.
Liu H, Zhu Y, Zhu H, Cai R, Wang KF, Song J, et al. Role of transforming growth factor β1 in the inhibition of gastric cancer cell proliferation by melatonin in vitro and in vivo. Oncol Rep. 2019;42(2):753–62.
León J, Casado J, Jiménez Ruiz SM, Zurita MS, González-Puga C, Rejón JD, et al. Melatonin reduces endothelin-1 expression and secretion in colon cancer cells through the inactivation of FoxO-1 and NF-κβ. J Pineal Res. 2014;56(4):415–26.
Bantounou M, Plascevic J, Galley HF. Melatonin and related compounds: antioxidant and anti-inflammatory actions. MDPI. 2022;11:532.
Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51(1):1–16.
Zhang HM, Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res. 2014;57(2):131–46.
Mortezaee K, Najafi M, Farhood B, Ahmadi A, Potes Y, Shabeeb D, et al. Modulation of apoptosis by melatonin for improving cancer treatment efficiency: An updated review. Life Sci. 2019;228:228–41.
Bizzarri M, Proietti S, Cucina A, Reiter RJ. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: a review. Expert Opin Ther Targets. 2013;17(12):1483–96.
Martín M, Macías M, León J, Escames G, Khaldy H, Acuña-Castroviejo D. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int J Biochem Cell Biol. 2002;34(4):348–57.
Ammar OA, El-Missiry MA, Othman AI, Amer ME. Melatonin is a potential oncostatic agent to inhibit HepG2 cell proliferation through multiple pathways. Heliyon. 2022;8(1): e08837.
Mihanfar A, Yousefi B, Ghazizadeh Darband S, Sadighparvar S, Kaviani M, Majidinia M. Melatonin increases 5-flurouracil-mediated apoptosis of colorectal cancer cells through enhancing oxidative stress and downregulating survivin and XIAP. Bioimpacts. 2021;11(4):253–61.
Bułdak RJ, Pilc-Gumuła K, Bułdak Ł, Witkowska D, Kukla M, Polaniak R, et al. Effects of ghrelin, leptin and melatonin on the levels of reactive oxygen species, antioxidant enzyme activity and viability of the HCT 116 human colorectal carcinoma cell line. Mol Med Rep. 2015;12(2):2275–82.
Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61(3):253–78.
Mehrzadi S, Hemati K, Reiter RJ, Hosseinzadeh A. Mitochondrial dysfunction in age-related macular degeneration: melatonin as a potential treatment. Expert Opin Ther Targets. 2020;24(4):359–78.
Fernandez-Gil BI, Guerra-Librero A, Shen YQ, Florido J, Martínez-Ruiz L, García-López S, et al. Melatonin enhances cisplatin and radiation cytotoxicity in head and neck squamous cell carcinoma by stimulating mitochondrial ROS generation. Apoptosis Autophagy. 2019;2019:7187128.
Trivedi PP, Jena GB, Tikoo KB, Kumar V. Melatonin modulated autophagy and Nrf2 signaling pathways in mice with colitis-associated colon carcinogenesis. Mol Carcinog. 2016;55(3):255–67.
Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–8.
Miura K, Kinouchi M, Ishida K, Fujibuchi W, Naitoh T, Ogawa H, et al. 5-fu metabolism in cancer and orally-administrable 5-fu drugs. Cancers. 2010;2(3):1717–30.
Sanduja M, Gupta J, Virmani T. Recent advancements in Uracil and 5-Fluorouracil hybrids as potential anticancer agents: a review. J Appl Pharm Sci. 2020;10(2):129–46.
Chalabi-Dchar M, Fenouil T, Machon C, Vincent A, Catez F, Marcel V, et al. A novel view on an old drug, 5-fluorouracil: an unexpected RNA modifier with intriguing impact on cancer cell fate. NAR Cancer. 2021;3(3):zcab032.
Zhang N, Yin Y, Xu SJ, Chen WS. 5-Fluorouracil: mechanisms of resistance and reversal strategies. Mol (Basel, Switzerland). 2008;13(8):1551–69.
Zhao X, Yu YT. Incorporation of 5-fluorouracil into U2 snRNA blocks pseudouridylation and pre-mRNA splicing in vivo. Nucleic Acids Res. 2007;35(2):550–8.
Wang W, Cassidy J, O’Brien V, Ryan KM, Collie-Duguid E. Mechanistic and predictive profiling of 5-Fluorouracil resistance in human cancer cells. Can Res. 2004;64(22):8167–76.
Denise C, Paoli P, Calvani M, Taddei ML, Giannoni E, Kopetz S, et al. 5-fluorouracil resistant colon cancer cells are addicted to OXPHOS to survive and enhance stem-like traits. Oncotarget. 2015;6(39):41706.
Mader RM, Müller M, Steger GG. Resistance to 5-fluorouracil. General Pharmacol Vasc Syst. 1998;31(5):661–6.
Sasada S, Miyata Y, Tsutani Y, Tsuyama N, Masujima T, Hihara J, et al. Metabolomic analysis of dynamic response and drug resistance of gastric cancer cells to 5-fluorouracil. Oncol Rep. 2013;29(3):925–31.
Linnekamp JF, Wang X, Medema JP, Vermeulen L. Colorectal cancer heterogeneity and targeted therapy: a case for molecular disease subtypes. Can Res. 2015;75(2):245–9.
Gil-Martín E, Egea J, Reiter RJ, Romero A. The emergence of melatonin in oncology: focus on colorectal cancer. Med Res Rev. 2019;39(6):2239–85.
Cutando A, Lopez-Valverde A, Arias-Santiago S, De Vicente J, De Diego RG. Role of melatonin in cancer treatment. Anticancer Res. 2012;32(7):2747–53.
Talib WH, Alsayed AR, Abuawad A, Daoud S, Mahmod AI. Melatonin in cancer treatment: current knowledge and future opportunities. Molecules. 2021;26(9):2506.
Asghari MH, Ghobadi E, Moloudizargari M, Fallah M, Abdollahi M. Does the use of melatonin overcome drug resistance in cancer chemotherapy? Life Sci. 2018;196:143–55.
Ju HQ, Li H, Tian T, Lu YX, Bai L, Chen LZ, et al. Melatonin overcomes gemcitabine resistance in pancreatic ductal adenocarcinoma by abrogating nuclear factor-κ B activation. J Pineal Res. 2016;60(1):27–38.
Uguz AC, Cig B, Espino J, Bejarano I, Naziroglu M, Rodríguez AB, et al. Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J Pineal Res. 2012;53(1):91–8.
Farez MF, Calandri IL, Correale J, Quintana FJ. Anti-inflammatory effects of melatonin in multiple sclerosis. BioEssays. 2016;38(10):1016–26.
Majidinia M, Reiter RJ, Shakouri SK, Mohebbi I, Rastegar M, Kaviani M, et al. The multiple functions of melatonin in regenerative medicine. Ageing Res Rev. 2018;45:33–52.
Mediavilla MD, Sanchez-Barcelo EJ, Tan DX, Manchester L, Reiter RJ. Basic mechanisms involved in the anti-cancer effects of melatonin. Curr Med Chem. 2010;17(36):4462–81.
Barni S, Lissoni P, Cazzaniga M, Ardizzoia A, Meregalli S, Fossati V, et al. A randomized study of low-dose subcutaneous lnterleukin-2 plus melatonin versus supportive care alone in metastatic colorectal cancer patients progressing under 5-fluorouracil and folates. Oncology. 1995;52(3):243–5.
Reiter RJ, Tan DX, Sainz RM, Mayo JC, Lopez-Burillo S. Melatonin: reducing the toxicity and increasing the efficacy of drugs. J Pharm Pharmacol. 2002;54(10):1299–321.
Büyükavci M, Özdemir Ö, Buck S, Ravindranath Y, Savaşan S. Effect of melatonin on the cytotoxicity of chemotherapeutic drugs in human leukemia cells. In Vivo. 2011;25(3):405–9.
Fan L-L, Sun G-P, Wei W, Wang Z-G, Ge L, Fu W-Z, et al. Melatonin and doxorubicin synergistically induce cell apoptosis in human hepatoma cell lines. World J Gastroenterol: WJG. 2010;16(12):1473.
Casado-Zapico S, Rodriguez-Blanco J, García-Santos G, Martín V, Sánchez-Sánchez AM, Antolín I, et al. Synergistic antitumor effect of melatonin with several chemotherapeutic drugs on human Ewing sarcoma cancer cells: potentiation of the extrinsic apoptotic pathway. J Pineal Res. 2010;48(1):72–80.
Lissoni P. Biochemotherapy with standard chemotherapies plus the pineal hormone melatonin in the treatment of advanced solid neoplasms. Pathol Biol (Paris). 2007;55(3–4):201–4.
Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95.
Negroni A, Cucchiara S, Stronati L. Apoptosis, necrosis, and necroptosis in the gut and intestinal homeostasis. Mediat Inflamm. 2015;2015.
Mihanfar A, Yousefi B, Darband SG, Sadighparvar S, Kaviani M, Majidinia M. Melatonin increases 5-flurouracil-mediated apoptosis of colorectal cancer cells through enhancing oxidative stress and downregulating survivin and XIAP. Bioimpacts. 2021;11(4):253.
Rigas A, Dervenis C, Giannakou N, Kozoni V, Shiff SJ, Rigas B. Selective induction of colon cancer cell apoptosis by 5-fluorouracil in humans. Cancer Invest. 2002;20(5–6):657–65.
Russo A, Saide A, Cagliani R, Cantile M, Botti G, Russo G. rpL3 promotes the apoptosis of p53 mutated lung cancer cells by down-regulating CBS and NFκB upon 5-FU treatment. Sci Rep. 2016;6(1):1–13.
Tsuruo T, Naito M, Tomida A, Fujita N, Mashima T, Sakamoto H, et al. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003;94(1):15–21.
Yousefi B, Azimi A, Majidinia M, Shafiei-Irannejad V, Badalzadeh R, Baradaran B, et al. Balaglitazone reverses P-glycoprotein-mediated multidrug resistance via upregulation of PTEN in a PPARγ-dependent manner in leukemia cells. Tumor Biol. 2017;39(10):1010428317716501.
Xu X, Zhang L, He X, Zhang P, Sun C, Xu X, et al. TGF-β plays a vital role in triple-negative breast cancer (TNBC) drug-resistance through regulating stemness, EMT and apoptosis. Biochem Biophys Res Commun. 2018;502(1):160–5.
Yang WZ, Zhou H, Yan Y. XIAP underlies apoptosis resistance of renal cell carcinoma cells. Mol Med Rep. 2018;17(1):125–30.
Cheung CHA, Chang Y-C, Lin T-Y, Cheng SM, Leung E. Anti-apoptotic proteins in the autophagic world: an update on functions of XIAP, Survivin, and BRUCE. J Biomed Sci. 2020;27(1):1–10.
Rauch A, Carlstedt A, Emmerich C, Mustafa A-HM, Göder A, Knauer SK, et al. Survivin antagonizes chemotherapy-induced cell death of colorectal cancer cells. Oncotarget. 2018;9(45):27835.
Neophytou CM, Trougakos IP, Erin N, Papageorgis P. Apoptosis deregulation and the development of cancer multi-drug resistance. Cancers. 2021;13(17):4363.
Shojaei F, Yazdani-Nafchi F, Banitalebi-Dehkordi M, Chehelgerdi M, Khorramian-Ghahfarokhi M. Trace of survivin in cancer. Eur J Cancer Prev. 2019;28(4):365–72.
Tabata M, Tsubaki M, Takeda T, Tateishi K, Tsurushima K, Imano M, et al. Dasatinib reverses drug resistance by downregulating MDR1 and Survivin in Burkitt lymphoma cells. BMC Complement Med Ther. 2020;20(1):1–9.
Kang DW, Choi CH, Park JY, Kang SK, Kim YK. Ciglitazone induces caspase-independent apoptosis through down-regulation of XIAP and survivin in human glioma cells. Neurochem Res. 2008;33(3):551–61.
Hehlgans S, Petraki C, Reichert S, Cordes N, Rödel C, Rödel F. Double targeting of Survivin and XIAP radiosensitizes 3D grown human colorectal tumor cells and decreases migration. Radiother Oncol. 2013;108(1):32–9.
Ruvolo P, Deng X, May W. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia. 2001;15(4):515–22.
Yin C, Knudson CM, Korsmeyer SJ, Dyke TV. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385(6617):637–40.
Pariente R, Bejarano I, Rodríguez AB, Pariente JA, Espino J. Melatonin increases the effect of 5-fluorouracil-based chemotherapy in human colorectal adenocarcinoma cells in vitro. Mol Cell Biochem. 2018;440(1):43–51.
Lin S, Li Y, Zamyatnin AA Jr, Werner J, Bazhin AV. Reactive oxygen species and colorectal cancer. J Cell Physiol. 2018;233(7):5119–32.
Simon H-U, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5(5):415–8.
Koh W, Jeong SJ, Lee HJ, Ryu HG, Lee EO, Ahn KS, et al. Melatonin promotes puromycin-induced apoptosis with activation of caspase-3 and 5′-adenosine monophosphate-activated kinase-alpha in human leukemia HL-60 cells. J Pineal Res. 2011;50(4):367–73.
Kim JH, Jeong SJ, Kim B, Yun SM, Choi DY, Kim SH. Melatonin synergistically enhances cisplatin-induced apoptosis via the dephosphorylation of ERK/p90 ribosomal S6 kinase/heat shock protein 27 in SK-OV-3 cells. J Pineal Res. 2012;52(2):244–52.
Lissoni P, Barni S, Mandala M, Ardizzoia A, Paolorossi F, Vaghi M, et al. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur J Cancer. 1999;35(12):1688–92.
Majsterek I, Gloc E, Blasiak J, Reiter RJ. A comparison of the action of amifostine and melatonin on DNA-damaging effects and apoptosis induced by idarubicin in normal and cancer cells. J Pineal Res. 2005;38(4):254–63.
Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang Z, et al. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI 3K/AKT and NF-κB/iNOS signaling pathways. J Pineal Res. 2017;62(2): e12380.
Shaked H, Hofseth LJ, Chumanevich A, Chumanevich AA, Wang J, Wang Y, et al. Chronic epithelial NF-κB activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. Proc Natl Acad Sci. 2012;109(35):14007–12.
Kim YI, Park SW, Yoon YK, Lee KW, Lee JH, Woo HJ, et al. Orostachys japonicus inhibits the expression of MMP-2 and MMP-9 mRNA and modulates the expression of iNOS and COX-2 genes in human PMA-differentiated THP-1 cells via inhibition of NF-κB and MAPK activation. Mol Med Rep. 2015;12(1):657–62.
Papaevangelou E, Whitley GS, Johnstone AP, Robinson SP, Howe FA. Investigating the role of tumour cell derived i NOS on tumour growth and vasculature in vivo using a tetracycline regulated expression system. Int J Cancer. 2016;138(11):2678–87.
Cianchi F, Cortesini C, Bechi P, Fantappiè O, Messerini L, Vannacci A, et al. Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology. 2001;121(6):1339–47.
Klimp AH, Hollema H, Kempinga C, van der Zee AG, de Vries EG, Daemen T. Expression of cyclooxygenase-2 and inducible nitric oxide synthase in human ovarian tumors and tumor-associated macrophages. Cancer Res. 2001;61(19):7305–9.
Yang L, Wang Y, Guo L, Wang L, Chen W, Shi B. The expression and correlation of iNOS and p53 in oral squamous cell carcinoma. BioMed Res Int. 2015;2015.
Siegert A, Rosenberg C, Schmitt W, Denkert C, Hauptmann S. Nitric oxide of human colorectal adenocarcinoma cell lines promotes tumour cell invasion. Br J Cancer. 2002;86(8):1310–5.
Kang YS, Kang YG, Park HJ, Wee HJ, Jang HO, Bae MK, et al. Melatonin inhibits visfatin-induced inducible nitric oxide synthase expression and nitric oxide production in macrophages. J Pineal Res. 2013;55(3):294–303.
Yi C, Zhang Y, Yu Z, Xiao Y, Wang J, Qiu H, et al. Melatonin enhances the anti-tumor effect of fisetin by inhibiting COX-2/iNOS and NF-κB/p300 signaling pathways. PLoS ONE. 2014;9(7): e99943.
Chang F, Lee J, Navolanic P, Steelman L, Shelton J, Blalock W, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17(3):590–603.
Beker MC, Caglayan B, Caglayan AB, Kelestemur T, Yalcin E, Caglayan A, et al. Interaction of melatonin and Bmal1 in the regulation of PI3K/AKT pathway components and cellular survival. Sci Rep. 2019;9(1):1–17.
Li Y, Guo Y, Fan Y, Tian H, Li K, Mei X. Melatonin enhances autophagy and reduces apoptosis to promote locomotor recovery in spinal cord injury via the PI3K/AKT/mTOR signaling pathway. Neurochem Res. 2019;44(8):2007–19.
Kim HS, Kim T-J, Yoo Y-M. Melatonin combined with endoplasmic reticulum stress induces cell death via the PI3K/Akt/mTOR pathway in B16F10 melanoma cells. PLoS ONE. 2014;9(3): e92627.
Li F, Cui H, Jin X, Gong X, Wang W, Wang J. Triptolide inhibits epithelial-mesenchymal transition and induces apoptosis in gefitinib-resistant lung cancer cells. Oncol Rep. 2020;43(5):1569–79.
Wang J-R, Gan W-J, Li X-M, Zhao Y-Y, Li Y, Lu X-X, et al. Orphan nuclear receptor Nur77 promotes colorectal cancer invasion and metastasis by regulating MMP-9 and E-cadherin. Carcinogenesis. 2014;35(11):2474–84.
Lu Y-X, Chen D-L, Wang D-S, Chen L-Z, Mo H-Y, Sheng H, et al. Melatonin enhances sensitivity to fluorouracil in oesophageal squamous cell carcinoma through inhibition of Erk and Akt pathway. Cell Death Dis. 2016;7(10):e2432-e.
Bartholomeusz C, Gonzalez-Angulo AM. Targeting the PI3K signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):121–30.
Li B, Li J, Xu WW, Guan XY, Qin YR, Zhang LY, et al. Suppression of esophageal tumor growth and chemoresistance by directly targeting the PI3K/AKT pathway. Oncotarget. 2014;5(22):11576.
Zhao H-J, Ren L-L, Wang Z-H, Sun T-T, Yu Y-N, Wang Y-C, et al. MiR-194 deregulation contributes to colorectal carcinogenesis via targeting AKT2 pathway. Theranostics. 2014;4(12):1193.
Wang C-Y, Deng J-Y, Cai X-W, Fu X-L, Li Y, Zhou X-Y, et al. High EGFR and low p-Akt expression is associated with better outcome after nimotuzumab-containing treatment in esophageal cancer patients: preliminary clinical result and testable hypothesis. Oncotarget. 2015;6(21):18674.
Hildebrandt MA, Yang H, Hung M-C, Izzo JG, Huang M, Lin J, et al. Genetic variations in the PI3K/PTEN/AKT/mTOR pathway are associated with clinical outcomes in esophageal cancer patients treated with chemoradiotherapy. J Clin Oncol. 2009;27(6):857.
Albanell J, Codony-Servat J, Rojo F, Del Campo JM, Sauleda S, Anido J, et al. Activated extracellular signal-regulated kinases: association with epidermal growth factor receptor/transforming growth factor α expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res. 2001;61(17):6500–10.
Zhang J, Zhi H, Zhou C, Ding F, Luo A, Zhang X, et al. Up-regulation of fibronectin in oesophageal squamous cell carcinoma is associated with activation of the Erk pathway. J Pathol J Pathol Soc Great Br Irel. 2005;207(4):402–9.
Yu S, Cai X, Wu C, Wu L, Wang Y, Liu Y, et al. Adhesion glycoprotein CD44 functions as an upstream regulator of a network connecting ERK, AKT and Hippo-YAP pathways in cancer progression. Oncotarget. 2015;6(5):2951.
Ebi H, Costa C, Faber AC, Nishtala M, Kotani H, Juric D, et al. PI3K regulates MEK/ERK signaling in breast cancer via the Rac-GEF, P-Rex1. Proc Natl Acad Sci. 2013;110(52):21124–9.
Pariente R, Bejarano I, Espino J, Rodríguez AB, Pariente JA. Participation of MT3 melatonin receptors in the synergistic effect of melatonin on cytotoxic and apoptotic actions evoked by chemotherapeutics. Cancer Chemother Pharmacol. 2017;80(5):985–98.
Lee JH, Yun CW, Han YS, Kim S, Jeong D, Kwon HY, et al. Melatonin and 5-fluorouracil co-suppress colon cancer stem cells by regulating cellular prion protein-Oct4 axis. J Pineal Res. 2018;65(4): e12519.
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–5.
Esch D, Vahokoski J, Groves MR, Pogenberg V, Cojocaru V, Vom Bruch H, et al. A unique Oct4 interface is crucial for reprogramming to pluripotency. Nat Cell Biol. 2013;15(3):295–301.
Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu P, Chen KK, et al. Octamer 4 (Oct4) mediates chemotherapeutic drug resistance in liver cancer cells through a potential Oct4–AKT–ATP-binding cassette G2 pathway. Hepatology. 2010;52(2):528–39.
Hu T, Liu S, Breiter DR, Wang F, Tang Y, Sun S. Octamer 4 small interfering RNA results in cancer stem cell–like cell apoptosis. Cancer Res. 2008;68(16):6533–40.
Wen K, Fu Z, Wu X, Feng J, Chen W, Qian J. Oct-4 is required for an antiapoptotic behavior of chemoresistant colorectal cancer cells enriched for cancer stem cells: effects associated with STAT3/Survivin. Cancer Lett. 2013;333(1):56–65.
Izumiya M, Kabashima A, Higuchi H, Igarashi T, Sakai G, Iizuka H, et al. Chemoresistance is associated with cancer stem cell-like properties and epithelial-to-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2012;32(9):3847–53.
Su SC, Hsieh MJ, Yang WE, Chung WH, Reiter RJ, Yang SF. Cancer metastasis: Mechanisms of inhibition by melatonin. J Pineal Res. 2017;62(1): e12370.
Martin-Lannerée S, Hirsch TZ, Hernandez-Rapp J, Halliez S, Vilotte J-L, Launay J-M, et al. PrPC from stem cells to cancer. Front Cell Dev Biol. 2014;2:55.
Wang Q, Qian J, Wang F, Ma Z. Cellular prion protein accelerates colorectal cancer metastasis via the Fyn-SP1-SATB1 axis. Oncol Rep. 2012;28(6):2029–34.
Santos TG, Lopes MH, Martins VR. Targeting prion protein interactions in cancer. Prion. 2015;9(3):165–73.
Du L, Rao G, Wang H, Li B, Tian W, Cui J, et al. CD44-positive cancer stem cells expressing cellular prion protein contribute to metastatic capacity in colorectal cancer PrPc+ metastatic cancer stem cells. Cancer Res. 2013;73(8):2682–94.
Cheng Y, Tao L, Xu J, Li Q, Yu J, Jin Y, et al. CD44/cellular prion protein interact in multidrug resistant breast cancer cells and correlate with responses to neoadjuvant chemotherapy in breast cancer patients. Mol Carcinog. 2014;53(9):686–97.
Lianos GD, Alexiou GA, Mangano A, Mangano A, Rausei S, Boni L, et al. The role of heat shock proteins in cancer. Cancer Lett. 2015;360(2):114–8.
Mirzaei MR, Arababadi MK, Asadi MH, Mowla SJ. Altered expression of high molecular weight heat shock proteins after OCT4B1 suppression in human tumor cell lines. Cell J (Yakhteh). 2016;17(4):608.
Lopes M, Santos T, Rodrigues B, Queiroz-Hazarbassanov N, Cunha I, Wasilewska-Sampaio A, et al. Disruption of prion protein–HOP engagement impairs glioblastoma growth and cognitive decline and improves overall survival. Oncogene. 2015;34(25):3305–14.
Lee J, Han Y, Yoon Y, Yun C, Yun S, Kim S, et al. Role of HSPA1L as a cellular prion protein stabilizer in tumor progression via HIF-1α/GP78 axis. Oncogene. 2017;36(47):6555–67.
Hong Y, Won J, Lee Y, Lee S, Park K, Chang KT, et al. Melatonin treatment induces interplay of apoptosis, autophagy, and senescence in human colorectal cancer cells. J Pineal Res. 2014;56(3):264–74.
Lee JH, Yoon YM, Han Y-S, Yun CW, Lee SH. Melatonin promotes apoptosis of oxaliplatin-resistant colorectal cancer cells through inhibition of cellular prion protein. Anticancer Res. 2018;38(4):1993–2000.
Akyuz C, Yasar NF, Uzun O, Peker KD, Sunamak O, Duman M, et al. Effects of melatonin on colonic anastomosis healing following chemotherapy in rats. Singapore Med J. 2018;59(10):545.
Semenzato G. Tumour necrosis factor: a cytokine with multiple biological activities. Br J Cancer. 1990;61(3):354.
Hillegass L, Griswold D, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990;24(4):285–95.
Sakatani A, Sonohara F, Goel A. Melatonin-mediated downregulation of thymidylate synthase as a novel mechanism for overcoming 5-fluorouracil associated chemoresistance in colorectal cancer cells. Carcinogenesis. 2019;40(3):422–31.
Carreras CW, Santi DV. The catalytic mechanism and structure of thymidylate synthase. Annu Rev Biochem. 1995;64:721–62.
Costi MP, Ferrari S, Venturelli A, Calo S, Tondi D, Barlocco D. Thymidylate synthase structure, function and implication in drug discovery. Curr Med Chem. 2005;12(19):2241–58.
Peters GJ, Backus H, Freemantle S, Van Triest B, Codacci-Pisanelli G, Van der Wilt C, et al. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta (BBA) Mol Basis Dis. 2002;1587(2–3):194–205.
Mirjolet J, Barberi-Heyob M, Merlin J, Marchal S, Etienne M, Milano G, et al. Thymidylate synthase expression and activity: relation to S-phase parameters and 5-fluorouracil sensitivity. Br J Cancer. 1998;78(1):62–8.
Pullarkat S, Stoehlmacher J, Ghaderi V, Xiong Y, Ingles S, Sherrod A, et al. Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. Pharmacogenomics J. 2001;1(1):65–70.
Johnston PG, Lenz H-J, Leichman CG, Danenberg KD, Allegra CJ, Danenberg PV, et al. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Can Res. 1995;55(7):1407–12.
Popat S, Matakidou A, Houlston RS. Thymidylate synthase expression and prognosis in colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2004;22(3):529–36.
Subbarayan PR, Lee K, Ardalan B. Arsenic trioxide suppresses thymidylate synthase in 5-FU-resistant colorectal cancer cell line HT29 In Vitro re-sensitizing cells to 5-FU. Anticancer Res. 2010;30(4):1157–62.
Gotanda K, Hirota T, Matsumoto N, Ieiri I. MicroRNA-433 negatively regulates the expression of thymidylate synthase (TYMS) responsible for 5-fluorouracil sensitivity in HeLa cells. BMC Cancer. 2013;13(1):1–8.
Li T, Gao F, Zhang X-P. miR-203 enhances chemosensitivity to 5-fluorouracil by targeting thymidylate synthase in colorectal cancer. Oncol Rep. 2015;33(2):607–14.
Lee SE, Kim SJ, Youn JP, Hwang SY, Park CS, Park YS. MicroRNA and gene expression analysis of melatonin-exposed human breast cancer cell lines indicating involvement of the anticancer effect. J Pineal Res. 2011;51(3):345–52.
Kim YD, Hwang SL, Lee EJ, Kim HM, Chung MJ, Elfadl AK, et al. Melatonin ameliorates alcohol-induced bile acid synthesis by enhancing miR-497 expression. J Pineal Res. 2017;62(2): e12386.
Kim S-J, Kang HS, Lee J-H, Park J-H, Jung CH, Bae J-H, et al. Melatonin ameliorates ER stress-mediated hepatic steatosis through miR-23a in the liver. Biochem Biophys Res Commun. 2015;458(3):462–9.
Lin Y, Jin Y, Xu T, Zhou S, Cui M. MicroRNA-215 targets NOB1 and inhibits growth and invasion of epithelial ovarian cancer. Am J Transl Res. 2017;9(2):466.
Yao J, Zhang P, Li J, Xu W. MicroRNA-215 acts as a tumor suppressor in breast cancer by targeting AKT serine/threonine kinase 1. Oncol Lett. 2017;14(1):1097–104.
Song B, Wang Y, Titmus MA, Botchkina G, Formentini A, Kornmann M, et al. Molecular mechanism of chemoresistance by miR-215 in osteosarcoma and colon cancer cells. Mol Cancer. 2010;9(1):1–10.
Boni V, Bitarte N, Cristobal I, Zarate R, Rodriguez J, Maiello E, et al. miR-192/miR-215 influence 5-fluorouracil resistance through cell cycle-mediated mechanisms complementary to its post-transcriptional thymidilate synthase regulation miR-192/miR-215 and FU chemoresistance. Mol Cancer Ther. 2010;9(8):2265–75.
Panchenko AV, Tyndyk ML, Maydin MA, Baldueva IA, Artemyeva AS, Kruglov SS, et al. Melatonin administered before or after a cytotoxic drug increases mammary cancer stabilization rates in HER2/Neu mice. Chemotherapy. 2020;65(1–2):42–50.
Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–49.
Ashok Kumar PV, Dakup PP, Sarkar S, Modasia JB, Motzner MS, Gaddameedhi S. It’s about time: advances in understanding the circadian regulation of DNA damage and repair in carcinogenesis and cancer treatment outcomes. Yale J Biol Med. 2019;92(2):305–16.
Walton ZE, Altman BJ, Brooks RC, Dang CV. Circadian clock’s cancer connections. Ann Rev Cancer Biol. 2018;2:133–53.
Sulli G, Lam MTY, Panda S. Interplay between circadian clock and cancer: new frontiers for cancer treatment. Trends Cancer. 2019;5(8):475–94.
Lin H-H, Farkas ME. Altered circadian rhythms and breast cancer: from the human to the molecular level. Front Endocrinol. 2018;9:219.
Ozturk N, Ozturk D, Halil Kavakli I, Okyar A. Molecular aspects of circadian pharmacology and relevance for cancer chronotherapy. Int J Mol Sci. 2017;18(10):2168.
Reiter RJ, Sharma R, Ma Q, Rosales-Corral S, Acuna-Castroviejo D, Escames G. Inhibition of mitochondrial pyruvate dehydrogenase kinase: a proposed mechanism by which melatonin causes cancer cells to overcome cytosolic glycolysis, reduce tumor biomass and reverse insensitivity to chemotherapy. Melatonin Res. 2019;2(3):105–19.
Zhang M, Zhang M, Li R, Zhang R, Zhang Y. Melatonin sensitizes esophageal cancer cells to 5-fluorouracil via promotion of apoptosis by regulating EZH2 expression. Oncol Rep. 2021;45(4):1.
Yamaguchi H, Hung M-C. Regulation and role of EZH2 in cancer. Cancer Res Treat Off J Korean Cancer Assoc. 2014;46(3):209–22.
Gautam N, Kaur M, Kaur S. Structural assembly of Polycomb group protein and Insight of EZH2 in cancer progression: a review. J Cancer Res Ther. 2021;17(2):311.
Lu W, Cao F, Feng L, Song G, Chang Y, Chu Y, et al. LncRNA Snhg6 regulates the differentiation of MDSCs by regulating the ubiquitination of EZH2. J Hematol Oncol. 2021;14(1):1–4.
Deng X, Kong F, Li S, Jiang H, Dong L, Xu X, et al. A KLF4/PiHL/EZH2/HMGA2 regulatory axis and its function in promoting oxaliplatin-resistance of colorectal cancer. Cell Death Dis. 2021;12(5):1–13.
Wang C, Li X, Zhang J, Ge Z, Chen H, Hu J. EZH2 contributes to 5-FU resistance in gastric cancer by epigenetically suppressing FBXO32 expression. Onco Targets Ther. 2018;11:7853–64.
Rastgoo N, Pourabdollah M, Abdi J, Reece D, Chang H. Dysregulation of EZH2/miR-138 axis contributes to drug resistance in multiple myeloma by downregulating RBPMS. Leukemia. 2018;32(11):2471–82.
Zheng X, Pang B, Gu G, Gao T, Zhang R, Pang Q, et al. Melatonin inhibits glioblastoma stem-like cells through suppression of EZH2-NOTCH1 signaling axis. Int J Biol Sci. 2017;13(2):245.
Chen X, Hao A, Li X, Du Z, Li H, Wang H, et al. Melatonin inhibits tumorigenicity of glioblastoma stem-like cells via the AKT–EZH2–STAT3 signaling axis. J Pineal Res. 2016;61(2):208–17.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The authors confirm contributions to this paper as follows; Investigation and database searching, writing, and draft preparation: AM, MR, NH, and SDH. Review and editing and validation: RJR, M-HA and ZA. Supervision and project administration: M-HA and ZA. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mafi, A., Rezaee, M., Hedayati, N. et al. Melatonin and 5-fluorouracil combination chemotherapy: opportunities and efficacy in cancer therapy. Cell Commun Signal 21, 33 (2023). https://doi.org/10.1186/s12964-023-01047-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-023-01047-x